Synergistic Leaching of Low-Grade Tungsten–Molybdenum Ore via a Novel KMnO4-Na2CO3-NaHCO3 Composite System Guided by Process Mineralogy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore Sample and Reagents
2.2. Characterization Methods
2.3. Leaching Experiments
3. Results and Discussion
3.1. Chemical Composition of LGTMO
3.2. Mineral Composition of LGTMO
3.3. Chemical Phase Analysis of LGTMO
3.4. Mineral Occurrence of Main Minerals
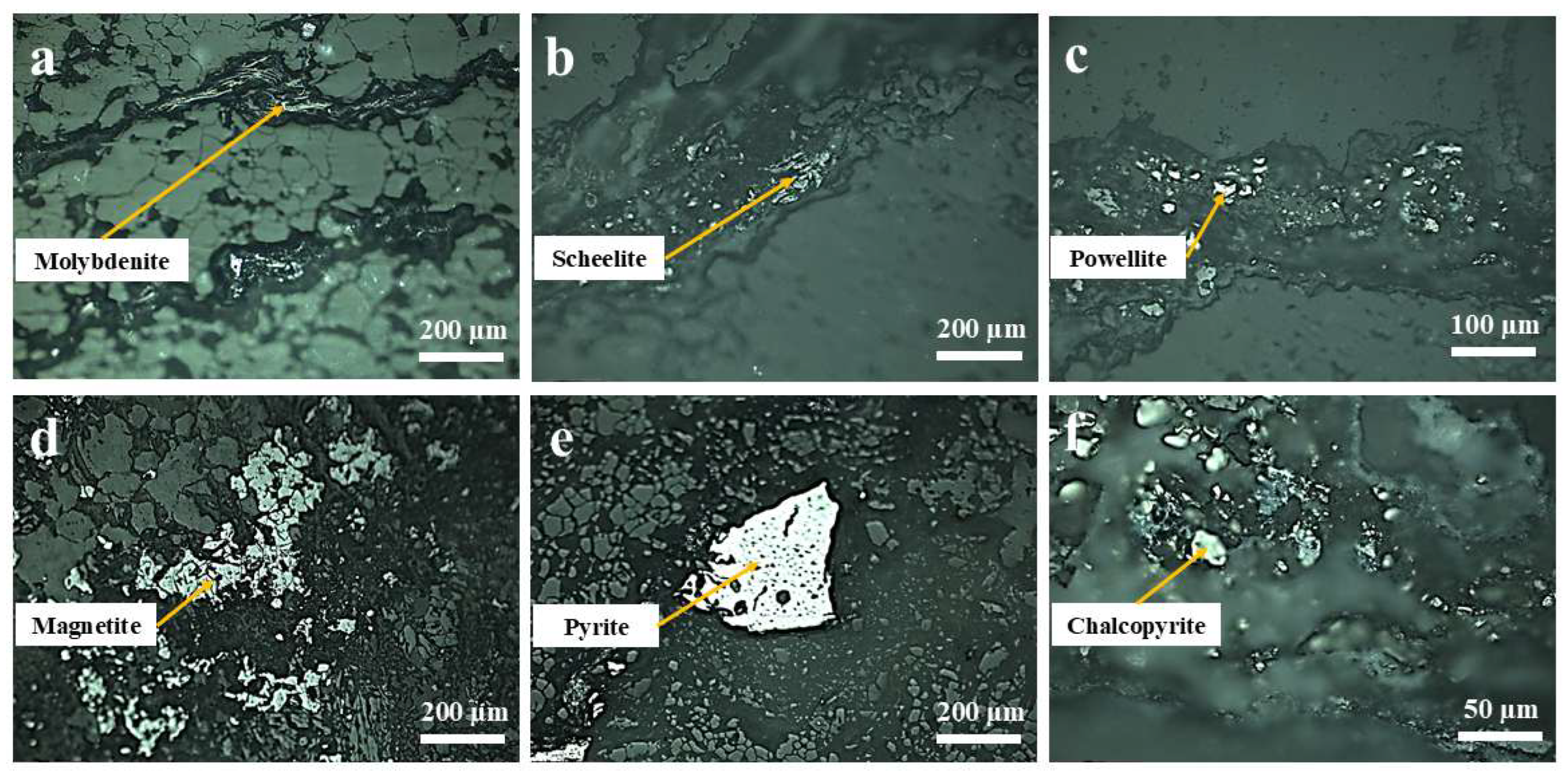
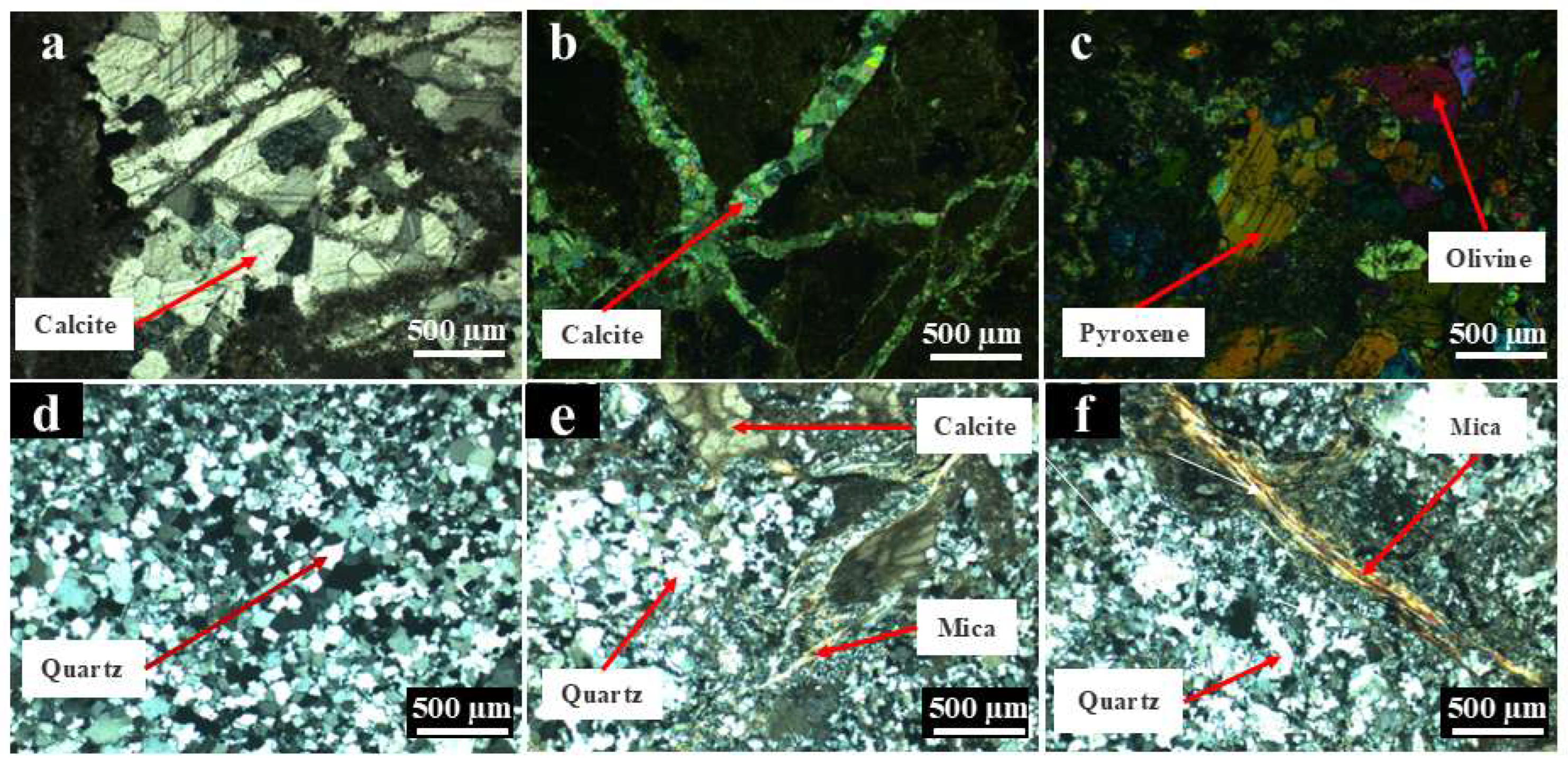
3.4.1. Analysis of Polarizing Microscope
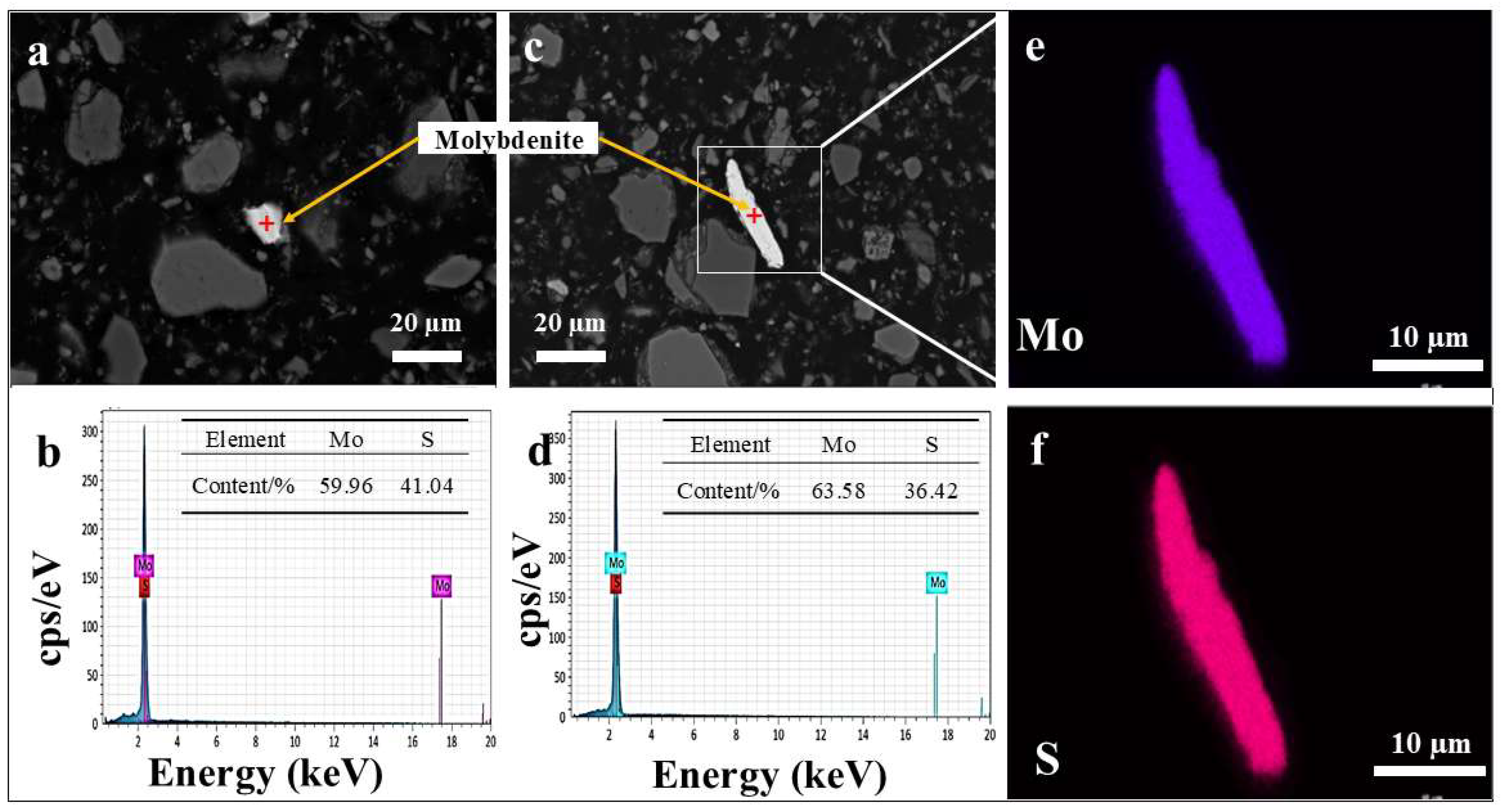
3.4.2. Occurrence of Molybdenite
3.4.3. Occurrence of Scheelite-Powellite
3.5. Properties and Embedding Characteristics of the Main Minerals
3.5.1. Dissociation of Main Minerals
3.5.2. Size Distribution of Main Minerals
3.5.3. Association of Main Minerals
3.5.4. Gangue Minerals
3.6. Mineralogical Factors Affecting Beneficiation
3.6.1. Mineralogical Factors Affecting Molybdenite Recovery
3.6.2. Mineralogical Factors Affecting Scheelite-Powellite Recovery
3.6.3. Other Influencing Factors
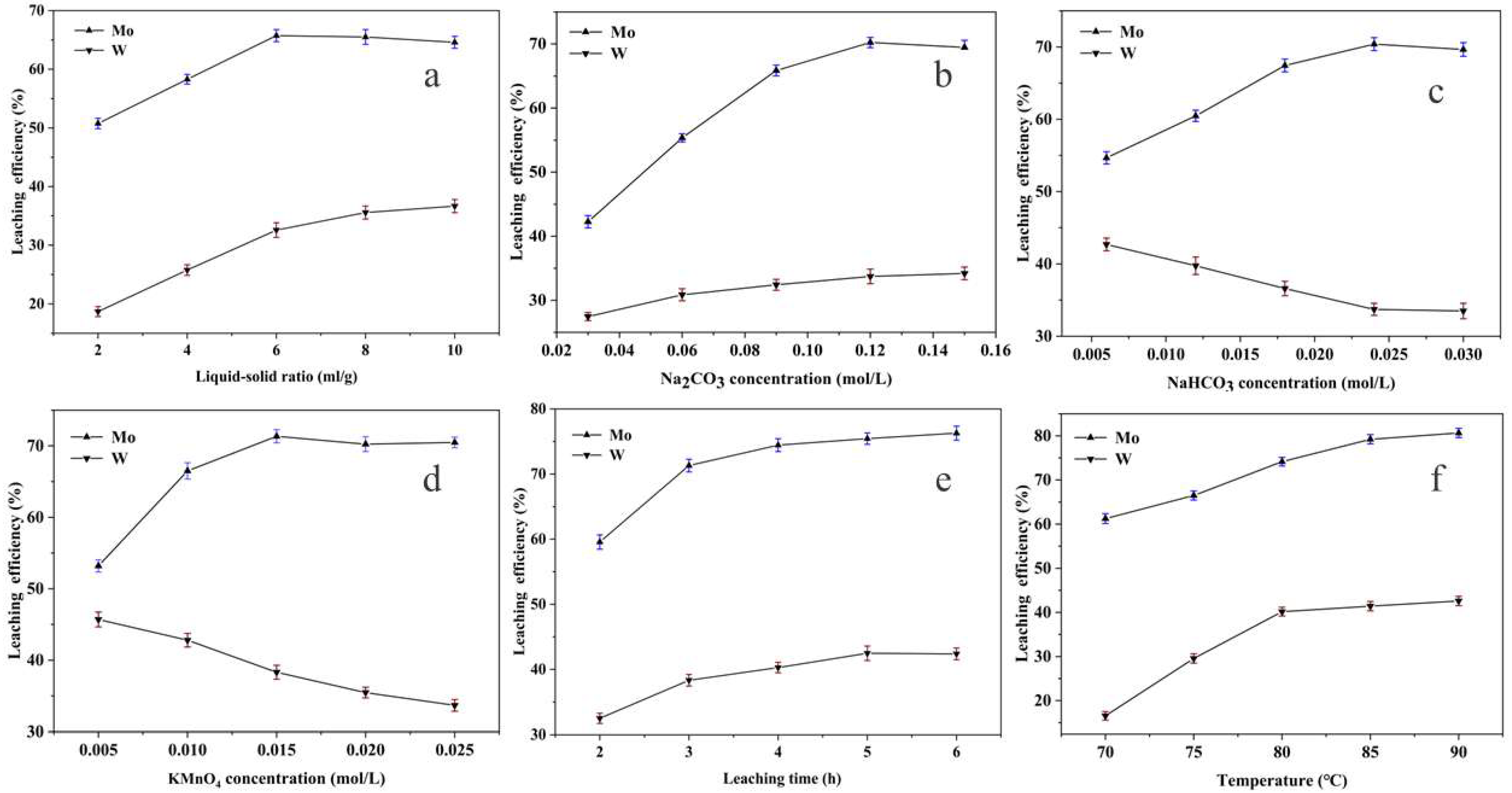
3.7. Leaching Experiments of Tungsten and Molybdenum
3.7.1. Dissolution Performance of LGTMO
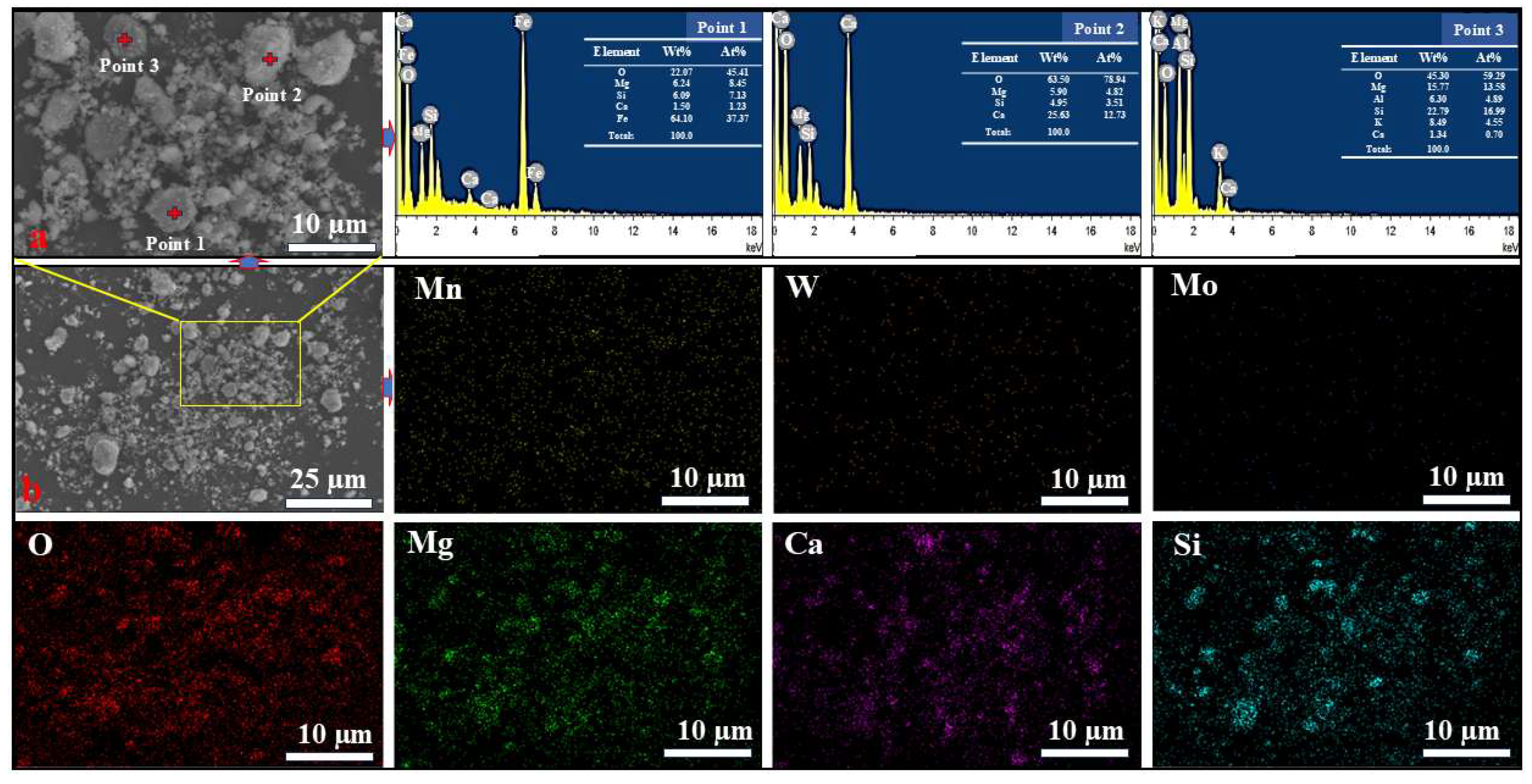
3.7.2. SEM-EDS Analysis of the Leaching Residue
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Zhang, L.; Huang, Y. A Novel Molybdenite Depressant for Efficient Selective Flotation Separation of Chalcopyrite and Molybdenite. Int. J. Min. Sci. Technol. 2024, 34, 1179–1196. [Google Scholar] [CrossRef]
- Wang, H. Efficient Dissolution of Tungstic Acid by Isopolytungstate Solution Based on the Polymerization Theory of Tungsten. Hydrometallurgy 2022, 209, 105835. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, L.; Yin, C.; Chen, A.; Chen, X.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Tungsten Extraction from Scheelite Hydrochloric Acid Decomposition Residue by Hydrogen Peroxide. Miner. Eng. 2022, 179, 107461. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2024; USGS: Reston, VA, USA, 2024. Available online: https://pubs.usgs.gov/publication/mcs2024 (accessed on 31 January 2024).
- Pan, W.; Li, S.; Zhu, Y.; Gao, L.; Ma, Z.; Cao, Y.; Chen, X.; Du, S. Application of a Novel Auxiliary Collector for Molybdenite Fines Recovery in Sustainable Froth Flotation Production: Combining DFT Calculations and Experiments. Colloids Surf. A 2025, 704, 135570. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Jin, J.; Gao, P.; Zhou, Z. Process Mineralogy Approach to Optimize Curing-Leaching in Vanadium-Bearing Stone Coal Processing Plants. Int. J. Min. Sci. Technol. 2023, 33, 123–131. [Google Scholar] [CrossRef]
- Li, N.; Yang, F.; Zhang, Z.; Yang, C. Geochemistry and Chronology of the Biotite Granite in the Xiaobaishitou W-(Mo) Deposit, Eastern Tianshan, China: Petrogenesis and Tectonic Implications. Ore Geol. Rev. 2019, 107, 999–1019. [Google Scholar] [CrossRef]
- Ji, Q.; Li, G.; Zhu, X.; Gao, T.; Qin, G.; Xu, C.; Cai, M.; Lu, Z.; Chen, Y.; Zhang, J.; et al. Process Mineralogy Automatic Detection System in Research of a Gold Polymetallic Mine. J. Phys. Conf. Ser. 2023, 2428, 12009. [Google Scholar] [CrossRef]
- Liu, J.; Xing, Z.; Liu, J.; Ding, X.; Xue, X. Evaluation of the Potential of Recovering Various Valuable Elements from a Vanadiferous Titanomagnetite Tailing Based on Chemical and Process Mineralogical Characterization. Environ. Sci. Pollut. Res. 2023, 30, 83991–84001. [Google Scholar] [CrossRef]
- Yi, G.; Macha, E.; Van Dyke, J.; Ed Macha, R.; McKay, T.; Free, M.L. Recent Progress on Research of Molybdenite Flotation: A Review. Adv. Colloid Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef]
- Khoshnevisan, A.; Yoozbashizadeh, H. Application of Artificial Neural Networks to Predict Pressure Oxidative Leaching of Molybdenite Concentrate in Nitric Acid Media. Miner. Process. Extr. Metall. Rev. 2012, 33, 292–299. [Google Scholar] [CrossRef]
- Chung, K.W.; Yoon, H.-S.; Kim, C.-J.; Jeon, H.-S. Selective Leaching of Molybdenum from Bulk Concentrate by Electro-Oxidation. Metals 2021, 11, 1904. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.-Y.; Tong, L.-L.; Zhu, J. Intensified Bioleaching of Low-Grade Molybdenite Concentrate by Ferrous Sulfate and Pyrite. Rare Met 2015, 34, 207–214. [Google Scholar] [CrossRef]
- Ganbari Arbat, A.; Asghari Fesaghandis, E.; Taghizadeh Tabrizi, A.; Aghajani, H. Comparison of the Effect of NaClO3 and H2O2 on the Molybdenum Leaching from Molybdenite Concentrate. Trans. Indian Inst. Met. 2020, 73, 2355–2360. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Wang, S.; Li, H.; Liu, M.; Sun, P.; Li, Y. Extracting Tungsten from Scheelite Concentrate with Caustic Soda by Autoclaving Process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Liu, X.; Chen, A.; Li, H. Sodium Hydroxide Digestion of Scheelite by Reactive Extrusion. Int. J. Refract. Met. Hard Mater 2011, 29, 739–742. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Liu, X.; Chen, X.; Zhao, Z. Sustainable and Efficient Recovery of Tungsten from Wolframite in a Sulfuric Acid and Phosphoric Acid Mixed System. ACS Sustain. Chem. Eng. 2020, 8, 13583–13592. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold Leaching from Oxide Ores in Alkaline Glycine Solutions in the Presence of Permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Cheng, T.C.; Soriano, C.; Jamieson, H.; Falck, H. Leaching Kinetics of Tungsten in Scheelite Tailings in Sodium Carbonate Solutions at Low Temperatures (25–75 °C). Can. Metall. Q. 2024, 63, 1484–1492. [Google Scholar] [CrossRef]
- Xu, C.; Chi, R.; Zhang, Y.; Zhong, C.; Ruan, Y.; Lyu, R.; Zhou, F. Process Mineralogy of Bayan Obo Rare Earth Ore by MLA. Physicochem. Probl. Miner. Process. 2020, 56, 737–745. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, C.; Lyu, R.; Ruan, Y.; Zhang, Z.; Chi, R. Process Mineralogy of Weishan Rare Earth Ore by MLA. J. Rare Earths 2019, 37, 334–338. [Google Scholar] [CrossRef]
- Gao, H.; Hu, Y.; Yang, H.; Meng, Q.; Tong, L.; Zhang, Q. Process Mineralogy and Leaching Toxicity of High-Sulfur Residue from Oxygen Pressure Zinc Leaching. JOM 2023, 75, 1068–1078. [Google Scholar] [CrossRef]
- Hu, W.; Tian, K.; Zhang, Z.; Guo, J.; Liu, X.; Yu, H.; Wang, H. Flotation and Tailing Discarding of Copper Cobalt Sulfide Ores Based on the Process Mineralogy Characteristics. Minerals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Hou, X.; Yang, Z.; Wang, Z. Occurrence State and Distribution Regularity of Key Metal Element Niobium in Bayan Obo Deposit, China. JOM 2023, 75, 2753–2762. [Google Scholar] [CrossRef]
- Huang, M.; Shao, Y.; Li, X.; Liu, D.; Ouyang, J.; Zhou, L.; Liu, Z. Process Mineralogical Study of U/Th Occurrence States and the Carrier Minerals in Refractory Tantalum Slag. Miner. Eng. 2024, 205, 108496. [Google Scholar] [CrossRef]
- Yang, H.; Shi, X.; Luo, C.; Wu, W.; Li, Y.; He, Y.; Zhong, K.; Wu, J. Mineral Composition of Prospective Section of Wufeng-Longmaxi Shale in Luzhou Shale Play, Sichuan Basin. Minerals 2021, 12, 20. [Google Scholar] [CrossRef]
- Dabek, P.; Chudy, K.; Nowak, I.; Zimroz, R. Superpixel-Based Grain Segmentation in Sandstone Thin-Section. Minerals 2023, 13, 219. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Wang, L.; Cao, C.; Nie, Y.; Liu, S. An Improved Mineral Image Recognition Method Based on Deep Learning. JOM 2023, 75, 2590–2602. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Nan, J.; Wang, X.; Ren, D. Characteristics and Genetic Mechanism of Chang Eight Low Permeability and Tight Reservoir of Triassic Yanchang Formation in Central-East Ordos Basin. Front. Phys. 2022, 9, 801264. [Google Scholar] [CrossRef]
- Han, Z.; Levett, A.; Edraki, M.; Jones, M.W.M.; Howard, D.; Southam, G. Microbially Influenced Tungsten Mobilization and Formation of Secondary Minerals in Wolframite Tailings. J. Hazard. Mater. 2023, 445, 130508. [Google Scholar] [CrossRef]
- Ge, B.; Liu, S.; Nie, Q.; Li, Q.; Zhu, C. Applying One-Stage Grinding and Flotation to Improving Copper Recovery of a Fine-Grained Cu-Mo Sulphide Ore. Sep. Sci. Technol. 2013, 48, 1900–1905. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Xu, S.; Jin, X.; Sun, W.; Gao, Z. Advanced Collector-Free Flotation of Typical Sulfide Minerals Using a Novel Heterocyclic Depressant. Miner. Eng. 2023, 199, 108120. [Google Scholar] [CrossRef]
- Dai, L.; Liu, J.; Li, D.; Hao, J.; Gao, H. A New Insight on a Novel Auxiliary Collector 4-MBA Synergize with BHA to Enhance Flotation of Scheelite. Sep. Purif. Technol. 2024, 346, 127412. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Deng, J.; Liu, S. Influence of Particle Size on Flotation Separation of Ilmenite, Olivine, and Pyroxene. Physicochem. Probl. Miner. Process. 2021, 57, 106–117. [Google Scholar] [CrossRef]
- Yu, P.; Ding, Z.; Miao, Y.; Yuan, J.; Yu, A.; Tang, Y.; Wen, S.; Bai, S. Synergism Mechanism of the Combined NaOL-SDBS Collector at Solid-Liquid Interface and Its Response to Flotation Separation of Ilmenite from Pyroxene. Surf. Interfaces 2024, 51, 104760. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Shu, K.; Wang, Z.; Xu, Y. Influence of Aluminum–Sodium Silicate on Olivine Flotation with Sodium Oleate. Miner. Eng. 2019, 143, 106008. [Google Scholar] [CrossRef]
- Yin, C.; Ji, L.; Chen, X.; Liu, X.; Zhao, Z. Efficient Leaching of Scheelite in Sulfuric Acid and Hydrogen Peroxide Solution. Hydrometallurgy 2020, 192, 105292. [Google Scholar] [CrossRef]
- Đorđević, N.; Mihajlović, S.; Vlahović, M.; Šajić, J.L.; Martinović, S. Thermal Analysis and Phase Changes of Mechanochemically Activated Sodium Carbonate. Thermochim. Acta 2022, 708, 179139. [Google Scholar] [CrossRef]
- Hesami, R.; Ahmadi, A.; Hosseini, M.R.; Torabi, M. Effect of Mechanical Activation on the Hypochlorite Leaching of Sarcheshmeh Molybdenite Concentrate. Sep. Sci. Technol. 2022, 57, 1966–1977. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, L.; Xi, X.; Nie, Z.; Nie, Z. Separation of Tungsten and Molybdenum Using Selective Precipitation with Manganese Sulfate Assisted by Cetyltrimethyl Ammonium Bromide (CTAB). Hydrometallurgy 2020, 198, 105494. [Google Scholar] [CrossRef]
- Saidi, M.; Kadkhodayan, H. Toxic Heavy Metal Removal from Sulfide Ores Using Potassium Permanganate: Process Development and Waste Management. J. Environ. Manag. 2020, 276, 111354. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Cao, C.; Huang, X.; Wan, L. Sustainable and Efficient Leaching of Tungsten from Scheelite Using the Mixture of Ammonium Phosphate, Ammonia and Calcium Fluoride. Hydrometallurgy 2022, 210, 105846. [Google Scholar] [CrossRef]


| Element | CaO | SiO2 | MgO | Fe2O3 | Al2O3 | MnO | K2O |
| Content | 36.935 | 32.822 | 19.84 | 5.975 | 1.421 | 1.123 | 0.744 |
| Element | MoO3 | WO3 | ZnO | TiO2 | P2O5 | CuO | PbO |
| Content | 0.204 | 0.097 | 0.091 | 0.063 | 0.034 | 0.025 | 0.024 |
| Mineral | Molecular | Content/% | Mineral | Molecular | Content/% |
|---|---|---|---|---|---|
| Magnetite | Fe3O4 | 3.29 ± 0.8 | Olivine | (Mg, Fe)2SiO4 | 19.11 ± 5.82 |
| Pyrite | FeS2 | 0.30 ± 0.25 | Dolomite | CaMg(CO3)2 | 16.63 ± 1.02 |
| Powellite | CaMoxW1−xO4 | 0.1 ± 0.1 | Calcite | CaCO3 | 12.09 ± 5.82 |
| Manganosite | MnO | 0.07 ± 0.04 | Hornblende | Ca2Mg5Si8O22(OH)2 | 5.88 ± 5.77 |
| Scheelite | CaWO4 | 0.08 ± 0.07 | Quartz | Si8O2 | 5.40 ± 1.53 |
| Molybdenite | MoS2 | 0.04 ± 0.03 | Rankinite | Ca3Si2O7 | 3.47 ± 2.86 |
| Chalcopyrite | CuFeS2 | 0.03 ± 0.03 | Fluorite | CaF2 | 2.28 ± 2.04 |
| Sphalerite | ZnS | 0.02 ± 0.02 | Mica | KAl2(AlSi3O10)(OH)2 | 2.19 ± 0.15 |
| Pyroxene | CaSi2O6 | 23.51 ± 9.48 | Feldspar | K(AlSi3O8) | 0.81 ± 0.09 |
| Element | Elemental Phase | Content | Distribution Ratio |
|---|---|---|---|
| Mo | Molybdenite | 0.100 | 63.30 |
| Powellite | 0.058 | 36.70 | |
| W | Scheelite | 0.073 | 75.26 |
| Powellite | 0.058 | 24.74 |
| Degree of Dissociation | Scheelite-Powellite | Molybdenite | ||
|---|---|---|---|---|
| Distribution Rate (%) | Cumulative Distribution Rate (%) | Distribution Rate (%) | Cumulative Distribution Rate (%) | |
| 0% | 1.70 | 100.00 | 1.30 | 100.00 |
| 0% < x ≤ 20% | 12.01 | 98.30 | 1.11 | 98.70 |
| 20% < x ≤ 40% | 0.55 | 86.29 | 2.78 | 97.59 |
| 40% < x ≤ 60% | 19.42 | 85.74 | 5.94 | 94.81 |
| 60% < x ≤ 80% | 0.45 | 66.32 | 0.00 | 88.87 |
| 80% < x < 100% | 38.73 | 65.87 | 0.00 | 88.87 |
| 100% | 27.14 | 27.14 | 88.87 | 88.87 |
| Particle Size Fraction (µm) | Scheelite–Powellite | Molybdenite | ||
|---|---|---|---|---|
| Distribution Rate (%) | Cumulative Distribution Rate (%) | Distribution Rate (%) | Cumulative Distribution Rate (%) | |
| 48 µm ≤ Particle size < 73 um | 0.00 | 0.00 | 0.00 | 0.00 |
| 23 µm ≤ Particle size < 48 um | 0.00 | 0.00 | 0.00 | 0.00 |
| 18 µm ≤ Particle size < 23 um | 26.58 | 26.58 | 0.00 | 0.00 |
| 13 µm ≤ Particle size < 18 um | 16.44 | 43.02 | 27.27 | 27.27 |
| 10 µm ≤ Particle size < 13 um | 22.37 | 65.39 | 0.00 | 0.00 |
| Particle size < 10 um | 34.61 | 100.00 | 72.73 | 100.00 |
| Sample | Monomer | Association | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Powellite | 74.56 | Olivine | Scheelite | Pyroxene | Dolomite | Calcite | Hornblende | Fluorite | Other |
| 9.92 | 3.66 | 3.46 | 1.12 | 0.74 | 0.81 | 0.15 | 5.58 | ||
| Molybdenite | 92.78 | Pyroxene | Calcite | Hornblende | Olivine | Mica | Dolomite | Fluorite | |
| 2.08 | 0.99 | 0.60 | 0.53 | 0.36 | 0.23 | 0.05 | |||
| No. | Liquid–Solid Ratio (mL/g) | Na2CO3 (mol/L) | NaHCO3 (mol/L) | KMnO4 (mol/L) | Time (h) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | 2, 4, 6, 8, 10 | 0.09 | 0.024 | 0.025 | 3 | 80 |
| 2 | 6 | 0, 0.03, 0.06, 0.09, 0.12, 0.15 | 0.024 | 0.025 | 3 | 80 |
| 3 | 6 | 0.12 | 0, 0.006, 0.012, 0.018, 0.024, 0.030 | 0.025 | 3 | 80 |
| 4 | 6 | 0.12 | 0.024 | 0, 0.005, 0.010, 0.015, 0.020, 0.025 | 3 | 80 |
| 5 | 6 | 0.12 | 0.024 | 0.015 | 2, 3, 4, 5, 6 | 80 |
| 6 | 6 | 0.12 | 0.024 | 0.015 | 4 | 70, 75, 80, 85, 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Tong, L.; Zhang, Q.; Zhao, H.; Wang, X.; Xiong, B.; Yang, H. Synergistic Leaching of Low-Grade Tungsten–Molybdenum Ore via a Novel KMnO4-Na2CO3-NaHCO3 Composite System Guided by Process Mineralogy. Minerals 2025, 15, 712. https://doi.org/10.3390/min15070712
Kang J, Tong L, Zhang Q, Zhao H, Wang X, Xiong B, Yang H. Synergistic Leaching of Low-Grade Tungsten–Molybdenum Ore via a Novel KMnO4-Na2CO3-NaHCO3 Composite System Guided by Process Mineralogy. Minerals. 2025; 15(7):712. https://doi.org/10.3390/min15070712
Chicago/Turabian StyleKang, Jian, Linlin Tong, Qin Zhang, Han Zhao, Xinyao Wang, Bin Xiong, and Hongying Yang. 2025. "Synergistic Leaching of Low-Grade Tungsten–Molybdenum Ore via a Novel KMnO4-Na2CO3-NaHCO3 Composite System Guided by Process Mineralogy" Minerals 15, no. 7: 712. https://doi.org/10.3390/min15070712
APA StyleKang, J., Tong, L., Zhang, Q., Zhao, H., Wang, X., Xiong, B., & Yang, H. (2025). Synergistic Leaching of Low-Grade Tungsten–Molybdenum Ore via a Novel KMnO4-Na2CO3-NaHCO3 Composite System Guided by Process Mineralogy. Minerals, 15(7), 712. https://doi.org/10.3390/min15070712





