1. Introduction
Quartz, second only to feldspar in crustal abundance, occurs in igneous, metamorphic, sedimentary, and hydrothermal rocks [
1]. Its crystallization spans a remarkable temperature range: in some hydrothermal veins, quartz can precipitate at ≈50 °C [
2], whereas melt-inclusion studies show that felsic magmas may stabilize quartz up to roughly 750–900 °C [
3]. Because the mineral is highly resistant to chemical alteration, it preserves fluid inclusions and stable-isotope signatures for millions of years, providing a dependable archive for reconstructing the thermal and chemical evolution of hydrothermal systems [
4].
Within hydrothermal systems, migrating hot fluids transport both heat and dissolved components [
5]. As these solutions move through the crust, evolving pressure, temperature, pH, and redox conditions govern the extent to which quartz sequesters trace and rare-earth elements—either in its lattice or in enclosed fluid inclusions. Element budgets shift systematically with fluid evolution: cathodoluminescence-guided LA-ICP-MS maps of vein quartz demonstrate that Al, Ti, and Li variations follow SEM-CL growth zoning [
6], while large datasets from porphyry- and epithermal-style deposits show stage-dependent Li/Al, Ti–Ge and As–Sb patterns in quartz that track ore-forming processes [
7]. Once incorporated, these elements serve as geochemical proxies, recording the physicochemical conditions prevailing during metal precipitation [
5,
8].
Recent advances in LA-ICP-MS now permit micrometer-scale measurement of trace and rare-earth elements in quartz with reproducibility better than ~10% RSD [
9,
10]. These datasets reveal systematic Al, Ti, Li, and Ge variations that correlate with cathodoluminescence (CL) growth zones and fluid-evolution stages across porphyry, epithermal, IOCG, orogenic-gold, and MVT deposits [
2]. Building on this framework, recent studies have distinguished successive vein generations via Al/Ti, Sb/Ti, and Ge/Ti ratios [
11] and tracked fluid evolution in the Bilihe porphyry Au deposit using combined Ti, Al, Li, and Ge mapping [
12]. Against this backdrop, a focused LA-ICP-MS investigation of quartz from the Tuztaşı low-sulfidation system can clarify its multi-stage mineralization history while providing new geochemical vectors for exploring analogous deposits in Western Türkiye.
The Tuztaşı gold mineralization is situated in Northwestern Türkiye, within the villages of Tuztaşı and Uzunalan (Ayvacık district), and Alakeçi, Yassıbağ, and Serhat (Bayramiç district), Çanakkale Province. Located in the Biga Peninsula, Tuztaşı represents a well-preserved example of a low-sulfidation epithermal system [
13,
14,
15]. This mineralization developed within amphibolite and schist lithologies of the Sütüven Formation, hosted along multiple generations of quartz veins that reflect distinct hydrothermal stages. Quartz samples were selected from the previously characterized gold-bearing vein system at Tuztaşı, but this study provides the first in-depth evaluation of their trace- and rare-earth-element (REE) chemistry using LA-ICP-MS.
The principal objective of this research is to elucidate the temporal evolution of the hydrothermal fluids responsible for the Tuztaşı gold deposit and to delineate the behavior of trace elements and REEs that reflect variations in the gold content of quartz. A concurrent aim is to evaluate the utility of quartz mineral chemistry as a tool in the exploration for similar deposit types. Accordingly, the trace-element content and REE fractionation patterns in quartz were meticulously examined, and the relationship between these chemical signatures and gold precipitation was assessed. Anomalies observed in highly redox-sensitive elements, notably cerium (Ce) and europium (Eu), have yielded insights into the oxidation–reduction conditions of the hydrothermal fluids, thereby contributing to an enhanced understanding of the mineralization processes. The findings from this work not only reveal the metallogenic characteristics of the Tuztaşı mineralization but also provide crucial data for a more precise classification of this system within the broader context of epithermal gold deposits.
In this respect, this study is among the earliest and more distinctive investigations in Türkiye to systematically appraise quartz mineral chemistry, integrating both REE and trace-element data, within low-sulfidation epithermal gold systems. The comprehensive approach, which considers multi-component fluid evolution and incorporates contributions from both clastic and magmatic sources, is expected to offer valuable direction for the exploration of other gold deposits in the region.
2. Geology of Study Area
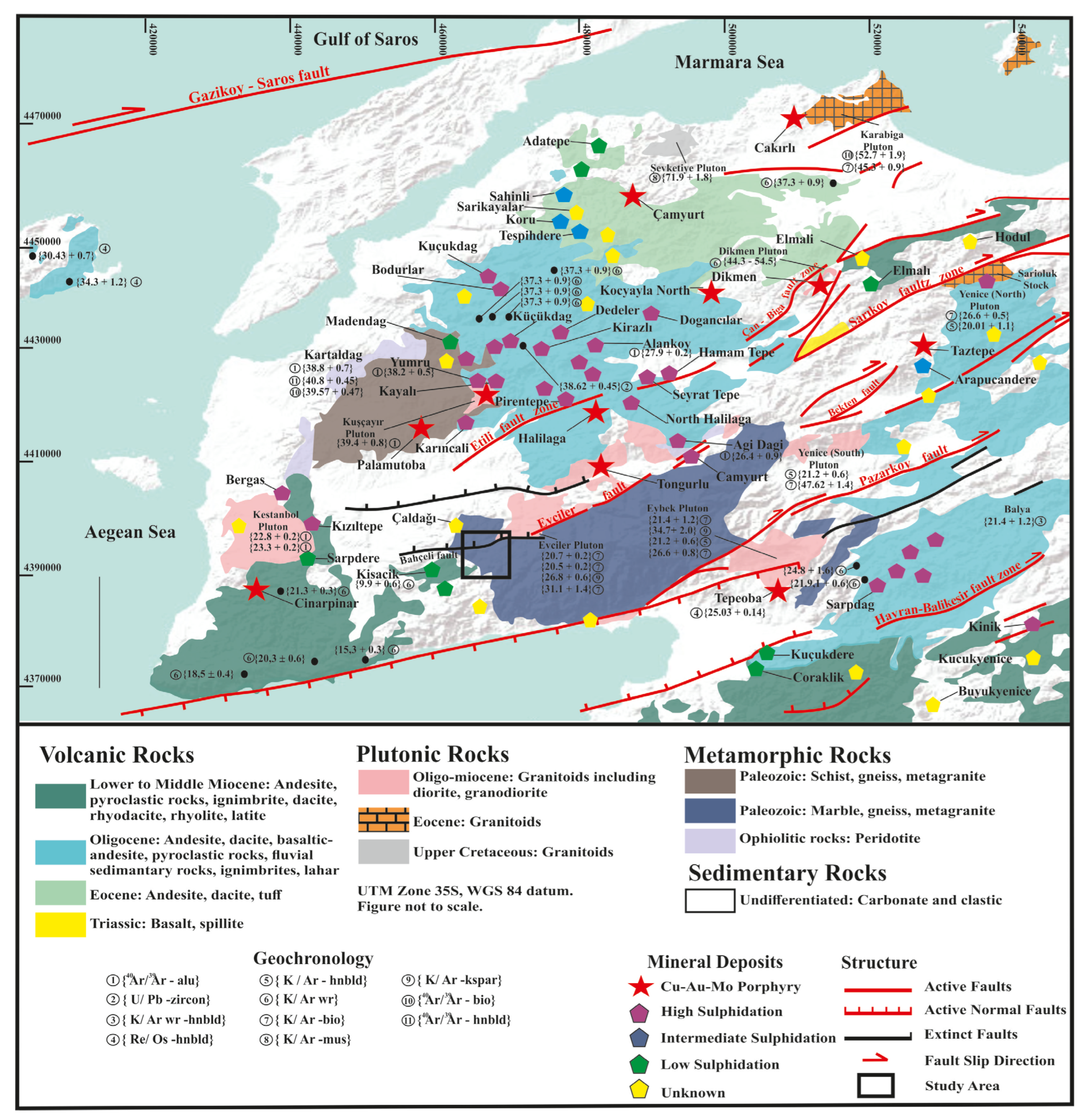
Western Anatolia’s Biga Peninsula is a major locus of Cenozoic magmatism and metallogeny within Türkiye. Geologically, it sits at the western tip of the Sakarya Zone, within the Alpine–Himalayan orogenic belt, and was assembled during Early Tertiary collisions of Paleozoic–Mesozoic terranes [
16,
17]. The region’s basement, the Kazdağı Massif, has recorded multiple episodes of metamorphism and magmatism since [
17] the Middle Carboniferous, with three main tectonostratigraphic zones trending NW–SE [
17]. High-K calc-alkaline to shoshonitic magmatism during the Oligo–Miocene was instrumental in forming major porphyry, epithermal, and skarn-type Au–Cu–polymetallic deposits in the Western Tethys Metallogenic Belt [
18,
19,
20]. The distribution of these systems is closely linked to Cenozoic post-collisional extension and crustal thickening. Gold and copper mineralization in Biga is mainly controlled by fault systems formed under alternating compressional and extensional regimes [
21]. High-sulfidation systems (e.g., Alanköy, Karaayı, and Kirazlı) are widespread [
22], whereas intermediate-sulfidation (e.g., Şahinli and Tespihdere) and low-sulfidation (e.g., Küçükdere and Madendağ) deposits are less common [
13,
23,
24].
A simplified regional geological map of the Biga Peninsula, highlighting major tectonic elements and known mineral deposits, is shown in
Figure 1. The Tuztaşı deposit lies along the boundary between metamorphic units of the Kazdağı Massif and intrusive granitoids of the Evciler Pluton.
The Tuztaşı gold mineralization developed along a northwest–southeast (NW–SE) trending fault zone that transects the upper levels of the Kazdağı Massif within the Biga Peninsula [
25]. The Sütüven Formation, which hosts the deposit, is mainly composed of amphibolite, gneiss, metagranite, and intercalated marble bands. Gneisses display pronounced foliation, while metagranites locally exhibit transitional granite–gneiss textures. Aplite veins within these units are rich in muscovite and plagioclase.
To the southwest, the Çetmi Ophiolitic Mélange forms a complex assemblage of serpentinite, diabase, gabbro, sandstone, and limestone blocks. The Evciler Pluton, of granitic composition, is intensely cataclysmically deformed along its contact with the Kazdağı Massif, displaying fractured and foliated granite textures.
The region is structurally shaped by NW-SE trending thrust and strike-slip faults. The Şelale Detachment Fault delineates the boundary between the Kazdağı Massif and the Çetmi Mélange. Concurrently, the northeast–southwest (NE–SW) trending Uzunalan Fault tectonically juxtaposes serpentinite and limestone blocks of the mélange with amphibolite, marble, and metagranite units of the Sütüven Formation. The Evciler Fault, exhibiting a dextral strike-slip component, creates a distinct structural separation between the Evciler Pluton and the metamorphic units of the massif. In addition, synthetic and antithetic faults locally offset quartz and aplite veins by several centimeters to meters (
Figure 2).
Gold mineralization is concentrated within the Main Quartz Vein (MQV), which trends approximately N50° E, dips\~40° NE, and reaches 2 km in length and 3–8 m in thickness. The MQV crosscuts sequences of amphibolite–marble and metagranite and is mainly composed of quartz, chalcedony, and opal, with localized development of hematite, goethite, pyrite, and marcasite. Surface samples show disseminated pyrite and free gold, reflecting post-vein oxidation processes.
3. Materials and Methods
The trace- and rare-earth-element (REE) concentrations in quartz from the Main Quartz Vein (MQV) were determined by LA-ICP-MS at the İstanbul University’s Cerrahpaşa Geochronology and Geochemistry Laboratory. Analyses were conducted on a Perkin Elmer NexION 2000 ICP-MS instrument (PerkinElmer, Waltham, MA, USA), coupled to an ESI NWR 213 nm solid-state laser ablation system (ESI, Portland, OR, USA), using a 50 µm spot size, 20 s of background acquisition, and 30 s of ablation; helium (0.5 L min−1) served as the carrier gas, with a laser fluence of 5 J/cm2, repetition rate of 10 Hz, and scan speed of 5 µm/s. External calibration employed BCR-2G for major elements (Al, K, and Ti), NIST SRM 612 for trace elements (As, Sb, Pb, and Zn), and NIST SRM 610 for REE, while silicon (Si, 29.76 wt%) was used as the internal standard. Detection limits were approximately 0.01 ppm for Au and Ag, 0.1–1 ppm for other trace elements, and 0.01–0.05 ppm for REE.
A total of 23 laser-ablation spots were targeted across five quartz samples. Among these, ten spots yielded reliable trace/REE signals and formed the basis for the geochemical interpretations discussed herein. These samples included not only crystalline quartz but also microcrystalline chalcedony and opaline silica phases in order to capture the full spectrum of silica paragenesis observed in the Main Quartz Vein.
Cerium (Ce) and europium (Eu) anomalies were calculated using chondrite-normalized values as follows:
where the subscript “n” denotes chondrite-normalized values, using the normalization constants of Sun and McDonough [
26]. Data reduction and quality control—including checks on signal stability, detection limits, and potential spectral interferences—were carried out using the SILLS v. 1.3.4 software package [
10].
Cathodoluminescence (CL) imaging was performed on quartz samples prepared as 3 × 2 × 1 cm polished blocks. These were mounted onto glass slides and ground to 80–120 µm thickness, followed by final polishing to obtain double-sided polished thin sections. CL and BSE images were obtained using a JEOL JXA-8900RL electron microprobe (ITU Department of Geological Engineering), equipped with a Technosyn 8200 Mk 4 Luminoscope and Alcatel vacuum pump, operating at 15 kV and 15 nA.
4. Results
4.1. Quartz Mineral Chemistry
Trace- and rare-earth-element concentrations were measured by LA-ICP-MS on five quartz samples from the Main Quartz Vein (MQV), representing both mineralized and barren domains. A total of 23 laser-ablation spots were positioned systematically to give representative coverage of the vein. Petrographic assignment of each spot to a distinct quartz stage relied on the CL textures illustrated in
Figure 3.
4.1.1. Trace-Element Contents
LA-ICP-MS analyses on 23 spots (
Figure 3e,f;
Table 1) reveal that gold varies from 0.03 to 22.34 ppm (mean 4.9 ± 8.6 ppm) and silver from 0.23 to 48.78 ppm (mean 15.2 ± 19.9 ppm). The highest Au–Ag values occur at spot 73-5v (Au 22.34 ppm; Ag 48.78 ppm), while most other spots record < 4 ppm Au. Concentrations of other elements show considerable scatter: As averages 2854 ± 6821 ppm; Sb, 57.8 ± 113 ppm; Se, 5.7 ± 12 ppm; Pb, 280 ± 632 ppm; Zn, 406 ± 902 ppm; Al, 2771 ± 1213 ppm; K, 498 ± 179 ppm; Ti, 26.5 ± 25.7 ppm; Ge, 1.72 ± 0.37 ppm; and Sn, 0.40 ± 0.44 ppm. Spot 73-4, located in an Fe-oxide-rich microfracture, is an outlier with 16,775 ppm As and 2245 ppm Zn; excluding this fracture-bound spot yields filtered means of As 70 ± 93 ppm and Zn 14 ± 14 ppm. Detailed spot-by-spot results are listed in
Table 1. As described in earlier petrographic studies of the Tuztaşı system [
15], the silica paragenesis comprises a transition from early opal and chalcedony to sparry quartz. (see
Figure 3g).
Element levels in barren quartz are much lower: gold sits at the detection limit (<0.04 ppm), silver averages about 4.4 ± 3.9 ppm, arsenic is 53 ± 47 ppm, antimony is 14 ± 6 ppm, aluminum is 6165 ± 3611 ppm, potassium is roughly 1780 ± 2114 ppm, and titanium is about 39 ± 51 ppm. The contrast underscores the different temperature–pH–redox regimes recorded by the mineralized and barren zones, and reveals how the As–Sb–Zn-rich fluids were unevenly distributed along the vein.
Arsenic displays a highly skewed distribution (
Figure 4). One mineralized spot (sample 73-4) yielded 16,775 ppm As—>10× higher than any other analysis—resulting in a raw mean of 2854 ± 6821 ppm for mineralized quartz (n = 10). Because this single outlier inflates the mean and standard deviation, we report both the raw statistics and a filtered dataset (As = 70 ± 93 ppm; n = 9) that excludes 73-4. Unless stated otherwise, discussion and comparative plots use the filtered values.
4.1.2. REE Distribution and Fractionation Parameters
Chondrite-normalized REE patterns were evaluated for the ten analytically robust laser-ablation spots (five mineralized and five barren) selected from different parts of the Main Quartz Vein. Total REE contents (ΣREE) range from 1.62 to 312.04 ppm, with a mean of 40.7 ppm. The light-to-heavy REE ratio (ΣLREE/ΣHREE) spans 0.21–10.84 (average = 2.9), indicating pronounced but heterogeneous LREE enrichment relative to HREE.
Cerium anomalies, calculated as δCe = Ce
n/√ (La
n × Pr
n), vary from 0.11 to 0.89 (mean = 0.29), whereas europium anomalies, calculated as δEu = Eu
n/√ (Sm
n × Gd
n), range from 0.63 to 9.31 (mean = 4.33). The strongly positive δEu values imply a significant Eu
2+ component, compatible with reducing conditions and/or feldspar breakdown. This relationship is illustrated in
Figure 5a.
Fractionation indices likewise vary widely:
Together, these data reveal marked REE heterogeneity within the vein and highlight zones that combine extreme LREE enrichment with pronounced positive Eu anomalies—features that may trace fluid pathways and redox gradients active during ore formation. Note that two barren analyses with exceptionally high Al (8718 ppm and 18,556 ppm) fall outside the plotting window of
Figure 5b. Although they are shown only by the off-scale arrow, both values are fully included in all statistical calculations (e.g., means and standard deviations).
As shown in
Figure 6a, inspection of the Pr/Pr*–Ce/Ce* diagram reveals that only one of the five mineralized analyses (sample 73-6) plots unequivocally in the “within” Ce-anomaly field, where both Pr/Pr* and Ce/Ce* exceed unity. All other mineralized and barren samples fall outside this quadrant, implying that their apparent Ce anomalies are likely pseudo-anomalies caused by chondrite normalization artifacts or mixed redox effects. This observation underscores the importance of using Pr-based discrimination to avoid over-interpreting weak Ce anomalies and highlights the need for integrated redox proxies when assessing ore-forming processes.
Figure 6a further illustrates the variability in REE fractionation by plotting chondrite-normalized (La/Yb)_N ratios against Y concentrations. The widespread (La/Yb)_N values, particularly among mineralized samples, reflect heterogeneous fluid compositions and suggest variable degrees of LREE enrichment and Y depletion, potentially linked to evolving redox and complexation conditions during quartz precipitation.
5. Discussion
5.1. Characteristics of the Ore-Forming Fluid: Integrated Evidence from REE and Oxygen Isotopes
The hydrothermal fluids that generated the Tuztaşı low-sulfidation epithermal deposit evolved through progressive interaction with felsic basement rocks and fluctuating redox conditions. These changes are recorded in the rare-earth-element (REE) signature of hydrothermal quartz [
6] and in its oxygen-isotope composition [
27,
28].
5.1.1. Evolution of Ore-Forming Fluids: REE and δ18O Evidence
A total of 23 LA-ICP-MS spot analyses were performed on both mineralized and barren quartz samples. In mineralized quartz, chondrite-normalized REE patterns exhibit a marked enrichment of light rare-earth elements (LREEs) relative to heavy rare-earth elements (HREEs). The (La/Yb)n ratio varies from 0.31 to 45.26, with an average of approximately 12.6, while the (La/Sm)n ratio ranges from 0.31 to 12.77, averaging around 3.83. In contrast, barren quartz displays flatter normalized profiles and significantly lower LREE/HREE ratios, indicating limited interaction with REE-rich lithologies.
Among the mineralized samples, the europium anomaly is calculated as follows:
The values range from 0.63 to 9.31, with a mean of approximately 4.33. These strong positive anomalies reflect the stabilization and retention of Eu
2+ under reducing conditions. In contrast, the cerium anomaly is defined as follows:
It varies between 0.11 and 0.89 (mean ≈ 0.29), indicating that Ce remained predominantly in the trivalent state (Ce
3+), with limited oxidative conversion to Ce
4+. The weak correlation between δEu and δCe (R
2 ≈ 0.05) implies that these anomalies developed independently under distinct redox conditions [
27,
29].
Although cathodoluminescence (CL) images were not obtained from the exact LA-ICP-MS mounts, sister thin sections from the same quartz vein system—previously described in Özbaş and Hanilçi [
15]—reveal clear contrasts between homogeneous, single-phase quartz and zoned, multi-generational textures (
Figure 3a–d). These petrographic features provide a robust framework for assigning ablation spots to distinct growth stages and interpreting the observed geochemical differences between mineralized and barren quartz.
Oxygen isotope analyses conducted on the same quartz samples yield δ
18O₍Qz₎ values between 6.6‰ and 8.6‰ [
15]. Using a quartz–water fractionation factor of approximately 9.4‰—appropriate for homogenization temperatures around 213 °C [
30,
31]—these values correspond to calculated fluid δ
18O₍H
2O₎ values between −6.8‰ and +0.7‰. These values are typical of low-salinity meteoric waters in low-sulfidation epithermal systems [
32] and were previously reported for Tuztaşı by Özbaş and Hanilçi [
15], providing a consistent isotopic baseline for the present study.
The combined REE patterns reported here, together with the δ
18O-fluid data previously established for the Tuztaşı vein system [
24], refine the earlier two-stage hydrothermal model as follows.
- I.
Meteoric influx stage: Early, low-salinity meteoric waters (δ
18O_H
2O = −6.8 to +0.7‰; [
15]) precipitated quartz with only modest LREE enrichment.
- II.
Deep circulation and felsic-rock interaction: As fluids penetrated deeper into the crust, they interacted with REE-bearing felsic lithologies (i.e., the Evciler Pluton and Sütüven Formation). New REE data from this study reveal pronounced LREE enrichment ((La/Yb)_N up to 45) and strong positive Eu anomalies. These features record deeper fluid circulation, extensive interaction with felsic basement rocks, and transient reducing conditions—processes only inferred but not previously documented quantitatively for Tuztaşı.
This geochemical evolution fostered conditions favorable for Au–Ag precipitation, particularly during late-stage cooling and localized redox fluctuations [
33,
34]. The Tuztaşı system thus reflects a transition from meteoric-dominated fluids to chemically evolved reducing fluids—a transformation tracked effectively by combined REE and δ
18O evidence.
Comparable geochemical trends have been observed in other systems. For instance, in the Shihu Au deposit (China), quartz exhibits only weak LREE enrichment and negative Eu–Ce anomalies, attributed to limited felsic-rock interaction and less evolved fluid compositions [
35]. In contrast, the Geyer Sn-skarn (Germany) and the Cerro de Pasco high-sulfidation system (Peru) both show quartz-hosted REE patterns that are sharply LREE-enriched and carry pronounced positive Eu anomalies—features interpreted as the result of direct magmatic input and prolonged fluid–rock interaction with felsic intrusions [
36,
37]. The Tuztaşı system exhibits a remarkably similar geochemical trajectory, highlighting the role of felsic host rocks in shaping the REE signature and redox conditions of ore-forming fluids.
5.1.2. Redox Control on Eu–Ce Anomalies
Anomalies in cerium and europium concentrations preserved in mineralized quartz offer valuable insights into redox conditions during ore formation at Tuztaşı. The europium anomaly, expressed as
ranges from 0.63 to 9.31, with an average value of 4.33. These elevated δEu values indicate significant stabilization of Eu
2+ under reducing conditions. In contrast, cerium shows a different behavior. The cerium anomaly, calculated as
varies from 0.11 to 0.89 (mean ≈ 0.29); such negative δCe values indicate that a significant fraction of Ce
3+ was oxidized to Ce
4+ and removed from the fluid phase, pointing to locally more oxidizing conditions during quartz growth [
8,
29].
Figure 4 shows Pathfinder δEu plotted against δCe; the negligible correlation (R
2 ≈ 0.05) indicates that europium and cerium anomalies did not form contemporaneously but record at least two distinct redox pulses. The strong positive Eu anomaly (δEu = 0.63–9.31) points to stabilization of Eu
2+ under transiently reducing conditions. Such Eu
2+ enrichment is commonly generated when acidic reducing fluids destabilize feldspar—especially plagioclase—liberating Eu that partitions into the fluid as Eu
2+ before being sequestered in quartz or fluorite [
8]. In contrast, the muted Ce anomaly (δCe = 0.11–0.89) implies that Ce remained largely trivalent, with only limited oxidation to Ce
4+ [
29]. In contrast, δCe values ranging from 0.11 to 0.89—significantly lower than 1—indicate that a substantial portion of Ce
3+ in the fluid phase was oxidized to Ce
4+ and subsequently removed through precipitation or adsorption. Thus, these anomalies record relatively short-lived oxidizing episodes during quartz precipitation [
29].
In some other deposits (e.g., Shihu, China), δCe and δEu values have been reported to increase and decrease in tandem, reflecting limited redox fluctuations during a single fluid pulse—specifically, partial oxidation of Ce and simultaneous partial reduction of Eu [
35]. In contrast, at Tuztaşı, there is no meaningful correlation between δEu and δCe (
Figure 4, R
2 ≈ 0.05), indicating that Eu
2+ enrichment occurred during short-lived reducing phases, whereas Ce behavior reflects more oxidizing intervals. This decoupling is further clarified by the Pr/Pr*–Ce/Ce* discrimination plot (
Figure 6a), which distinguishes “true” Ce anomalies from artefacts caused by chondrite normalization or irregular Pr behavior. Among the ten samples analyzed, only one mineralized spot (sample 73-6) plots within the “true Ce anomaly” field, where both Pr/Pr* and Ce/Ce* exceed unity. The remaining samples, including other mineralized ones, fall outside this field, suggesting that their Ce anomalies may be pseudo-anomalies or the product of complex redox overprints. This highlights the importance of using Pr-based correction to assess the reliability of Ce anomalies and avoid overinterpreting redox conditions from Ce behavior alone.
Taken together, these redox-sensitive anomalies are consistent with the broader hydrothermal evolution inferred from REE fractionation patterns and δ
18O data. Prolonged fluid–rock interaction with felsic basement rocks likely maintained reducing conditions that favored Eu
2+ enrichment [
8,
29]. In contrast, the absence of a δEu–δCe correlation, along with the presence of distinct Ce anomalies, points to intermittent oxidative inputs—possibly triggered by transient shifts in fluid pathways or minor mixing with shallow, oxidized groundwater. These localized redox oscillations likely created micro-environments favorable for Au–Ag precipitation [
34]. Taken together, the decoupled behavior of δEu and δCe in mineralized quartz points to complex, stage-specific redox dynamics during fluid evolution. When interpreted alongside REE distribution and oxygen isotope signatures, these anomalies provide a nuanced geochemical record of the conditions that governed ore precipitation in the Tuztaşı epithermal system
5.2. REE Fractionation Mechanisms
Mineralized quartz from the Tuztaşı low-sulfidation epithermal system shows a pronounced enrichment of light REEs over heavy REEs, as reflected by high chondrite-normalized (La/Yb)
n and (La/Sm)
n ratios—an outcome best explained by ligand-controlled complexation coupled with ongoing fluid–rock interaction [
29,
38].
LA-ICP-MS spot analyses targeting quartz growth zones yielded the following averages in mineralized quartz: (La/Yb)
n = 0.31–45.26 (mean = 12.6) and (La/Sm)
n = 0.31–12.77 (mean = 3.83). Such pronounced LREE enrichment implies that La, Ce, and Nd must have been efficiently mobilized into the hydrothermal fluid. The feldspar- and mica-rich gneisses and metagranites of the Sütüven Formation and the adjacent Evciler Pluton contain accessory allanite and monazite, which are plausible primary LREE reservoirs and therefore the most likely solid sources for the liberated REE budget during hydrothermal alteration [
28].
Thermodynamic context: Experimental data show that at moderate-to-high temperatures (≈200–400 °C) and slightly acidic pH (4–6), LREEs form stable chloride (LREE–Cl) and carbonate (LREE–CO
3) complexes that greatly enhance their solubility [
38]. As the fluids cool or pH rises, these complexes break down, promoting LREE precipitation into quartz or micro-inclusions [
39]. Fluid-inclusion salinities of 2–5 wt% NaCl eq. in Tuztaşı quartz [
15] are likewise consistent with the predominance of neutral LREE–Cl
0 and carbonate LREE–CO
3− species [
40].
Conversely, HREEs preferentially form complexes with phosphate, sulfate, or fluorine ligands—minerals that are scarce in Tuztaşı wall rocks. Consequently, HREEs remain largely immobile or are locked in resistant accessory phases, accounting for their depletion in quartz (no monazite-hosted HREE phases were observed in Sütüven samples).
Elevated (La/Yb)
n and (La/Sm)
n ratios in Tuztaşı quartz therefore record a dynamic fractionation regime in which early fluid–rock interaction mobilized LREEs, whereas subsequent cooling and redox shifts drove their precipitation. Grouped frequency plots of these ratios
Figure 6b for (La/Yb)
n clearly separate “Mineralized” from “Barren” quartz populations. A similar LREE-rich signature has been reported from the Geyer Sn-skarn in Germany and from quartz veins of the Potosí district in Bolivia, where sustained interaction with felsic crust governs REE geochemistry [
41].
5.3. Significance of REE and Trace-Element Distributions in Ore-Forming Processes
Comparison of mineralized and barren quartz from the Tuztaşı vein reveals statistically robust contrasts in both trace elements and REE contents, shedding light on the fluid conditions that controlled gold precipitation. These contrasts define reliable geochemical criteria for distinguishing ore-bearing zones from barren alteration halos.
Aluminum provides the clearest discriminator. Mineralized quartz contains 2771 ± 1213 ppm Al, whereas barren quartz reaches values as high as 6165 ppm. High Al in barren material likely reflects (i) higher crystallization temperatures and/or (ii) stronger pH buffering, either of which enhances Al
3+ incorporation via H
+-substitution mechanisms in the quartz lattice [
42,
43]. Conversely, lower Al in mineralized quartz implies precipitation from cooler, chemically evolved fluids—conditions favorable for Au deposition (see
Table 2). This Al depletion aligns with higher As, K, and Sb contents, reinforcing the link between reduced Al uptake and ore-forming fluid evolution.
Titanium contents in mineralized quartz average 26.5 ± 25.7 ppm, slightly lower than in barren quartz (39 ± 51 ppm). However, due to internal variability, e.g., mineralized spots 73-5v and 73-6 exceed 50 ppm, this parameter alone does not robustly distinguish ore-bearing domains. Previous work on Tuztaşı quartz [
15] suggested that lower Ti, particularly when coupled with reduced Al, reflects late-stage crystallization from cooler fluids. Building on that model, the present study integrates Ge/Si and REE systematics to refine interpretations of redox conditions and metal deposition.
Despite overlapping Ti contents, the Ti/Al ratio—a reliable proxy for growth temperature and redox state—remains distinctly lower in mineralized quartz (0.0041 ± 0.0004) than in barren samples (0.0084 ± 0.0008), supporting crystallization from chemically evolved, cooler fluids (
Table 2). For clarity,
Figure 5b truncates the
x-axis at 6000 ppm; the two off-scale barren samples are shown with arrows and remain fully included in all statistical analyses.
REE fractionation patterns reinforce this contrast. Mineralized quartz shows markedly higher (La/Yb)
n ratios (mean ≈ 12.6), reflecting strong LREE enrichment, whereas barren quartz displays flatter chondrite-normalized patterns and lower LREE/HREE slopes. These differences likely stem from prolonged interaction between hydrothermal fluids and REE-bearing felsic host rocks under reducing conditions [
41].
Redox-sensitive anomalies provide further insight. δCe values remain slightly negative in mineralized quartz (mean ≈ 0.29), while δEu anomalies are strongly positive (mean ≈ 4.33). This pattern suggests that gold precipitation occurred under moderately reducing conditions sufficient to stabilize Eu
2+ but insufficient for extensive oxidation of Ce
3+ to Ce
4+ [
8,
29]. These trends are summarized in
Table 2.
Trace elements such as As, K, and Sb show marked enrichment in ore-bearing quartz. After exclusion of the single high-As outlier, arsenic still averages ~70 ± 93 ppm, roughly seven times higher than in barren quartz, while potassium reaches ~500 ± 180 ppm. In addition, the As/Sb ratio clusters around 31 in mineralized spots but remains <5 in barren quartz, underscoring a pronounced chemical break across the vein. Although no community-wide threshold has been codified, studies of quartz from other low-sulfidation and intermediate-sulfidation systems document similar enrichment of As relative to Sb in late, ore-proximal stages (e.g., Gao et al. [
7]). In the Tuztaşı dataset, ratios > 25 consistently coincide with textural evidence for fluid conduits and therefore constitute a practical geochemical vector toward the Au-Ag deposition front
Table 2.
Collectively, these results support a multi-element geochemical model for identifying productive quartz veins. The data suggest that ore-forming fluids at Tuztaşı evolved from meteoric waters toward chemically mature systems with enhanced LREE content and Eu
2+ stability under reducing conditions, enabling gold precipitation in structurally favorable zones. It is important to note that the CL images (
Figure 3a–d) were taken from sister sections rather than from the laser-ablated mounts themselves. After LA-ICP-MS, the ablated surface is pitted and charge-prone, which prevents reliable CL acquisition; repolishing would erase the analytical coordinates. The reference CL data therefore serve as an indirect but robust petrographic analogue, allowing us to correlate trace-element signatures with single-phase versus multi-phase quartz growth observed along the same vein system.
5.4. Quartz Chemistry as a Vectoring Tool for Gold Exploration
Trace elements such as As, K, and Sb are markedly enriched in ore-bearing quartz from the Main Quartz Vein: the filtered averages are As = 70 ± 93 ppm, K = 498 ± 179 ppm, and Sb = 57.8 ± 3.4 ppm, compared with much lower levels in barren samples (
Table 2). In addition, the mean As/Sb ratio of ≈ 31 is well above the >25 threshold that characterizes fluid up-flow zones in many epithermal and porphyry systems; comparable As–Sb enrichments (and their use as exploration vectors) are documented in the global compilation of quartz trace-element data by Gao et al. [
7].
Potassium enrichment probably reflects tiny sericite/illite or K-feldspar inclusions introduced during wall-rock alteration; this mechanism—together with the overall Al-substitution control on quartz trace-element budgets—has been demonstrated for hydrothermal veins at Butte and other porphyry Cu systems by Rusk et al. [
47].
The single outlier that contains >16,000 ppm As (sample 73-4) may signify a short-lived input of magmatic vapor, which can selectively concentrate semi-metals such as As by several orders of magnitude; selective metal partitioning into magmatic vapor versus brine was quantified in the Yankee Lode study of Audétat et al. [
51].
Taken together, the thresholds As > 700 ppm, As/Sb > 25, and elevated K provide a reliable geochemical vector that distinguishes mineralized segments of the Tuztaşı vein from barren quartz.
The As/Sb ratio—which averages ≈ 31 ± 3 at Tuztaşı—stands out as a practical exploration filter: values > 25 commonly mark fluid up-flow zones and ore-proximal quartz [
7] (Gao et al., 2022). When this ratio is paired with petrographic criteria such as red cathodoluminescence (CL) zoning, it delineates high-priority drill or surface targets far more effectively than single-element thresholds alone (
Table 2).
5.4.1. Physicochemical Indicators: Ti/Al and Ge/Si Ratios
The Ti/Al ratio in quartz reflects growth temperature, whereas its combined behavior with Ge/Si is sensitive to fluid redox state. Mineralized Tuztaşı quartz records a significantly lower Ti/Al = 0.0041 ± 0.0004 than barren quartz (0.0084 ± 0.0008), signifying crystallization from cooler fluids, consistent with the temperature-dependent Ti solubility calibrated by Wark and Watson [
45] and refined for pressure effects by Thomas et al. [
52].
Concurrently, Ge/Si is higher in mineralized samples (0.14–0.65 µmol mol
−1) than in barren ones (≤0.12 µmol mol
−1). The combination of low Ti/Al and elevated Ge/Si matches the trend documented by Rusk et al. [
42], who showed that Ge enrichment and Ti depletion coincide with reduced Au-depositional stages in epithermal quartz. Together, these ratios therefore constitute a robust vector toward Au–Ag ore, pinpointing cooler, mildly reducing fluid pathways within the Tuztaşı vein. Likewise, the Ge/Si ratio—0.14–0.65 µmol mol
−1 in mineralized quartz versus 0.03–0.12 µmol mol
−1 in barren quartz—indicates selective Ge enrichment under chemically evolved fluid conditions. Because germanium and gold can be co-transported in low-salinity, slightly reducing fluids, elevated Ge/Si values are a useful proxy for zones of enhanced metal-carrying capacity ([
42];
Table 2). These element patterns benchmark Tuztaşı against other deposit styles and emphasize its distinctive low-sulfidation epithermal signature (
Figure 7).
5.4.2. Redox-Sensitive Anomalies: δCe and δEu
The contrasting magnitudes of the two anomalies give an additional exploration vector. Strongly positive δEu (mean ≈ 4.3) implies sustained reducing conditions capable of stabilizing Eu
2+, whereas slightly negative δCe (mean ≈ 0.29) indicates that Ce remained largely trivalent and was not oxidized to Ce
4+. The resulting Eu–Ce decoupling—well documented as a redox proxy in hydrothermal quartz by [
8,
29]—pinpoints segments of the vein where Eu
2+-favorable conditions promoted gold precipitation, while Ce chemistry was essentially unaffected (see
Table 2).
5.4.3. Additional Indicators: Sn, Fluid-Inclusion Data
Mineralized quartz also contains an average of 0.40 ppm Sn—modest, yet significant when considered alongside As and LREE enrichment. This may indicate deep interaction with tin-bearing granitic sources. Fluid-inclusion data further support the interpretation of chemically evolved fluids: average homogenization temperatures of ~213 °C and low salinities (~2–5 wt.% NaCl eq.) are consistent with LS epithermal models [
15].
5.4.4. Exploration Guidelines and Practical Recommendations
Based on the geochemical signatures observed in the Tuztaşı low-sulfidation epithermal system, the following vectoring criteria are proposed for field application. These indicators integrate trace-element geochemistry, redox-sensitive anomalies, and fluid-inclusion characteristics to support efficient drill targeting and geochemical mapping.
- I.
Prioritize zones where arsenic exceeds 700 ppm, antimony is greater than 50 ppm, and the As/Sb ratio exceeds 25—especially in samples displaying red cathodoluminescent (CL) zoning or fluid-inclusion trails.
- II.
Target quartz veins with Ti/Al ratios below 0.005 and Ge/Si ratios greater than 0.15 µmol mol−1. These thresholds mark intervals affected by cool, metal-bearing, and chemically evolved hydrothermal fluids.
- III.
Map δEu and δCe anomalies as redox tracers. Strongly positive δEu values accompanied by neutral to slightly negative δCe values are characteristic of fluid pulses associated with ore deposition fronts.
- IV.
Use Al and Ti concentrations as mineralogical proxies for growth conditions. Quartz with Al < 3000 ppm and Ti < 30 ppm typically represents low-temperature, late-stage mineralizing phases.
- V.
Integrate δ18O and fluid-inclusion data, where available, to constrain meteoric water input and fluid evolution trends throughout the hydrothermal system.
These combined criteria—derived from detailed analytical results (
Section 5.1,
Section 5.2,
Section 5.3 and
Section 5.4) and supported by the compositional trends in
Table 2, highlight the utility of quartz geochemistry as a sensitive, cost-effective exploration tool. When used in conjunction with petrographic and structural observations, these geochemical vectors can greatly improve targeting precision and reduce the risk of false anomalies during early-stage exploration in epithermal terranes.
6. Conclusions
This investigation demonstrates that the trace- and rare-earth-element (REE) chemistry of hydrothermal quartz offers an incisive window into the physicochemical evolution of the Tuztaşı low-sulfidation epithermal Au–Ag system and provides a practical geochemical toolkit for exploration in similar terranes. High-resolution LA-ICP-MS analyses, integrated with quartz δ18O data, reveal a two-stage fluid history: (i) an early influx of low-salinity meteoric waters, and (ii) subsequent deep circulation and prolonged interaction with felsic basement rocks of the Kazdağı Massif and Evciler Pluton. This transition is recorded by pronounced but heterogeneous LREE enrichment ((La/Yb)n up to 45.3) and strongly positive Eu anomalies (δEu up to 9.3), in concert with slightly negative Ce anomalies. The negligible Ce–Eu covariance (r2 ≈ 0.05) indicates that Eu2+ stabilization and Ce oxidation occurred during discrete redox pulses, underscoring episodic fluid mixing and fluctuating redox states during ore precipitation.
Systematic contrasts between mineralized and barren quartz confirm the sensitivity of specific trace-element and elemental-ratio proxies to these evolving conditions. Mineralized specimens are characterized by elevated As (filtered avg. 70 ± 93 ppm; raw median 759 ppm; locally > 16,000 ppm in a single spot) and Sb, high K (~500 ppm on average in the filtered dataset; raw median ≈ 836 ppm), low Ti/Al (<0.005), and enriched Ge/Si (>0.15 µmol mol−1), all diagnostic of chemically evolved, reducing fluids capable of transporting and precipitating precious metals. In parallel, markedly positive δEu and depleted δCe values pinpoint zones where Eu2+-stabilizing, Au-favorable redox conditions prevailed. Collectively, these parameters delineate a robust geochemical footprint that distinguishes ore-bearing veins from barren equivalents.
From an exploration standpoint, this study validates quartz geochemistry as a rapid, cost-effective vectoring method during early-stage prospect evaluation. Threshold values such as As > 700 ppm, As/Sb > 25, Ti/Al < 0.005, and δEu ≫ 1 emerge as reliable indicators of proximity to mineralized zones, especially in the present study, with fluid-inclusion microthermometry and structural mapping. Adoption of these criteria will sharpen drill-hole targeting, reduce exploration risk, and complement conventional pathfinder-element assays in LS epithermal terrains.
Beyond its practical implications, this work represents one of the first systematic applications of integrated trace-element and REE analysis of quartz to LS epithermal deposit. The approach clarifies the metallogenic evolution of the Biga Peninsula and provides a transferable template for assessing fluid pathways, redox controls, and metal precipitation mechanisms in analogous systems worldwide. Future research combining in situ isotopic analyses (e.g., δD and δ18O of fluid inclusions), high-spatial-resolution CL imaging, and reactive-transport modeling will further refine our understanding of quartz-hosted geochemical records and enhance their utility in mineral exploration.
Author Contributions
Conceptualization, methodology, investigation, formal analysis, validation, data curation, resources, visualization, software, writing—original draft preparation, writing—review and editing, supervision, project administration, and funding acquisition, F.Ö.; scientific review and intellectual input, A.T. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All relevant data supporting the findings of this study are presented within the article. No supplementary datasets were deposited in public repositories.
Acknowledgments
The authors extend their sincere gratitude to Nurullah Hanilçi for his valuable scientific insights and constructive feedback, which greatly contributed to the interpretation and contextualization of the results. We also thank Namık Aysal, Gönenç Göçmengil, and Fatma Şişman Tükel for their kind support during the LA-ICP-MS analyses. During the preparation of this manuscript, the authors used ChatGPT ver. GPT-3.5 (o3) (OpenAI, San Francisco, CA, USA) for language refinement, assistance in drafting selected figures, and support in the organization and interpretation of data analyses. The authors have thoroughly reviewed and edited all AI-assisted content and take full responsibility for the final interpretations, methods, and conclusions presented herein. We further acknowledge the handling editor and anonymous reviewers for their constructive comments and valuable suggestions, which helped to improve the overall clarity and scientific quality of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Götze, J.; Möckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Morey, G.W.; Fournier, R.O.; Rowe, J.J. The Solubility of Quartz in Water in the Temperature Interval from 25° to 300° C. Geochim. Cosmochim. Acta 1962, 26, 1029–1043. [Google Scholar] [CrossRef]
- Webster, J.D.; Thomas, R. Silicate Melt Inclusions in Felsic Plutons: A Synthesis and Review. Melt Incl. Plutonic Rocks 2006, 36, 165–188. [Google Scholar]
- Wilkinson, J.J. Fluid Inclusions in Hydrothermal Ore Deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Redmond, P.B.; Einaudi, M.T.; Inan, E.E.; Landtwing, M.R.; Heinrich, C.A. Copper Deposition by Fluid Cooling in Intrusion-Centered Systems: New Insights from the Bingham Porphyry Ore Deposit, Utah. Geology 2004, 32, 217–220. [Google Scholar] [CrossRef]
- Landtwing, M.R.; Pettke, T. Relationships between SEM-Cathodoluminescence Response and Trace-Element Composition of Hydrothermal Vein Quartz. Am. Mineral. 2005, 90, 122–131. [Google Scholar] [CrossRef]
- Gao, S.; Zou, X.; Hofstra, A.H.; Qin, K.; Marsh, E.E.; Bennett, M.M.; Li, G.; Jiang, J.; Su, S.; Zhao, J.; et al. Trace Elements in Quartz: Insights into Source and Fluid Evolution in Magmatic-Hydrothermal Systems. Econ. Geol. 2022, 117, 1415–1428. [Google Scholar] [CrossRef]
- Monecke, T.; Kempe, U.; Götze, J. Genetic Significance of the Trace Element Content in Metamorphic and Hydrothermal Quartz: A Reconnaissance Study. Earth Planet. Sci. Lett. 2002, 202, 709–724. [Google Scholar] [CrossRef]
- Flem, B.; Larsen, R.B.; Grimstvedt, A.; Mansfeld, J. In Situ Analysis of Trace Elements in Quartz by Using Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Chem. Geol. 2002, 182, 237–247. [Google Scholar] [CrossRef]
- Guillong, M.; Hametner, K.; Reusser, E.; Wilson, S.A.; Günther, D. Preliminary Characterisation of New Glass Reference Materials (GSA-1G, GSC-1G, GSD-1G and GSE-1G) by Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry Using 193 Nm, 213 Nm and 266 Nm Wavelengths. Geostand. Geoanal. Res. 2005, 29, 315–331. [Google Scholar] [CrossRef]
- Rottier, B.; Casanova, V. Trace Element Composition of Quartz from Porphyry Systems: A Tracer of the Mineralizing Fluid Evolution. Miner. Depos. 2021, 56, 843–862. [Google Scholar] [CrossRef]
- Hong, J.; Zhai, D.; Keith, M. Quartz Texture and the Chemical Composition Fingerprint of Ore-Forming Fluid Evolution at the Bilihe Porphyry Au Deposit, NE China. Am. Mineral. 2024, 109, 1203–1219. [Google Scholar] [CrossRef]
- Yılmaz, H. Stream Sediment Geochemical Exploration for Gold in the Kazdaǧ Dome in the Biga Peninsula, Western Turkey. Turk. J. Earth Sci. 2007, 16, 33–55. [Google Scholar]
- Özbaş, F. Tuztaşı (Çanakkale) Altın Cevherleşmesini Oluşturan Çözeltilerin Kökeni ve Karakteristikleri; Istanbul Üniversitesi-Cerrahpaşa: Istanbul, Türkiye, 2023. [Google Scholar]
- Özbaş, F.; Hanilçi, N. Quartz Textures, Mineral Chemistry and Fluid Inclusion Features of Tuztaşı Low-Sulphidation Au Mineralization: Implication to It’s Formation. Geochemistry 2024, 85, 126220. [Google Scholar] [CrossRef]
- Siyako, M.; Burkan, K.A.; Ve Okay, A.I. Biga ve Gelibolu Yarımadalarının Tersiyer Jeolojisi ve Hidrokarbon Olanakları. TPJD Bülteni 1989, 1, 183–199. [Google Scholar]
- Okay, A.I.; Siyako, M.; Bürkan, K.A. Biga Yarımadası’nın Jeolojisi ve Tektonik Evrimi. TPJD Bülteni 1990, 2, 83–121. [Google Scholar]
- Altunkaynak, Ş.; Genç, Ş.C. Petrogenesis and Time-Progressive Evolution of the Cenozoic Continental Volcanism in the Biga Peninsula, NW Anatolia (Turkey). Lithos 2008, 102, 316–340. [Google Scholar] [CrossRef]
- Yigit, O. A Prospective Sector in the Tethyan Metallogenic Belt: Geology and Geochronology of Mineral Deposits in the Biga Peninsula, NW Turkey. Ore Geol. Rev. 2012, 46, 118–148. [Google Scholar] [CrossRef]
- Aysal, N. Mineral Chemistry, Crystallization Conditions and Geodynamic Implications of the Oligo–Miocene Granitoids in the Biga Peninsula, Northwest Turkey. J. Asian Earth Sci. 2015, 105, 68–84. [Google Scholar] [CrossRef]
- Sánchez, M.G.; McClay, K.R.; King, A.R.; Wijbrams, J.R. Cenozoic Crustal Extension and Its Relationship to Porphyry Cu-Au-(Mo) and Epithermal Au-(Ag) Mineralization in the Biga Peninsula, Northwestern Turkey. 2016. Available online: https://pubs.geoscienceworld.org/segweb/books/edited-volume/1387/chapter-abstract/107045996/Cenozoic-Crustal-Extension-and-Its-Relationship-to?redirectedFrom=fulltext (accessed on 12 August 2015).
- Leroux, G.M. Stratigraphic and Petrographic Characterization of HS Epithermal Au-Ag Mineralization at the TV Tower District, Biga Peninsula, NW Turkey. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
- Bozkaya, G.; Banks, D.A.; Ozbas, F.; Wallington, J. Fluid Processes in the Tesbihdere Base-Metal-Au Deposit: Implications for Epithermal Mineralization in the Biga Peninsula, NW Turkey. Cent. Eur. J. Geosci. 2014, 6, 148–169. [Google Scholar] [CrossRef]
- Yigit, O. Mineral Deposits of Turkey in Relation to Tethyan Metallogeny: Implications for Future Mineral Exploration. Econ. Geol. 2009, 104, 19–51. [Google Scholar] [CrossRef]
- Duru, M.; Pehlivan, Ş.; Şentürk, Y. New Results on the Lithostratigraphy of the Kazdağ Massif in Northwest Turkey. Turk. J. Earth Sci. 2004, 13, 177–186. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral Textures and Fluid Inclusion Petrography of the Epithermal Ag-Au Deposits at Guanajuato, Mexico: Application to Exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. Hydrothermal Mobilization of Pegmatite-Hosted REE and Zr at Strange Lake, Canada: A Reaction Path Model. Geochim. Cosmochim. Acta 2013, 122, 324–352. [Google Scholar] [CrossRef]
- Bau, M. Controls on the Fractionation of Isovalent Trace Elements in Magmatic and Aqueous Systems: Evidence from Y/Ho, Zr/Hf, and Lanthanide Tetrad Effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Compilation of Stable Isotope Fractionation Factors of Geochemical Interest; United States Government Printing Office: Washington, DC, USA, 1977.
- Taylor, H. Oxygen and Hydrogen Isotope Relationships in Hydrothermal Mineral Deposits. In Geochemistry of Hydrothermal Ore Deposits; John Wiley & Sons, Inc.: New York, NY, USA, 1997; pp. 236–277. [Google Scholar]
- Hedenquist, J.W.; Lowenstern, J.B. The Role of Magmas in the Formation of Hydrothermal Ore Deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Simmons, S.F.; White, N.C.; John, D.A. Geological Characteristics of Epithermal Precious and Base Metal Deposits; GeoScienceWorld: McLean, VA, USA, 2005. [Google Scholar]
- Heinrich, C.A. Fluid-Fluid Interactions in Magmatic-Hydrothermal Ore Formation. Rev. Mineral. Geochem. 2007, 65, 363–387. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Yao, M.; Zhang, H. Significance of Quartz REE Geochemistry, Shihu Gold Deposit, Western Hebei Province, North China, Using LA-ICP-MS. Front. Earth Sci. China 2010, 4, 337–344. [Google Scholar] [CrossRef]
- Mlynarczyk, M.S.J.; Williams-Jones, A.E. The Role of Collisional Tectonics in the Metallogeny of the Central Andean Tin Belt. Earth Planet. Sci. Lett. 2005, 240, 656–667. [Google Scholar] [CrossRef]
- Meyer, N.; Burisch, M.; Gutzmer, J.; Krause, J.; Scheibert, H.; Markl, G. Mineral Chemistry of the Geyer SW Tin Skarn Deposit: Understanding Variable Fluid/Rock Ratios and Metal Fluxes. Miner. Depos. 2024, 60, 85–111. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal Transport and Deposition of the Rare Earth Elements by Fluorine-Bearing Aqueous Liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, S.B.; Zheng, Y.F.; Wu, S.T.; Li, W.C.; Sun, F.Y.; Liang, T. Mobilization and Fractionation of HFSE and REE by High Fluorine Fluid of Magmatic Origin during the Alteration of Amphibolite. Lithos 2022, 420–421, 106701. [Google Scholar] [CrossRef]
- Wood, S.A. The Aqueous Geochemistry of the Rare-Earth Elements and Yttrium. 2. Theoretical Predictions of Speciation in Hydrothermal Solutions to 350°C at Saturation Water Vapor Pressure. Chem. Geol. 1990, 88, 99–125. [Google Scholar] [CrossRef]
- Romer, R.L.; Kroner, U. Phanerozoic Tin and Tungsten Mineralization-Tectonic Controls on the Distribution of Enriched Protoliths and Heat Sources for Crustal Melting. Gondwana Res. 2016, 31, 60–95. [Google Scholar] [CrossRef]
- Rusk, B.G.; Lowers, H.A.; Reed, M.H. Trace Elements in Hydrothermal Quartz: Relationships to Cathodoluminescent Textures and Insights into Vein Formation. Geology 2008, 36, 547–550. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Habermann, D. Origin, Spectral Characteristics and Practical Applications of the Cathodoluminescence (CL) of Quartz—A Review. Mineral. Petrol. 2001, 71, 225–250. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and Mineral Chemistry of Quartz: A Review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Wark, D.A.; Watson, E.B. TitaniQ: A Titanium-in-Quartz Geothermometer. Contrib. Mineral. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and Minor Elements in Sphalerite: A LA-ICPMS Study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Rusk, B. Cathodoluminescent Textures and Trace Elements in Hydrothermal Quartz. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–329. [Google Scholar]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of Hydrothermal Magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Catchpole, H.; Kouzmanov, K.; Putlitz, B.; Seo, J.H.; Fontboté, L. Zoned Base Metal Mineralization in a Porphyry System: Origin and Evolution of Mineralizing Fluids in the Morococha District, Peru. Econ. Geol. 2015, 110, 39–71. [Google Scholar] [CrossRef]
- Monnier, L.; Salvi, S.; Pochon, A.; Melleton, J.; Béziat, D.; Lach, P.; Bailly, L. Antimony in Quartz as a Vector to Mineralization: A Statistical Approach from Five Variscan Sb Occurrences (France). J. Geochem. Explor. 2021, 221, 106705. [Google Scholar] [CrossRef]
- Audétat, A.; Günther, D.; Heinrich, C.A. Formation of a Magmatic-Hydrothermal Ore Deposit: Insights with LA-ICP-MS Analysis of Fluid Inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [CrossRef]
- Thomas, J.B.; Watson, E.B.; Spear, F.S.; Shemella, P.T.; Nayak, S.K.; Lanzirotti, A. TitaniQ under Pressure: The Effect of Pressure and Temperature on the Solubility of Ti in Quartz. Contrib. Mineral. Petrol. 2010, 160, 743–759. [Google Scholar] [CrossRef]
- Altunkaynak, Ş.; Sunal, G.; Aldanmaz, E.; Genç, C.Ş.; Dilek, Y.; Furnes, H.; Foland, K.A.; Yang, J.; Yildiz, M. Eocene Granitic Magmatism in NW Anatolia (Turkey) Revisited: New Implications from Comparative Zircon SHRIMP U-Pb and 40Ar-39Ar Geochronology and Isotope Geochemistry on Magma Genesis and Emplacement. Lithos 2012, 155, 289–309. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).