Zr-Th-REE Mineralization Associated with Albite–Aegirine-Bearing Rocks of the Burpala Alkaline Intrusion (North Baikal Region, South Margin of the Siberian Craton)
Abstract
1. Introduction
2. Materials and Methods
3. Geological and Mineralogical Background
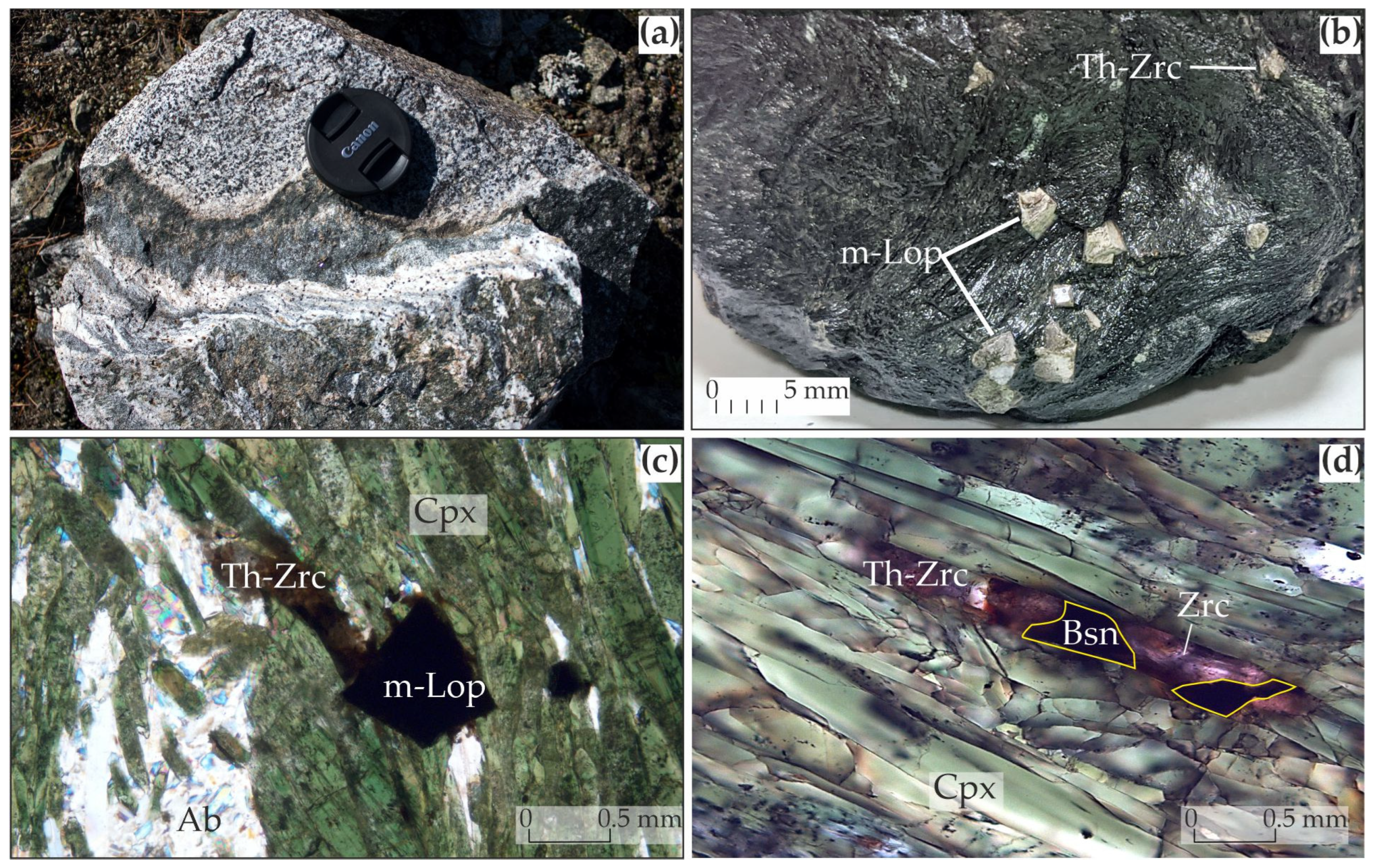
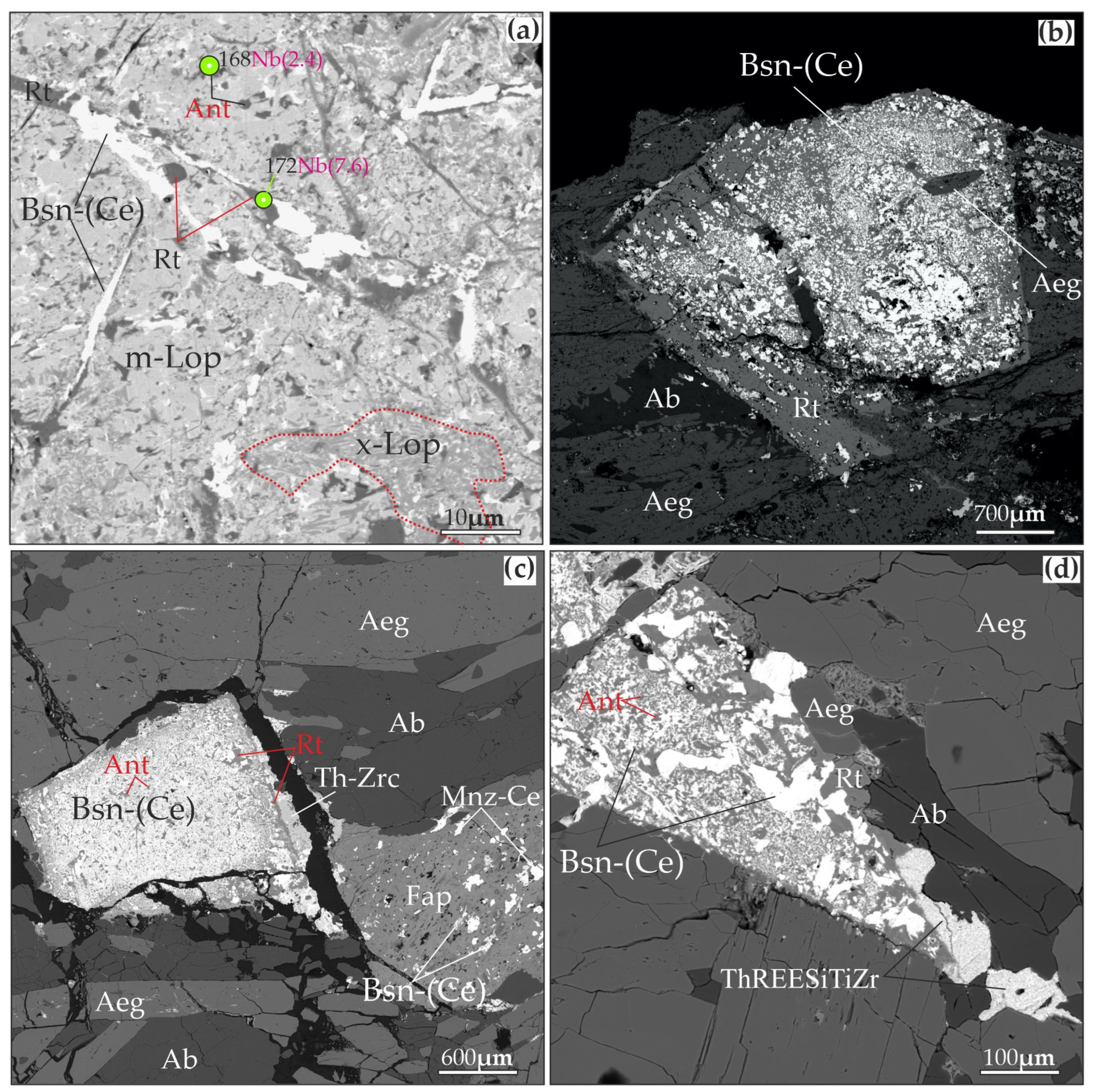
4. Results of the Study of Albite–Aegirine Rocks
Mineral Chemistry
5. Discussion
5.1. Genesis of Albite–Aegirine Rocks of the Burpala Alkaline Intrusion: The Role of Magmatic and Hydrothermal Processes
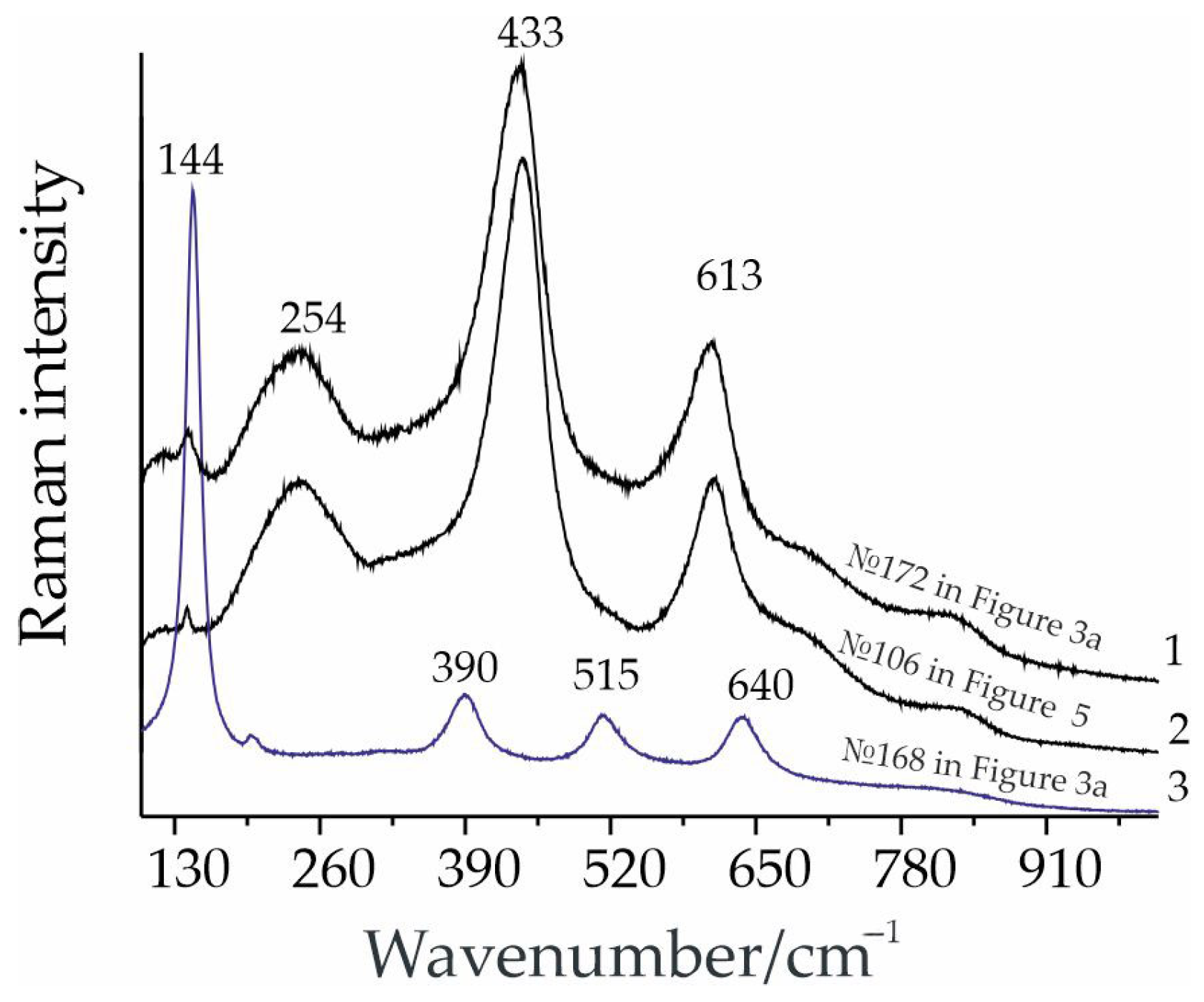
5.2. Formation of “Metaloparite” Under Hydrothermal Conditions
5.3. Zircon Formation in Albite–Aegirine Rocks
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheard, E.R.; Williams-Jones, A.E.; Heiligmann, M.; Pederson, J.C.; Trueman, D.L. Controls on the Concentration of Zirconium, Niobium, and the Rare Earth Elements in the Thor Lake Rare Metal Deposit, Northwest Territories, Canada. Econ. Geol. 2012, 107, 81–104. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E.; Collins, P. Lithogeochemical Vectors for Hydrothermal Processes in the Strange Lake Peralkaline Granitic REE-Zr-Nb Deposit. Econ. Geol. 2016, 111, 1241–1276. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Mitchell, R.H.; Ren, M.; Pal, S.; Pal, S.; Sen, A.K. Nb–Zr–REE Re-Mobilization and Implications for Transitional Agpaitic Rock Formation: Insights from the Sushina Hill Complex, India. J. Petrol. 2018, 59, 1899–1938. [Google Scholar] [CrossRef]
- Liu, S.; Fan, H.-R.; Yang, K.-F.; Hu, F.-F.; Rusk, B.; Liu, X.; Li, X.-C.; Yang, Z.-F.; Wang, Q.-W.; Wang, K.-Y. Fenitization in the Giant Bayan Obo REE-Nb-Fe Deposit: Implication for REE Mineralization. Ore Geol. Rev. 2018, 94, 290–309. [Google Scholar] [CrossRef]
- Cuney, M.; Émertz, A.; Mercadier, J.; Mykchaylov, V.; Shunko, V.; Yuslenko, A. U Deposits Associated with Sodium Metasomatism from Central Ukraine: A Review of Some of the Major Deposits and Genetic Constraints. Ore Geol. Rev. 2012, 44, 82–106. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Sotnikova, I.A. Petrology, Geochemistry and Source Characteristics of the Burpala Alkaline Massif, North Baikal. Geosci. Front. 2017, 8, 711–719. [Google Scholar] [CrossRef]
- Semenov, E.I.; Es’kova, E.M.; Kapustin, Y.L.; Khomyakov, A.P. Mineralogy of Alkaline Massifs and Their Deposits; Nauka: Moscow, Russia, 1974; p. 251. [Google Scholar]
- Portnov, A.M. Burpala—A Mineralogical Reserve? Priroda 2018, 5, 73–82. [Google Scholar]
- Arkhangel’skaya, V.V. Rare-Metal Alkaline Complexes of the Southern Margin of the Siberian Platform; Nedra: Moscow, Russia, 1974; p. 128. [Google Scholar]
- Portnov, A.M. Calcium Catapleiite—A New Variety of Catapleiite. Dokl. Akad. Nauk USSR 1964, 154, 603–609. [Google Scholar]
- Portnov, A.M.; Nikolaeva, L.V.; Stolyarova, T.I. Calcium Seidocerite—A New Variety of Seidocerite. Dokl. Akad. Nauk USSR 1964, 156, 338–340. [Google Scholar]
- Portnov, A.M.; Nikolaeva, L.V.; Stolyarova, T.I. Landauite, a New Titanium Mineral. Dokl. Akad. Nauk USSR 1966, 166, 1420–1421. [Google Scholar]
- Merlino, S.; Perchiazzi, N.; Khomyakov, A.P.; Pushcharovskii, D.Y.; Kulikova, I.M.; Kuzmin, V.I. Burpalite, a New Mineral from Burpalinskii Massif, North Transbaikalia, USSR: Its Crystal Structure and OD Character. Eur. J. Miner. 1990, 2, 177–185. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H.; Pankov, A.V.; Chukanov, N.V. Loparite and ‘Metaloparite’ from the Burpala Alkaline Complex, Baikal Alkaline Province (Russia). Miner. Mag. 1999, 63, 519–534. [Google Scholar] [CrossRef]
- Macdonald, R.; Baginski, B.; Kartashov, P.; Zozulia, D.; Dzierzanowski, P. Chevkinite-Group Minerals from Russia and Mongolia: New Data from Fenites, Metasomatites and Ore Deposits. Miner. Mag. 2012, 76, 535–550. [Google Scholar] [CrossRef]
- Stachowicz, M.; Bagiński, B.; Macdonald, R.; Kartashov, P.M.; Oziębło, A.; Wożniak, K. Structure of Sr-Zr-Bearing Perrierite-(Ce) from the Burpala Massif, Russia. Miner. Mag. 2014, 78, 1647–1659. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chakhmouradian, A.R. Th-Rich Loparite from the Khibina Alkaline Complex, Kola Peninsula: Isomorphism and Paragenesis. Miner. Mag. 1998, 62, 341–353. [Google Scholar] [CrossRef]
- Skublov, S.G.; Li, S.-H. Anomalous Geochemistry of Zircon from the Yastrebetskoye Rare-Metal Deposit (SIMS and TOF Study). Zap. Gorn. Inst. 2016, 222, 798–802. [Google Scholar]
- Hoskin, P.W.O.; Schaltegger, U. The Composition of Zircon and Igneous and Metamorphic Petrogenesis. Rev. Miner. Geochem. 2003, 53, 25–104. [Google Scholar] [CrossRef]
- Vlach, S.R.F. On the Morphology and Geochemistry of Hydrothermal Crypto- and Microcrystalline Zircon Aggregates in a Peralkaline Granite. Minerals 2022, 12, 628. [Google Scholar] [CrossRef]
- Abd El-Naby, H.H. High and Low Temperature Alteration of Uranium and Thorium Minerals, Um Ara Granites, South Eastern Desert, Egypt. Ore Geol. Rev. 2009, 35, 436–446. [Google Scholar] [CrossRef]
- Abd El-Naby, H.H. Alteration and Non-Formula Elements Uptake of Zircon from Um Ara Granite, South Eastern Desert, Egypt. Minerals 2024, 14, 834. [Google Scholar] [CrossRef]
- Starikova, A.E.; Doroshkevich, A.G.; Sklyarov, E.V.; Donskaya, T.V.; Gladkochub, D.P.; Shaparenko, E.O.; Zhukova, I.A.; Semenova, D.V.; Yakovenko, E.S.; Ragozin, A.L. Magmatism and Metasomatism in the Formation of the Katugin Nb-Ta-REE-Zr-Cryolite Deposit, Eastern Siberia, Russia: Evidence from Zircon Data. Lithos 2024, 472–473, 107557. [Google Scholar] [CrossRef]
- Griffin, W.L.; Powell, W.J.; Pearson, N.J.; O’Reilly, S.Y. GLITTER: Data Reduction Software for Laser Ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Mineralogical Association of Canada, Short Course Series; Sylvester, P., Ed.; Mineralogical Association of Canada: Québec City, QC, Canada, 2008; Volume 40, pp. 307–311. [Google Scholar]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The Application of Laser Ablation-Inductively Coupled Plasma Mass Spectrometry to In Situ U-Pb Zircon Geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Yuan, H.-L.; Gao, S.; Dai, M.-N.; Zong, C.-L.; Günther, D.; Fontaine, G.H.; Liu, X.-M.; Diwu, C.R. Simultaneous Determination of U-Pb Age, Hf Isotopes and Trace Element Compositions of Zircon by Excimer Laser-Ablation Quadrupole and Multiple-Collector ICP-MS. Geostand. Geoanal. Res. 2008, 32, 405–419. [Google Scholar] [CrossRef]
- Piazolo, S.; Belousova, E.; La Fontaine, A.; Corcoran, C.; Cairney, J.M. Trace Element Homogeneity from Micron- to Atomic Scale: Implications for the Suitability of Zircon GJ-1 as a Reference Material. Chem. Geol. 2017, 456, 10–18. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC Approved Mineral Symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Jahn, B.M.; Litvinovsky, B.A.; Zanvilevich, A.N.; Reichow, M. Peralkaline Granitoid Magmatism in the Mongolian–Transbaikalian Belt: Evolution, Petrogenesis and Tectonic Significance. Lithos 2009, 113, 521–539. [Google Scholar] [CrossRef]
- Litvinovsky, B.A.; Tsygankov, A.A.; Jahn, B.M.; Katzir, Y.; Be’eri-Shlevin, Y. Origin and Evolution of Overlapping Calc-Alkaline and Alkaline Magmas: The Late Palaeozoic Post-Collisional Igneous Province of Transbaikalia (Russia). Lithos 2011, 125, 845–874. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Ripp, G.S.; Izbrodin, I.A.; Savatenkov, V.M. Alkaline Magmatism of the Vitim Province, West Transbaikalia, Russia: Age, Mineralogical, Geochemical and Isotope (O, C, D, Sr and Nd) Data. Lithos 2012, 152, 157–172. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Burmakina, G.N.; Khubanov, V.B.; Buyantuev, M.D. Geodynamics of Late Paleozoic Batholith-Forming Processes in Western Transbaikalia. Petrology 2017, 25, 396–418. [Google Scholar] [CrossRef]
- Izbrodin, I.; Doroshkevich, A.G.; Rampilov, M.; Elbaev, A.; Ripp, G. Late Paleozoic Alkaline Magmatism in Western Transbaikalia, Russia: Implications for Magma Sources and Tectonic Settings. Geosci. Front. 2020, 11, 1289–1303. [Google Scholar] [CrossRef]
- Andreev, A.A.; Rytsk, E.Y.; Velikoslavinskii, S.D.; Tolmacheva, E.V.; Bogomolov, E.S.; Lebedeva, Y.M.; Fedoseenko, A.M. Age, Composition, and Tectonic Setting of the Formation of Late Neoproterozoic (Late Baikalian) Complexes in the Kichera Zone, Baikal-Vitim Belt, Northern Baikal Area: Geological, Geochronological, and Nd Isotope Data. Petrology 2022, 30, 345–378. [Google Scholar] [CrossRef]
- Andreev, G.V. Petrology of the Formation of Potassic, Nepheline, and Alkaline Syenites; Nauka: Novosibirsk, Russia, 1981; p. 85. [Google Scholar]
- Pak, A.S.; Mikov, N.A.; Bushuev, V.P.; Mikhalev, D.F.; Tokar’, N.V.; Yudovskii, A.G. Report on the Results of Geological Exploration Work by the Sol’ Party in the Maigunda River Basin for 1960–1961; Buryat Geological Department: Ulan-Ude, Russia, 1962; p. 213. [Google Scholar]
- Kotov, A.B.; Vladykin, N.V.; Yarmolyuk, V.V.; Sal’nikova, E.B.; Sotnikova, I.A.; Yakovleva, S.Z. Permian Age of the Burpala Alkaline Intrusion (Northern Baikal Area): Geodynamic Implications. Dokl. Akad. Nauk 2013, 453, 295–299. [Google Scholar]
- Izbrodin, I.A.; Doroshkevich, A.G.; Malyutina, A.V.; Semenova, D.V.; Radomskaya, T.A.; Kruk, M.N.; Prokopev, I.R.; Starikova, A.E.; Rampilov, M.O. Geochronology of the Rocks of the Burpala Alkaline Complex (Northern Baikal Area): New U-Pb Data. Geodin. Tektonofiz. 2024, 15, 741. [Google Scholar] [CrossRef]
- Starikova, A.E.; Malyutina, A.V.; Izbrodin, I.A.; Doroshkevich, A.G.; Radomskaya, T.A.; Isakova, A.T.; Semenova, D.V.; Korsakov, A.V. Mineralogical-Petrographic and Geochemical Characteristics of Zircon as an Indication of Its Formation Conditions Based on Zircons from Rocks of the Burpalinsky Massif, Northern Baikal Region. Geodin. Tektonofiz. 2024, 15, 787. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Sotnikova, I.A.; Kotov, A.B.; Yarmolyuk, V.V.; Sal’nikova, E.B.; Yakovleva, S.Z. Structure, Age, and Ore Potential of the Burpala Rare-Metal Alkaline Massif (Northern Baikal Area). Geol. Rudn. Mestorozhd. 2014, 56, 272–290. [Google Scholar]
- Malyutina, A.V.; Doroshkevich, A.G.; Starikova, A.E.; Izbrodin, I.A.; Prokop’ev, I.R.; Radomskaya, T.A.; Kruk, M.N. Composition of Mafic Rock-Forming Minerals in the Rocks of the Burpala Alkaline Massif (Northern Baikal Area). Russ. Geol. Geophys. 2025, 66, 299–315. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Savatenkov, V.M.; Malyutina, A.V.; Izbrodin, I.A.; Prokopiev, I.R.; Starikova, A.E.; Radomskaya, T.A. Petrogenesis and Sources for Rocks of the Alkaline Rare-Metal Burpala Intrusion (Northern Baikal Region). Petrology 2025, 33, 45–67. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Sacco, A.; Mandrile, L.; Tay, L.-L.; Itoh, N.; Raj, A.; Moure, A.; Del Campo, A.; Fernandez, J.F.; Paton, K.R.; Wood, S.; et al. Quantification of Titanium Dioxide (TiO2) Anatase and Rutile Polymorphs in Binary Mixtures by Raman Spectroscopy: An Interlaboratory Comparison. Metrologia 2023, 60, 05501. [Google Scholar] [CrossRef]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the Apatite Supergroup Minerals. Eur. J. Miner. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Rodgers, K.A. Raman Spectral Shift in the Isomorphous Series (Zr1−xHfx)SiO4. Eur. J. Solid State Inorg. Chem. 1996, 33, 1111–1121. [Google Scholar]
- Nasdala, L.; Irmer, G.; Wolf, D. The Degree of Metamictization in Zircon: A Raman Spectroscopic Study. Eur. J. Miner. 1995, 7, 471–478. [Google Scholar] [CrossRef]
- Zhang, M.; Salje, E.K.H.; Farnan, I.; Graeme-Barber, A.; Daniel, P.; Ewing, R.C.; Clark, A.M.; Leroux, H. Metamictization of Zircon: Raman Spectroscopic Study. J. Phys. Condens. Matter 2000, 12, 1915–1925. [Google Scholar] [CrossRef]
- Zamyatin, D.A. Application of Raman Spectroscopy for Studying Shocked Zircon from Terrestrial and Lunar Impactites: A Systematic Review. Minerals 2022, 12, 969. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y. Magmatic–Hydrothermal Zircons in Syenite: A Record of Nb-Ta Mineralization Processes in the Emeishan Large Igneous Province, SW China. Chem. Geol. 2022, 589, 120675. [Google Scholar] [CrossRef]
- Kostyleva-Labuntsova, E.E.; Borutskii, B.E.; Sokolova, M.N.; Shlyukova, Z.V.; Dorfman, M.D.; Dudkin, O.B.; Kozyreva, L.V. Mineralogy of the Khibiny Intrusion; Nauka: Moscow, Russia, 1978; Volume 2, p. 586. [Google Scholar]
- Feng, Y.; Samson, I. Replacement Processes Involving High Field Strength Elements in the T Zone, Thor Lake Rare-Metal Deposit. Can. Miner. 2015, 53, 31–60. [Google Scholar] [CrossRef]
- Giebel, R.J.; Gauert, C.D.K.; Marks, M.A.W.; Costin, G.; Markl, G. Multi-Stage Formation of REE Minerals in the Palabora Carbonatite Complex, South Africa. Am. Miner. 2017, 102, 1218–1233. [Google Scholar] [CrossRef]
- Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V. Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Intrusion, Russia). Geosciences 2018, 8, 281. [Google Scholar] [CrossRef]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of Zircon in Aqueous Fluids and Melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Corfu, F.; Hanchar, J.; Hoskin, P.W.O.; Kinny, P. Atlas of Zircon Textures. Rev. Miner. Geochem. 2003, 53, 469–500. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Bronswijk, W.; Kurtz, R. Transport of Uranium, Thorium, and Lead in Metamict Zircon under Low-Temperature Hydrothermal Conditions. Chem. Geol. 2002, 191, 141–154. [Google Scholar] [CrossRef]
- Hoskin, P.W.O. Trace-Element Compositions of Hydrothermal Zircon and the Alteration of Hadean Zircon from the Jack Hills. Geochim. Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Förster, H.J. Composition and Origin of Intermediate Solid Solutions in the System Thorite–Xenotime–Zircon–Coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.; Chen, X.; Wang, D. Th-Rich Zircon from Peralkaline A-Type Granite: Mineralogical Features and Petrological Implications. Chin. Sci. Bull. 2005, 50, 809–817. [Google Scholar]
- Yin, R.; Sun, X.-M.; Wang, S.-W.; Ru-Cheng, W.; Ran, M.-L.; Wu, B.; Huang, X.-L. Zircons in NYF-Type Pegmatites in the Emeishan Large Igneous Province, SW China: A Record of Nb and REE Mineralization Processes. Ore Geol. Rev. 2023, 162, 105700. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Roy, R. Hydrothermal Stability Studies of the Zircon-Thorite Group. Geochim. Cosmochim. Acta 1961, 21, 217–238. [Google Scholar] [CrossRef]
- Burnham, A.D.; Berry, A.J. An Experimental Study of Trace Element Partitioning Between Zircon and Melt as a Function of Oxygen Fugacity. Geochim. Cosmochim. Acta 2012, 95, 196–212. [Google Scholar] [CrossRef]
- Trail, D.; Bruce, E.; Tailby, N.D. Ce and Eu Anomalies in Zircon as Proxies for the Oxidation State of Magmas. Geochim. Cosmochim. Acta 2012, 97, 70–87. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Frost, B.R. On the Controls of Oxygen Fugacity in the Generation and Crystallization of Peralkaline Melts. J. Petrol. 2010, 51, 1831–1847. [Google Scholar] [CrossRef]
- Skublov, S.G. Geochemistry of Rare Earth Elements in Rock-Forming Minerals of Metamorphic Rocks; Nauka: St. Petersburg, Russia, 2005; p. 147. [Google Scholar]
- Bouvier, A.-S.; Ushikubo, T.; Kita, N.T.; Cavosie, A.J.; Kozdon, R.; Valley, J.W. Li Isotopes and Trace Elements as a Petrogenetic Tracer in Zircon: Insights from Archean TTGs and Sanukitoids. Contrib. Miner. Petrol. 2012, 163, 745–768. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Lissenberg, C.J.; Brooker, R.A.; Hinton, R.W.; Trail, D.; Hellebrand, E. Hydrogen Incorporation and Charge Balance in Natural Zircon. Geochim. Cosmochim. Acta 2014, 141, 472–486. [Google Scholar] [CrossRef]
- Hay, D.C.; Dempster, T.J. Zircon Alteration, Formation and Preservation in Sandstones. Sedimentology 2009, 56, 2175–2191. [Google Scholar] [CrossRef]
- Hoshino, M.; Watanabe, Y.; Murakami, H.; Kon, Y.; Tsunematsu, M. Formation Process of Zircon Associated with REE-Fluorocarbonate and Niobium Minerals in the Nechalacho REE Deposit, Thor Lake, Canada. Resour. Geol. 2012, 63, 1–26. [Google Scholar] [CrossRef]
- Breiter, K.; Förster, H.-J.; Škoda, R. Extreme P-, Bi-, Nb-, Sc-, U- and F-Rich Zircon from Fractionated Perphosphorous Granites: The Peraluminous Podlesí Granite System, Czech Republic. Lithos 2006, 88, 15–34. [Google Scholar] [CrossRef]
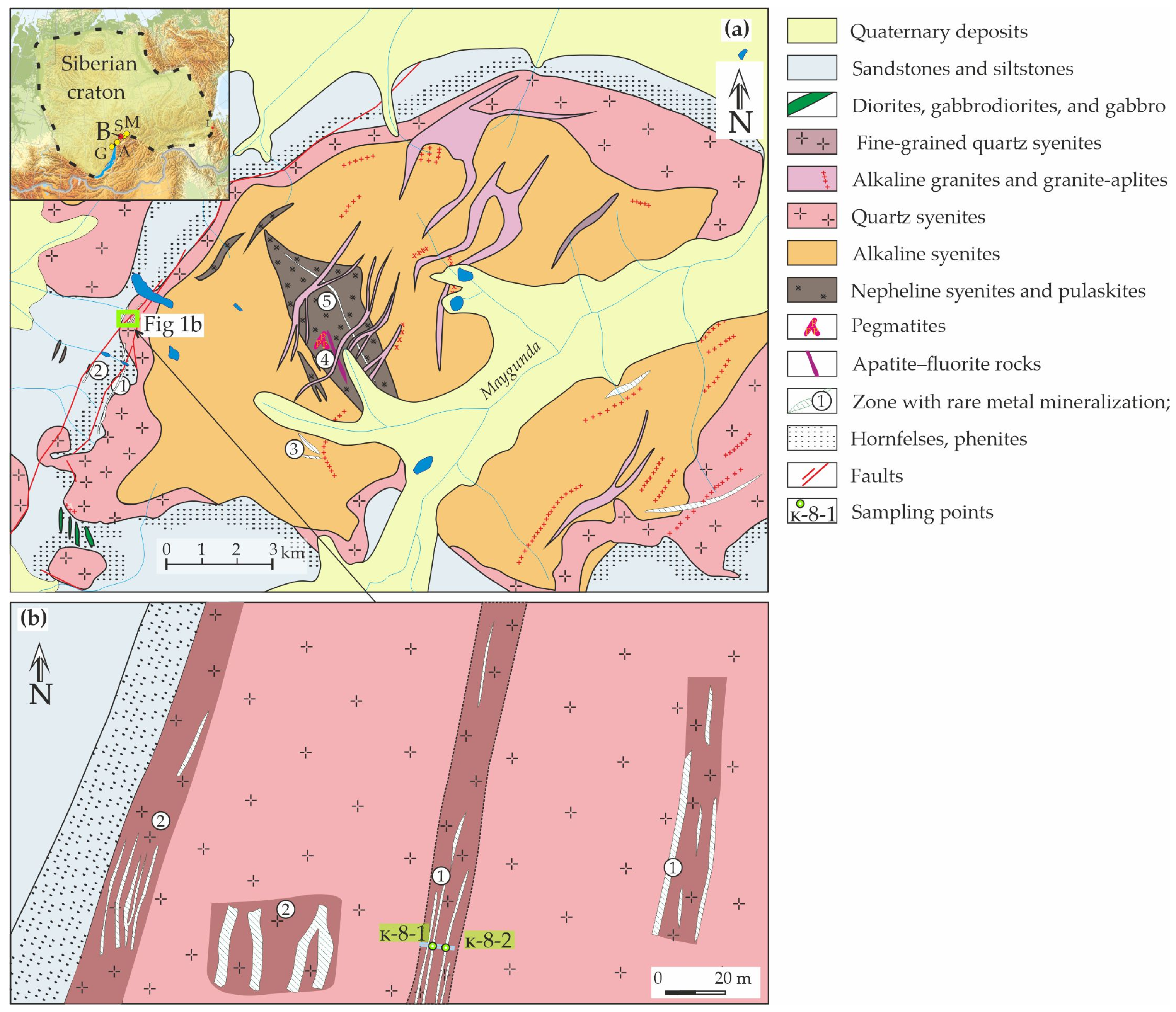
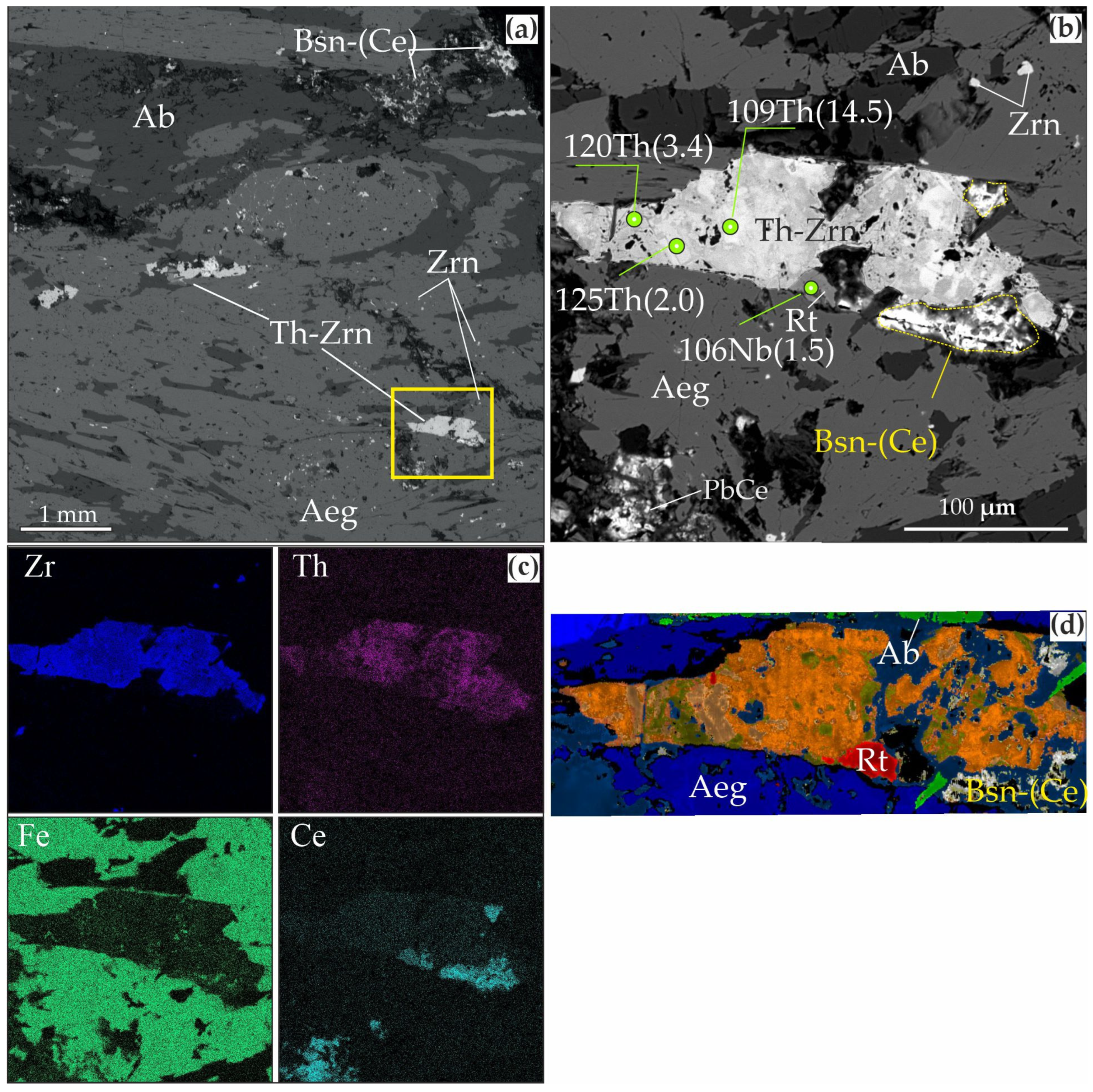
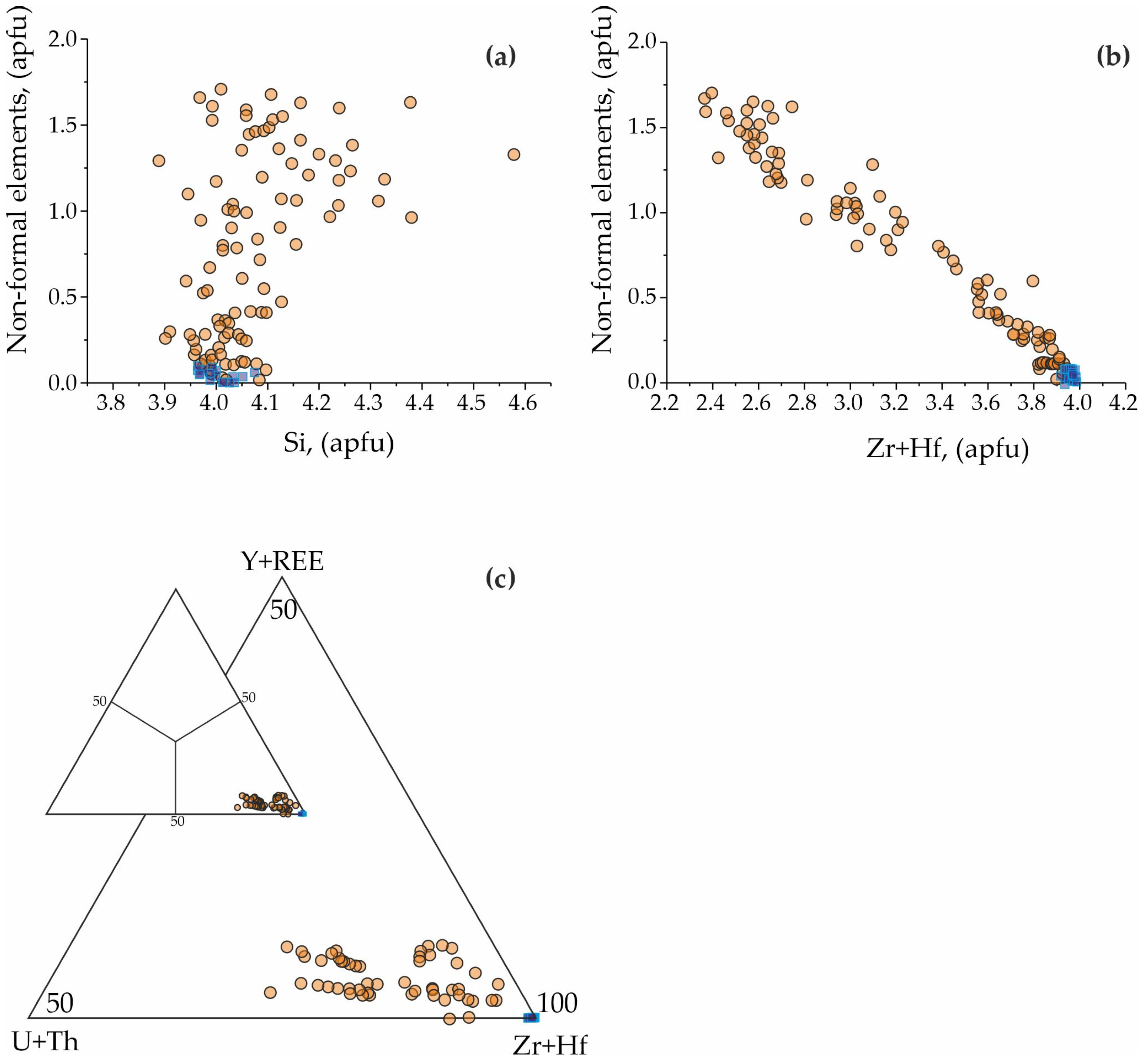

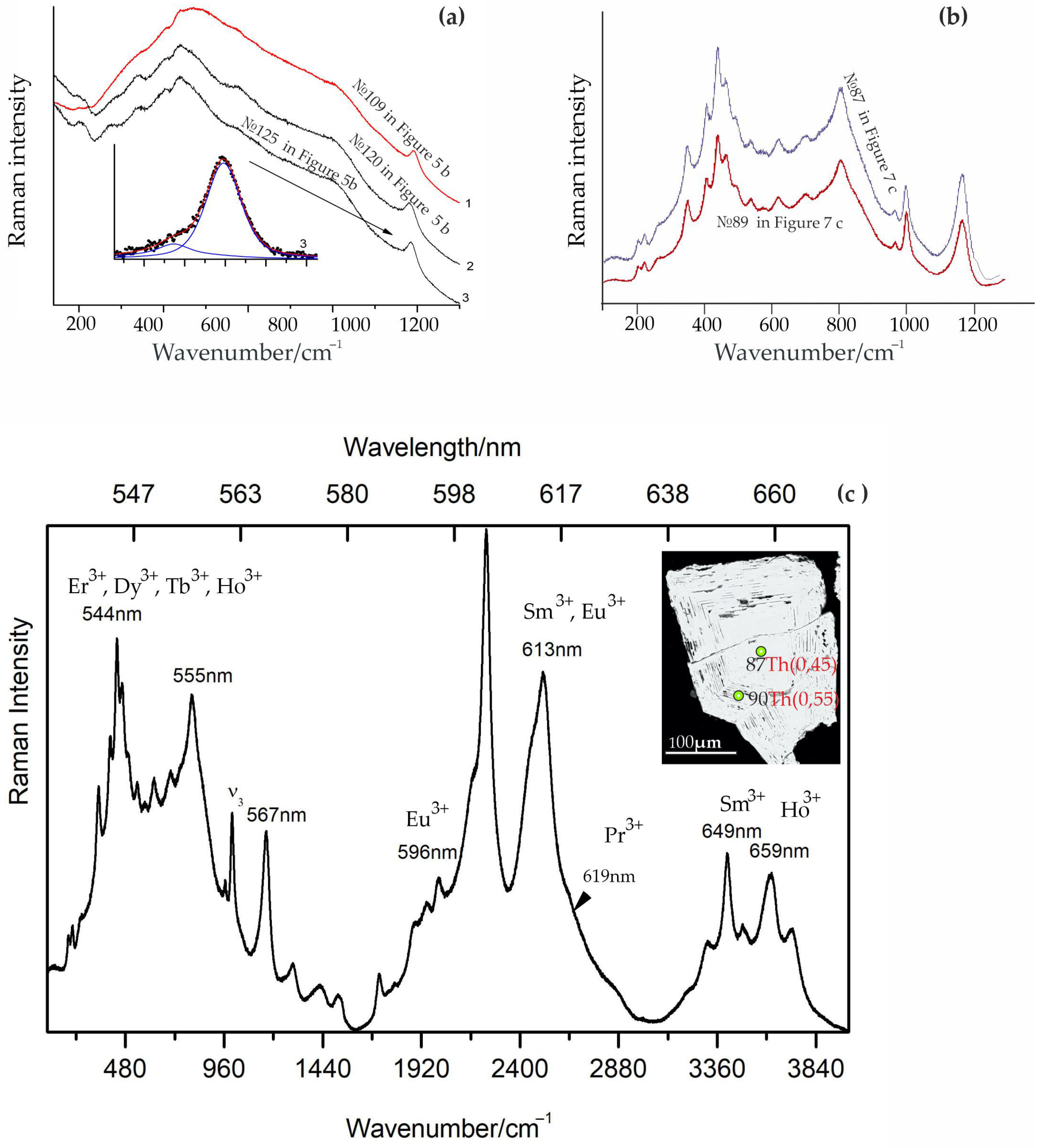
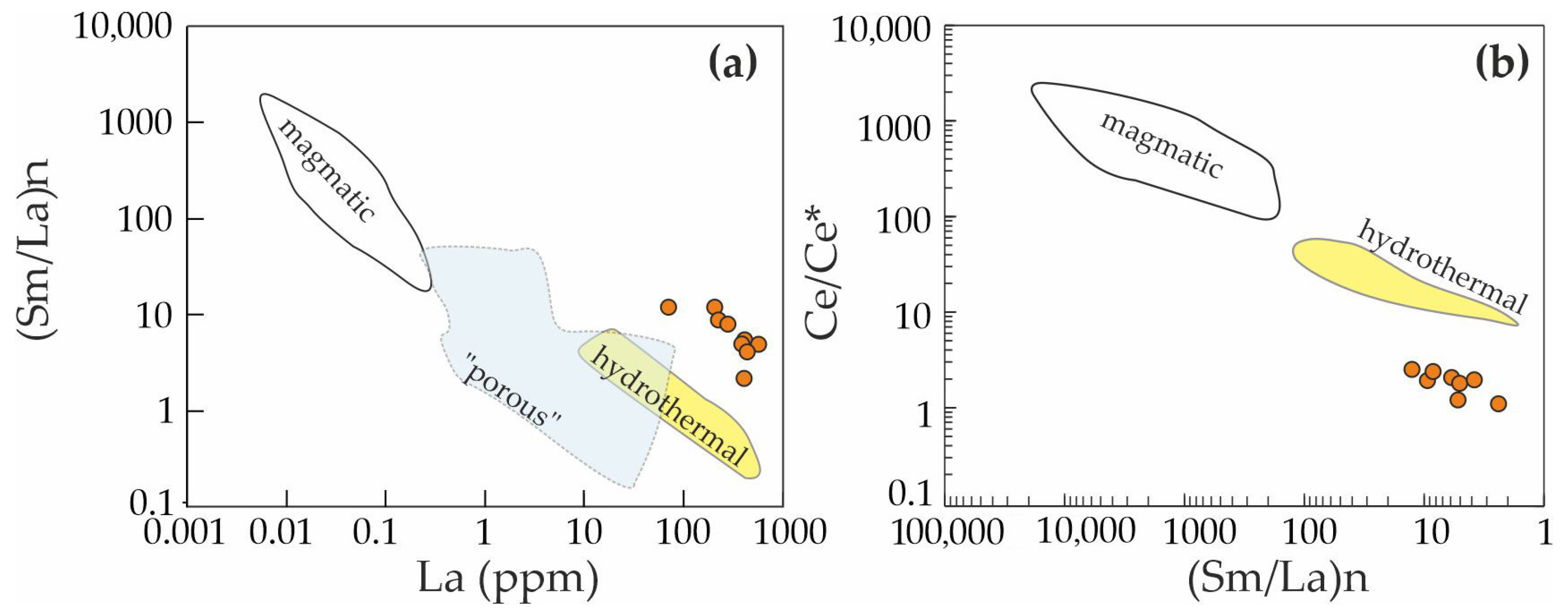
| Ore Zone | Type of Rocks | Ore Minerals Associated | Size | Age, Ma (References) |
|---|---|---|---|---|
| №5 | Nepheline syenites | Zircon, thorite, loparite-(Ce), chevkinite-(Ce), bastnäsite-(Ce), and apatite-(Ce), | The total thickness of the zone is more than 100 m, with a length of more than 2 km. | 296 ± 2 [38] |
| №4 | Dyke-like fluorite-apatite body, fluoritized, and albitized syenites | Apatite-(Ce), fluorite, and leucophanite | The thickness is 4–5 m, with a length of up to 150 m and eluvial landfills on an area of 150 × 40 m | |
| №3 | Pegmatoid alkaline syenites and pegmatites | Eudialyte, lamprophyllite, catapleite, lovenite, vlasovite, ramsite, astrophyllite, tainiolite, ilmenite, pyrophanite, magnetite, chalcopyrite, chalcosine, pyrite, wolframite, apatite-(Ce), britholite-(Ce), and titanite | The thickness is 1–2 m, up to 30 m, and the length is 100–200 m | 283 ± 8 [37] |
| №2 | Fenites AND albite–aegirine rocks | Zircon, thorite, catapleiite, lovenite, astrophyllite, aeschynite, calcium seidoserite, ilmenite, rutile, loparite-(Ce), “metaloparite”, melanocerite-(Ce), chevkinite-(Ce), bastnäsite-(Ce), monazite-(Ce), apatite-(Ce), perrierite, leucophanite, burpalite, landauite, U-pyrochlore, and fluorite | The thickness of the fenitization zone is 10–30 m, with albite–aegirine metasomatites 1–5 m, extending up to 4 km | 295 ± 3 [39] |
| №1 | Albite–aegirine and aegirine–microcline rocks | Zircon, thorite, catapleiite, lovenite, astrophyllite, aeschynite, calcium seidoserite, ilmenite, rutile, loparite-(Ce), “metaloparite”, melanocerite-(Ce), chevkinite-(Ce), bastnäsite-(Ce), monazite-(Ce), apatite-(Ce), perrierite, leucophanite, burpalite, landauite, U-pyrochlore, and fluorite | The thickness is 1–5 m in length with interruptions up to 4 km |
| № | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | k8-1 | k8-1 | k8-1a | k8-2 | k8-1 | k8-1a | k8-1 | k8-1 | k8-1 | k8-1 | k8-1 |
| SiO2 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.60 | 9.69 | 8.32 | 8.90 | 9.65 |
| CaO | 0.92 | 1.04 | 0.74 | 0.15 | 0.13 | 0.14 | 0.32 | 2.14 | 1.37 | 2.14 | 2.01 |
| SrO | 1.47 | 0.43 | 0.78 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.69 |
| BaO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 2.49 | 2.13 | b.d. | b.d. |
| La2O3 | 12.65 | 13.90 | 14.05 | 14.73 | 13.74 | 12.29 | 13.74 | 4.44 | 8.47 | b.d. | 2.54 |
| Ce2O3 | 20.22 | 21.07 | 20.87 | 23.12 | 21.89 | 21.46 | 22.03 | 12.04 | 16.49 | 6.44 | 8.48 |
| Pr2O3 | 1.16 | 1.43 | 1.40 | 1.19 | 1.58 | 1.49 | 1.64 | 0.94 | 1.29 | b.d. | 1.04 |
| Nd2O3 | 3.56 | 3.67 | 3.38 | 3.32 | 4.15 | 4.30 | 3.78 | 3.18 | 2.90 | 2.69 | 3.23 |
| ThO2 | 4.89 | 4.16 | 4.56 | 3.80 | 5.13 | 7.03 | 5.87 | 7.17 | 4.75 | 19.67 | 19.08 |
| UO2 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 1.17 |
| PbO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 8.81 | 4.19 |
| MnO | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 2.00 | 1.30 | 1.98 | 1.61 |
| TiO2 | 45.70 | 44.65 | 44.27 | 43.80 | 43.49 | 43.24 | 42.90 | 35.35 | 34.25 | 23.62 | 24.07 |
| Fe2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.51 | 0.45 | b.d. | b.d. |
| Nb2O5 | 2.53 | 4.38 | 3.73 | 4.71 | 4.66 | 4.08 | 3.96 | 5.11 | 4.46 | 7.30 | 7.85 |
| Total | 93.10 | 94.73 | 93.78 | 94.82 | 94.77 | 94.03 | 94.84 | 85.06 | 86.18 | 84.37 | 85.61 |
| The formula is calculated on the basis of two cations at the B site | |||||||||||
| Si | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | ||||
| Ca | 0.06 | 0.06 | 0.05 | 0.01 | 0.01 | 0.01 | 0.02 | ||||
| Sr | 0.05 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| La | 0.26 | 0.29 | 0.30 | 0.31 | 0.29 | 0.26 | 0.30 | ||||
| Ce | 0.42 | 0.43 | 0.44 | 0.48 | 0.46 | 0.46 | 0.47 | ||||
| Pb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Pr | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | ||||
| Nd | 0.07 | 0.07 | 0.07 | 0.07 | 0.09 | 0.09 | 0.08 | ||||
| Th | 0.06 | 0.05 | 0.06 | 0.05 | 0.07 | 0.09 | 0.08 | ||||
| U | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| ∑ | 0.94 | 0.96 | 0.96 | 0.94 | 0.95 | 0.94 | 1.02 | ||||
| Ti | 1.94 | 1.89 | 1.90 | 1.88 | 1.88 | 1.89 | 1.90 | ||||
| Fe3+ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Nb | 0.07 | 0.11 | 0.10 | 0.12 | 0.12 | 0.11 | 0.11 | ||||
| ∑ | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | ||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 1.25 | 0.15 | b.d. | b.d. | b.d. | 0.20 | b.d. | b.d. | 0.35 | b.d. | b.d. |
| TiO2 | b.d. | b.d. | b.d. | b.d. | 0.82 | 0.72 | b.d. | 0.73 | 0.57 | b.d. | b.d. |
| SrO | b.d. | b.d. | b.d. | 0.72 | b.d. | b.d. | b.d. | 0.48 | b.d. | b.d. | b.d. |
| ThO2 | 1.35 | 1.63 | 1.13 | 3.03 | 1.41 | 0.53 | 0.83 | 1.66 | b.d. | b.d. | b.d. |
| La2O3 | 28.17 | 21.88 | 26.22 | 25.03 | 25.73 | 25.95 | 21.72 | 24.31 | 27.23 | 23.97 | 21.98 |
| Ce2O3 | 30.55 | 34.87 | 35.14 | 36.11 | 36.72 | 36.73 | 36.74 | 36.75 | 37.12 | 37.16 | 37.46 |
| Pr2O3 | 2.13 | 2.12 | 1.95 | 1.99 | 1.95 | 2.50 | 2.29 | 1.94 | 1.72 | 2.21 | 2.48 |
| Nd2O3 | 6.14 | 7.22 | 4.98 | 4.91 | 4.98 | 5.16 | 7.07 | 5.18 | 4.11 | 5.77 | 5.61 |
| F | 8.60 | 7.90 | 8.74 | 8.86 | 8.96 | 9.20 | 8.74 | 8.87 | 7.92 | 8.55 | 8.59 |
| Total | 78.19 | 75.77 | 78.16 | 80.65 | 80.57 | 80.99 | 77.39 | 79.92 | 79.02 | 77.66 | 76.12 |
| –O=F2 | 3.61 | 3.32 | 3.67 | 3.72 | 3.76 | 3.87 | 3.67 | 3.73 | 3.33 | 3.59 | 3.61 |
| Total | 74.58 | 72.45 | 74.49 | 76.93 | 76.81 | 77.12 | 73.72 | 76.19 | 75.69 | 74.07 | 72.51 |
| № | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | k8-1 | k8-1a | k8-2 | k8-1a | k8-2 | k8-1 | k8-1 | k8-1 | k8-1 | k8-1a | k8-1 |
| SiO2 | 33.75 | 33.16 | 33.73 | 30.44 | 30.74 | 25.65 | 28.13 | 23.94 | 23.47 | 29.03 | 28.35 |
| ZrO2 | 65.73 | 64.97 | 63.76 | 59.38 | 57.69 | 41.81 | 51.02 | 29.66 | 29.18 | 49.9 | 43.06 |
| HfO2 | 1.01 | 1.89 | 1.76 | 0.8 | 1.2 | b.d. | 1.36 | 0.87 | b.d. | 1.06 | 0.99 |
| ThO2 | b.d. | b.d. | b.d. | 1.83 | 2.00 | 2.57 | 3.2 | 15.54 | 17.69 | 4.04 | 9.02 |
| UO2 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.59 | b.d. | b.d. | b.d. | b.d. | b.d. |
| TiO2 | b.d. | b.d. | b.d. | b.d. | 0.38 | 0.87 | 0.43 | 1.03 | 1.02 | 0.53 | 0.95 |
| Al2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | 0.42 | 0.21 | b.d. | b.d. | b.d. | 0.28 |
| Ce2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | 1.17 | b.d. | 1.04 | 1.19 | 0.49 | 0.8 |
| Nd2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.79 | 0.79 | 0.58 | 0.78 |
| Y2O3 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 1.84 | 1.93 |
| FeO | b.d. | b.d. | b.d. | b.d. | 1.04 | 0.90 | 1.07 | 2.50 | 1.83 | 1.81 | 1.83 |
| Fe2O3 | b.d. | b.d. | b.d. | b.d. | 1.16 | 1.00 | 1.19 | 2.78 | 2.03 | 2.01 | 2.03 |
| MnO | b.d. | b.d. | b.d. | b.d. | 0.43 | 0.56 | 0.34 | 0.7 | 1.01 | 0.5 | 0.74 |
| CaO | b.d. | b.d. | b.d. | b.d. | 0.24 | 0.67 | 0.32 | 1.05 | 1.12 | 0.62 | 0.83 |
| Na2O | b.d. | b.d. | b.d. | 0.63 | 0.36 | 1.24 | 0.42 | b.d. | b.d. | 0.35 | 0.42 |
| Nb2O5 | b.d. | b.d. | b.d. | b.d. | b.d. | 2.03 | 1.32 | 1.39 | 1.86 | b.d. | 1.30 |
| Total | 100.49 | 100.02 | 99.25 | 93.08 | 95.24 | 79.48 | 89.01 | 81.29 | 81.19 | 92.76 | 93.31 |
| Cation proportions on the basis of 16 oxygen atoms | |||||||||||
| Si | 3.95 | 3.91 | 3.95 | 4.02 | 4.03 | 4.02 | 3.98 | 4.10 | 4.06 | 4.01 | 4.00 |
| Zr | 3.87 | 3.89 | 3.87 | 3.82 | 3.68 | 3.20 | 3.52 | 2.48 | 2.46 | 3.36 | 2.96 |
| Hf | 0.04 | 0.07 | 0.06 | 0.03 | 0.04 | 0.00 | 0.05 | 0.04 | 0.00 | 0.04 | 0.04 |
| Th | 0.00 | 0.00 | 0.00 | 0.05 | 0.06 | 0.09 | 0.10 | 0.61 | 0.70 | 0.13 | 0.29 |
| U | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.10 | 0.05 | 0.13 | 0.13 | 0.06 | 0.10 |
| Al | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.03 | 0.00 | 0.00 | 0.00 | 0.05 |
| Ce | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.07 | 0.08 | 0.02 | 0.04 |
| Nd | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.05 | 0.03 | 0.04 |
| Y | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.14 |
| Fe | 0.00 | 0.00 | 0.00 | 0.05 | 0.11 | 0.12 | 0.13 | 0.36 | 0.26 | 0.21 | 0.22 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.07 | 0.04 | 0.10 | 0.15 | 0.06 | 0.09 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.11 | 0.05 | 0.19 | 0.21 | 0.09 | 0.13 |
| Na | 0.00 | 0.00 | 0.00 | 0.16 | 0.09 | 0.38 | 0.12 | 0.00 | 0.00 | 0.09 | 0.11 |
| Nb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.08 | 0.11 | 0.15 | 0.00 | 0.08 |
| Component | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| P | 155 | 159 | 168 | 230 | 428 | 390 | 268 | 204 | 120 |
| Ca | 1531 | 1225 | 1137 | 625 | 3020 | 1235 | 999 | 553 | 352 |
| Ti | 238 | 333 | 356 | 139 | 1357 | 928 | 292 | 140 | 36 |
| Rb | 5.3 | 110 | 24 | 42 | 17 | 28 | 66 | 50 | 1.5 |
| Sr | 142 | 103 | 104 | 39 | 262 | 73 | 64 | 37 | 16 |
| Y | 10,110 | 6428 | 8150 | 6832 | 8379 | 6950 | 6815 | 6491 | 5201 |
| Nb | 860 | 1227 | 1319 | 458 | 2437 | 1503 | 745 | 489 | 118 |
| Ba | 186 | 156 | 175 | 74 | 283 | 210 | 130 | 69 | 21 |
| La | 46 | 91 | 92 | 62 | 125 | 90 | 86 | 50 | 16 |
| Ce | 711 | 1691 | 977 | 1285 | 752 | 283 | 888 | 965 | 174 |
| Pr | 76 | 226 | 118 | 148 | 143 | 43 | 121 | 138 | 15 |
| Nd | 668 | 1365 | 648 | 1177 | 832 | 235 | 849 | 975 | 109 |
| Sm | 374 | 338 | 226 | 337 | 418 | 133 | 284 | 299 | 130 |
| Eu | 115 | 87 | 71 | 86 | 111 | 42 | 81 | 79 | 59 |
| Gd | 495 | 325 | 311 | 308 | 445 | 219 | 302 | 293 | 412 |
| Tb | 126 | 75 | 84 | 75 | 119 | 74 | 73 | 71 | 96 |
| Dy | 1113 | 674 | 850 | 703 | 1070 | 809 | 715 | 669 | 828 |
| Ho | 304 | 184 | 264 | 214 | 292 | 244 | 207 | 188 | 217 |
| Er | 1226 | 809 | 1163 | 959 | 1213 | 1026 | 939 | 864 | 752 |
| Tm | 278 | 189 | 292 | 238 | 280 | 243 | 226 | 204 | 143 |
| Yb | 2178 | 1520 | 2371 | 1953 | 2209 | 1889 | 1813 | 1646 | 906 |
| Lu | 346 | 248 | 389 | 326 | 361 | 311 | 299 | 273 | 136 |
| Hf | 7197 | 8090 | 6870 | 8185 | 7402 | 7610 | 7270 | 7783 | 4901 |
| Ta | 33 | 42 | 55 | 17 | 46 | 33 | 21 | 16 | 1.7 |
| Th | 5722 | 2851 | 2523 | 4974 | 6513 | 5407 | 4002 | 3049 | 3368 |
| U | 474 | 1042 | 943 | 790 | 2004 | 1327 | 1075 | 897 | 2930 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izbrodin, I.A.; Doroshkevich, A.G.; Starikova, A.E.; Malyutina, A.V.; Moroz, T.N.; Sharygin, I.S. Zr-Th-REE Mineralization Associated with Albite–Aegirine-Bearing Rocks of the Burpala Alkaline Intrusion (North Baikal Region, South Margin of the Siberian Craton). Minerals 2025, 15, 742. https://doi.org/10.3390/min15070742
Izbrodin IA, Doroshkevich AG, Starikova AE, Malyutina AV, Moroz TN, Sharygin IS. Zr-Th-REE Mineralization Associated with Albite–Aegirine-Bearing Rocks of the Burpala Alkaline Intrusion (North Baikal Region, South Margin of the Siberian Craton). Minerals. 2025; 15(7):742. https://doi.org/10.3390/min15070742
Chicago/Turabian StyleIzbrodin, Ivan Aleksandrovich, Anna Gennadievna Doroshkevich, Anastasia Evgenyevna Starikova, Alexandra Vladislavovna Malyutina, Tatyana Nikolaevna Moroz, and Igor Sergeevich Sharygin. 2025. "Zr-Th-REE Mineralization Associated with Albite–Aegirine-Bearing Rocks of the Burpala Alkaline Intrusion (North Baikal Region, South Margin of the Siberian Craton)" Minerals 15, no. 7: 742. https://doi.org/10.3390/min15070742
APA StyleIzbrodin, I. A., Doroshkevich, A. G., Starikova, A. E., Malyutina, A. V., Moroz, T. N., & Sharygin, I. S. (2025). Zr-Th-REE Mineralization Associated with Albite–Aegirine-Bearing Rocks of the Burpala Alkaline Intrusion (North Baikal Region, South Margin of the Siberian Craton). Minerals, 15(7), 742. https://doi.org/10.3390/min15070742






