Characteristics of Laterite Soil for Potential Geopolymer Applications
Abstract
1. Introduction
2. Materials and Analytical Techniques
2.1. X-Ray Fluorescence (XRF)
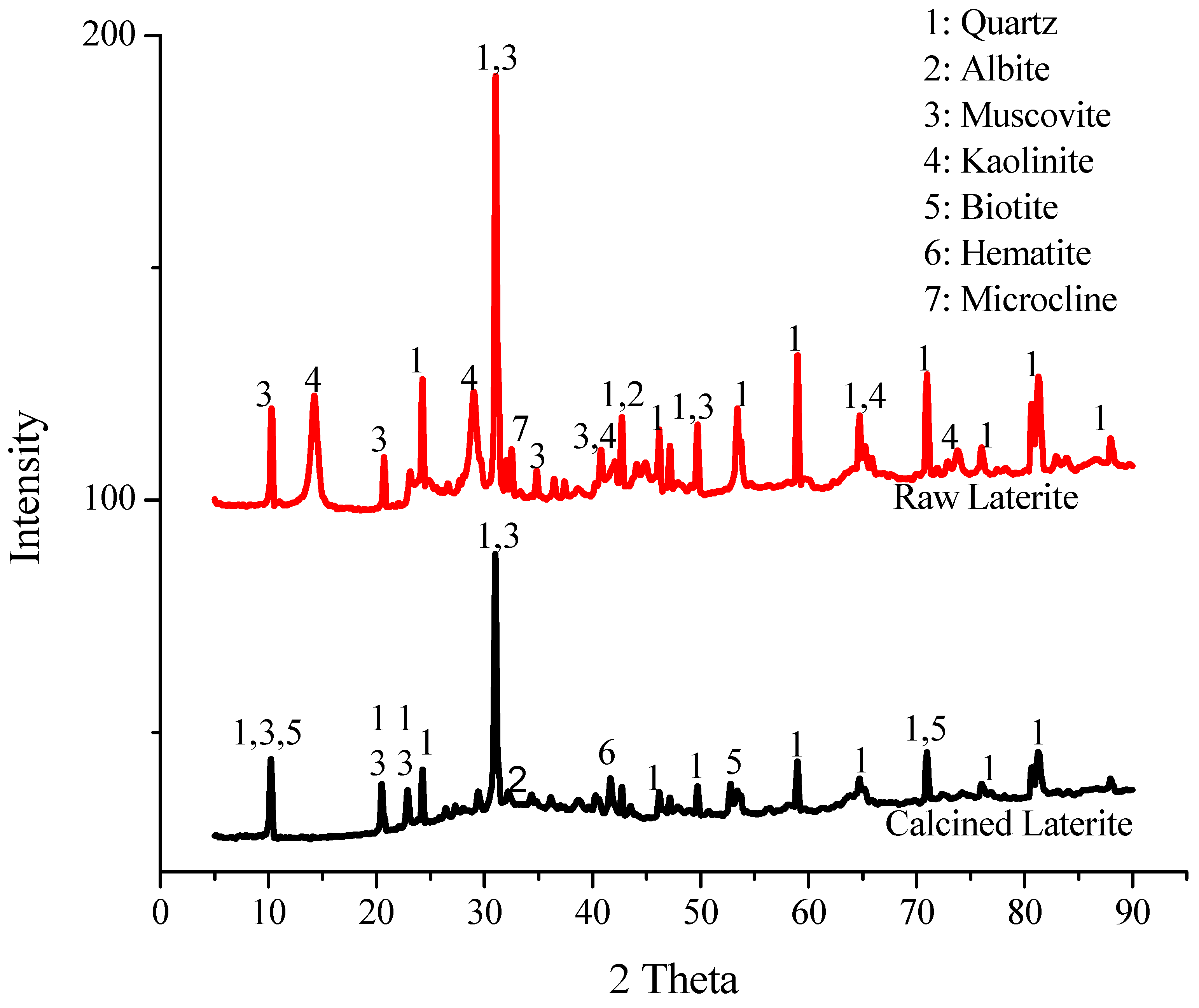
2.2. X-Ray Diffraction (XRD)
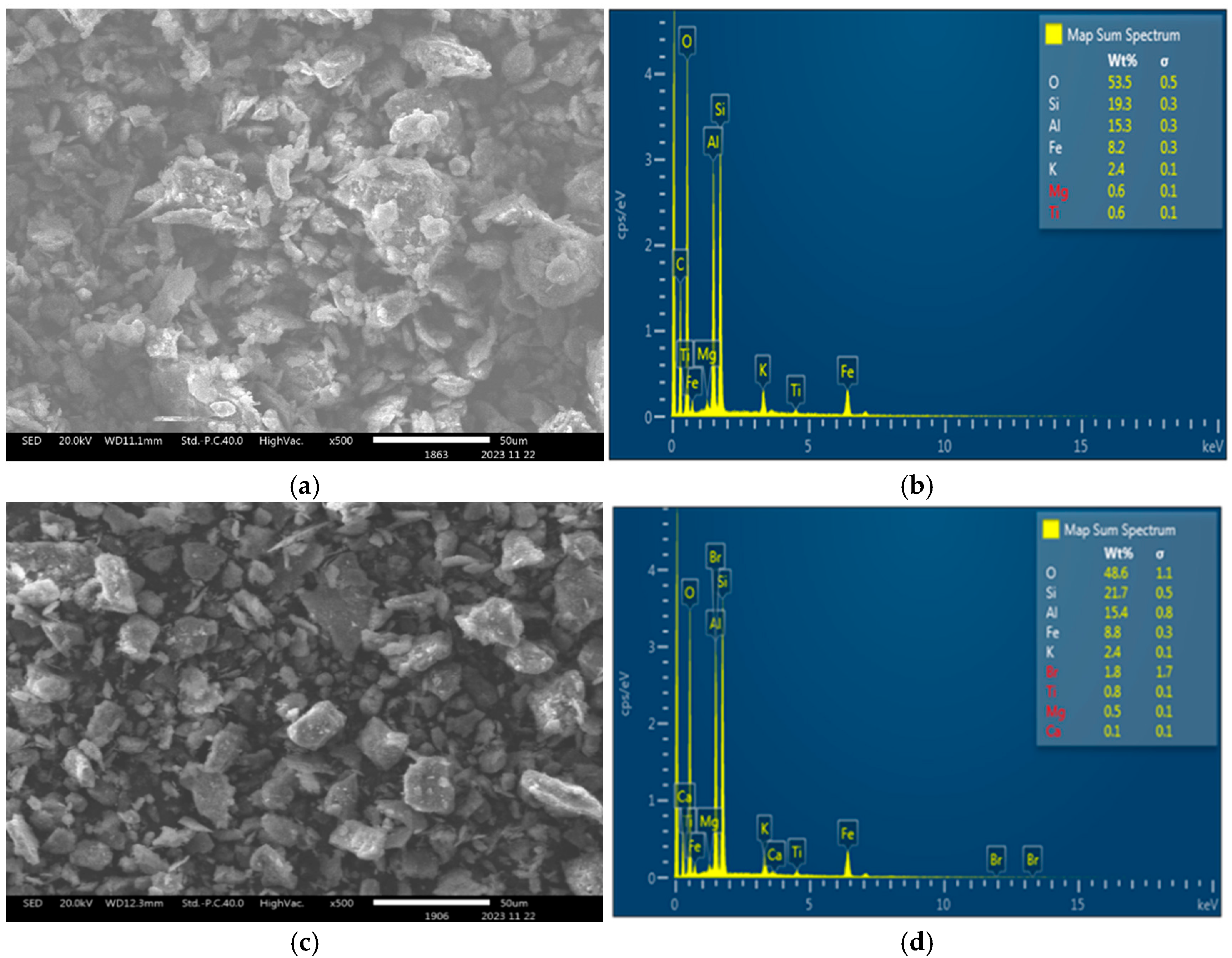
2.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
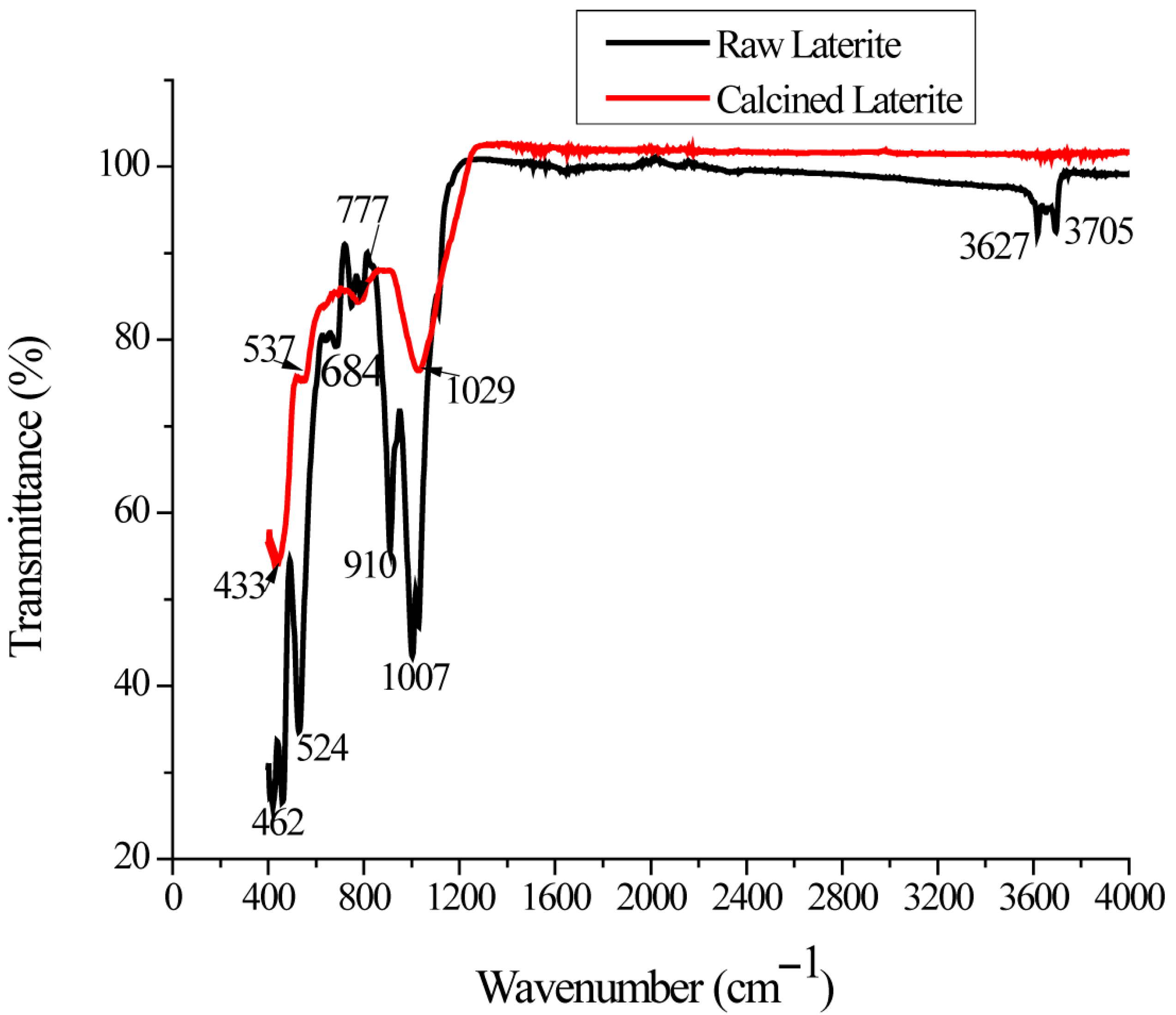
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
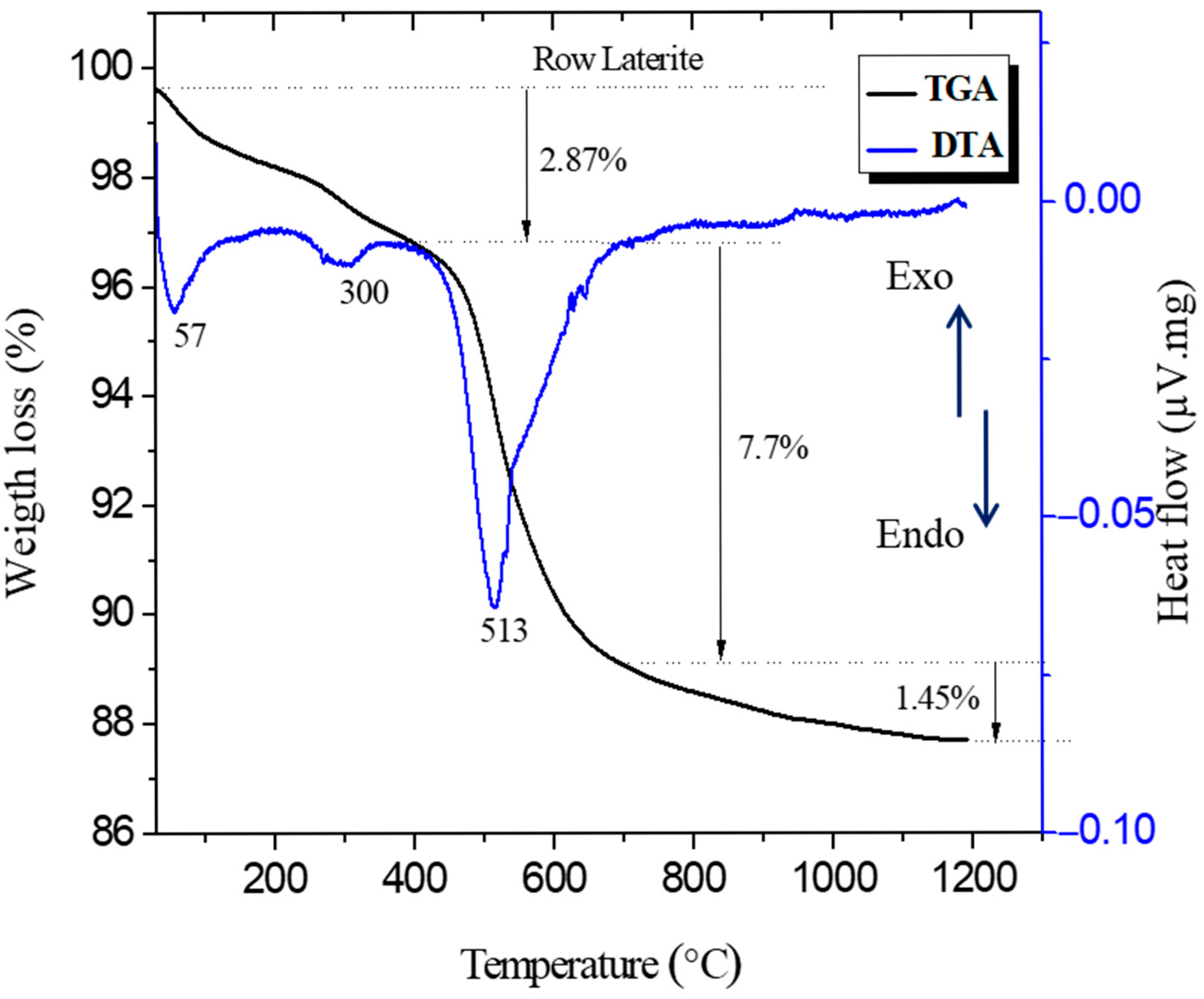
2.5. Thermogravimetric (TGA) and Differential Thermal (DTA) Analyses
2.6. pH Test
3. Results and Discussion
3.1. XRF
3.2. XRD Analysis
3.3. SEM and EDS Analysis
3.4. FTIR Analysis
3.5. TGA and DTA Analysis
3.6. pH of Laterite Soil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, F.H. A Journey from Madras Through the Countries of Mysore, Canara, and Malabar; Cadell, T., Davies, W., Eds.; Black, Parry, and Kingsbury: London, UK, 1807; Volume 3. [Google Scholar]
- Thurston, E. The Madras Presidency with Mysore, Coorg and the Associated States, Provincial Geographies of India; Cambridge University Press: London, UK, 1913; Volume 1. [Google Scholar]
- Maignien, R. Review of Research on Laterites; Natural Resources Research IV; The United Nations Education, Scientific and Cultural Organization Vaillant-Carmanne: Paris, France, 1966; Volume 4, pp. 1–156. [Google Scholar]
- Kheoruenromne, I.R.B. Red and Yellow Soil and Laterite Formation in the Northeast Plateau, Thailand. Chem. Geol. 1987, 60, 319–326. [Google Scholar]
- Lyle, A.; John, C. Genesis and Hardening of Laterite in Soils; US Department of Agriculture: Washington, DC, USA, 1962; Volume 1282. [Google Scholar]
- Martin, F.J.; Doyne, H.C. Laterite and Lateritic Soils in Sierra Leone. J. Agric. Sci. 1927, 17, 530–547. [Google Scholar] [CrossRef]
- Pendleton, R.L.; Sharasuvana, S. Analyses of Some Siamese Laterites. Soil. Sci. 1946, 62, 423–440. [Google Scholar]
- Mahalinga-Iyer, U.; Williams, D.J. Properties and Performance of Lateritic Soil in Road Pavements. Eng. Geol. 1997, 46, 71–80. [Google Scholar]
- Gidigasu, M.D. The Importance of Soil Genesis in the Engineering Classification of Ghana Soils. Eng. Geol. 1971, 5, 117–161. [Google Scholar]
- Mahalinga-Iyer, U.; Williams, D.J. Engineering Properties of a Lateritic Soil Profile. Eng. Geol. 1991, 31, 45–58. [Google Scholar]
- Nuru, Z.K.; Kearsley, E.P.; Elsaigh, W.A.; Student, P.D. Application of Laterite-Based Geopolymer Mortar for Masonry Bedding. Mater. Sci. Forum 2024, 1137, 81–86. [Google Scholar] [CrossRef]
- Kaze, R.C.; Lemougna, P.N.; Alomayri, T.; Assaedi, H.; Adesina, A.; Kumar Das, S.; Lecomte-Nana, G.L.; Elie, K.; Melo, U.C.; Cristina, L. Characterization and Performance Evaluation of Laterite Based Geopolymer Binder Cured at Different Temperatures. Constr. Build. Mater. 2021, 270, 121443. [Google Scholar] [CrossRef]
- Subaer; Haris, A.; Irhamsyah, A.; Akifah, N.; Amalia, N.S. Physico-Mechanical Properties of Geopolymer Based on Laterite Deposit Sidrap, South Sulawesi. J. Phys. Conf. Ser. 2019, 1244, 012037. [Google Scholar]
- Tantono, S.N.; Belibi Belibi, P.D.; Baenla, J.; Elimbi, A. Alkaline and Acid Activations of Calcined Laterites: A Comparative Study. Silicon 2023, 15, 2797–2810. [Google Scholar] [CrossRef]
- Poudeu, R.C.; Ekani, C.J.; Djangang, C.N.; Blanchart, P. Role of Heat-Treated Laterite on the Strengthening of Geopolymer Designed with Laterite as Solid Precursor. Ann. Chim. Sci. Des. Mater. 2019, 43, 359–367. [Google Scholar] [CrossRef]
- Metekong, J.V.S.; Kaze, C.R.; Adesina, A.; Nemaleu, J.G.D.; Djobo, J.N.Y.; Lemougna, P.N.; Alomayri, T.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Influence of Thermal Activation and Silica Modulus on the Properties of Clayey-Lateritic Based Geopolymer Binders Cured at Room Temperature. Silicon 2022, 14, 7399–7416. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal Treatment of Kaolin: The Effect of Mineralogy on the Pozzolanic Activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar]
- Joseph, D. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2020; Volume 5, ISBN 9782954453118. [Google Scholar]
- Nuru, Z.K.; Elsaigh, W.A.; Kearsley, E.P. The Influence of Curing Method on Properties of Laterite Soil Based Geopolymer Cement. Constr. Build. Mater. 2025, 483, 141768. [Google Scholar]
- ISO 10390; Soil, Treated Biowaste and Sludge-Determination of PH. ISO: Geneva, Switzerland, 2021.
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Townsend, F.C.; Manke, P.G.; Parcher, J. V The Influence of Sesquioxides on Lateritic Soil Properties. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 1970. Volume 374. [Google Scholar]
- Abomo, T.; Cyriaque Kaze, R.; Cengiz, O.; Alomayri, T.; Pefouo Wilson, T.; Eko Robert, M.; Naghizadeh, A.; Kamseu, E. Impact of the Depth of a Lateritic Profile on the Physicochemical, Mechanical and Microstructural Properties of Geopolymer Binders. Constr. Build. Mater. 2023, 403, 133138. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of Calcination Temperature of Kaolinite Clays on the Properties of Geopolymer Cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Carvalheiras, J.; Novais, R.M.; Labrincha, J.A. Metakaolin/Red Mud-Derived Geopolymer Monoliths: Novel Bulk-Type Sorbents for Lead Removal from Wastewaters. Appl. Clay Sci. 2023, 232, 106770. [Google Scholar]
- Townsend, F.C. The Influence of Sesquioxides on Some Physico-Chemical and Engineering Properties of a Lateritic Soil; Oklahoma State University: Stillwater, OK, USA, 1970. [Google Scholar]
- Ghani, U.; Hussain, S.; Noor-ul-Amin; Imtiaz, M.; Khan, S.A. Role of Calcination on Geopolymerization of Lateritic Clay by Alkali Treatment. J. Saudi Chem. Soc. 2021, 25, 101198. [Google Scholar] [CrossRef]
- Mimboe, A.G.; Abo, M.T.; Djobo, J.N.Y.; Tome, S.; Kaze, R.C.; Deutou, J.G.N. Lateritic Soil Based-Compressed Earth Bricks Stabilized with Phosphate Binder. J. Build. Eng. 2020, 31, 101465. [Google Scholar] [CrossRef]
- Akhmal Saadon, S.; Rahim Mohd Yusoff, A.; Yusop, Z.; Azman, S.; Uy Achmad Syafiuddin, D. Heated Laterite as a Low-Cost Adsorbent for Arsenic Removal from Aqueous Solution. Malays. J. Fundam. Appl. Sci. 2018, 14, 1–8. [Google Scholar]
- Bewa, C.N.; Tchakouté, H.K.; Rüscher, C.H.; Kamseu, E.; Leonelli, C. Influence of the Curing Temperature on the Properties of Poly (Phospho-Ferro-Siloxo) Networks from Laterite. SN Appl. Sci. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar]
- Song, X.; Boily, J.F. Surface and Bulk Thermal Dehydroxylation of FeOOH Polymorphs. J. Phys. Chem. A 2016, 120, 6249–6257. [Google Scholar] [CrossRef]
- Lecomte-Nana, G.; Goure-Doubi, H.; Smith, A.; Wattiaux, A.; Lecomte, G. Effect of Iron Phase on the Strengthening of Lateritic-Based “Geomimetic” Materials. Appl. Clay Sci. 2012, 70, 14–21. [Google Scholar] [CrossRef]
- Caballero, L.R.; de Moraes, E.; Fairbairn, R.; Dias, R.; Filho, T.; Civil, D.E.; de Janeiro, R. Thermal, Mechanical and Microstructural Analysis of Metakaolin Based Geopolymers 2. Exp. Proced. 2019, 22, 1–8. [Google Scholar]
- Hughes, B.; Davenport, D.; Dohle, L. Standard Soil Test Methods and Guidelines for Interpretation of Soil Results; Government of South Australia: Adelaide, Australia, 1996. [Google Scholar]
- Anburuvel, A. The Role of Activators in Geopolymer-Based Stabilization for Road Construction: A State-of-the-Art Review. Multiscale Multidiscip. Model. Exp. Des. 2023, 6, 41–59. [Google Scholar]
- Khale, D.; Chaudhary, R. Mechanism of Geopolymerization and Factors Influencing Its Development: A Review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Risdanareni, P.; Puspitasari, P.; Januarti Jaya, E. Chemical and Physical Characterization of Fly Ash as Geopolymer Material. In Proceedings of the MATEC Web of Conferences, Sibiu, Romania, 7–9 June 2017; EDP Sciences: Les Ulis, France, 2017; Volume 97. [Google Scholar]
- Saride, S.; Jallu, M. Effect of Fly Ash Geopolymer on Layer Coefficients of Reclaimed Asphalt Pavement Bases. J. Transp. Eng. Part. B Pavements 2020, 146, 04020033. [Google Scholar] [CrossRef]
| Oxide | Mass (%) | Oxide | Mass (%) |
|---|---|---|---|
| SiO2 | 61.09 | MnO | 0.04 |
| Al2O3 | 20.04 | P2O5 | 0.04 |
| Fe2O3 | 6.64 | Nb2O5 | 0.03 |
| K2O | 1.92 | Cr2O3 | 0.02 |
| MgO | 0.67 | V2O5 | 0.01 |
| TiO2 | 0.57 | Na2O | <0.01 |
| ZrO2 | 0.18 | LOI | 8.54 |
| CaO | 0.09 |
| Quartz | Albite | Microcline | Muscovite | Kaolinite | Biotite | Hematite | |
|---|---|---|---|---|---|---|---|
| LN | 52.1 | 4 | 0.7 | 14.6 | 28.6 | - | - |
| LC | 49.9 | 0.0 | 9 | 24.9 | - | 5.5 | 10.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuru, Z.K.; Elsaigh, W.A.; Kearsley, E.P. Characteristics of Laterite Soil for Potential Geopolymer Applications. Minerals 2025, 15, 719. https://doi.org/10.3390/min15070719
Nuru ZK, Elsaigh WA, Kearsley EP. Characteristics of Laterite Soil for Potential Geopolymer Applications. Minerals. 2025; 15(7):719. https://doi.org/10.3390/min15070719
Chicago/Turabian StyleNuru, Zeyneb K., Walied A. Elsaigh, and Elsabe P. Kearsley. 2025. "Characteristics of Laterite Soil for Potential Geopolymer Applications" Minerals 15, no. 7: 719. https://doi.org/10.3390/min15070719
APA StyleNuru, Z. K., Elsaigh, W. A., & Kearsley, E. P. (2025). Characteristics of Laterite Soil for Potential Geopolymer Applications. Minerals, 15(7), 719. https://doi.org/10.3390/min15070719








