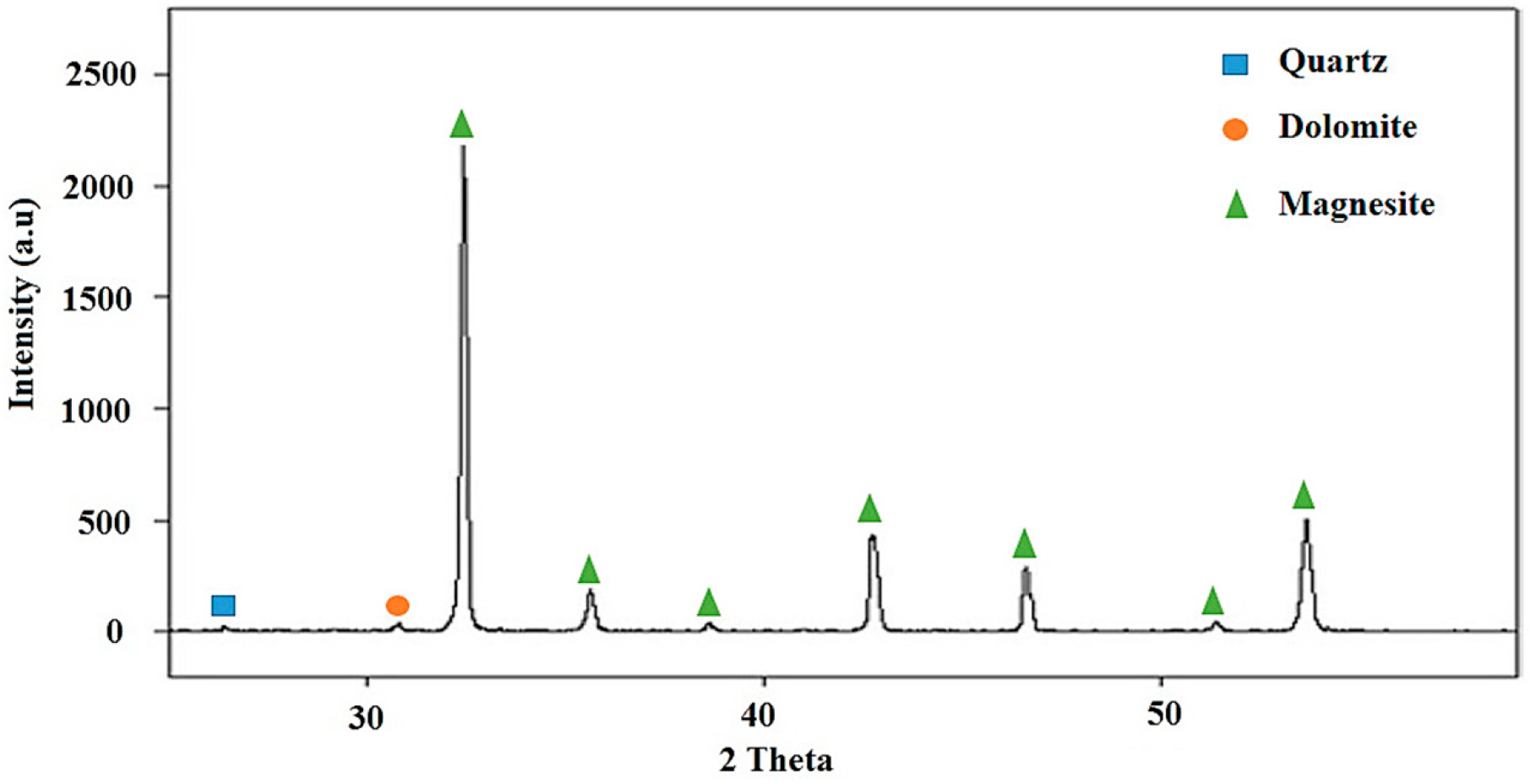
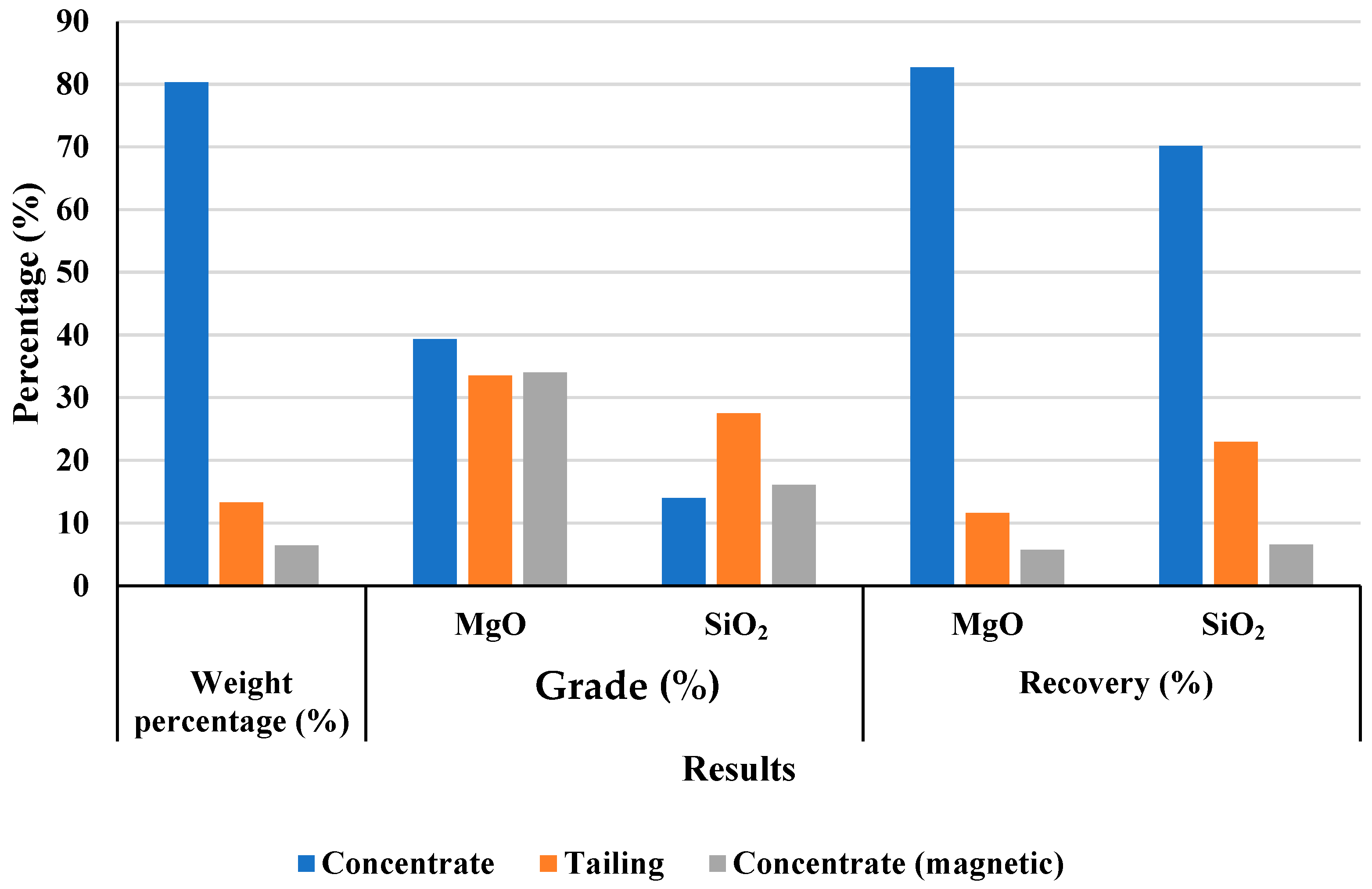1. Introduction
Magnesite (MgCO
3) is a carbonate mineral, also referred to as a salt-type mineral, with substantial commercial importance attributed to its unique refractory properties [
1,
2]. Recognized for its critical role in various industrial applications, magnesium, derived primarily from magnesite, is classified as a critical mineral by the U.S. Geological Survey due to concerns over supply concentration and import reliance. Globally, magnesium production is heavily concentrated in a few countries, particularly China, which dominates the market, followed by Russia, Kazakhstan, and Turkey [
3]. This geographic concentration raises concerns about supply chain stability and strategic resource security. Magnesium’s applications span a wide range of industries: it plays a vital role in the production of refractory materials due to its high melting point and thermal stability, serves as a crucial component in steelmaking and construction (e.g., cement and fireproof materials), and is used in the chemical industry as a catalyst and pH regulator. In addition, magnesium is employed in the manufacturing of glass, ceramics, and heat-resistant consumer goods. These widespread and critical uses underscore the strategic importance of developing efficient beneficiation methods to upgrade low-grade magnesite ores for commercial use [
4,
5]. Magnesite ores typically contain key gangue minerals, including iron oxides, silicates, and carbonates, with common examples being limonite, talc, quartz, and dolomite [
6]. Iron impurities can react with carbonates to form low-melting-point materials, which reduce the thermal stability and fire resistance of the final products [
7].
The chemical composition of magnesite, particularly the MgO content and impurity levels (CaO, SiO
2, Fe
2O
3, Al
2O
3), critically influences its suitability for industrial applications and the quality after calcination. Commercial-grade magnesite for refractory use typically requires a MgO content between 40% and 47% (equivalent to 84.0%–99.0% MgCO
3), CaO between 0% and 3.5%, SiO
2 from 0% to 7%, and combined Fe
2O
3 and Al
2O
3 between 0% and 3% [
8]. In the beneficiation process, the presence of magnesium-associated minerals with similar physical and chemical characteristics poses significant challenges [
9]. Therefore, comprehensive research is essential to gain deeper insights into the physicochemical properties of the associated minerals, optimize processing conditions and technologies, and provide the necessary technical support to ensure the effective development and utilization of magnesite resources [
10]. To enhance flotation efficiency and reduce costs, Zhang et al. [
11] mixed collectors of sodium oleate (NaOL) and monohydric alcohols were used for magnesite and dolomite flotation. Compared to NaOL alone, mixtures with alcohols (butanol, isobutanol, octanol, isooctanol) improved mineral recovery, especially with longer carbon chains and isomeric alcohols. However, magnetic separation processes can be effectively applied in the iron removal from magnesite but processing non-magnetic minerals is difficult and in some cases is more complicated [
11]. Wasmuth applied a high-intensity dry magnetic field (3.2 T) for magnesite ore (4–100 mm particle size, containing 20% SiO
2 and 4% Fe
2O
3) to achieve a substantial reduction in impurities, lowering SiO
2 to below 1.5% and Fe
2O
3 to under 0.3%. This demonstrates the effectiveness of dry magnetic separation in significantly upgrading magnesite quality for industrial applications [
12]. Atasoy [
13] recently investigated the application of wet high-intensity magnetic separation (WHIMS) to magnesite ore, which initially contained 77.69% MgCO
3 and 3.14% Fe
2O
3. The process increased the MgCO
3 content to 91.03% and reduced Fe
2O
3 to 0.32%, illustrating the potential of WHIMS in magnesite upgrading [
13]. It is important to note that magnetization roasting is a technique commonly employed to enhance the magnetic susceptibilities of materials [
13,
14]. Magnetization roasting is a widely utilized technique to improve the magnetic susceptibility of materials. Magnesite middlings, initially comprising 52.70% MgO, 2.06% SiO
2, 0.61% Fe
2O
3, 1.36% CaO, 0.07% Al
2O
3, and 43.20% LOI, underwent a series of beneficiation processes including calcination, classification, and magnetic separation. These processes resulted in a weight recovery of 47%. The final caustic-calcined MgCO
3 product achieved an impressive MgO content of 89.57%, with reduced impurities: SiO
2 at 0.96%, Fe
2O
3 at 0.43%, CaO at 2.35%, Al
2O
3 at 0.05%, and LOI at 6.64% [
14]. During magnetization roasting, hematite and limonite are typically reduced to magnetite, enhancing their magnetic properties. For example, limonite exhibits a significant increase in magnetism with temperature, achieving maximum saturation magnetization at 700 °C [
15]. Similarly, hematite undergoes partial reduction to magnetite, further improving its magnetic susceptibility [
16,
17].
Flotation is considered the most cost-effective and efficient method for magnesite beneficiation, allowing for the precise separation of valuable minerals from impurities [
18]. Given magnesite’s semi-soluble characteristics, the interaction between collectors and minerals during flotation is influenced by the dissolved species present in the system. Processes such as precipitation, adsorption, and hydrolysis can occur, leading to the formation of new complexes that interfere with the selective interaction between mineral surfaces and reagents [
19,
20]. Furthermore, the dissolution kinetics of minerals significantly influence the extent of precipitation, with faster dissolution rates promoting quicker precipitation. Bulk precipitation, in particular, can adversely affect the flotation of Mg carbonates, as the formation of bulk species reduces the availability of collectors, thereby hindering effective surface interactions [
21].
Direct and reverse flotation are well-established methods for effectively separating impurities from carbonate minerals [
22]. In a reverse flotation approach, separation is achieved by selectively depressing the valuable minerals. Depressants commonly utilized in this process consist of both organic and inorganic compounds, including sodium carbonate, sodium silicate (water glass), pyrophosphate, sodium hexametaphosphate (SHMP), starch, carboxymethyl cellulose (CMC), quebracho, and tannin derivatives [
23]. A thorough assessment of various depressants revealed that EDTA, citric acid, and tannic acid exhibited the most significant depressant efficacy in magnesite flotation. In contrast, sodium silicate and tartaric acid showed minimal inhibitory effects, proving less effective in the flotation process of magnesite [
6]. In the direct flotation of magnesite and calcium-bearing carbonates, challenges arise from the similarities in surface characteristics between these minerals. This overlap can cause depressants to target both valuable and gangue minerals, hindering effective separation. Therefore, selecting an optimal depressant is crucial for achieving efficient separation in Mg-carbonate flotation, particularly when a collector is used [
24]. Effective collectors, such as oleates, hydroxamates, sulfosuccinates, and sarcosinates, are commonly employed in the flotation of calcium-bearing minerals. Additionally, various studies have shown that the combination of different collectors can exhibit a synergistic effect, enhancing the overall process efficiency [
25,
26]. In reverse flotation for impurity removal, cationic collectors, particularly amine-based types, are widely used to target silicon impurities like talc and quartz. The novel cationic surfactant KD-1 (Hunan Mingzhu Flotation Reagents Co.) has been shown to significantly improve reverse flotation efficiency for magnesite and quartz [
19,
27]. pH plays a crucial role in the beneficiation of Mg-carbonate ores. While both acidic and alkaline conditions can be employed, acidic environments tend to cause the dissolution of carbonate lattices and promote excessive collector adsorption. Due to the challenges associated with this dissolution, an alkaline pH range (7–10) is generally favored to optimize flotation performance [
28].
Carbonate minerals, such as calcite and dolomite, are regarded as significant impurities in magnesite beneficiation processes. In flotation, magnesite, dolomite, and calcite exhibit similar responses to reagents, as they are classified as semi-soluble salt-type minerals containing (Ca
2+/Mg
2+) cations, which results in their analogous chemical compositions and behavior during the flotation process. In the absence of depressants, the collector demonstrates comparable adsorption behavior toward both magnesite and calcium carbonate gangue minerals. This leads to nearly identical recovery rates, thereby reducing the selectivity and efficiency of the flotation process [
23]. Sodium hexametaphosphate is a commonly used depressant in flotation processes, particularly for suppressing calcium-containing minerals. With the chemical formula (NaPO
3)
6, SHMP is a cyclic metaphosphate featuring a P-O-P backbone structure that undergoes hydrolysis in aqueous solutions, decomposing into lower phosphate species like Na
3PO
4, Na
2HPO
4, and NaH
2PO
4 [
29]. In flotation systems, the hydrolytic products, H
2PO
4− and HPO
42−, act as active species. These species donate electrons to metal ions such as Ca
2+, Mg
2+, Al
3+, and Fe
3+, facilitating adsorption onto mineral surfaces through electrostatic attraction or chelation. Another potential adsorption mechanism involves the formation of a strong hydrogen bonding network between the hydrolytic species of hexametaphosphate and water molecules surrounding calcium-containing mineral particles, resulting in the generation of insoluble calcium polyphosphate and calcium phosphate. The optimal pH range for maximizing the depressant function of SHMP appears to be between 2 and 12, as the active species H
2PO
4− and HPO
42− dominate within this range. Consequently, flotation tests were conducted at pH 10 [
30,
31].
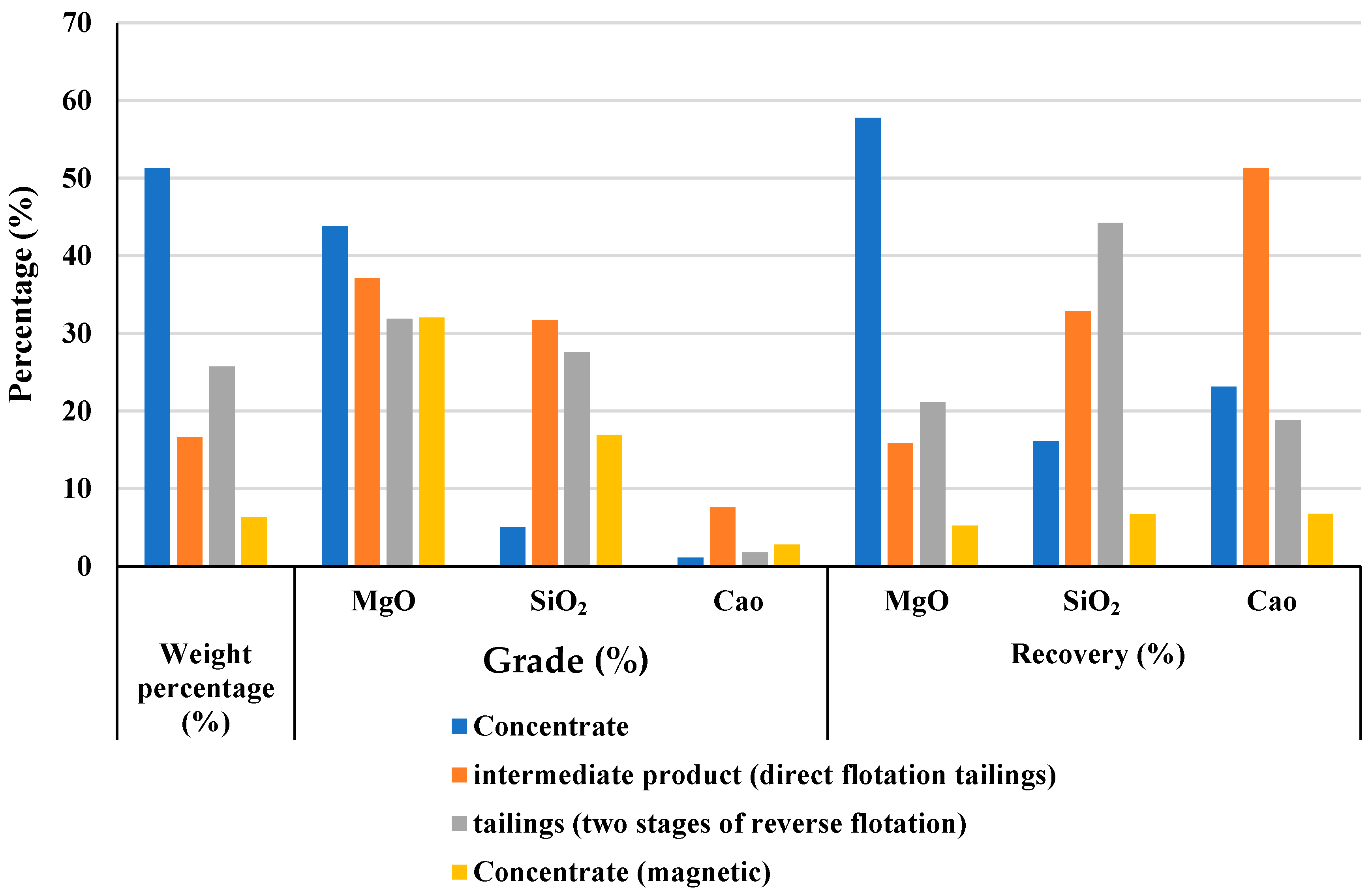
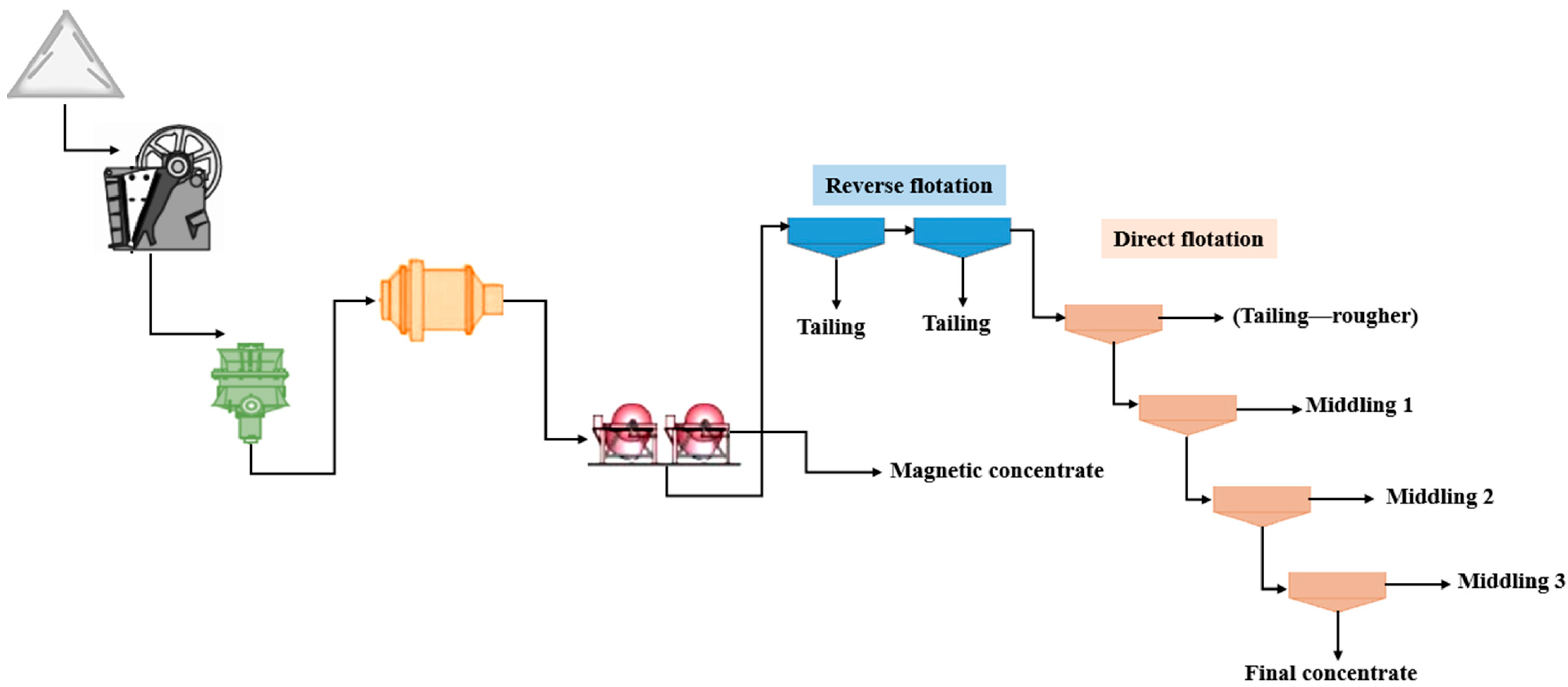
This study advances the beneficiation of low-grade magnesite ore by introducing a fully integrated and experimentally optimized processing strategy that aligns with current industrial specifications for refractory applications. Unlike prior research, which often focuses on isolated separation methods or limited reagent systems, our work proposes a multi-stage flowsheet combining medium-intensity magnetic separation, two-stage reverse flotation, and four-stage direct flotation. The proposed method achieves a MgO grade exceeding 46% while reducing SiO2, CaO, and Fe2O3 levels to below 2.5%, meeting international standards for caustic-calcined magnesite. This contribution is particularly relevant for the economic exploitation of low-grade magnesite deposits, offering a scalable solution for industries facing declining availability of high-purity resources.
4. Conclusions
Magnesium is increasingly recognized as a critical metal for high-tech industries due to its unique properties and broad range of advanced applications. However, the depletion of high-grade, high-purity magnesite deposits, combined with rising demand, highlights the urgent need to process lower-grade magnesite resources.
This study focused on the beneficiation of low-grade magnesite ore (~70% MgCO3), which contains high levels of impurities (Fe2O3 + Al2O3 + SiO2 + CaO > 20%). The major challenge was the separation of magnesite from gangue minerals with similar physico-chemical characteristics.
To address this, magnetic separation and flotation methods were systematically evaluated. Magnetic separation at 5000 Gauss was found to be optimal, reducing Fe2O3 content in the non-magnetic product to 0.8% while achieving a high MgO recovery rate of over 94%. These results were consistent across repeated trials, confirming process reliability.
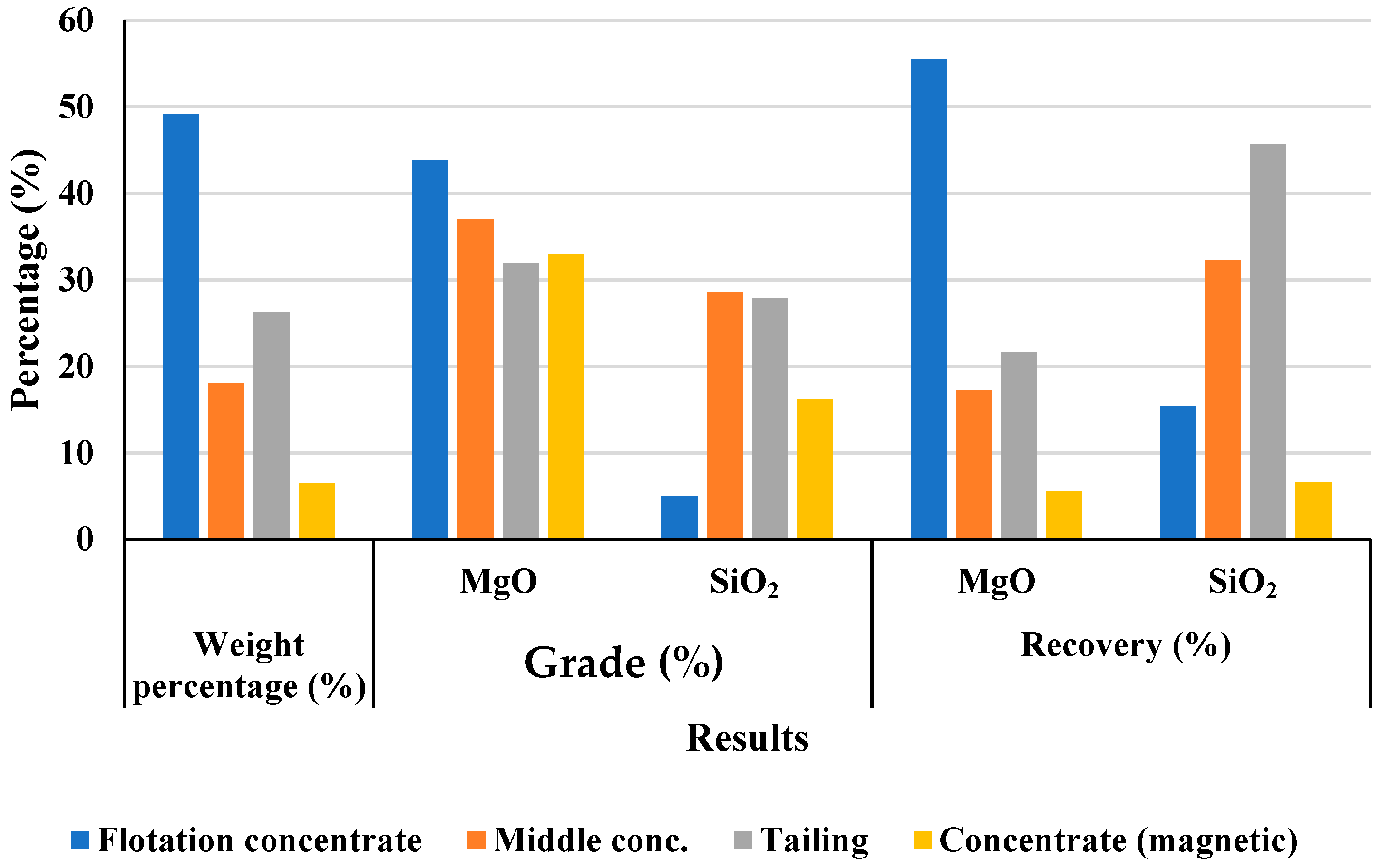
In parallel, flotation experiments using a combined approach—two-stage reverse flotation with dodecylamine (DDA) for silicates, followed by four-stage direct flotation for magnesite—achieved effective removal of over 94% of silica and other impurities. This process yielded a magnesite concentrate with MgO content exceeding 46%, meeting commercial standards for refractory applications.
Overall, this study demonstrates that a combination of optimized magnetic separation and flotation techniques can successfully upgrade low-grade magnesite to a high-purity concentrate, supporting more sustainable utilization of magnesite resources.