Abstract
Spodumene-bearing pegmatites are geochemically anomalous among crystalline rocks and important critical mineral resources in the green energy transition. However, prospecting is challenging due to their small size and the fact that they are often covered by soil and vegetation. This study demonstrates that, rather than being a hindrance, soil cover can enhance geochemical exploration, at least at the prospect scale. This study examines the dispersion pathways of lithium (Li) and its pathfinder elements (Rb, B, Ga, and Sn) from pegmatites (<10 m thick) into metamorphic host rocks and further into overlying undisturbed soils in heavily forested, postglaciated terrain of northeastern Wisconsin, USA. Soil-sample traverses over the world-renowned, lepidolite-type Animikie Red Ace pegmatite and two nearby dikes reveal pronounced <20 m anomalies with up to 1400 ppm of Li, 450 ppm of Rb, 3100 ppm of B, 40 ppm of Ga, and 60 ppm of Sn, greatly exceeding the control soil concentrations from nonmineralized granite and pegmatites. Soils mirror both the magmatic fractionation and alteration of pegmatite bedrock and metasomatic halos in parent host rocks. Metasomatized amphibolite revealed the presence of a holmquistite-ferro-holmquistite mineral. This greenfield pilot exploration led to lithium-rich pegmatite discoveries within the district and demonstrates the applicability of proximal sensors for soil exploration in Wisconsin and beyond.
1. Introduction
Lithium (Li) and other valuable commodities critical to the green energy transition and advanced technologies are largely sourced from “group 1” pegmatites in the recent classification by Wise et al. [1] of lithium–cesium–tantalum (LCT) affiliation [2,3,4]. LCT pegmatites currently account for nearly half of global Li production [5,6]. To meet increasing demand, the continued exploration and discovery of new deposits are required. While soil geochemistry is a well-established exploration method for many mineral resources, its potential in targeting Li-rich pegmatites remains underutilized [7,8,9,10,11]. Moreover, although the use of handheld analytical instruments such as portable X-ray fluorescence (pXRF) and laser-induced breakdown spectroscopy (pLIBS) is growing rapidly in the exploration and extractive industries, their application to identifying lithogeochemical anomalies and soil signatures associated with LCT pegmatite has only recently begun to be documented [12,13,14,15,16,17].
In this study, rock and soil samples collected from the mineralized Florence County pegmatite (FCP) field in northeastern Wisconsin [18,19,20] are compared to control samples from the barren Dickinson County pegmatite field (DCP), located ~30 km northeast of the FCP field, in Michigan’s Upper Peninsula. Random forest, support vector machine, and neural network predictive algorithms applied to the concentration of Li and a suite of 12 other major and trace elements [21] on the same set of 112 soil samples collected from the FCP and DCP demonstrated that Li concentrations and bedrock type can be accurately predicted based solely on the pXRF analysis of bulk soil geochemistry. A coefficient of determination (R2) of 0.90 and an RMSE of 40 ppm were achieved for Li prediction using neural networks, while an accuracy of 0.88 was obtained for bedrock-type prediction using a random forest model [21]. These results are encouraging for the future use of soil geochemistry in lithium exploration.
Although geophysics and remote sensing are increasingly used to identify pegmatite bodies at the province or district scale, these methods are challenging to apply in heavily forested terrains [11]. Moreover, distinguishing between barren and mineralized pegmatites (e.g., spodumene-bearing) still requires boots-on-the-ground methods. At the prospect scale, exploration in these terrains typically involves costly and invasive methods: constructing access roads for heavy equipment, clearing vegetation (e.g., cutting down trees), bulldozing soil cover to expose shallow bedrock, and trenching pegmatite bodies indiscriminately for whole-rock analyses, often before the mineralization potential is even assessed. In contrast, early-stage soil prospecting would offer a minimally disruptive, inexpensive alternative, particularly if soil geochemistry can be analyzed directly in the field using portable equipment [11,21].
To complement the pXRF-based soil study of Pierangeli et al. [21], we investigate the spatial distribution of LCT geochemical anomalies along soil and rock sample traverses placed across known pegmatite dikes. This prospect-scale study focuses on the transfer of Li and associated pathfinder elements: (1) from the pegmatite body to the host rocks during emplacement and cooling, and (2) into the bedrock-overlying soil during weathering and pedogenesis. Pathfinder elements related to lithium Li-pegmatites include Li, Rb, Cs, Be, B, P, S, F, Ga, As, Ta, Sn, W, and Tl [12,22,23,24,25,26,27]. A better understanding of the mechanisms that form dispersion halos during the magmatic–hydrothermal and weathering stages may validate the future use of handheld techniques (pXRF and pLIBS) to quantify Li and associated LCT-pathfinder elements in soils. This would enable the faster, less invasive early-stage exploration of concealed pegmatite bodies, aligned with the growing demand for more sustainable exploration and extraction practices [25,26,28].
2. Pegmatite-Related Geochemical Dispersion Halos
2.1. Magmatic–Hydrothermal Metasomatic Halos
Granitic pegmatites form as intrusive bodies either through the partial melting of crustal rocks or as fractionation products of larger granitic plutons [1]. However, few pegmatites are mineralized. Their prospectivity is linked to emplacement depth and magma undercooling [29,30]. Pegmatite fields highly prospective for critical minerals are emplaced at shallow crustal levels, at or above the ductile-to-brittle transition, where they experience significant temperature contrasts with the host rocks, typically metamorphosed in the upper-greenschist to lower amphibolite facies [2,31,32]. These temperature contrasts promote disequilibrium crystallization and fractionation due to undercooling, ultimately facilitating rare-element ore formation [30,32].
It is widely accepted that granitic-pegmatite systems are enriched in fluxing components, especially water [32,33,34]. Regardless of when fluid saturation occurs, hydrothermal fluids, along with their dissolved components—including those remobilized during the alteration of magmatic pegmatite minerals (e.g., [35])—tend to escape the intrusion and infiltrate the surrounding host rocks during and immediately after pegmatite solidification [36,37]. The resulting metasomatic (or exomorphic) halos serve as key geochemical indicators in rare-element pegmatite exploration [24,38,39]. The following literature review is presented below to document the connection between the host rock geochemical anomalies and those in the overlying soils, which has not been comprehensively addressed.
The interaction between pegmatitic fluids and metamorphic host rocks typically results in (1) a thin exocontact band (0.01–1 m) of completely metasomatized host rock adjacent to the pegmatite; (2) an outer, partially metasomatized exocontact zone, where metasomatic minerals occur in thin fractures or as disseminations within the well-preserved primary rock texture; and (3) a broader, cryptic, geochemical dispersion halo extending tens of meters from the pegmatite contact (e.g., [32,37,38], Table 1). For Li-rich pegmatites wider than 50 m emplaced in mafic host rocks, dispersion halos extending at ≥200 m have been documented [39]. In the distal zone, the sinks of Li and Li pathfinders are the original metamorphic minerals (e.g., hornblende, biotite, muscovite, staurolite, etc.) that were compositionally modified via interaction with the pegmatite fluids rather than newly formed metasomatic minerals [19,24].

Table 1.
Examples of metasomatic-mineral assemblages reported to occur in the dispersion halos of various pegmatites. Mineral abbreviations: Ab—albite, Aby—amblygonite, Bt—biotite, Ep—epidote, Hlm—holmquistite, Lph-Trp—lithiophilite-triphylite, Ms—muscovite, Pl—plagioclase, Ptl—petalite, Spd—spodumene, Ttn—titanite, Tur—Tourmaline, and Znw—zinnwaldite [40].
Beyond the distance from the pegmatite body and the chemistry of the pegmatitic fluids, the pathfinder-sink mineralogy is largely controlled by the host rock type (Table 1). Metasomatic tourmaline is reported in most studies, regardless of host rock composition, whereas biotite and holmquistite (a Li-rich ortho-amphibole) are reported in amphibolites (metagabbros or metabasalts) adjacent to relatively large LCT pegmatites (Table 1). Less frequently observed neo-formed phases include zinnwaldite (a Li-Fe-rich mica), epidote and epidote-group minerals, and titanite formed through the destabilization of metamorphic hornblende, plagioclase, and ilmenite, respectively, in mafic lithologies [36,43]. Other factors influencing mineralogy, geochemistry, and the extent of the dispersion halos include host rock structure and the composition, size, emplacement history of the pegmatite magma, and the stages of fluid exsolution [19,25,46].
2.2. Geochemical Dispersion Halos of Soils Related to Weathering and Pedogenesis
While soil geochemistry is increasingly recognized as a valuable exploration tool for LCT pegmatites [8,24,26,39,49], the processes governing the behavior and mobility of Li and its pathfinder elements in overlying soils during the weathering of pegmatites and their host rocks remain poorly understood. Beyond the influence of the parent material, factors such as geomorphology, climate, and vegetation also shape the degree of weathering, pedogenesis, and resulting soil geochemistry (e.g., [49,50]). The following literature review focuses on soil formation processes in postglacial landscapes with humid, temperate to subarctic continental climates, such as those found in northern Wisconsin and northern Michigan, USA.
Pegmatite prospecting in wet temperate to subarctic climates is particularly challenging due to the presence of soil, glacial till, and vegetation cover [11,26]. To our knowledge, only a few pegmatites in the Northern Hemisphere have been the focus in pedogenetic and soil geochemistry investigations. These include the Aclare pegmatite, Leinster pegmatite group, in southeast Ireland [51], and Herbb No. 2 pegmatite, Powhatan County, Virginia, USA [52]. Broader exploration programs incorporating soil geochemistry are more common, including reports on the Archean Dibs pegmatite [9,53] and the Wekusko Lake pegmatite field in the Paleoproterozoic Flin Flon–Snow Lake greenstone belt [54], Manitoba, Canada; as well as the Little Nahanni pegmatite field, Yukon Territory, Canada [55].
In soil formed directly on bedrock (residuum), conventional soil geochemistry has proven effective as an exploration tool, but primarily where the soil cover is relatively thin, typically less than a few meters thick. For instance, Luecke [51] identified apical anomalies of Rb, Sn, and other trace elements in the B horizon of 5–6 m thick soils overlying spodumene pegmatite hosted in granite and mica schist, using wet chemical analysis combined with energy-dispersive XRF on bulk soil samples. The study suggested that the broader dispersion of Li downslope, into soils formed on Li-poor host rocks, was due to the higher mobility of Li ions compared to larger alkalis, which were more effectively retained by clay minerals and Fe, Mn, and Al oxyhydroxides.
In soils developed on glacial sediments, nontraditional partial leaching techniques successfully revealed Li-rich deposits obscured beneath 10s to even 100s of meters of glacial overburden [7,9]. However, detecting low-ppb-level apical anomalies of Li, Cs, Rb, etc., requires ICP-MS instrumentation with very low detection limits. The upward migration of these pathfinder ions through thick overburden has been attributed to diffusion, capillarity, electrochemical forces, and redox gradients [7].
Pierangeli et al. [21] used machine learning to predict Li concentrations in soil and underlying parent material from northern Wisconsin, USA, based on p-XRF bulk geochemical data requiring minimal to no soil sample preparation. The study suggested that the geochemistry of thin soils formed directly on bedrock can serve as an exploration indicator for Li-rich pegmatite, allowing discrimination among metamorphic country rock, granite, non-mineralized pegmatites, and mineralized pegmatite. Furthermore, the multivariate statistical analysis revealed significant anomalies not only in soils above the mineralized pegmatites but also in those covering adjacent host rocks. However, even a thin (<1 m) layer of glaciofluvial sediment masked the Li and Li pathfinder signature of the underlying bedrock in the soil cover.
Several questions remain regarding the applicability of soil geochemistry for exploring Li-rich pegmatites in regions with wet, cold temperate climates. To what extent do regolith materials mirror the distribution and magnitude of the dispersion haloes around LCT pegmatites? What factors influence the extent and intensity of geochemical anomalies in overlying soils? What elements can portable analytical equipment (e.g., pXRF) detect, and what are the limitations imposed by soil thickness? What are the potential hosts of Li and its soluble or insoluble pathfinder elements? How do weathering-resistant detrital particles contribute to the overall geochemical signature of the regolith? To address some of these gaps, this pilot study integrates whole-rock geochemistry and mineralogy with soil geochemistry to investigate the release and dispersion pathways of Li and its pathfinder elements from mineralized bodies to host rocks and weathering products.
3. Geological Setting
The Florence County pegmatites (FCPs) include several highly fractionated LCT pegmatites that have been relatively well studied but never explored systematically or mined [18,19,20,56]. The pegmatites form ~2–3 m thick, subparallel/branching dikes discontinuously exposed in a heavily wooded, hummocky terrain in northeastern Wisconsin [21]. Four mineralized (spodumene-bearing) pegmatites were included in this study: the Animikie Red Ace (ARA), King’s-X (KX), King’s-X2 (KX2), and Price Lake (PL) (Figure 1). Control samples were collected from the unmineralized Dickinson County pegmatite (DCP) field, located in northern Michigan, within ~30 km of FCP. Although, as detailed below, they belong to two different tectonic provinces separated by a major tectonic suture (Figure 1a), the two pegmatite groups intrude similar Paleoproterozoic metamorphic rocks, dominated by amphibolites. Because the two areas are relatively close to each other, they share similar geomorphology, forest cover, glacial history, and soil characteristics. The geologic and pedologic features for each study areas are summarized below.
3.1. The Florence County Pegmatites (FCPs)
The FCPs are located in northeastern Wisconsin, USA, in close proximity to the NW-SE trending Niagara fault zone, the major suture between the Superior Province and Wisconsin magmatic terranes accreted during the 1875–1835 Ma Penokean orogeny (Figure 1) [57]. The pegmatites occur as subparallel, thin, N to NW trending discordant to subconcordant dikes with a 45–60° westerly dip. They intrude the Paleoproterozoic metavolcanics and minor metasediments of the Quinnesec Formation within the Pembine-Wausau terrane [58,59,60,61]. The peak metamorphic conditions reached garnet-zone amphibolite facies in the area south of the Niagara fault zone, where the pegmatites are located [58]. The Bush Lake granite situated < 3 km southeast of the mineralized dikes is the potential parental intrusion for the FCPs based on its peraluminous biotite (±muscovite) composition; the external pegmatite–aplite dikes directly emanating from it; and the internal pegmatitic segregations with accessory Mn-rich garnet, tourmaline, and beryl and enrichments in Rb, Cs, and Li [60,62].
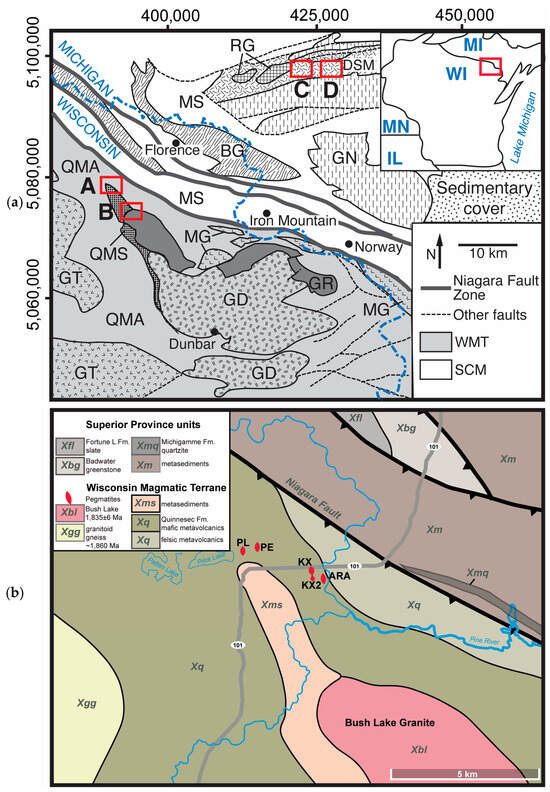
Figure 1.
Geologic sketches of studied areas: (a) Regional geology at the border between Wisconsin (WI) and Michigan (MI) shown in the upper left inset, after the studies of [57,59,60]. Wisconsin magmatic terrane (WMT) units: BL—Bush Lake granite, GR—other granite, DG—Dunbar gneiss, GT—undifferentiated granite and tonalite, MG—metagabbro, QMA—Quinnesec mafic amphibolite, and QMS—Quinnesec mica schist; Superior Craton Margin units: GN—gneiss; MS—Michigamme Schist; DSM—Dickinson Six-Mile amphibolite; RG—porphyritic red granite; BG—Badwater Greenstone. Red outlines are the sampling areas: A—Florence County pegmatite (FCP), B—Bush Lake granite (BL), C—Sturgeon River Quarry, and D—Six-Mile pegmatite. (b) Local geology of Florence County pegmatites after the studies of [59,60]: ARA—Animikie Red Ace, KX—King’s X, KX2—King’s X2, PL—Price Lake, and PE—Price Lake East, recently discovered, not included in this study.
Although not directly dated, the Bush Lake granite and associated aplite and pegmatite dikes have been considered the latest pulse of felsic magmatism in the area [60]. The FCP dikes seem to postdate the 1875–1835 Ma Penokean orogeny [57] because they crosscut the foliation of their metamorphic host rocks, lack pervasive deformation fabric or metamorphism, and are nearly perpendicular to the ca. 1860 Ma, south-dipping Niagara fault (Figure 1b) [20]. They clearly postdate the syntectonic, 1845 ± 7 My old, tonalitic Dunbar gneiss [61] and may postdate the 1835 ± 6 My old Spikehorn Creek granite, the youngest granitoid in the area [60,63,64,65]. The FCPs and the Bush Lake granite might also postdate the ca. 1785 My felsic magmatic event during the Yavapai accretion, based on the recent zircon-age compilation of Zi et al. [61]. An overprinting thermal event may have also affected the area, possibly related to the ~1630 Ma Baraboo orogeny [66,67] or the 1484–1468 Ma Wolf River Batholith [68].
The soil samples collected from the Bush Lake granite—the potential parent granite for the FCPs—are included here for comparison with the soils developed on the mineralized FCPs. An anatectic origin has also been proposed for FCP dikes [69,70]. However, without direct geochronology on the pegmatites and the Bush Lake granite, it is difficult to discern between granitic or direct anatectic origins at this time. Regardless, the origin of the pegmatite magmas does not directly influence the formation of the dispersion halos in their host rocks and soil cover, which is the focus of this study.
The literature describes the spodumene-bearing FCP dikes as <5 m in width and <600 m in length [18]. Their location within the Wild Pine-Popple River State Park has prohibited industrial exploration and mining in the FCP field. Our recent studies uncovered additional lithium-rich dike systems reaching <10 m in width within an ~10 km2 field area (Figure 1b) [21]. The most fractionated FCP is the lepidolite subtype Animikie Red Ace (ARA), best known for its striking rubellite tourmaline (pink elbaite var.) and the only rhodizite (K,Cs)Al4Be4(B,Be)12O28 occurrence in the United States [18]. The ARA was recognized during geological mapping focused on the early Proterozoic greenschist to amphibolite-facies metamorphic rocks of the Quinnesec Formation [58]. Further field characterization dedicated to ARA was reported [71,72], but the ARA mineralogy and geochemistry were not described systematically until Falster et al. [18]. Fluid and melt inclusions and apatite geochemistry suggested the rapid, undercooled crystallization of ARA and documented the occurrence of diffusion-controlled chemical boundary layers [20,48]. The unusual occurrence of Li-rich and exotic minerals in the outer zones of the pegmatite, the presence of columbite–tantalite minerals in all zones of the pegmatite, reversed compositional zoning of elbaite tourmaline, and other signs of reversed internal zoning [18] suggest that ARA melt might represent residual melt expulsed from a larger fractionating pegmatite body.
Liu et al. [19] proposed that the Li dispersion halo within the quartz-mica and hornblende schists in the ARA and KX contact aureoles expanded as far as 30 m on either side of the dikes and resulted in a significant diffusion-controlled Li isotope via fluid diffusion and advection. The heterogeneous permeability structure related to lithological differences and the orientation of pegmatite dikes relative to the foliation were found to largely influence the size and distribution of the anomalies. The Li concentration gradients in the dikes’ contact halos range from <500 ppm of Li in the schist at the contact with ARA and <350 ppm of Li in amphibolite in contact with KX, to the 10–40 ppm of Li characteristic of regional felsic schists and amphibolites [19]. However, the mineral hosts for Li and Li pathfinders in the contact halos remained understudied.
The preliminary mineralogy of King’s X2 (KX2), another highly differentiated, spodumene pegmatite dike, and its location and attitude in the field suggest that it is a possible extension of KX. Additional spodumene and petalite occurrences north of Price and Patten Lakes were mentioned, but their location remained unspecified [69,73]. Therefore, FCP is an understudied, unmined pegmatite field but with opportunities for further expansion, as new spodumene-bearing dikes have been recently discovered. Except for some outcrop disturbance at the ARA pegmatite caused by mineral collectors, the absence of mining and the protection offered by the natural wild river preserve have kept the overlying soils in a near-natural state, free of major disruptions. Therefore, this area is an optimal target for studying the release of Li and its pathfinders, as well as the formation of geochemical anomalies within the metamorphic host rocks and soil cover during weathering in a humid-temperate climate of glaciated terrains.
3.2. The Dickinson County Pegmatites (DCPs)
Control samples of rocks and soils were collected from the mineralogically simple to beryl-type Dickinson County pegmatites (DCPs), in Michigan (Figure 1a), to constrain the background geochemistry of soils associated with Li poor pegmatites. The DCP field is located within the southern edge of the Superior Craton, ~30 Km northeast of the FCP field and ~25 km north of the Niagara fault suture. Although more widespread than the FCP field [73,74,75], the mineralization potential of the DCP field remained underexplored. The pegmatites selected in this study intrude the Paleoproterozoic Dickinson Group rocks dominated by the Six-Mile Lake amphibolite and are likely associated with a nearby 2.1 By old Red Granite, which was dated via U-Pb zircon [76,77]. The pegmatite area is a mix of forests, public and private land, and small villages. It lacks significant mining activities, except for historic dimension stone and aggregate quarrying of the largest pegmatites, such as the Sturgeon River Quarry pegmatite (about 40 m in apparent depth and over 1000 m in length).
4. Soils Formed on Rock Outcrops
In the two study areas, soils belong mainly to two soil orders, spodosols (Orthod suborder) and alfisols (Udalf suborder) [78,79]. To better characterize the weathering products of local bedrock within the resolution limits of the available analytical equipment, soil formed on glacial till was intentionally avoided. Available soil surveys were used to selectively investigate areas classified as soils developed on crystalline rock outcrops (inceptisols) [80]. Soil mapping units (complexes) are delineated by topographic slopes. For soil classification maps, with sampling site locations and additional soil classification per U.S. Soil Taxonomy, readers are directed to Pierangeli et al. [21]. Typical soils are sandy to fine sandy loams, approximately 10–50 cm deep, and even shallower on and near outcrops. They consist of a mixture of mineral particles, small lithoclasts (<1 cm in size), small amounts of authigenic minerals (e.g., clays, oxides), organic matter, and potentially mobile/soluble elements temporarily adsorbed onto particle surfaces.
The formation of these soils is largely influenced by the current humid continental, mild summer, wet-all-year climate Köppen zone Dfb of northern Wisconsin and Michigan states [80,81,82] with broad temperature variations from summer to winter seasons. During the cold winter months, the chemical processes of soil formation are slow, and physical freezing and thawing processes weather the bedrock mechanically. In the warmer summer months (average air temperature in the hottest month < 22 °C and >10 °C for >4 months), the udic moisture regime (soils moist for at least 180 cumulative days per year) and thick forest vegetation leads to relatively large amounts of percolating water and organic material available for rapid soil development [78,82]. The inceptisols selected in this study developed directly on bedrock and were found in areas with steep slopes, ridges, and minimal glacial deposition. Glacial polish, striations, and grooves were commonly observed near the sampling sites or underneath soil/moss cover, consistent with the scouring of older saprolitic material and the typical absence of a C horizon (Supplementary Figure S1). The sampled O and A horizons (rarely B) are rich in organic matter and detrital particles but low in clay content.
The Florence County pegmatite field lies within the Pine-Popple River basin, a hummocky landscape shaped by glacial and postglacial processes during major Holocene climate shifts. Soil formation began around 11,700 years ago during Early Holocene warming and continued with podzolization as coniferous vegetation expanded during the Mid-Holocene (8200–4200 years ago). These shallow inceptisols have been affected by bioturbation and freeze–thaw cycles to the present day (cryoturbation) [83]. The study area relief is ~60 m (e.g., 445 m elevation at PL pegmatite vs. 385 m at the Pine River near ARA pegmatite), with similar variation (30–75 m) in the control areas. Postglacial hillslope processes initially modified the periglacial topography, but these slowed as mixed forests stabilized after 4200 years ago [83]. At slopes > 15%, additional soil processes likely include slopewash erosion, gelifluction-related creep, and tree throw [84]. Stream activity also played a role; for example, the flat area north of the KX pegmatite likely represents an ancient Pine River floodplain. Thus, KX soils do not reflect the underlying bedrock, despite local glacial polish on the outcrop [21].
A national-scale soil survey established baseline concentrations for a broad suite of chemical elements and the mineralogical composition in the A- and C-horizons across the conterminous United States [85]. This low-density study (one sample per 1600 Km2) provides a regional geochemical overview rather than site-specific details. Soils in the study area are reported to have remarkably low concentrations of Li and related elements (Be, Ga, Rb, Nb, etc.) typically enriched in fractionated pegmatites. These soils are predominantly quartz–feldspar-rich with low clay and carbonate content, indicating glacial till as the dominant parent material [85]. To our knowledge, our study, along with [21], represents the first published geochemical investigation of soils in this region pertinent to pegmatite exploration.
5. Materials and Methods
5.1. Sampling Strategies
In the FCP area, rock and soils were sampled at four LCT pegmatite outcrop locations: Animikie Red Ace (ARA), Kings-X2 (KX2), Kings-X (KX), and a newly discovered Price Lake (PL) pegmatite. The Bush Lake Granite (BL), the potential parental granite of FCPs, was also sampled. In the DCP area, two mineralogically simple pegmatites and their soils were sampled at the inactive Sturgeon River Quarry (SRQ) and Six-Mile Lake pegmatite (6-Mi). A total of 116 soil samples were collected from the surface horizons (O, A, rarely B), typically 30–40 cm in depth, from all localities along three types of point traverses (Figure 2, Figure 3, and Figure S1). See additional details in Figure S1.
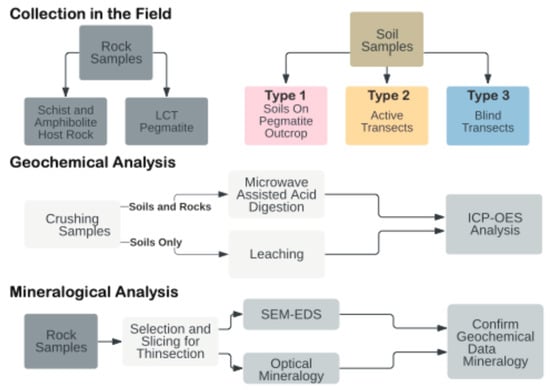
Figure 2.
Sample collection, preparation, and analysis.
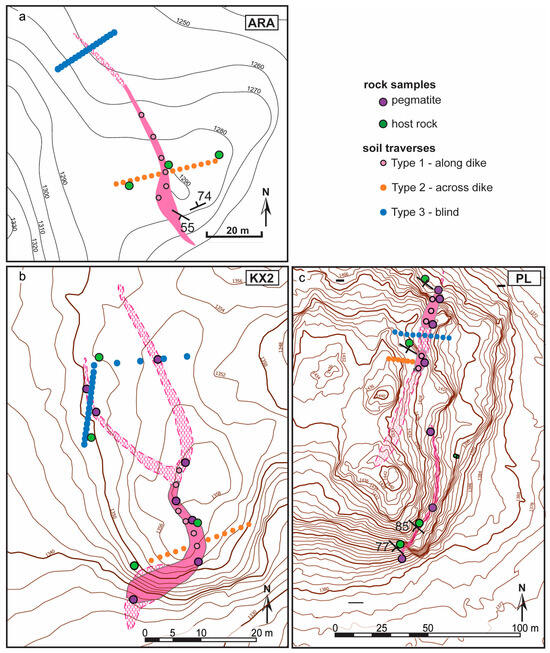
Figure 3.
Mapped sketches of Florence County pegmatite dikes with rock and soil sample locations included in this study: (a) Animikie Red Ace (ARA); (b) King’s X2 (KX2); and (c) Price Lake (PL). Pink areas: nearly continuous pegmatite outcrop. Pink hachured areas: hidden pegmatite, inferred from shallowly buried outcrop, boulder lineaments, and soil geochemistry. Surrounding metamorphic rocks left white. Topography: ESRI, Esri Community Map Contributors and other public domain sources.
- Type 1 soil samples—soils that formed directly on pegmatite outcrop, sampled along dike strike. Four to seven samples were collected for each pegmatite. In some cases, only shallow, <20 cm deep, and H horizon soils were available. The Type 1 samples were spaced 3–30 m apart depending on the length of the pegmatite outcrop and soil availability.
- Type 2 soil samples—collected along traverses cutting across the strike of outcropping dikes and extending in soil overlying the surrounding host rock. Up to 14 Type 2 samples per LCT pegmatite were collected. Representative nearby host rock samples were also sampled when available, at some of the soil sampling points.
- Type 3 soil samples—collected along ‘blind’ traverses cutting across hypothetical extensions of pegmatite dikes concealed under thicker soils. Eleven to twenty Type 3 samples were collected for each LCT pegmatite. The ‘blind’ traverses were located 10 to 20 m from the last pegmatite exposure (Figure 3).
For Type 2 and 3 transects, the distance between points varied from 1 to 4 m, depending on the soil availability, to a total length of approximately 7 to 40 m. The distance from the contact to the pegmatite was recorded. Soils that formed on top of glacial till or fluvial sediment were recognized by the presence of subangular-rounded pebbles of diverse lithologies (glacial till) and were avoided because they were not representative of local bedrock at all localities except for KX. Several samples of relatively fresh pegmatite and host rock samples were selected from each location for a total of 20 rock samples for comparative geochemical analysis between the bulk chemical signature of the parent material and the weathering products (Figure 3 and Figure 4) and to investigate the mineral carriers of Li and Li pathfinders. A few weathered pegmatite samples were also collected with care to preserve the loose alteration materials and any moss/lichen attached to weathered surfaces and fractured.
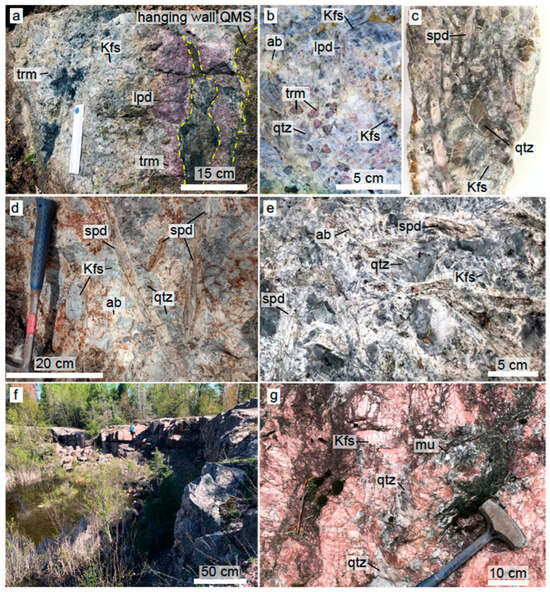
Figure 4.
(a) Animikie Red Ace (ARA) outcrop with pink elbaite tourmaline in its wall zone; dark blue tourmaline in its interior; and lepidolite mass surrounding metasomatized raft of host rock. Yellow dashed line: contact with quartz mica-schist (QMS) host rock and raft. (b) “Watermelon” elbaite tourmaline in the inner zone of ARA (c) slabbed of spodumene-rich, hydrothermally altered sample from ARA. (d) Price Lake (PL) slender, slightly brecciated spodumene rimmed by albite. (e) King’s X2 (KX2) spodumene-rich mineral assemblage. (f,g) Sturgeon River Quarry common pegmatite. Overview (f) and typical mineral assemblage (g). Mineral abbreviations: ab—albite, Kfs—K feldspar, lpd—lepidolite, mu—muscovite, qtz—quartz, spd—spodumene, and trm—tourmaline.
5.2. Sample Preparation
Soil aliquots were dried in an oven at ~65 °C, disintegrated using a mortar and pestle, and sieved to eliminate organic fragments >2 mm. A sample splitter was used to divide the sample into four equal aliquots. One aliquot was pulverized in a shatter box for complete homogenization. Soil leachates were obtained from the pulverized soil for understanding soluble element mobility in the soil profile. Leaching was performed in three steps: (1) 24 h shaking of 5 g soil with 20 g of distilled and deionized water (>18.6 MΩ), mixed in 50 mL centrifuge vials; (2) centrifuging for 30 min at 2000 RPM; and (3) filtering the decanted liquid by passing through 0.45 μm particle size nylon filter. The filtered solutions were stored at room temperature and acidified prior to Inductively Coupled Plasma—Optical Emission Spectroscopy (ICP-OES) (Figure 2).
After slicing in two duplicates and photographic documentation, one set of rock duplicates was pulverized and homogenized in a shattering ring mill for bulk chemical analysis. The other set of rock and mineral samples was cut into billets for polished sections 30 μm in thickness to be studied in transmitted light and scanning electron microscopy. A subset of rock samples was also prepared in thicker sections (200 μm). In total, 115 pulverized soils and 20 rocks were digested at 210 °C in an Ethos Milestone high-pressure, 12-vessel carousel microwave operated at 1800 W. To assess the digestion recoveries for various elements, NIST 2710a or OREAS 47a certified reference soils were included for each soil digestion batch, and OREAS 751 certified reference Li pegmatite was included with each rock digestion batch. Reagents added to pre-weighted 0.25 g of solid sample and Teflon vessels included 1 mL of hydrogen peroxide (H2O2), 3 mL of hydrofluoric acid (HF), 2 mL of hydrochloric acid (HCl), and 6 mL of nitric acid (HNO3). After the microwave-assisted digestion, the samples were recovered with 10 mL of nanopure H2O, poured into Savillex evaporation vials, and evaporated at 95 °C in 2–3 days to a viscous gel bead. The gel beads were then re-dissolved in 2% HNO3 and stored in 15 mL vials for ICP-OES analysis.
5.3. Petrography
Transmitted and reflected light microscopy was performed using an Olympus BX51. Photomicrographs were collected with a DP74 camera operated by Olympus Stream Essentials software, desktop version. Cathodoluminescence (CL) was performed on thin sections and mineral grains mounted in epoxy using an ELM-4 Luminoscope cold-cathode electron emission gun, (RELION, Bellefonte, PA, USA) attached to a petrographic microscope. Spodumene CL imaging was conducted at a vacuum pressure of ~90 mTorr, current of 1.49 mA, voltage ranging from 4 to 6 kV, and solenoid leak valve set to 1.2. Prior to scanning electron microscopy (SEM) analysis, microprobe-quality polished thin sections were gold-sputtered. Backscattered electron (BSE) imaging and energy-dispersive X-ray spectroscopy (SEM-EDS) were carried out on a Hitachi S-3400N II Scanning Electron Microscope (Hitachi High-Technologies Corporation, Tokyo, Japan) equipped with a 5-element backscattered electron detector and a Thermo-Noran System Seven energy-dispersive x-ray spectrometer with a silicon-drift detector. The BSE images were captured typically at a working distance of 10 mm, with an accelerating voltage of 15 kV, a probe current of 70 nA, and a 50 µm aperture. The SEM-EDS spectra were collected with an acquisition time of 60–120 s and used for mineral identification. To confirm the mineral holmquistite (orthorhombic Li amphibole), the unknowns were mounted alongside and compared to a reference holmquistite from the Big Whopper pegmatite, Separation Rapids, Ontario, Canada [46]. For further compositional confirmation of the holmquistite mineral species, standardless EDS compositions were used to calculate the ionic site assignments and to construct the amphibole formula following Locock et al. [86] (Supplementary Table S1). Applying this analytical strategy to the Big Whopper holmquistite (used here as a check standard) yielded a composition and classification that matched published values within analytical error [46].
5.4. Inductively Coupled Plasma—Optical Emission Spectroscopy (ICP-OES)
A ThermoScientific ICAP 7400 Inductively Coupled Plasma—Optical Emission Spectrometer (ICP-OES) was used to analyze the soil leachates, acid-digested bulk soils, and acid-digested bulk rocks. The analytes with concentrations consistently above detection limits in leachates and bulk solutions that had satisfactory post-digestion recoveries were B, Fe, Ga, K, Li, Mn, Na, Nb, P, Rb, Sn, and Ti. For example, Li had an average of 0.8 recovery for NIST 2710a (24.57 ppm). Digestion-related issues were assessed by calculating the recoveries of a soil standard (NIST 2710a) processed for each of the 12 digestion batches. Si, Al, Ca, Mg, Sr, and Ba had highly variable, low recoveries and are excluded from results.
6. Results
6.1. Field and Petrographic Characterization
As soil geochemistry is largely controlled by the parental rock, in this section, we describe the structures, textures, and mineralogy of pegmatites and their host rocks based on field, hand sample, and microscopic observations. To address the extent and pathways of Li and Li pathfinder mobility from pegmatite bodies to the surrounding host rocks during primary magmatic and secondary magmatic–hydrothermal processes, a special emphasis is placed here on the alteration and metasomatic mineral assemblages, within the pegmatite and at the contact between pegmatite and host rocks, both internal (endocontact) and external (exocontact) relative to the pegmatite body.
6.1.1. Pegmatites
The Animikie Red Ace (ARA) pegmatite system consists of a main dike, 3 m in width at its largest known exposure, and thinner offshoots [19,71] emplaced discordantly to sub-concordantly in a fine-grained quartz mica schist and amphibolite schist, belonging to the Paleoproterozoic Quinnesec Formation [65]. Although prior researchers reported a length of ~600 m [18], the ARA dike could be traced with certainty along strike for only ~100 m (see simplified sketch in Figure 3a). The main dike thins out to <0.5 m in width at both southern and northern ends before being completely covered by soil and probably pinching out. The dominant Li minerals are lepidolite occurring in decimeter-sized pods and replacement masses, comb-texture pink/blue elbaite tourmaline, occasionally showing reversed chemical zoning, in the border and wall zone, dark-blue tourmaline rosettes in the core and along late fractures (Figure 4a,b), and discontinuous spodumene clusters in the core, core margin, and, occasionally, in the hanging wall zone (Figure 4c).
The ARA spodumene forms randomly oriented to subparallel elongated crystals, commonly 1–15 cm long. Locally, spodumene makes up less than 20% of an assemblage that includes smoky quartz, gray K-feldspar, pink elbaite tourmaline, and lepidolite (Figure 4a–c). The albite–muscovite ± lepidolite replacement of spodumene (“cymatolitization”) is recognized by white, tan, or pink concentric bands around remnant or completely pseudomorphosed crystals (Figure 4c and Figure 5a,b). Other minerals found to replace spodumene include zinnwaldite, cookeite, and a clay mineral, tentatively identified as illite. In general, ARA spodumene is nearly obliterated by hydrothermal alteration, but relatively fresh prisms < 15 cm long are present in the southern segment of the ARA dike. Notably, weathering rinds > 1 cm thick cover boulder fragments broken by geologists/collectors within the past 50 years (Figure 4c), suggesting rapid weathering of the Li-rich assemblage.
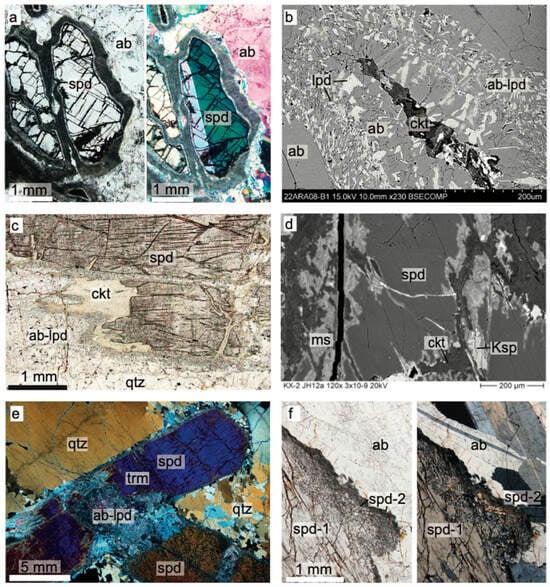
Figure 5.
Typical primary and secondary Li assemblages in FCPs in transmitted light photomicrographs and back-scatter electron images (b,d). (a) Animikie Red Ace (ARA) spodumene partially replaced by albite-mica rim (‘cymatolite). (b) ARA spodumene completely replaced by albite-lepidolite intergrowth and cookeite. (c,d) King’s X2 (KX2) spodumene replaced by albite, micas, K-feldspar, and cookeite along edges and fractures. (e) Price Lake (PL) deformed primary spodumene-quartz assemblage, (f) PL primary spodumene (spd-1) with dissolved and recrystallized margins (spd-2). Mineral abbreviations same as in Figure 4.
King’s-X2 (KX2) is a zoned, fractionated pegmatite of variable width ranging 0.5 to 4 m and variable attitude emplaced discordantly to subconcordantly in amphibolite. It crops out nearly continuously for around 35 m, but host rock and soil geochemical evidence developed in this study revealed that the main dike branches out and extends for an additional >25 m (Figure 3b). The contacts with the host rock are sharp, and large fragments of entrapped amphibolite xenoliths (rafts) are common. The mineral zones are discontinuous. The main minerals are blocky dark gray to black K-feldspar, quartz, coarse albite (cleavelandite), schorl tourmaline, with accessory muscovite, silvery to purple lepidolite mica, and white beryl. Spodumene and amblygonite-montebrasite are the main lithium minerals. The mineralization is very sporadic. Uncommon coarse tabular-bladed clusters with >50% spodumene, by volume, and amblygonite ovoid masses or crystals can be found along the footwall zone of the KX2. Although albitization is common, the main Li minerals, spodumene and amblygonite–montebrasite, are better preserved and fresher than at ARA (Figure 4e). Alteration minerals include albite–lepidolite intergrowths, muscovite, and cookeite (Figure 5c,d). Additionally, primary spodumene is commonly affected by brittle and plastic deformation, dissolution, and recrystallization (Figure 5e). Biochemical processes also affected spodumene and other silicates and enhanced the mechanical weathering rates (Figure S3). Kings-X (KX) pegmatite is a spodumene–amblygonite-bearing dike exposed in a Highway 101 roadcut. Mineralogically, it resembles KX2 and seems to be its northern extension; thus, we will not characterize it further (see also description in Liu et al. [19]).
The Price Lake (PL) pegmatite is a poorly zoned spodumene-bearing dike, with mineralization occurring sporadically along >100 m, partially covered length. At its widest spot, it exceeds >10 m in width. Similarly to better-studied ARA and KX2 pegmatites, PL encloses large fragments of amphibolite host rock and/or branches out in smaller veins (outline in Figure 3c is simplified). The dike has a simple granitic mineralogy with accessory bluish-gray tourmaline, white to purplish mica, and spodumene. Although it occurs only sporadically, slender spodumene crystals > 50 cm in length form radiating aggregates nucleating in the hanging wall zone of the dike (Figure 4d) [21]. Mineralized zones can reach ~10% spodumene. Compared to the other FCPs, PL appears to have been affected by widespread albitization and some brittle deformation. Locally, PL suffered minor faulting associated with brecciation and albitization. Coarse, primary PL spodumene (spd-1) is bounded by irregular dissolution and reprecipitation to spodumene—quartz ± albite symplectitic intergrowths and fine-grained secondary spodumene (spd-2, Figure 5f).
6.1.2. Host Rocks and Contacts Between Pegmatites and Host Rocks
The FCPs intrude the Quinnesec Formation, which consists mainly of mafic metavolcanic amphibolites and hornblende schists, depending on the hornblende-to-plagioclase and the presence of foliation. Locally, the ARA is also hosted by a foliated meta-porphyritic quartz-mica schist derived from a felsic to intermediate volcanic protolith. The host rocks were metamorphosed to upper-greenschist to amphibolite facies, as shown by garnet occurrence and absence of staurolite or other high-temperature indicators.
Background amphibolites consist of hornblende, typically as coarse poikiloblasts, and interstitial plagioclase, with variable amounts of quartz, calcite, and ilmenite. Minor and accessory minerals include biotite, chlorite, clinozoisite, garnet, and titanite. Typical quartz-mica schist hosting parts of the ARA pegmatite consist mainly of muscovite, quartz, and feldspars, with subordinate amounts of biotite, chlorite, epidote–clinozoisite, titanite, and ilmenite. The quartz-mica schist foliation is variable as a consequence of the variable abundance of micas. Quartz, plagioclase, and K-feldspar phenocrysts are preserved in some units.
Distinct mineral assemblages within 0 to 2 m from the contact with the FCP dikes were interpreted to have a metasomatic origin (Figure 6). The most common effect of metasomatism is tourmaline forming selvages (0.5–5 cm wide) in the pegmatite’s exocontacts in both mafic and felsic metavolcanic host rocks. The felsic host rock along the contacts with ARA is hardened by silicification and/or feldspathization and becomes non-foliated. In the endocontact of ARA, a typical border zone pegmatite assemblage includes tourmaline nucleating directly on the contact as fine-grained, acicular radiating blue aggregates, fine-grained ovoidal apatite, interstitial quartz, and patchy K feldspar-albite fine-grained intergrowths. Some of the blue tourmaline (Fe-rich) in the border zone coarsens and transitions to colorless or pink (Li-rich) elbaites of the wall zone, the hallmark feature of the ARA pegmatite. In the exocontact with the mica schist, the blue tourmaline transitions to brown-green (Mg-rich), which is often noticed to replace biotite. Besides tourmaline, other minerals in the exocontact include apatite, patchy alkali feldspar, and chlorite (Figure 6a,b) [20,48].
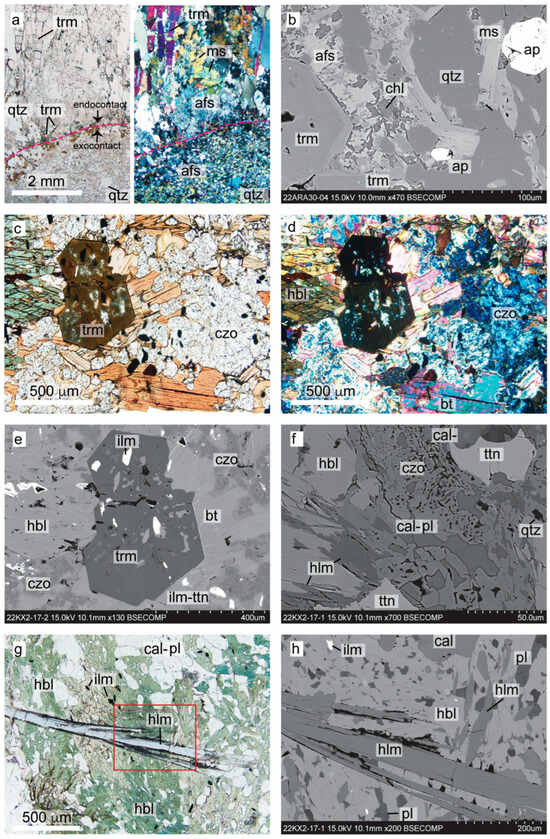
Figure 6.
Typical mineral assemblages at the contact between Florence County pegmatites and host rocks in transmitted light photomicrographs (a,c,d,g) and backscatter electron images (b,e,f,h): (a) Animikie Red Ace (ARA) endo- and exocontact contact with heavily metasomatized quartz mica schist host rock. (b) ARA exocontact mineral assemblage. (c–e) Typical metasomatic assemblage replacing the hornblende amphibolite host rock of King’s X2 (KX2). (f–h) Holmquistite-bearing assemblage replacing hornblende amphibolite, in the metasomatic halo of KX2; see also Figure S2. Mineral abbreviations as in Table 1.
Metasomatic biotite, clinozoisite–epidote, tourmaline, apatite, and titanite at the expense of ilmenite and remobilization of calcite are common occurrences in amphibolite enclaves or host rocks within <1 m from contact with pegmatites (Figure 6c–f). In addition, a holmquistite mineral species was provisionally documented in the exocontact of KX2, replacing hornblende in the assemblage with clinozoisite, calcite, and titanite (Figure 6f–h). The habit and optical properties (parallel extinction, the unique sky blue to violet to colorless pleochroism, birefringence, sign of elongation, 2V angle, etc.) confirmed that it is an orthorhombic amphibole, most likely holmquistite. The Fe contents (16.31 to 17.10 wt% FeO) exceeded the Fe of the reference holmquistite from the Big Whopper pegmatite (12.41 ± 0.6 wt% FeO), suggesting that the KX2 metasomatic mineral may be an Fe-rich member of the holmquistite-ferro-holmquistite solid solution [42]. Classification as possible ‘ferro-holmquistite’ was determined using a well-established amphibole-classification program [86], which constrained the unknowns (Li2O, H2O as OH−, and Fe3+/ΣFe) by optimizing the ionic site occupancies (Table S1). The same program was applied to the Big Whopper holmquistite concurrently analyzed with the KX2 sample. The reference material formula derived through this method was within analytical error. Rigorous classification would require additional quantification and was beyond the scope of this study.
6.2. Whole-Rock Geochemistry
Twenty rock samples were analyzed to
- Constrain the geochemistry of the parent materials including the mineralized FCP dikes, their host rocks, the parental BL granite, and barren DCP pegmatites;
- Better understand the extent of Li and Li pathfinder metasomatic halos;
- Further relate to the geochemistry of weathering products.
The concentrations of Li and potential Li pathfinders such as Rb, Sn, B, and Ga in the pegmatites are, in general, elevated compared to reference values (Figure 7). However, the contents in pegmatites are highly variable, due to the mineralogical heterogeneity and coarse grain size (Table S2). Noticeably, one spodumene-bearing sample from the newly discovered PL dike contained 23,864 ppm of Li and relatively high Sn and Ga contents of 126 and 101 ppm, respectively. For the ARA pegmatite, the whole-rock concentrations are compiled from the literature [18,19]. Samples from the Bush Lake granite and DCP common pegmatites are also plotted, for comparison (Figure 7).
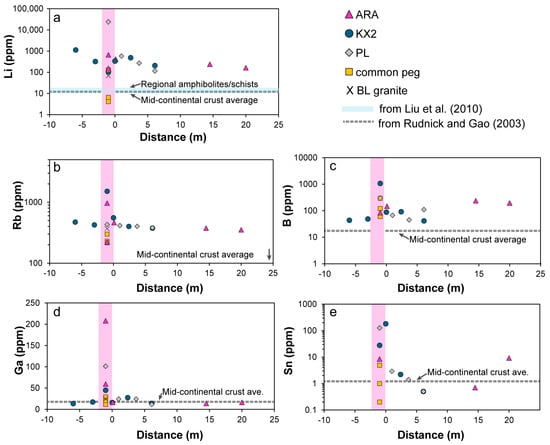
Figure 7.
Whole-rock trace elements (ppm) for pegmatites and their host rocks plotted against distance (in meters) from the contact with pegmatite: (a) lithium, (b) rubidium, (c) boron, (d) gallium, and (e) tin. Positive distance values represent westerly points, and negative values are easterly points. ARA pegmatite Li concentrations and other elements are from the studies of [18,19]. ARA host rocks and all other pegmatites, granite, and host rocks are determined in this study. Background concentrations are shown as a solid blue line for Li in the Quinnesec regional amphibolites/schists from the study of [19] and as a dashed gray line for the other elements, representing the mid-continental crust values from the study of [84].
In the FCP host rocks, the LCT pathfinders that best reveal significant metasomatic halos are Li, Rb, B, and Sn, with values generally decreasing with increasing distance from the contact but maintaining above-average mid-continental-crust concentrations (Figure 7) [87]. In contrast, Ga lacks a substantial dispersion halo in the host rocks, based on our limited number of rock samples (Figure 7d). Despite limited visible mineralogical changes in the host rocks (Section 6.1), Li concentrations remain above the regional amphibolite/schist background, consistent with results of around 30 m wide anomalies previously documented for KX and ARA dikes [19]. Notably, the holmquistite-bearing amphibolite sampled at 6 m west from the initially projected trend of the dike and ~30 m from the known KX2 outcrop contained a record Li value of 1134 ppm, suggesting the proximity to the buried pegmatite dike possibly branching out from the main dike. This hypothesis was confirmed during the following field campaign (Figure 3b). Similarly, the quartz-mica schist samples collected 14.5 and 20 m from the ARA main dike contained anomalously high concentrations of Li, B, and/or Sn, suggesting proximity to a hidden mineralized pegmatite, which could be confirmed by future studies.
6.3. Bulk Soil and Leachate Geochemistry
The bulk soil geochemical results represent the cumulative concentrations of fixed constituents of lithic/mineral fragments, authigenic minerals, and organic matter and mobile elements, temporarily absorbed on the particle surfaces. Conversely, soil leachate results, represent only the water-soluble elements released within 24 h during leaching experiments. By comparing the two signals, the chemical elements can be separated in a solid, fixed load and a water-soluble (transient) load.
The list of measured elements, which, in addition to Li, had high digestion recoveries and concentrations above detection limits in all soil samples, falls into two categories: elements that correlate positively with Li: Na, K, Rb, B, Sn, Ga, and P and those that correlate negatively with Li: Fe, Mn, and Ti [21]. However, Na, K, P, Mn, and Ti were excluded here because their distribution in soils fluctuated, possibly due to biological ± redox processes masking the relation to the bedrock (Table S3). Comparative boxplots showing bulk results of Type 1 soils formed on mineralized FCPs vs. control locations are in Figure 8. Data for all three FCP soil types are plotted against distance from contact (Figure 9) and on element-element binary plots (Figure 10). Leachate plots are in Figure 11.
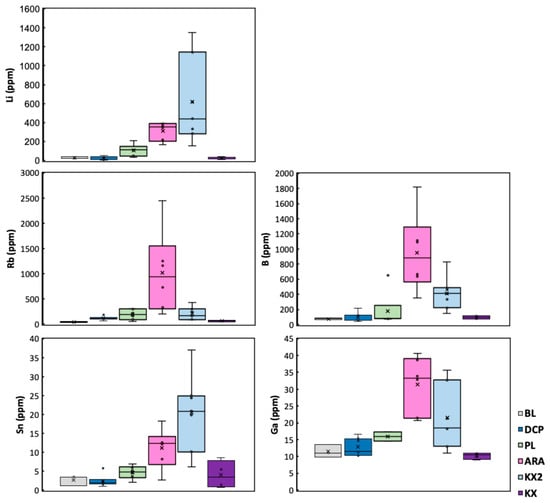
Figure 8.
Elemental concentrations in Type 1 soils overlying Florence County pegmatites (FCPs) and control locations (data in Table S3). BL—Bush Lake granite; DCPs—Dickinson County pegmatites are soils formed directly on the Sturgeon River Quarry and Six-Mile Lake common pegmatites; FCPs are PL—Price Lake, ARA—Animikie Red Ace, KX2—King’s X2, and KX—King’s X.
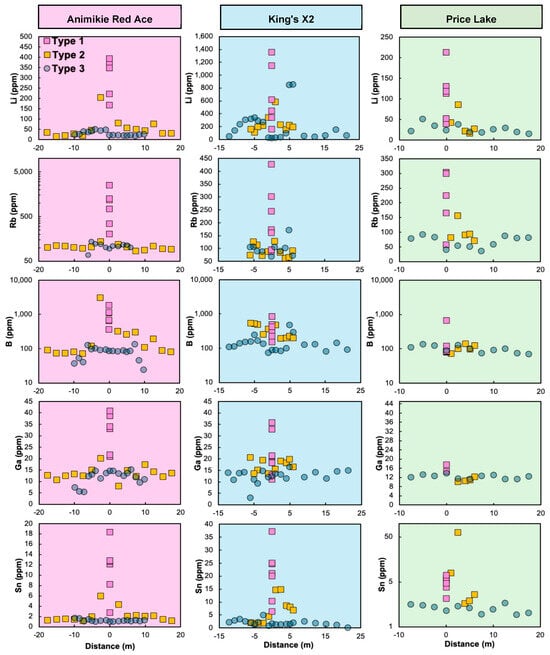
Figure 9.
Bulk soil concentrations (in ppm) for the Florence County pegmatites and their host rocks, plotted against distance (in meters) from the westerly contact with pegmatite. Positive distance values represent westerly points, and negative values are easterly points. The background color is pegmatite-specific: ARA—pink, left panel; KX2—blue, middle panel; and PL—green, right panel. Note the logarithmic vertical axis for Rb (ARA), B (all), and Sn (PL). Legend in top left panel: Type 1—soils on pegmatite outcrop, plotted at 0 m distance, regardless of the dike width. Type 2—soil traverses across exposed pegmatite. Type 3—soil samples along ‘blind’ traverses.
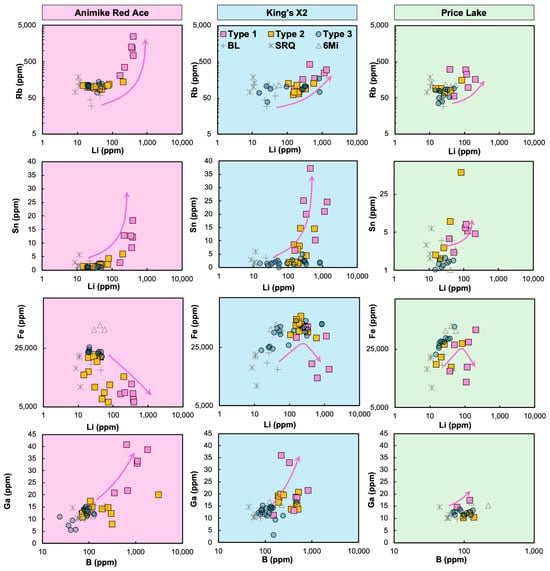
Figure 10.
Cross-plots of elemental concentrations (in ppm) in soils overlying Florence County pegmatites (FCPs) and their host rocks. Legend in top middle panel: FCP soil traverse types same as in Figure 9; control soils: BL—Bush Lake granite, SRQ—Sturgeon River Quarry pegmatite, and 6Mi—Six-Mile common pegmatite, Dickinson County, Michigan. See the logarithmic scale for Rb, Li, B, and Fe axes in all plots and for the Sn axis in the Sn vs. Li plot (PL only). Arrows: FCP soil enrichment patterns mirroring bedrock’s magmatic fractionation.
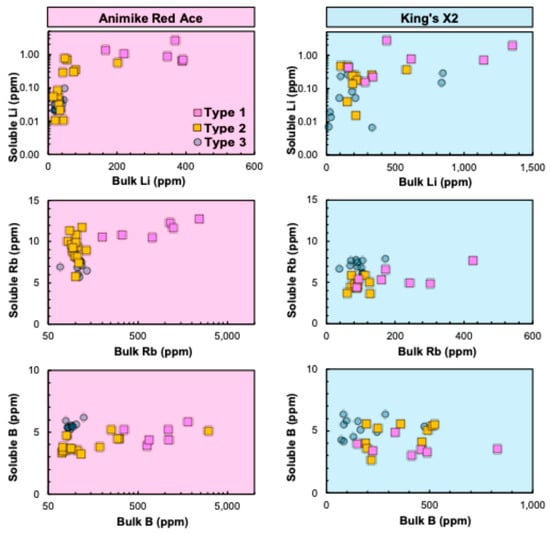
Figure 11.
Cross-plots of mobile vs. bulk elemental concentrations (in ppm) in soils overlying the Animikie Red Ace (ARA) and King’s X2 (KX2) pegmatites and their host rocks. Legend in top left panel: FCP soil types as described in Figure 9 and in text. See the logarithmic scale for soluble Li (both ARA and KX2 plots) and for bulk Rb and B (ARA plots only).
6.3.1. Bulk Soil Geochemistry
Type 2 geochemical traverses across exposed FCPs show Li, Rb, B, and Sn contents declining rapidly within ~5 m from the contacts with the dikes (Figure 9). As an exception, trace element contents along the 35 m long Type 2 profile at ARA (orange square symbols, left panel, Figure 9) reach a plateau at distances exceeding ~15 m. Although more spurious, Ga contents are generally more elevated in the soils formed on pegmatites and decline away from them, similar to the other trace elements. Concentrations along Type 3 (blind soil traverses) are generally more uniform and lower (e.g., Li < 50 ppm), with some notable anomalies. For instance, two prominent enrichments in Li, Rb, B, ±Ga were observed around ±5 m from the anticipated extension of the KX2 dike. One of these spikes correlated with the presence of a holmquistite-‘ferro-holmquistite’ mineral species in the nearby amphibolite outcrop (Figure 9). Two shallowly buried branches of the KX2 (Figure 4b) were validated in the following field campaign using this soil geochemistry information.
Despite general similarities among the geochemical traits of soils formed on the three FCPs, significant differences can be observed on concentration crossplots (Figure 10). Additionally, these plots were used to compare the soils formed on mineralized FCP outcrops vs. the control soils from common DCP pegmatites and parental BL granite and provide insights into the preservation of the bedrock geochemical signature, including its degree of magmatic fractionation and mineralization potential.
The cross-plots at ARA (Figure 10) illustrate positive correlations among Li, Rb, B, Sn, and Ga, along with a negative correlation between Li and Fe. A first-order observation is that soil geochemistry mirrors well the enrichment patterns related to magmatic fractionation from the parental granite to FCPs. Type 1 soils at all three FCPs show higher Li pathfinder levels than most Type 3 soils. In contrast, the Type 2 soils contain intermediate concentrations, depending on their distance to the pegmatite.
Additionally, Li pathfinder contents in Type 1 FCP soils are greater than in Type 1 control samples (BL granite and DCP common pegmatites). Upon closer examination, the Rb vs. Li trend has a steeper slope for Type 1 soils at ARA, with the highest Rb concentration of 2453 ppm corresponding to a Li concentration of 393 ppm and a shallower slope for KX2 Type 1 soils (e.g., 302 ppm of Rb corresponding to 1354 ppm of Li). The PL soils show the least enrichment (Li < 213 ppm, Rb < 300 ppm), with concentrations partly overlapping with the control soils. Iron is generally depleted in Type 1 soils, particularly at ARA, which is surrounded by felsic metavolcanics. Meanwhile, Type 1 soils at KX2 and PL, surrounded by mafic metavolcanics, are, in part, Fe-rich.
6.3.2. Leachate Geochemistry
Leachate analysis provides an assessment of the mobility of Li pathfinders (Table S4). In the leaching experiments, leachate concentrations represent the soluble fraction of a species released after 24 h in nanopure water, relative to the total (bulk) soil concentrations. In contrast, bulk soil concentrations obtained via microwave-assisted acid digestion encompass both the soluble and insoluble fractions of an element, including those contained within detrital or authigenetic mineral structures, bound to other weathering products (e.g., amorphous oxy-hydroxides), and/or absorbed onto organic matter.
The percentages of soluble pathfinders in a subset of 51 dry soil samples from ARA and KX2 are generally very low, but not insignificant. The percentage of soluble Li ranges from 0.002 to 5.29%, with an average of only 0.40%; soluble Rb ranges from 0.41 to 17.42% (on average 6.66%); and soluble B ranges from 0.16 to 7.63%, with an average of 3.18% (Table S4). The compositions of ARA and KX2 soil samples are differentiated by traverse type to describe the relative mobility of Li, Rb, and B (Figure 11). The concentration of soluble Li tends to correlate positively with the bulk contents and typically increases from distal Type 3 to proximal Type 1 at ARA. The behavior of soluble Rb at ARA is similar to Li behavior. In contrast, soluble Rb at KX2 in Type 3 distal soils is higher than for Types 1 and 2, even for lower or overlapping contents of bulk Rb. Moreover, the soluble and bulk B contents do not correlate at ARA and have a slight negative correlation at KX2.
7. Discussion
Soils can serve as effective exploration tools if their geochemical composition accurately reflects their parent materials. The following discussion evaluates the extent to which the LCT signature is transferred from pegmatites and their metasomatic halos to pegmatite-derived soils (Type 1) and host rock-derived soils (Types 2 and 3). The subsequent sections examine the formation mechanisms of metasomatic halos in host rocks and the dispersion halos in soils. The final section summarizes the effectiveness of soil geochemistry in Li pegmatite exploration.
7.1. Pegmatite-Derived (Type 1) Soil Geochemistry
Results herein show that, despite high variability, Li, Rb, B, Ga, and Sn concentrations in FCP Type 1 soils generally exceed those in control sites. Distinct differences among the three FCP soil datasets reflect variations in pegmatite fractionation and alteration, both important in Li exploration (Figure 8, Figure 9, Figure 10 and Figure 11). While all three dikes are spodumene-bearing LCT pegmatites, PL is the least fractionated, whereas ARA underwent the most extensive hydrothermal alteration, with spodumene nearly fully replaced (Figure 5c and Figure 6a,b).
The soil geochemical differences mirror the distinct dominant mineralogy in the pegmatites. King’s X2 soils reach the highest Li concentrations due to the abundance of fresh spodumene in KX2. In contrast, ARA soils have the highest B levels, attributed to the high elbaite tourmaline abundance, whereas lepidolite contributes to ARA soils, ranking second in Li levels. The high lepidolite and Rb-rich K-feldspar content at ARA [18] likely explains its elevated Ga and Rb concentrations in soils (Figure 8). These mineral enrichments reflect the degree of magmatic fractionation and drive the strong positive correlations among Li, Rb, B, Sn, and Ga and a negative correlation between Li and Fe. The elevated Fe in some of the Type 1 soils at KX2 and PL is a consequence of cross-contamination with detrital particles from surrounding mafic metavolcanic outcrops, as previously hypothesized [21] (Figure 10).
Distinct data trends of varying lengths and slopes, highlighted by arrows in Figure 10, also indicate the extent of fractionation from the parent granite and alteration. For example, at ARA, the steep Rb vs. Li trend likely results from the high abundance of blocky, Rb-rich feldspar, coupled with a lower abundance of spodumene than at KX2, due to Li loss into the host rock via hydrothermal alteration (Figure 10). In contrast, the PL pegmatite is less fractionated, at least in the sampled portion of the dike, resulting in the lowest pathfinder element concentrations (shortest arrows in Figure 10), partially overlapping with control site values (Figure 8). Finally, although the KX pegmatite is a highly fractionated spodumene–amblygonite type [19], its soils do not reflect the underlying pegmatite due to an intervening layer of glacio-fluvial sediment [21] and will not be further discussed (Figure 8).
7.2. Host Rock Halos and Types 2 and 3 Soil Anomalies
Although spatially limited, the mineralogical and geochemical halos observed in the felsic and mafic metavolcanic host rocks of the FCPs appear to directly impact the soil geochemical anomalies along Type 2 and 3 soil traverses. Therefore, the host rock mineralogical and geochemical data are examined first in the context of the relevant literature to obtain insights into the dispersion mechanisms and the mineral carriers of the pathfinders, before considering other pedogenetic factors that influence soil geochemistry.
7.2.1. Characteristics, Formation Mechanisms, and Variability of the FCP Halos
Based on the mineralogical and geochemical data collected in this study, the host rock adjacent to the mineralized FCPs can be divided into three main zones:
- A metasomatic alteration zone typically within <1 m from the pegmatite contact, characterized by abundant metasomatic minerals;
- A transitional zone, spanning 1–10 m from the contact, with disseminated metasomatic minerals;
- A cryptic geochemical halo, typically extending beyond 10 m from the contact.
The Li-Rb-B-Sn halo zones associated with the FCPs align with the proximal-to-distal subdivisions of lithogeochemical halos described in previous studies [39] (Table 1). In contrast to the Li-rich FCPs, common DCP pegmatites lack obvious metasomatic minerals in their exocontacts, and their overlying soil shows no enrichment in Li pathfinder elements (Figure 7).
Metasomatic halos around pegmatite dikes emplaced in the upper-crustal host rocks at 2–3.5 kbar are described as relatively small, commensurate with the typical dike thickness of only a few meters and the short-lived heating and fluid release [27,32,88]. For this study, this observation is validated by the conductive cooling model for the ARA dike using the Heat3D software, constrained by fluid inclusion entrapment temperatures [20,89], which predicted a rapid temperature drop from 720 °C to 410 °C within 10 cm of the host rock contact in just 14 days and complete cooling of the ~2.5 m dike to below 350 °C within ~60 days. Simultaneously, the host rock temperature rose from 220° to <400 °C, with heat and exsolved hydrothermal fluids propagating only a few meters away from the contact, unless discreet conduits such as foliation planes or fractures facilitated further fluid infiltration [19].
The three sampled FCPs show significant variability in the extent of their Li-B-Rb ± Sn litho- and pedo-geochemical dispersion halos (Figure 7, Figure 8, Figure 9 and Figure 10). The metasomatic halo differences between the three dikes arise from magmatic factors such as variations in magma sheet thickness, composition, and cooling dynamics, as well as from differences in host rock type [27,47]. The halos are also influenced by the local host rock lithology and relationships between the dike orientation and host rock foliation [19,39], as well as the timing of fluid exsolution and the intensity of auto-metasomatic alteration at the magmatic hydrothermal transition.
For instance, the extensive internal metasomatism at ARA, evidenced by the pervasive albite—lepidolite ± muscovite replacement of primary minerals (e.g., Figure 3h in [20]; Figure 5c) likely explains the broad Li, B, and Rb metasomatic halos extending beyond 25 m from the contact (Figure 7). In this scenario, the alteration of spodumene and elbaite tourmaline would release Li and B to the metasomatizing fluid, whereas the albitization of K-feldspar would release significant amounts of Rb. As the Li-B-Rb ± Sn-rich aqueous fluid further infiltrates and reacts with the host rock, it generates a metasomatic halo. This type of step-wise mass transfer and dispersion from the altered pegmatite to the host rocks was proposed for other mineralized pegmatite systems [24,35,39,45,90]. These broad host rock halos at ARA are mirrored by the geochemical anomalies of the Type 2 soil traverse (Figure 9), which extend more than 15 m from the ARA dike, at least on its east side.
In contrast, the wider KX2 and PL dikes contain relatively fresh, albeit sporadic, spodumene. While the host amphibolite could not be sampled beyond 6.1 m (Figure 7), leaving the full extent of the metasomatic aureole uncertain, the anomalous Li, B, Rb, ±Sn concentrations in the soil traverses tend to decline abruptly within 4 m from the contact, leveling off at baseline (blind traverse) levels beyond that point (Figure 9). Based on the available rock samples, the occurrence of post-deformational dravite-schorl tourmaline with up to 10.89 wt% B2O3 (stoichiometric) ± holmquistite with an average of 3.43 wt% Li2O (estimated) metasomatic assemblages at KX2 (Figure 6c–h; Table S1) suggests that the metasomatizing fluid carried enough B and Li to form intense anomalies but apparently across a shorter distance than at ARA. This might be due to the lower permeability of amphibolite at KX2 compared to mica schist at ARA, as pointed out by previous research [19] and the enhanced reactivity of hornblende, the dominant mineral in the amphibolite, as a source of Mg and Fe, essential for the tourmaline- and holmquistite-forming reactions.
7.2.2. Mineral Hosts of Li Pathfinder Elements
The lithogeochemical halos are hosted by 1) newly formed metasomatic minerals characteristic of proximal zones 1 and 2 (e.g., tourmaline, biotite, and holmquistite) and 2) pre-existing metamorphic mineral structures. In the latter case, Li pathfinders occur as solid-solution substituents (e.g., Li in hornblende) [19]. These minerals co-exist with the metasomatic minerals in proximal zones 1 and 2 but serve as the sole pathfinder-element traps in distal zone 3, where concentrations gradually decline to background levels.
Within the first 1–2 m from the FCP contact, metasomatic minerals include post-deformational tourmaline ± biotite ± holmquistite–titanite–clinozoisite assemblages (Figure 6), resembling proximal overprints described in previous studies (Table 1). Additionally, narrow (1–2 decimeters thick) zones of intense tourmalinization, biotitization, ± feldspathization along exocontacts and within enclaves provide compelling evidence of a direct link to pegmatite intrusions. The correlation between mineralogical changes and whole-rock enrichments in Li, B, Rb, and Sn further supports an LCT pegmatite metasomatic event rather than an unrelated overprinting event.
The presence of a holmquistite-series mineral in the amphibolite adjacent to KX2 is a significant discovery as it represents, to our knowledge, the first recorded occurrence in the midwestern United States and an important exploration indicator for Li pegmatites hosted by mafic to ultramafic host rocks [36,39,44,91]. The holmquistite at KX2 occurs exclusively as a partial replacement of hornblende. It is post-deformational, as it clearly crosscuts foliation (Figure 6f–h). The hornblende to holmquistite exchange reactions involve two main coupled substitutions [44]. For each Ca2+ on the M4 site in the hornblende structure that is replaced by Li+ from the aqueous fluid, either one Mg2+ from the M3, M2, or M1 sites is replaced by Al3+, or one Al3+ in tetrahedral coordination (T site) is replaced by Si4+:
Ca2+M4 Mg2+M3,M2,M1 = Li+M4 Al3+M3,M2,M1,
Ca2+M4 Al3+T = Li+M4 Si4+T
As part of the metasomatic holmquistite-producing mineral reactions, clinozoisite-epidote minerals intergrown with quartz are a product of plagioclase alteration. The breakdown of hornblende and plagioclase also produces calcite in the presence of CO2 or titanite at the expense of ilmenite (Figure 6c–h). These retrograde metamorphic reactions are caused by the interactions between the amphibolite-grade metamorphic rocks and the H2O-CO2 fluid released by the crystallizing pegmatite [20,44].
Although holmquistite from the FCP field is the only metasomatic mineral for which Li is an essential constituent and the main Li trap in KX2 amphibolites with >1000 ppm of Li, it is relatively rare. Instead, the likely most common Li hosts in amphibolites are newly formed and/or compositionally modified biotite and hornblende. In felsic metasedimentary lithologies, biotite, white micas, or chlorite are the most common Li carriers [19,25,27,44]. These minerals are the likely Li and Rb carriers in quartz-mica schists situated at 0.1 m from the contact with the main ARA dike, which contain 420 ppm of Li and 462 ppm of Rb (whole-rock). These interpretations are aligned with prior studies for similar lithologies [25,27]. Additionally, mottled alkali feldspar may incorporate some of the Rb in the ARA exocontact (Figure 6b). Metasomatic B and P are likely added to the host rock as newly formed tourmaline and apatite, respectively (Figure 6a–e), which are abundant in Zone 1. In distal zones, distinguishing between the metamorphic and metasomatic generations of tourmaline and apatite is challenging without mineral chemistry data. Based on limited whole-rock data available, B and P distributions in the metasomatic halo appear irregular, possibly due to initial heterogeneities in B and P contents of the Quinnesec Formation. The mineral host for Sn, which forms a pronounced, albeit short, dispersion halo extending <5 m into the host rock (Figure 7e), has not been identified; however, it could be cassiterite ± secondary mica. Also, high-spatial-resolution 2D compositional mapping of whole-rock and mineral chemistry data would be necessary for validating these interpretations.
7.2.3. Relationships Between Host Rock and Soil Geochemical Anomalies
The lithology of the parent material is the primary control on overlying soil geochemistry. This is evident from the overall Fe enrichment in Type 2 and 3 soils overlying mafic host rocks Fe (KX2, PL, and 6Mi) compared to those on felsic rocks (ARA, BL granite) (Figure 10).
Moreover, the LCT trace element cargo of Type 2 and 3 soils reflects their proximity to the FCPs. Although limited outcrop availability impeded systematic observations, the few rock samples linked to adjacent soil samples suggest that metasomatized bedrock, rather than weathering pegmatite, is the primary source of Type 2 and 3 soil enrichments. For example, a quartz-mica schist located 14.5 m from the ARA contains ~240 ppm of Li, 240 ppm of B, and 375 ppm of Rb—significantly higher than the average Li concentrations in the regional amphibolites and schists (13 to 51 ppm [19]) and average middle continental crust (12 ppm of Li, 17 ppm of B, and 65 ppm of Rb [87]; Figure 7). Two Type 2 soil samples collected near this outcrop (12.5 and 15 m from the contact) also contain relatively high contents of Li (30–75 ppm), Rb (94–107 ppm), and B (88–191 ppm), exceeding the baseline defined by Type 3 soil blind traverse at ARA (Figure 9), where the soil cover was too thick or too far from the northern pinch-out of ARA, placing it outside the soil dispersion halo (Figure 3a). Another example is the holmquistite-bearing amphibolite recorded initially at −6 m (west side or hanging wall) of the hypothetical extension of KX2. This rock contains notably high concentrations of 1134 ppm of Li and 424 ppm of Rb. The adjacent soil samples, initially classified as Type 3 due to the absence of nearby pegmatite outcrop at the time of sample collection, contain record-high 840–850 ppm of Li, 72–171 ppm of Rb, and 287–475 ppm of B (Figure 9).
Although these observations strongly suggest that much of the soil geochemical signature is inherited from the local bedrock, the Ga anomaly along Type 2 ARA and KX2 traverses does not correspond to significant host rock anomalies, which are negligible (Figure 7, Figure 9, and Figure 10). Therefore, the influence of detrital mineral particles transported from the pegmatite on Type 2 soil geochemistry within a certain distance cannot be ruled out, a topic explored further in the next sections.
7.3. Geomorphologic and Pedogenetic Controls on Soil Geochemical Halos
Gaining deeper insights into the distribution of Li and its pathfinders requires considering the impacts of parental material type and weatherability, soil thickness and age, paleoclimate, paleohydrology, topography, and vegetation. Knowledge of geomorphologic changes and weathering processes is relevant for exploration studies, but holistic approaches are rare in the literature. A promising multidisciplinary effort has been conducted in the Canary Islands Archipelago, Spain, where researchers integrated structural and erosional landform analysis with sedimentology, petrography, geochronology, mineralogy, and geochemistry of recent hotspot volcanics and volcanogenic sediments as markers of REE-Nb-F-Li-Be mineralization in immature landscapes [92]. Furthermore, integrated-applied geomorphology—mineralogical approaches have been proposed and implemented specifically for pegmatite exploration worldwide [49,93]. Primary features of pegmatites are their distinct shape (e.g., inclined, thin dikes in the current study) and silica-rich compositions with typical massive quartz—feldspar ± spodumene zones. This mineralogical makeup imparts a high resistance to mechanical weathering, especially in contrast to their foliated metamorphic wall rocks [11,49]. Outcropping and subcropping pegmatites from periglacial or recently glaciated terrains typically form conspicuous topographic highs, information that can be further utilized in remote sensing-based exploration methods [94]. Moreover, the distinctive mineralogy and metasomatic halo chemistry of pegmatites have been effectively leveraged in such terrains through soil and stream sediment geochemical ± mineralogical surveys [95,96].
According to the CMS (chemical composition—mineral assemblage—structural) classification scheme of [49,97], the mineralized rock bodies in this investigation are classified as pegmatitic, tabular, zoned to poorly zoned, meter-sized, and Li-Cs-Ta rich (spodumene ± elbaite ± lepidolite ± amblygonite–montebrassite). Furthermore, consistent with their morpho-climatic location within a glacial to periglacial landscape, the relatively high resistance to mechanical and chemical weathering leads to pure erosional landforms (i.e., erosional cross-section Type I [49]), typically forming ridges or knobs smoothened by glacial erosion, where exposures are available. However, because glacial till dominates the area, a large portion of the Florence County pegmatite swarm is likely to be concealed by sediments and/or host rocks and the soil blanket (i.e., Type V [49]).
To reduce the number of controlling variables, this pilot study focused on the geochemistry of young soils and their parent materials associated with Type I outcropping pegmatites and their nearby hypothetical buried extensions. The goal was to assess the accuracy and limitations of this method as a rapid, seasonal, prospect-scale exploration tool. This approach led to the discovery of previously unknown spodumene-bearing pegmatites in the study area. The simplified pedogenetic context enhanced our interpretations and provided a clear framework for refining geochemical exploration strategies in more complex weathering terrains. Ultimately, a comprehensive, multidisciplinary approach is required to constrain hidden mineralized bodies—such as Type V concealed pegmatites—at the district and province scale [49].
In northeastern Wisconsin and northern Michigan, Holocene spodosols and alfisoils derived from crystalline parent material are relatively thin, immature, and rich in detrital mineral particles [21,50]. The present wet, temperate climate with hot summers and cold winters supports mixed deciduous–evergreen forests with organic-rich soils. Glacial erosion has scraped and polished bedrock surfaces, slowing soil formation in many elevated areas, including pegmatite outcrops. However, over at least the past 8000 years, starting with the Middle Holocene warming interval and the establishment of the forest ecosystem [83,84], mechanical weathering processes such as frost-wedging, tree rooting and uprooting, and interactions with the microbiome have progressively altered these rock surfaces. These processes initiated the mechanical and chemical weathering of pegmatite minerals, including spodumene, leading to the release of soluble Li into surficial water, and incorporation into the regolith either as detrital particles or clays. Microscopy revealed minor amounts of cookeite and illitic clay associated with moss and lichen filaments (Figure S3). Nonetheless, field observations indicate a generally low degree of supergene alteration. Although a systematic size-fraction analysis was not conducted, the soil matrix was observed to contain abundant unweathered or partially weathered lithoclasts and mineral fragments, high organic matter content, and relatively low clay content.
Lithoclast transport and accumulation on relatively steep slopes with small local catchments may explain the anomalies observed in certain Type 2 soil profiles. For example, Li pathfinder concentrations in host rocks on the east side of KX2 and PL are very similar, declining to background levels within 7 m from the contact (Figure 7). At KX2, the Sn values in the Type 2 soils are below the Type 1 values. However, PL Type 2 soils within 2.5 m from the dike are anomalously enriched in Sn, with one outlier amounting to 61.5 ppm of Sn, far exceeding the average of 5 ppm of Sn of Type 1 PL soils (Figure 9 and Figure 10). This suggests that these samples may represent a local catchment of cassiterite (SnO2) grains transported from the pegmatite itself rather than a weathering product of the underlying metamorphic bedrock. This may also explain some of the elevated concentrations on the east side of ARA, which has a more prominent downslope (Figure 3a and Figure 9). As mentioned in Section 7.1, local topography may also lead to the detrital particle cross-contamination of Type 1 soils from nearby high-standing host rocks with contrasting composition. These effects are local, however.
To better understand the formation and longevity of the soil dispersion halos, the ratio between the mobile (soluble) and fixed (insoluble) loads of Li, Rb, and B in the ARA and KX2 soils was explored via leachate vs. bulk concentration plots (Figure 11). Sn was excluded because it was below detection limits in the leachates. The data points plot far to the right of the 1:1 line (not shown), indicating that the fixed load is approximated well by the bulk concentration, and it significantly exceeds the mobile load for these three components. Type 1 and some Type 2 samples stand out as being most enriched in insoluble Li, Rb, and B at both locations (Figure 11) because they contain higher amounts of unweathered mineral carriers, such as spodumene ± elbaite tourmaline ± lepidolite, and K-feldspar, compared to most Type 2 and 3 soils. Remarkably, the highest concentrations of soluble Rb at KX2 and B at both ARA and KX2 were found in Type 3 soils, exceeding the levels observed in both Type 1 and Type 2 soils, even in samples with low bulk Rb and B. This suggests that, at least for amphibolite-host pegmatites, mobile (extractable) Rb and B may signal hidden pegmatites or their metasomatic halos, even in soil layers thicker than 1 m and measurable at relatively high detection levels of ICP-OES. This is consistent with previous exploration studies using mobile components [9,54]. However, additional leaching experiments are needed to establish background levels of mobile components.
Future studies investigating vertical compositional profiles of soils deeper than 1 m, incorporating both bulk and extractable analyses (e.g., leaching followed by ICP-MS), as well as the role of vegetation in the short-term storage and recycling of pathfinder elements, will further advance the understanding of physical, chemical, and biological element mobility, enhancing the applicability of soil geochemistry to Li exploration.
7.4. Bulk Soil Geochemistry as an Exploration Tool of Li Pegmatites
The prospect-scale (<25 Km2) [11] comparative characterization of dispersion halos in soils overlying highly fractionated pegmatites and their host rocks, contrasted with those from control field sites (granite and common pegmatites), provides insights for vectoring toward Li pegmatites in other areas with similar pedogenesis and topography. Comparing bulk soil compositions from three distinct LCT pegmatites—emplaced in typical quartz-mica schist or amphibolite host rocks—each exhibiting varying degrees of magmatic fractionation and hydrothermal alteration, as a function of distance from their parent material, provides a framework for future investigations.
The A-horizon soils from the mineralized pegmatites of Florence County (FCP), Wisconsin, yield anomalous bulk Li concentrations (>100 ppm) compared to the common pegmatites of Dickinson County (DCP), Michigan. These values are attributed to indicate proximity to a target for two reasons: (1) Li concentrations in all control soils (parental granite and nonmineralized pegmatites) were below 100 ppm, with the sum of Li + Rb + B + Ga + Sn concentrations not exceeding 420 ppm (Figure 12) and (2) all soils from mineralized pegmatites with Li and Li pathfinder elements that exceeded these thresholds were less than ~1 m thick and located within 6 m of a spodumene pegmatite outcrop. Therefore, Li > 100 ppm and Σ(Li + Rb + B + Ga + Sn) > 420 ppm could serve as conservative cutoff values for future studies in similar immature, shallow, undisturbed postglacial soils.
Figure 12.
Li concentration (ppm) vs. the sum of Li pathfinder elements (Li, Rb, B, Ga, and Sn) in acid-digested bulk soils analyzed via ICP-OES. Blue squares represent all soil types from mineralized pegmatite locations, while gray circles represent all soil types from nonmineralized locations.
These soil Li contents are consistent with studies that uncovered buried dikes using, in part, bulk soil geochemistry at Jiajika, a major lithium deposit [98]. The Jiajika orefield soils contained Li (11–1774 ppm, with an average of 96 ppm), B (20–4380 ppm), and Rb (100–400 ppm). However, some of the anomalies at Jiajika were caused by contamination from ore transport. A recent greenfield discovery of concealed Li pegmatite orebodies (Bandeira project) in northeastern Minas Gerais, Brazil, part of the emerging “Lithium Valley”, was triggered by geochemical anomalies in tropical residual soils with Li exceeding 153 ppm [99].
Results herein are also compared to regional background levels from a low-resolution soil geochemical mapping database based on 4813 data points across the U.S. [85]. Regional soils contain <1 to 11 ppm of Li, ranking among the lowest 20% Li ranges in the U.S. Control DCP alfisols averaged 10.2 ppm at the Archean Sturgeon River Quarry and 55.5 ppm of Li at the Proterozoic Six-Mile pegmatite. In contrast, FCP spodosols averaged 157 ppm of Li across all three soil types, with a maximum of 1354.2 ppm, far surpassing the national maximum of 315 ppm. Even the minimum Li concentration (14 ppm) in FCP soils on crystalline bedrock exceeded the regional background range. Similar trends were observed for Rb, Ga, and Sn (B was not included in the national database).
This pronounced deviation from regional background levels suggests that a broader, district-scale soil anomaly may exist in Florence County, Wisconsin, reflecting the LCT signature across the entire dike swarm. The soluble load is likely the principal carrier of this broader dispersion halo as suggested by Type 3 leachate results, but a systematic grid study (~25–50 m spacing) is needed to confirm the extent of the anomalies. Scaling up an investigation from prospect to district scale could be significantly accelerated by using handheld analytical instrumentation.
Portable XRF, though widely used in soil science for its simple sample preparation, affordability, and accuracy, cannot detect Li directly [100]. Nevertheless, Li can be inferred from proxy elements, much like pH [101]. Pierangeli et al. [21] showed positive correlations between Li (analyzed via ICP-OES) and Rb, P, Zn, and Mn (determined via pXRF). In addition, a strong negative correlation between Li and the K/Rb ratio was documented, mirroring the well-established fractionation trend of granites and pegmatites [23]. These multivariate relationships allowed the accurate predictions of Li contents in soils through machine learning techniques applied to pXRF data. Results of this study (Figure 9 and Figure 10) further show that Sn, B, and Ga (determined via ICP-OES) positively correlate with Li and thus serve as useful Li pathfinder elements in soil exploration. However, recent studies show these Li concentrations can be quantified directly—along with K, Rb, and other major and minor elements—using LIBS on pressed-soil pellets, provided that the LIBS instrument is equipped with suitable, matrix-specific calibrations [10].
Using a simple pellet press, Li and Li pathfinder elements can be quickly analyzed on-site with pLIBS and pXRF. This rapid data acquisition allows for real-time anomaly mapping that can further guide prospecting and exploration activities. However, the accuracy of these results depends on the rigor of custom calibrations optimized for the specific materials and concentration ranges and validated using traditional geochemical methods in specialized laboratories. Additionally, the integrated soil data can inform machine learning predictions of the parent rock types, potentially broadening the known extent of pegmatite dike swarms, and supporting prospecting in new areas with similar soil type, climates, and vegetation.
8. Conclusions
New petrographic data and geological sketches are presented for previously published Li pegmatites (Animikie Red Ace and King’s X), a less-studied spodumene pegmatite (King’s X2), and a newly identified spodumene-bearing pegmatite (Price Lake) and their host rocks from the Florence County pegmatite field. Soil and host rock geochemistry revealed shallowly covered extensions of the KX2 and PL dike systems and led to the discovery of additional Li-bearing pegmatites (Price Lake East, not included in this study). The insights gained from this comparative, prospect-scale pilot study can be applied to other regions with similar geology and pedogenetic history.
The identification of holmquistite (classification pending additional quantitative analysis) in amphibolites hosting the FCPs is a novel finding for the midwestern United States, consistent with occurrences in the metasomatic halos of world-class Li ore bodies such as Tanco, Canada; Volta Grande, Brazil; or Greenbushes, Australia. Cautiously, these findings support the existence of a more extensive LCT pegmatite swarm in Florence County, Wisconsin, as suggested by Falster et al. [18,70].
Elements enriched in the O and A horizons of shallow podzolic soils in postglaciated terrains with the wet temperate climate of northern Wisconsin—that correlated to high Li prospectivity and were quantifiable via ICP-OES—included Li, Rb, B, Ga, and Sn, with concentrations far exceeding those of the control granite and unmineralized pegmatites as well as the regional background levels [85]. The results complement a pXRF study that also included K measurements [21].
In summary, here are responses to the main research questions listed at the end of Section 2 of this article.
- Soil geochemical anomalies in mineralized pegmatites mirror well the distribution and magnitude of the metasomatic halos in their host rocks but extend to only roughly half of the distance from pegmatites compared to the host rock anomalies, due to dilution and dispersion.
- Soil dispersion halos are influenced by glacio-fluvial cover, soil thickness, topographic relief, pegmatite size/composition, and metasomatic halo extent, for areas with the same climate, vegetation cover, and minimal human impact.
- The soil anomalies exceed the detection limits of portable instruments (LIBS for Li and K:Rb ratios and portable XRF [10,21]. Integrated pXRF plus machine learning methods on soil geochemistry successfully predict Li-rich pegmatites [21].
- Lithium concentrations exceeding ~100 ppm, Li pathfinder elements (Li, Rb, B, Ga, and Sn) totaling > 420 ppm, and K:Rb > 275 indicate proximity to mineralized dikes.
- For the detection limits utilized here, soil thickness greater than ~1 m or intervening till or glaciofluvial sediment mask the presence of hidden pegmatite bodies.
- Leaching experiments targeting mobile components show promise for detecting concealed mineralization in thicker soils. However, soils developed on glacial till should be avoided, unless more sensitive instrumentation is utilized.
- In soils less than 1 m thick, the principal carriers of Li pathfinders are unweathered mineral particles, either in situ or transported for short distances.
With improved detection capabilities of handheld devices (pXRF and pLIBS) using matrix-specific custom calibrations and by applying the general guidelines developed in this study, soil geochemistry can provide a promising pathway for the rapid, cost-effective, and environmentally friendly exploration of Li and other critical minerals.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/min15060615/s1, Figure S1: Field photos during soil-sample collection; Figure S2: SEM documentation of holmquistite in metasomatized host rock amphibolite at King’s X2 pegmatite; Figure S3: Examples of weathering products on spodumene from King’s X2 pegmatite. Table S1: Compositions and cation occupancy calculations for KX2 ‘ferro-holmquistite’; Table S2: Whole-rock ICP-OES compositions; Table S3: Bulk soil ICP-OES compositions; and Table S4: Li, B, and Rb concentrations in soil leachates and their proportions relative to bulk soils.
Author Contributions
Conceptualization, M.-L.C.S., T.R.B. and D.C.W.; field documentation and sampling, M.-L.C.S., T.R.C., L.M.P.P. and J.O.Y.; sample preparation and analysis, M.-L.C.S., T.R.C., L.M.P.P. and J.O.Y.; resources, M.-L.C.S., T.R.B. and D.C.W.; data curation, M.-L.C.S., T.R.C. and L.M.P.P.; writing—original draft preparation, M.-L.C.S. and T.R.C.; writing—review and editing, M.-L.C.S., T.R.B., D.C.W. and L.M.P.P.; visualization, M.-L.C.S., T.R.C. and J.O.Y.; supervision, M.-L.C.S.; project administration, M.-L.C.S.; funding acquisition, M.-L.C.S., T.R.B., D.C.W., T.R.C., L.M.P.P. and J.O.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project 22-0034, Lithium Americas Corporation, Vancouver, BC V6C 1E5, Canada, to M-L.C.S.; the Office of Research and Graduate Studies at Central Michigan University, Mount Pleasant, MI, 48859, USA to T.C. and J.Y.; and the Brazilian Coordination for Improvement of Higher Education Personal (CAPES) and Mineralogy Geochemistry Petrology and Volcanology (MGPV) division of the Geological Society of America, Chantilly VA 20151-1110 USA grants to L.P.
Data Availability Statement
Data are contained within this article and the Supplementary Materials.
Acknowledgments
We thank the Wisconsin Department of Natural Resources for their guidance in accessing the Florence County study area and the organizers of the Pegmatite Symposium PEG 24 for facilitating access to the Big Whopper pegmatite, which enabled the collection of reference holmquistite samples. We sincerely thank the Academic Editor and four anonymous reviewers for their thoughtful suggestions.
Conflicts of Interest
T.R.B. is an employee of Lithium Argentina AG (formerly Lithium Americas Corporation), that funded in part this academic project. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
References
- Wise, M.A.; Müller, A.; Simmons, W.B. A Proposed New Mineralogical Classification System for Granitic Pegmatites. Can. Miner. 2022, 60, 229–248. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Miner. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegmatites. Part I: Anatomy and internal evolution of pegmatite deposits. Geosc. Can. 1991, 18, 49–67. [Google Scholar]
- Černý, P. Fertile Granites of Precambrian Rare-Element Pegmatite Fields—Is Geochemistry Controlled by Tectonic Setting or Source Lithologies? Precambrian Res. 1991, 51, 429–468. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; de los Hoyos, C.R.; Declercq, J. Classification and Characteristics of Natural Lithium Resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Benson, T.R.; Jowitt, S.M.; Simon, A.C. Special Issues on the Geology and Origin of Lithium Deposits—Introduction: Lithium Deposit Types, Sizes, and Global Distribution. Econ. Geol. 2025; In press. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Liang, B.; Geng, Y.; Liu, T.; Wang, Q.B. Extraction of soils above concealed lithium deposits for rare metal exploration in Jiajika area: A pilot study. Appl. Geochem. 2019, 107, 142–151. [Google Scholar] [CrossRef]
- Steiner, B.M. Tools and Workflows for Grassroots Li-Cs-Ta (LCT) Pegmatite Exploration. Minerals 2019, 9, 499. [Google Scholar] [CrossRef]
- Galeschuk, C.R.; Vanstone, P.J. Exploration Techniques for Rare-Element Pegmatite in the Bird River Greenstone Belt, Southeastern Manitoba. In Proceedings of the Exploration 07: Fifth Decennial International Conference on Mineral Exploration, Toronto, ON Canada, 9–12 September 2007; pp. 823–839. [Google Scholar]
- Wise, M.A.; Harmon, R.S.; Curry, A.; Jennings, M.; Grimac, Z.; Khashchevskaya, D. Handheld LIBS for Li exploration: An example from the Carolina tin-spodumene belt, USA. Minerals 2022, 12, 77. [Google Scholar] [CrossRef]
- Müller, A.; Brönner, M.; Menuge, J.; Williamson, B.; Haase, C.; Tassis, G.; Pohl, C.; Brauch, K.; Saalmann, K.; Teodoro, A.; et al. The GREENPEG Project Toolset to Explore for Buried Pegmatites Hosting Lithium, High-Purity Quartz, and Other Critical Raw Materials. Econ. Geol. 2025, 120, 745–778. [Google Scholar] [CrossRef]
- Balaram, V.; Sawant, S.S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [Google Scholar] [CrossRef]
- Fabre, C.; Ourti, N.E.; Ballouard, C.; Mercardier, J.; Cauzid, J. Handheld LIBS analysis for in situ quantification of Li and detection of the trace elements (Be, Rb and Cs). J. Geochem. Explor. 2022, 236, 106979. [Google Scholar] [CrossRef]
- Dias, F.; Ribeiro, R.; Gonçalves, F.; Lima, A.; Roda-Robles, E.; Martins, T. Calibrating a Handheld LIBS for Li Exploration in the Barroso-Alvao Aplite-Pegmatite Field, Northern Portugal: Textural Precautions and Procedures When Analyzing Spodumene and Petalite. Minerals 2023, 13, 470. [Google Scholar] [CrossRef]
- Fabre, C.; Ourti, N.E.; Mercadier, J.; Cardoso-Fernandes, J.; Dias, F.; Perrotta, M.N.; Koerting, F.; Lima, A.; Kaestner, F.; Koellner, N.; et al. Analyses of Li-Rich Minerals Using Handheld LIBS Tool. Data 2021, 6, 68. [Google Scholar] [CrossRef]
- Harmon, R.S.; Lawley, C.J.M.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-Induced Breakdown Spectroscopy-An emerging analytical tool for mineral exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef]
- Korbel, C.; Mezoued, N.; Demeusy, B.; Fabre, C.; Cauzid, J.; Filippova, I.V.; Filippov, L.O. Quantification of lithium using handheld instruments: Application of LIBS and XRF spectroscopy to assay the lithium content of mineral processing products. J. Anal. At. Spectrom. 2024, 39, 1838–1853. [Google Scholar] [CrossRef]
- Falster, A.U.; Simmons, W.B.; Webber, K.L. The mineralogy and geochemistry of the Animikie Red Ace pegmatite, Florence County, Wisconsin. Recent Res. Dev. Mineral. 1996, 1, 7–67. [Google Scholar]
- Liu, X.-M.; Rudnick, R.L.; Hier-Majumder, S.; Sirbescu, M.-L. Processes controlling lithium isotopic distribution in contact aureoles: A case study of the Florence County pegmatites, Wisconsin. Geochem. Geophys. Geosystems 2010, 11, 21. [Google Scholar] [CrossRef]
- Sirbescu, M.L.C.; Hartwick, E.E.; Student, J.J. Rapid crystallization of the Animikie Red Ace Pegmatite, Florence county, northeastern Wisconsin: Inclusion microthermometry and conductive-cooling modeling. Contrib. Miner. Pet. 2008, 156, 289–305. [Google Scholar] [CrossRef]
- Pierangeli, L.M.P.; Sirbescu, M.-L.C.; Silva, S.H.G.; Weindorf, D.C.; Benson, T.R.; Cury, N. Soil geochemistry towards lithium pegmatite exploration: Building a machine-learning predictive algorithm via portable-XRF. Econ. Geol. 2025, 120. [Google Scholar]
- Selway, J.B.; Breaks, F.W.; Tindle, A.G. A review of rare-element (Li-Cs-Ta) pegmatite exploration techniques for the Superior Province, Canada, and large worldwide tantalum deposits. Explor. Min. Geol. 2005, 14, 1–30. [Google Scholar] [CrossRef]
- Trueman, D.; Černý, P. Exploration for rare-element granitic pegmatites. In Pegmatites in Science and Industry; Cerny, P., Ed.; Mineralogical Association of Canada: Toronto, ON, Canada, 1982; pp. 463–494. [Google Scholar]
- Barros, R.; Kaeter, D.; Menuge, J.F.; Fegan, T.; Harrop, J. Rare Element Enrichment in Lithium Pegmatite Exomorphic Halos and Implications for Exploration: Evidence from the Leinster Albite-Spodumene Pegmatite Belt, Southeast Ireland. Minerals 2022, 12, 981. [Google Scholar] [CrossRef]
- Errandonea-Martin, J.; Garate-Olave, I.; Roda-Robles, E.; Cardoso-Fernandes, J.; Lima, A.; Ribeiro, M.d.A.; Teodoro, A.C. Metasomatic effect of Li-bearing aplite-pegmatites on psammitic and pelitic metasediments: Geochemical constraints on critical raw material exploration at the Fregeneda–Almendra Pegmatite Field (Spain and Portugal). Ore Geol. Rev. 2022, 150, 105155. [Google Scholar] [CrossRef]
- Müller, A.; Reimer, W.; Wall, F.; Williamson, B.; Menuge, J.; Brönner, M.; Haase, C.; Brauch, K.; Pohl, C.; Lima, A. GREENPEG–exploration for pegmatite minerals to feed the energy transition: First steps towards the Green Stone Age. In The Green Stone Age: Exploration and Exploitation of Minerals for Green Technologies; Smelror, M., Hanghøj, K., Schiellerup, H., Eds.; Geological Society, London, Special Publications: London, UK, 2023; Volume 526, pp. 193–218. [Google Scholar]
- Garate-Olave, I.; Roda-Robles, E.; Santos-Loyola, N.; Martins, T.; Lima, A.; Errandonea-Martin, J. Crystallization Sequence of the Spodumene-Rich Alijó Pegmatite (Northern Portugal) and Related Metasomatism on Its Host Rock. Minerals 2024, 14, 701. [Google Scholar] [CrossRef]
- Pell, R.; Tijsseling, L.; Goodenough, K.; Wall, F.; Dehaine, Q.; Grant, A.; Deak, D.; Yan, X.Y.; Whattoff, P. Towards sustainable extraction of technology materials through integrated approaches. Nat. Rev. Earth Envrion. 2021, 2, 665–679. [Google Scholar] [CrossRef]
- Černý, P. Rare-element granitic pegmatites. Part II: Regional to global environments and petrogenesis. In Ore Deposit Models; Sheahan, P.A., Cherry, M.E., Eds.; Geoscience Canada—Geological Association of Canada, Reproduction Series: Ottawa, ON, Canada, 1993; Volume 18, pp. 49–67. [Google Scholar]
- McCaffrey, D.M.; Jowitt, S.M. The crystallization temperature of granitic pegmatites: The important relationship between undercooling and critical metal prospectivity. Earth-Sci. Rev. 2023, 244, 104541. [Google Scholar] [CrossRef]
- Breaks, F.W.; Selway, J.B.; Tindle, A.G. Fertile peraluminous granites and related rare element pegmatites, Superior Province of Ontario. In Rare-Element Geochemistry and Mineral Deposits; Linnen, R., Sampson, I., Eds.; Geological Association of Canada: Ottawa, ON, Canada, 2005; Volume 17, pp. 87–127. [Google Scholar]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Nabelek, P.I.; Whittington, A.; Sirbescu, M.-L. The role of H2O in rapid emplacement and crystallization of granite pegmatites: Resolving the paradox of large crystals in highly undercooled melts. Contrib. Miner. Pet. 2010, 160, 313–325. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Muchez, P. Tracing fluid saturation during pegmatite differentiation by studying the fluid inclusion evolution and multiphase cassiterite mineralisation of the Gatumba pegmatite dyke system (NW Rwanda). Lithos 2020, 354, 105285. [Google Scholar] [CrossRef]
- Chen, J.Z.; Zhang, H.; Tang, Y.; Lv, Z.H.; An, Y.; Wang, M.T.; Liu, K.; Xu, Y.S. Lithium mineralization during evolution of a magmatic-hydrothermal system: Mineralogical evidence from Li-mineralized pegmatites in Altai, NW China. Ore Geol. Rev. 2022, 149, 105058. [Google Scholar] [CrossRef]
- Morgan, G.B.; London, D. Alteration of Amphibolitic Wallrocks around the Tanco Rare-Element Pegmatite, Bernic Lake, Manitoba. Am. Miner. 1987, 72, 1097–1121. [Google Scholar]
- Shearer, C.K.; Papike, J.J.; Simon, S.B.; Laul, J.C. Pegmatite—wallrock interactions, Black Hills, South Dakota: Interactions between pegmatite-derived fluids and quartz-mica schist wallrock. Am. Miner. 1986, 71, 518–539. [Google Scholar]
- Martins, T.; Linnen, R.L.; Fedikow, M.A.F.; Singh, J. Whole-rock and mineral geochemistry as exploration tools for rare-element pegmatite in Manitoba: Examples from the Cat Lake–Winnipeg River and Wekusko Lake pegmatite fields (parts of NTS 52L6, 63J13). Manit. Geol. Survey Rep. Act 2017, 5, 42–51. [Google Scholar]
- Sweetapple, M.T.; Vanstone, P.J.; Lumpkin, G.R.; Collins, P.L.F. A review of lithogeochemical dispersion haloes of LCT pegmatites, and their application to rare metal exploration, with special reference to lithium in an Australian context. Aust. J. Earth Sci. 2024, 71, 1050–1084. [Google Scholar] [CrossRef]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Frost, M.T.; Tsambourakis, G.; Davis, J. Holmquistite-Bearing Amphibolite from Greenbushes, Western Australia. Mineral. Mag. 1987, 51, 585–591. [Google Scholar] [CrossRef]
- Cámara, F.; Oberti, R. The crystal-chemistry of holmquistites: Ferroholmquistite from Greenbushes (Western Australia) and hints for compositional constraints in BLi amphiboles. Am. Miner. 2005, 90, 1167–1176. [Google Scholar] [CrossRef]
- Lagache, M.; Quemeneur, J. The Volta Grande Pegmatites, Minas Gerais, Brazil; an example of rare-element granitic pegmatites exceptionally enriched in lithium and rubidium. Can. Miner. 1997, 35, 153–165. [Google Scholar]
- Shearer, C.K.; Papike, J.J. Pegmatite-Wallrock Interaction—Holmquistite-Bearing Amphibolite, Edison Pegmatite, Black Hills, South-Dakota. Am. Miner. 1988, 73, 324–337. [Google Scholar]
- Selway, J.B.; Novák, M.; Cerny, P.; Hawthorne, F.C. The Tanco pegmatite at Bernic Lake, Manitoba.: XIII.: Exocontact tourmaline. Can. Miner. 2000, 38, 869–876. [Google Scholar] [CrossRef]
- Bodeving, S.; Vasyukova, O.V.; Williams-Jones, A.E. Controls on the origin and evolution of wall-rock alteration at the Big Whopper Li-Cs-Ta pegmatite, Canada. Econ. Geol. 2025, 120, 689–714. [Google Scholar] [CrossRef]
- Keyser, W.; Müller, A.; Augland, L.E.; Steiner, R. Rare-metal halos of lithium pegmatite at the Wolfsberg deposit, Austria, and their implications for exploration. Ore Geol. Rev. 2024, 171, 106179. [Google Scholar] [CrossRef]
- Sirbescu, M.L.C.; Leatherman, M.A.; Student, J.J.; Beehr, A.R. Apatite Textures and Compositions as Records of Crystallization Processes in the Animikie Red Ace Pegmatite Dike, Wisconsin, USA. Can. Miner. 2009, 47, 725–743. [Google Scholar] [CrossRef]
- Dill, H.G. An overview of the pegmatitic landscape from the pole to the equator—Applied geomorphology and ore guides. Ore Geol. Rev. 2017, 91, 795–823. [Google Scholar] [CrossRef]
- Schaetzl, R.J.; Thompson, M.L. Soils: Genesis and Geomorphology, 2nd ed.; Cambridge University Press: New York, NY, USA, 2015; p. 778. [Google Scholar]
- Luecke, W. Soil Geochemistry Above a Lithium Pegmatite Dyke at Aclare, Southeast Ireland. Ir. J. Earth Sci. 1984, 6, 205–211. Available online: https://www.jstor.org/stable/30002472 (accessed on 15 April 2025).
- Marshall, B.T.; Herman, J.S. Trace element distribution in the soils above deeply weathered pegmatites, Virginia, U.S.A.: Implications for exploration. Appl. Geochem. 1986, 1, 681–690. [Google Scholar] [CrossRef]
- Galeschuk, C.R.; Vanstone, P. Exploration for Buried Rare Element Pegmatites in the Bernic Lake Region of Southeastern Manitoba. In Rare-Element Geochemistry and Mineral Deposits; Linnen, R., Sampson, I., Eds.; Geological Association of Canada, Short Course Notes: Ottawa, ON, Canada, 2005; Volume 17, pp. 159–173. [Google Scholar]
- Fedicow, M.; Zelligan, S. NI 43-101 Technical Report on the Zoro Lithium Project, Snow Lake, Manitoba. Unpublished Report Prepared for Far Resources Ltd. 2018, p. 187. Available online: https://sedar-filings-backup.thecse.com/00032046/1809051817578606.pdf (accessed on 15 April 2025).
- Turner, D.; Young, I. Geological Assessment Report on the Selwyn 1–10 Claims, Located at NTS 105I02 Latitude 62° 05′N.; Longitude 125° 51′W in the Yukon Territory; Unpublished Report Prepared for War Eagle Mining Company; 2008; p. 65. Available online: https://yma.gov.yk.ca/095100.pdf (accessed on 15 April 2025).
- Bradley, D.C.; McCauley, A.; Stillings, L.M. Mineral-deposit models for lithium-cesium-tantalum pegmatites. In U.S. Geological Survey Scientific Investigations Report 2010-5070-0; U.S. Geological Survey: Reston, VA, USA, 2017; p. 48. [Google Scholar] [CrossRef]
- Holm, D.; Medaris, L.G.; McDannell, K.T.; Schneider, D.A.; Schulz, K.; Singer, B.S.; Jicha, B.R. Growth, overprinting, and stabilization of Proterozoic provinces in the southern Lake Superior region. Precambrian Res. 2020, 339, 105587. [Google Scholar] [CrossRef]
- Dutton, C.E. Geology of the Florence Area, Wisconsin and Michigan. Scale 1:24,000; U. S. Geological Survey Professional Paper 633: Reston, VA, USA, 1971; p. 54. [Google Scholar] [CrossRef]
- Sims, P.K.; Schulz, K.J.; DeWitt, E.; Brasaemle, B. Petrography and geochemistry of early Proterozoic granitoid rocks in Wisconsin magmatic terranes of Penokean Orogen, northern Wisconsin. U.S. Geol. Surv. Bull. 1993, 1904-J, 1–31. [Google Scholar] [CrossRef]
- Sims, P.K.; Schulz, K.J.; Peterman, Z.E. Geology and Geochemistry of Early Proterozoic Rocks in the Dunbar Area, Northeastern Wisconsin; U.S. Geological Survey Professional Paper 1517: Reston, VA, USA, 1992; p. 65. [Google Scholar] [CrossRef]
- Zi, J.-W.; Sheppard, S.; Muhling, J.R.; Rasmussen, B. Refining the Paleoproterozoic tectonothermal history of the Penokean Orogen: New U-Pb age constraints from the Pembine-Wausau terrane, Wisconsin, USA. GSA Bull. 2022, 134, 776–790. [Google Scholar] [CrossRef]
- Koehler, S.R. Geological setting and geochemistry of the Bush Lake Granite in relation to rare-element pegmatites, Florence county, Wisconsin. In Proceedings of the 36th Annual Meeting, Institute of Lake Superior Geology, Thunder Bay, ON, Canada, 9–12 May 1990; pp. 51–52. [Google Scholar]
- Sims, P.K.; Peterman, Z.E.; Schulz, K.J. The Dunbar Gneiss-Granitoid Dome—Implications for Early Proterozoic Tectonic Evolution of Northern Wisconsin. Geol. Soc. Am. Bull. 1985, 96, 1101–1112. [Google Scholar] [CrossRef]
- Schulz, K.J. Field Trip 4: Granitoid rocks of the Pembine-Wasau terrane in Northeastern Wisconsin. In Proceedings of the 64th Annual Meeting, Institute of Lake Superior Geology, Iron Mountain, MI, USA, 15–18 May 2018. [Google Scholar]
- Schulz, K.J.; Cannon, W.F. The penokean orogeny in the lake superior region. Precambrian Res. 2007, 157, 4–25. [Google Scholar] [CrossRef]
- Geiger, C.A.; Guidotti, C.V. Precambrian metamorphism in the southern Lake Superior region and its bearing on crustal evolution. Geosci. Wis. 1989, 13, 1–33. [Google Scholar] [CrossRef]
- Daniel, C.G.; Indares, A.; Medaris, L.G., Jr.; Aronoff, R.; Malone, D.; Schwartz, J. Linking the Pinware, Baraboo, and Picuris orogens: Recognition of a trans-Laurentian ca. 1520–1340 Ma orogenic belt. In Turning Points in the Evolution of a Continent: Geological Society of America Memoir 220; Whitmeyer, S.J., Williams, M.L., Kellett, D.A., Tikoff, B., Eds.; Geological Society of America: Boulder, CO, USA, 2023; Volume 220, pp. 175–190. [Google Scholar]
- Dewane, T.J.; Van Schmus, W.R. U-Pb geochronology of the Wolf River batholith, north-central Wisconsin: Evidence for successive magmatism between 1484 Ma and 1468 Ma. Precambrian Res. 2007, 157, 215–234. [Google Scholar] [CrossRef]
- Falster, A.U.; Simmons, W.B.; Webber, K.L. Origin of the pegmatites in the Hoskin Lake pegmatite field, Florence Co., Wisconsin. In Proceedings of the Crystallization Processes in Granitic Pegmatites, International Meeting, Cavoli, Elba Island, Italy, 23–28 May 2005. [Google Scholar]
- Falster, A.U.; Simmons, W.B.; Webber, K.L. Anatectic origin of the post-Penokean Li-Cs-Ta-enriched pegmatites in Florence County, Wisconsin, USA. In Proceedings of the XXII International Mineralogical Association meeting, Melbourne, Australia, 13–17 August 2018. [Google Scholar]
- Gerla, P.J. Occurrence of a Heterogeneous Pegmatite in Florence County, Wisconsin; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 1988; p. 13. [Google Scholar]
- Koehler, S.R. Geological Setting, Mineralogy, and Geochemistry of Four Rare Element Pegmatites from the Penokean Volcanic Belt, Florence County, Wisconsin; University of Wisconsin-Extension, Geological and Natural History Survey: Madison, WI, USA, 1991; p. 27. [Google Scholar]
- Buchholz, T.; Falster, A.U.; Simmons, W.B.; Webber, K.L.; Van Daalen, C.M. A comparison of two Paleoproterozoic pegmatite districts and nearby granite bodies in Florence County, Wisconsin and Marquette County, Michigan. Can. Miner. 2019, 57, 719–721. [Google Scholar] [CrossRef]
- James, H.L.; Clark, L.D.; Lamey, C.A.; Pettijohn, F.J. Geology of Central Dickinson County, Michigan. Geol. Surv. Prof. Pap. 1961, 310, 172. [Google Scholar] [CrossRef]
- Van Daalen, C.M. Mineralogy and Geochemistry of Late Archean and Paleoproterozoic Granites and Pegmatites in the Northern Penokean Terrane of Marquette and Dickinson Counties, Michigan. Master’s Thesis, University of New Orleans, New Orleans, LA, USA, 2015. [Google Scholar]
- Cannon, W.F.; Schulz, K.J.; Ayuso, R.A.; Mroz, T.H. Field Trip 1: Archean and Paleoproterozoic geology of the Felch district, Central Dickinson County, Michigan. In Proceedings of the 64th Annual Meeting, Institute of Lake Superior Geology, Iron Mountain, MI, USA, 15–18 May 2018. [Google Scholar]
- Ayuso, R.A.; Schultz, K.J.; Cannon, W.F.; Woodruff, L.G.; Vasquez, J.A.; Foley, N.K.; Jackson, J. New U-Pb Zircon Ages for Rocks from the Granite-Gneiss Terrane in Northern Michigan: Evidence for Events at ~3750, 2750, and 1850 Ma. In Proceedings of the 64th Annual Meeting, Institute on Lake Superior Geology, Iron Mountain, MI, USA, 15–18 May 2018; pp. 7–8. [Google Scholar]
- Soil Survey Staff, Natural Resources Conservation Service. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; U.S. Department of Agriculture Handbook: Washington, DC, USA, 1999. Available online: https://www.nrcs.usda.gov/resources/guides-and-instructions/soil-taxonomy (accessed on 15 April 2025).
- California-Soil-Resource-Lab. SoilWeb Earth: SSURGO Soils in Google Earth. n.d. Available online: https://casoilresource.lawr.ucdavis.edu/soilweb/ (accessed on 15 April 2025).
- Boelter, J.M.; Elg, A.M. Soil survey of Florence County, Wisconsin; Natural Resources Conservation Service: Washington, DC, USA, 2004. Available online: https://purl.fdlp.gov/GPO/LPS94964 (accessed on 15 April 2025).
- CEC. Guide to drought indices and indicators used in North America. In Project Publication© Commission for Environmental Cooperation; CEC: Montreal, QC, Canada, 2021. [Google Scholar]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef]
- Webb, T.I.; Bartlein, P.J.; Harrison, S.P.; Anderson, K.H. Vegetation, lake levels, and climate in eastern North America for the past 18,000 years. In Global Climates Since the Last Glacial Maximum; Wright, H.E., Jr., Kutzbach, J.E., Webb, T., III, Ruddiman, W.F., Street-Perrott, F.A., Bartlein, P.J., Eds.; University of Minnesota Press: Minneapolis, MN, USA, 1993; pp. 415–467. [Google Scholar]
- Clayton, L. Pleistocene geology of Florence County, Wisconsin. In Wisconsin Geological and Natural History Survey Information Circular; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 1986; Volume 51. [Google Scholar]
- Smith, D.B.; Solano, F.; Woodruff, L.G.; Cannon, W.F.; Ellefsen, K.J. Geochemical and Mineralogical Maps, with Interpretation, for Soils of the Conterminous United States; United Stages Geological Survey: Reston, VA, USA, 2019. [Google Scholar]
- Locock, A.J. An Excel spreadsheet to classify chemical analyses of amphiboles following the IMA2012 recommendations. Comput. Geosci. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust; Elsevier-Pergamon: Oxfordshire, UK, 2003. [Google Scholar] [CrossRef]
- Zhou, J.S.; Wang, Q.; Wang, H.; Ma, J.L.; Zhu, G.H.; Zhang, L. Pegmatite lithium deposits formed within low-temperature country rocks. Nat. Commun. 2025, 16, 447. [Google Scholar] [CrossRef]
- Wohletz, K. HEAT3D: Magmatic Heat Flow Calculation 2007. Available online: https://kware-heat3d.software.informer.com/4.1/ (accessed on 15 April 2025).
- London, D.; Morgan, G.B.; Wolf, M.B. Boron in granitic rocks and their contact aureoles. Rev. Miner. 1996, 33, 299–330. [Google Scholar]
- London, D. Holmquistite as a guide to pegmatitic rare metal deposits. Econ. Geol. 1986, 81, 704–712. [Google Scholar] [CrossRef]
- Dill, H.G.; Buzatu, A.; Balaban, S.I.; Rüsenberg, K.A. A mineralogical-geomorphological terrain analysis of hotspot volcanic islands—The missing link between carbonatite- and pegmatite Nb-F-Zr-Li-Be-bearing REE deposits and new tools for their exploration (Canary Islands Archipelago, Spain). Ore Geol. Rev. 2023, 163, 105702. [Google Scholar] [CrossRef]
- Dill, H.G. The Hagendorf-Pleystein Province: The Center of Pegmatites in an Ensialic Orogen. In Modern Approaches in Solid Earth Sciences; Pirajno, F., Ed.; Springer: Cham, Switzerland, 2015; Volume 15, p. 475. [Google Scholar] [CrossRef]
- Cardoso-Fernandes, J.; Lima, J.; Lima, A.; Roda-Robles, E.; Köhler, M.; Schaefer, S.; Barth, A.; Knobloch, A.; Gonçalves, M.A.; Gonçalves, F.; et al. Stream sediment analysis for Lithium (Li) exploration in the Douro region (Portugal): A comparative study of the spatial interpolation and catchment basin approaches. J. Geochem. Explor. 2022, 236, 106978. [Google Scholar] [CrossRef]
- Dill, H.G.; Weber, B.; Melcher, F.; Wiesner, W.; Müller, A. Titaniferous heavy mineral aggregates as a tool in exploration for pegmatitic and aplitic rare-metal deposits (SE Germany). Ore Geol. Rev. 2014, 57, 29–52. [Google Scholar] [CrossRef]
- Kaeter, D.; Menuge, J.F. Columbite-tantalite and cassiterite as indicator minerals for lithium pegmatites: Implications from geospatial and mineralogical analyses of stream sediments in southeast Ireland. Miner. Depos. 2025. [Google Scholar] [CrossRef]
- Dill, H.G. The CMS classification scheme (Chemical composition—Mineral assemblage—Structural geology)—linking geology to mineralogy of pegmatitic and aplitic rocks. J. Miner. Geochem. 2016, 193, 231–263. [Google Scholar] [CrossRef]
- Huang, T.; Fu, X.; Ge, L.; Zou, F.; Hao, X.; Yang, R.; Xiao, R.; Fan, J. The genesis of giant lithium pegmatite veins in Jiajika, Sichuan, China: Insights from geophysical, geochemical as well as structural geology approach. Ore Geol. Rev. 2020, 124, 103557. [Google Scholar] [CrossRef]
- Evangelista Silva, C.J.; Santos Rocha, L.S.; Bergman, P. NI 43-101 Technical Report—Mineral Resource Update on Bandeira Project, Araçuaí and Itinga, Minas Gerais State, Brazil; Lithium Ionic Corp.: Toronto, ON, Canada, 2024; Bandeira Project, GE 21 Project No 240403; 202p, Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=f66e55cdb876bf130d06292fd2b1a47b2c8b2238d3c5bc88523bf54dd3212921 (accessed on 15 April 2025).
- Weindorf, D.C.; Chakraborty, S. Portable X-ray fluorescence spectrometry analysis of soils. Soil Sci. Soc. Am. J. 2020, 84, 1384–1392. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Man, T.; Aldabaa, A.A.A.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 3. Soil reaction (pH). Geoderma 2014, 232, 141–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).