Abstract
This study aims to describe the triphylite (LiFe2+PO4) from Li-bearing pegmatites from the Conțu-Negovanu area (Southern Carpathians, Romania). Thus, for the first time in this area, using four analytical methods, i.e., electron micro-probe analysis (EMPA), polarized optical microscopy (POM), Fourier transform infrared spectroscopy (FTIR), and powder X-ray diffraction (p-XRD), the authors have succeeded in isolating the triphylite from the isomorphous triphylite–lithiophilite series. In addition, in the Conțu-Negovanu area, two new minerals were identified and described for the first time in pegmatites from this area: Fe-rich gatehouseite and wolfeite. The use of EMPA allowed for the tentative calculation of empirical formulae for these secondary phosphate minerals.
1. Introduction
This study focuses on the mineralogical and geochemical characterization of triphylite (LiFe2+PO4) within the lithium pegmatites of the Conțu-Negovanu area. The identification of Fe-rich gatehouseite and its relationship with gatehouseite provides new insights into Fe-Mn phosphate transformation within pegmatitic environments. The paragenetic relationships between triphylite, wolfeite, and Fe-rich gatehouseite also contribute to a broader understanding of lithium-bearing pegmatite.
According to the European Critical Raw Materials Act, which led to the establishment of a framework to help secure the supply of such raw materials in Europe, it is stipulated that at least 10% of the EU’s annual consumption should come from the exploitation of raw materials in Europe. Consequently, there has been a spike in the demand for raw materials and the rate of the development of new technologies for their exploitation and utilization, leading to the revitalization of research on ores which had previously been abandoned.
In this context, this study focuses on research on some lithium-bearing minerals found within the pegmatites of the Conțu-Negovanu area, which represent the most important pegmatite sources in Romania discovered so far that contain lithium and rare-earth element (REE) mineralization. Notably, these pegmatites contain cerium in monazite, uranium in uraninite, and niobium and tantalum within the columbite–tantalite series.
Starting from the regional and local geological framework, from the presentation of the pegmatite deposit in Conțu-Negovanu, this study aims to both qualitatively and quantitatively characterize an accessory mineral present in the pegmatite mass—namely, triphylite, a mineral used as a material for lithium battery electrodes—for the first time in Romania. Besides triphylite, the Conțu-Negovanu pegmatites contain other Li-bearing minerals like spodumene, which have economic importance. Lithium from spodumene can be used in the ceramics industry, with others uses in grease, polymers, air treatment, etc. [1].
In the Conțu-Negovanu area, the lithiophilite isomorphic series was identified by the authors of [2]. For the very first time, the authors of that paper managed to isolate triphylite from the isomorphic series with the help of an electron microprobe analyzer (EMPA). Additional analytical methods were employed to supplement EMPA, including polarized optical microscopy (POM), Fourier-transform infrared spectroscopy (FTIR), and powder X-ray diffraction (p-XRD). This paper summarizes the results of these investigations and certifies the existence of triphylite in the Conțu-Negovanu area. At the same time, this study brings new data related to isomorphic series of lithium phosphates with iron and manganese existing in the pegmatites of Conțu-Negovanu. This study only details the occurrence of a mineral from the triphylite–lithiophilite series, with the composition of the Fe-rich end-member triphylite being indicated by the higher proportion of iron at the expense of manganese in pegmatites from Conțu-Negovanu.
Additionally, newly identified secondary minerals were observed within triphylite fissures: wolfeite and Fe-rich gatehouseite. These findings, confirmed via EMPA, p-XRD, and POM, are novel in the context of the literature on Conțu-Negovanu pegmatites.
2. Description of Lithium-Bearing Deposits in Conțu-Negovanu
2.1. Geological Background
The Conțu-Negovanu area is located in the Lotru-Cibin (Cindrel) Mountains, part of the Southern Carpathians, Romania. The Lotru-Cibin Mountains belong to the Getic Domain and are made up of pre-Hercynian metamorphic rocks grouped under the name of the Șebes–Lotru Series. This series resulted from the transformation in amphibolitic facies of predominantly pelitic sedimentary formations, incorporating quartzite intercalations and products of mafic volcanism. The crystalline schists, which are the result of high-grade metamorphism, are represented mainly by paragneisses in the lower part and by micaschists in the upper part. In both stacks, from the base to the top, the following unit compositions were identified [2]:
The lower unit consists mainly of paragneisses, particularly biotitic gneisses containing a sillimanite–biotite assemblage.
The middle unit is subdivided into several lithological sequences, arranged from bottom to top as follows: an amphibolite unit; a high-grade gneiss complex; a strongly migmatized gneiss complex, which contains numerous small lenses of eclogite and ultramafic rocks; a mafic–terrigenous complex; and a meta-igneous alkaline gneiss unit.
The uppermost unit, known as the Negovanu Mare Formation, is composed of micaschists that contain abundant relicts of a kyanite–muscovite–staurolite–almandine–rutile assemblage preserved in porphyroclasts.
The Conțu-Negovanu area, where the Negovanu Mare formation outcrops occur, is perhaps the only site containing lithium–pegmatite with REEs in Romania. To outline areas with economic prospects for pegmatites with micas, feldspar, rare elements (Li, Be), kyanite, etc., geological studies (on the prospecting, exploration, and experimental exploitation of spodumene) have been carried out in the Lotru-Cibin mountains since 1955. These studies have significantly enhanced our understanding of the region’s structural, genetic, and economic framework.
Pegmatites with spodumene outcrops near the Conțu brook occur in two distinct pegmatite bodies: Conțu Superior and Conțu Inferior (Figure 1). These pegmatites are hosted within micaschists, amphibolites, and paragneisses. Here, there is the orthogneiss–granitepegmatite system, rich in P, Li, and Sn, with common occurrences of almandine, tourmalines, and primary and secondary phosphates [3].
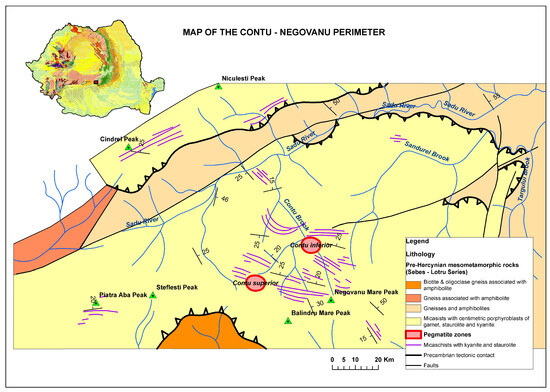
Figure 1.
Geological map of lithium pegmatite Conțu-Negovanu with the two pegmatite bodies: Conțu Superior and Conțu Inferior.
2.2. Geological and Mineralogical Characteristics of the Conțu-Negovanu Pegmatites
The pegmatites in the Conțu-Negovanu area occur as tabular veins, irregular lenses, or massive bodies, generally following the schistosity of the meso-metamorphic host rocks. These pegmatitic veins align with the northwest–southeast orientation of the surrounding formations and dip toward the north and northeast. Their thickness varies between 2 and 12 m in the central area, with lengths ranging from 175 to 475 m. The veins are affected by transverse and directional fractures, typically exhibiting minor displacements (0.5–5 m), which are characteristic for the area containing spodumene-bearing pegmatites. Most pegmatitic bodies outcrop on the western side of the Conțu Valley, where they range in thickness from a few tens of centimeters to several meters. Pegmatitic bodies appear as veins that are discordant with the host formations, while the pegmatitic lenses maintain the same orientation as the formations. These pegmatites are highly fractured and fissured, displaying significant weathering [4]. Notably, tavorite and apatite result from supergene processes [5]. Fracturing is caused by strong deformation of the pegmatitic bodies, which exhibit either sharp intrusive contacts or gradual to tectonically mixed contacts with the host rocks.
The structural framework of the Conțu pegmatitic field is characterized by fold and fault tectonics, indicating an E–W or NW–SE orientation of the crystalline formations, with predominantly northward dips. The Șebeș-Lotru Group has been geochronologically constrained through multiple dating methods. K-Ar dating suggests an age of approximately 480 million years [6], while 39Ar–40Ar dating indicates Variscan tectonic events (320–295 million years) [7], revealing evidence of both Caledonian and Variscan metamorphic processes.
2.3. Pegmatite Types and Mineral Assemblages
Conțu-Negovanu pegmatites are classified into two types: albite–spodumene pegmatites, and feldspathic pegmatites with muscovite.
The albite–spodumene pegmatites consist primarily of spodumene (15–25%), feldspars (45–50%), quartz (20–30%), and micas (2–10%) [4]. These pegmatites also contain a diverse suite of accessory minerals such as phosphates, including triphylite, ferrisicklerite, heterosite, Fe-rich gatehouseite, maricite, wolfeite, amblygonite–montebrasite, monazite, vivianite, apatite, fluorapatite, and hydroxylapatite, as well as microcline, microcline–perthite, beryl, cassiterite, columbite-group minerals, lepidolite, rutile, scarce schorl, titanite, sillimanite, spessartine, topaz, and uraninite [4,8,9,10,11,12,13]. In this study, gatehouseite is identified as Fe2+-dominant [10,11,12,13]. The feldspathic–muscovite-type pegmatites include a mineralogical association consisting of feldspar, quartz, muscovite, biotite, and accessory minerals.
The lithium-bearing pegmatites from the Conțu-Negovanu field host independent or associated lithium minerals, including spodumene (Li-silicate), triphylite (Li-Fe-Mn phosphate), ferrisicklerite (Li-Fe phosphate), amblygonite–montebrasite (Li-Al phosphate), and lepidolite (Li-mica).
The phosphates in the Conțu-Negovanu lithium pegmatites appear as crystals ranging from a few millimeters to 5 cm in diameter, exhibiting dark green to purple-brown hues due to isomorphic transformations [10]. The primary phosphate, triphylite (Figure 2), appears to be associated with spodumene, suggesting its crystallization from the Li-silicate melt [10], characteristic of these pegmatites.


Figure 2.
Macroscopic associations of minerals (8 images): triphylite, of centimeter-size and green and brown in color, occurs alongside long prismatic green spodumene, albite (almost pure), cleavelandite (white variety), green muscovite, and gray quartz from the Conțu-Negovanu pegmatites. 1—mineralogical association: triphylite, albite, quartz and muscovite; 2—mineralogical association: quartz, spodumene, albite, triphylite; 3—mineralogical association: muscovite, spodumene, quartz, albite, triphylite; 4—mineralogical association: muscovite, spodumene, albite, triphylite, quartz; 5—mineralogical association: spodumene, albite, triphylite, quartz; 6—mineralogical association: muscovite, albite, quartz, triphylite; 7—mineralogical association: albite, muscovite, spodumene, quartz, triphylite; 8—mineralogical association: triphylite, muscovite, albite and quartz. Trp = triphylite; Ab = albite–cleavelandite variety; Qtz = quartz; Sp = spodumene; and Ms = muscovite).
2.4. Genesis of the Pegmatites
The origin of the Lotru-Cibin pegmatites has been the subject of extensive research. Several authors [14,15,16] have proposed an anatectic origin. Anatexis is the process of partially melting rocks. The relationship between the pegmatites and the meso-metamorphic host rocks, as well as the lack of magmatic bodies, led those authors to the conclusion that these pegmatites are metamorphic and formed under metasomatic and anatexis conditions, crystallizing from a pegmatitic melt [15]. Other authors [17], considering the complex mineralogy—including rare minerals—along with aeromagnetic data indicating deep-seated faults and the presence of basic and ultrabasic rocks at depth, have drawn parallels with the pegmatites from the Cataracte area in the Lotru Mountains. Cataracte, also representing the Sebeș-Lotru metamorphic series, is adjacent to the Conțu-Negovanu study area. Cataracte pegmatite is a large magmatic body measuring 3.5 km in length and 1 km in width. Based on field research, corroborated by the results of mineralogical analyses, the present authors are in favor of the theory regarding the magmatic origin of pegmatites as part of a larger granitic system (fractionation pegmatites).
Thus, in this study on the genesis of the Lotru Mountain pegmatites, six stages of genetic processes have been identified, characterized by the complex geochemical evolution of pegmatitic fluids, leading to the crystallization of specific minerals [16].
These stages define a zonation pattern within pegmatite bodies, best illustrated in the Cataracte area. From the marginal to the central zone, the following stages have been identified [16]:
Marginal Zone
- ○
- Stage I is the calc–alkaline stage (Ca, Na), referring to enrichment in anorthite and garnets (andradite ± grossular). Calcium is sourced from micasschists and paragneisses.
External Zone
- ○
- Stage II is alkaline stage 1 (potassic and sodic phase), in which the dominance of potassium (K) and sodium (Na) in pegmatites leads to the development of quartz–feldspar graphic textures typical of feldspar-rich pegmatites, as observed in the Cataracte area.
Intermediate Zone
- ○
- Stage III is alkaline stage 2 (potassic phase), comprising the formation of massive microcline.
- ○
- Stage IV is the hydrolysis stage (H2O influence), referring to the transformation of potassic feldspar and biotite into muscovite, along with the crystallization of rare-metal-bearing minerals (Li, Be, Nb).
- ○
- Stage V is alkaline stage 3 (sodic phase), which pertains to the massive development of cleavelandite, spodumene, and other lithium-bearing minerals.
Central Zone (Core)
- ○
- Stage VI is the acid stage, characterized by the dominance of quartz in the pegmatite core.
On a regional scale, the Cataracte pegmatite body can be considered a model, as it exhibits all six genetic stages [18]. It is assumed to be the closest to the granitic melt.
With increasing distance from the potential parental granite, the genesis and composition of the pegmatite bodies changes. Consequently, the Conțu Superior and Conțu Inferior pegmatites are the only Li-bearing albite spodumene-type [19,20] pegmatites in the area. With increasing distance from the granitic source (Cataracte—Voineasa, Lotru mountains), the degree of fractionation of the pegmatitic melt is more advanced. Located 10 km from Cataracte, in Conțu-Negovanu Li-pegmatite bodies, three zones have been identified: the marginal, intermediary, and central zones (or the border, intermediate, and core zones, according to [18]).
The marginal zone (or the border zone, according to [18]) is up to 1 m thick. From a mineralogical point of view, it is composed of spodumene, quartz, plagioclase feldspars, and muscovite. At the pegmatites’ point of contact with metamorphic rocks, ferromagnesian minerals appear such as tourmaline (scarce schorl variety), biotite, and garnets; the crystal size ranges from a few millimeters to 3 cm.
The intermediary zone (referred to in the same way in [18]) indicated an alkaline stage with the development of almost pure albite (cleavelandite variety), along with green spodumene (hiddenites, a pale to-emerald green variety of spodumene). There was also a triphylite series in the pegmatite bodies from the Conţu area [16]; this stage is characterized by the perthite structure of feldspar and the crystallization of beryl, columbite–tantalite, and cassiterite [16]. The presence of perthite in the microcline is linked to a high sodium content. The central area (also known as the core zone [18]) is represented by large amounts of quartz.
The most prominent zone in the Conțu Superior and Conțu Inferior localities is the intermediate zone.
3. Analytical Methods Used
3.1. Powder X-Ray Diffraction (p-XRD)
X-ray diffraction analysis was performed using a Bruker D8 ADVANCE (Bruker AXS GmbH, Karlruhe, Germany), diffractometer with CuKα radiation (λ = 1.54056 Å), within the Geological Institute of Romania, National Geological Museum branch. The instrument operated at an accelerating voltage of 40 kV and a beam current of 40 mA. Data analysis was conducted using the PDF-2/Release 2007 database, upgraded to the PDF-2/Release 2013 database, with phase identification performed via the EVA 13 software (ICDD database). The D8 ADVANCE is perfectly suited for X-ray powder diffraction. The D8 ADVANCE allows one to measure all sample types –from thin films to solid blocks, from liquids to powders –with a single instrument. The p-XRD techniques are the most important tools for material characterization. The DIFFRAC.SUITE software Version 2.0704 provides support for p-XRD methods, aiding the identification of both crystalline and amorphous phases and the determination of specimen purity; indexing, crystal determination, and crystal structure refinement; microstructure analysis (crystallite size, microstrain, disorder); and texture analysis (preferred orientation analysis).
3.2. Polarized Optical Microscopy (POM)
Mineral identification in thin sections was carried out using polarizing optical microscopy (POM). The instruments employed were Jenapol U and Leica-type polarizing microscopes (from the Laboratoire de Minéralogie et Cristallochimie Department de Geologie, Université de Liège, Liège, Belgium).
3.3. Electron Micro-Probe Analysis (EMPA)
Electron microprobe analysis was performed using a CAMECA SX-50, operating in wavelength-dispersion mode, at Ruhr University, Bochum, Germany. The instrument was set at an accelerating voltage of 15 kV and a beam current of 20 mA. Calibration was conducted using the following mineral standards: topaz (Al2SiO4(F,OH)2), titanite (CaTiSiO5), jadeite (NaAlSi2O6), graftonite (Fe2+,Mn,Ca)3(PO4)2), andradite (Ca3Fe2(SiO4)3), pyrope (Mg3Al2(SiO4)3), and spessartine (Mn3Al2(SiO4)3). For lithium, we used the calculation methods described in [21]. A range of 0.1–0.2 wt.% is hypothesized for EMPA in general.
3.4. Infrared Transmittance Spectroscopy (ITS)
Infrared transmittance spectroscopy was conducted using a Bruker TENSOR 27 spectrometer, equipped with a Fourier transform module, and the results were analyzed using the OPUS 6.5 software, at the Geological Institute of Romania, National Geological Museum. ”FTIR” is the abbreviation for Fourier transform infrared spectroscopy. The spectra obtained represent the molecular transmission, like a fingerprint of the sample. FTIR can be used to identify unknown materials, determine the quality or consistency of a sample, and determine the quantities of various components in a mixture.
4. Triphylite LiFe2+(PO)4
Triphylite crystallizes in the orthorhombic system, space group Pnma. It typically exhibits a nodular habit, though prismatic forms are sometimes observed. The mineral has a dark green color with a greasy vitreous luster and is translucent. Triphylite has well-developed cleavage, specifically (001)—perfect cleavage, (010)—good cleavage and (011)—poor cleavage [10]. It has a Mohs hardness of 4 and belongs to the triphylite group, while also being isostructural with olivine. In the lithium pegmatites of the Conțu-Negovanu, triphylite occurs as dark green nodules ranging in size from a few millimeters to 5 cm in diameter [10].
4.1. Triphylite (p-XRD)
Six triphylite samples were analyzed using p-XRD. The diffractograms were calculated using the ICDD reference pattern 01-077-0178 for lithiophilite, as the two minerals form an isomorphic series and share the same crystal structure. The obtained diffraction values correspond well with data from the literature [22,23,24]. A representative X-ray diffractogram illustrating triphylite in association with fluorapatite, wolfeite, gatehouseite, and quartz is shown in Figure 3.
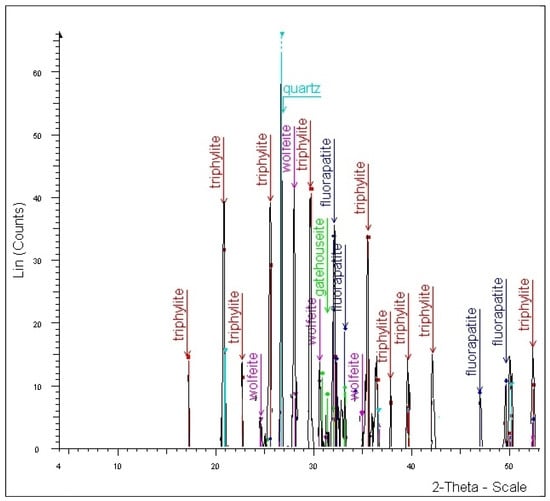
Figure 3.
Powder X-ray diffraction, for a representative sample of triphylite in association with wolfeite, gatehouseite, fluorapatite, and quartz from the Conţu-Negovanu lithium pegmatites.
The powder X-ray diffraction data are presented in Table 1, where the unit cell parameters of the six triphylite samples are compared with values reported in the literature.

Table 1.
Values of elementary cell parameters for six triphylite Conțu-Negovanu samples compared with data from the literature.
4.2. Triphylite (POM)
Three samples of lithium pegmatites from Conțu-Negovanu were analyzed. Under polarizing optical microscopy with parallel nicols, triphylite exhibits high relief, lacks pleochroism, appears colorless, and displays well-developed cleavage. When observed under crossed nicols, triphylite shows straight extinction, occasionally exhibiting slight undulatory extinction, possibly influenced by the presence of quartz. Quartz is commonly associated with phosphates, circulating through microcracks and fissures in lithium pegmatites, as observed in triphylite thin sections examined under crossed nicols. The birefringence of triphylite was measured, yielding values between 0.005 and 0.008. The mineral is anisotropic and exhibits a negative biaxial interference pattern (Figure 4).
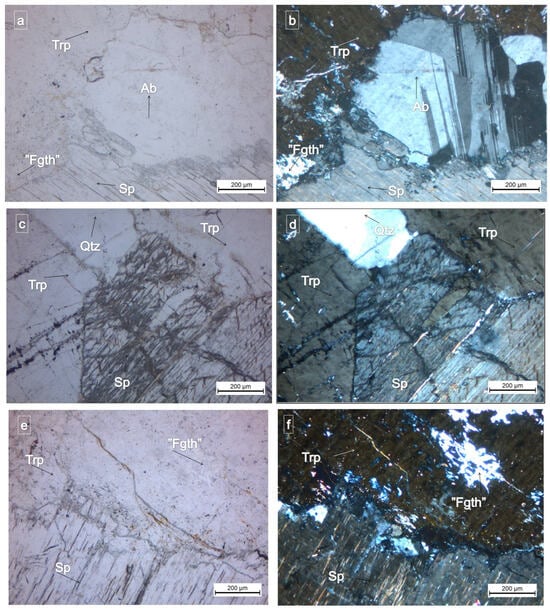
Figure 4.
(a) = (N II) and (b) = (N+), showing triphylite in association with plagioclase, spodumene, and Fe-rich gatehouseite with weak birefringence. (c) = (N II) and (d) = (N+), depicting microscope images of triphylite associated with spodumene and quartz. (e) = (N II) and (f) = (N+), showing the association between triphylite and spodumene with quartz. Between spodumene and triphylite, we have quartz and Fe-rich gatehouseite with weak birefringence on triphylite cleavage (plane-polarized light = N II; crossed-polarized light = N+). Trp = triphylite; Ab = albite; Qtz = quartz; Sp = spodumene; and Fgth = Fe-rich gatehouseite.
4.3. Triphylite (EMPA)
EMPA allowed for the identification of triphylite within the triphylite–lithiophilite isomorphic series. A total of 14 chemical analyses were conducted on triphylite samples (Table 2 and Table 3).

Table 2.
Representative results for fourteen triphylite samples from Conțu-Negovanu Li–pegmatites.

Table 3.
Relevant chemical empirical formulas for fourteen triphylite samples from Conțu-Negovanu Li–pegmatites.
Fe apfu had the highest values in samples CS1 and CS8 (0.660) and the lowest in sample CS14 (0.566). Mn apfu had the highest value in sample CS12 (0.429) and the lowest in sample CS5 (0.324). Mg apfu had the highest values in samples CS1, CS3, and CS8 (0.011) and the lowest in samples CS12, CS13, and CS14 (0.002). Ca apfu had a value of 0.001 or was completely absent.
The results, along with the calculated chemical formulas, were based on one (PO4) group per formula unit.
In the formula M1M2PO4 = (LiFePO4 − triphylite), M1 = Li + Na + K (sometimes Ca), and M2 = Fe + Mn + Mg.
Li apfu = occupancy M1 − (Na + K + Ca), apfu [21]. In sample CS2, Ca = 0.001.
Indeed, the chemical formulas do not close perfectly, except for the one for triphylite sample CS2, with a deduced chemical formula of (Li0.998Ca0.001)0.999(Fe0.663Mn0.329Mg0.010)1.002PO4. It is possible that the cumulate analytical precision was 0.1 to 0.2 wt%, which would indicate that the values are approximate, as this fact indicated a Li2O content greater than the possible stoichiometric maximum Li2O in the two end-members (triphylite (9.47) and lithiophilite (9.53)).
4.4. Triphylite (ITS)
Triphylite was identified using Fourier transform infrared transmittance spectrometry (ITS) (Figure 5) in a sample from Conțu-Negovanu. The recorded spectra displayed absorption bands characteristic of the antisymmetric stretching vibrations of the (PO4)3− structural group, which were observed at 1085.90 cm−1 and 962.46 cm−1. Additionally, planar deformation vibrations were recorded at 651.06 cm−1, 761.33 cm−1, and 787.95 cm−1, values corresponding to data from the specialized literature [25].
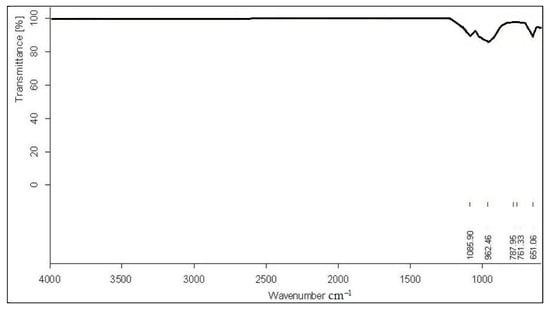
Figure 5.
Infrared transmittance spectra of the triphylite sample from Conţu-Negovanu lithium pegmatites.
5. Alteration of Triphylite to Secondary Phosphate (Wolfeite—(Fe2+,Mn2+)2(PO4)(OH)) and Fe-Rich Gatehouseite—(Fe,Mn,Mg)5(PO4)2(OH)4)
5.1. Wolfeite–(Fe2+,Mn2+)2(PO4)(OH)
5.1.1. Wolfeite (p-XRD)
Four diffractograms were indexed using the ICDD 01-072-3634 reference file for wolfeite, confirming that it crystallizesd in the monoclinic system, with space group P21/a (14). The obtained data are presented in Table 4, alongside reference values from the literature [26,27], showing a close correlation between the measured and reference values.

Table 4.
Values of elementary cell parameters for four samples of wolfeite from Conțu-Negovanu Li-pegmatites, including reference data.
The paragenesis of wolfeite in association with amblygonite, apatite, and vivianite observed in the lithium pegmatites of the Conțu-Negovanu area was similar to that reported in the Panasqueira deposit [28].
5.1.2. Wolfeite (POM)
Under plane-polarized light microscopy, wolfeite exhibits weak pleochroism, displaying white–yellow tones, along with a high relief. It is an anisotropic, biaxially positive mineral with a maximum birefringence value of 0.049. Within the triphylite mineral, wolfeite occurs with a fibrous habit, with dimensions on the order of 50 µm (Figure 6 and Figure 7).
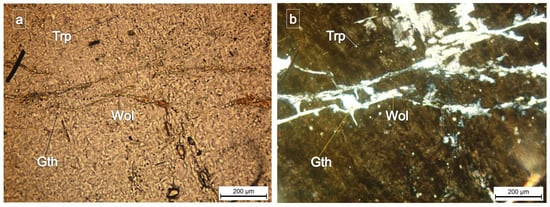
Figure 6.
Samples of wolfeite (Wol) and gatehouseite—(Gth) = (Fe,Mn,Mg)5(PO4)2(OH)4. Fe-rich gatehouseite present in the mass of triphylite (Trp), (plane-polarized light—(a); crossed nicols—(b)), from the Conțu-Negovanu Li–pegmatites. Weak pleochroism of wolfeite can be seen in triphylite.
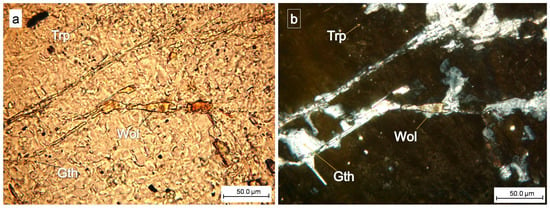
Figure 7.
Close-up images of wolfeite (Wol) and gatehouseite—(Gth) = (Fe,Mn,Mg)5(PO4)2(OH)4 Fe-rich gatehouseite in phosphate: triphylite (Trp) (plane-polarized light—(a), crossed nicols—(b)).
The correlation of optical microscope images taken under parallel and crossed nicols was essential in confirming the identity of the analyzed mineral, which appeared within the primary phosphate triphylite.
5.1.3. Wolfeite (EMPA)
We performed a chemical analysis of 13 wolfeite samples; the results are presented in Table 5 and Table 6. The results, along with the calculated chemical formulas, are based on one (PO4) group per formula unit.

Table 5.
Representative results for 13 samples of wolfeite from the Conțu-Negovanu Li–pegmatites.

Table 6.
Relevant chemical empirical formulas resulting from 13 samples of wolfeite from Conțu-Negovanu Li–pegmatites.
Data derived from our analysis of the wolfeite from the Li-pegmatites were compared with data present in the literature. The composition of sample CS2 was as follows: P2O5 = 32.34%, Al2O3 = 0.14%, FeO = 39.06%, MnO = 24.26%, MgO = 0.09%, CaO = 0.48%, and H2O = 4.10%, with a total of 100.47%. For the reference literature sample [26], the composition is as follows: P2O5 = 31.75%, FeO = 37.53%, MnO = 25.16%, H2O = 4.60%, Na2O = 0.06%, MgO = 0.43%, ZnO = 0.43%, and CaO = 0.04%, with a total of 100.00%.
Fe apfu had the highest value in sample CS13 (1.477) and the lowest value in sample CS1 (1.047). Mn apfu had the highest value in sample CS1 (0.967) and the lowest in sample CS7 (0.570). Mg apfu had the highest values in samples CS11 and CS4 (0.023) and the lowest in sample CS3 (0.003). Ca apfu had the highest value in sample CS3 (0.033) and the lowest in samples CS6 and CS13 (0.001). Al apfu had the highest values in samples CS2 and CS7 (0.006) and no values in samples CS3 and CS4. The values for H2O were deduced. The deduced water in wolfeite ranged from 3.89 to 4.31 wt% H2O. ”If H2O has not been determined or is thought to be unreliable the mineral formula can be calculated on an anhydrous basis assuming the (OH) content to be ideal” [29]. In our case, for wolfeite, we can consider that OH = 1.
In backscattered images, wolfeite appears in white tones, contrasting with the gray tones of the surrounding triphylite mass (Figure 8).
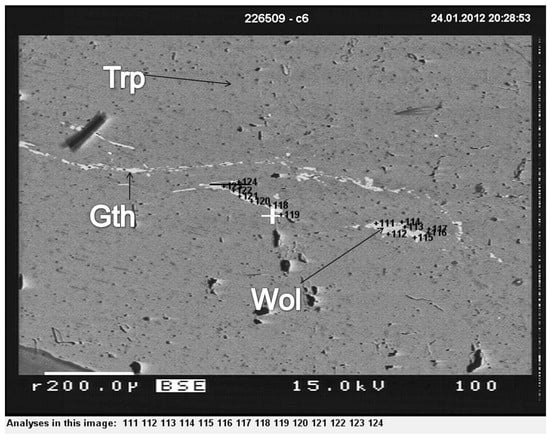
Figure 8.
Backscattered image of the sample in Figure 6 showing the association of triphylite (Trp) and wolfeite (Wol), as well as gatehouseite—(Gth) = (Fe,MnMg)5(PO4)2(OH)4 -Fe-rich gatehouseite Conțu-Negovanu Li–pegmatites.
5.2. Fe-Rich Gatehouseite–(Fe,Mn,Mg)5(PO4)2(OH)4
5.2.1. Fe-Rich Gatehouseite (p-XRD)
X-ray diffractometry analysis identified gatehouseite in three diffractograms. The diffraction patterns were indexed using the ICDD 01-070-0516 data sheet, which confirmed the orthorhombic system, space group P212121 (19) of gatehouseite.
The obtained diffraction values are presented in (Table 7), showing a close correlation with previously reported data in the literature [30,31].

Table 7.
Values of elementary cell parameters for three gatehouseite samples from the Li–pegmatite from Conțu-Negovanu compared with data from the literature.
According to the diffractograms in Figure 3, gatehouseite constituted 2.4% of the analyzed sample and was associated with fluorapatite (25.6%), triphylite (40.7%), wolfeite (9.0%), and quartz (22.4%).
5.2.2. Fe-Rich Gatehouseite (POM)
Polarized optical microscopy revealed that gatehouseite was aligned along the cleavage direction of triphylite and occurred as xenomorphic disseminations. The mineral exhibits high relief, is colorless and anisotropic, and lacks pleochroism. It displays straight extinction and weak birefringence, appearing in white tones (Figure 9 and Figure 10).
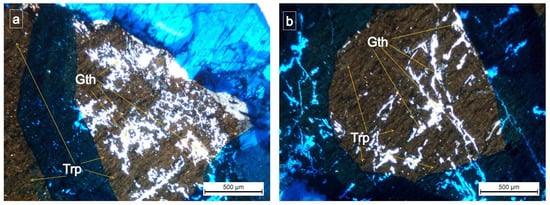
Figure 9.
(a,b) triphylite (Trp) with Gatehouseite—(Gth) = (Fe,Mn,Mg)5(PO4)2(OH)4 Fe-rich gatehouseite from Conțu-Negovanu Li–pegmatites (crossed nicols = (a,b); the blue ink marks the areas of interest).
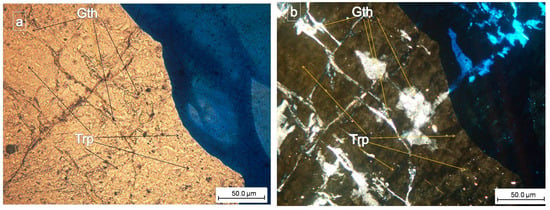
Figure 10.
(a,b) Detailed view of the phosphates: Gatehouseite—(Gth) = (Fe,Mn,Mg)5(PO4)2(OH)4 Fe-rich gatehouseite, is arranged in the cleavage direction of the primary phosphate, triphylite (Trp) (plane-polarized light = (a); crossed nicols = (b)). The secondary phosphate has a length of about 30 μm and a width of 20 μm, and the blue ink marks the areas of interest.
5.2.3. Fe-Rich Gatehouseite–(EMPA)
The results of ten chemical analyses of an unknown phosphate which was identified as gatehouseite, along with the calculated chemical formulas based on there being 2PO4 groups per formula unit, are presented in Table 8 and Table 9.

Table 8.
Representative results for 10 samples of gatehouseite from the Conțu-Negovanu Li–pegmatites.

Table 9.
Relevant empirical chemical formulas found for 10 samples of gatehouseite from the Li–pegmatite from Conţu-Negovanu.
Chemical analysis of the Fe-rich gatehouseite revealed the following composition for sample CS2: P2O5 = 26.76%, FeO = 38.97%, MnO = 27.15%, MgO = 0.41%, CaO = 0.05%, and H2O = 6.79%, with a total of 100.13%. ”If Fe3+ has not been determined, total iron is usually presented as an FeO equivalent. There are many ways of gaining an estimate Fe3+ and Fe2+, most which are referred to in” [32]. The method does not provide accurate estimates unless the analyses are of high quality. EMPA is inadequate for estimating trace amounts of Fe3+ in ferrous iron-rich minerals like olivine [32].
This composition closely resembled that reported for gatehouseite in the literature [27]: P2O5 = 22.18%, MnO = 64.42%, FeO = 0.19%, CuO = 0.03%, ZnO = 0.03%, PbO = 0.05%, Al2O3 = 0.10%, V2O5 = 0.38, As2O5 = 3.58, and H2O = 6.44%, with a total of 97.40%. With the value of 97.4 for gatehouseite [31], we can say that this empirical chemical formula was related to a percentage of 100%, the ideal closing value of the empirical formula. In this case, there was a difference of –2.60%, but we can say with certainty that those values, requiring minus 3 and plus 3 to add to 100%, can be taken into account. Fe apfu is variable in the analyzed samples of Fe-rich gatehouseite, even though contamination with host triphylite was avoided by analyzing coarse particles. The values of Fe, Mn, and Mg apfu were higher than they were in triphylite, which suggests that the analysis was performed on Fe-rich gatehouseite.
Fe apfu had the highest value in sample CS7 (3.716) and the lowest value in sample CS5 (2.735). Mn apfu had the highest value in sample CS2 (2.030) and the lowest in sample CS7 (1.252). Mg apfu had the highest value in sample CS3 (0.055) and the lowest in sample CS1 (0.021). Ca apfu had the highest value in sample CS1 (0.009) and was non-existent in samples CS3, CS6, CS7, CS8, and CS9. The values for H2O were deduced.
The calculation was based on two PO4 apfu. For the CS1 sample, the percentage obtained by the electronic microprobe analyzer for P2O5 had a value equal to 26.60%. To calculate the molecular % of P2O5, we used the following strategy: the value 26.60% was divided by the sum (number of P atoms × 2) + (number of oxygen atoms × 5), which had a value of 141.9446 g/mol, leading to a value of 0.1873.
The cationic percentage of P2O5 was the result of molecular % P2O5 × 2, leading to a value of 0.3747.
We divided the calculations based on two PO4 apfu by the cationic percentage of P2O5 (0.3747), and the result was 2/0.3747 = 5.3376.
We assumed that the OH group = 4 in the stoichiometric formula (FeMn)5(PO4)2(OH)4, which we divided by 5.3376, and we obtained the cationic percentage of H2O, which had the value 0.7495. The molecular percentage of H2O resulted from the cationic percentage of H2O/2 = 0.3747.
The percentage of H2O deduced in a theoretical composition (weight percent) results from the molecular % of H2O (0.3747) multiplied by the molecular weight of H2O (18.0154 g/mol). The percentage of H2O deduced, in the case of sample CS1, was 6.752 wt% (Table 8).
“If H2O has not been determined or is thought to be unreliable the mineral formula can be calculated on an anhydrous basis assuming the (OH) content to be ideal” [29]. In our case, for gatehouseite, we can consider OH to equal 4. For gatehouseite, the deduced values were between 6.73 and 7.37 wt% H2O.
In the case of gatehouseite, in sample CS7, the best conclusion was the deduced chemical formula Ca0.005(Fe3.717Mn1.252Mg0.027)4.996(PO4)2(OH)4. From this formula, we can say that we were probably dealing with a new mineral species, much closer to iron than manganese.
Gatehouseite = Fe-rich gatehouseite was identified via electron microprobe analysis (EMPA), appearing with a light gray color tone within the gray triphylite mass (Figure 11).

Figure 11.
Backscattered image showing gatehouseite—(Gth) = (Fe,Mn,Mg)5(PO4)2(OH)4 Fe-rich gatehouseite in triphylite—(Trp) (primary phosphate).
6. Discussions
6.1. Genetic Considerations
Due to oxidation conditions and the metasomatic replacement of chemical elements, triphylite (LiFe2+(PO4)) undergoes transformation into ferrisicklerite (LiFe2+Fe3+(PO4)), which subsequently loses lithium and converts into heterosite (Fe3+(PO4)). A particularly interesting aspect arises when analyzing the chemical composition of ferrisicklerite, as it contains a higher proportion of Fe2+ than Fe3+. The presence of Fe is considered a geochemical indicator for beryl crystallization [33]; this aspect, together with the presence of uraninite [34,35], suggests a strong genetic link to a magmatic reservoir. Granitic pegmatites serve as important raw material sources for Li, Be, REEs, Sn, U, Cs, Ta, and Nb [36].
Triphylite is the primary phosphate phase in the Conțu-Negovanu pegmatites, with a crystal structure similar to olivine. Triphylite formation is primarily associated with magmatic–hydrothermal processes [37].
During the genetic evolution of the pegmatites, the triphylite-ferr isicklerite-heterosite series develops under oxidizing conditions and undergoes magmatic (for primary phosphate triphylite) and hydrothermal transformation [38,39,40,41,42,43].
The presence of triphylite as the primary phosphate in the Conțu-Negovanu pegmatites is consistent with observations from d’Angarf-Sud pegmatites [39] and the Sapucaia pegmatite [44].
The triphylite–lithiophilite series Li(Fe,Mn)PO4 occurs in evolved granitic pegmatites, enriched in lithium and phosphorus [45]. Wolfeite forms through high-temperature hydrothermal alteration of the primary triphylite [45]. This mineral is a result of metasomatic processes, with OH groups in wolfeite indicating water involvement during pegmatite formation. Wolfeite is often associated with maricite, and vivianite [45].
The chemical composition (Fe,Mn,Mg)5(PO4)2(OH)4 results from the substitution of Mn2+ in the phosphate by Fe2+, leading to the formation of Fe-rich gatehouseite. Gatehouseite was first discovered in 1993 by Bryan Michael Kenneth Cummings Gatehouse at the Monarch Iron ore quarry, Australia [31]. The mineral gatehouseite (Mn5(PO4)2(OH)4) is a phosphate found in Fe-Mn sedimentary deposits, particularly in South Australia, specifically on the Eyre Peninsula [31].
No iron–manganese phosphate with four OH groups has been reported in the literature, although the manganese equivalent is well documented in Fe-Mn-rich sedimentary formations.
The Conțu-Negovanu pegmatites are classified as magmatic in origin [46]. The triphylite–ferrisicklerite–heterosite series (known as the Quensel–Mason series) represents an oxidation process of phosphates within granitic pegmatites [39,47,48]. In the Conțu-Negovanu pegmatite zone, both ferrisiklerite and heterosite were found [10,11,12,13]. In this study, only theoretical reference was made to these two minerals, but they exist, and based on this, the oxidation process was discussed [10,11,12,13].
The Li-Fe and Li-Mn phosphate minerals—such as triphylite–lithiophilite, ferrisicklerite–sicklerite, and heterosite–purpurite—are common lithium-bearing minerals in granitic pegmatites [21]. These Li-rich pegmatites originate either from the anatexis of Li-bearing metasediments or through the extensive differentiation of granitic magma [37].
The Fe/Mn ratio of minerals usually reflects the fractionation degree of the pegmatitic melt and is supported by the Fetot/(Fetot + Mn) ratio, as observed in the Boca Rica pegmatite and Sapucaia pegmatite from Minas Gerais, Brazil [42]. Boca Rica triphylite, enriched in Fe and Mg, has a Fetot/(Fetot + Mn) ratio of 0.74, and triphylite, richer in Mn, has a ratio of Fetot/(Fetot + Mn) = 0.58. Sapucaia triphylite has a ratio of Fetot/(Fetot + Mn) = 0.71. In Conțu-Negovanu pegmatite, triphylite has a Fetot/(Fetot + Mn) ratio of 0.66 for CS1 sample. The ratio of triphylite from Boca Rica and Sapucaia, Minas Gerais, Brazil [42], resembles that of triphylite from the Conțu-Negovanu pegmatite, further confirming their genetic similarities.
Over time, researchers have discussed various isomorphic phosphate series [24], including triphylite, ferrisicklerite–sicklerite, and heterosite–purpurite. Based on 37 chemical analyses carried out via EMPA, a diagram regarding the variation in the Fe/(Fe + Mn)*100, in atomic proportions, was constructed to illustrate the triphylite–wolfeite–Fe-rich gatehouseite association in this research (Figure 12).
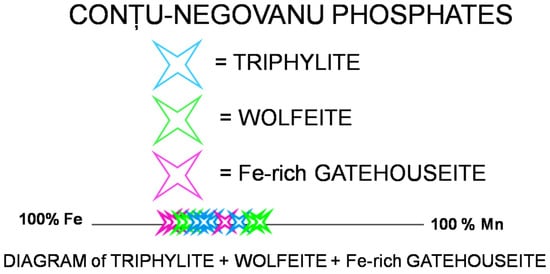
Figure 12.
Plot of Fe/(Fe+Mn)*100 in the Conțu-Negovanu phosphates, calculated on an atomic proportions basis. Triphylite = Li(Fe,Mn)PO4; wolfeite = (Fe,Mn)2(PO4)(OH); Fe-rich gatehouseite = (Fe,Mn,Mg)5(PO4)2(OH)4.
This diagram demonstrates that, in all analyzed samples, the concentration of iron is significantly higher than that of manganese.
6.2. Triphylite for LFP Batteries
LFP is synthesized from Li, which originates mainly from spodumene or Li brines that are subsequently converted to LiOH or Li2CO3 and, only after that, to an LFP battery [49].
Triphylite (LiFePO4), in particular, is of significant interest because it is part of the mineralogical basis of LFP battery chemistry.
Although it is not directly synthesized from natural triphylite at present, we note that LFP (LiFePO4) is a type of rechargeable lithium ion battery that utilizes lithium iron phosphate as its cathode material [50,51,52,53,54,55]. Among all electrode materials, LiFePO4 has become one of the most extensively studied [45]. The solid solutions of the triphylite series (LiMPO4, where M = Fe, Mn, Co, Ni) have attracted the greatest interest from industrial applications [56]. The advantages of LiFePO4 are as follows:
- It has high resistance to aging (if discharged at a maximum of 80%);
- It has excellent thermal stability, with minimal degradation at high temperatures;
- It has a durability of 2000 to 10,000 complete charge–discharge cycles;
- It has stable voltage performance.
In summary, from an economic perspective, lithium phosphates and lithium silicates (such as spodumene, LiAlSi2O6) from Conțu-Negovanu pegmatites represent possible sources of lithium, a critical raw material, for electric vehicles and glass ceramics.
7. Conclusions
In this study, we identified the main lithium-bearing mineral from the triphylite–lithiophilite isomorphic series. Triphylite (LiFePO4) is a mineral that, if found in large quantities and with efficient benefication, might be used as a direct source for LFP batteries (or, at least, as a Li resource), along with other Li phosphates and spodumene.
Wolfeite and gatehouseite (in particular, Fe-rich gatehouseite) are secondary minerals formed due to alteration of primary triphylite.
We can say with certainty that the minerals presented in this paper exist because they wereidentified by at least three methods of analysis and calculated from EMPA. Many chemical formulas included components that were not analyzed but calculated (e.g., Li, H2O). The calculations still yielded strong evidence for the presence of the mineral, but it is not unequivocal proof. Given this context, the Conțu-Negovanu pegmatites could represent a domestic source of lithium in Romania. The presence of triphylite (LiFePO4) and spodumene (LiAlSi2O6) suggests that these pegmatites may hold economic potential for future lithium extraction. We mentioned that, in the past, lithium was extracted from spodumene in an experimental project in this area. The isomorphic series triphylite–lithiophilite and spodumene, taken together, may be upgraded from a lithium occurrence to a lithium ore deposit.
This study establishes a foundation for future mineralogical and geochemical research into lithium-bearing pegmatites in Romania. The results indicate that the Conțu-Negovanu occurrence holds potential not only for scientific investigation, but also for identifying possible domestic lithium sources.
Author Contributions
Conceptualization N.C. and C.C.; method, formal analysis and writing original draft, N.C.; writing-proofreading and review, C.C. and V.C.; data analysis, data curation, and cartography, D.P.; supervision and funding acquisition, V.C.; V.P. and D.P. provided assistance in the writing–original draft preparation and the verification and validation of the methodology. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this paper was partially funded by the Romanian Ministry of Research, Innovation, and Digitization through the Core Program (project PN 23-39-03-01–ProGeo-RO, contract 34N/2023).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank Ruhr University, Bochum, Germany, for the EPMA; and André-Mathieu Fransolet, Frédéric Hatert, and Maxime Baijot of the Laboratoire de Minéralogie et Cristallochimie Department de Geologie, Université de Liège, Liège, Belgium, for their help in carrying out optical microscope analyses, the acquisition and interpretation of chemical analyses, and providing financial support for chemical analyses. We thank Emil Constantinescu, Laurențiu Lucian Petrescu, Marin Șeclăman, and Gheorghe Udubașa from the University of Bucharest, Faculty of Geology and Geophysics, for their part in discussions on pegmatites. We also extend our gratitude to the Scientific Researchers grade I Delia-Georgeta Dumitraș and Ștefan Marincea from the Geological Institute of Romania for their role in discussions on the lithium-bearing pegmatites from Conțu-Negovanu.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pöllmann, H.; König, U. Monitoring of Lithium Contents in Lithium Ores and Concentrate–Assessment Using X-ray Diffraction (XRD). Minerals 2021, 11, 1058. [Google Scholar] [CrossRef]
- Săbău, G. Relationships Among Eclogite Bodies and Host Rocks in the Lotru Metamorphic Suite (South Carpathians, Romania): Petrological Evidence for Multistage Tectonic Emplacement. Int. Geol. Rev. 2003, 45, 225–262. [Google Scholar]
- Vácha, J.; Škoda, R. Harrisonite, A Rare Silicate-Phosphate From Granitic Pegmatites of the Bohemian Massif. Acta Musei Morav. Sci. Geol. 2022, 107, 21–32. [Google Scholar]
- Androne, D.A.-M. Geochemistry and Metallogenetic Potential of the Conțu–Negovanu Pegmatitic Field (Lotru-Cibin Mountains); Tehnopress Publishing House: Iasi, Romania, 2005; 259p. (In Romanian) [Google Scholar]
- Roda, E.; Fontan, F.; Pesquera, A.; Keller, P. The Fe–Mn phosphate associations from the Pinilla de Fermoselle pegmatite, Zamora, Spain: Occurrence of Kryzhanovskite and natrodufrénite. Eur. J. Miner. 1998, 10, 155–167. [Google Scholar]
- Kräutner, H.; Berza, T.; Dimitrescu, R. South Carpathians In: Precambrian in Younger Fold Belts; Zoubek, V., Ed.; Wiley & Sons: London, UK, 1988; pp. 633–664. [Google Scholar]
- Dallmeyer, R.D.; Neubauer, F.; Handler, R.; Fritz, H.; Müller, W.; Pană, D.; Putiš, M. Tectonothermal evolution of the unternal Alps and Carpathians: 40Ar/39Ar mineral and whole rock data. Eclogae Geol. Helv. 1996, 89, 203–277. [Google Scholar]
- Androne, D.A.-M.; Juvale, D.; Kasper, H.U. Preliminary data on some phosphates from Conțu–Negovanu pegmatites (Lotru–Cibin Mts.). Rom. J. Miner. Depos. 2006, 82, 161–166. [Google Scholar]
- Androne, D.A.-M.; Buzgar, N.; Dorohoi, D.-O.; Kasper, H.U. Complex Investigation Data from Granitic pegmatites. Rev. Chim. 2009, 60, 356–359. [Google Scholar]
- Călin, N.; Constantinescu, E. Mineralogy of Li–Pegmatites from the Conțu Basin, Cindrel Mountains, Romania. Ph.D. Thesis, Faculty of Geology and Geophysics, Bucharest, Romania, 20 September 2012; 206p. (In Romanian). [Google Scholar]
- Călin, N.; Fransolet, A.-M.; Baijot, M.; Marincea, Ș.; Dumitraș, D.-G.; Hatert, F.; Anason, A.-M.; Iancu, A.-M. A possible new mineral species, ‘ferrogatehouseite’ (Fe,Mn)5(PO4)2(OH)4 from Conțu Pegmatite, Romania. In Proceedings of the 21st General Meeting of IMA, Johannesburg, South Africa, 1–5 September 2014. [Google Scholar] [CrossRef]
- Călin, N.; Constantina, C.; Bălășcuță, E.R. Spodumene and beryl from Conțu lithium pegmatite, Cindrel (Cibin) Mountains, Central Group, South Carpathians, Romania. Carpathians J. Earth Environ. Sci. 2023, 18, 183–194. [Google Scholar] [CrossRef]
- Călin, N.; Constantina, C.; Ciobotea-Barbu, O.-C.; Iancu, A.-M.; Ion, A.-M.; Dumitraș, D.-G.; Perșa, D.; Marincea, Ș.; Rădoi, O.-G.; Anason, M.A. Mineral with Economic Potential, present in the Pegmatites from Conțu, Cindrel Mountains, (Romania). In Selected Studies in Geomorphology, Sedimentology, and Geochemistry, Proceedings of the CAJG 2020; Advances in Science, Technology & Innovation; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Hann, H.P. Pegmatites from the Southern Carpathians; Publishing House of the Academy of the Socialist Republic of Romania: Bucharest, Romania, 1987; p. 141. (In Romanian) [Google Scholar]
- Săbău, G.; Bindea, G.; Hann, H.P.; Ricman, C.; Pană, D.I. The metamorphic evolution of the low pressure terrain in the central south carpathians (Getic Nappe). Geol. Zb.—Geol. Carpathica 1987, 38, 735–754. [Google Scholar]
- Murariu, T.; Răileanu, M.; Calcan, C.D. The geochemical role of the alkalinity of crystallization environment in the genesis of pegmatites from the Carpathian province, Romania. Analele Științifice Univ. Al Cuza din Iași. Sect. 2 Geologie 2008, 54, 39–45. [Google Scholar]
- Diaconu, F.; Ghețaru, A.; Vulpescu, D. The Geological Structure of the Getic Crystalline Formations Is the Genesis of the Pegmatites in the NW Part of the Lotru Mountains and the Southern Part of the Cibin Mountains. In The Petrology of Metamorphic Rocks; Dări de Semă ale Ședințelor Vol. LXII; Institutul Geologic al României: Bucharest, Romania, 1976; pp. 215–232. (In Romanian) [Google Scholar]
- Cameron, E.N.; Jahns, H.; McNoir, A.H.; Page, L.R. Internal Structure of Granitic Pegmatites; Economic Geology Monograph Series; Society of Economic Geologists, Economic Geology Publishing Co.: Urbana, IL, USA, 1949; Volume 2. [Google Scholar]
- Černý, P. Rare-element Granitic Pegmatites. Part 1: Anatomy and Internal Evolution of Pegmatite Deposits. Geosci. Can. 1991, 18, 49–67. Available online: https://journals.lib.unb.ca/index.php/GC/article/view/3722 (accessed on 28 May 2025).
- Černý, P.; Ercit, T.S. The Classification of Granitic Pegmatites Revisited. Can. Mineral. 2005, 43, 2005–2006. [Google Scholar] [CrossRef]
- Lyalina, L.M.; Selivanova, E.A.; Hatert, F. Nomenclature of the triphylite group of minerals. Eur. J. Miner. 2023, 35, 427–437. [Google Scholar] [CrossRef]
- Săbău, G.; Apostoloiu, A.; Urcan, T. Mineral assemblages within the lithium pegmatites at Conțu, Lotru Mts (Central South Carpathians) and their genetical bearing. Mineral. Petrol. Geochim. 1989, 74, 251–262. [Google Scholar]
- Losey, A.; Rakovan, J.; Huges, J.M.; Francis, C.A.; Dyar, M.D. Structural variation in the lithiophilite–triphylite series and other olivine group structures. Can. Miner. 2004, 42, 1105–1115. [Google Scholar]
- Kmječ, T.; Kohout, J.; Dopita, M.; Veverka, M.; Kuriplach, J. Mössbauer Spectroscopy of triphylite (LiFePO4) et Low Temperatures. Condens. Matter 2019, 4, 86. [Google Scholar] [CrossRef]
- Nazarov, E.E.; Dembitskiy, A.D.; Trussov, I.A.; Tyablikov, O.A.; Glazkova, I.S.; Alexey, S.V.; Presniakov, I.A.; Mikheev, I.V.; Morozov, A.V.; Nikitina, V.A.; et al. A Li-rich strategy towards advanced Mn-doped triphylite cathodes for Li-ion batteries. Energy Adv. 2023, 2, 328–337. [Google Scholar] [CrossRef]
- Antenucci, D.; Fontan, F.; Fransolet, A.-M. X-ray Powder Diffraction Data for Wolfeite: (Fe0.59Mn0.40Mg0.01)2PO4(OH). Powder Diffr. 1989, 4, 34–35. [Google Scholar] [CrossRef]
- Hatert, F. FeII2(PO4)(OH), a synthetic analogue of wolfeite. Acta Crystallogr. 2007, 63, 119–121. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Santos, L.A.; Ribeiro, J.; Coelho, P.; Pedro, A.M.G. Influence of Environmental Condition on the Behaviour of Tailings from Tungsten Mining for Sustainable Geotechnical Application and Storage. Sustainability 2024, 16, 10987. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Forming Minerals, 3rd ed.; Berforts Information Press: Hertfordshire, UK, 2013; ISBN 978-0903056-33-5.549. [Google Scholar] [CrossRef]
- Karanović, L.; Dordović, T. The crystal Structure and Crystal Chemistry of Mineral–like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclastite and Gatehouseite. Minerals 2022, 12, 1601. [Google Scholar] [CrossRef]
- Pring, A.; Birch, W.D. Gatehouseite, a new manganese hydroxyl phosphate from Iron Monarch, South Australia. Miner. Mag. 1993, 57, 309–313. [Google Scholar]
- Droop, G.T.R. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef]
- Fan, Z.-W.; Xiong, Y.-Q.; Shao, Y.-J.; Wen, C.-H. Textural and chemical characteristics of beryl from the Baishawo Be-Li-Nb-Ta pegmatite deposit, Jiangnan Orogen: Implication for rare metal pegmatite genesis. Ore Geol. Rev. 2022, 149, 105094. [Google Scholar] [CrossRef]
- Wu, B.; Bonnetti, C.; Liu, Y.; Zhang, Z.-S.; Guo, G.-L.; Li, G.-L.; Hu, Y.-Q.; Yan, Z.-Y. Uraninite from the Guangshigou Pegmatite- Type Uranium Deposit in the North Qinling Orogen, Central China: Its Occurrence, Alteration and Implications for Post-caledonian Uranium Circulation. Minerals 2021, 11, 729. [Google Scholar] [CrossRef]
- Pohjolainen, E. Uranium deposits of Finland. In Mineral Deposits of Finland; Elsevier: Amsterdam, The Netherlands, 2015; pp. 781–792. [Google Scholar]
- London, D.; Kontak, D.J. Granitic Pegmatites: Scientific Wonders and Economic Bonanzas. Elements 2012, 8, 257–261. [Google Scholar]
- Nazarov, E.E.; Aksyonov, D.A.; Antipov, E.V.; Fedotov, S.S. A Comprenhensive Review of Defects Genealogy in Olivines: From the Mineral World to Modern Electrode Materials. Energies 2023, 16, 5083. [Google Scholar] [CrossRef]
- Fransolet, A.-M.; Antenucci, D.; Speetjens, J.-M.; Tarte, P. An X-ray determinative method for the divalent cation ratio in the triphylite-lithiophilite series. Miner. Mag. 1984, 48, 373–381. [Google Scholar]
- Fransolet, A.-M.; Abraham, K.; Speetjens, J.-M. Evolution génétique et signification des associations de phosphates de la pegmatite d, Angarf-Sud, plaine de Tazenakht Anti-Atlas, Maroc. Bull. Miner. 1985, 108, 551–574. [Google Scholar]
- Fransolet, A.-M.; Keller, P.; Fontan, F. The phosphate mineral associations of the Tsaobismund pegmatite, Namibia. Contrib. Mineral. Petrol. 1986, 92, 502–517. [Google Scholar]
- Fransolet, A.-M. L’huréaulite: Ses propriétés minéralogiques et son rôle dans l’évolution génétique des phases Li(Fe,Mn)PO4. Bull. Soc. Fr. Minéral. Cristallogr. 1976, 99, 261–273. [Google Scholar]
- Baijot, M.; Hatert, F.; Philippo, S.; Cassedanne, J.; Fransolet, A.-M. Mineralogy and Petrography of phosphate minerals from Sapucaia and Boca Rica Pegmatites, Minas Gerais, Brazil. Estud. Geol. 2009, 19, 47–51. [Google Scholar]
- Fransolet, A.-M. The eosphorite-childrenite series associated with the Li-Mn-Fe phosphate minerals from the Buranga pegmatite, Rwanda. Miner. Mag. 1980, 43, 1015–1023. [Google Scholar]
- Baijot, M.; Hatert, F.; Philippo, S. Mineralogy and Geochemistry of Phosphates and Silicates in the Sapucaia Pegmatite, Minas Gerais, Brazil: Genetic Implications. Can. Miner. 2012, 50, 1531–1554. [Google Scholar] [CrossRef]
- Melgarejo, J.C.; Martin, R.F. Atlas of Non-Silicate Minerals in Thin Section. Can. Miner. 2011, 7, 522. [Google Scholar]
- Maieru, O.; Superceanu, C.; Apostoloiu, A. Neue Spodumen und Beryllpegmatite im Mittleren Südkarpatischen Schiefergebirge (Rumänien), Geologie Jahrgang 17; Akademie Verlag: Berlin, Germany, 1968; pp. 388–397. [Google Scholar]
- Quensel, P. The Paragenesis of the Varuträsk Pegmatite. Geol. Mag. 1952, 89, 49–60. [Google Scholar] [CrossRef]
- Mason, B. Minerals of the Varuträsk pegmatite. XXIII. Some iron–manganese phosphate minerals and their alteration products, with special reference to material from Varuträsk. Geol. Fören. Förhandl. 1941, 63, 117–175. [Google Scholar]
- Lu, Y.; Zhu, T. Status and prospects of lithium iron phosphate manufacturing in the lithium battery industry. MRS Commun. 2024, 14, 888–899. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Gong, R.; Li, C.; Meng, Q.; Dong, P.; Zhang, Y.; Zhang, B.; Yan, J.; Li, Y. A sustainable closed-loop method of selective oxidation leaching and regeneration for lithium iron phosphate catode materials from spent batteries. J. Environ. Manag. 2022, 319, 115740. [Google Scholar] [CrossRef]
- Yakubovich, O.; Khasanova, N.; Antipov, E. Review Mineral–Inspired Materials: Synthetic Phosphate Analogues for Battery Applications. Minerals 2020, 10, 254. [Google Scholar] [CrossRef]
- Zhao, T.; Mahandra, H.; Marthi, R.; Ji, X.; Zhao, W.; Chae, S.; Traversy, M.; Li, W.; Yu, F.; Choi, Y.; et al. An overview on the life cycle of lithium iron phosphate: Synthesis, modification, application, and recycling. Chem. Eng. J. 2024, 485, 149923. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, Y.; Liu, C. Annual operating characteristics analysis of photovoltaic-energy storage microgrid based on retired lithium iron phosphate batteries. J. Energy Storage 2022, 45, 103769. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, T.-R.; Yougbaré, S.; Lin, L.-Y. Design of LiFePO4 and porous carbon composites with excellent High-Rate charging performance for Lithium-Ion secondary battery. J. Colloid Interface Sci. 2022, 607 Pt 2, 1457–1465. [Google Scholar] [CrossRef]
- Nazarov, E.E.; Tyablikov, O.A.; Nikitina, V.A.; Antipov, E.V.; Fedotov, S.S. Polyacrylonitrile-Derived Carbon Nanocoating for Long-Life High-Power Phosphate Electrodes. Appl. Nano 2023, 4, 25–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).