Abstract
Research on dolomite has long been central in geoscience, yet understanding the origin of Middle Ordovician dolomite in the northeast of the North China Platform remains limited. Based on this, this study focuses on dolomite of Majiagou Formation in Liujiang Basin, and analyzes its genetic process. The research is based on the measured geological section and conducts high-precision analysis and testing, encompassing major and trace elements, rare earth elements, stable carbon and oxygen isotopes, strontium isotopes, crystal structure parameters, and micro-area elements of dolomite. Analysis of V/(V + Ni), Th/U, Sr/Ba, Mn/Sr, (Eu/Eu*) N, (Ce/Ce*) N, and the dolomite crystal parameters indicates that the formation of dolomite is related to evaporation. Furthermore, REE and micro-area characteristics of dolomite, as well as the significant negative deviation of δ13C and δ18O, in conjunction with 87Sr/86Sr deviating from the standard values of Ordovician seawater, suggest an origin of the dolomite in this formation with mixed-water dolomitization and burial dolomitization. A comprehensive assessment of dolomite formation suggests three distinct stages: early-stage evaporation dolomitization, subsequent mixed-water dolomitization, and later-stage burial dolomitization. The research further corroborated that dolomite formation is a complex outcome resulting from the interplay of various geological processes over space and time.
1. Introduction
Dolomite genesis, a significant sedimentary mineral process, has been a focal point in earth sciences research. Unlike modern carbonate sediments primarily composed of calcite and aragonite, dolomite, although scarce and slow to form at surface temperatures below 80 °C [1,2], is prevalent in ancient marine carbonate sequences. This intriguing paradox, dating back to the 18th century, has captivated the geological community. Despite advancements in thermodynamics, the origins of dolomite remain contentious due to gaps in understanding chemical, hydrological, and kinetic processes [3]. Various diagenetic models have been proposed, including seepage–reflux dolomitization, capillary-concentration dolomitization, sabkha dolomitization, mixed-water dolomitization, adjusted dolomitization, buried dolomitization, seawater thermal convection dolomitization, and tectonically controlled hydrothermal dolomitization [4]. These models highlight specific geological conditions during penecontemporaneous or burial processes, yet a standardized classification based on diagenetic models is lacking [5]. Recent research indicates that extensive massive dolomites form in shallow, hypersaline subtidal, and specific subsurface environments, with mixed-water dolomitization and refluxed sabkha dolomitization models offering significant explanatory power [6].
Research on dolomite formation in the Middle Ordovician Majiagou Formation of the North China Platform has predominantly centered on the Ordos Basin in the platform’s western region [7,8,9,10]. Studies in this area have shown that dolomitizing fluids were highly saline and closely linked to seawater. Diagenesis in this region was characterized by significant penecontemporaneous dolomitization, later overlaid by burial dolomitization. In contrast, investigations in the northeastern part of the North China Platform are limited. Feng Z.Z. et al. [11] examined Lower Paleozoic dolomites on the platform, identifying mud-silt-sized crystalline dolomites and saccharoid dolomites as the primary types. These dolomites, akin to penecontemporaneous supratidal dolomites of the Bahama Platform, formed under the influence of hypersaline seawater in supratidal settings. Further studies on Middle Ordovician dolomites in the central–northern North China Platform indicated that sea-level fluctuations control the formation of dolomite. Penecontemporaneous evaporative dolomitization, seepage–reflux dolomitization, and burial dolomitization collectively contributed to dolomite development in diverse depositional environments [12]. This discovery implies that dolomitization is influenced not by a singular mechanism but by the combined effects of multiple mechanisms in time and space. Recent examinations of the Majiagou Formation in the Liujiang Basin unveiled a shift from early evaporative dolomitization to subsequent modification through multistage burial dolomitization [13]. This model sheds light on the variations in dolomite genesis among different tectonic units of the North China Platform.
Geochemical methods have become essential for investigating the origins of dolomite and establishing its formation mechanisms [14,15]. Sedimentary geochemistry enables the analysis of elemental content and mineral composition of dolomite sheds light on its origins, sedimentary environment characteristics, and rock evolution processes [16,17,18]. Isotope geochemistry provides insights into the environmental conditions during dolomite formation, including fluid properties. The combined analysis of carbon, oxygen, and strontium isotopes in carbonate rocks is particularly valuable for understanding paleoenvironmental and paleoclimatic changes [19,20,21,22]. Parameters related to dolomite crystal structure, such as orderliness and cell dimensions, are crucial microscopic indicators for studying dolomite formation environments and mechanisms [23,24]. Additionally, Electron Probe Microanalysis (EPMA), employing advanced microanalytical techniques, can accurately characterize the ring-band structure and trace element distribution within dolomite crystals, facilitating the reconstruction of multi-phase diagenetic fluid histories and providing direct evidence [25]. This paper discusses the sedimentary environment changes, geochemical properties of dolomite fluids, and origin of dolomite in the Majiagou Formation of the Liujiang Basin. This is achieved through polarized microscopy observations, tests of stable carbon and oxygen isotopic composition and strontium isotopic composition, major and trace element, the crystal structure of dolomite, and EPMA. The research results hold significant implications for understanding the origin of dolomite in the northeastern area of the North China Platform.
2. Geological Setting
The North China Platform, a prominent stable craton in China, features an inverted triangular shape, broad in the north and tapering southward. It spans 14 provinces and autonomous regions, including the Bohai and Yellow Seas, marking it as a distinctive geotectonic unit in China [11,26,27]. The Liujiang Basin in Hebei Province, situated at the northeastern edge of the North China Platform, is a composite basin reshaped by Meso-Cenozoic tectonic activity against a backdrop of negative topography due to Paleozoic tilts extending south–southeast. Its oblique core comprises the Jurassic System, with nearly monoclinic stratigraphy on the eastern side (Figure 1a). During the Early Paleozoic, the North China Platform was predominantly deposited in a shallow marine environment, with the abundance of dolomites and dolomitic limestone is about equal to that of limestone [11].
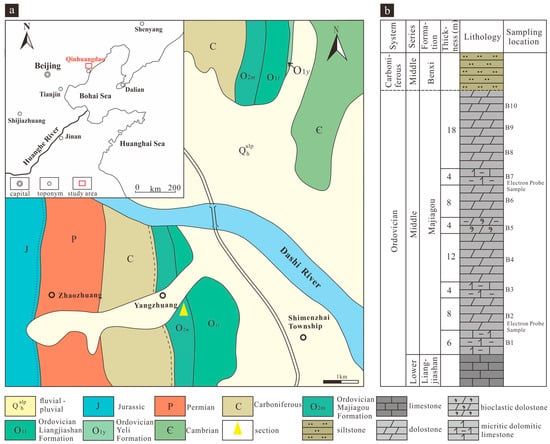
Figure 1.
Geological setting map of study area: (a) location and geological map of Liujiang Basin, Hebei; (b) stratigraphic column and sampling locations of the Majiagou Formation in Liujiang Basin.
The Middle Ordovician Majiagou Formation in the Liujiang Basin, Hebei Province, is best exposed in the Liangjiashan section, with a stratigraphic thickness of approximately 66 m (Figure 1b). The formation primarily consists of micritic dolomitic limestone and mud-silt-sized crystalline dolomites, with some layers containing chert nodule dolomitic limestone and bioclastic dolomite interbedded with bioclastic limestone (Figure 2a). The formation has yielded cephalopod fossils such as Ormoceras and Orthoceras (Figure 2b), as well as gastropod fossils including Ecculiomphalus (Figure 2c) and Maclurites. The formation is in consolidated contact with the underlying Lower Ordovician Liangjia Mountain Formation and in parallel unconformable contact with the overlying Middle Carboniferous Benxi Formation. The North China Platform was generally uplifted and exposed during the late Ordovician Caledonian orogeny, and did not receive sedimentary deposition again until the Middle Carboniferous, resulting in a hiatus in the stratigraphic record from the Upper Ordovician to the Lower Carboniferous.

Figure 2.
The dolomites and fossils of the Majiagou Formation in the Liujiang Basin: (a) field outcrop of the Majiagou Formation; (b) cephalopod fossils, B5; (c) gastropod fossils, B5.
3. Methods
Samples from the Liangjiashan section in the Liujiang Basin were collected based on distinct lithologies, with infilling conducted for lithologies of considerable thickness. The primary targets for collection comprised micritic dolomitic limestone and mud-silt-sized crystalline dolomites, resulting in a total of 10 samples. The specific location of each sampling point is delineated in Figure 1b.
Whole-rock analysis methods involved X-ray fluorescence (XRF) analysis using a Shimadzu XRF-1800 instrument for major elements, ensuring data accuracy within 1%. Trace elements were analyzed via inductively coupled plasma mass spectrometry (ICP-MS) using an Analytic Jena M90 instrument. Validation against standard samples demonstrated data accuracy within 5%, with analysis errors below 10% for volatile and trace elements.
X-ray powder diffraction analysis was conducted on dolomite samples to examine their mineral composition, degree of order, and unit cell parameters. The analysis was performed using a Bruker D4 high-speed XRD diffractometer with a LynxEye high-energy detector, covering a detection range from 0.1% to 100%. These analyses were carried out at ALS Minerals Laboratory
Isotope analysis was carried out as follows: Carbon, oxygen, and strontium isotopes underwent testing at the Analytical and Testing Research Center of the Beijing Research Institute of Uranium Geology. Carbon and oxygen isotopes were assessed using a MAT-253 gas isotope mass spectrometer and results were standardized against VPDB, reported as δ13C and δ18O. Strontium isotope analysis utilized a Phoenix thermal surface ionization mass spectrometer, with ratios compared to standard values. Instrument measurements were adjusted for accuracy and precision of isotope ratio determination.
Backscattered electron (BSE) imaging of dolomite and micro-area component analysis of minerals were conducted at the Electron Probe Laboratory of the Hebei Key Laboratory of Earthquake Dynamics, Institute of Disaster Prevention. A JEOL JEE-420 carbon coater was utilized for sample surface conductivity enhancement. The analysis was performed using a JEOL JXA-8230 electron probe under the following conditions: accelerating voltage of 15 kV, current of 10 nA, electron beam spot diameter of 10 μm, and an analysis error of less than 1%.
4. Results
4.1. Petrological Characteristics
The Middle Ordovician Majiagou Formation mainly consists of a set of dolomite-bearing carbonate rocks. From bottom to top, it includes micritic dolomitic limestone, bioclastic dolomitic limestone, and mud-silt-sized crystalline dolomite. Among these, powdery-fine crystalline dolomite is the most prevalent type. The fresh rock surfaces exhibit hues ranging from yellowish to blackish, displaying distinct knife-cut structures.
The matrix of the micritic dolomitic limestone is composed of micritic calcite with an exceptionally fine grain size, rendering the individual mineral grains indistinguishable. The dolomite content within the matrix is approximately 20%, occurring as powder-grade authigenic crystals (30–50 μm) with a scattered distribution (Figure 3a). Bioclastic debris are embedded in the micritic matrix of micritic dolomitic limestone (Figure 3b), with few complete fossils; most are detritus and composed of calcium. Fine-powder crystal dolomite (Figure 3c,d) is predominantly high purity (content > 90%), with grain sizes ranging from 50 to 100 μm, primarily composed of fine, rhombic euhedral crystals. The backscattered electron images reveal a distinct zonal structure within the dolomite crystals, characterized by alternating light and dark bands (Figure 3e,f). Calcite cement frequently fills the spaces between these crystals, with some cements exhibiting signs of dissolution, such as irregular pits or notches at the edges. The vertical sequence of the dolomite in the Majiagou Formation displays a clear evolutionary trend. From the bottom to the top, the dolomite content generally increases, the grain size becomes larger, the degree of self-deformation in the crystal form intensifies, and the zonal structure develops more prominently.
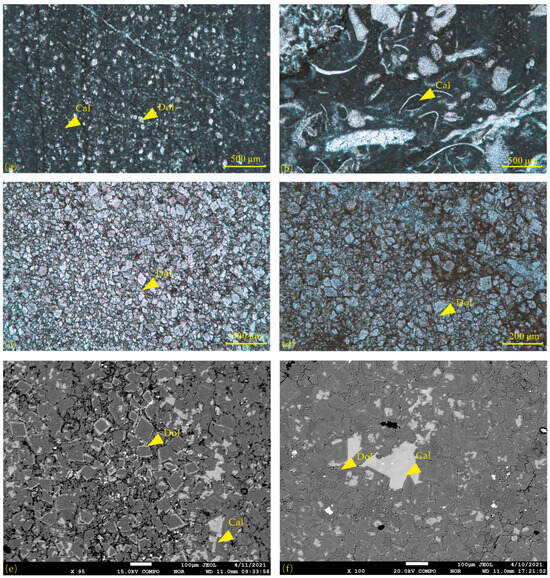
Figure 3.
The microscopic characteristics of dolomite in the Majiagou Formation: (a) micritic dolomitic limestone, B1; (b) micritic dolomitic limestone, B3; (c) mud-silt-sized crystalline dolomites, B8; (d) mud-silt-sized crystalline dolomites, B9; (e) mud-silt-sized crystalline dolomites, B2; (f) mud-silt-sized crystalline dolomites interlayers in micritic dolomitic limestone, B7.
X-ray powder diffraction analysis (Figure 4a–d) reveals that the Majiagou Formation’s carbonate rocks primarily consist of dolomite and calcite, with minor quartz and halite. The dolomite diffraction pattern is characterized by a prominent (104) crystal plane peak, while the (015) and (110) peaks are weaker. Significant peaks in these two planes suggest a highly ordered crystal structure; hence, the dolomite in the study area is low order. Calcite, quartz, and halite exhibit characteristic peaks at the (104), (101), and (200) planes, respectively. The intensity of these peaks varies across strata, influenced by mineral grain size, content, and crystallization degree. Notably, halite peaks at B2 and B6 are more intense (Figure 4b), suggesting a period of salinization driven by evaporation, linked to a regional sea regression event.
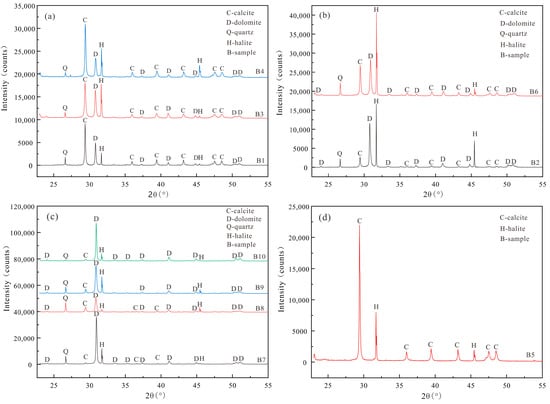
Figure 4.
The X-ray diffraction pattern of dolomites in the Majiagou Formation: (a) the X-ray diffraction pattern of B1, B3 and B4; (b) the X-ray diffraction pattern of B2 and B6; (c) the X-ray diffraction pattern of B7, B8, B9 and B10; (d) the X-ray diffraction pattern of B5.
Quartz, a typical terrigenous clastic indicator, suggests a nearshore shallow-water environment during sedimentation when found in carbonate rocks. Evaporite minerals like halite and gypsum are key indicators of sedimentary environments dominated by evaporation under arid conditions. When seawater evaporation surpasses supply, high-salinity brine crystallizes, forming these minerals. The co-occurrence of dolomite, quartz, and halite in the Majiagou Formation’s carbonate rocks illustrates a dual environment: “shallow-water terrigenous clastic input” and “high-salinity brine concentration” offering crucial evidence for the penecontemporaneous origin of dolomite.
4.2. Geochemical Characteristics of Elements
In carbonate rocks, the presence of elements such as Al, Ti, Si, K, Cr, Ni, Cu, and Ba is influenced by terrigenous materials, while elements like Ca, Mg, and Sr are governed by the carbonate minerals [28]. Al2O3 and K2O are typically abundant in clay minerals or the fluids of compacted clastic rocks [12,29]. The Al2O3 content in dolomites from the Majiagou Formation ranges from 0.41% to 2.26%, averaging 1.12%, with K2O ranging from 0.01% to 0.82%, averaging 0.33% (Table 1). These dolomites exhibit generally low Al2O3 and K2O contents, showing a strong positive linear correlation between them (Figure 5a). This suggests that Al2O3 and K2O in the carbonates of this formation primarily originate from clay minerals or terrigenous materials, with limited terrigenous input. The presence of Si, Al, and K indicates some influence of terrigenous materials on the deposition of the Majiagou Formation, although not significant. SiO2 content shows a correlation with sea-level fluctuations, ranging from 1.42% to 17.68%, averaging 7.78% in the Majiagou Formation (Table 1). Particularly at the upper part of the Majiagou Formation, the average of B7~B10 is 11.39%, compared to 5.91% in the underlying Liangjiashan Formation, suggesting an overall sea-level decline during the Early to Middle Ordovician in the Liujiang Basin. Despite some influence of terrigenous materials on the sedimentation process, it was minor and did not alter the primary marine sedimentary characteristics of the study area. Additionally, a significant regression occurred during the later stages of deposition in this formation.

Table 1.
The major element (%) and trace element (ppm) date of the carbonate rock in the Majiagou Formation.
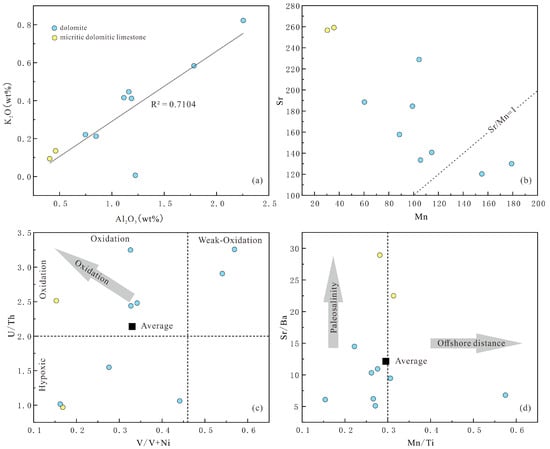
Figure 5.
The sedimentary environment discrimination map of the Majiagou Formation: (a) cross-plot of Al2O3 and K2O values; (b) cross-plot of Mn and Sr values; (c) cross-plot of V/V+Ni and U/Th values; (d) cross-plot of Mn/Ti and Sr/Ba values.
The contents of strontium Sr and Mn elements can not only serve as important bases for discriminating sedimentary environments, but also are geochemical markers for sample screening and fluid characteristic discrimination. Research indicates that carbonate rocks with Mn/Sr ratios below 10 typically preserve primary δ13C values. In the Majiagou Formation, Mn/Sr ratios range from 0.19 to 1.38, averaging 0.64, suggesting the δ13C values of the analyzed samples have retained their primary characteristics. Typical marine carbonate rocks exhibit Mn/Sr ratios near 1. When the diagenetic fluid is affected by atmospheric fluid, Sr is exsolved from marine carbonate minerals while Mn is absorbed, causing the Mn/Sr ratio to increase, without affecting another major element chemistry [30]. The Mn/Sr ratio increases in the upper strata of the Majiagou Formation (Figure 5b), likely due to meteoric freshwater involvement in dolomitization.
Redox-sensitive elements, mainly including U, V, and Mo, have their distribution, cycling, and differentiation in ocean water bodies and sediments governed by their chemical properties and prevailing redox conditions [31]. These elements hold geological significance for paleoceanographic investigations in modern oceans. For instance, a study in the Ross Sea, Antarctica, demonstrated that Mn, Mo, Ni, and Co can help discern the redox status of marine sediments [32]. Hatch et al. [33] suggested that the V/(V + Ni) ratio serves as an indicator of sediment redox conditions, with values between 0.84 and 0.89 indicating an anoxic environment, 0.54 to 0.82 suggesting weak oxygenation, and 0.46 to 0.6 signifying oxygen depletion. Higher values correspond to increased anoxia levels. Wignall et al. [34] proposed the use of the Th/U ratio for assessing sediment redox conditions. Under anoxic conditions, U forms insoluble uranium fluoride complexes (U4+), while in oxygenated settings, it converts to soluble uranyl carbonate (U6+), leading to higher U concentrations in anoxic sediments. Th, being redox-insensitive, remains insoluble. The Th/U ratio in anoxic sediments is below 2, contrasting with values ranging from 2 to 7 in oxygenated marine sediments. In the Majiagou Formation, V/(V + Ni) ratios range from 0.15 to 0.57, averaging 0.33, and Th/U ratios range from 0.97 to 3.26, averaging 2.14. A comprehensive assessment of redox proxies indicates that this formation was deposited in a weakly oxygenated to oxidizing environment (Figure 5c), reflecting a shallowing trend in the sedimentary water body transitioning from a shallow sea to littoral.
In the investigation of modern sediment elemental geochemistry, a relationship has been observed between element accumulation/dispersion and basin depth (distance from shore), primarily linked to element differentiation during sedimentation [35]. For instance, in the muddy region of the East China Sea’s continental shelf, Ca and Sr concentrations exhibit an increasing pattern with distance from shore (notably enriched in Sr), while Ba content is negatively correlated with the distance from the shore, leading to a gradual rise in Sr/Ba values with increasing water depth [36]. Despite their similar chemical properties, Sr exhibits greater mobility than Ba, being transported to the deep ocean by water flow, whereas Ba tends to concentrate in near-shore sediments, with minimal entry into deep-sea environments. Sr/Ba ratio below 1 typically indicates a freshwater setting, while a ratio above 1 signifies a saline environment [37,38]. This ratio correlates positively with paleosalinity, serving as a crucial geochemical marker for paleosalinity reconstruction [39]. In carbonate rocks, Mn content commonly rises with increasing distance from shore. Al and Ti are used as terrigenous tracers, the ratio (Mn/Ti) is closely tied to distance from shore. For instance, in Pacific Ocean sediments, the ratio below 0.1 suggests deposition on the near-shore continental shelf (distance < 100 km), while a ratio of 0.1~0.3 indicates an offshore environment over 300 km from shore [35]. Analysis of Sr/Ba and Mn/Ti indicators reveals that samples from the Majiagou Formation in the study area exhibit Sr/Ba values ranging from 5.1 to 28.93, averaging 12.08, well above the saline environment threshold of 1, and Mn/Ti values ranging from 0.15 to 0.57, averaging 0.29, falling within the offshore deposition range. Based on these discriminant indicators (Figure 5d), the Majiagou Formation was deposited in a distant epicontinental sea with high salinity characteristics.
4.3. Characteristics of Rare Earth Elements
Rare earth elements (REEs) exhibit unique geochemical properties and chemical stability. Throughout the Phanerozoic, their distribution in seawater has remained relatively constant, with marine authigenic minerals preserving this pattern without significant deviation from contemporary seawater [40]. REEs are crucial for studying sediment provenance, sedimentary environments, paleo-seawater characteristics, paleo-marine environments, and tectonic settings. In particular, REEs in carbonate rocks are valuable indicators for tracing the geochemical and physicochemical conditions of water bodies [41].
The arithmetic and geometric mean calculation methods are two primary approaches for quantifying rare earth element anomalies [42,43]. When adjacent elements exhibit anomalies and the results from the two methods diverge substantially, the geometric mean calculation is preferred [43]. In this study, the geometric mean approach was employed. Rare earth element normalization was conducted using the average mass fraction of the Australian Post-Archean Shale (PAAS) as the reference [44]. The calculation formula is as follows [43,45]:
(Ce/Ce*) N = CeN/PrN × (PrN/NdN),
(Eu/Eu*) N = EuN/(SmN2 × TbN) 1/3
Values with the subscript N are PAAS-normalized. Due to the frequent positive anomaly of La in carbonate rocks, PrN/TbN can substitute for LREEN/MREEN, PrN/YbN for LREEN/HREEN, and TbN/YbN for MREEN/HREEN [43,46].
The ΣREE in the Majiagou Formation samples ranges from 10.56 × 10−6 to 31.73 × 10−6 (Table 2), indicating a relatively low total rare earth element (REE) content, with an average of 19.1 × 10−6. This is significantly lower than the averages for Australian Post-Archean Shale (184.773 × 10−6) and North American Shale Composite (173.2 × 10−6). The average LREEN/MREEN ratio is 0.814, suggesting a slight depletion of LREE compared to MREE. The average LREEN/HREEN ratio is 1.06, indicating a slight enrichment of LREE relative to HREE. The average MREEN/HREEN ratio is 1.296, showing a slight enrichment of MREE over HREE. Thus, the Majiagou Formation exhibits slight MREE enrichment and slight LREE and HREE depletion. REE distribution patterns in carbonate rocks not only reflect the depletion and enrichment of elements but also indicate paleo-oceanic conditions and sedimentary processes. The REE patterns in the Majiagou Formation samples are generally consistent, displaying a gentle overall pattern with negative Ce and Eu anomalies forming a “V” shape (Figure 6). Normal marine carbonates typically show LREE depletion and HREE enrichment [46]. Diagenetic alteration of carbonate rocks can result in flat or middle rare earth element (MREE)-enriched distribution patterns. The REE distribution pattern of the Majiagou Formation is characterized by flat and MREE-enriched patterns, indicative of the effects of burial diagenesis on marine carbonate rocks.

Table 2.
The rare earth element (ppm) date of the carbonate rock in the Majiagou Formation.
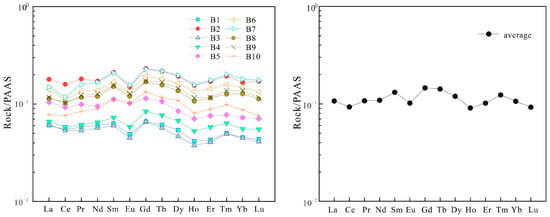
Figure 6.
The rare earth element distribution pattern diagram of the Majiagou Formation.
Eu and Ce anomaly indices can elucidate sedimentary environments. In the contemporary ocean, Eu primarily exists as Eu3+, with Eu2+ occurring only in highly reducing conditions. Eu enrichment is linked to reduction, though typical sedimentary and diagenetic environments rarely achieve the reducing conditions necessary for Eu3+ [46,47]. Ce anomaly indicates changes in sedimentary conditions. In a marine environment, Ce3+ is transformed into Ce4+ through oxidation reaction. Ce4+readily forms insoluble oxides or colloids in seawater, leading to their adsorption onto particle surfaces and subsequent settling, thereby reducing Ce concentration in the water, A negative Ce anomaly suggests that ancient seawater was predominantly under oxidizing conditions, whereas a positive Ce anomaly indicates periods of water being under anoxic reducing conditions [48]. For the Majiagou Formation samples, (Eu/Eu*) N ranges from 0.7 to 0.92, averaging 0.80, indicating a negative anomaly, while (Ce/Ce*) N ranges from 0.77 to 1.06, averaging 0.91, suggesting a weak negative anomaly. These Eu and Ce anomalies imply that the Majiagou Formation was deposited in a marginal sea with relatively shallow water depth and was subject to oxidation.
Typically, the Y/Ho ratio in marine chemical sediments ranges from 44 to 72, and in modern river or estuarine waters, it ranges from 25 to 28 [43]. In contrast, samples from the Majiagou Formation exhibit Y/Ho ratios between 27.48 and 35.12, averaging 30.78. The Y/Ho ratios in samples from the Majiagou Formation exceed those of modern river waters but are significantly lower than those in marine chemical sediments. This suggests that the dolomite in the Majiagou Formation was influenced by a mixture of atmospheric freshwater and seawater.
4.4. Isotope Geochemical Characteristics
Stable isotopes in carbonate rocks can provide evidence for reconstructing their intricate formation history. Oxygen isotopes are particularly important for tracing geological fluid sources, analyzing diagenetic mechanisms, and reconstructing paleoclimate changes. Carbon isotopes are essential for reconstructing paleoclimate evolution, identifying sea-level fluctuations, and characterizing carbon sources during diagenesis [19,49,50].
Since the Cambrian period, the carbon isotopic composition in marine carbonate rocks has remained relatively stable, while oxygen isotopes exhibit a gradual increasing trend with decreasing stratigraphic age. This phenomenon is likely attributed to oxygen exchange between sediments and non-original environmental water or recrystallization during diagenesis [21]. Marine fossil studies indicate that δ13C and δ18O values of seawater during the Ordovician period ranged from −2.0‰ to +0.5‰ and −6.6‰ to −4.0‰, respectively [12,22]. Specifically, δ13C values of micritic dolomitic limestone in the Majiagou Formation range from −3.5‰ to −2.1‰, averaging −2.8‰, and δ18O values range from −16.8‰ to −12.5‰, averaging −14.7‰; δ13C values of dolomitic range from −6.4‰ to −2.0‰, averaging −3.2‰, and δ18O values range from −17.3‰ to −6.8‰, averaging −9.6‰ (Table 3), both displaying significant negative offsets. In freshwater diagenetic settings, both δ13C and δ18O values tend to shift towards higher negative ranges. Dolomites of mixed-water origin typically exhibit lower δ13C and δ18O values [19]. Moreover, a strong positive correlation exists between δ18O values and burial depth, with recrystallization causing a noticeable negative offset of δ18O in buried dolomite [51]. Dolomite formed under burial conditions exceeding 50 °C is termed high-temperature dolomite [52]. Fluid inclusion homogenization temperatures in fine-powdery dolomite from the Majiagou Formation in the Liujiang Basin range from 76.4 °C to 95.1 °C and 112.1 °C to 137.1 °C [13]. The samples from the Majiagou Formation exhibit high negative values and a weak correlation between carbon and oxygen isotopes (Figure 7a), suggesting a dual process of mixed-water dolomitization and burial dolomitization.

Table 3.
The data of C-O-Sr isotopes, order degree, and crystal parameters of dolomites in the Majiagou Formation.
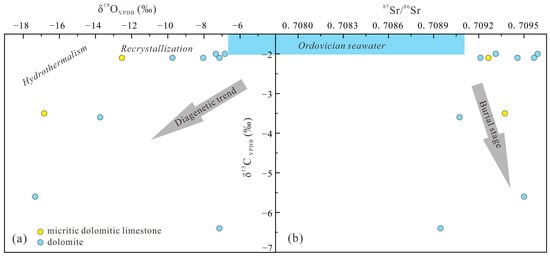
Figure 7.
The C-O-Sr isotope convergence map of the Majiagou Formation: (a) cross-plot of δ18O and δ13C values; (b) cross-plot of 87Sr/86Sr and δ13C.
Carbon and oxygen isotopes play a crucial role in differentiating the salinity of sedimentary water, as indicated by the discriminant value Z in the following formula [21]:
Z = 2.048 × (δ13C + 50) + 0.498 × (δ18O + 50)
Z value of ≥120 indicates marine sedimentation, whereas Z value of <120 signifies freshwater sedimentation. The Z values for samples from the Majiagou Formation range from 107.21 to 119.82, averaging 115.56, all below 120 (Table 3). This implies that the dolomites in this formation are substantially influenced by freshwater, and the diagenetic fluid is closely associated with mixed water.
Strontium isotopes, compared to carbon and oxygen isotopes, are influenced by fewer factors, directly reflecting fluid isotopic characteristics [21]. The 87Sr/86Sr ratio serves as a valuable indicator for discerning fluid source and properties during dolomitization. Research indicates that the 87Sr/86Sr ratio of seawater in the Ordovician period ranged from 0.707850 to 0.709100 [22], while dolomite in the Majiagou Formation exhibited a range of 0.708946 to 0.709591, averaging 0.709330 (Table 3), notably higher than contemporary seawater ratios. Various explanations exist for the elevated 87Sr/86Sr ratio compared to seawater. Some scholars suggest that weathering of Precambrian basement rocks releases groundwater with radiogenic strontium, which, upon mixing with seawater and accumulating in local tectonic units, elevates the fluid’s 87Sr/86Sr ratio above that of seawater [14]. Others propose that non-marine diagenetic influences, such as aluminosilicate mineral dissolution during burial diagenesis, meteoric freshwater effects during epigenetic diagenesis, and deep-seated fluid impacts, contribute to the deviation of marine carbonate rock 87Sr/86Sr ratios from seawater [53,54]. Additionally, seepage reflux of density-driven hypersaline evaporative brines circulating in overlying siliceous strata may increase the 87Sr/86Sr ratio [55]. The weak correlation between 87Sr/86Sr and δ13C values in Majiagou Formation samples (Figure 7b), both significantly differing from Ordovician seawater, suggests independent or decoupled carbon and strontium isotopes in terms of material source, control mechanism, and evolution. Diagenetic alteration of marine carbonates is characterized by Mn enrichment and Sr loss, making Mn and Sr contents and the Mn/Sr ratio effective indicators of diagenetic alteration intensity and seawater information representativeness [30,50]. The intensity of diagenetic alteration increases from Zone I to V, with Majiagou Formation samples mainly in Zone III, indicating multiple-stage burial diagenesis transformation (Figure 8a). A positive correlation exists between 87Sr/86Sr and Z values (Figure 8b). The presence of siliceous components in Majiagou Formation carbonate rocks, covered by Carboniferous clastic rocks in an unconformable contact relationship, suggests that dissolution products of aluminosilicate minerals and meteoric freshwater infiltration during burial diagenesis significantly shift the 87Sr/86Sr ratio of carbonate rocks in the Majiagou Formation.
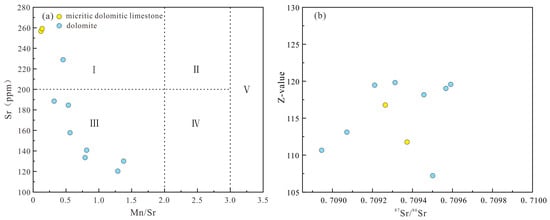
Figure 8.
The diagenesis discrimination map of the Majiagou Formation: (a) cross-plot of Mn/Sr and Sr values; (b) cross-plot of 87Sr/86Sr and Z values.
4.5. Crystal Structure Characteristics of Dolomite
The crystal structure of dolomite retains information about its formation environment, crystallization rate, crystal growth, and fluid properties [56]. Specifically, the unit cell parameters and degree of order are crucial compositional indicators. The unit cell parameter, representing the smallest crystal unit, directly reflects crystal characteristics. For ideal dolomite, these parameters are a = b = 4.8069 (Å), c = 16.0034 (Å) [23]. The deviation of actual parameters from ideal parameters can serve as an important basis for determining their formation environment. The degree of dolomite ordering is strongly influenced by formation time, fluid salinity, temperature, and burial depth [24,57]. Typically, variations in cation order over time and space indicate local dolomitization conditions near the surface and mineral changes during burial. Surface environmental conditions may play a more crucial role in dolomitization than oceanic chemistry and tectonics [58]. The degree of order in dolomite crystals varies significantly depending on the genetic mechanisms controlling their formation: dolomite from capillary concentration shows the lowest order, dolomite from hypersaline lagoons has a relatively low order, while dolomite from mixed-water dolomitization approaches full order [57]. From a fluid chemistry standpoint, a high Mg/Ca ratio in high-salinity sedimentary water accelerates nucleation and crystallization, reducing cation arrangement regularity. When freshwater dilutes the system, the Mg/Ca ratio and ion concentrations decrease, slowing crystallization and promoting a more ordered cation arrangement in the crystal lattice.
The findings indicate that higher values of parameters a and c result in the formation of dolomite that is linked with calcite, characterized by a high calcium content, and deposited in coastal environments. Conversely, lower values of parameters a and c lead to the formation of dolomite associated with magnesite, rich in magnesium, and deposited in arid regions [59]. Larger unit-cell parameters indicate faster but less complete crystallization, while smaller parameters suggest slower but more complete crystallization. Dolomite is contained in all layers of Majiagou Formation, a0 ranges from 4.803 to 4.812 (Å), averaging 4.809 (Å), slightly above the ideal. c0 ranges from 15.984 to 16.119 (Å), with an average of 16.030 (Å), significantly exceeding the ideal (Table 3). This suggests that the unit-cell parameters of dolomite in the Majiagou Formation are above ideal values, reflecting a relatively weak crystallization degree (Figure 9a).
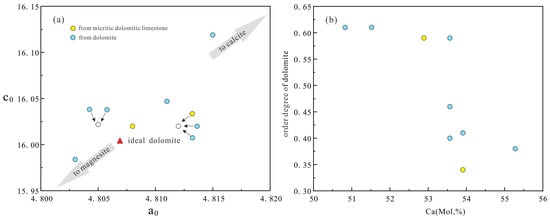
Figure 9.
The dolomite crystal parameters convergence map of the Majiagou Formation: (a) cross-plot of unit cell parameters of dolomite; (b) cross-plot of order degree of dolomite and mole fraction of Ca.
Dolomite’s crystal structure orderliness increases as its order degree approaches 1. Values above 0.8 indicate high order, 0.6 to 0.8 medium order, 0.4 to 0.6 low order, and below 0.4 disorder [60]. In the Majiagou Formation, order degree of dolomite spans 0.34 to 0.61, averaging 0.49 (Table 3), placing it between low and medium order. Correlation analysis reveals a negative relationship between order degree and mole fraction of Ca (Figure 9b), suggesting a moderately low order level. A comprehensive evaluation of dolomite’s crystal structure parameters indicates that dolomitization in the Majiagou Formation is closely linked to penecontemporaneous evaporative dolomitization.
4.6. Microstructural Features
Research in the field of mineralogy indicates that the linear relationship between MgO and CaO in dolomite can distinguish the extent of dolomite replacing calcite. A positive correlation typically suggests a sedimentary origin and rapid crystallization, whereas a negative correlation often implies replacement recrystallization [12,61]. To examine the material composition of dolomite’s zonal structure, the team employed electron probe technology on specimens B2 (Figure 10a,b) and B7 (Figure 10c,d), collecting 40 data points.
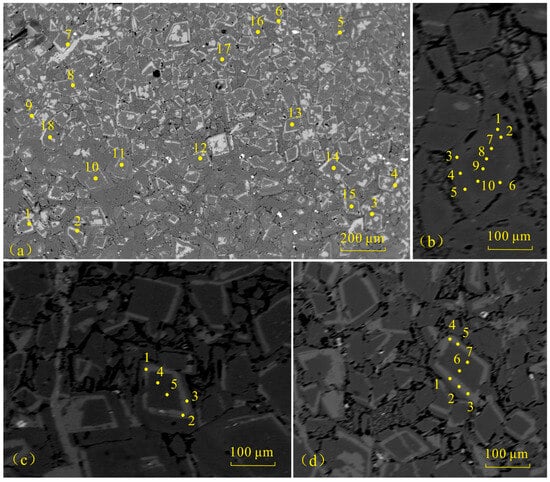
Figure 10.
The EPMA testing point locations of dolomite in the Majiagou Formation: (a) mud-silt-sized crystalline dolomites, 1–18 correspond to electron probe analysis points, B2; (b) mud-silt-sized crystalline dolomites, 1–10 correspond to electron probe analysis points, B2; (c) mud-silt-sized crystalline dolomites, 1–5 correspond to electron probe analysis points, B7; (d) mud-silt-sized crystalline dolomites, 1–7 correspond to electron probe analysis points, B7.
The analysis of data reveals that the CaO content at the dolomite edges varies from 34.14% to 63.51%, averaging 42.97%, while the MgO content ranges from 0.75% to 21.94%, averaging 14.87%. Internally, the CaO content of dolomite ranged from 34.25% to 38.03%, averaging at 36.11%, and the MgO content ranged from 18.88% to 21.93%, averaging 20.06% (Table 4). Compared to ideal dolomite, which has CaO at 30.40% and MgO at 21.90%, the samples show high CaO and low MgO levels. The CaO content at the edge of dolomite is uneven and the content difference is obvious, while the CaO inside is relatively more uniform. The difference in MgO content between the edge and the interior is relatively small (Figure 11a). Meanwhile, the negative correlation between MgO and CaO is more pronounced at the dolomite edges than in the interiors, and the contents of MgO and CaO inside are closer to the ideal composition (Figure 11b). Previous studies have suggested that Ca-rich dolomite can remain stable for an extended period, and the presence of calcareous dolomite indicates formation in a solution with a relatively low Mg/Ca ratio [62]. The dolomite in the Majiagou Formation is characterized by high calcium and low magnesium, suggesting a genesis linked to dolomitization via meteoric freshwater. Qualitative elemental analysis reveals, besides Ca and Mg, a minor presence of Si (Figure 12), aligning with the infiltration of dissolved aluminosilicate minerals indicated by the 87Sr/86Sr ratio.

Table 4.
CaO and MgO contents (%) in dolomite samples in the Majiagou Formation.
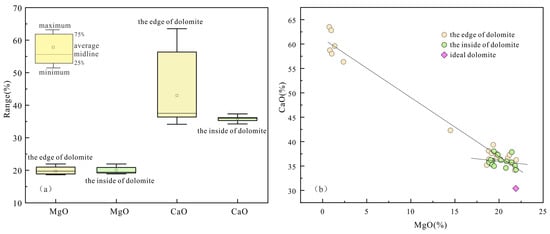
Figure 11.
The box-plot and cross-plot of MgO and CaO contents in dolomites of the Majiagou Formation: (a) CaO and MgO contents of the inside and edge of dolomite; (b) cross-plot of CaO and MgO values.
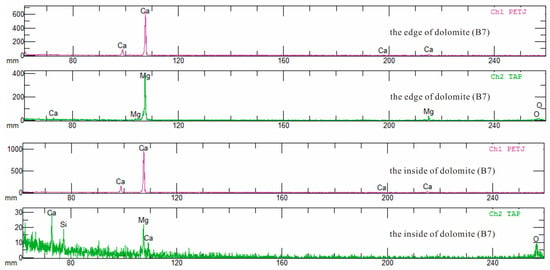
Figure 12.
The qualitative analysis spectrogram of mineral whole elements (B7).
Comprehensive analysis indicates that the formation of this group of dolomites is the result of the combined action of multiple stages and multiple genetic mechanisms. Initial dolomitization was driven by evaporative conditions, resulting in rapid crystal growth. As burial proceeds, meteoric freshwater participates in gradual metasomatism from the mineral edges inward, leading to more pronounced transformation at the edges while preserving original crystallization features at the core. This phenomenon offers crucial mineralogical evidence for understanding fluid–mineral interactions during the diagenetic evolution of carbonate rocks.
5. Discussion
5.1. Sedimentary Environment
The dolomite process in the Majiagou Formation is closely linked to the evolution of its sedimentary environment. Variations in the levels of CaO, SiO2, TiO2, Al2O3, Sr, and δ13C serve as significant indicators of environmental changes. Increases in SiO2, Al2O3, and TiO2 suggest a drop in sea level, while rises in CaO and Sr indicate sea-level elevation. Sea-level rise results in expanded seawater accommodation, fostering plankton blooms. Plankton photosynthesis depletes significant amounts of 12C, whereas marine carbonate sediments are enriched in 13C. Consequently, δ13C values in marine carbonate rocks will rise in response to sea-level elevation [63]. Vertical analysis of the Majiagou Formation’s geochemical data reveals a distinct zigzag pattern, with SiO2, TiO2, and Al2O3 generally showing an inverse relationship with CaO, Sr, and δ13C variations (Figure 13). Research indicates that the North China Craton underwent three distinct transgressive–regressive sedimentary cycles during the Middle Ordovician [27]. X-ray diffraction analysis indicates that three large-scale sea regressions occurred at the stratigraphic horizons represented by B2, B6, and B8 during the deposition of the Majiagou Formation in the Middle Ordovician of the Liujiang Basin. Geochemical proxies, such as V/(V + Ni), Th, Sr/Ba, Mn/Sr, and the anomaly characteristics of Eu and Ce, suggest that the Majiagou Formation was primarily deposited in an epicontinental sea environment with relatively low terrigenous input, elevated seawater salinity, and weakly oxygenated conditions. The overall sea-level trend during this period was regressive, providing the necessary shallow-water, oxidizing, and high-salinity conditions for penecontemporaneous evaporative dolomitization.
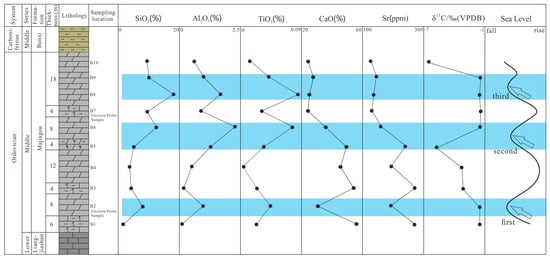
Figure 13.
Sea-level changes in Majiagou Formation: the blue area represents the range of marine regression, and the arrows indicate the lowest position of sea level during regression.
5.2. Characteristics of Dolomitizing Fluids
The dolomitizing fluids of the Majiagou Formation in the Liujiang Basin are primarily associated with a mix of meteoric freshwater and seawater. Despite the influence of multiple stages of non-marine diagenetic fluids and potential recrystallization, the rare earth elements (REEs) in carbonate rocks effectively reflect seawater REE characteristics [64]. The Y/Ho ratios in the samples are generally lower than those in marine chemical sediments. Furthermore, the δ13C and δ18O values exhibit highly negative shifts, the 87Sr/86Sr ratios deviate from standard Ordovician seawater values, and the dolomite shows high Ca and low Mg characteristics. These observations indicate the influence of atmospheric freshwater during the epigenetic diagenetic process. Collectively, this evidence suggests that mixed-water fluids played a crucial role in the dolomitization of this formation. In particular, when sea levels are stable or rising, water circulation becomes more uniform, leading to increased mixing between freshwater and seawater. This enhanced mixing creates favorable conditions for mixed-water dolomitization.
5.3. Dolomitization Mechanism
Analysis of sedimentary environment changes and fluid characteristics suggests that the dolomite in the study area experienced three primary stages of dolomitization: early evaporative dolomitization was followed by mixed-water dolomitization and finally, burial dolomitization.
Following each marine regression, the Majiagou Formation was predominantly in a supratidal sedimentary environment, characterized by an arid, evaporative climate with high salinity. Seawater, a prevalent fluid in dolomite strata, is often altered by evaporation or microbial activity [14]. As evaporation intensifies, Ca2+ becomes supersaturated, resulting in CaCO3 precipitation, while the Mg/Ca ratio in pore water rises. Penecontemporaneous evaporation facilitates the dolomitization of early carbonate sediments (Figure 14a). The resulting dolomite exhibits weak crystallization and moderately low ordering. During this phase, infiltration reflux may also occur. Ongoing sea-level variations and sedimentary processes facilitate the formation of new carbonate sediments that eventually cover the pre-existing dolomite. The infiltration of atmospheric precipitation, groundwater, and residual pore water generates a mixed fluid of freshwater and seawater underground. This mixed fluid triggers metasomatic alteration and freshwater dissolution of the dolomite, leading to a distinctive zonal structure, prominent calcareous cements, and dissolution pores (Figure 14b). Sedimentary minerals, typically metastable, recrystallize into more stable forms over time and with environmental changes [65]. Shallow burial dolomitization occurs with variations in burial time and depth. The Caledonian orogeny caused the uplift of the North China Platform strata, during which meteoric water infiltration led to significant dissolution of dolomite. Following the Carboniferous period, the region experienced marine-continental interactive sedimentation, resulting in the unconformable deposition of substantial clastic rocks over the Majiagou Formation. This period saw the formation of a high-temperature closed system within the Majiagou Formation (Figure 14c), propelling the pre-existing dolomite into a burial phase. As burial continued, metasomatism progressed from the mineral edges inward. This stage primarily involved burial dolomitization, with contributions from the dissolution of aluminosilicate minerals and the influx of meteoric freshwater. Concurrently, intensified recrystallization led to a reduction in the dolomite matrix, decreased voids, and enlarged crystal grains.
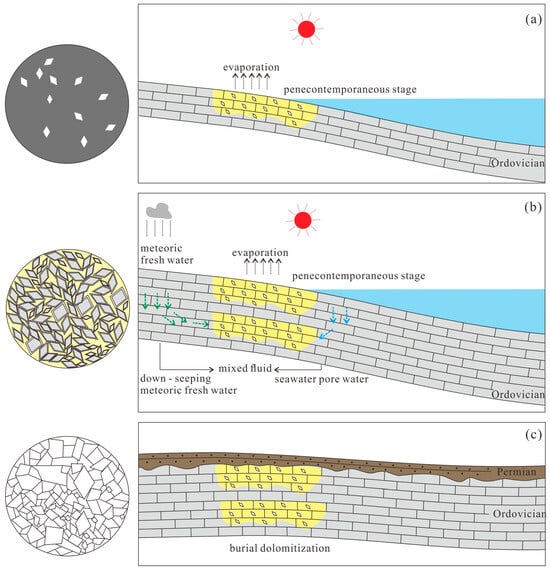
Figure 14.
Formation mechanism of dolomites in the Majiagou Formation.: (a) evaporative dolomitization; (b) mixed dolomitization; (c) burial dolomitization.
6. Conclusions
- The Middle Ordovician Majiagou Formation in the Liujiang Basin, experienced three transgressive–regressive sedimentation stages, primarily in a distal coastal environment. During regression, terrigenous clastics influenced the area, increasing seawater salinity. The environment was predominantly weakly oxygenated to oxidized, with a general decline in sea level throughout sedimentation. The changes in sedimentary environment provided key geological conditions such as shallow water, oxidation, and high salinity for penecontemporaneous evaporative dolomitization.
- Geochemical and isotopic analyses reveal that the dolomitizing fluids of the Majiagou Formation are closely linked to meteoric freshwater. The interaction of meteoric freshwater with seawater creates a mixed fluid system, establishing the necessary material and physicochemical conditions for mixed-water dolomitization.
- The dolomite in the Majiagou Formation has a complex genesis, shaped by the interplay of multiple geological processes over time. Three distinct dolomitization stages can be identified: initially dominated by evaporative dolomitization, transitioning to mixing dolomitization during the diagenetic period, and eventually evolving into burial dolomitization at the sedimentary burial stage. These successive dolomitization events, each with its own characteristics, have collectively contributed to the unique mineralogical and petrological properties of the dolomite in this formation.
- The findings of this study provide valuable insights into the formation of multi-stage dolomite. Subsequent research endeavors may integrate isotope dating and cathodoluminescence techniques to investigate crucial factors like fluid migration and diagenetic conditions that influence dolomite formation, and further unveil the origin of dolomite.
Author Contributions
Conceptualization, H.X. and J.Q.; methodology, H.X.; software, H.X.; validation, H.X. and J.Q.; formal analysis, H.X.; investigation, H.X. and W.X.; data curation, H.X.; writing—original draft preparation, H.X.; writing—review and editing, J.Q.; visualization, H.X. and W.X.; supervision, J.Q.; project administration, H.X.; funding acquisition, J.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number 41263003).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jinghwa, H.S.; Siegenthaler, C. Preliminary experiments on hydrodynamic movement induced by evaporation and their bearing on the dolomite problem. Sedimentology 2010, 12, 11–25. [Google Scholar]
- Roberts, J.A. The problem with dolomite. Nat. Geosci. 2024, 17, 716. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization: A critical reappraisal. Geol. Soc. Spec. Publ. 2004, 235, 7–63. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Shen, A.J.; Qiao, Z.F.; Pan, L.Y.; Hu, A.P.; Zhang, J. Genetic types and distinguished characteristics of dolomite and the origin of dolomite reservoirs. Pet. Explor. Dev. 2018, 45, 983–997. [Google Scholar] [CrossRef]
- Huang, S.G.; Hou, M.C.; Chen, A.Q.; Xu, S.L.; Zhang, B.J.; Deng, Y.W.; Yu, Y. Fluid properties and genesis of dolomites in the devonian Guanwushan Formation of Upper Yangtze Platform, SW China. Minerals 2022, 12, 317. [Google Scholar] [CrossRef]
- Machel, H.G.; Mountjoy, E.W. Chemistry and environments of dolomitization-a reappraisal. Earth Sci. Rev. 1986, 23, 175–222. [Google Scholar] [CrossRef]
- Yu, Z.; Luo, X.R.; Wei, L.B.; Shi, P.P.; Yang, N.; Wu, D.X. Genetic mechanism and development model of dolostone reservoirs of the second member of the Ordovician Majiagou Formation in the central-eastern Ordos Basin. Acta Geol. Sin. 2024, 98, 1–18. [Google Scholar]
- Wu, D.X.; Yu, J.; Zhou, J.G.; Wu, X.N.; Yu, Z.; Ding, Z.C.; Wang, S.Y.; Li, W.L.; Cai, J. Sedimentary characteristics and reservoir controlling effect of the Member 4 of Ordovician Majiagou Formation in Ordos Basin. J. Palaeogeogr. (Chin. Ed.) 2021, 23, 1140–1157. [Google Scholar]
- Liu, G.Q.; Su, Z.T.; Hao, Z.L.; Wei, L.B.; Ren, J.F.; Liao, H.H.; Wu, H.W. Microfacies and depositional model of a carbonate-evaporite symbiotic system of the Majiagou Formation in Ordos Basin. J. Palaeogeogr. (Chin. Ed.) 2024, 26, 279–295. [Google Scholar]
- Su, Z.T.; Chen, H.D.; Xu, F.Y.; Wei, L.B.; Li, J. Geochemistry and dolomitization mechanism of Majiagou dolomites in Ordovician, Ordos, China. Acta Petrol. Sin. 2011, 27, 2230–2238. [Google Scholar]
- Feng, Z.Z.; Jin, Z.K. Types and origin of dolostones in the Lower Palaeozoic of the North China Platform. Sediment. Geol. 1994, 93, 279–290. [Google Scholar]
- Xiang, P.F. Sedimentary Microfacies and Dolomitization Mechanism of Ordovician Carbonates in the North-Central North China Platform. Ph.D. Thesis, China University of Petroleum, Qingdao, China, 2022. [Google Scholar]
- Feng, S.H.; Li, H.; Jiang, J.J.; Lei, Y.; Niu, Y.Z.; Yang, R.; Liu, Y.J. The multiple dolomitizations in Ordovician Majiagou carbonate rocks in Liujiang Basin, Qinhuangdao area, North China. Acta Sedimentol. Sin. 2017, 35, 664–680. [Google Scholar]
- Compton, J.S.; Harris, C.; Thompson, S. Pleistocene dolomite from the Namibian Shelf: High 87Sr/86Sr and δ18O values indicate an evaporative, mixed-water origin. J. Sediment. Res. 2001, 71, 800–808. [Google Scholar] [CrossRef]
- Rameil, N. Early diagenetic dolomitization and dedolomitization of Late Jurassic and earliest Cretaceous platform carbonates: A case study from the Jura Mountains (NW Switzerland, E France). Sediment. Geol. 2008, 212, 70–85. [Google Scholar] [CrossRef]
- Veizer, J.; Demovic, R. Strontium as a tool in facies analysis. J. Sediment. Res. 1974, 44, 93–115. [Google Scholar]
- Calvo, J.P.; Jones, B.F.; Bustillo, M.; Fort, R.; Alonso Zarza, A.M.; Kendall, C. Sedimentology and geochemistry of carbonates from lacustrine sequences in the Madrid Basin, central Spain. Chem. Geol. 1995, 123, 173–191. [Google Scholar] [CrossRef]
- Vincent, B.; Rambeau, C.; Emmanuel, L.; Loreau, J.P. Sedimentology and trace element geochemistry of shallow-marine carbonates: An approach to paleoenvironmental analysis along the Pagny-sur-Meuse Section (Upper Jurassic, France). Facies 2006, 52, 69–84. [Google Scholar] [CrossRef]
- Chen, R.K. Application of Stable Oxygen and Carbon isotope in the research of carbonate diagenetic environment. Acta Sedimentol. Sin. 1994, 4, 11–21. [Google Scholar]
- Huang, S.J.; Huang, Y.; Lan, Y.F.; Huang, K.K. A comparative study on strontium isotope composition of dolomites and their coeval seawater in the Late Permian-Early Triassic, NE Sichuan Basin. Acta Petrol. Sin. 2011, 27, 3831–3842. [Google Scholar]
- Keith, M.L.; Weber, J.N. Carbon and oxygen isotopic composition of selected limestones and fossils. Geochim. Cosmochim. Acta 1964, 28, 1787–1816. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.L.; Buhl, D.; Bruhn, F.; Carden, A.F.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86 Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.Y. Crystal structure characterization and X-ray study of dolomite. Miner. Rocks 1988, 8, 28–33. [Google Scholar]
- Zhong, Q.Q.; Huang, S.J.; Zhou, M.L.; Tong, H.P.; Huang, K.K.; Zhang, X.H. Controlling factors of order degree of dolomite in carbonate rocks: A case study from Lower Paleozoic in Tahe Oilfield and Triassic in northeastern Sichuan Basin. Lithol. Reservoirs 2009, 21, 50–55. [Google Scholar]
- Xiao, K.; Shi, Z.Q.; Wu, B.; Li, Z.Y.; Duan, X. Electron microprobe analysis of the Permian Sinian Dengying Formation in well Lin-1, southeastern Sichuan: Element concentration changing during the hydrothermal dolomitization. Bull. Mineral. Petrol. Geochem. 2017, 36, 289–298. [Google Scholar]
- Wang, Q. North China Craton and global tectonics. Geol. Bull. China 2011, 30, 1–18. [Google Scholar]
- Meng, X.H.; Ge, M.; Tucker, M.E. Sequence stratigraphy, sea-level changes and depositional systems in the Cambro-Ordovician of the North China carbonate platform. Sediment. Geol. 1997, 114, 189–222. [Google Scholar] [CrossRef]
- Scott, M.; Mclennan, B.J.; Fryer, B.J.; Young, G.M. The geochemistry of the carbonate-rich Espanola Formation (Huronian) with emphasis on the rare earth elements. Can. J. Earth Sci. 1979, 16, 230–239. [Google Scholar]
- Esmat, A.A. Composition and origin of the dolostones of Um Bogma Formation, Lower Carboniferous, West Central Sinai, Egypt. Carbonates Evaporites 2014, 29, 239–250. [Google Scholar]
- Marquillas, R.; Sabino, I.; Sial, A.N.; Papa, C.; Ferreira, V.; Matthews, S. Carbon and oxygen isotopes of Maastrichtian–Danian shallow marine carbonates: Yacoraite Formation, northwestern Argentina. J. S. Am. Earth Sci. 2007, 23, 304–320. [Google Scholar] [CrossRef]
- Chang, H.J.; Chu, X.L.; Feng, L.J.; Huang, J.; Zhang, Q.R. Redox sensitive trace elements as paleoenvironments proxies. Geol. Rev. 2009, 55, 91–99. [Google Scholar]
- Wang, J.K.; Li, T.G.; Xiong, Z.F.; Chang, F.M.; Qin, B.B.; Wang, L.M.; Jia, Q. Sedimentary geochemical characteristics of the redox-sensitive elements in Ross Sea, Antarctica: Implications for paleoceanography. Mar. Geol. Quatern. Geol. 2018, 38, 112–121. [Google Scholar]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) stark shale member of the dennis limestone, Wabaunsee County, Kansas, U.S.A. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Wignall, P.B.; Twitchett, R.J. Oceanic anoxia and the End Permian mass extinction. Science 1996, 272, 1155–1158. [Google Scholar] [CrossRef]
- Deng, H.W.; Qian, K. Sedimentary Geochemistry and Environmental Analysis, 1st ed.; Gansu Science and Technology Press: Lanzhou, China, 1993; pp. 28–29. [Google Scholar]
- Guo, Z.G.; Yang, Z.S.; Fan, D.J.; Li, B.L. The geochemical characteristics of Ca, Sr, Ba in the surface sediments of the middle continental shelf mud area and its ambient areas in the East China Sea. J. Ocean Univ. Qingdao 1998, 28, 481–488. [Google Scholar]
- Guo, X.Q.; Li, H.B.; Wei, R.Z.; Dong, A.G.; Du, Y.W.; Yang, J.C. Characteristics of elemental geochemistry of the Cambrian carbonate rocks and their palaeoenvironmental implication in western margin of Qinshui Basin, Shanxi Province. J. Palaeogeogr. (Chin. Ed.) 2020, 22, 349–366. [Google Scholar]
- Tian, Y.; Zhao, X.M.; Wang, L.Z.; Tu, B.; Xie, G.G.; Zeng, B.F. Geochemical Characteristics and its paleoenvironmental implication of Permian Qixia Formation in Shizhu, Chongqing. Acta Sedimentol. Sin. 2014, 32, 1035–1045. [Google Scholar]
- Hou, E.G.; Gao, J.H.; Wang, X.L.; Wang, G.H.; Hu, X.R.; Ma, Z.C. Geochemical characteristics and environmental significance of the Upper Triassic Riganpeicuo Formation in Gaize, Tibet. Bull. Mineral. Petrol. Geochem. 2015, 34, 556–563. [Google Scholar]
- Shields, G.A.; Webb, G.E. Has the REE composition of seawater changed over geological time? Chem. Geol. 2004, 204, 103–107. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wei, W.; Santosh, M.; Hu, J.; Wei, H.T.; Yang, J.; Liu, S.; Zhang, G.L.; Yang, D.D.; Li, S.Z. A review of retrieving pristine rare earth element signatures from carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 586, 110765. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Su-pergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Greig, A.; Collerson, K.D.; Kamber, B.S. Rare Earth Element and Yttrium variability in south east Queensland Waterways. Aquat. Geochem. 2006, 12, 39–72. [Google Scholar] [CrossRef]
- Mclennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 1989, 21, 169–200. [Google Scholar]
- Liang, J.T.; Karem, A.; Li, K.Y.; Liu, S.B.; Li, L.P.; Zhou, G.; Qiu, Y.C.; Li, W.Z.; He, Y.; Wen, H.G. Petrographic and geochemical characteristics of the Middle-Upper Cambrian Xixiangchi dolomites in the central Sichuan Basin, southwest China: Implications for dolomite origins and dolomitization mechanisms. Mar. Pet. Geol. 2023, 586, 110765. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Li, S.Z.; Li, D.; Guo, L.L.; Dai, L.M.; Tao, J.L. Rare Earth Element geochemistry of carbonate and its paleoenvironmental implications. Geotect. Metallogen. 2019, 43, 141–167. [Google Scholar]
- Yin, H.S.; Lin, J.H.; Zhao, X.X.; Zhou, K.K.; Li, J.P.; Huang, H.G. Geochemistry of Rare Earth Elements and origin of positive Europium anomaly in Miocene-Oligocene lacustrine cabonates from Tuotuohe Basin of Tibetan Plateau. Acta Sedimentol. Sin. 2008, 26, 1–10. [Google Scholar]
- Li, J.; Sang, S.X.; Lin, H.X.; Chen, S.Y.; Miao, Y.; Yang, Y. REE characteristics and its geological significance of the Permo-Carboniferous in Bohawian basin. Acta Sedimentol. Sin. 2007, 25, 589–596. [Google Scholar]
- Huang, S.J.; Huang, K.K.; Lü, J.; Lan, Y.F. Carbon isotopic composition of Early Triassic marine carbonates, eastern Sichuan Basin, China. Sci. China Earth Sci. 2012, 42, 1508–1522. [Google Scholar] [CrossRef]
- Li, X.D.; Wei, Z.Y.; He, Y.B.; Zhong, J.W. Strontium isotope and restricted marine basin analysis from thinbedded limestone at the top of Xujiajuan Formation, Xiangshan Group in Ningxia, China. Acta Geol. Sin. 2024, 98, 1229–1243. [Google Scholar]
- Prather, B.E.; Goldstein, R.H.; Kopaska-Merkel, D.C.; Heydari, E.; Gill, K.; Minzoni, M. Dolomitization of reservoir rocks in the Smackover Formation, southeastern Gulf Coast, U.S.A. Earth-Sci. Rev. 2023, 244, 104512. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhao, L.; Chen, X.B.; Fan, Z.F.; He, L. Geochemical characteristics and genetic model of dolomite reservoirs in the eastern margin of the Pre-Caspian Basin. Petrol. Sci. 2012, 9, 161–169. [Google Scholar] [CrossRef]
- Huang, S.J.; Liu, S.G.; Li, G.R.; Zhang, M.; Wu, W.H. Strontium isotope composition of marine carbonate and the influence of diagenetic fluid on it in Ordovician. J. Chengdu Univ. Technol. Sci. Technol. Ed. 2004, 31, 1–7. [Google Scholar]
- Li, Y.S.; Liu, G.D.; Song, Z.Z.; Sun, M.L.; Tian, X.W.; Yang, D.L.; Wang, Y.L.; Zhu, L.Q.; You, F.L. Constraints of C-O-Sr isotope and elemental geochemistry on the origin of dolomite of the deeply buried Ediacaran sedimentary succession, central Sichuan Basin (SW China). J. Asian Earth Sci. 2023, 255, 105780. [Google Scholar] [CrossRef]
- Peryt, T.M. The origin of Upper Permian basinal dolomites in SW Poland: Impact of ascending brines. Terra Nova 2021, 33, 483–493. [Google Scholar] [CrossRef]
- Zhang, J.; Shou, J.F.; Zhang, T.F.; Pan, L.Y.; Zhou, J.G. New approach on the study of dolomite origin: The crystal structure analysis of dolomite. Acta Sedimentol. Sin. 2014, 32, 550–559. [Google Scholar]
- Huang, S.J. The degree of order and forming conditions of the dolomite of the third and fourth members of lower Triassic Jialingjiang Formation in Longmenxia, Quxian, Sichuan. Miner. Rocks 1985, 5, 57–62. [Google Scholar]
- Manche, C.J.; Kaczmarek, S.E. A global study of dolomite stoichiometry and cation ordering through the Phanerozoic. J. Sediment. Res. 2021, 91, 520–546. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth-Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Wang, H.; Shen, A.J.; Luo, X.Y.; Zhao, C.; Kun, D. Genesis of dolomite reservoir in Ediacaran Chigbrak Formation of Tarim basin, NW China: Evidence from U–Pb Dating, isotope and element Geochemistry. Minerals 2023, 13, 725. [Google Scholar] [CrossRef]
- Zheng, J.F.; Shen, A.J.; Liu, Y.F.; Chen, Y.Q. Multi-parameter comprehensive identification of the genesis of Lower Paleozoic dolomite in Tarim basin, China. Acta Petrol. Sin. 2012, 33, 145–153. [Google Scholar]
- Krmac, M.Z.; Akdag, K. Origin of dolomite in the Late Cretaceous Paleocene limestone turbidites, eastern Pontides, Turkey. Sediment. Geol. 2005, 181, 39–57. [Google Scholar] [CrossRef]
- Zhao, G.W. Middle-Late Ordovician Sea-Level Changes in the Bachu Area, Tarim Basin, Xinjiang: Carbon, Oxygen and Strontium Isotope Records. Ph.D. Thesis, Jilin University, Changchun, China, 2013. [Google Scholar]
- Banner, J.L.; Hanson, G.N.; Meyers, W.J. Rare earth element and Nd isotopic variations in regionally extensive dolomites from the Burlington-Keokuk Formation (Mississippian); implications for REE mobility during carbonate diagenesis. J. Sediment. Res. 1988, 58, 415–432. [Google Scholar]
- Tribble, J.S.; Arvidson, R.S.; Lane III, M.; Mackenzie, F.T. Crystal chemistry, and thermodynamic and kinetic properties of calcite, dolomite, apatite, and biogenic silica: Applications to petrologic problems. Sediment. Geol. 1995, 95, 11–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).