Multiscale Flotation Testing for the Recovery of REE-Bearing Fluorapatite from a Finnish Carbonatite Complex Deposit Using Conventional Collectors and Lignin Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore Characterization
2.2. Lignin Production and Properties
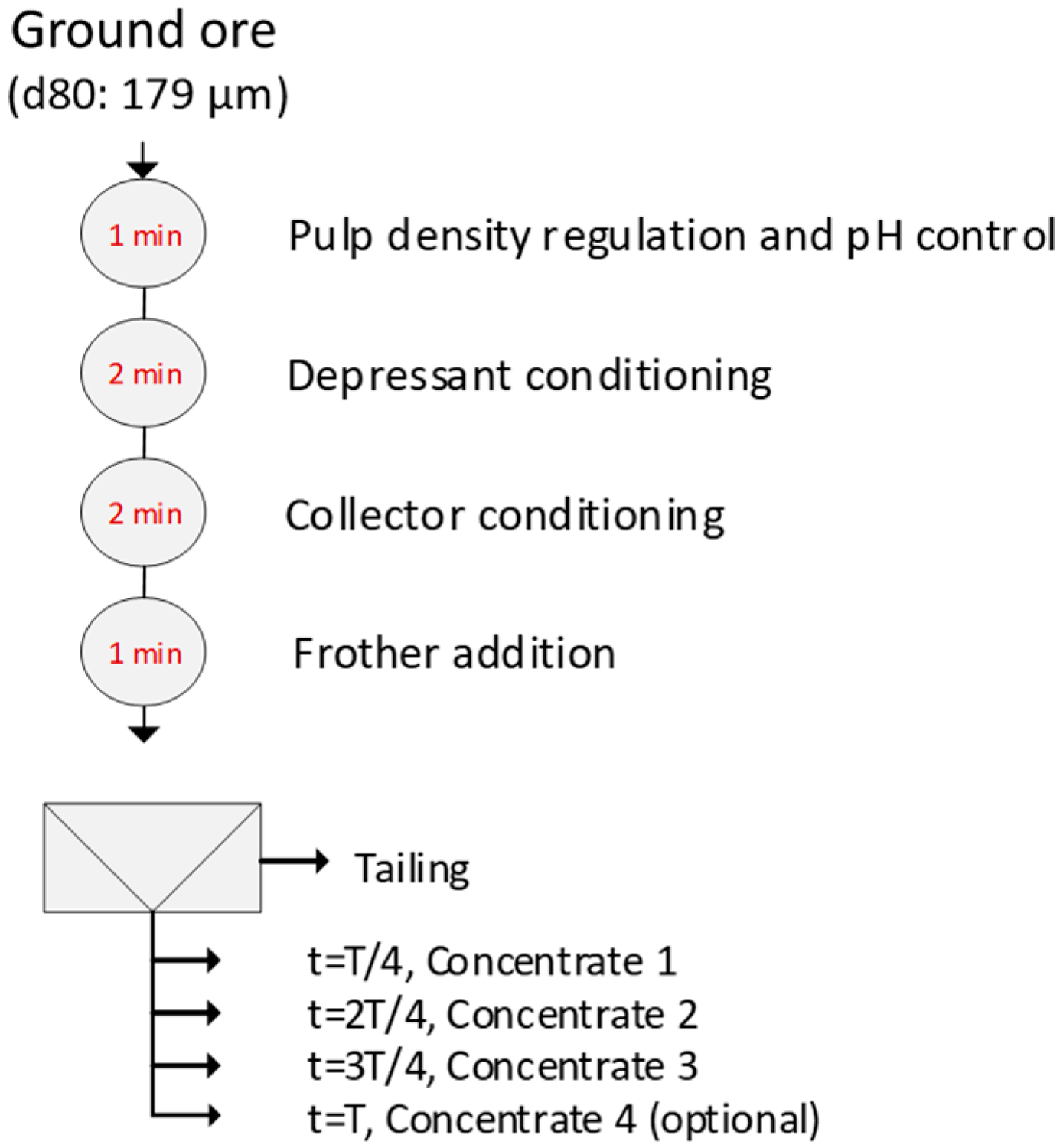
2.3. Flotation Tests
3. Results and Discussion
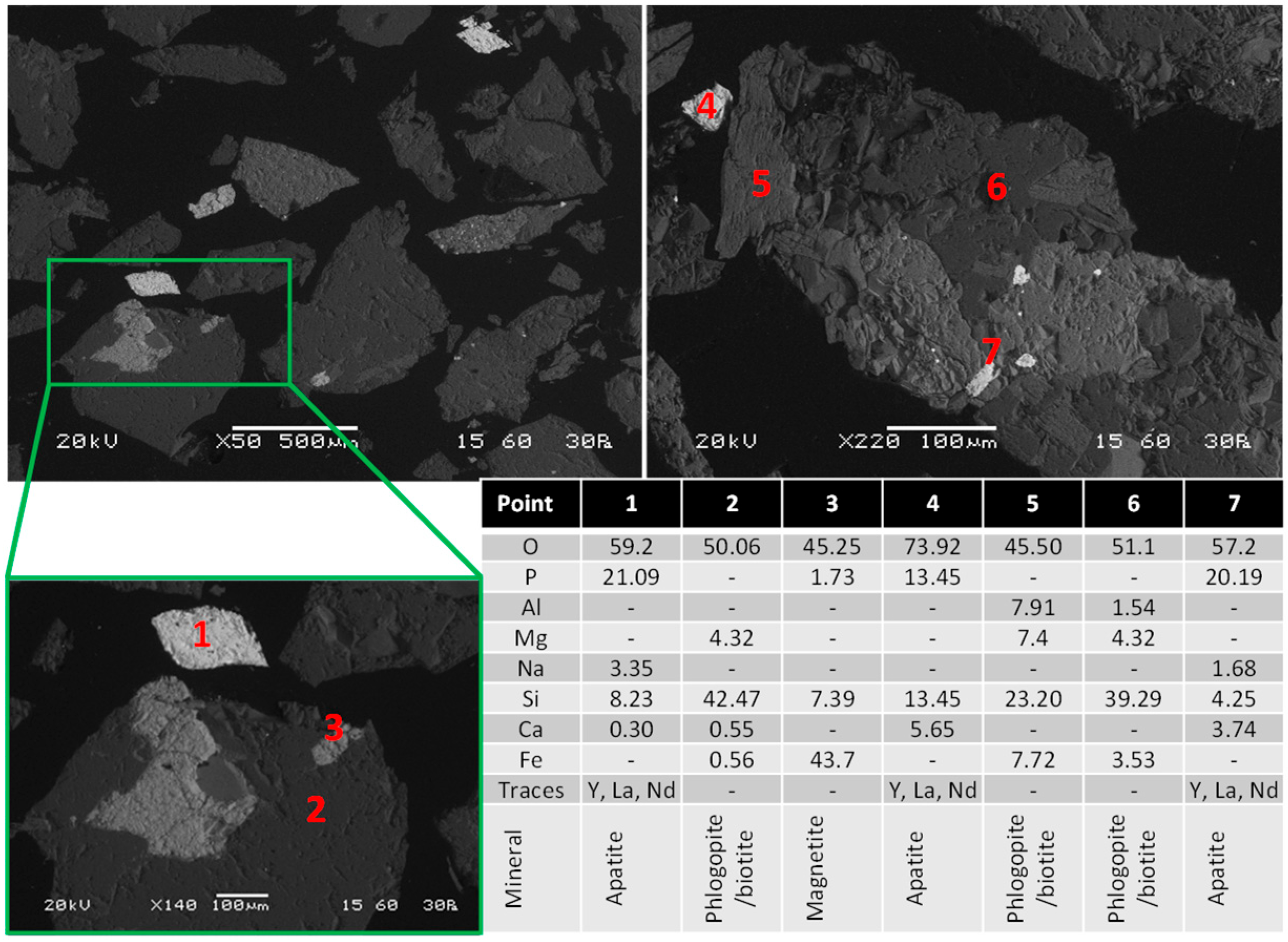
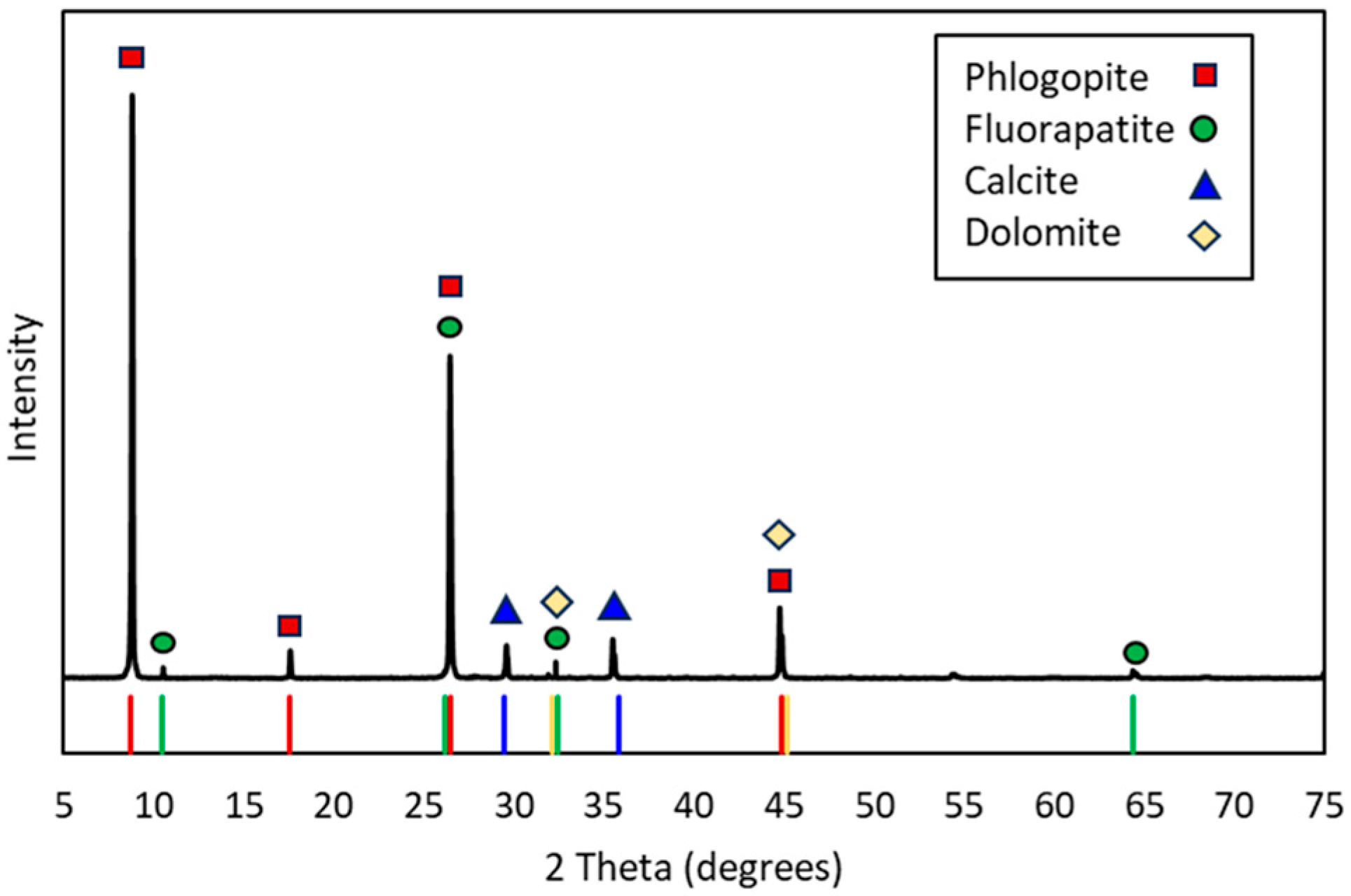
3.1. Feed Properties
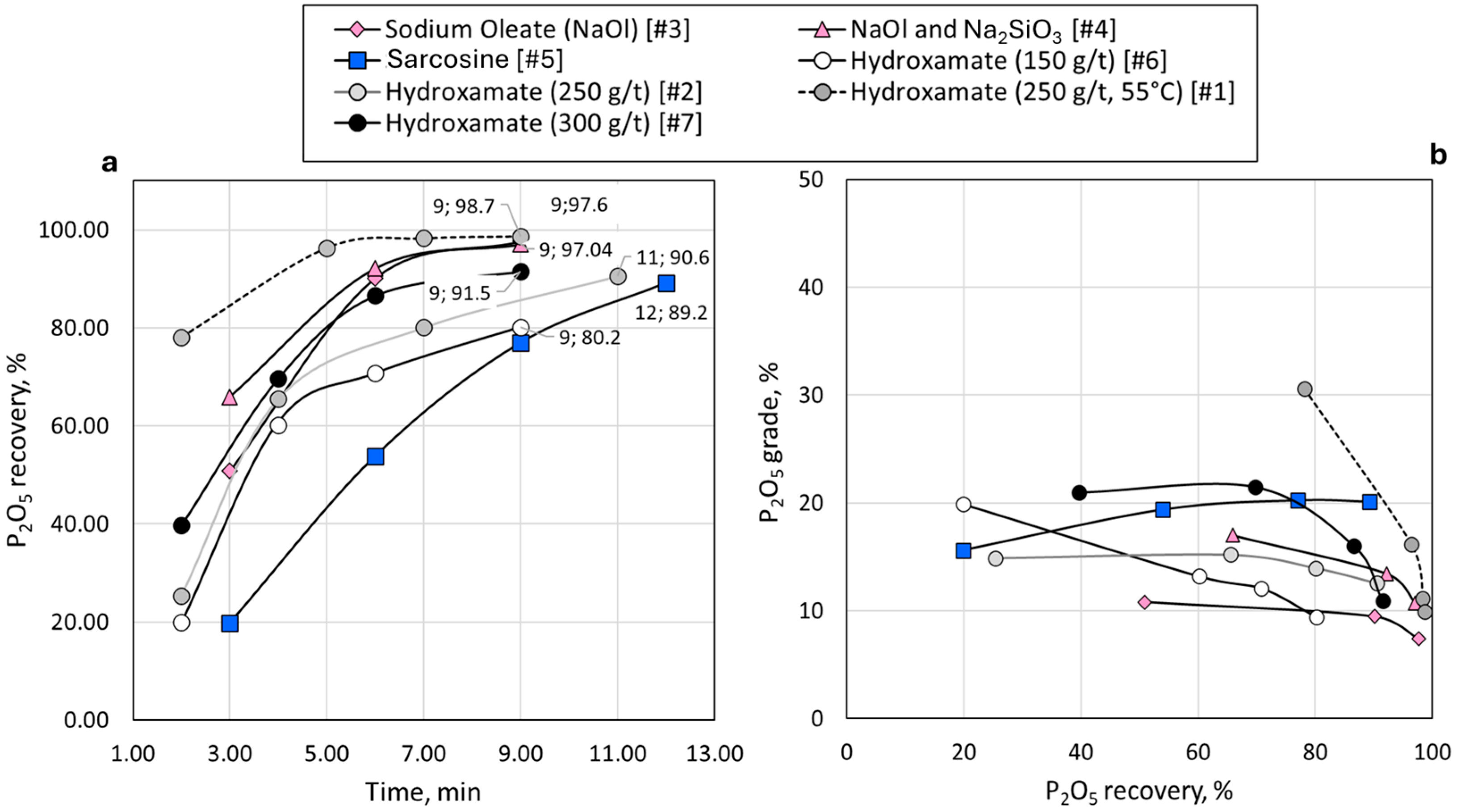
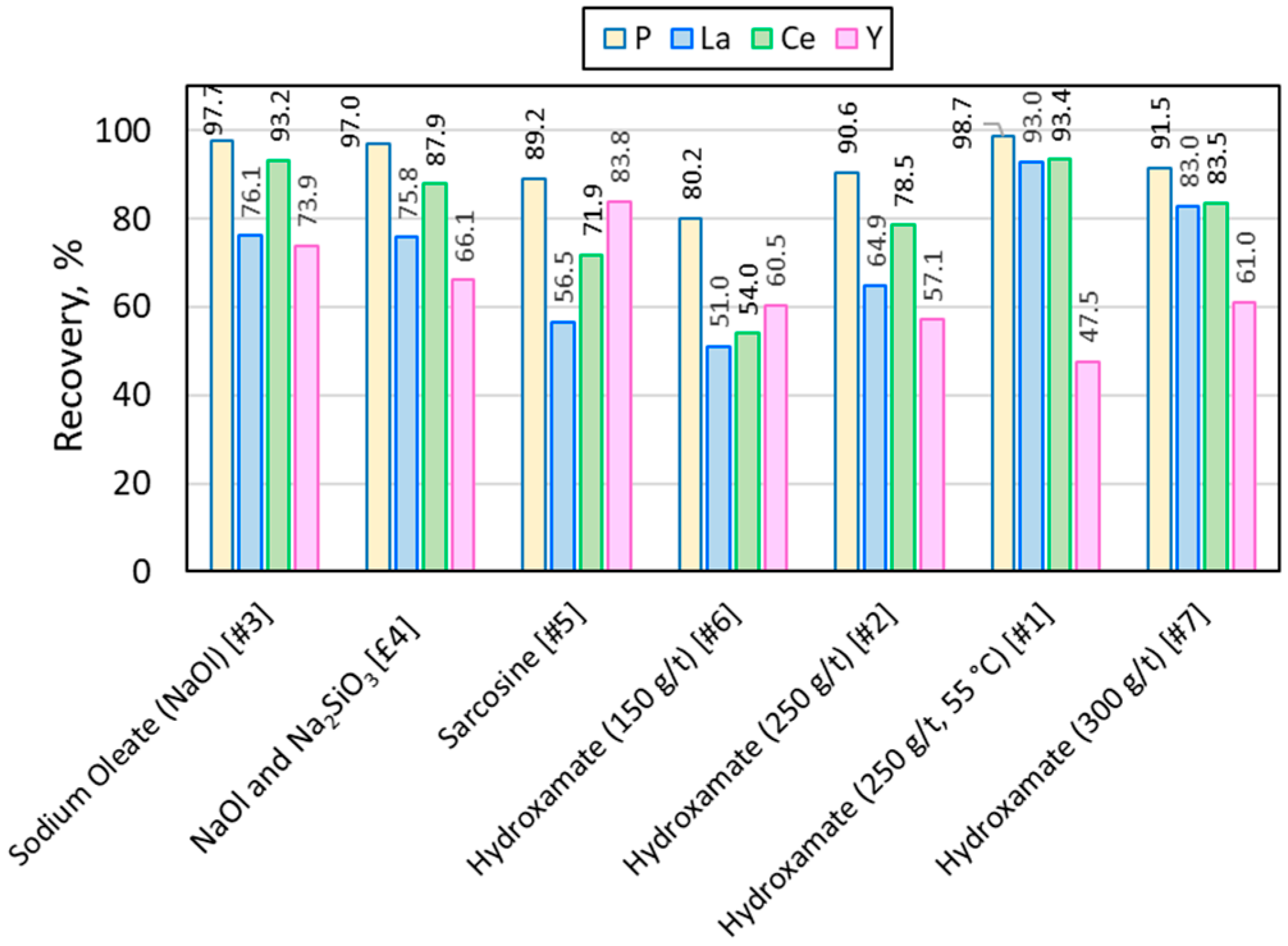
3.2. Lab-Scale Flotation with Conventional Collectors
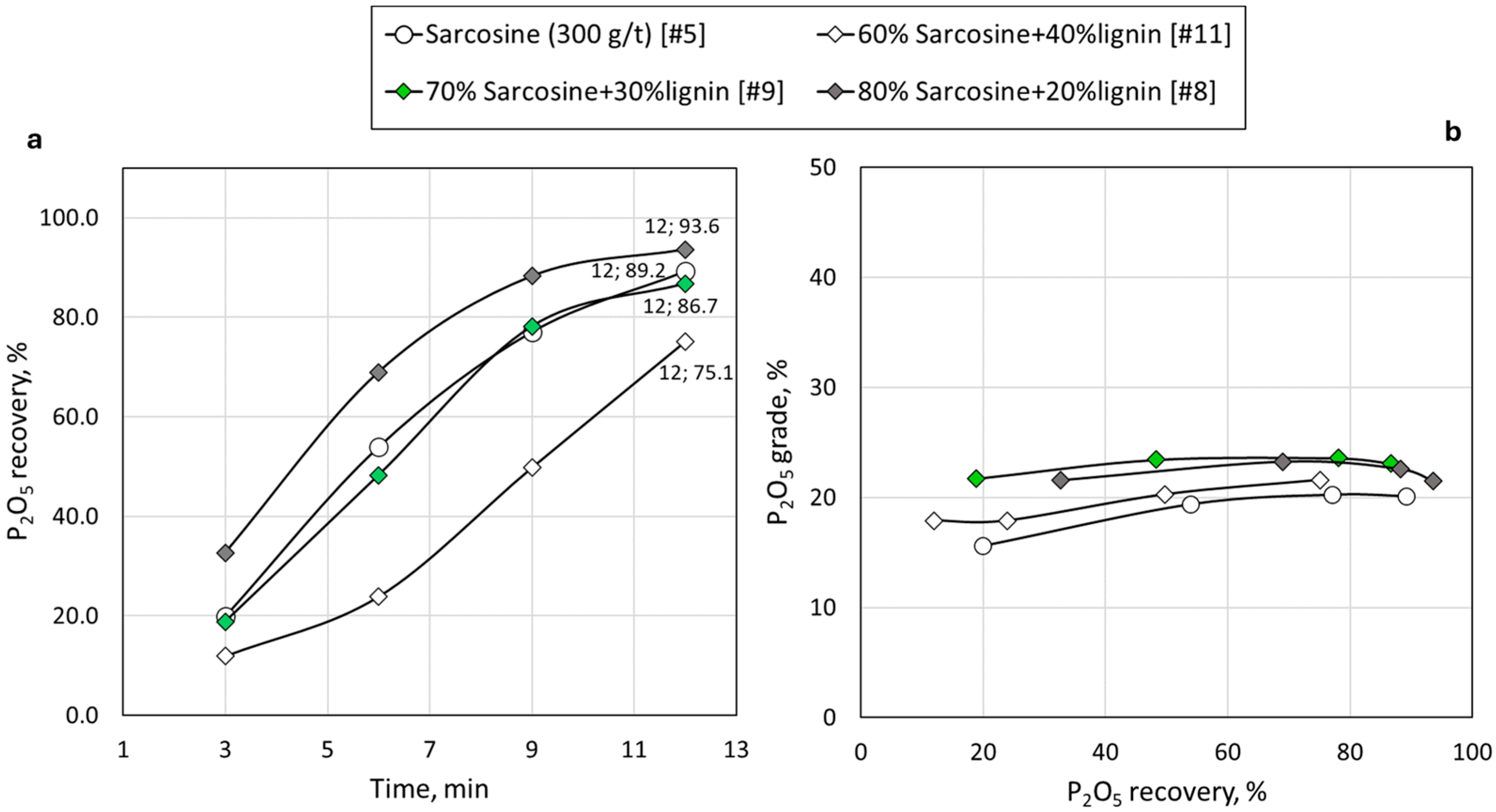
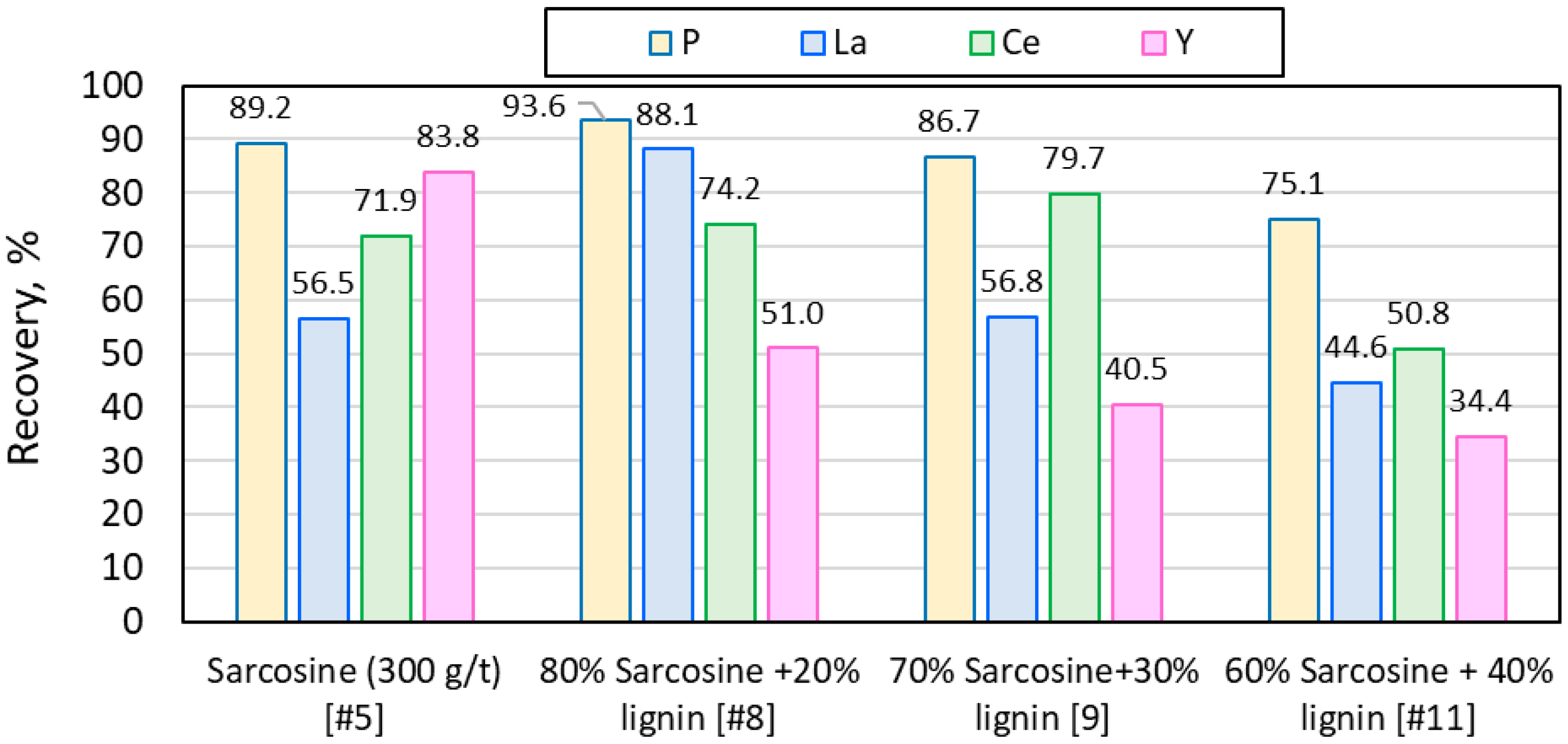
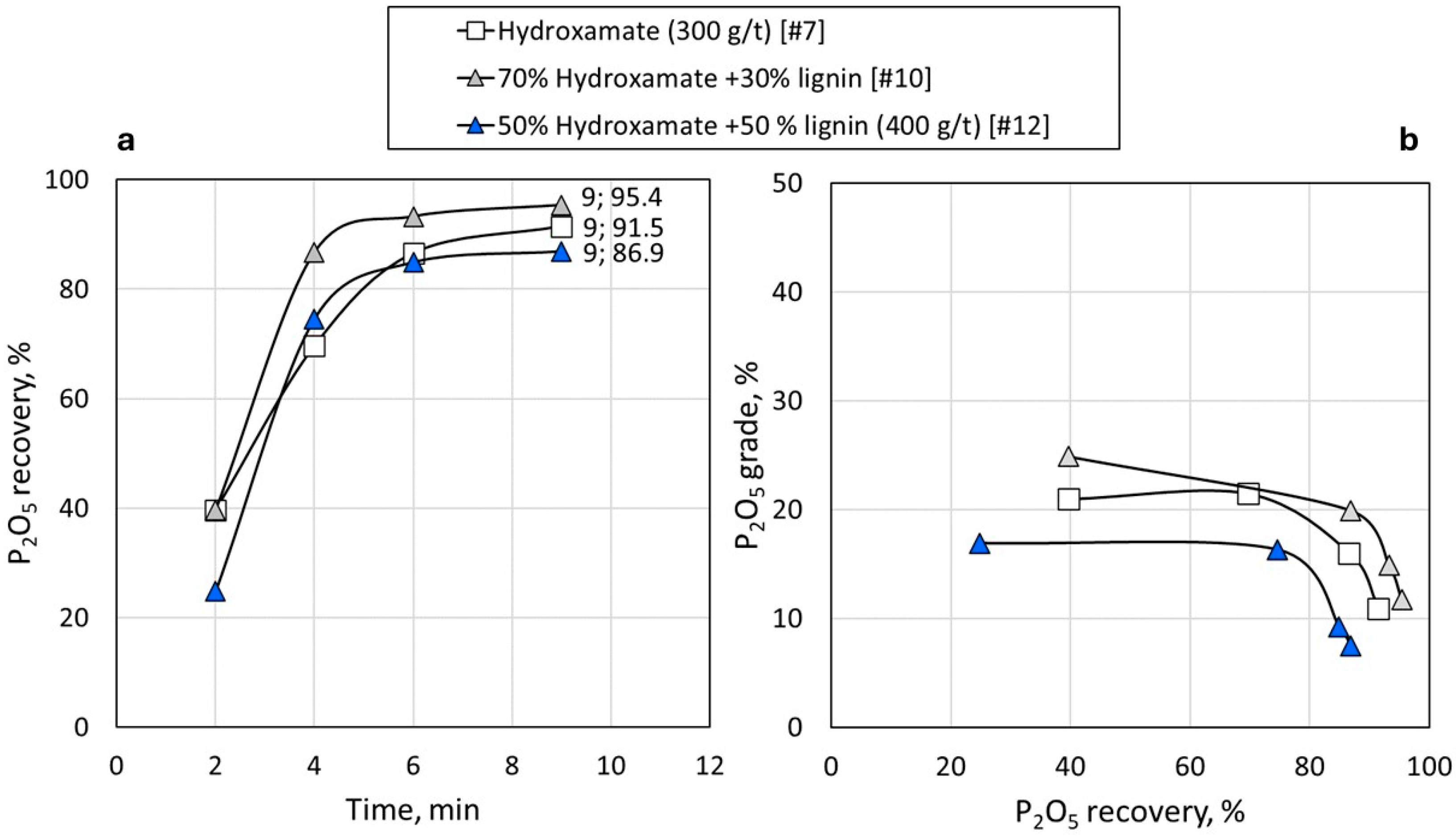
3.3. Synergy of Lignin with Conventional Reagents
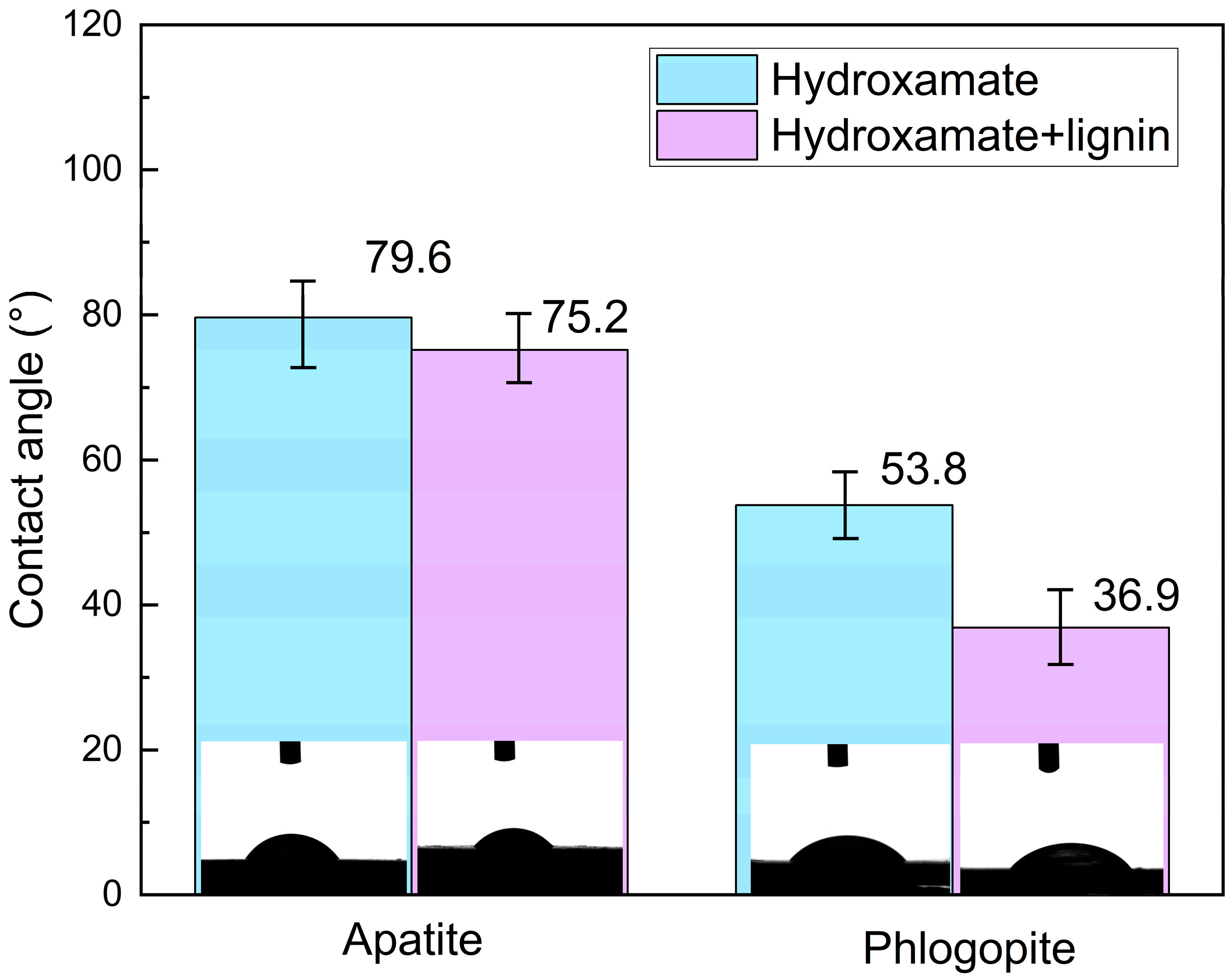
3.4. Separation Efficiency
3.5. Bench-Scale Flotation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of Rare Earth Elements Adsorbed on Clay Minerals: I. Desorption Mechanism. Hydrometallurgy 2012, 117–118, 71–78. [Google Scholar] [CrossRef]
- Mokoena, B.K.; Mokhahlane, L.S.; Clarke, S. Effects of Acid Concentration on the Recovery of Rare Earth Elements from Coal Fly Ash. Int. J. Coal Geol. 2022, 259, 104037. [Google Scholar] [CrossRef]
- Dushyantha, N.P.; Ratnayake, N.P.; Premasiri, H.M.R.; Ilankoon, I.M.S.K.; Hemalal, P.V.A.; Jayawardena, C.L.; Chandrajith, R.; Rohitha, L.P.S.; Abeysinghe, A.M.K.B.; Dissanayake, D.M.D.O.K.; et al. Leaching of Rare Earth Elements (REEs) from Lake Sediments around Eppawala Phosphate Deposit, Sri Lanka: A Secondary Source for REEs. Hydrometallurgy 2021, 205, 105751. [Google Scholar] [CrossRef]
- Cordier, D.U.S. Geological Survey, 2023, Rare Earths. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-molybdenum.pdf (accessed on 7 September 2024).
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s Rare Earth Element Resource Potential: An Overview of REE Metallogenetic Provinces and Their Geodynamic Setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- EuRare Project Webpage. Available online: https://www.eurare.org/contactUs.html (accessed on 14 November 2024).
- Bhattacharjee, M.; Behera, K.K.; Swain, S.P. Apatite-Hosted REE Mineralization in the Eastern Ghats Mobile Belt: A Future Prospect for REE. Geosystems Geoenvironment 2024, 3, 100290. [Google Scholar] [CrossRef]
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite Enrichment by Rare Earth Elements: A Review of the Effects of Surface Properties. Adv. Colloid Interface Sci. 2019, 265, 14–28. [Google Scholar] [CrossRef]
- Xiqiang, L.; Hui, Z.; Yong, T.; Yunlong, L. REE Geochemical Characteristic of Apatite: Implications for Ore Genesis of the Zhijin Phosphorite. Minerals 2020, 10, 1012. [Google Scholar] [CrossRef]
- Ihlen, P.M.; Schiellerup, H.; Gautneb, H.; Skår, Ø. Characterization of Apatite Resources in Norway and Their REE Potential—A Review. Ore Geol. Rev. 2014, 58, 126–147. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Mariano, A.N. Rare-Earth-Element Ordering and Structural Variations in Natural Rare-Earth-Bearing Apatites. Am. Mineral. 1991, 76, 1165–1173. [Google Scholar]
- Pan, Y.; Fleet, M.E. Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- O’Brien, H.; Heilimo, E.; Heino, P. Chapter 4.3—The Archean Siilinjärvi Carbonatite Complex. In Mineral Deposits of Finland; Maier, W.D., Lahtinen, R., O’Brien, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 327–343. ISBN 978-0-12-410438-9. [Google Scholar]
- Decrée, S.; Savolainen, M.; Mercadier, J.; Debaille, V.; Höhn, S.; Frimmel, H.; Baele, J.M. Geochemical and Spectroscopic Investigation of Apatite in the Siilinjärvi Carbonatite Complex: Keys to Understanding Apatite Forming Processes and Assessing Potential for Rare Earth Elements. Appl. Geochem. 2020, 123, 104778. [Google Scholar] [CrossRef]
- Puustinen, K.; Kauppinen, H. The Siilinjärvi Carbonatite Complex, Eastern Finland. In Phosphate Deposits of the World. Volume 2: Phosphate Rock Resources; Notholt, A.J.G., Sheldon, R.P., Davidsoneditors, D.F., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 394–397. ISBN 0-521-67333-X. [Google Scholar]
- Al Ani, T. Mineralogy and Petrography of Siilinjärvi Carbonatite and Glimmerite Rocks, Estern Finland; Geological Survey of Finland: Espoo, Finland, 2013. [Google Scholar]
- Vaziri Hassas, B.; Rezaee, M.; Pisupati, S.V. Precipitation of Rare Earth Elements from Acid Mine Drainage by CO2 Mineralization Process. Chem. Eng. J. 2020, 399, 125716. [Google Scholar] [CrossRef]
- Patil, A.B.; Struis, R.P.W.J.; Ludwig, C. Opportunities in Critical Rare Earth Metal Recycling Value Chains for Economic Growth with Sustainable Technological Innovations. Circ. Econ. Sustain. 2023, 3, 1127–1140. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Yang, X.; Heino, N.; Pakkanen, L. Beneficiation Studies of a Rare Earth Ore from the Olserum Deposit. Nat. Resour. 2019, 10, 346–357. [Google Scholar] [CrossRef]
- Alsabbagh, A.H.; Mustafa, R.M. Wet Gravity Separation and Froth Floatation Techniques for Rare Earth Elements Beneficiation from Monazite Ore in Jordan. Heliyon 2023, 9, e19597. [Google Scholar] [CrossRef]
- Quast, K. Flotation of Hematite Using C6-C18 Saturated Fatty Acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Li, D.; Nie, G.; Wang, Z.; Li, J.; Li, J. Flotation Separation of Dolomite from Fluorapatite Using NaF as an Activator. Surf. Interface Anal. 2021, 53, 860–866. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation Mechanism of Oleic Acid Amide on Apatite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 127–131. [Google Scholar] [CrossRef]
- Eskanlou, A.; Arnold, B.J.; Foucaud, Y.; Badawi, M.; Dzade, N.Y. Optimizing Flotation Separation of Fluorapatite from Florida Waste Clay Using a Multiscale Approach. Appl. Surf. Sci. 2024, 662, 160067. [Google Scholar] [CrossRef]
- Karlkvist, T.; Patra, A.; Rao, K.H.; Bordes, R.; Holmberg, K. Flotation Selectivity of Novel Alkyl Dicarboxylate Reagents for Apatite–Calcite Separation. J. Colloid. Interface Sci. 2015, 445, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Y.; Weng, X.; Jin, Y.; Kasomo, R.M.; Ao, S. Flotation Separation of Dolomite from Fluorapatite Using Sodium Dodecyl Benzene Sulfonate as the Efficient Collector under Low Temperature. Minerals 2022, 12, 228. [Google Scholar] [CrossRef]
- Wang, L.; Tian, M.; Khoso, S.A.; Hu, Y.; Sun, W.; Gao, Z. Improved Flotation Separation of Apatite from Calcite with Benzohydroxamic Acid Collector. Miner. Process. Extr. Metall. Rev. 2019, 40, 427–436. [Google Scholar] [CrossRef]
- Paulsen Thoresen, P.; Lange, H.; Crestini, C.; Rova, U.; Matsakas, L.; Christakopoulos, P. Characterization of Organosolv Birch Lignins: Toward Application-Specific Lignin Production. ACS Omega 2021, 6, 4374–4385. [Google Scholar] [CrossRef]
- Hrůzová, K.; Kolman, K.; Matsakas, L.; Nordberg, H.; Christakopoulos, P.; Rova, U. Characterization of Organosolv Lignin Particles and Their Affinity to Sulfide Mineral Surfaces. ACS Appl. Nano Mater. 2023, 6, 17387–17396. [Google Scholar] [CrossRef]
- Qian, H.; Bao, J.; Shen, C.; Wu, D.; Wang, J.; Hao, H.; Zhang, Y. Improved Flotation Separation of Scheelite from Calcite by Sulfomethylated Kraft Lignin. Materials 2023, 16, 4690. [Google Scholar] [CrossRef]
- Vidal, C.; Carrillo-Varela, I.; Reyes-Contreras, P.; Gutiérrez, L.; Mendonça, R.T. Sulfomethylation of Radiata Pine Kraft Lignin and Its Use as a Molybdenite Depressant in Selective Chalcopyrite-Molybdenite Separation by Flotation. Bioresources 2021, 16, 5646–5666. [Google Scholar] [CrossRef]
- Hrůzová, K.; Matsakas, L.; Sand, A.; Rova, U.; Christakopoulos, P. Organosolv Lignin Hydrophobic Micro- and Nanoparticles as a Low-Carbon Footprint Biodegradable Flotation Collector in Mineral Flotation. Bioresour. Technol. 2020, 306, 123235. [Google Scholar] [CrossRef]
- Witecki, K.; Szkurat, M.; Hruzova, K. Organosolv Lignin Particles as an Ecological Reagent in the Kupfershiefer Copper Ore Flotation. Physicochem. Probl. Miner. Process. 2023, 59, 174363. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Anastassakis, G.; Kountouris, N.; Taxiarchou, M.; Koutsotheodorou, E.; Pefkos, T.; Klepkos, V.; Samara, C.; Mprokos, G. Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials. Mater. Proc. 2023, 15, 81. [Google Scholar]
- Bazar, J.A.; Hrůzová, K.; Jolsterå, R.; Matsakas, L.; Rova, U.; Christakopoulos, P. Enhancing Froth Flotation Performance of Iron Oxide Apatite Ore Tailings through Synergistic Utilization of Organosolv Lignin Particles and Tall Oil Fatty Acid-Based Collector. Miner. Eng. 2024, 216, 108868. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Matsakas, L.; Aspden, J.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules 2018, 23, 1647. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Gerber, M.; Yu, L.; Rova, U.; Christakopoulos, P. Preparation of Low Carbon Impact Lignin Nanoparticles with Controllable Size by Using Different Strategies for Particles Recovery. Ind. Crops Prod. 2020, 147, 112243. [Google Scholar] [CrossRef]
- Valderrama, L.; Gómez, O.; Pavez, O.; Santander, M. Recovery of Apatite from Magnetic Concentration Tailings by Flotation. Minerals 2024, 14, 441. [Google Scholar] [CrossRef]
- Pålsson, B.I.; Martinsson, O.; Wanhainen, C.; Fredriksson, A. Unlocking Rare Earth Elements from European Apatite-Iron Ores. In Proceedings of the 1st European Rare Earth Resources Conference (ERES2014), Milos, Greece, 4–7 September 2014; pp. 211–220. [Google Scholar]
- Karlkvist, T. Selectivity in Calcium Mineral Flotation—An Analysis of Novel and Existing Approaches. Ph.D. Thesis, Lulea University of Technology, Luleå, Sweden, 2017. [Google Scholar]
- Karvinen, S.; Heinonen, A.; Beier, C.; Jöns, N. The Composition of Apatite in the Archean Siilinjärvi Glimmerite-Carbonatite Complex in Eastern Finland. Bull. Geol. Soc. Finl. 2024, 96, 5–34. [Google Scholar] [CrossRef]
- Salo, A. Geology of the Jaakonlampi Area in the Siilinjärvi Carbonatite Complex; University of Oulu: Oulu, Finland, 2016; p. 27. [Google Scholar]
- Su, F.; Hanumantha Rao, K.; Forssberg, K.S.E.; Samskog, P.O. The Influence of Temperature on the Kinetics of Apatite Flotation from Magnetite Fines. Int. J. Miner. Process. 1998, 54, 131–145. [Google Scholar] [CrossRef]
- Cooke, S.R.B.; Iwasaki, I.; Choi, H. Effect of Temperature in Soap Flotation of Iron Ore. Trans. AIME 1960, 217, 76–83. [Google Scholar]
- O’Connor, C.T.; Mills, P.J.T. The Effect of Temperature on the Pulp and Froth Phases in the Flotation of Pyrite. Miner. Eng. 1990, 3, 615–624. [Google Scholar] [CrossRef]
- Pradip; Fuerstenau, D.W. Adsorption of Hydroxamate Collectors on Semi-Soluble Minerals Part II: Effect of Temperature on Adsorption. Colloids Surf. 1985, 15, 137–146. [Google Scholar]
- Li, M.; Gao, K.; Zhang, D.; Duan, H.; Ma, L.; Huang, L. The Influence of Temperature on Rare Earth Flotation with Naphthyl Hydroxamic Acid. J. Rare Earths 2018, 36, 99–107. [Google Scholar] [CrossRef]
- Pavez, O.; Peres, A.E.C. Effect of Sodium Metasilicate and Sodium Sulphide on the Floatability of Monazite-Zircon-Rutile with Oleate and Hydroxamates. Miner. Eng. 1993, 6, 69–78. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; Li, R.; Rudolph, M.; Waters, K.E. An Evaluation of Hydroxamate Collectors for Malachite Flotation. Sep. Purif. Technol. 2017, 183, 258–269. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; Chaves, A.P.; Ferreira, W.R. Effect of Alkyl Chain Length of Amines on Fluorapatite and Aluminium Phosphates Floatabilities. J. Mater. Res. Technol. 2019, 8, 3623–3634. [Google Scholar] [CrossRef]
- Balan, E.; Delattre, S.; Roche, D.; Segalen, L.; Morin, G.; Guillaumet, M.; Blanchard, M.; Lazzeri, M.; Brouder, C.; Salje, E.K.H. Line-Broadening Effects in the Powder Infrared Spectrum of Apatite. Phys. Chem. Miner. 2011, 38, 111–122. [Google Scholar] [CrossRef]
- Sammons, R.J.; Harper, D.P.; Labbe, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of Organosolv Lignin Using Thermal and FT-IR Spectroscopic Analysis. Bioresources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Šontevska, V.; Jovanovski, G.; Makreski, P.; Raškovska, A.; Šoptrajanov, B. Minerals from Macedonia. XXI. Vibrational Spectroscopy as Identificational Tool for Some Phyllosilicate Minerals. Acta Chim. Slov. 2008, 55, 757–766. [Google Scholar]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, X.; Yang, D.; Yi, C.; Qiu, X. Pi Stacking in Lignosulfonates. BioResources 2012, 7, 1145–1156. [Google Scholar] [CrossRef]
- Gaudin, A.M. Flotation, 2nd ed.; McGraw-Hill: New York, NY, USA, 1957. [Google Scholar]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780080970530. [Google Scholar]







| Trade Name and Formula | Molecular Structure of the Functional Group |
|---|---|
| Aero 6494© (Syensqo SA, Brussels, Belgium) anionic, alkyl hydroxamate-based collector | 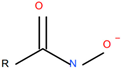 |
| Sodium Oleate (NaOL) (Merck KGaA, Darmstadt, Germany), anionic, sodium salt of oleic acid | 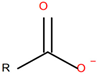 |
| Berol A3© (Nouryon, Amsterdam, Netherlands), sarcosine, a carboxylic acid coupled to a methylated nitrogen | 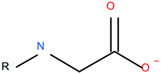 |
| Number | Reagents (g/t) | pH | Time (m) | Cell Size (L) | Pulp Density (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxamate (Aero 6494®) | Long-Chain Fatty Acid (NaOL) | Sarcosine (Berol A3®) | Organosolv Nanosized Lignin | Replacement ** | Na2SiO3 | Conditioning | Flotation | ||||
| 1 * | 250 | 800 | 10 | 5 | 9 | 2.5 | 300 | ||||
| 2 | 250 | 800 | 10 | 5 | 9 | 2.5 | 300 | ||||
| 3 | 300 | 10.5 | 5 | 6 | 2.5 | 300 | |||||
| 4 | 350 | 1400 | 10.5 | 5 | 9 | 2.5 | 300 | ||||
| 5 | 300 | 11 | 5 | 9 | 2.5 | 300 | |||||
| 6 | 150 | 800 | 10 | 5 | 12 | 2.5 | 300 | ||||
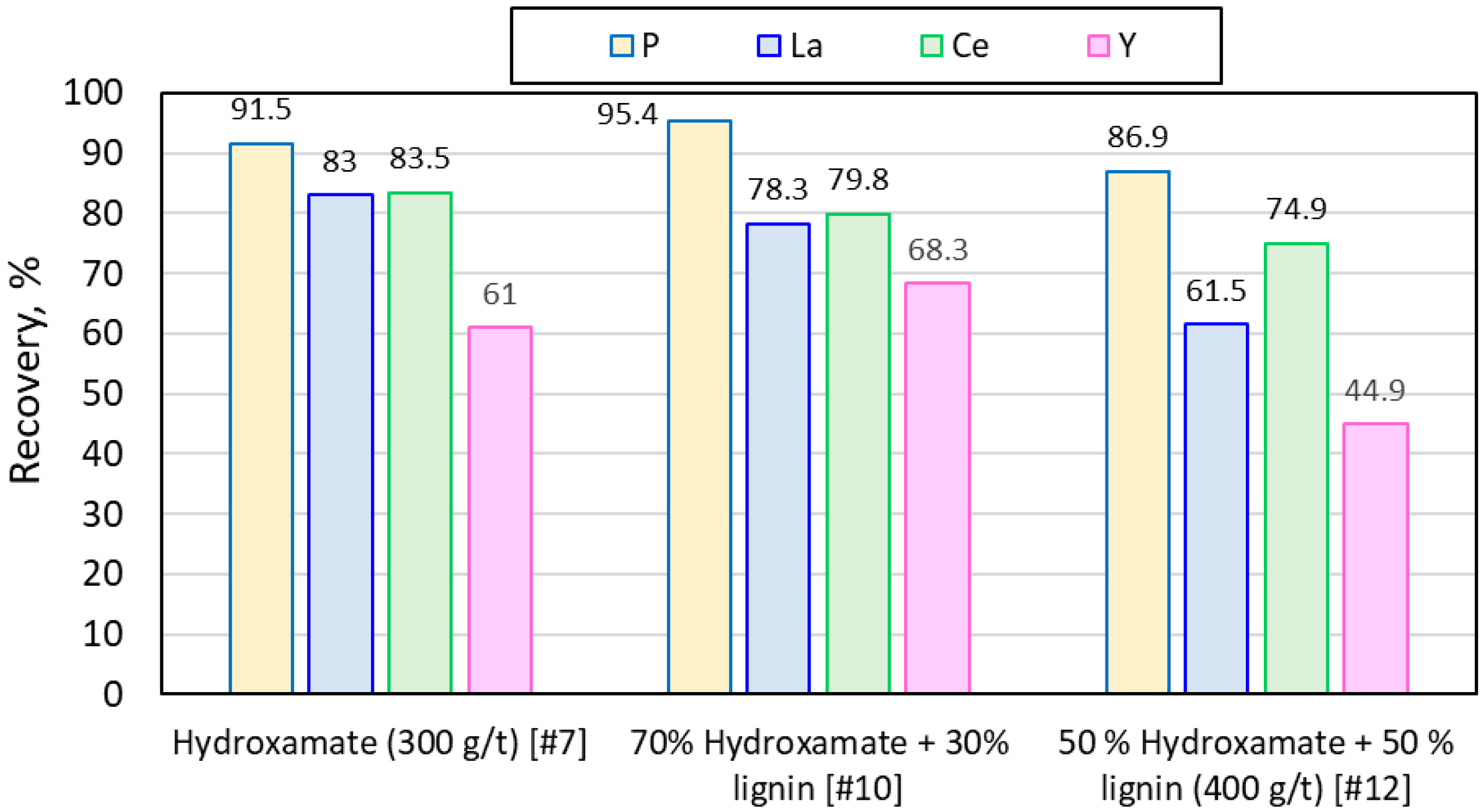
| 7 | 300 | 800 | 10 | 5 | 9 | 2.5 | 300 | ||||
| 8 | 240 | 60 | 20 | 11 | 5 | 9 | 2.5 | 300 | |||
| 9 | 210 | 90 | 30 | 11 | 5 | 9 | 2.5 | 300 | |||
| 10 | 210 | 90 | 30 | 800 | 10 | 5 | 9 | 2.5 | 300 | ||
| 11 | 180 | 120 | 40 | 11 | 5 | 9 | 2.5 | 300 | |||
| 12 | 200 | 200 | 50 | 800 | 10 | 5 | 9 | 2.5 | 300 | ||
| Oxide | Content (wt. %) |
|---|---|
| SiO2 | 32.4 |
| MgO | 17.90 |
| CaO | 17.17 |
| Al2O3 | 7.52 |
| FeO | 6.88 |
| K2O | 6.51 |
| P2O5 | 3.75 |
| Na2O3 | 0.40 |
| C | 3.04 |
| La | 0.01 |
| Ce | 0.019 |
| Y | 0.002 |
| Mineral | Content (wt. %) |
|---|---|
| K-feldspar | 2.28 |
| Phlogopite (KMg3(AlSi3O10)(OH)2) | 57.37 |
| Biotite (K(Mg,Fe)3(AlSi3)O10(OH)2) | 3.18 |
| Apatite (Ca5(PO4)3(OH, F, Cl) | 8.87 |
| Calcite (CaCO3) | 16.68 |
| Dolomite (CaMg(CO3)2 | 2.45 |
| Magnetite (Fe3O4) | 0.74 |
| Flotation Experiment | REE Grade in Final Concentrate, % | ||
|---|---|---|---|
| La | Ce | Y | |
| Sodium Oleate (NaOl) [#3] | 0.0192 | 0.0413 | 0.0031 |
| NaOl and Na2SiO3 [#4] | 0.0237 | 0.0550 | 0.0037 |
| Sarcosine [#5] | 0.0402 | 0.0926 | 0.0054 |
| Hydroxamate (150 g/t) [#6] | 0.0498 | 0.1046 | 0.0041 |
| Hydroxamate (250 g/t) [#2] | 0.0265 | 0.0627 | 0.0038 |
| Hydroxamate (250 g/t, 55 °C) [#1] | 0.0257 | 0.0550 | 0.0032 |
| Hydroxamate (300 g/t) [#7] | 0.0299 | 0.0595 | 0.0037 |
| In the feed | 0.0098 | 0.0190 | 0.0020 |
| Number | Reagents | Collector Dosage Reduction (%) | P rec. in Conc. (%) | Mg rec. in Conc. (%) | Mg rec. in Tail (%) | SI | SE |
|---|---|---|---|---|---|---|---|
| 1 | Hydroxamate diluted at 55 °C | - | 98.7 | 13.6 | 86.4 | 5.2 | 85.1 |
| 2 | Hydroxamate | - | 90.6 | 13.7 | 86.3 | 0.7 | 76.9 |
| 3 | Fatty acid | - | 97.7 | 38.3 | 61.7 | 0.9 | 59.4 |
| 4 | Fatty acid, Na2SiO3 | - | 97 | 46.2 | 53.8 | 0.5 | 50.8 |
| 5 | Sarcosine | - | 89.2 | 6.7 | 93.3 | 1.3 | 82.5 |
| 6 | Hydroxamate, Na2SiO3 | - | 80.2 | 59.3 | 40.7 | 0.0 | 20.9 |
| 7 | Hydroxamate, Na2SiO3 | - | 91.5 | 8.9 | 91.1 | 1.2 | 82.6 |
| 8 | Sarcosine, lignin | 20 | 93.6 | 4.8 | 95.2 | 3.1 | 88.8 |
| 9 | Sarcosine, lignin | 30 | 86.7 | 5.5 | 94.5 | 1.2 | 81.2 |
| 10 | Hydroxamate, lignin, Na2SiO3 | 30 | 95.4 | 18.6 | 81.4 | 1.0 | 76.8 |
| 11 | Sarcosine, lignin | 40 | 75.1 | 4.7 | 95.3 | 0.7 | 70.4 |
| 12 | Hydroxamate, lignin, Na2SiO3 | 50 | 86.9 | 5.9 | 94.1 | 1.2 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, P.M.; Yang, X.S.; Anastassakis, G.; Koukoulis, N.; Christakopoulos, P.; Taxiarchou, M. Multiscale Flotation Testing for the Recovery of REE-Bearing Fluorapatite from a Finnish Carbonatite Complex Deposit Using Conventional Collectors and Lignin Nanoparticles. Minerals 2025, 15, 614. https://doi.org/10.3390/min15060614
Angelopoulos PM, Yang XS, Anastassakis G, Koukoulis N, Christakopoulos P, Taxiarchou M. Multiscale Flotation Testing for the Recovery of REE-Bearing Fluorapatite from a Finnish Carbonatite Complex Deposit Using Conventional Collectors and Lignin Nanoparticles. Minerals. 2025; 15(6):614. https://doi.org/10.3390/min15060614
Chicago/Turabian StyleAngelopoulos, Panagiotis M., Xiao Sheng Yang, Georgios Anastassakis, Nikolaos Koukoulis, Paul Christakopoulos, and Maria Taxiarchou. 2025. "Multiscale Flotation Testing for the Recovery of REE-Bearing Fluorapatite from a Finnish Carbonatite Complex Deposit Using Conventional Collectors and Lignin Nanoparticles" Minerals 15, no. 6: 614. https://doi.org/10.3390/min15060614
APA StyleAngelopoulos, P. M., Yang, X. S., Anastassakis, G., Koukoulis, N., Christakopoulos, P., & Taxiarchou, M. (2025). Multiscale Flotation Testing for the Recovery of REE-Bearing Fluorapatite from a Finnish Carbonatite Complex Deposit Using Conventional Collectors and Lignin Nanoparticles. Minerals, 15(6), 614. https://doi.org/10.3390/min15060614










