Abstract
Rare-earth elements (REEs), including lanthanides, scandium, and yttrium, are important for advanced technologies such as renewable energy systems, electronics, medical diagnostics, and precision agriculture. Despite their relative crustal abundance, REE extraction is impeded by complex geochemical behavior, dispersed distribution, and environmental challenges. This review presents a comprehensive overview of REE geochemistry, mineralogy, and major deposit types including carbonatites, alkaline igneous rocks, laterites, placer deposits, coal byproducts, and marine sediments. It also highlights the global distribution and economic potential of key REE projects. The integration of machine learning has further enhanced exploration by enabling deposit classification and geochemical modeling, especially in data-limited regions. Environmental and health challenges associated with REE mining, processing, and electronic waste (e-waste) recycling are studied, along with the expanding use of REEs in agriculture and medicine. Some recycling efforts offer promise for supply diversification, but significant technological and economic barriers remain. Ensuring a secure and sustainable REE supply will require integrated approaches combining advanced analytics, machine learning, responsible extraction, and coordinated policy efforts. The present review offers a general overview that can be useful for informing future studies and resource-related discussions.
1. Introduction
REEs comprise 17 elements, including the fifteen lanthanides—lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu)—along with scandium (Sc) and yttrium (Y), which are classified with lanthanides because of their similar ionic sizes and chemical properties [1]. REEs are commonly categorized into two sub-groups according to their atomic numbers and characteristics: light rare-earth elements (LREEs), which include La, Ce, Pr, Nd, Pm, Sm, and Eu, and heavy rare-earth elements (HREEs), consisting of Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu, along with Y and Sc [2]. The distinctive magnetic, catalytic, optical, and electronic properties of REEs have made them important for a broad spectrum of advanced technologies. LREEs and HREEs find use in permanent magnets (Nd, Dy, Tb), phosphors for LED displays and lighting (Eu, Tb, Y), petroleum refining catalysts (Ce, La), and emerging applications in green energy, biomedicine, and quantum computing [3,4,5,6,7]. Figure 1 highlights the wide range of industrial uses of REEs, demonstrating the contribution of individual elements to fields such as magnets, phosphors, metal alloys, catalysts, glass and ceramics, and other advanced technologies.
Figure 1.
Key topics related to REEs, including geological resources, mineral hosts, industrial applications, market dynamics, secondary sources and recycling, and associated environmental concerns [3,5].
These widespread applications underscore the essential role of REEs in driving technological innovation and supporting global energy transitions. Despite their relatively high crustal abundance ranging from 130 to 240 µg/g [8], economically recoverable REE deposits are limited and geographically concentrated. Over the past decades, China has dominated the global supply chain, contributing 70–90% of global REE production [9,10,11]. To protect its domestic reserves and mitigate environmental impacts, China has implemented strict export controls, production quotas, and licensing systems [12,13,14,15,16]. These regulatory actions have heightened international interest in diversifying REE sources through exploration, recycling, and material substitution [17,18,19,20].
In response to growing concerns over supply vulnerability, several nations including the United States, Canada, Australia, and members of the European Union have designated REEs as critical or strategic materials. The 2021 U.S. White House 100-Day Supply Chain Review emphasized the urgency of reducing dependence on foreign REE sources by encouraging domestic production and advancing technological innovation [21,22]. These efforts reflect a broader geopolitical contest surrounding REE access and underscore the importance of sustainable resource management. Table 1 shows the average concentrations of REEs (in µg/g) within the Earth’s crust and their corresponding chondritic abundances, providing a reference point for understanding their natural distribution. An overview of their natural abundance helps illustrate why REEs are both strategically important and geopolitically contested. Table 1 displays the average REE concentrations (in µg/g) in the Earth’s crust alongside their chondritic abundances, serving as a baseline for interpreting their natural distribution. To better understand the strategic importance and extraction challenges of REEs, it is essential to examine their geochemical behavior and mineral hosts. Chondritic abundances refer to the concentration of elements in chondrite meteorites, which are considered primitive, undifferentiated materials representing the bulk composition of the early solar system. These values serve as a reference baseline in geochemistry for evaluating elemental enrichment or depletion in Earth’s crust and mantle.

Table 1.
Comparison of average REEs concentrations in the Earth’s crust (µg/g) from three different geochemical studies and their corresponding chondritic abundances. Differences among the crustal values reflect variations in sampling sources, geological coverage, and analytical methods. Crustal values are based on Taylor and McLennan [23], Wedepohl [24], and Abu El-Anwar [25]. Chondritic values are based on Wakita et al. [26] and Pourmand et al. [27].
2. Geochemistry and Mineralogy of REEs
2.1. Behavior in the Crust and Mantle
Due to their high reactivity, REEs are not found as native metals in nature. Instead, they are considered lithophile elements, tending to concentrate in the Earth’s silicate fraction, characterized by large ionic radii and a mainly trivalent oxidation state under crustal conditions [28,29,30]. Their geochemical behavior is largely governed by ionic size and charge, which allows them to substitute for major cations such as Ca2+, Y3+, and Th4+ in a wide range of mineral lattices [31].
The average total REE concentration in the continental crust is estimated at approximately 169.1 µg/g, consisting of ~137.8 µg/g LREEs and ~31.3 µg/g HREEs [32]. This value falls within the range of REE concentrations reported in Table 1, which compiles data from different authors to illustrate the variability in crustal estimates across studies. Among these, Ce (~63 µg/g), La (~31 µg/g), Nd (~27 µg/g), and Y (~21 µg/g) are the most abundant. These concentrations are comparable to, or even exceed, those of several commonly used industrial metals such as copper (~60 µg/g), zinc (~70 µg/g), and cobalt (~25 µg/g), suggesting that the term “rare” is somewhat misleading [30]. Nevertheless, REEs rarely occur in pure mineral phases and are typically dispersed in low concentrations within accessory minerals, which presents significant challenges for their economic extraction [31,33,34,35]. As a result, they are commonly dispersed in a wide range of minerals rather than forming large, concentrated deposits.
2.2. Common REE-Bearing Minerals
While more than 250 minerals are known to contain REEs, only a few have notable economic importance [29]. REE-bearing minerals span several mineralogical classes, including phosphates, carbonates, silicates, oxides, and mixed phases. Among the most commercially important are monazite, bastnäsite, and xenotime, which collectively serve as the primary global sources of REEs [36,37]. Monazite is particularly enriched in LREEs and often contains appreciable concentrations of thorium and uranium, raising radiological concerns in certain deposits [38,39,40,41]. In contrast, xenotime is a valuable host of HREEs, such as Y, Dy, Er, and Yb, and is typically associated with granitic pegmatites and high-grade metamorphic terrains [42]. Table 2 lists key REE-bearing minerals and their chemical formulas, based on data from Dostal et al. [43]. The minerals have been reordered based on a qualitative assessment of their relative abundance in REE-bearing deposits and their historical economic significance in commercial REE production, and their corresponding rare-earth oxide (REO) contents are also shown.

Table 2.
Key REE-bearing minerals, listed based on a qualitative assessment of their geological abundance and historical economic importance in global REE extraction. Chemical formulas and rare-earth oxide (REO) weight percentages are also provided.
3. Deposit Types
3.1. Alkaline Igneous Complexes
The global push toward energy transition and decarbonization has significantly intensified the demand for REEs. This rising demand is placing considerable strain on existing supply chains, which remain heavily dominated by China [5,44,45]. Although recycling is anticipated to contribute more significantly to future REE supply, it is currently inadequate to satisfy short- and mid-term demand forecasts [46,47,48]. Consequently, the exploration and development of primary REEs resources particularly those hosted in alkaline–silicate igneous complexes and carbonatites are becoming increasingly critical [49]. Alkaline igneous rocks, formed through partial melting of mantle-derived materials, are typically rich in incompatible elements including REEs, Zr, Nb, F, Sc, and P [50,51,52,53]. The geodynamic environments that host these complexes are typically intraplate and extensional in nature, encompassing continental rift zones and post-collisional settings [54,55]. Geochemically, alkaline complexes can host both LREEs and HREEs, with mineralization commonly found in layered intrusions, pegmatites, dykes, and volcanic sequences [56].
Several globally significant REEs deposits are associated with alkaline igneous systems. The Mountain Pass deposit in the United States and the Bayan Obo deposit in China are among the most prominent examples; the latter is the largest known REEs deposit globally and contributes approximately 80% of the world’s LREEs production [57]. In India, a number of alkaline complexes have demonstrated considerable REEs potential, including the carbonatites of the Sung Valley complex in Meghalaya and the Saranu and Kamthai deposits in Rajasthan [58,59]. At Kamthai, LREE concentrations as high as 17.31% have been reported, with average grades of 3.33% extending to depths of 84 m [60]. The Amba Dongar carbonatite complex in Gujarat also hosts REE-bearing minerals such as bastnäsite and parisite [61]. Additionally, the Neoproterozoic peralkaline Siwana granite within the Malani Igneous Suite has recorded REE concentrations ranging from 2% to 2.5%, offering high potential for low-cost, open-pit extraction due to favorable topographic and metallurgical conditions [56]. Despite their mineral potential, alkaline–silicate complexes present notable exploration challenges due to their geometallurgical complexity, multi-phase intrusions, variable REE mineralogy, and alteration overprints that obscure surface indicators; these factors complicate both resource estimation and metallurgical processing. Effective exploration requires integrated approaches that combine geophysical surveys (e.g., magnetic, radiometric), geochemical vectoring, and structural mapping; recent efforts also include the application of hyperspectral imaging, machine learning algorithms, and 3D geometallurgical modeling to improve target prediction and resource evaluation in these complex systems. Nonetheless, their broad global distribution, deep mantle origins, and enrichment in critical elements continue to make them high-priority targets for expanding and diversifying future REE supply chains [57].
3.2. Residual and Lateritic Deposits
Residual REE deposits develop through extensive tropical chemical weathering of REE-rich igneous rocks like pegmatites, carbonatites, and iron-oxide copper–gold (IOCG) systems. During this in situ weathering process, chemically mobile elements such as Na, Mg, and Ca are leached out, while relatively immobile elements such as Fe and Al are retained, leading to the concentration of REEs when the parent rock is sufficiently enriched [62,63,64]. Deep weathering of carbonatite and peralkaline intrusive bodies can produce high-grade residual REE enrichment. For instance, lateritic regoliths developed over carbonatites may exhibit REO concentrations ranging from 10% to 25% [65]. A prominent example is the Mount Weld deposit in Western Australia, where weathering enhanced the REO concentration from an original 0.1–0.2% in the unaltered carbonatite to up to 18.0% within the laterite profile produced by weathering [66,67]. Ion-adsorption deposits (IADs) represent a significant type of residual deposit, where REEs are weakly attached to clay minerals in weathered profiles. These deposits are mainly located in southern China, in areas like Hunan, Jiangxi, Fujian, and Guangdong, where they result from the weathering of REE-rich granitic rocks [68,69]. In these systems, REEs are adsorbed onto clay minerals including kaolinite, illite, halloysite, montmorillonite, vermiculite, and chlorite [70,71]. Similar IADs have also been identified outside of China, such as in Mt Clere, Western Australia [72], as well as in parts of Southeast Asia and Japan [73,74]. Additionally, iron–manganese oxides may enhance REEs accumulation particularly of Ce by promoting its oxidative transformation from Ce3+ to Ce4+, resulting in Ce-enriched surface horizons [71,75]. Another potential REEs source is bauxite, which forms primarily through the intense weathering of aluminosilicate-rich rocks. Bauxite residue, also called red mud, a byproduct of the Bayer process, has recently gained attention as a secondary source of REEs due to its residual enrichment [76,77].
3.3. Heavy Mineral Placer Deposits
Placer deposits, formed by the accumulation of heavy, durable minerals transported and concentrated by coastal or river processes, represent an important sedimentary source of REEs [78,79]. These detrital deposits are derived from the weathering and erosion of REE-rich parent rocks, especially high-grade metamorphic and granitic lithologies, and often contain minerals enriched in Ti, Zr, and REEs. To date, more than 360 REE-enriched placer deposits have been identified worldwide [80].
Placer deposits occur in a range of depositional settings and across various geological periods, including Tertiary, Quaternary, and even Precambrian formations in the form of paleo-placers. These can be unlithified (recent or modern deposits) or lithified (ancient, compacted deposits). Lithified paleo-placers have often been buried under significant overburden for hundreds of millions to billions of years, such as the Archaean placers in the Witwatersrand Basin of South Africa. The most economically important REE-bearing placers are typically marine in origin, found along both present-day and ancient shorelines [81]. An important example is the REE-enriched marine placer deposits along the Australian coastline. Monazite is the most common REE-bearing mineral in these placers, though xenotime, euxenite, fergusonite, allanite, samarskite, knopite, pyrochlore, and loparite are also present [28].
3.4. Coal Deposits
Due to the growing global gap between supply and demand for REEs, it is now strategically necessary to look for alternative sources, especially for nations that depend on imports. Among these unconventional sources, coal and its byproducts especially coal fly ash and coal discard have gained attention as promising candidates for REE recovery [82]. Although REE concentrations in raw coal are typically comparable to those found in average sedimentary rocks, the vast quantity of available material enhances its potential significance. On a global scale, the average REE concentration in coal is approximately 68 μg/g [83], with regional variability observed: 138 μg/g in China [84], 116 μg/g in Turkey, 77 μg/g in North Korea, and 62 μg/g in the United States [85].
More notably, REEs tend to become enriched in combustion byproducts particularly fly ash during high-temperature processing. This enrichment is influenced by factors such as the composition of the parent coal, combustion conditions, and the resulting ash mineralogy. On average, global coal ash contains around 403.5 μg/g of REEs, while U.S. fly ash may contain approximately 513 μg/g, with some samples reaching concentrations as high as 1358 μg/g, including Y [86]. In this context, the U.S. Department of Energy has set a baseline of 300 μg/g REEs (on a dry coal basis) as the threshold for considering a coal source to be a viable REE resource [87].
Several studies have demonstrated that coal-based materials may exhibit relative enrichment in HREEs compared to traditional mineral deposits. For instance, Laudal et al. [88] reported HREEs enrichment in certain coal sources, while Zhang and Honaker [89] found that coal combustion byproducts are often enriched in both HREEs and critical REEs (CREEs) elements such as Nd, Eu, Tb, Dy, and Y that are essential for advanced technologies and considered vulnerable to supply risks relative to LREEs [89]. Moreover, REE recovery efforts are not limited to solid combustion residues; significant concentrations have also been identified in coal mine drainage (CMD) and associated treatment system precipitates [90,91]. The widespread occurrence of REEs in fly ash has been confirmed by numerous investigations, including that of Blissett et al. [92], further supporting the viability of coal-derived materials as alternative REE resources.
3.5. REEs in Marine Sediments and Ocean Resources
Eroded materials from land are transported and deposited in offshore environments, so the mineral resources found on the continental shelf often resemble those on nearby land. These resources include beach placer deposits and deeper marine sediments. One of the most important offshore sedimentary resources is phosphorite, or phosphate rock, which is valuable not only as a source of phosphorus but also for its potential to host REEs. Phosphorite is a non-detrital sedimentary rock that usually forms in shallow marine settings on continental shelves through the chemical precipitation of phosphate-rich waters. This process is often associated with regions of strong oceanic upwelling, which lead to the accumulation of phosphate minerals such as carbonate fluorapatite (francolite). These minerals are capable of incorporating REEs via ionic substitution for Ca2+, due to their similar ionic radii [93,94]. Currently, marine sedimentary phosphorites account for approximately 95% of global phosphate reserves [95,96]. Because fluorapatite can structurally host REEs, these deposits are increasingly viewed as viable unconventional REE resources. Numerous REE-enriched phosphorite occurrences have been documented across regions, for example, Australia, the United States, Venezuela, Tunisia, Algeria, and northwestern Saudi Arabia [97,98,99,100]. Reported total REE concentrations in sedimentary phosphate deposits vary widely from several hundred to more than 18,000 µg/g with HREEs’ concentrations reaching up to 7000 µg/g [97], making certain phosphorite bodies comparable in grade to conventional REE ores.
Beyond phosphorites, several deep-sea mineral resources have attracted attention due to their REE content, including polymetallic nodules, Co-rich ferromanganese crusts, and deep-sea muds [101,102,103]. Polymetallic nodules are found on sediment-covered abyssal plains at depths ranging from 4500 to 6000 m, particularly within the Pacific and Indian Oceans [104]. Co-rich ferromanganese crusts develop on hard substrates such as seamounts and ocean ridges and are notably enriched in REEs through sorption onto ferromanganese oxides and associated clay minerals [105,106]. These crusts grow extremely slowly at rates of just 1 to 7 mm per million but are also rich in other critical elements such as Co, Ti, Ni, Pt, and Mo.
Ferromanganese crusts are typically categorized as either hydrogenous or hydrothermal based on their formation processes, with each type exhibiting distinct REE patterns [107]. Some crusts and associated marine sediments have shown REE concentrations exceeding 2500 µg/g, with Ce alone accounting for up to 60% of total REE content [103]. Similar REE-rich crusts have been reported in the Indian Ocean, particularly around the Afanasy Nikitin Seamount [108,109]. Another major marine REE resource is deep-sea mud. Geochemical surveys by Yasukawa et al. [110] and Takaya et al. [111] revealed that deep-sea sediments from regions such as the western North Pacific Ocean can contain REE concentrations exceeding 5000 µg/g. In particular, enriched biogenic calcium phosphate grains have been found to contain up to 22,000 µg/g of REEs, underscoring the significant potential of these deposits. According to Kato et al. [102], a single 2.3 km wide deposit of REE-rich mud near Minamitorishima Island could theoretically meet global REE demand for an entire year. Moreover, simple acid leaching methods have proven effective in recovering REEs from these muds, demonstrating the technical feasibility of extraction. Table 3 shows the average of REEs in different oceans sediments. Despite their promising resource potential, deep-sea mining efforts face considerable technological and environmental barriers. These include the lack of cost-effective deep-sea extraction technology, challenges in transporting and processing materials at extreme depths, and concerns over ecological impacts such as sediment plume generation, habitat destruction, and biodiversity loss. Figure 2 and Figure 3 show the global locations and types of major REE deposits, and deposit types, respectively.
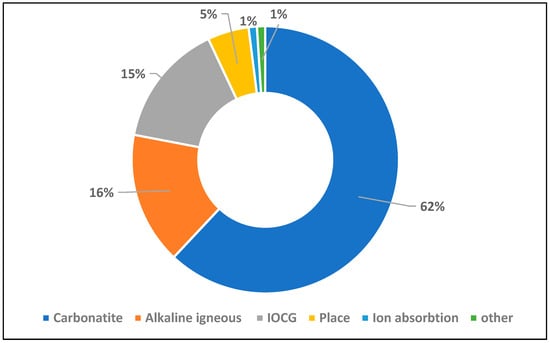
Figure 2.
The distribution of global rare-earth deposit type.

Table 3.
Reported concentrations of total rare-earth elements (ΣREE or ΣREY*, in μg/g) in various marine sediment types and crusts across different oceanic regions: Balaram et al. [108], Cui et al. [112], Nath et al. [113], Gonzalez et al. [114], Li et al. [115], Kato et al. [102], Yasukawa et al. [110], Bu et al. [116], Piper [117], Sattarova et al. [118], Sa et al. [119], Yamazaki et al. [120], and Takaya et al. [111].
Table 3.
Reported concentrations of total rare-earth elements (ΣREE or ΣREY*, in μg/g) in various marine sediment types and crusts across different oceanic regions: Balaram et al. [108], Cui et al. [112], Nath et al. [113], Gonzalez et al. [114], Li et al. [115], Kato et al. [102], Yasukawa et al. [110], Bu et al. [116], Piper [117], Sattarova et al. [118], Sa et al. [119], Yamazaki et al. [120], and Takaya et al. [111].
| Matrix Type | Ocean/Location | ΣREE/ΣREY (μg/g) | Reference |
|---|---|---|---|
| Cobalt crusts | Afanasy Nikitin Seamount (Eastern Indian Ocean) | 1727–2511 | Balaram et al. (2012) [108] |
| Cobalt-rich crusts | Mid-Pacific Seamount | 2084 | Cui et al. (2009) [112] |
| Ferromanganese crusts | Indian Ocean | 928–1570 | Nath et al. (1992) [113] |
| Ferromanganese crusts | Scotia Sea | 3400 | Gonzalez et al. (2010) [114] |
| Deep-sea sediments/mud | Indian Ocean | 399.92–875.27 | Li et al. (2023) [115] |
| Deep-sea sediments/mud | Eastern South Pacific | 1000–2230 | Kato et al. (2011) [102] |
| Deep-sea sediments/mud | North Pacific (Hawaii) | 400–1000 | Kato et al. (2011) [102] |
| Marine mud | Indian Ocean | up to 920 | Yasukawa et al. (2015) [110] |
| Fe-Mn nodules | Mid-Pacific Ocean | 1178–1434 | Bu et al. (2003) [116] |
| Deep nodules | Pacific Ocean | 1326 | Piper (1974) [117] |
| Shallow nodules | Pacific Ocean (shallow water) | 1398 | Piper (1974) [117] |
| Bottom sediments | East Siberian Arctic Shelf | 104 to 220 | Sattarova et al. (2023) [118] |
| Siliceous sediments | Central North Pacific Ocean | 810.4 | Sa et al. (2018) [119] |
| REE-rich mud | Minamitorishima Island in the western North Pacific | >1446.2 (REE+Y) | Yamazaki et al. (2021) [120] |
| Deep-sea mud | North Pacific Ocean near Minamitorishima Island, Japan | >5000 (REE+Y) | Takaya et al. (2018) [111] |
* Note: ΣREE or ΣREY represents the total concentration of measured rare-earth elements.
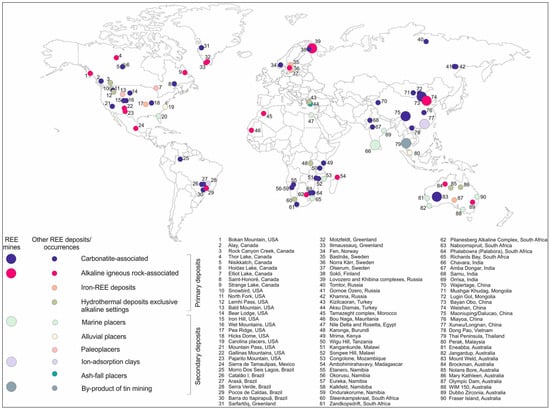
Figure 3.
Global distribution and classification of major REE mines and deposits (NERC 2017) [121].
4. Global REE Projects
Although REE exploration and development projects are widespread globally, most of the resource value is concentrated in Asia and Greenland. By contrast, Australia, although possessing approximately 22% of global REE resources, accounts for only 4% of the total value, as many of its deposits are dominated by low-value LREEs such as La and Ce [7]. Ionic clay deposits in South China and parts of Southeast Asia continue to serve as major sources of HREEs, owing to their favorable leaching properties despite relatively low grades (~0.02 wt%) [7]. In response to China’s dominant position in REE processing which constitutes 70–90% of global capacity at least 67 processing plants are either operational or under development outside of China (Table 4) [7]. These facilities are intended to form integrated international supply chains, linking production centers in Africa and Australia to processing hubs in Europe, North America, and Southeast Asia. In parallel, some countries, including the United States, are also pursuing independent supply chains—such as MP Materials’ initiative to fully process REEs domestically at the Mountain Pass site [7]. If successfully implemented, they are projected to deliver over 75,000 tons of rare-earth oxides (REOs) annually by 2030, partially compensating for the expected 41% rise in global REE demand [7]. The annual production of REEs by country from 1994 to 2050 includes both historical data (1994–2025) and projections (2025–2050), based on trend extrapolations assuming steady global demand growth, policy support for diversified supply chains, and technological advancement in extraction. These projections are sensitive to variables such as geopolitical dynamics, environmental regulations, and market demand, which may shift over time [122].

Table 4.
Top twenty REE projects by estimated value, calculated using total contained REO tonnage multiplied by average REO price per ton, based on the methodology from Sustainability (2024) [7].
5. Machine Learning Applications in REE Research
5.1. Overview of Machine Learning Techniques in REE Research
Machine learning (ML) is increasingly being applied in REE research to address challenges in data interpretation, the prediction of elemental concentrations, and exploration of unconventional resources. ML techniques offer robust solutions for handling nonlinear relationships, high-dimensional datasets, and incomplete measurements factors commonly encountered in geochemical studies.
Bishop and Robbins [123] applied a suite of ML methods to predict REE concentrations in sedimentary strata using a dataset compiled from the Saskatchewan Geological Survey, the Alberta Geological Survey, and the Sedimentary Geochemistry and Paleoenvironments Project (SGP). Bishop and Robbins [123] incorporated both unsupervised and supervised learning approaches. Unsupervised methods including principal component analysis (PCA), correlation matrices, and hierarchical clustering revealed consistent associations between REEs and incompatible trace elements such as Th, Nb, and P. In the supervised learning domain, regression-based models including random forest (RF), AdaBoost, and Gradient Boosting were trained to predict total REE concentrations, with the highest-performing models consistently identifying P and Th as key predictive variables. Bishop and Robbins emphasized that these ML workflows not only improved predictive accuracy but also provided insights into the geochemical proxies most indicative of REE enrichment in sedimentary environments [123].
In another example of ML applied to geochemically sparse datasets, Chatterjee et al. [124] explored REE potential in Pennsylvanian coals from Indiana using data from the Indiana Coal Quality Database. Given the limited number of samples containing complete REE measurements, the authors employed two data augmentation strategies, Random Over-Sampling Examples (ROSE) and the Synthetic Minority Over-Sampling Technique (SMOTE), to improve class balance and model performance. The study tested four classification algorithms: linear discriminant analysis (LDA), support vector machine (SVM), RF, and artificial neural networks (ANNs). The SVM model trained with SMOTE augmentation yielded the highest classification accuracy of 95% in distinguishing between coal samples with “promising” and “unpromising” REE outlook coefficients. Chatterjee et al. [124] concluded that ML models trained on conventional coal quality parameters (e.g., Al2O3, Fe2O3, S, ash yield, P, Zn, As) can serve as proxies for REE enrichment and screening, thus reducing the need for extensive laboratory testing in early-stage exploration [124].
The integration of ML in REEs research is rapidly advancing, particularly in exploration and geochemical modeling. As global REE demand surges due to their critical role in clean energy and high-tech industries, ML offers a powerful solution to manage and interpret the rapidly growing and complex geochemical datasets generated through exploration and monitoring programs.
Machine learning algorithms (MLAs) have been successfully deployed to analyze large, multivariate datasets, revealing hidden patterns and associations among elemental variables. In REE-focused studies, unsupervised learning methods like correlation matrices, PCA, and structured cluster analysis have been used to classify samples, reduce dimensionality, and uncover relationships between major, trace, and REE variables within sedimentary formations [125,126]. These methods allow researchers to identify geochemical signatures indicative of REE enrichment and to understand the processes controlling REE mobility and deposition in complex environments such as coal seams, phosphorites, and formation waters [30,127].
Supervised ML models including RF, SVM, Gradient Boosting (GB), and Ridge Regression (RR) have also shown great promise in predicting REE concentrations based on input variables such as major oxides, trace metals, and lithological characteristics [128]. For instance, the RF model, known for handling high-dimensional geochemical data, has been used to classify pyrite compositions and predict hydrothermal alteration zones relevant to REE prospecting [129,130]. These techniques are particularly valuable in data-rich but less studied sedimentary environments, where historical exploration and energy development have already generated extensive datasets suitable for ML analysis. A recent example applied ML to public datasets from the Sedimentary Geochemistry and Paleoenvironments Project (SGP), Alberta Geological Survey, and Saskatchewan Geological Survey to predict REE abundances in sedimentary lithologies [131,132,133].
5.2. LIBS and ML for REE Detection
Laser-Induced Breakdown Spectroscopy (LIBS) is increasingly recognized as a powerful tool for rapid, multi-elemental analysis of REEs, particularly due to its minimal sample preparation, real-time feedback, and field-portability. Beyond detection, LIBS is also capable of providing reasonable estimates of mineral composition, although its effectiveness is often hindered by matrix effects, spectral overlaps, and nonlinear behavior in complex mineralogical environments. Recent advances have shown that coupling LIBS with ML can significantly enhance both qualitative and quantitative REE analysis by improving data interpretation and reducing error propagation in spectral datasets.
A transformer-based deep learning model, termed iTBi-LIBS, was developed to quantitatively analyze La, Ce, and Nd in rare-earth ores using LIBS [134]. The model integrates a transformer architecture with bidirectional long short-term memory (BiLSTM) to capture both local and global dependencies in spectral features. To further improve robustness, an ensemble model (iTBi-RF-LIBS) was constructed by combining the iTBi neural network with an RF regressor. Their results demonstrated outstanding accuracy, achieving coefficients of determination (R2) up to 0.996 and mean absolute errors as low as 0.053 wt% for Nd. This model outperformed traditional multivariate approaches such as BiLSTM, RF, and backpropagation neural networks, particularly in overcoming matrix effects and spectral line interference [134].
In a related study, Tian et al. [135] introduced a novel ML-LIBS framework for classifying e-waste phosphors containing REEs such as Gd, Y, and Yb. Their system employed PCA for dimensionality reduction, followed by a supervised backpropagation artificial neural network (BP-ANN) classify three types of phosphors. The resulting model achieved a near-perfect recognition rate of 99.9%. The study not only highlighted LIBS’s applicability in REE detection but also demonstrated how ML models can discriminate between chemically similar substances by extracting subtle spectral differences that are not readily apparent to the human eye or classical statistical techniques [135].
5.3. ML in Ore Classification and Exploration
ML techniques have become increasingly valuable in REE exploration and mineral classification, especially for delineating prospective zones, handling imbalanced geospatial data, and interpreting multivariate geochemical signals. Two recent studies exemplify the integration of ML into REE-related resource identification: one at the regional geological scale and the other at the reaction process level.
A supervised ML framework for early-stage exploration of Fe-Ti-P-REE mineralization in the Southern Oslo Rift, Norway, was applied [136]. Their methodology incorporated geological field expertise and domain-specific sampling strategies to generate training datasets from 400+ field rock samples, which were supported by high-resolution airborne magnetic, radiometric, and topographic data. To address class imbalance typical in mineral exploration datasets they used a spatial buffering strategy and weighted cost functions. Two classifiers were evaluated: RF and SVM. The RF model outperformed the SVM in precision and robustness, identifying 0.3% of the mapped region as promising for mineralization. This prediction was validated by alignment with legacy drilling and field surveys, demonstrating the model’s geological relevance. The authors emphasized the role of geophysical and geochemical contrast (e.g., P2O5, Fe2O3, TiO2) between mineralized zones and host rocks, which was well captured by the ML models and informed through domain knowledge [136].
In a complementary study at the process-scale level, REE mineralization mechanisms were investigated using a novel approach based on hydrothermal synthesis data from functional materials [137]. The authors constructed a dataset of over 1200 REE crystallization reactions typically used for synthesizing phosphates and carbonates under hydrothermal conditions and repurposed this dataset to train ML models. K-nearest neighbor (KNN), RF, and Extreme Gradient Boosting (XGB) algorithms were tested to predict phase and elemental composition of REE-bearing products. The XGB model showed the best performance, achieving 97.4% accuracy in element prediction and 96.0% in phase prediction. Feature importance analysis revealed that thermodynamic descriptors (e.g., formation enthalpy, ionic radius, pKa of additives) were critical predictors, underscoring the mechanistic control of crystallization processes. Furthermore, the XGB regression models were able to predict crystallization temperature and pH from precursors and expanded chemical descriptors with high confidence (R2 = 0.87). This model-to-experiment approach demonstrates that material science synthesis data can effectively be leveraged to inform natural REE mineralization scenarios [137].
6. REE Recycling from E-Waste
Given the rising demand and constrained supply, recycling REEs has become a critical strategy for ensuring long-term resource sustainability. The extraction and separation of REEs from primary ores remain both economically inefficient and ecologically damaging, driving increasing interest in secondary sources such as electronic waste (e-waste) and industrial discards [138,139]. Key secondary materials including sintered NdFeB magnets, fluorescent lamps, and Ni–metal hydride batteries contain substantial quantities of Nd, Pr, La, and Ce, which can be efficiently recovered through targeted recycling efforts [140]. The recycling of electronic components addresses both environmental concerns and supply chain vulnerabilities, as devices like smartphones, lighting systems, and displays contain REEs essential for their luminescent, magnetic, and electrochemical functions [141]. Upon disposal, these devices form a concentrated secondary REE reservoir; for instance, printed circuit boards (PCBs) often contain metal concentrations exceeding those found in natural ores [142].
Among secondary materials, NdFeB magnets used in hard drives, wind turbines, and electric vehicles offer the highest potential for REE recovery due to their high Nd and Pr content (typically 25–35% by weight) and relatively straightforward recycling via hydrogen decrepitation or wet chemical leaching [47]. Fluorescent lamps, though more diffuse and less concentrated in REEs like Eu, Tb, and Y, are frequently collected through municipal e-waste programs and have proven recycling routes, albeit with lower yield and higher handling costs due to mercury content. Nickel–metal hydride (NiMH) batteries contain lanthanum, cerium, and neodymium, and although their REE concentrations are lower than in magnets, they remain a valuable source due to rising usage in hybrid vehicles. Among these, NdFeB magnets are currently the most promising target due to both volume and ease of processing.
Recycling typically involves two major stages: pre-processing and end-processing. The pre-processing phase includes dismantling, sorting, and shredding, while end-processing involves chemical transformations to extract target metals [143]. A variety of advanced metallurgical techniques including pyrometallurgy, hydrometallurgy, and bioleaching have been implemented to recover REEs from complex waste streams [144,145]. Despite their effectiveness, these methods often face limitations pyrometallurgy is typically energy-intensive, while hydrometallurgical approaches may require large quantities of chemical reagents; both can be inefficient in recovering trace-level REEs from complex matrices [47].
Furthermore, e-waste contains hazardous substances such as brominated flame retardants, dioxins, and heavy metals, which pose significant environmental and health risks if improperly managed [146]. Informal recycling and uncontrolled incineration can release toxic emissions, including polycyclic aromatic hydrocarbons (PAHs) and dioxins, contaminating air, soil, and water [147]. Consequently, regulated and safe recycling practices are essential to mitigate these impacts. Such practices include the use of closed-loop recycling systems, advanced mechanical separation methods, controlled atmosphere thermal treatment, and acid-free hydrometallurgical extraction techniques. For instance, formal recycling centers in Europe and Japan utilize automated dismantling, emissions filtration systems, and proper hazardous waste disposal protocols to reduce pollutant release [148]. Additionally, ISO-certified recycling facilities follow international standards (e.g., ISO 14001 [149]) that ensure worker safety and minimize environmental emissions. The absence of appropriate infrastructure and training during manual dismantling further elevates risks to both human health and ecosystems, highlighting the need for standardized procedures and trained personnel.
The absence of appropriate infrastructure including formal collection systems, licensed recycling facilities, and secure processing technologies significantly hampers safe e-waste handling, especially in developing countries. This deficiency often leads to the proliferation of informal recycling sectors where hazardous substances are released into the environment. For example, in regions lacking formal infrastructure, manual dismantling and open burning are frequently used to extract valuable metals, posing risks to both workers and surrounding communities [147].
Recent developments have emphasized cost-effective and environmentally responsible recovery technologies. For instance, controlled thermal pre-treatment followed by solvent-based extraction has shown promise in enhancing recovery efficiency while reducing environmental impact [47]. Despite these advancements, the field still lacks a fully integrated and scalable framework for REE recycling. While some studies have addressed environmental or economic aspects individually [150,151], most current approaches focus on isolated techniques. As a result, they often fall short of combining technical feasibility, ecological responsibility, economic viability, and regulatory considerations into a unified system suitable for large-scale implementation.
Although conventional methods such as precipitation and hydrometallurgy remain in use, they are often inadequate for recovering REEs with low chemical affinity and offer limited scalability [47]. Precipitation typically involves converting dissolved REEs into insoluble compounds (e.g., oxalates or hydroxides) using chemical agents; while it is simple and low-cost, it lacks selectivity and often co-precipitates impurities. Hydrometallurgy, on the other hand, relies on acid or alkaline leaching followed by solvent extraction or ion exchange to selectively separate REEs. Although more effective, hydrometallurgy requires careful pH control, multiple stages, and the use of hazardous chemicals, which limit its environmental and economic viability on a scale. As a result, the development of integrated strategies that optimize recovery efficiency, economic feasibility, and environmental safety is imperative. Current research is increasingly aligned with global sustainability frameworks, particularly the United Nations Sustainable Development Goals (SDGs), which consist of 17 interconnected objectives designed to address urgent global challenges such as poverty, climate change, and environmental degradation. In the context of REE recycling, SDG 12 (Responsible Consumption and Production), SDG 9 (Industry, Innovation, and Infrastructure), and SDG 13 (Climate Action) are especially relevant. These goals promote clean technology development, resource-efficient practices, and environmentally sound waste management principles that directly support the advancement of sustainable and scalable REE recovery strategies [152]. Figure 4 illustrates global trends in e-waste generation, highlighting both regional disparities and future projections in total and per capita volumes [147]. Figure 4a shows that global e-waste generation has been increasing steadily, driven by rising sales of electronic equipment and shorter product lifecycles. The bar plots represent annual totals (in Mt), while the line plot overlays projected growth trends through 2030 based on EEE consumption patterns. In Figure 4b, regional disparities in both total and per capita e-waste generation are highlighted for the year 2019. The green bars reflect total e-waste volumes, and the magenta line corresponds to per capita generation. While Asia leads in total generation, Europe and Oceania exhibit the highest per capita figures (16.2 and 16.1 kg/person, respectively), reflecting differences in consumption habits and economic development levels [153].
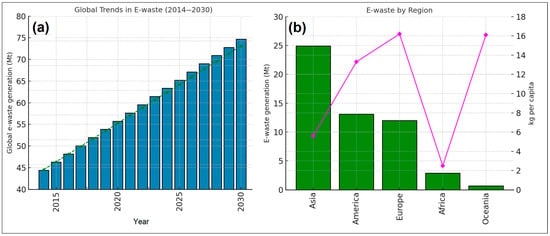
Figure 4.
(a) Global e-waste generation from 2014 to projected values in 2030, illustrating both historical data and future estimates based on trends in electronic and electrical equipment (EEE) consumption. Bar plots indicate total annual e-waste generation (in million metric tons), while the overlaid line represents the projected growth trajectory. (b) Total and per capita e-waste generation by region for the year 2019. Green bars show total generation (Mt), and the magenta line denotes per capita values (kg/person), highlighting regional disparities in consumption and waste generation. Data adapted from Thakur and Kumar [153].
7. Environmental and Health Hazards of REE Exposure
The mining, processing, and extensive industrial use of REEs have resulted in their widespread release into various environmental compartments, raising significant ecological and public health concerns. Elevated REE concentrations have been documented in soils, surface waters, and airborne particulate matter, particularly in proximity to mining and smelting operations. Inhalation of airborne REEs especially in the form of fine particulate matter (PM2.5) is recognized as a major exposure pathway. The health risks associated with REE inhalation near mining areas in Baotou, northern China, were quantified [154], while occupational and environmental exposures in both residential and industrial settings were reported [155,156], with some regions registering annual inhalation doses exceeding 400 µSv/year. Once absorbed, REEs can accumulate in vital organs such as the lungs, liver, kidneys, and brain, potentially inducing respiratory inflammation, oxidative stress, neurotoxicity, and reproductive toxicity [157]. Moreover, studies have shown that REEs can cross the placental barrier, thereby posing developmental risks to fetuses [158]. In addition to direct human health impacts, REE extraction and processing contribute significantly to environmental degradation. REE mining often produces large volumes of radioactive tailings, particularly from monazite-bearing ores, and acidic wastewater that can contaminate local water systems and soils [159,160]. Improper containment of these wastes poses long-term environmental hazards, including elevated radiation levels and heavy metal mobility [161]. Furthermore, open-pit mining and deforestation associated with REE operations lead to erosion, habitat destruction, and in regions such as southern China have been linked to land subsidence and desertification [162].
Improper disposal of waste electrical and electronic equipment (WEEE), a rapidly growing global source of REEs, also contributes significantly to environmental contamination. These e-wastes are largely non-biodegradable and possess long environmental half-lives, facilitating their dispersion through air and water [163,164,165].
Collectively, these findings highlight the complex and persistent nature of REE exposure across environmental and biological systems. They also emphasize the pressing need for comprehensive toxicological evaluations and regulatory policy development. While awareness of REE-related health risks is increasing, further research is essential to fully understand the mechanisms of toxicity and the long-term impacts on human populations.
8. Agricultural Applications of REEs
REEs, particularly La and Ce, have gained increasing attention for their potential to enhance agricultural productivity. Initial applications of REEs in agriculture began in China, where they were extensively utilized as micronutrient fertilizers and plant growth regulators [166]. Subsequent research has expanded to investigate the physiological and biochemical effects of REEs across various crop and wild plant species, revealing a complex, dose-dependent response pattern.
At low concentrations typically in the micromolar range REEs such as La3+ and Ce3+ have demonstrated a range of beneficial effects on plant physiology. These include enhanced chlorophyll synthesis, improved photosynthetic efficiency, increased P uptake, and modulation of antioxidant enzyme activity [167,168]. For example, in soybean (Glycine max), La concentrations ranging from 5 to 160 µM stimulated biomass production, photosynthesis, and mitotic activity [167], while in rice (Oryza sativa), low levels of La3+ reduced reactive oxygen species (ROS) and activated antioxidant defenses [169,170].
However, at higher concentrations typically exceeding 0.2 mM, REEs begin to exhibit phytotoxic effects [171], with significant reductions in shoot biomass observed in Zea mays and Vigna radiata exposed to La and Ce concentrations between 1 and 5 mM. Toxic responses have also included inhibited seed germination, reduced root elongation, and chromosomal abnormalities [172,173]. Furthermore, soil applications of REE oxides such as CeO2, La2O3, Gd2O3, and Yb2O3 have demonstrated cytotoxic and genotoxic effects in Allium cepa and other plant species [174,175]. This biphasic response, which refers to a two-phase effect where low concentrations promote growth and high concentrations cause toxicity, is characteristic of hormesis, a phenomenon where low doses stimulate growth while high doses inhibit it. This behavior in REE-plant systems was reviewed, confirming the growth-promoting effects of REEs under control conditions [176,177]. These effects may result from REE-induced alterations in membrane transport, enzyme function, DNA integrity, and cell signaling pathways [178,179,180].
Field studies have supported these laboratory findings. Foliar applications of La and Ce were shown to significantly improve the growth and yield of Chinese cabbage (Brassica chinensis) [181], while season-dependent yield improvements in both cabbage and turnip crops were also reported [182]. The use of REE-containing fertilizers in maize was investigated, showing increased accumulation in roots and leaves with negligible residues in grains even at application rates up to 10 kg/ha [183]. This observation is consistent with findings by Liang et al. [184], who reported that foliar-applied REEs primarily accumulate in non-edible plant tissues.
A noteworthy regulatory milestone was achieved when the European Commission approved REE citrate (“Lancer”) as a feed additive for piglets, signaling broader international acceptance of REE use in agriculture [185]. Nevertheless, environmental accumulation and food chain transfer continue to raise important safety concerns. In Citrus sinensis (navel orange), REE accumulation was found to be highest in roots and lowest in edible pulp, with concentrations remaining well below Chinese safety thresholds, supporting the potential for safe REE use underregulated agricultural practices [186].
9. Medical Applications of REEs
REEs, particularly the lanthanides, have gained significant importance in medical science due to their distinctive magnetic, optical, and radioactive properties. Gd is widely employed as a contrast agent in magnetic resonance imaging (MRI), where it enhances image resolution by accelerating magnetic relaxation processes, offering high diagnostic clarity with minimal toxicity [187,188]. Ce and Eu are utilized in positron emission tomography (PET) imaging and various bioassays, owing to their favorable scintillation and luminescent characteristics [189]. Eu chelates play a crucial role in genetic labeling applications [190].
Lutetium-based radioisotopes, such as Lu, are actively used in clinical settings for targeted therapies, including prostate cancer treatment [191]. Other lanthanide radioisotopes such as 153Sm, 149Tb, and 165Dy are employed in diverse therapeutic contexts, including palliative care for metastatic bone pain, targeted oncological treatments, and the management of joint effusions [192,193,194]. In addition, Ho and Er are integrated into laser technologies used in minimally invasive surgical procedures, such as kidney stone disintegration and dental operations [195,196]. The growing reliance on REEs for both diagnostic and therapeutic medical applications underscore the urgent need for sustainable sourcing and effective recycling strategies to ensure continued availability for the healthcare sector [197,198].
10. Advanced Engineering Applications of REEs
REEs are vital to modern technologies due to their unique magnetic, optical, and electronic properties [199]. The largest application is in permanent magnets, especially NdFeB and SmCo types, used in wind turbines, electric vehicles, and electronic devices. These magnets offer high coercivity and energy density, enabling miniaturization and improved efficiency in clean energy systems [200,201,202,203,204,205,206,207].
Optically, REEs like Eu3+, Tb3+, and Yb3+ are essential in phosphors for LEDs, displays, lasers, and advanced bioimaging. Their 4f-4f and 5d-4f transitions produce stable luminescence across the UV–NIR spectrum [208,209]. Recent advances in nanotechnology have led to REE-doped nanoparticles used in photodynamic therapy, cancer diagnostics, and multiplexed detection [210,211,212].
REEs are also employed in sensors (pH, oxygen, ammonia) and high-resolution imaging due to their upconversion capabilities and environmental stability [213]. Despite efforts to find alternatives, their superior properties make them irreplaceable in many high-tech and energy-related applications [199,201].
11. Conclusions
- REEs play a central role in renewable energy, electronics, and advanced technologies, but their extraction remains constrained by geochemical and environmental challenges.
- This review provides a comprehensive synthesis of REE-bearing deposit types, mineralogy, and emerging unconventional sources such as coal ash, marine sediments, and red mud.
- Machine learning is increasingly applied in REE exploration and analysis, offering solutions for data-limited environments and geochemical modeling.
- REE recycling from e-waste shows promise but is limited by technological, economic, and regulatory challenges that require integrated global strategies.
- Future research and policy should focus on responsible extraction, sustainable recycling, and the use of advanced analytical and computational tools to ensure long-term REE supply security.
Author Contributions
Conceptualization, M.R.; methodology, M.R.; validation, M.R.; formal analysis, M.R.; investigation, M.R., G.S.-L. and O.A.; resources, M.R.; data curation, M.R.; writing—original draft preparation, M.R.; writing—review and editing, M.R., G.S.-L. and O.A.; visualization, G.S.-L.; supervision, M.R.; project administration, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there are no conflicts of interest related to this work.
References
- Damhus, T.; Hartshorn, R.; Hutton, A. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Atwood, D.A. The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Zhou, B.; Li, Z.; Chen, C. Global potential of rare earth resources and rare earth demand from clean technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Liu, S.L.; Fan, H.R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.F.; Li, X.C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Zepf, V. Rare Earth Elements: What and Where They Are. In Rare Earth Elements; Springer Theses (Recognizing Outstanding Ph.D. Research); Springer: Berlin/Heidelberg, Germany, 2013; pp. 11–39. [Google Scholar]
- Mancheri, N.A. Chinese monopoly in rare earth elements: Supply–demand and industrial applications. China Rep. 2012, 48, 449–468. [Google Scholar] [CrossRef]
- Liang, T.; Li, K.; Wang, L. State of rare earth elements in different environmental components in mining areas of China. Environ. Monit. Assess. 2014, 186, 1499–1513. [Google Scholar] [CrossRef]
- Patil, A.B.; Paetzel, V.; Struis, R.P.; Ludwig, C. Separation and recycling potential of rare earth elements from energy systems: Feed and economic viability review. Separations 2022, 9, 56. [Google Scholar] [CrossRef]
- Hayes-Labruto, L.; Schillebeeckx, S.J.; Workman, M.; Shah, N. Contrasting perspectives on China’s rare earths policies: Reframing the debate through a stakeholder lens. Energy Policy 2013, 63, 55–68. [Google Scholar] [CrossRef]
- Wübbeke, J. Rare earth elements in China: Policies and narratives of reinventing an industry. Resour. Policy 2013, 38, 384–394. [Google Scholar] [CrossRef]
- Han, A.P.; Ge, J.P.; Lei, Y.L. An adjustment in regulation policies and its effects on market supply: Game analysis for China’s rare earths. Resour. Policy 2015, 46, 30–42. [Google Scholar] [CrossRef]
- Lei, S.; Na, W.; Shuai, Z.; Li, G. Overview on China’s rare earth industry restructuring and regulation reforms. J. Resour. Ecol. 2017, 8, 213–222. [Google Scholar] [CrossRef]
- Hu, X.; Sun, B.; Wang, C.; Lim, M.K.; Wang, P.; Geng, X.; Yao, C.; Chen, W.-Q. Impacts of China’s exports decline in rare earth primary materials from a trade network-based perspective. Resour. Policy 2023, 81, 103321. [Google Scholar] [CrossRef]
- Van Gosen, B.S.; Verplanck, P.L.; Seal II, R.R.; Long, K.R.; Gambogi, J. Rare-Earth Elements. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey Professional Paper 1802; U.S. Geological Survey: Reston, VA, USA, 2017; pp. O1–O31. [Google Scholar]
- Ciacci, L.; Vassura, I.; Cao, Z.; Liu, G.; Passarini, F. Recovering the “new twin”: Analysis of secondary neodymium sources and recycling potentials in Europe. Resour. Conserv. Recycl. 2019, 142, 143–152. [Google Scholar] [CrossRef]
- Humphries, M. Critical Minerals and US Public Policy; Report No. R45810; Congressional Research Service: Washington, DC, USA, 2019.
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- White, H. 100-Day Reviews Under Executive Order 14017: Building Resilient Supply Chains, Revitalizing American Manufacturing, and Fostering Broad-Based Growth; The White House: Washington, DC, USA, 2021.
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to build a circular economy for rare-earth elements. Nature 2023, 619, 248–251. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publication: Oxford, UK, 1985; p. 312. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A. Geochemical Evaluation of the Rare Earth and Trace Elements in the Upper Eocene Carbonate of Abu Rimth Formation, Southern Galala Plateau, Eastern Desert, Egypt. J. Umm Al-Qura Univ. Appl. Sci. 2025, 1–11. [Google Scholar] [CrossRef]
- Wakita, H.; Rey, P.; Schmitt, R.A. Abundances of the 14 Rare Earth Elements and 12 Other Elements in Apollo 12 Samples. In Proceedings of the Second Lunar Science Conference; Levinson, A.A., Ed.; MIT Press: Cambridge, MA, USA, 1971; pp. 1319–1329. [Google Scholar]
- Pourmand, A.; Dauphas, N.; Ireland, T.J. A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI chondrite and Post-Archean Australian Shale (PAAS) abundances. Chem. Geol. 2012, 291, 38–54. [Google Scholar] [CrossRef]
- Möller, P. Rare Earth Mineral Deposits and Their Industrial Importance. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 171–188. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements–Critical Resources for High Technology; U.S. Geological Survey Fact Sheet 087–02; U.S. Geological Survey: Reston, VA, USA, 2002.
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Rezaei, M.; Gabitov, R.; Sadekov, A. Crystallographic Influence on Elemental Uptake by Calcite from Artificial Seawater. In Proceedings of the GSA Connects 2023, Pittsburgh, PA, USA, 15–18 October 2023; Geological Society of America: Boulder, CO, USA, 2023. Paper No. 254-11. [Google Scholar]
- Rezaei, M.; Gabitov, R.; Sadekov, A.; Perez-Huerta, A.; Borrelli, C.; Stiles, A. Elemental uptake by different calcite crystal faces: An in situ study. Crystals 2024, 14, 442. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Sadekov, A.; Perez-Huerta, A.; Borrelli, C.; Rezaei, M. Elemental uptake by individual calcite crystals. In Proceedings of the 2022 Goldschmidt Conference, Honolulu, HI, USA, 11–15 July 2022. [Google Scholar]
- Meshram, P.; Pandey, B. Perspective of availability and sustainable recycling prospects of metals in rechargeable batteries–A resource overview. Resour. Policy 2019, 60, 9–22. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Andreoli, M.; Smith, C.; Watkeys, M.; Moore, J.; Ashwal, L.; Hart, R. The geology of the Steenkampskraal monazite deposit, South Africa; implications for REE-Th-Cu mineralization in charnockite-granulite terranes. Econ. Geol. 1994, 89, 994–1016. [Google Scholar] [CrossRef]
- Hussain, A.; Zhao, K.-D.; Arif, M.; Palmer, M.R.; Chen, W.; Zhang, Q.; Li, Q.; Jiang, S.-Y.; Girei, M.B. Geochronology, mineral chemistry and genesis of REE mineralization in alkaline rocks from the Kohistan Island Arc, Pakistan. Ore Geol. Rev. 2020, 126, 103749. [Google Scholar] [CrossRef]
- Su, J.-H.; Zhao, X.-F.; Li, X.-C.; Su, Z.-K.; Liu, R.; Qin, Z.-J.; Chen, M. Fingerprinting REE mineralization and hydrothermal remobilization history of the carbonatite-alkaline complexes, Central China: Constraints from in situ elemental and isotopic analyses of phosphate minerals. Am. Mineral. 2021, 106, 1545–1558. [Google Scholar] [CrossRef]
- Dostal, J.; Gerel, O. Rare earth element deposits in Mongolia. Minerals 2023, 13, 129. [Google Scholar] [CrossRef]
- Harben, P. The Industrial Mineral Handy Book—A Guide to Markets, Specifications and Prices, 4th ed.; Industrial Mineral Information: Worcester Park, UK, 2002; Volume 412. [Google Scholar]
- Dostal, J. Rare earth element deposits of alkaline igneous rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Cox, C.; Kynicky, J. The rapid evolution of speculative investment in the REE market before, during, and after the rare earth crisis of 2010–2012. Extr. Ind. Soc. 2018, 5, 8–17. [Google Scholar] [CrossRef]
- European Commission. Critical Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; European Union Technical Report; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Wall, F.; Williams, C.T.; Woolley, A.R.; Stanley, C.J. Pyrochlore in Niobium Ore Deposits. In Mineral Deposits: Processes to Processing, Proceedings of the SGA Biennial Meeting (5th) and IAGOD Quadrennial Symposium (10th), London, UK, 22–25 August 1999; A.A. Balkema: Rotterdam, The Netherlands, 1999; pp. 687–690. [Google Scholar]
- Mitchell, R.H. Primary and secondary niobium mineral deposits associated with carbonatites. Ore Geol. Rev. 2015, 64, 626–641. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Vasyukova, O.V. The economic geology of scandium, the runt of the rare earth element litter. Econ. Geol. 2018, 113, 973–988. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Siegfried, P.R.; Wall, F.; O’Neill, M.; Brooker, R.A.; Fallon, E.K.; Pickles, J.R.; Banks, D.A. The origin and composition of carbonatite-derived carbonate-bearing fluorapatite deposits. Miner. Depos. 2020, 56, 863–884. [Google Scholar] [CrossRef]
- Hou, Z.; Tian, S.; Xie, Y.; Yang, Z.; Yuan, Z.; Yin, S.; Yi, L.; Fei, H.; Zou, T.; Bai, G.; et al. The Himalayan Mianning–Dechang REE belt associated with carbonatite-alkaline complexes, eastern Indo-Asian collision zone, SW China. Ore Geol. Rev. 2009, 36, 65–89. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Deady, E.A.; Beard, C.D.; Broom-Fendley, S.; Elliott, H.A.L.; van den Berg, F.; Öztürk, H. Carbonatites and alkaline igneous rocks in post-collisional settings: Storehouses of rare earth elements. J. Earth Sci. 2021, 32, 1332–1358. [Google Scholar] [CrossRef]
- Bhushan, S.K.; Somani, O.P. Rare earth elements and yttrium potentials of Neoproterozoic peralkaline Siwana granite of Malani igneous suite, Barmer district, Rajasthan. J. Geol. Soc. India 2019, in press. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Van Gosen, B.S.; Seal, R.R.; McCafferty, A.E. A Deposit Model for Carbonatite and Peralkaline Intrusion-Related Rare Earth Element Deposits; U.S. Geological Survey Scientific Investigations Report 2010-5070-J; U.S. Geological Survey: Reston, VA, USA, 2014; p. 58.
- Singh, H.; Sadiq, M.; Sharma, B.B. Exploration for rare earth elements in North East India. Curr. Sci. 2014, 107, 178–180. [Google Scholar]
- Bhushan, S.K.; Kumar, A. First carbonatite-hosted REE deposit from India. J. Geol. Soc. India 2013, 81, 41–60. [Google Scholar] [CrossRef]
- Bhushan, S.K. Geology of the Kamthai rare earth deposit. J. Geol. Soc. India 2015, 85, 537–546. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Oliveira, S.M.B.; Imbernon, R.A.L. Weathering alteration and related REE concentration in the Catalão I carbonatite complex, central Brazil. J. S. Am. Earth Sci. 1998, 11, 379–388. [Google Scholar] [CrossRef]
- Witt, W.; Hammond, D.; Hughes, M. Geology of the Ngualla carbonatite complex, Tanzania, and origin of the weathered bastnaesite zone REE ore. Ore Geol. Rev. 2019, 105, 28–54. [Google Scholar] [CrossRef]
- Zhukova, I.A.; Stepanov, A.S.; Jiang, S.-Y.; Murphy, D.; Mavrogenes, J.; Allen, C.; Chen, W.; Bottrill, R. Complex REE systematics of carbonatites and weathering products from uniquely rich Mount Weld REE deposit, Western Australia. Ore Geol. Rev. 2021, 139, 104539. [Google Scholar] [CrossRef]
- Walter, A.; Lusty, P.; Chetmyn, C.; Hill, A. Rare Earth Elements; British Geological Survey, Natural Environment Research Council; Centre of Sustainable Mineral Development: Cornwall, UK, 2010. [Google Scholar]
- Castor, S.B.; Hendrik, J.B. Rare Earth Elements. In Industrial Minerals and Rocks: Commodities, Markets, and Uses, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 769–792. [Google Scholar]
- Green, C.; Simandl, G.J.; Paradis, S.; Katay, F.; Hoshino, M.; Kon, Y.; Kodama, S.; Graf, C. Geological setting of the Rock Canyon Creek REE-fluorite deposit, British Columbia, Canada. In Geological Fieldwork 2016; Report 2017-1; British Columbia Geological Survey, Ministry of Energy and Mines: Victoria, BC, Canada, 2017; pp. 195–203. [Google Scholar]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408, 1339–1343. [Google Scholar] [CrossRef]
- Yang, X.J.; Lin, A.J.; Li, X.L.; Wu, Y.D.; Zhou, W.B.; Chen, Z.H. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Huang, J.; Tan, W.; Liang, X.; He, H.; Ma, L.; Bao, Z.; Zhu, J. REE fractionation controlled by REE speciation during formation of the Renju regolith-hosted REE deposits in Guangdong Province, South China. Ore Geol. Rev. 2021, 134, 104172. [Google Scholar] [CrossRef]
- Zhu, X.P.; Zhang, B.; Ma, G.T.; Pan, Z.W.; Hu, Z.G.; Zhang, B.T. Mineralization of ion-adsorption type rare earth deposits in Western Yunnan, China. Ore Geol. Rev. 2022, 148, 104984. [Google Scholar] [CrossRef]
- Krakatoa Resources Ltd. MT Clere Rare Earth Project: 9 October 2020; Company Report; Krakatoa Resources Ltd.: Perth, WA, Australia, 2020. [Google Scholar]
- Li, X.C.; Zhou, M.F. Hydrothermal alteration of monazite-(Ce) and chevkinite-(Ce) from the Sin Quyen Fe-Cu-LREE-Au deposit, northwestern Vietnam. Am. Mineral. J. Earth Planet. Mater. 2017, 102, 1525–1541. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Honda, T.; Tanaka, M.; Tanaka, K.; Takahashi, Y. Discovery of ion-adsorption type deposits of rare earth elements (REE) in Southwest Japan with speciation of REE by extended X-ray absorption fine structure spectroscopy. Geochem. J. 2018, 52, 415–425. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Z. Geochemistry of mineralization with exchangeable REY in the weathering crusts of granitic rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Valeton, I. Developments in Soil Science 1. In Bauxites; Elsevier: Amsterdam, The Netherlands, 1972; ISBN 978-0-444-40888-4. [Google Scholar]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare earth element phases in bauxite residue. Minerals 2018, 8, 32. [Google Scholar] [CrossRef]
- Komar, P.D. The entrainment, transport and sorting of heavy minerals by waves and currents. Dev. Sedimentol. 2007, 58, 3–48. [Google Scholar]
- Amalan, K.; Ratnayake, A.S.; Ratnayake, N.P.; Weththasinghe, S.M.; Dushyantha, N.; Lakmali, N.; Premasiri, R. Influence of nearshore sediment dynamics on the distribution of heavy mineral placer deposits in Sri Lanka. Environ. Earth Sci. 2018, 77, 1–13. [Google Scholar] [CrossRef]
- Orris, G.J.; Grauch, R.I. Rare Earth Element Mines, Deposits, and Occurrences; U.S. Geological Survey Open File Report 02-189; U.S. Geological Survey: Reston, VA, USA, 2002.
- Cronan, D.S. Handbook of Marine Mineral Deposits; CRC Marine Science Series; CRC Press: Boca Raton, FL, USA, 1999; Volume 17, ISBN 0-8493-8429-X. [Google Scholar]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef]
- Ketris, M.Á.; Yudovich, Y.E. Estimations of Clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Dai, S.; Li, D.; Chou, C.-L.; Zhao, L.; Zhang, Y.; Ren, D.; Ma, Y.; Sun, Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu surface mine, Jungar coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Finkelman, R.B. Trace and Minor Elements in Coal. In Organic Geochemistry: Principles and Applications; Springer: Dordrecht, The Netherlands, 1993; pp. 593–607. [Google Scholar]
- Hower, J.C.; Granite, E.J.; Mayfield, D.B.; Lewis, A.S.; Finkelman, R.B. Notes on contributions to the science of rare earth element enrichment in coal and coal combustion byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A review of rare earth elements and yttrium in coal ash: Content, modes of occurrences, combustion behavior, and extraction methods. Prog. Energy Combust. Sci. 2021, 88, 100954. [Google Scholar] [CrossRef]
- Laudal, D.A.; Benson, S.A.; Addleman, R.S.; Palo, D. Leaching behavior of rare earth elements in Fort Union lignite coals of North America. Int. J. Coal Geol. 2018, 191, 112–124. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q. Rare earth elements recovery using staged precipitation from a leachate generated from coarse coal refuse. Int. J. Coal Geol. 2018, 195, 189–199. [Google Scholar] [CrossRef]
- Hedin, B.C.; Capo, R.C.; Stewart, B.W.; Hedin, R.S.; Lopano, C.L.; Stuckman, M.Y. The evaluation of critical rare earth element (REE) enriched treatment solids from coal mine drainage passive treatment systems. Int. J. Coal Geol. 2019, 208, 54–64. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.-F. Recovery potential of rare earth elements from mining and industrial residues: A review and case studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Jarvis, I. Phosphorite geochemistry: State-of-the-art and environmental concerns. Eclogae Geol. Helv. 1994, 87, 643–700. [Google Scholar]
- Auer, G.; Reuter, M.; Hauzenberger, C.A.; Piller, W.E. The impact of transport processes on rare earth element patterns in marine authigenic and biogenic phosphates. Geochim. Cosmochim. Acta 2017, 203, 140–156. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Groat, L.A. Sedimentary and igneous phosphate deposits: Formation and exploration: An invited paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Wang, Z.; Hill, R.; Williams, G.; Dwyer, G.S.; Hu, J.; Schnug, E.; Bol, R.; Sun, Y.; Coleman, D.S.; Liu, X.-M. Lead isotopes and rare earth elements geochemistry of global phosphate rocks: Insights into depositional conditions and environmental tracing. Chem. Geol. 2023, 639, 121715. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Buccione, R.; Kechiched, R.; Mongelli, G.; Sinisi, R. REEs in the North Africa P-bearing deposits, paleoenvironments, and economic perspectives: A review. Minerals 2021, 11, 214. [Google Scholar] [CrossRef]
- Linares, E.; Velasquez, G.; Manrique, J.; Monsalve, J.; Mónaco, S.L.; Shumlyanskyy, L. REE + Y signatures of the Navay phosphate deposit, SW Venezuela: Seawater paleoredox conditions and diagenetic implications. J. S. Am. Earth Sci. 2023, 129, 104532. [Google Scholar] [CrossRef]
- Ahmed, A.; Aseri, A.; Ali, K. Geological and geochemical evaluation of phosphorite deposits in northwestern Saudi Arabia as a possible source of trace and rare-earth elements. Ore Geol. Rev. 2022, 144, 104854. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.A.; Staudigel, H. Seamount mineral deposits, a source of rare-metals for high technology industries. Oceanography 2010, 23, 184–189. [Google Scholar] [CrossRef]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 535–539. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.; Gonzalez, F.J.; Zheng, X.; Li, G.; Luo, Y.; Mo, A.; Xu, A.; Wang, S. Rare earth elements and yttrium in ferromanganese deposits from the South China Sea: Distribution, composition and resource considerations. Acta Oceanol. Sin. 2018, 37, 41–54. [Google Scholar] [CrossRef]
- Nath, B.N.; Roelandts, I.; Sudhakar, M.; Plüger, W.L.; Balaram, V. Cerium anomaly variations in ferromanganese nodules and crusts from the Indian Ocean. Mar. Geol. 1994, 120, 385–400. [Google Scholar] [CrossRef]
- Banakar, V.K.; Borole, D.V. Depth profiles of 230Th excess, transition metals and mineralogy of ferromanganese crusts of the Central Indian basin and implications for paleo-oceanographic influence on crust genesis. Chem. Geol. 1991, 94, 33–44. [Google Scholar] [CrossRef]
- Sander, S.G.; Koschinsky, A. Metal flux from hydrothermal vents increased by organic complexation. Nat. Geosci. 2011, 4, 145–150. [Google Scholar] [CrossRef]
- Prakash, L.S.; Ray, D.; Paropkari, A.L.; Mudholkar, A.V.; Satyanarayanan, M.; Sreenivas, B.; Chandrasekharam, D.; Kota, D.; Raju, K.A.K.; Kaisary, S.; et al. Distribution of REE and yttrium among major geochemical phases of marine Fe-Mn-oxides: Comparative study between hydrogenous and hydrothermal deposits. Chem. Geol. 2012, 312–313, 127–137. [Google Scholar] [CrossRef]
- Balaram, V.; Banakar, V.K.; Subramanyam, K.S.V.; Roy, P.; Satyanarayanan, M.; Mohan, M.R.; Sawant, S.S. Yttrium and rare earth element contents in seamount cobalt crusts in the Indian Ocean. Curr. Sci. 2012, 103, 1334–1338. [Google Scholar]
- Krishna, K.S.; Bull, J.M.; Ishizuka, O.; Scrutton, R.A.; Jaishankar, S.; Banakar, V.K. Growth of the Afanasy Nikitin Seamount and its relationship with the 85°E ridge, northeastern Indian Ocean. J. Earth Syst. Sci. 2014, 123, 33–47. [Google Scholar] [CrossRef]
- Yasukawa, K.; Nakamura, K.; Fujinaga, K.; Machida, S.; Ohta, J.; Takaya, Y.; Kato, Y. Rare-earth, major, and trace element geochemistry of deep-sea sediments in the Indian Ocean: Implications for the potential distribution of REY-rich mud in the Indian Ocean. Geochem. J. 2015, 49, 621–635. [Google Scholar] [CrossRef]
- Takaya, Y.; Yasukawa, K.; Kawasaki, T.; Fujinaga, K.; Ohta, J.; Usui, Y.; Nakamura, K.; Kimura, J.; Chang, Q.; Hamada, M.; et al. The tremendous potential of deep-sea mud as a source of rare earth elements. Sci. Rep. 2018, 8, 8. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J.; Ren, X.; Shi, X. Geochemistry of rare earth elements in cobalt-rich crusts from the Mid-Pacific M seamount. J. Rare Earths 2009, 27, 169–176. [Google Scholar] [CrossRef]
- Nath, B.N.; Balaram, V.; Sudhakar, M.; Pluger, W.L. Rare earth element geochemistry of ferromanganese deposits from the Indian Ocean. Mar. Chem. 1992, 38, 185–208. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Somoza, L.; Maldonado, A.; Lunar, R.; Martinez-Frias, J.; Martin-Rubi, J.A.; Carrion, M.C. High technology elements in Co-rich ferromanganese crusts from the Scotia Sea. Rev. Soc. Esp. Mineral 2010, 13, 113–114. [Google Scholar]
- Li, J.; Shi, X.; Huang, M.; Yu, M.; Bi, D.; Song, Z.; Shen, F.; Liu, J.; Zhang, Y.; Wang, H.; et al. The transformation and accumulation mechanism of rare earth elements in deep-sea sediments from the Wharton Basin, Indian Ocean. Ore Geol. Rev. 2023, 161, 105655. [Google Scholar] [CrossRef]
- Bu, W.R.; Shi, X.F.; Peng, J.T. Geochemical characteristics of seamount ferromanganese nodules from mid-Pacific Ocean. Chin. Sci. Bull. 2003, 48, 98–105. [Google Scholar] [CrossRef]
- Piper, D.Z. Rare earth elements in ferromanganese nodules and other marine phases. Geochim. Cosmochim. Acta 1974, 38, 1007–1022. [Google Scholar] [CrossRef]
- Yakovenko, V.G.; Yakovenko, N.M.; Rusanov, M.S.; Val’tsiferov, D.A. Formation Conditions and Characteristics of Synsedimentary Diagenetic Apatite in Phosphorites of the Eocene–Oligocene Dnipro–Donets Depression (Ukraine). Russ. Geol. Geophys. 2023, 64, 1733–1747. [Google Scholar]
- Sa, R.; Sun, X.; He, G.; Xu, L.; Pan, Q.; Liao, J.; Zhu, K.; Deng, X. Enrichment of Rare Earth Elements in Siliceous Sediments under Slow Deposition: A Case Study of the Central North Pacific. Ore Geol. Rev. 2018, 94, 12–23. [Google Scholar] [CrossRef]
- Yamazaki, T.; Nakatani, N.; Arai, R.; Sekimoto, T.; Katayama, H. Combined Mining and Pulp-Lifting of Ferromanganese Nodules and Rare-Earth Element-Rich Mud around Minamitorishima Island in the Western North Pacific: A Prefeasibility Study. Minerals 2021, 11, 310. [Google Scholar] [CrossRef]
- European Parliament. European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU; European Parliament: Brussels, Belgium, 2017. [Google Scholar]
- El Azhari, H.; Cherif, E.K.; El Halimi, R.; Azzirgue, E.M.; Ou Larbi, Y.; Coren, F.; Salmoun, F. Predicting the Production and Depletion of Rare Earth Elements and Their Influence on Energy Sector Sustainability through the Utilization of Multilevel Linear Prediction Mixed-Effects Models with R Software. Sustainability 2024, 16, 1951. [Google Scholar] [CrossRef]
- Bishop, B.A.; Robbins, L.J. Using machine learning to identify indicators of rare earth element enrichment in sedimentary strata with applications for metal prospectivity. J. Geochem. Explor. 2024, 258, 107388. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mastalerz, M.; Drobniak, A.; Karacan, C.Ö. Machine learning and data augmentation approach for identification of rare earth element potential in Indiana coals, USA. Int. J. Coal Geol. 2022, 259, 104054. [Google Scholar] [CrossRef]
- Lindsay, J.J.; Hughes, H.S.R.; Yeomans, C.M.; Andersen, J.C.Ø.; McDonald, I. A machine learning approach for regional geochemical data: Platinum-group element geochemistry vs. geodynamic settings of the North Atlantic Igneous Province. Geosci. Front. 2021, 12, 101098. [Google Scholar] [CrossRef]
- Hu, B.; Zeng, L.-P.; Liao, W.; Wen, G.; Hu, H.; Li, M.Y.H.; Zhao, X.-F. The origin and discrimination of high-Ti magnetite in magmatic-hydrothermal systems: Insight from machine learning analysis. Econ. Geol. 2022, 117, 1613–1627. [Google Scholar] [CrossRef]
- Montross, S.N.; Bagdonas, D.; Paronish, T.; Bean, A.; Gordon, A.; Creason, C.G.; Thomas, B.; Phillips, E.; Britton, J.; Quillian, S.; et al. On a unified core characterization methodology to support the systematic assessment of rare earth elements and critical minerals bearing unconventional carbon ores and sedimentary strata. Minerals 2022, 12, 1159. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Yang, M.; Ren, Z.J. Machine learning in environmental research: Common pitfalls and best practices. Environ. Sci. Technol. 2023, 57, 17671–17689. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.D.; Cracknell, M.J.; Large, R.R.; McGoldrick, P.; Kuhn, S.; Maslennikov, V.V.; Baker, M.J.; Fox, N.; Belousov, I.; Figueroa, M.C.; et al. Distinguishing ore deposit type and barren sedimentary pyrite using laser ablation-inductively coupled plasma-mass spectrometry trace element data and statistical analysis of large data sets. Econ. Geol. 2019, 114, 771–786. [Google Scholar] [CrossRef]
- Schnitzler, N.; Ross, P.S.; Gloaguen, E. Using machine learning to estimate a key missing geochemical variable in mining exploration: Application of the random forest algorithm to multi-sensor core logging data. J. Geochem. Explor. 2019, 205, 106344. [Google Scholar] [CrossRef]
- Lopez, G.P.; Rokosh, C.D.; Weiss, J.A.; Pawlowicz, J.G. Inorganic Geochemistry of Bulk Samples from Selected Alberta Geological Units (Tabular Data, Tab-Delimited Format); Alberta Energy Regulator/Alberta Geological Survey, AER/AGS Digital Data 2019, 2019-0021; Alberta Energy Regulator: Calgary, AB, Canada, 2019. [Google Scholar]
- Jensen, G.K.S.; Pollard, A.; Rostron, B.J. Lithium Concentration in the Duperow Formation: Preliminary Results of Geochemical Analysis of Core Samples from Two Wells in Southeastern Saskatchewan. In Summary of Investigations 2020; Miscellaneous Report 2020-4.1, Paper A-2; Saskatchewan Geological Survey, Saskatchewan Ministry of Energy and Resources: Regina, SK, Canada, 2020; Volume 1. [Google Scholar]
- Farrell, Ú.C.; Samawi, R.; Anjanappa, S.; Klykov, R.; Adeboye, O.O.; Agic, H.; Ahm, A.C.; Boag, T.H.; Bowyer, F.; Brocks, J.J.; et al. The sedimentary geochemistry and paleoenvironments project. Geobiology 2021, 19, 545–556. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Zhang, Z.; Qian, D.; Sun, S.; Liu, X.; Liu, Z. Transformer-based deep learning models for quantification of La, Ce, and Nd in rare earth ores using laser-induced breakdown spectroscopy. Talanta 2025, 292, 127937. [Google Scholar] [CrossRef]
- Tian, L.; Shen, L.; Tian, D.; Ge, Y.; Sun, Z.; Liu, Y. Rare earth metals detection and recognition based on laser-induced breakdown spectroscopy and machine learning. Opt. Express 2023, 31, 20545–20558. [Google Scholar] [CrossRef]
- Wang, Y.; Coint, N.; Mansur, E.T.; Acosta-Gongora, P.; Miranda, A.C.R.; Nasuti, A.; Baranwal, V.C. Leveraging domain expertise in machine learning for critical metal prospecting in the Oslo Rift: A case study for Fe-Ti-P-Rare Earth Element mineralization. Minerals 2024, 14, 377. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Feng, Y.; Wang, Z.; Rosso, K.M.; Guo, X.; Zhang, X. Data-driven insights into rare earth mineralization: Machine learning applications using functional material synthesis data. arXiv 2025, arXiv:2504.07007. [Google Scholar]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.-M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Bai, R.; Yang, F.; Meng, L.; Zhao, Z.; Guo, W.; Cai, C.; Zhang, Y. Polyethylenimine functionalized and scaffolded graphene aerogel and the application in the highly selective separation of thorium from rare earth. Mater. Des. 2021, 197, 109195. [Google Scholar] [CrossRef]
- Batinic, B.; Vaccari, M.; Savvilotidou, V.; Kousaiti, A.; Gidarakos, E.; Marinkovic, T.; Fiore, S. Applied WEEE pre-treatment methods: Opportunities to maximizing the recovery of critical metals. Glob. Nest J. 2018, 20, 706–711. [Google Scholar]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Rare Earths in Rechargeable Batteries. In Rare Earths; Elsevier: Oxford, UK, 2015; pp. 167–180. [Google Scholar]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.-H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Jadhao, P.R.; Ahmad, E.; Pant, K.K.; Nigam, K.D.P. Environmentally friendly approach for the recovery of metallic fraction from waste printed circuit boards using pyrolysis and ultrasonication. Waste Manag. 2020, 118, 150–160. [Google Scholar] [CrossRef]
- de Oliveira, R.P.; Benvenuti, J.; Espinosa, D.C.R. A review of the current progress in recycling technologies for gallium and rare earth elements from light-emitting diodes. Renew. Sust. Energ. Rev. 2021, 145, 111090. [Google Scholar] [CrossRef]
- Pokhrel, P.; Lin, S.-L.; Tsai, C.-T. Environmental and economic performance analysis of recycling waste printed circuit boards using life cycle assessment. J. Environ. Manag. 2020, 276, 111276. [Google Scholar] [CrossRef]
- Sepúlveda, A.; Schluep, M.; Renaud, F.G.; Streicher, M.; Kuehr, R.; Hagelüken, C.; Gerecke, A.C. A review of the environmental fate and effects of hazardous substances released from electrical and electronic equipment during recycling: Examples from China and India. Environ. Impact Assess. Rev. 2010, 30, 28–41. [Google Scholar] [CrossRef]
- Parajuly, K.; Fitzpatrick, C.; Muldoon, O.; Kuehr, R. End-of-life management of electronics: Back to the future. Resour. Conserv. Recycl. 2020, 158, 104800. [Google Scholar]
- ISO 14001:2015; Environmental Management Systems—Requirements with Guidance for Use. 3rd ed. Confirmed 2025. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Abrahami, S.T.; van den Boogaart, K.G.; van den Berg, G.; Stolle, A.; Ondruschka, B. Recovery of rare earth elements from Nd–Fe–B magnets using acetic acid and ultrasound. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar]
- Chancerel, P.; Rotter, V.S. Recovery of rare and precious metals from waste electrical and electronic equipment (WEEE): A review of current literature. Waste Manag. Res. 2009, 27, 535–545. [Google Scholar]
- Vanessa, F.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global e-Waste Monitor 2020; United Nations University (UNU), International Telecommunication Union (ITU) & International Solid Waste Association (ISWA): Bonn, Germany; Geneva, Switzerland; Rotterdam, The Netherlands, 2020; Volume 120. [Google Scholar]
- Thakur, P.; Kumar, S. Evaluation of e-waste status, management strategies, and legislations. Int. J. Environ. Sci. Technol. 2022, 19, 6957–6966. [Google Scholar] [CrossRef]
- Li, K.; Liang, T.; Wang, L.; Tian, S. Inhalation exposure and potential health risk estimation of lanthanides elements in PM2.5 associated with rare earth mining areas: A case of Baotou city, northern China. Environ. Geochem. Health 2018, 40, 2795–2805. [Google Scholar] [CrossRef]
- Qiao, X.; Cui, W.; Gao, S.; Zhi, Q.; Li, B.; Fan, Y.; Liu, L.; Gao, J.; Tan, H. Occupational exposure to rare earth elements: Assessment of external and internal exposure. Environ. Pollut. 2022, 309, 119801. [Google Scholar] [CrossRef]
- Dai, L.; Ge, J.; Wang, L.; Wan, X.; Guo, G.; Liang, T.; Bolan, N.; Rennert, T.; Rinklebe, J. Hair-biomonitoring assessment of rare-earth-element exposure in residents of the largest rare-earth mining and smelting area of China. Environ. Int. 2023, 179, 108177. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, H.; Cui, Y.; Zhang, L.; Zhou, W.; Huang, M.; Xia, W.; Xu, S.; Li, Y. Associations of maternal urinary rare earth elements individually and in mixtures with neonatal size at birth. Environ. Pollut. 2024, 343, 123163. [Google Scholar] [CrossRef]
- Pietsch, T.J.; Guo, Z. Environmental impact of rare earth mining and processing. Environ. Geochem. Health 2021, 43, 2925–2940. [Google Scholar]
- Klinger, J.M. Rare earth elements: Development, sustainability, and policy issues. Resour. Policy 2015, 46, 118–131. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Ali, S.H.; Giurco, D.; Arndt, N.; Nickless, E.; Brown, G.; Demetriades, A. Mineral supply for sustainable development requires resource governance. Nature 2017, 543, 367–372. [Google Scholar] [CrossRef]
- Freitas, R.; Cardoso, C.E.D.; Costa, S.; Morais, T.; Moleiro, P.; Lima, A.F.D.; Soares, M.; Figueiredo, S.; Águeda, T.L.; Rocha, P.; et al. New insights on the impacts of e-waste towards marine bivalves: The case of the rare earth element Dysprosium. Environ. Pollut. 2020, 260, 113859. [Google Scholar] [CrossRef]
- Odnevall, I.; Brookman-Amissah, M.; Stábile, F.; Ekvall, M.T.; Herting, G.; Bermeo Vargas, M.; Messing, M.E.; Sturve, J.; Hansson, L.A.; Isaxon, C.; et al. Characterization and toxic potency of airborne particles formed upon waste from electrical and electronic equipment waste recycling: A case study. ACS Environ. Au 2023, 3, 370–382. [Google Scholar] [CrossRef]
- Cashion, W.; Weisbord, S.D. Radiographic contrast media and the kidney. Clin. J. Am. Soc. Nephrol. 2022, 17, 1234–1242. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. 2002, 9, 143–148. [Google Scholar] [CrossRef]
- Oliveira, C.; Ramos, S.J.; Siqueira, J.O.; Faquin, V.; de Castro, E.M.; Amaral, D.C.; Techio, V.H.; Coelho, L.C.; Silva, P.H.; Schnug, E.; et al. Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotoxicol. Environ. Saf. 2015, 122, 136–144. [Google Scholar] [CrossRef]
- Emmanuel, E.S.C.; Anandkumar, B.; Natesan, M.; Maruthamuthu, S. Efficacy of rare earth elements on the physiological and biochemical characteristics of Zea mays L. Aust. J. Crop Sci. 2010, 4, 289–294. [Google Scholar]
- Liu, D.; Zheng, S.; Wang, X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci. Rep. 2016, 6, 31860. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, R.L.; Zhang, P.; Sun, L.L.; Xu, J. Involvement of reactive oxygen species in lanthanum-induced inhibition of primary root growth. J. Exp. Bot. 2016, 67, 6149–6159. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Effects of lanthanum and cerium on the growth and mineral nutrition of corn and mung bean. Ann. Bot. 2008, 101, 971–982. [Google Scholar] [CrossRef]
- Kotelnikova, A.; Fastovets, I.; Rogova, O.; Volkov, D.S.; Stolbova, V. Toxicity assay of lanthanum and cerium in solutions and soil. Ecotoxicol. Environ. Saf. 2019, 167, 20–28. [Google Scholar] [CrossRef]
- Liman, R.; Acikbas, Y.; Ciğerci, İ.H. Cytotoxicity and genotoxicity of cerium oxide micro and nanoparticles by Allium and Comet tests. Ecotoxicol. Environ. Saf. 2019, 168, 408–414. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- Tang, X.; Sun, Y.; Xia, M.; Wen, C.; Zhang, Z. Ecological effects of low dosage mixed rare earth elements accumulation on major soil microbial groups in a yellow cinnamon soil. Ying Yong Sheng Tai Xue Bao 2004, 15, 2137–2141. [Google Scholar]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormetic dose responses induced by lanthanum in plants. Environ. Pollut. 2019, 244, 332–341. [Google Scholar] [CrossRef]
- Nascarella, M.A.; Calabrese, E.J. Hazard Assessment and the Evaluation of Rare Earth Element Dose–Response Relationships. In Rare Earth Elements in Human and Environmental Health: At Crossroads Between Toxicity and Safety; Pagano, G., Ed.; Pan Stanford Ltd.: Singapore, 2016; pp. 184–194. ISBN 978-981-4745-00-0. [Google Scholar]
- Wang, C.; Zhang, K.; He, M.; Jiang, C.; Tian, L.; Tian, Y.; Wang, X. Mineral nutrient imbalance, DNA lesion and DNA-protein crosslink involved in growth retardation of Vicia faba L. seedlings exposed to lanthanum ions. J. Environ. Sci. 2012, 24, 214–220. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, Q.; Yang, G.; Ding, X.L.; Li, X.; Cai, C.X.; Zhang, Z.; Wei, H.Y.; Lu, T.H.; et al. Rare earth elements activate endocytosis in plant cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12936–12941. [Google Scholar] [CrossRef]
- Daumann, L.J. Essential and ubiquitous: The emergence of lanthanide metallobiochemistry. Angew. Chem. Int. Ed. Engl. 2019, 58, 12795–12802. [Google Scholar] [CrossRef]
- Ma, J.J.; Ren, Y.J.; Yan, L.Y. Effects of spray application of lanthanum and cerium on yield and quality of Chinese cabbage (Brassica chinensis L.) based on different seasons. Biol. Trace Elem. Res. 2014, 160, 427–432. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, X.; Ma, J.; Yan, L. Effects of mixed rare earth fertilizer on yield and nutrient quality of leafy vegetables during different seasons. J. Rare Earths 2016, 34, 638–643. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, W.; Wang, Z.; Witkamp, G.J. Distributions of rare earths and heavy metals in field-grown maize after application of rare earth-containing fertilizer. Sci. Total Environ. 2002, 293, 97–105. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, S.; Wang, L.; Kung, H.T.; Wang, Y.; Hu, A.; Ding, S. Environmental biogeochemical behaviors of rare earth elements in soil–plant systems. Environ. Geochem. Health 2005, 27, 301–311. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Implementing Regulation (EU) L 319/5 of 1 October 2020; Official Journal of the European Union: Luxemburg, 2020. [Google Scholar]
- Cheng, J.; Ding, C.; Li, X.; Zhang, T.; Wang, X. Rare earth element transfer from soil to navel orange pulp (Citrus sinensis Osbeck cv. Newhall) and the effects on internal fruit quality. PLoS ONE 2015, 10, e0120618. [Google Scholar] [CrossRef]
- Atar, E. Gadolinium-based magnetic resonance imaging contrast agents in interventional radiology. Isr. Med. Assoc. J. 2004, 6, 412–414. [Google Scholar]
- Mitsumori, L.M.; Bhargava, P.; Essig, M.; Maki, J.H. Magnetic resonance imaging using gadolinium-based contrast agents. Top. Magn. Reson. Imaging 2014, 23, 51–69. [Google Scholar] [CrossRef]
- Tu, D.; Zheng, W.; Huang, P.; Chen, X. Europium-activated luminescent nanoprobes: From fundamentals to bioapplications. Coord. Chem. Rev. 2019, 378, 104–120. [Google Scholar] [CrossRef]
- Nishioka, T.; Yuan, J.; Yamamoto, Y.; Sumitomo, K.; Wang, Z.; Hashino, K.; Matsumoto, K. New luminescent europium (III) chelates for DNA labeling. Inorg. Chem. 2006, 45, 4088–4096. [Google Scholar] [CrossRef]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium-177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- English, R.J.; Zalutsky, M.; Venkatesan, P.; Sledge, C.B. Therapeutic application of Dysprosium-165-FHMA in the treatment of rheumatoid knee effusions. J. Nucl. Med. Technol. 1986, 14, 18–20. [Google Scholar]
- Allen, B.J.; Blagojevic, N. Alpha- and beta-emitting radiolanthanides in targeted cancer therapy: The potential role of terbium-149. Nucl. Med. Commun. 1996, 17, 40–47. [Google Scholar] [CrossRef]
- Sartor, O. Overview of Samarium Sm-153 lexidronam in the treatment of painful metastatic bone disease. Rev. Urol. 2004, 6, 3–12. [Google Scholar]
- Sandhu, A.S.; Srivastava, A.; Madhusoodanan, P.; Sinha, T.; Gupta, S.K.; Kumar, A.; Khanna, R. Holmium: YAG laser for intracorporeal lithotripsy. Med. J. Armed Forces India 2007, 63, 48–51. [Google Scholar] [CrossRef]
- Diaci, J.; Gaspirc, B. Comparison of Er:YAG and Er,Cr:YSGG lasers used in dentistry. J. Laser Health Acad. 2012, 2012, 1–13. [Google Scholar]
- Kostova, I. Lanthanides as anticancer agents. Curr. Med. Chem. Anticancer Agents 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Abeynayake, N.S.; Le, N.; Sanchez-Lecuona, G.; Donnadieu, B.; Webster, C.E.; Montiel-Palma, V. Unexpected alkyl isomerization at the silicon ligand of an unsaturated Rh complex: Combined experiment and theory. Dalton Trans. 2023, 52, 17168–17176. [Google Scholar] [CrossRef]
- Dent, P.C. Rare earth elements and permanent magnets (invited). J. Appl. Phys. 2012, 111, 07A721. [Google Scholar] [CrossRef]
- Garside, M. (Ed.) Distribution of Rare Earth Element Consumption Worldwide in 2020, by End Use; Statista GmbH: Hamburg, Germany, 2022. [Google Scholar]
- Smith Stegen, K. Heavy rare earths, permanent magnets, and renewable energies: An imminent crisis. Energy Policy 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Zijlstra, H. Application of permanent magnets in electromechanical power converters; the impact of Nd-Fe-B magnets. J. Phys. Colloq. 1985, 46, C6-3–C6-8. [Google Scholar] [CrossRef]
- Nakamura, H. The current and future status of rare earth permanent magnets. Scr. Mater. 2018, 154, 273–276. [Google Scholar] [CrossRef]
- Sugimoto, S. Current status and recent topics of rare-earth permanent magnets. J. Phys. D Appl. Phys. 2011, 44, 064001. [Google Scholar] [CrossRef]
- Tripathy, P.K.; Mondal, K.; Khanolkar, A.R. One-step manufacturing process for neodymium-iron (magnet grade) master alloy. Mater. Sci. Energy Technol. 2021, 4, 249–255. [Google Scholar] [CrossRef]
- Goonan, T.G. Rare Earth Elements—End Use and Recyclability; Scientific Investigations Report 2011-5094; U.S. Geological Survey: Reston, VA, USA, 2011.
- Keane, E. Neodymium Magnets Provide Key to Understanding Rare Earth Trends; Seeking Alpha: New York, NY, USA, 2009. [Google Scholar]
- Li, L.Z.; Yan, B.; Lin, L.-X.; Zhao, Y. Solid state synthesis, microstructure and photoluminescence of Eu3+ and Tb3+ activated strontium tungstate. J. Mater. Sci. Mater. Electron. 2011, 22, 1040–1045. [Google Scholar] [CrossRef]
- Misiak, M.; Prorok, K.; Bednarkiewicz, A. Biological application of lanthanide doped nanomaterials. Wiad. Chem. 2012, 66, 5–6. [Google Scholar]
- Bednarkiewicz, A.; Mączka, M.; Strek, W.; Hanuza, J.; Karbownik, M. Size dependence on infrared spectra of NaGdF4 nanocrystals. Chem. Phys. Lett. 2006, 418, 75. [Google Scholar] [CrossRef]
- Park, Y.I.; Kim, J.H.; Lee, K.T.; Jeon, K.S.; Na, H.B.; Yu, J.H.; Hyeon, T. Nonblinking and nonbleaching upconverting nanoparticles as an optical imaging nanoprobe and T1 magnetic resonance imaging contrast agent. Adv. Mater. 2009, 21, 4467. [Google Scholar] [CrossRef]
- Sun, L.N.; Peng, H.; Stich, M.I.; Achatz, D.; Wolfbeis, O.S. pH sensor based on upconverting luminescent lanthanide nanorods. Chem. Commun. 2009, 33, 5000–5002. [Google Scholar] [CrossRef]
- Mader, H.S.; Wolbies, O.S. Optical ammonia sensor based on upconverting luminescent nanoparticles. Anal. Chem. 2010, 82, 5002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).