Recovery of Fine Rare Earth Minerals from Simulated Tin Tailings by Carrier Magnetic Separation: Selective Heterogeneous Agglomeration with Coarse Magnetite Particles
Abstract
1. Introduction
2. Materials and Methods
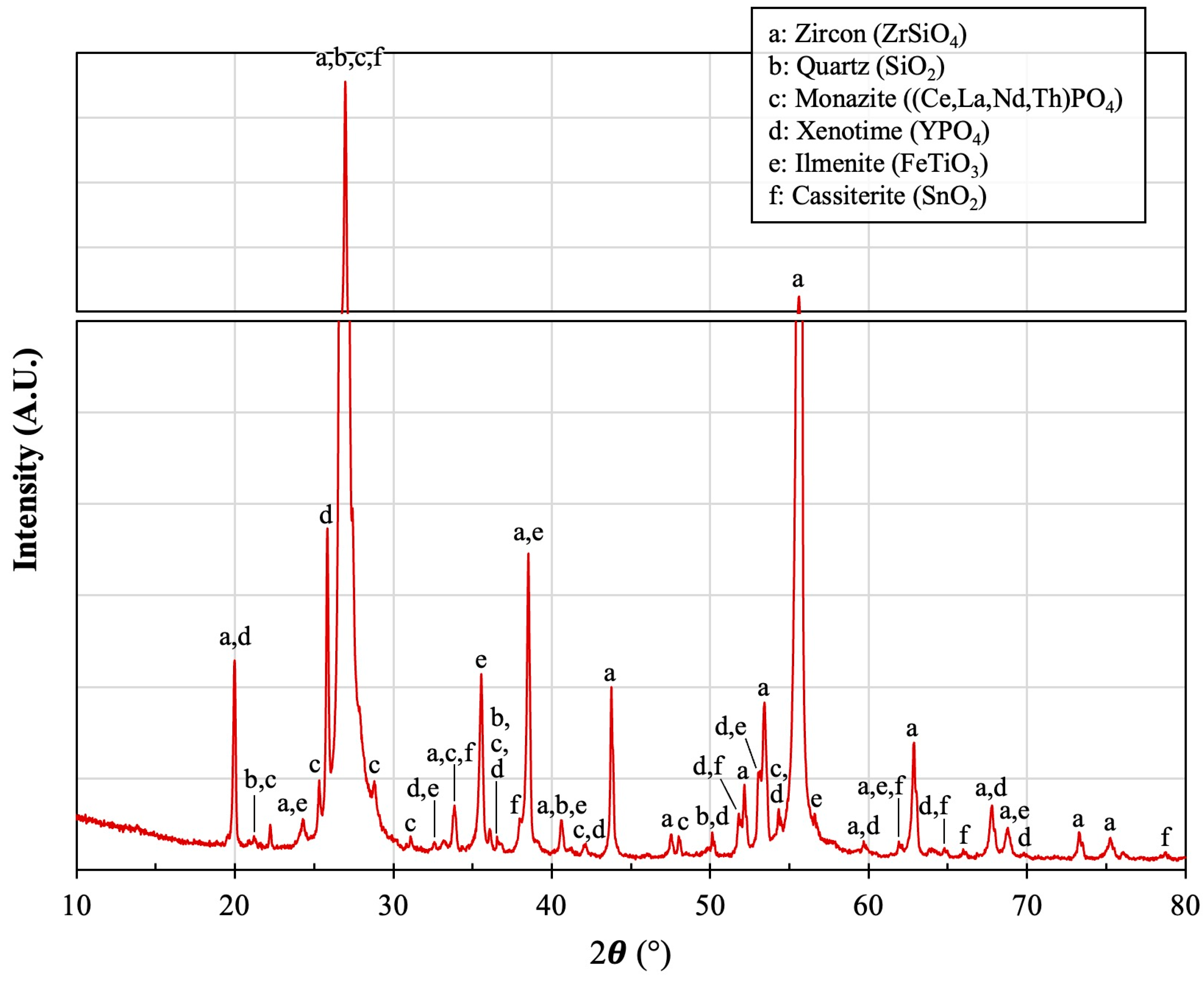
2.1. Materials
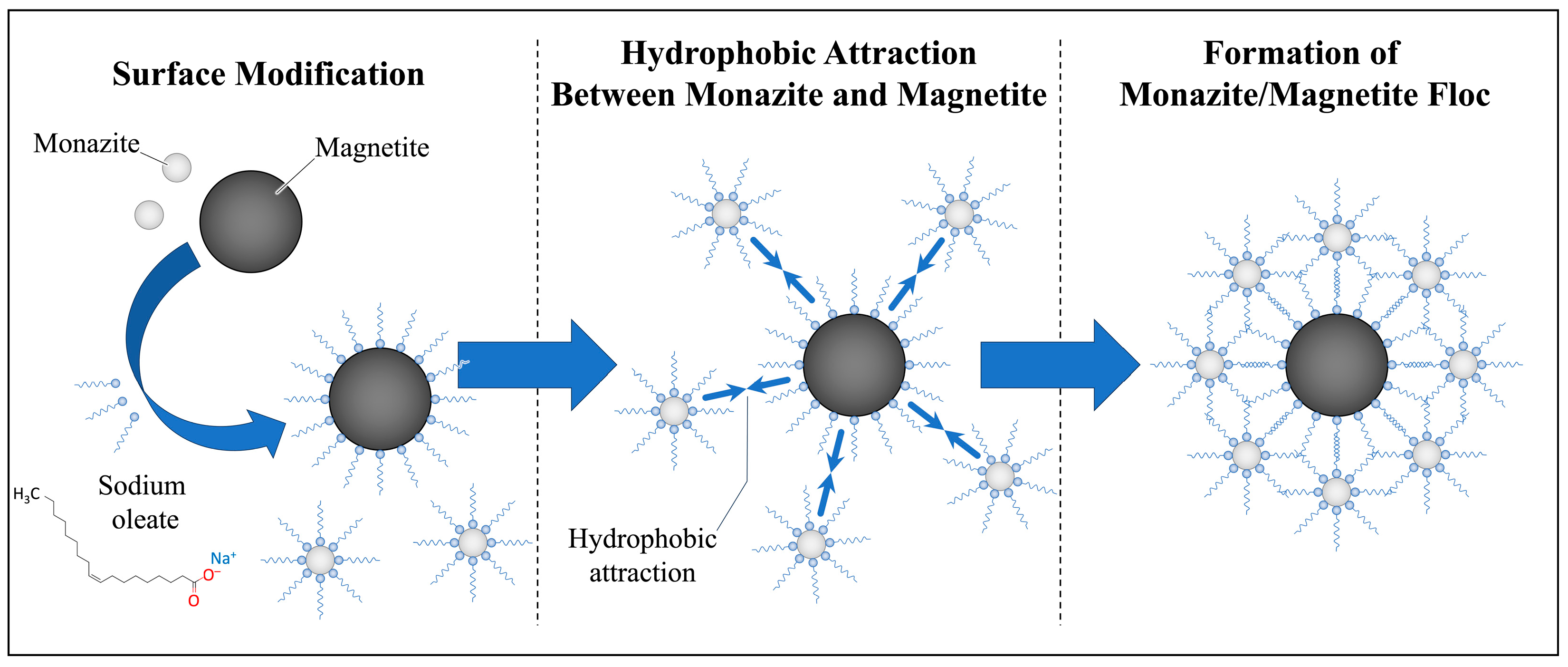
2.2. Surface Modifications for the Attachment of Fine Monazite to Coarse Magnetite
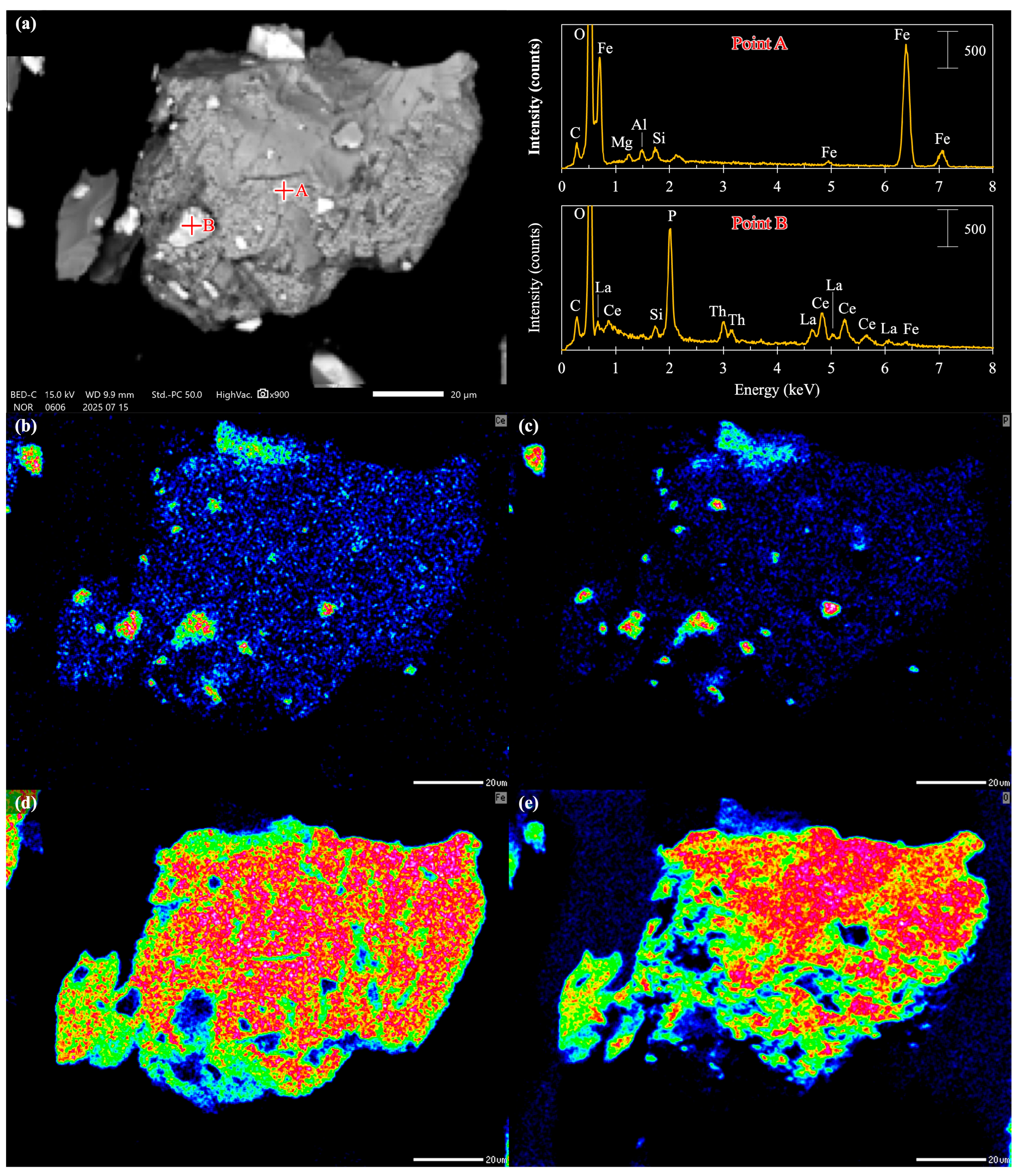
2.3. Surface Characterization and Morphological Analysis
2.4. Magnetic Separation Using a Hand-Held Magnet
2.5. Wet High-Intensity Magnetic Separation
2.6. Theoretical Calculation of Particle Aggregation via EDLVO Theory
3. Results and Discussion
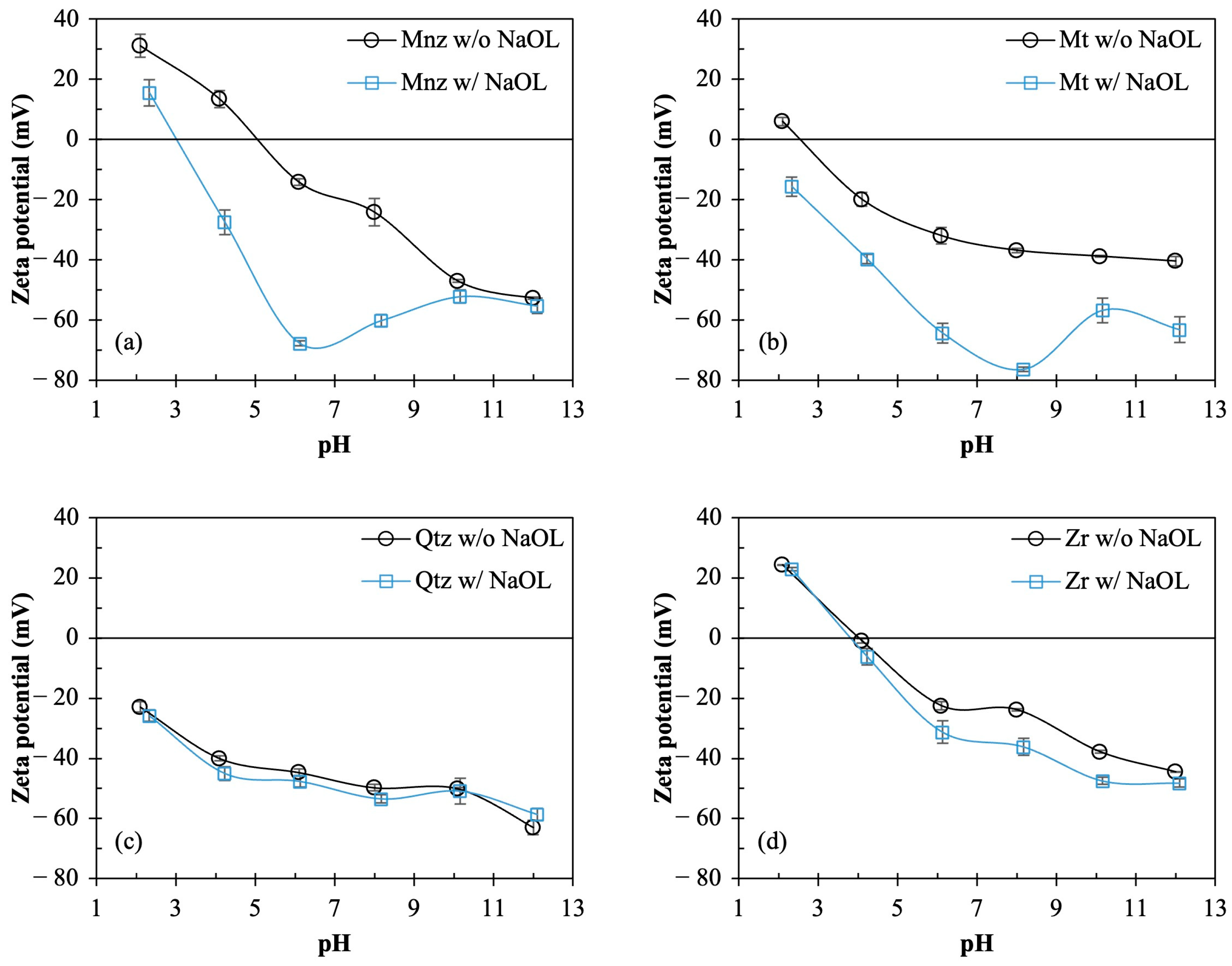
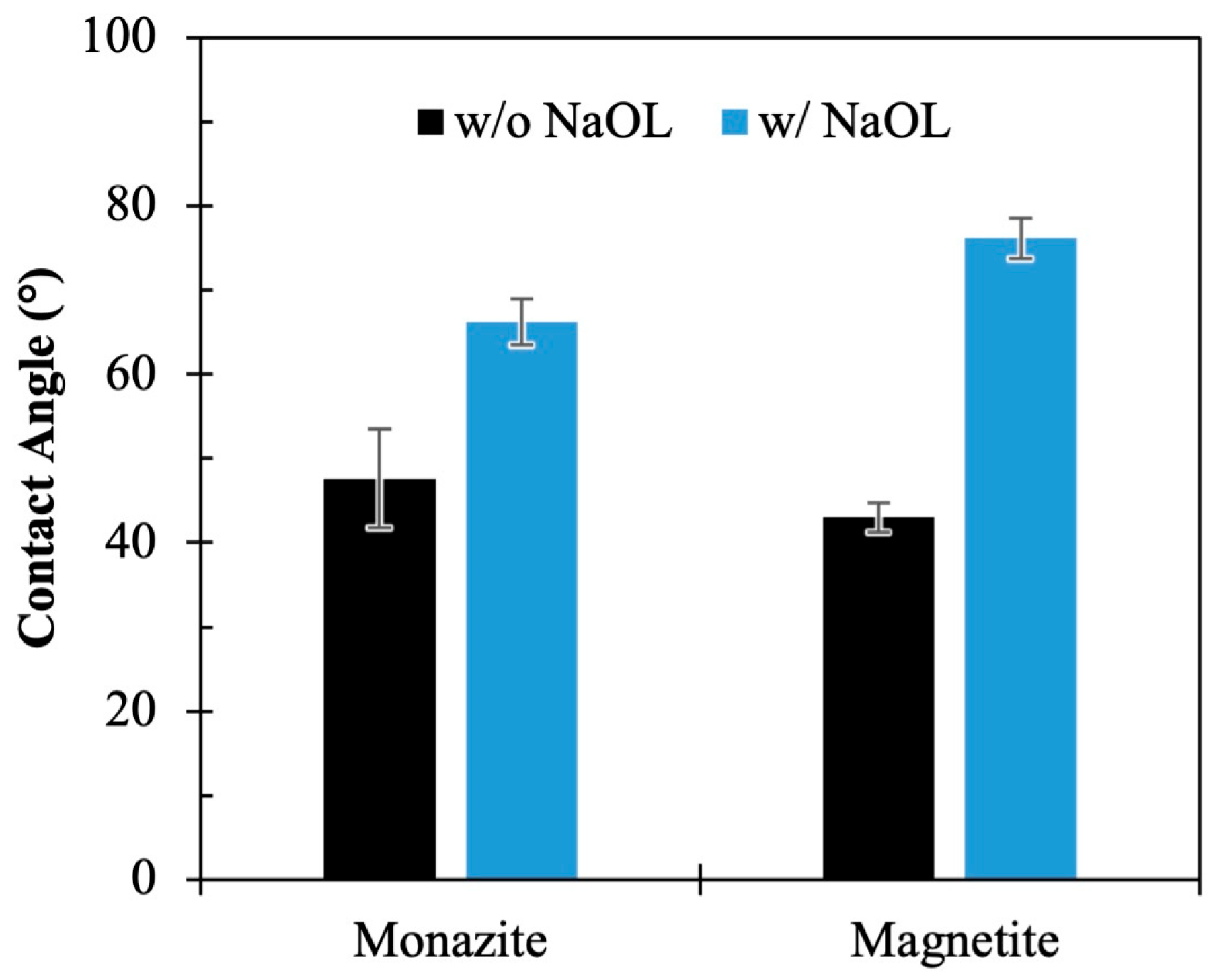
3.1. Surface Modification of Monazite and Magnetite Using Sodium Oleate
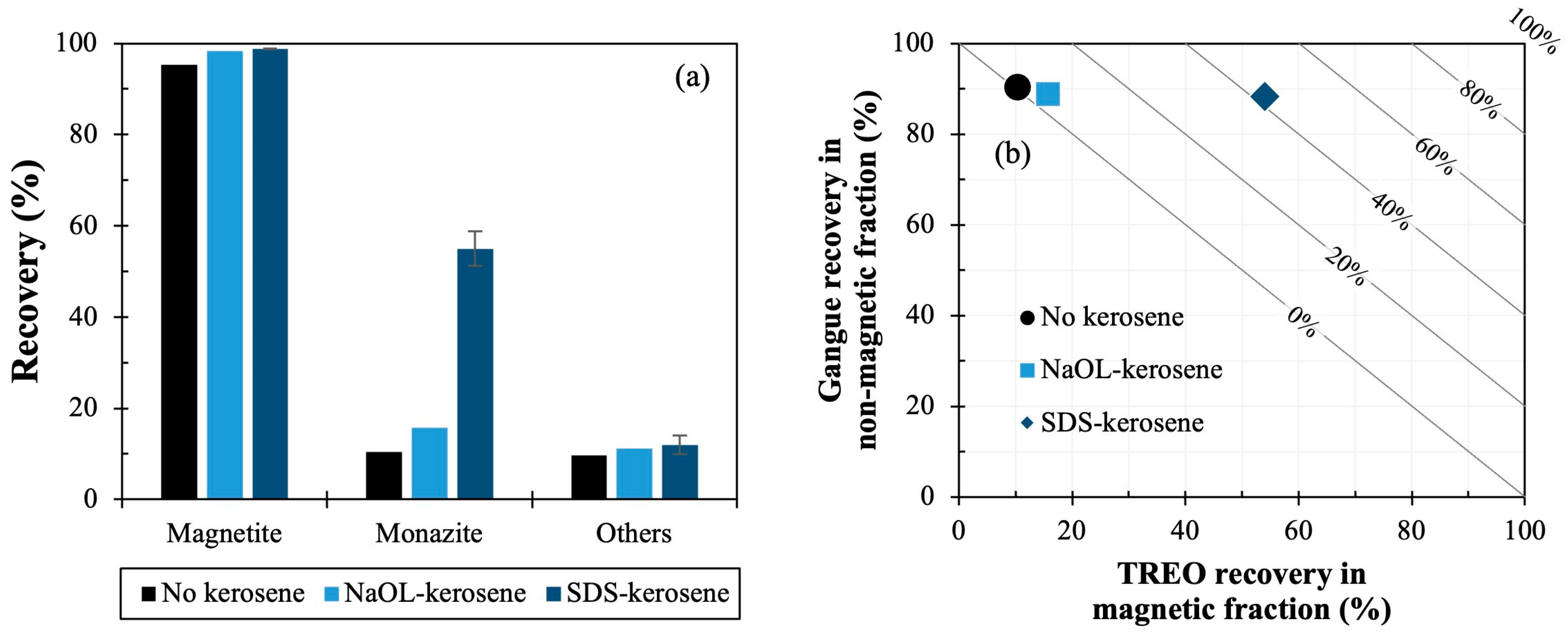
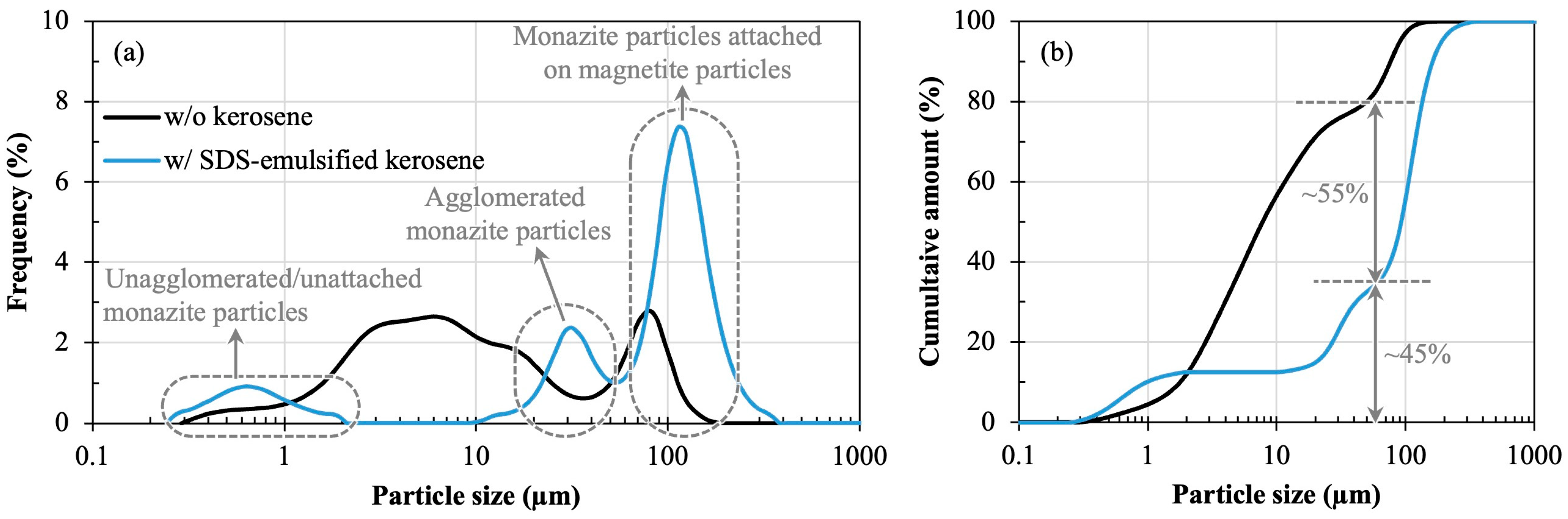
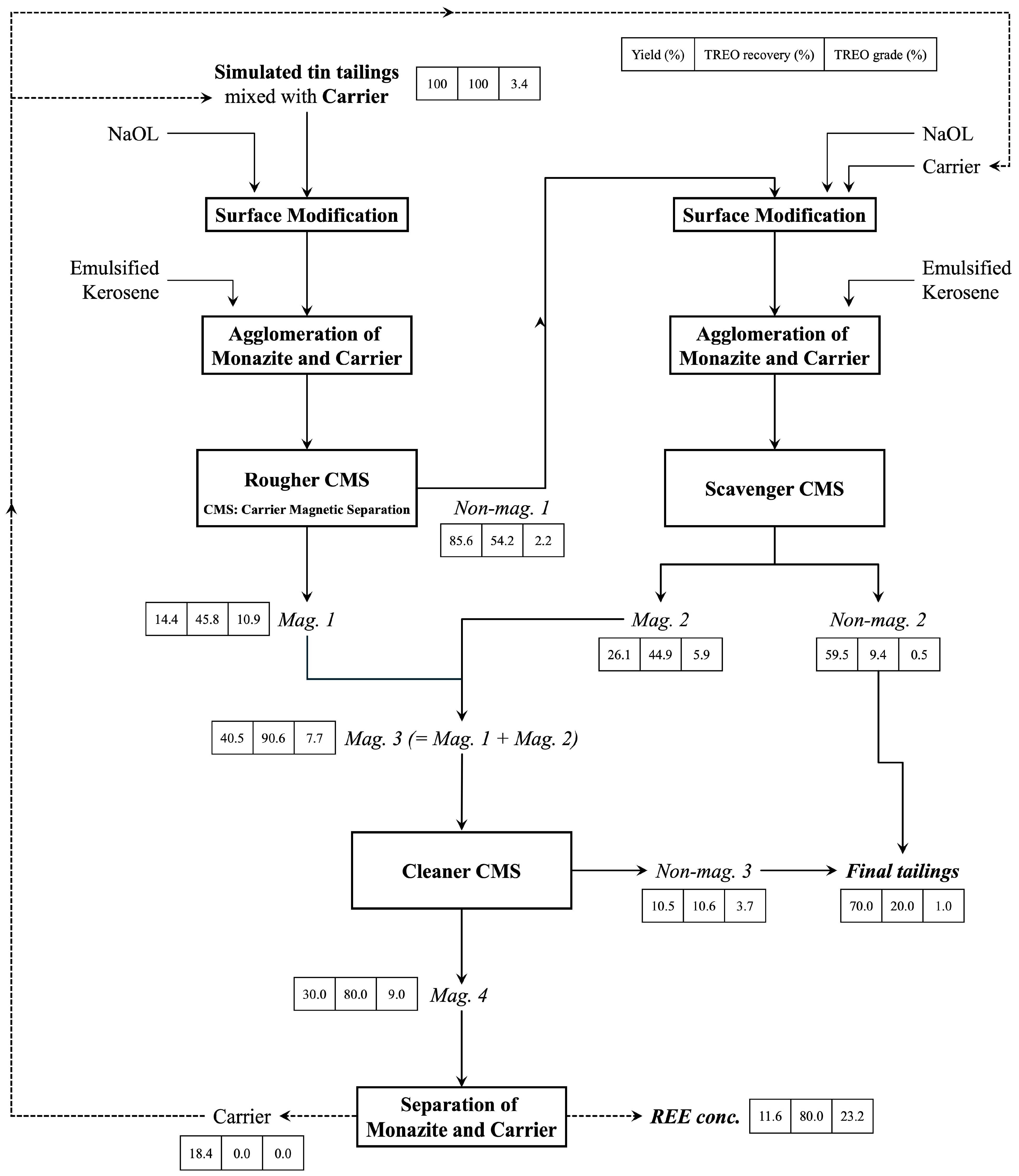
3.2. Carrier Magnetic Separation to Recover Monazite from Simulated Tailings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villocorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Mellawati, J.; Nurtjahya, E. Natural radionuclide content in horticulture plants from former tin mining land and health risk assessment: A case study on Bangka Belitung Island, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2024, 1297, 012097. [Google Scholar] [CrossRef]
- Nurtjahya, E.; Mellawati, J.; Pratama, D.; Syahrir, S. Study of soil–to–plant transfer factors (TFs) of 226Ra, 232Th, and 40K on plants cultivated on ex–tin mining land in Bangka Belitung, Indonesia. J. Environ. Radioact. 2023, 261, 107144. [Google Scholar] [CrossRef] [PubMed]
- Syarbaini, S.; Setiawan, A. Terrestrial Gamma Radiation Exposure in Bangka-Belitung Islands, Indonesia. At. Indones. 2015, 41, 41–45. [Google Scholar] [CrossRef][Green Version]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Maestro, P.; Huguenin, D. Industrial applications of rare earths: Which way for the end of the century. J. Alloys Compd. 1995, 225, 520–528. [Google Scholar] [CrossRef]
- Park, I.; Kanazawa, Y.; Sato, N.; Galtchandmani, P.; Jha, M.K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Beneficiation of Low-Grade Rare Earth Ore from Khalzan Buregtei Deposit (Mongolia) by Magnetic Separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Mineral Commodity Summaries 2025. Available online: https://pubs.usgs.gov/publication/mcs2025 (accessed on 16 April 2025).
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Zglinicki, K.; Szamalek, K.; Wolkowicz, S. Critical Minerals from Post-Processing Tailings. A Case Study from Bangka Island, Indonesia. Minerals 2021, 11, 352. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Qu, L. Recovery Enhancement of Ultrafine Wolframite through Hydrophobic Flocs Magnetic Separation. Miner. Process. Extr. Metall. Rev. 2017, 38, 298–303. [Google Scholar]
- Roy, S. Recovery Improvement of Fine Magnetic Particles by Floc Magnetic Separation. Miner. Process. Extr. Metall. Rev. 2012, 33, 170–179. [Google Scholar] [CrossRef]
- Song, S.; Lu, S.; Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 2002, 15, 415–422. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Akishino, R.; Bu, X.; Hassan, F.U.; Park, I.; Jeon, S.; Aikawa, K.; Hiroyoshi, N. Heterogenous carrier flotation technique for recovering finely ground chalcopyrite particles using coarse pyrite particles as a carrier. Miner. Eng. 2022, 180, 107518. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of Six Flotation Kinetic Models Used in the Conventional Flotation and Carrier Flotation of −74 µm Coal Fines. ASC Omega 2020, 5, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Goode, J.R. Rare Earth Elements. In SME Mineral Processing & Extractive Metallurgy Handbook; Dunne, R.C., Kawatra, S.K., Young, C.A., Eds.; Society of Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019; Volume 2, pp. 2049–2075. [Google Scholar]
- Cheng, T.-W.; Holtham, P.N.; Tran, T. Froth Flotation of Monazite and Xenotime. Miner. Eng. 1993, 6, 341–3351. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Lin, Z.; Li, P.; Hou, D.; Kuang, Y.; Wang, G. Aggregation mechanism of particles: Effect of Ca2+ and polyacrylamide on coagulation and flocculation of coal slime water containing illite. Minerals 2017, 7, 30. [Google Scholar] [CrossRef]
- van Oss, C.J. Acid—Base interfacial interactions in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Azizi, D.; Sarvaramini, A.; Larachi, F. Liquid-liquid mineral separation via ionic-liquid complexation of monazite and bastnäsite—An alternate route for rare-earth mineral beneficiation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 301–323. [Google Scholar] [CrossRef]
- Jung, M.; Tadesse, B.; Dick, C.; Logan, A.; Dyer, L.; Albijanic, B. Rheological properties of rare earth minerals flotation pulp in the presence of anions. J. Rare Earths 2024, 42, 2172–2182. [Google Scholar] [CrossRef]
- Lu, J.; Yuan, Z.; Liu, J.; Li, L.; Zhu, S. Effects of magnetite on magnetic coating behavior in pentlandite and serpentine system. Miner. Eng. 2015, 72, 115–120. [Google Scholar] [CrossRef]
- Li, D.; Yin, W.; Liu, Q.; Cao, S.; Sun, Q.; Zhao, C.; Yao, J. Interactions between fine and coarse hematite particles in aqueous suspension and their implications for flotation. Miner. Eng. 2017, 114, 74–81. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Liu, Q.; Liu, J.-T. Extended DLVO theory applied to coal slime-water suspensions. J. Cent. S. Univ. 2012, 19, 3558–3563. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Ito, M.; Hassan, F.U.; Aikawa, K.; Jeon, S.; Hiroyoshi, N. Carrier Flotation Using Coarse Pyrite for Improving the Recovery of Finely Ground Chalcopyrite: Development of Post-Process of Carrier Flotation to Separate Finely Ground Chalcopyrite Particles from Coarse Pyrite Particles. Minerals 2023, 13, 916. [Google Scholar] [CrossRef]



| Elements | Size Fractions (µm) | |||||
|---|---|---|---|---|---|---|
| +425 | −425 +300 | −300 +212 | −212 +106 | −106 +75 | −75 | |
| TREO | 15.7 | 19.0 | 20.3 | 12.8 | 6.2 | 3.7 |
| Fe2O3 | 10.4 | 14.7 | 13.9 | 11.9 | 13.4 | 12.5 |
| TiO2 | 19.0 | 28.1 | 32.6 | 31.4 | 10.1 | 11.4 |
| ZrO2 | 9.4 | 3.8 | 6.4 | 15.8 | 35.0 | 32.4 |
| SiO2 | 19.3 | 16.3 | 13.6 | 17.8 | 17.2 | 17.6 |
| Al2O3 | 11.1 | 4.7 | 2.4 | 4.3 | 6.9 | 3.8 |
| Others | 15.2 | 13.3 | 10.9 | 6.0 | 11.3 | 18.6 |
| Monazite | Zircon | Quartz | Magnetite | ||||
|---|---|---|---|---|---|---|---|
| Elements | Contents (%) | Elements | Contents (%) | Elements | Contents (%) | Elements | Contents (%) |
| La | 12.8 | Zr | 70.3 | Si | 99.2 | Fe | 79.1 |
| Ce | 33.2 | Si | 22.0 | Al | 0.3 | Si | 8.3 |
| Nd | 13.9 | Al | 4.2 | P | 0.4 | Al | 4.3 |
| Other REEs | 12.7 | Mg | 2.5 | Others | 0.1 | Ti | 4.5 |
| P | 8.9 | Others | 1.0 | Others | 3.8 | ||
| Th | 16.2 | ||||||
| Others | 2.2 | ||||||
| Liquid | γL (mJ∙m−2) | γLd (mJ∙m−2) | γL+ (mJ∙m−2) | γL− (mJ∙m−2) |
|---|---|---|---|---|
| Water | 72.8 | 21.8 | 25.5 | 25.5 |
| Glycerol | 64.0 | 34.0 | 4.92 | 57.4 |
| Formaldehyde | 58.0 | 39.0 | 2.28 | 39.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Gumilang, T.S.; Pratama, R.Y.; Jeon, S.; Tabelin, C.B.; Phengsaart, T.; Bilal, M.; Kawamura, Y.; Ito, M. Recovery of Fine Rare Earth Minerals from Simulated Tin Tailings by Carrier Magnetic Separation: Selective Heterogeneous Agglomeration with Coarse Magnetite Particles. Minerals 2025, 15, 757. https://doi.org/10.3390/min15070757
Park I, Gumilang TS, Pratama RY, Jeon S, Tabelin CB, Phengsaart T, Bilal M, Kawamura Y, Ito M. Recovery of Fine Rare Earth Minerals from Simulated Tin Tailings by Carrier Magnetic Separation: Selective Heterogeneous Agglomeration with Coarse Magnetite Particles. Minerals. 2025; 15(7):757. https://doi.org/10.3390/min15070757
Chicago/Turabian StylePark, Ilhwan, Topan Satria Gumilang, Rinaldi Yudha Pratama, Sanghee Jeon, Carlito Baltazar Tabelin, Theerayut Phengsaart, Muhammad Bilal, Youhei Kawamura, and Mayumi Ito. 2025. "Recovery of Fine Rare Earth Minerals from Simulated Tin Tailings by Carrier Magnetic Separation: Selective Heterogeneous Agglomeration with Coarse Magnetite Particles" Minerals 15, no. 7: 757. https://doi.org/10.3390/min15070757
APA StylePark, I., Gumilang, T. S., Pratama, R. Y., Jeon, S., Tabelin, C. B., Phengsaart, T., Bilal, M., Kawamura, Y., & Ito, M. (2025). Recovery of Fine Rare Earth Minerals from Simulated Tin Tailings by Carrier Magnetic Separation: Selective Heterogeneous Agglomeration with Coarse Magnetite Particles. Minerals, 15(7), 757. https://doi.org/10.3390/min15070757










