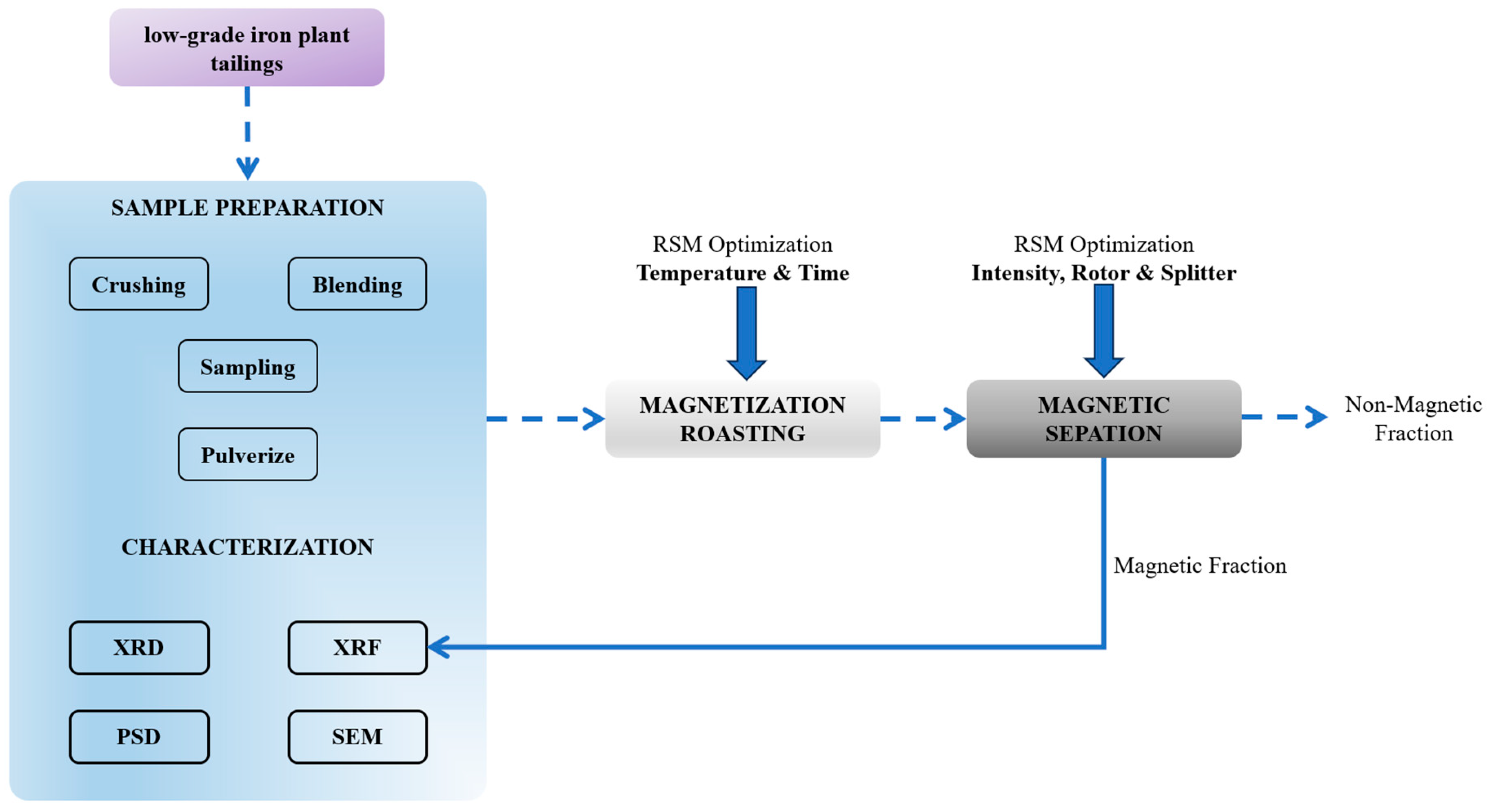
3.1. The As-Received Sample Characterization
The chemical composition of the sample, detailed in
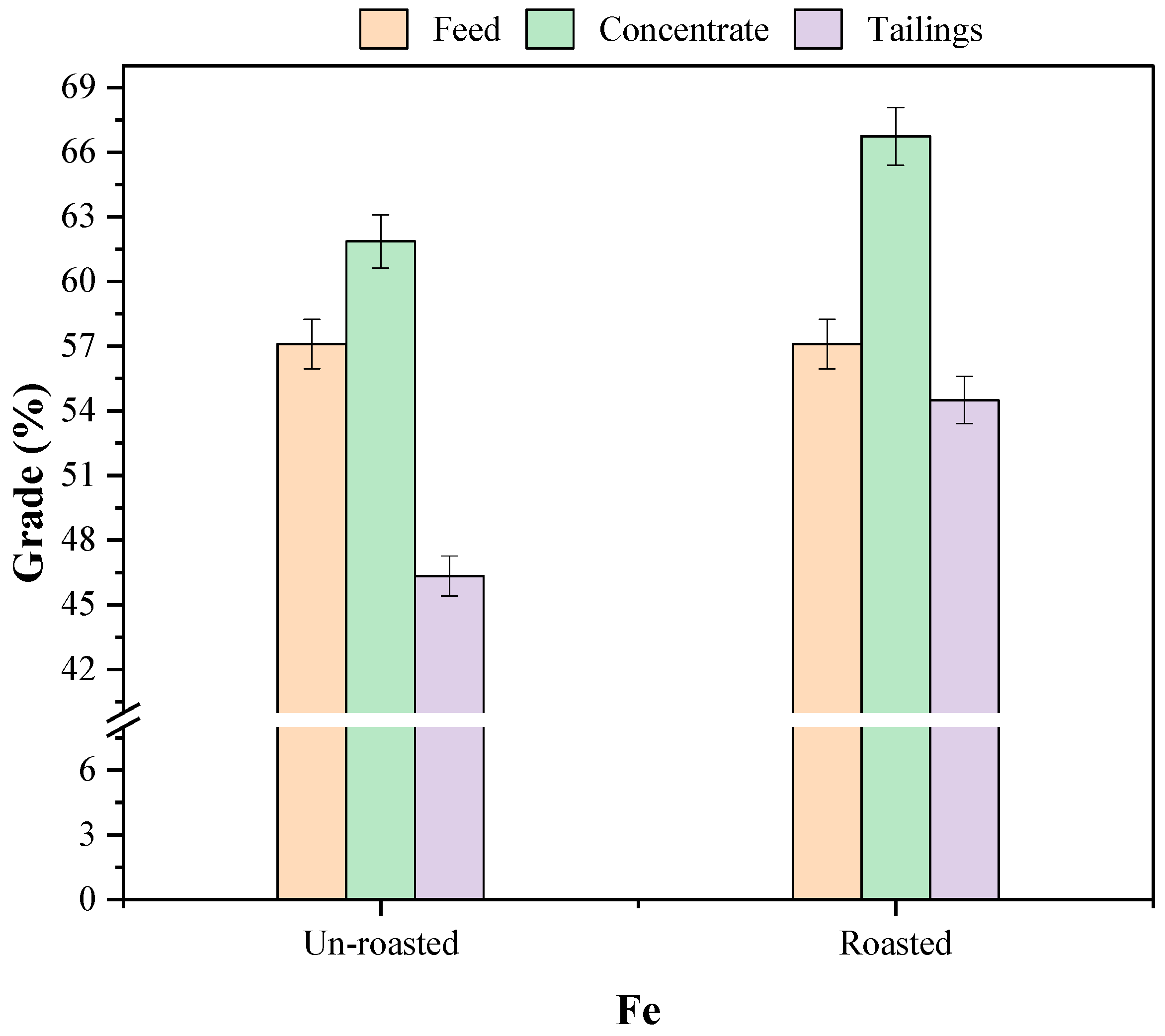
Table 3, reveals that the head sample contains 82.76% Fe
2O
3, corresponding to a total iron (Fe) of 57.1%. Although the iron concentration in the iron plant tailings is substantial, it cannot be utilized directly for economic purposes due to significant impurities. The sample contains approximately 4.1% Al
2O
3 (2.2% Al) and 10.9% SiO
2 (5.11% Si). The assigned objective was upgrading the material to achieve at least 65% Fe. Additionally, the combined average of Al
2O
3 and SiO
2 must not exceed 5% to satisfy the required market specifications.
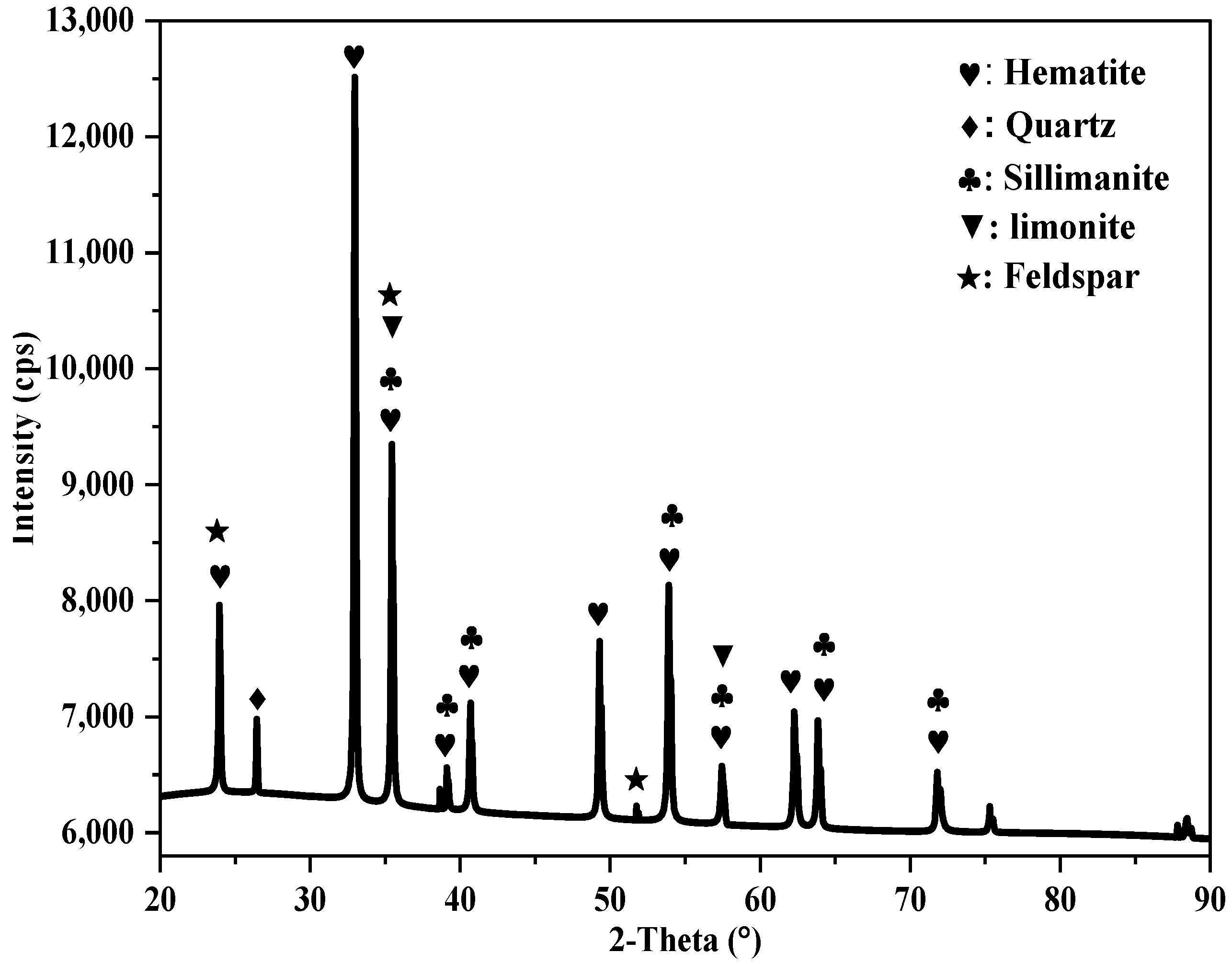
The analysis of the XRD spectrum for the iron tailings sample, as shown in
Figure 2, revealed that the sample was dominated by the presence of Hematite occurring with traces of limonite characterized by paramagnetic behavior. Further examination indicated that Si, Al, and other elements were found in the form of quartz (SiO
2), sillimanite (Al
2SiO
5), and traces of feldspar (Na, K, Ca), Al (Al, Si)
3O
8. According to Srivastava et al. (2001) [
12], quartz and sillimanite can result in a high slag volume, which may lead to the formation of viscous slag during the iron-making process. Therefore, it is crucial to reduce these impurities through beneficiation to enhance the productivity of iron-making operations.
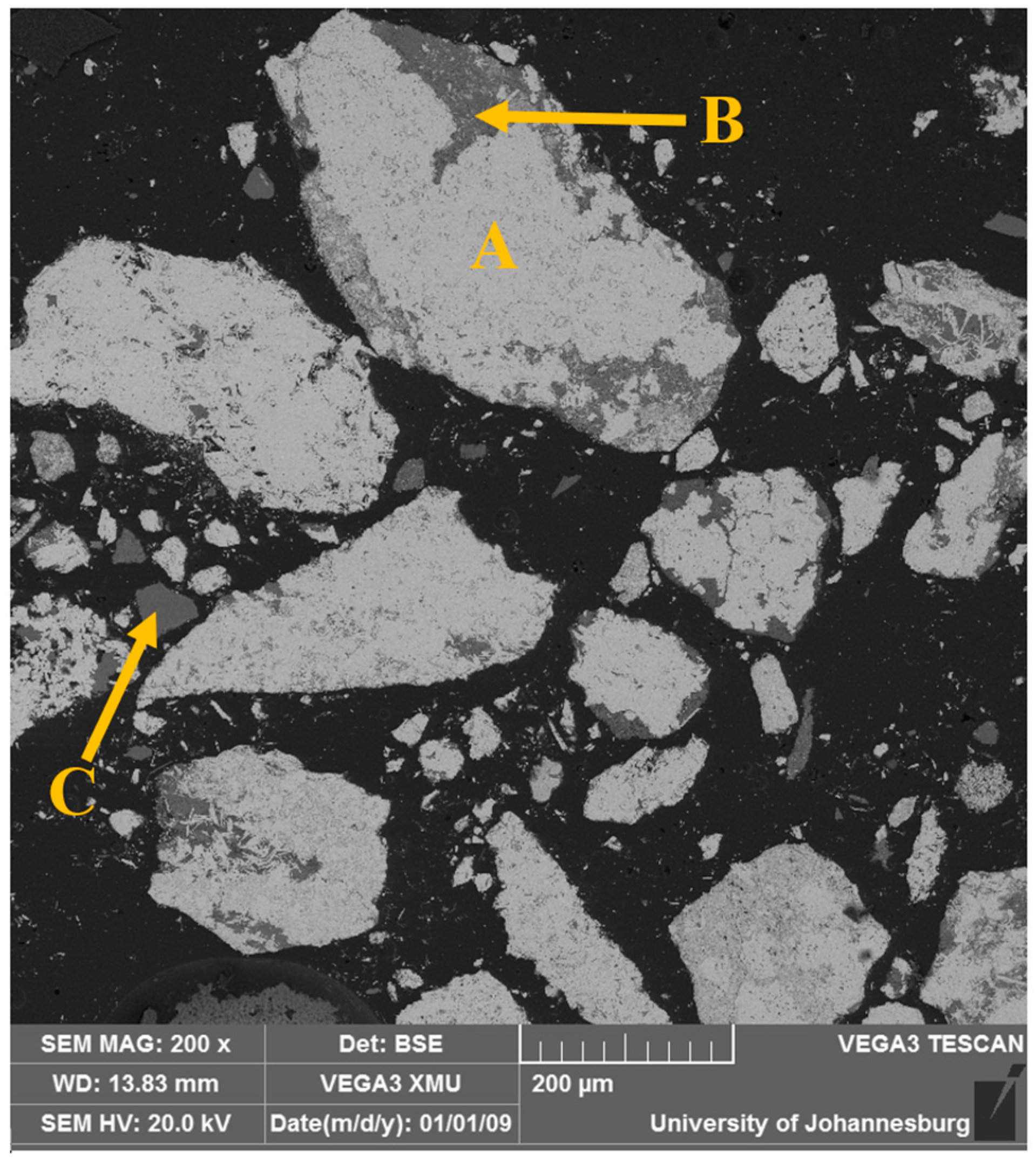
The morphology of the as-received sample was analyzed using SEM, while the chemical composition of the selected areas was determined using EDS. The micrograph results are shown in
Figure 3, and the EDS spectra analysis results are provided in
Table 4. The micrograph indicates that the sample is characterized by two main contrasts, indicating that the hematite phase (light gray) is interlocked into the silicate one (dark gray) and vice versa. It should be noted that the occurrence of the magnetite makes separation challenging. Therefore, a fine milling process was required to liberate Fe phases.
The bright spots that indicate heavier compounds are identified as Fe
2O
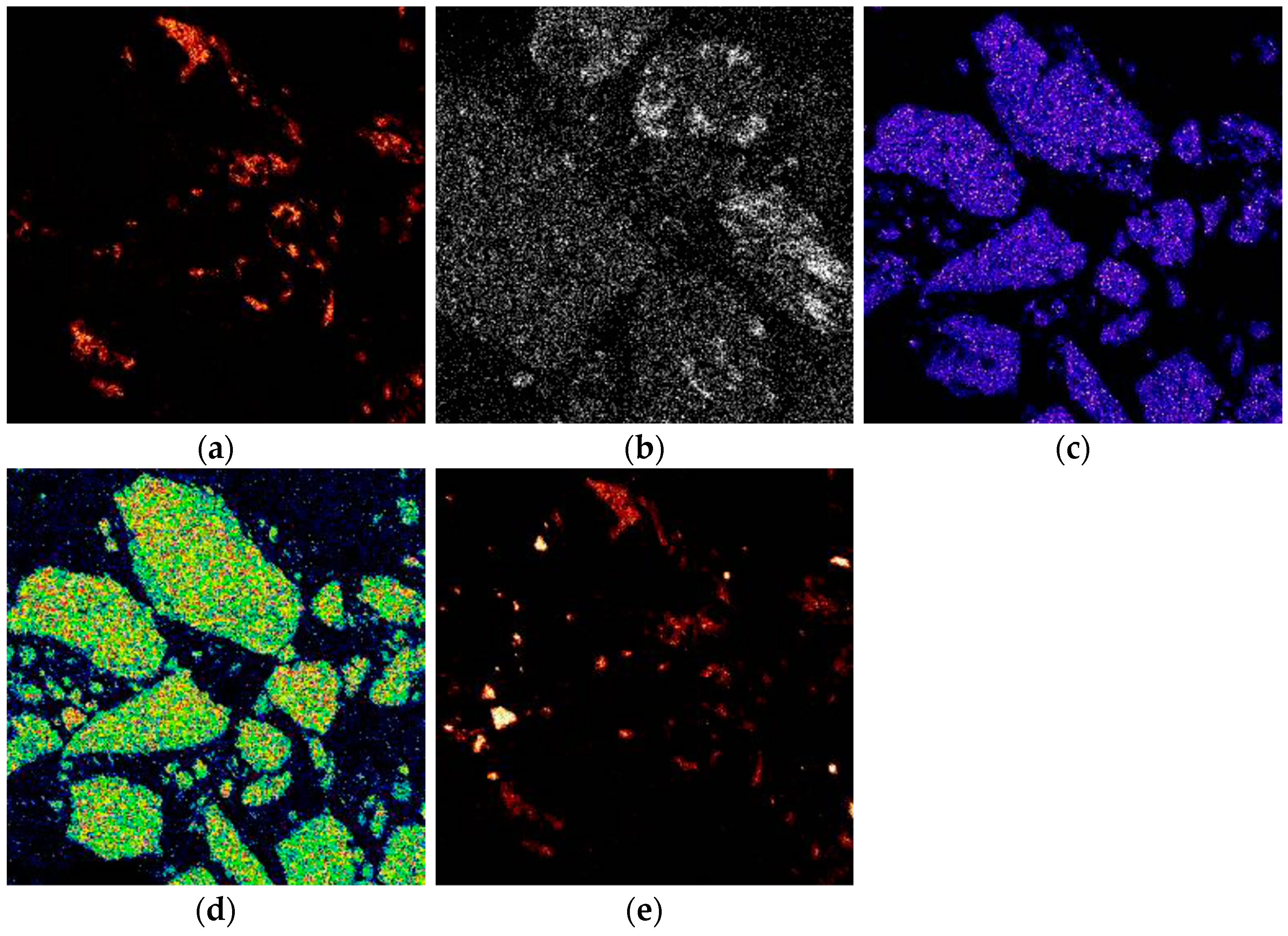
3, whereas the gray spots, predominantly composed of lighter compounds, reveal the presence of minerals such as quartz and sillimanite. The EDS analysis confirmed that the sample primarily comprises hematite, sillimanite, and quartz, consistent with the XRD results. Furthermore, it was noted that silica is distributed throughout the hematite matrix. The circled areas in the mapping, as shown in
Figure 4, confirm the evidence of the interlinked Al-, Fe-, K-, and Si-bearing minerals.
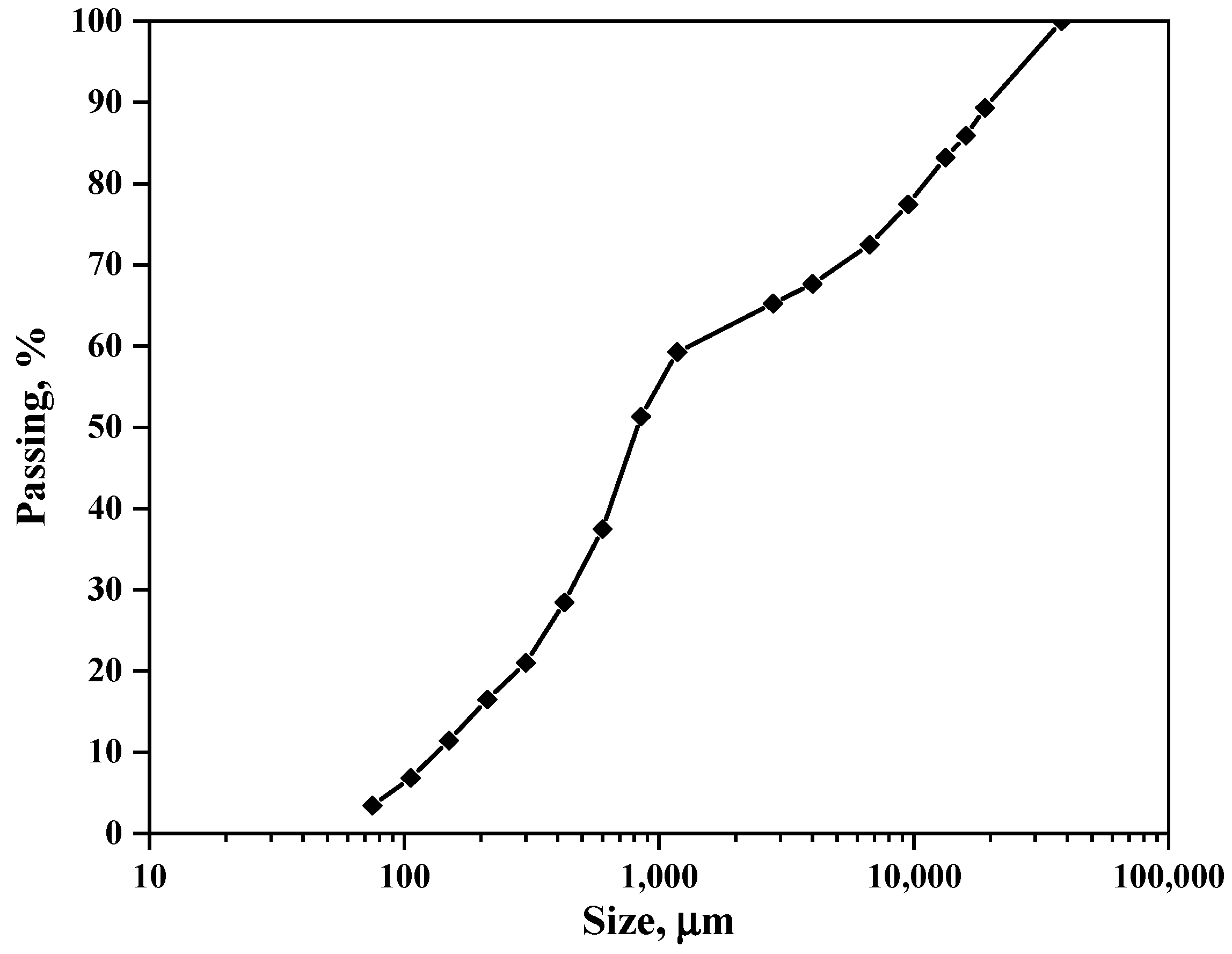
The particle size distribution of the as-received iron plant tailings was analyzed using wire mesh sieves, and the results are illustrated in
Figure 5. The size distribution curve exhibits a bimodal profile, with sections covering the size ranges of 40,000–1180 µm and 1180–75 µm, corresponding to 40% and 60% splits, respectively. This suggests that the iron plant tailing sample originates from a processing route that employs various beneficiation techniques with different size specifications, similar to those described by Das and Rath (2020) [
29]. The analysis further indicated that the F
80 of the iron tailing sample was 11,300 µm, encompassing a wide range of size fractions from 75 to 4000 µm, which points to a mixture of fine and coarse particles. Given that the primary objective of this project involves magnetic separation, it is crucial to compare the size distribution results of the as-received sample with the recommended particle size distribution for the effective operation of an induced dry-roll magnetic separator. This optimal range lies between 100 µm and 3000 µm, as Wills and Napier-Munn (2006) [
30] specified. Therefore, the iron plant tailings must undergo comminution before processing to conform to these specifications.
3.3. Optimization of the Magnetization Roasting Process Parameters
A two-factor five-level RSM optimal design was employed to assess the significance of roasting temperature (
A) and time (
B) and their interactions on the Fe grade and recovery achieved through magnetization roasting. A total of 12 experimental runs were proposed using Design-Expert
® software version 13.0.5.0.
Table 5 presents an overview of the experimental design and the observed responses for optimizing magnetization roasting.
Two statistical models were developed for each response variable, i.e., Fe grade and Fe recovery. A linear first-order model was utilized to represent the relationship between Fe grade and each input factor derived from the experimental data [
31]. Conversely, a quadratic second-order polynomial model described the relationship between controllable variables and Fe grade and recovery. The empirical coded equations for the Fe grade and Fe recovery are shown in Equations (4) and (5), respectively.
where the Fe grade and recovery are given in percentage, roasting temperature (
A) is in °C, and roasting time (
B) is in minutes.
The analysis of variance (ANOVA) was utilized at a 5% significant level to validate the adequacy of the developed regression models for Fe grade and recovery for a
p-value ≤ 0.05.
Table 6 and
Table 7 present the ANOVA analysis conducted for Fe grade and Fe recovery during the magnetization roasting optimization.
Table 6 shows that a fissure test value (F-value) of 5.96 implies that the Fe grade model is significant. There is only a 2.25% chance that an F-value this large could occur due to noise.
The value of Adequate Precision (Adeq Precision) serves as a signal-to-noise ratio to evaluate the sufficiency of a model. Two hypotheses were formulated to assess the sufficiency [
32].
The “null hypotheses—H0” suggests that the “Adeq Precision” value is >4: the predicted model fits the data and indicates adequate model discrimination.
The “alternate hypotheses—Ha” implies that the value of “Adeq Precision” is <4, suggesting that the predicted model does not adequately fit the data and exhibits insufficient model discrimination.
With an “Adeq Precision” ANOVA value of 6.7872, which exceeds 4, we can conclude that the model is sufficient and can be reliably used to navigate the design space [
33].
The values of p-values below 0.05 indicate that the model terms are statistically significant. In the context of Fe grade, the results reveal that term B is significant. The F-value assesses the main effects and their interactions, with larger F-values indicating greater significance. The order of significance for the main effects related to Fe grade was determined to be B > A.
Table 7 shows that the F-value of 23.64 signifies the significance of the Fe recovery model, with only a 0.07% probability that such a large F-value could arise from random noise. Additionally, the “Adeq Precision” ANOVA value of 11.6 exceeds 4, demonstrating that the model is sufficient for navigating the design space.
The analysis indicated that the terms A and A2 were significant in the Fe recovery model (p-value < 0.05). According to the F-value, the order of significance for the main effects and interactions influencing Fe recovery is as follows: A > A2 > A × B > B2 > B.
The comparison of actual and predicted Fe grade and recovery values is derived from the regression model Equations (4) and (5), which are presented in
Table 8.
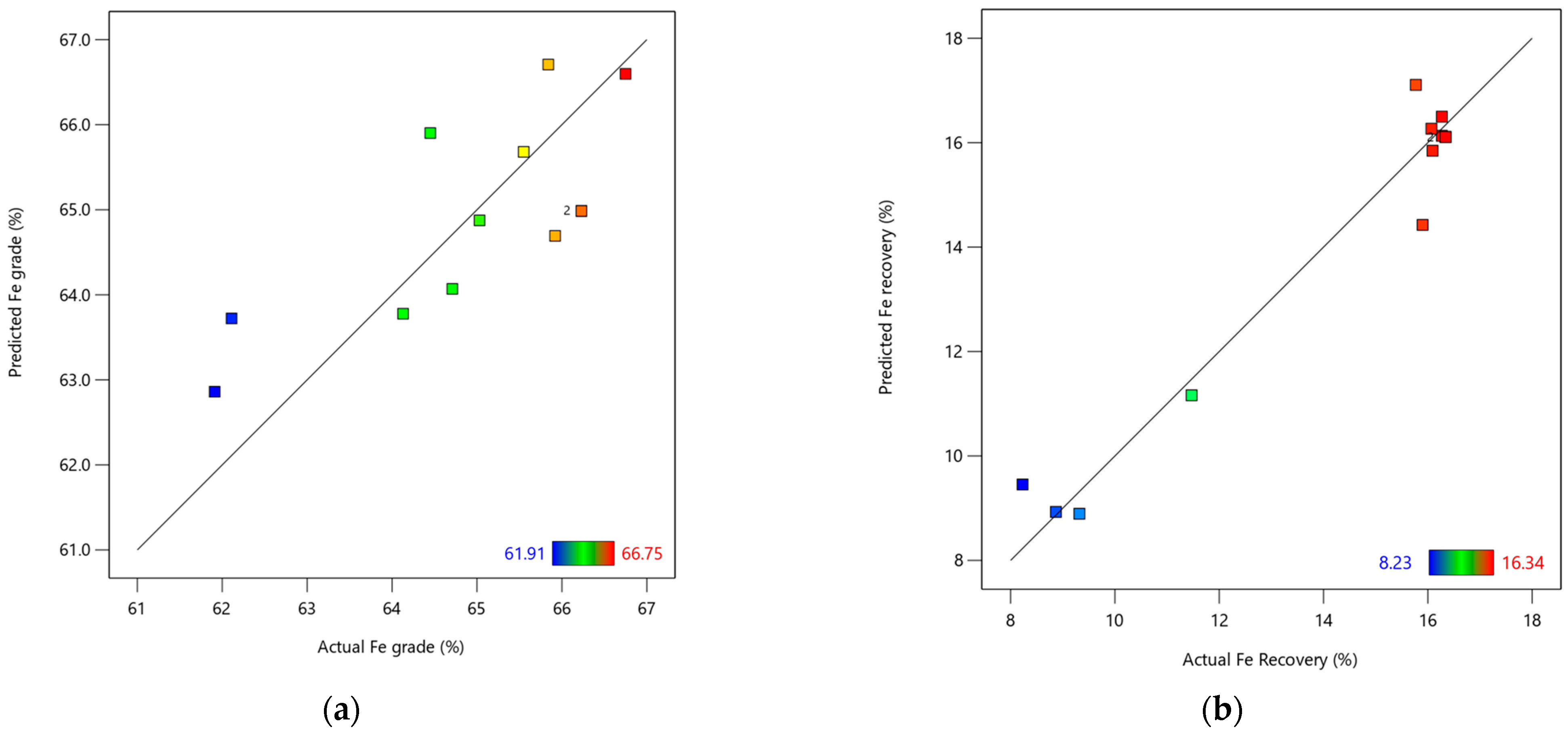
Figure 7a,b also illustrates the relationship between the actual and predicted Fe grade and recovery, respectively.
According to
Figure 7a, a moderately good fit was observed between the actual and predicted Fe, with a correlation R
2 value of 0.56. However, additional investigations may be necessary to refine the regression model. In contrast,
Figure 7b illustrates a strong correlation between the actual and predicted values of Fe recovery, as evidenced by an R
2 value of 0.95. This indicates that the developed iron recovery model is effective and reliable.
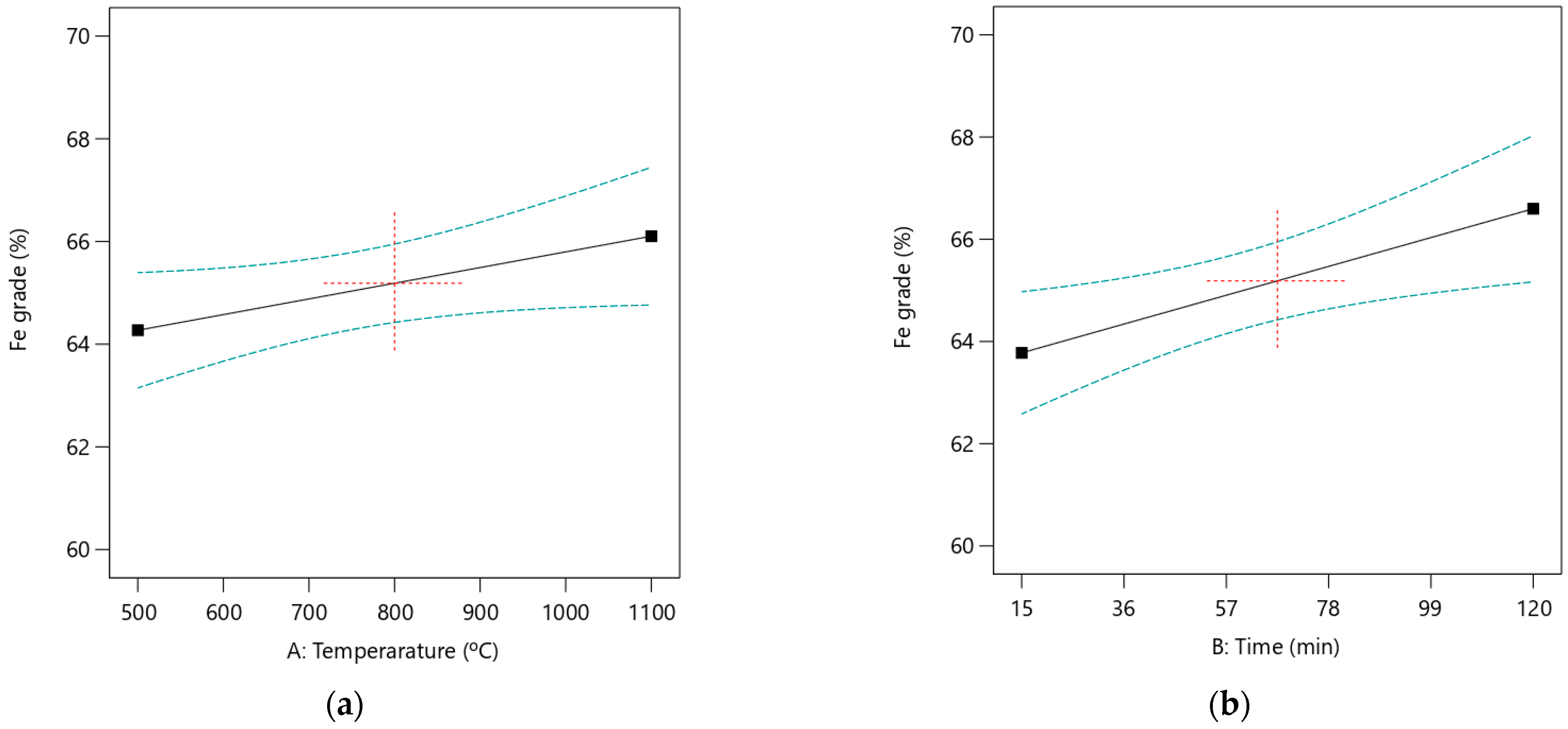
The steep slope observed in
Figure 8 indicates a more substantial influence of the main effect on the Fe grade, as previously predicted with the ANOVA analysis. Furthermore, based on a first-order linear model developed for Fe grade, there is no evidence of a two-way interaction between the input factors affecting Fe grade.
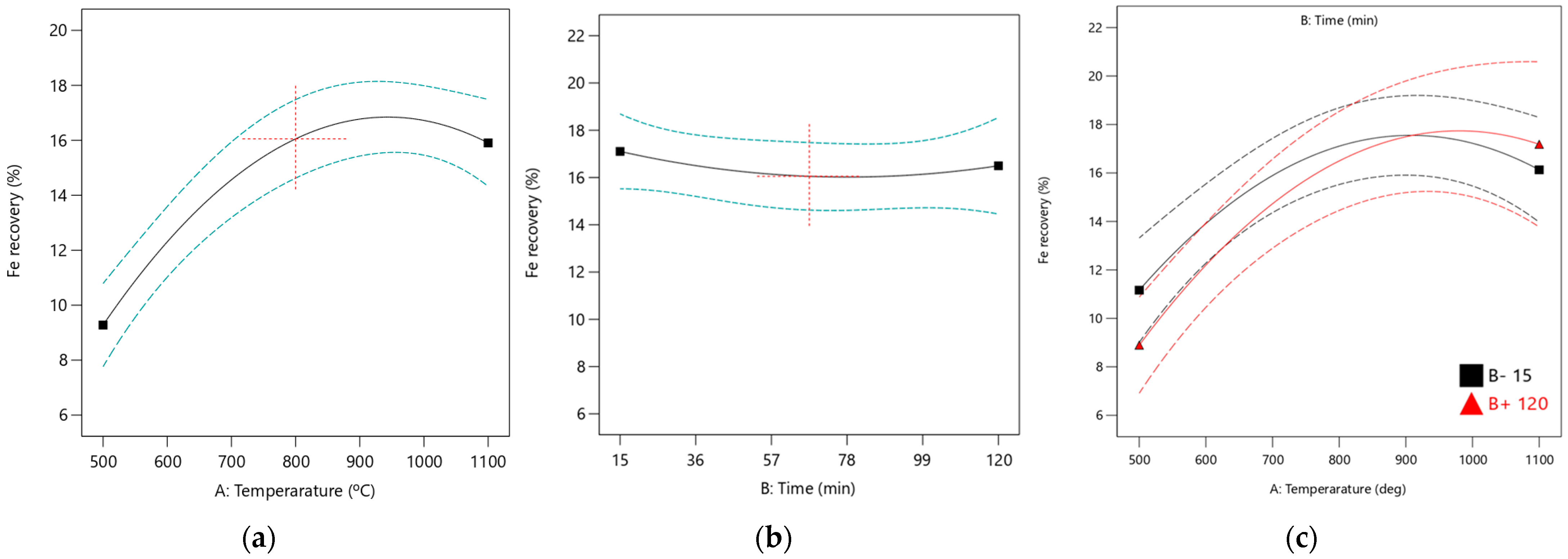
It was observed that the roasting time is nearly parallel to the horizontal axis, as illustrated in
Figure 9b. This indicates that the effect of roasting time on Fe recovery is minimal and consistent with the predictions made with the ANOVA analysis. The interaction plot between roasting temperature and time, also shown in
Figure 9c, evaluates the effects of these independent factors. Increasing the roasting temperature improves Fe recovery, irrespective of whether the roasting time is high or low. However, the interaction between roasting temperature and time has a low impact on Fe recovery, as indicated by the ANOVA results. The quadratic effects of temperature and time were not significant, reflected in their low F-values.
Three-dimensional response surface and contour plots are essential for comprehending the main and interaction effects of the independent factors. These plots can support decision-making in selecting suitable input conditions to yield the optimal combination for the target response [
14,
33,
34]. The combined contour and 3D surface plots, which display the interactions of the variables on Fe grade and Fe recovery, are presented in
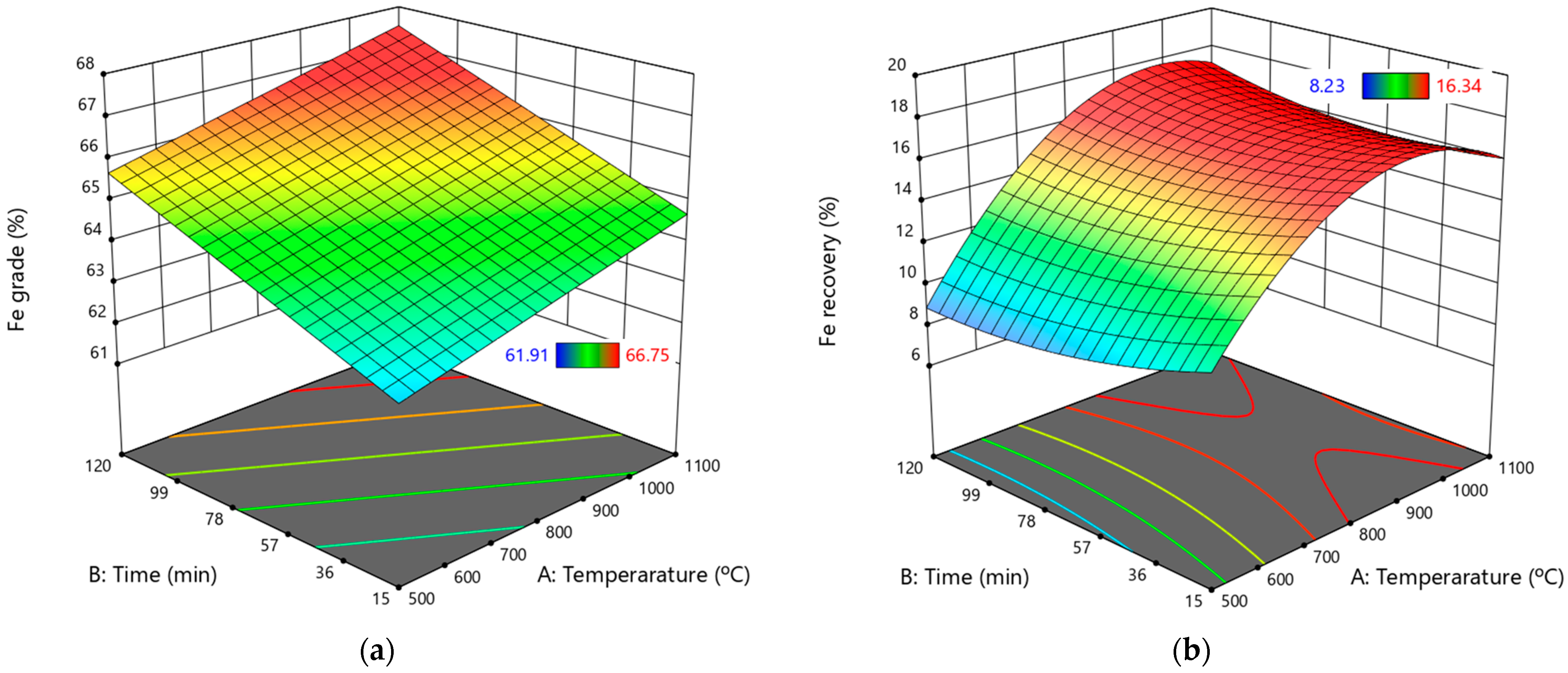
Figure 10a,b.
Utilizing the findings illustrated in
Figure 10a,b, the optimum working zone (optimized formulation region) can be easily identified [
35].
Figure 10a illustrates that the Fe grade increases with the roasting temperature and time. In contrast, the 3D surface plot regarding Fe recovery indicates that only increased roasting temperature has a positive effect, while additional roasting time does not contribute to improved recovery. Therefore, a shorter roasting time that does not compromise the Fe grade can be chosen to save production time and costs.
In mineral processing, grade and recovery are essential parameters for evaluating the performance of any unit process. As such, the model responses were optimized using the Design-Expert
® prediction profiler. During the magnetization roasting process, the optimization aimed to achieve a maximum Fe grade of at least 65%, which is crucial for economic viability.
Table 9 summarizes the optimal solution values for variables calculated by the model to achieve the desired Fe grade and Fe recovery. In this context, the desirability function uses a value of “0” to represent an undesirable response and a value of “1” to indicate the most ideal and desirable response.
The results in
Table 9 indicate that operating at optimal roasting temperatures of 1050 °C and a duration of 67 min yields an Fe grade of 66.8%, but only results in a recovery rate of 16.9%. Notably, these roasting conditions lead to the lowest levels of Fe recovery. Therefore, optimizing the next stage, which involves the induced dry-roll magnetic separator, is crucial.
The optimized operating conditions were used to assess the suitability of the developed model in predicting response values. To validate these optimal conditions, an experiment was conducted, resulting in an Fe recovery rate of 17.63% at an Fe grade of 67.45%. The experimental response values aligned well with the predicted values, as illustrated in
Table 9. The model is reliable and accurate in forecasting outcomes.
3.4. Optimization of the Induced Dry-Roll Magnetic Separator Process Parameters
The sample generated at the optimum roasting temperature of 1050 °C and a roasting time of 98 min was used to prepare a bulk material for optimizing the induced dry-roll magnetic separation. RSM was also utilized to evaluate the influence of magnetic field intensity (A), rotor speed (B), and product splitter position (C) on iron (Fe) grade and Fe recovery. After conducting the experiments, the outcomes of the experimental design and the observed responses for optimizing induced dry-roll magnetic separation are presented in
Table 10.
Two statistical models were developed for each response: Fe grade and Fe recovery. A quadratic second-order polynomial was used to describe the relationship between the input factors and the output responses. The empirical coded equations for both Fe grade and Fe recovery are provided in Equations (6) and (7), respectively.
where the Fe grade and recovery are given in percentage, magnetic field intensity (
A) is in T, rotor speed (
B) is in Hz, and product splitter position (
C) is in mm.
The ANOVA analysis was utilized at a 5% significance level to evaluate the adequacy of the developed polynomial regression models for Fe grade and Fe recovery, in which the significance was determined for
p-values ≤ 1.
Table 11 and
Table 12 present the ANOVA analysis for Fe grade and recovery, respectively. As shown in
Table 11, the F-value of 3.8 indicates that the Fe grade model is statistically significant, with only a 2.46% probability that such an F-value could arise from random noise. Additionally, the “Adeq Precision” ANOVA value of 7.03 exceeds the threshold of 4, suggesting that the model is sufficient and can be effectively used to explore the design space.
The significant factors affecting Fe grade were C, A × B, and C2 (p-value < 0.05). Based on the F-values, the order of significance for the main and interaction effects on Fe grade is as follows: A × B > C2 > C > B × C > A > B > A × C > B2 > A2.
The results from
Table 12 indicate that the F-value of 40.16 demonstrates the significance of the developed model for Fe recovery. There is only a 0.01% chance that an F-value this high could occur due to random noise. Furthermore, the “Adeq Precision” ANOVA value of 19.69 surpasses the threshold of 4, confirming that the model is adequate and can effectively guide the design space.
The analysis revealed that the terms A, B, C, A2, and C2 are significant (p-value < 0.05). The order of significance of the main and interaction effects was B > A > A2 > C > C2 > A × B > A × C > B × C > B2.
The actual and predicted Fe grade and Fe recovery values were compared using the quadratic polynomial Equations (6) and (7), which are given in
Table 13.
Figure 11a,b illustrates the relationships between the actual and predicted Fe grade and Fe recovery, respectively.
Figure 11a indicates a good fit between the actual and predicted Fe grade values, as evidenced by an R
2 value of 0.77. Meanwhile,
Figure 11b suggests a strong correlation between the predicted and experimental data for Fe recovery. This indicates that the Fe grade and recovery models are effective and reliable.
However, it is essential to note that one data point (run 8) in the Fe grade analysis lies outside the acceptable range, indicating a residual more significant than 4. This suggests that run 8 is an outlier, and repeating this run could enhance the model’s accuracy.
The main and interaction effect plots were developed to illustrate the relationship between the input parameters (magnetic field intensity, rotor speed, and product splitter position) and the output parameters (Fe grade and Fe recovery). The generated plots are presented in
Figure 12 and
Figure 13 for Fe grade and Fe recovery, respectively.
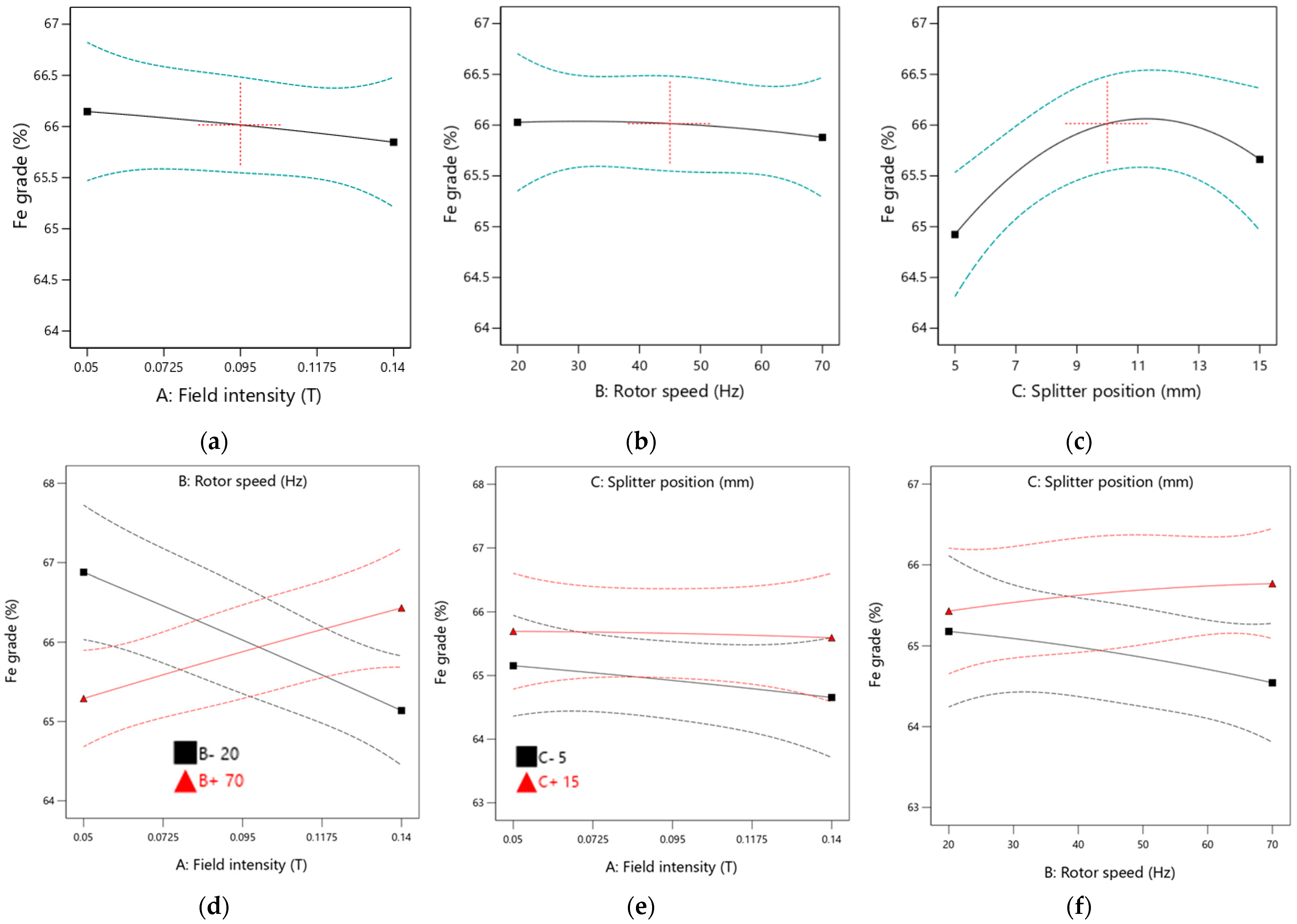
The results indicate that the product splitter position positively impacts the obtained Fe grade, as evidenced by the steep slopes observed in the main plot for the splitter position. In contrast, the main plots for magnetic field intensity and rotor speed are nearly parallel to the horizontal axis, suggesting that increases in these parameters have an insignificant effect on the Fe grade. This aligns with the ANOVA analysis, which found the p-values for field intensity and rotor speed to be insignificant.
The interaction effects involving field intensity and rotor speed, field intensity and splitter position, and rotor speed and splitter position on Fe grade are illustrated in
Figure 12. It was observed that the Fe grade increases only with higher field intensity and rotor speed. Lower rotor speeds resulted in poor Fe grades. The interaction between field intensity and splitter position contributed minimally to the Fe grade, as indicated by a plot that is parallel to the horizontal axis. However, operating the equipment at a higher splitter position leads to better Fe grades. Additionally, increasing rotor speed while using a lower splitter position negatively affects the Fe grade. The interactions between field intensity and splitter position and those between rotor speed and splitter position demonstrate minimal impact on the Fe grade due to low F-values, as shown in the ANOVA analysis.
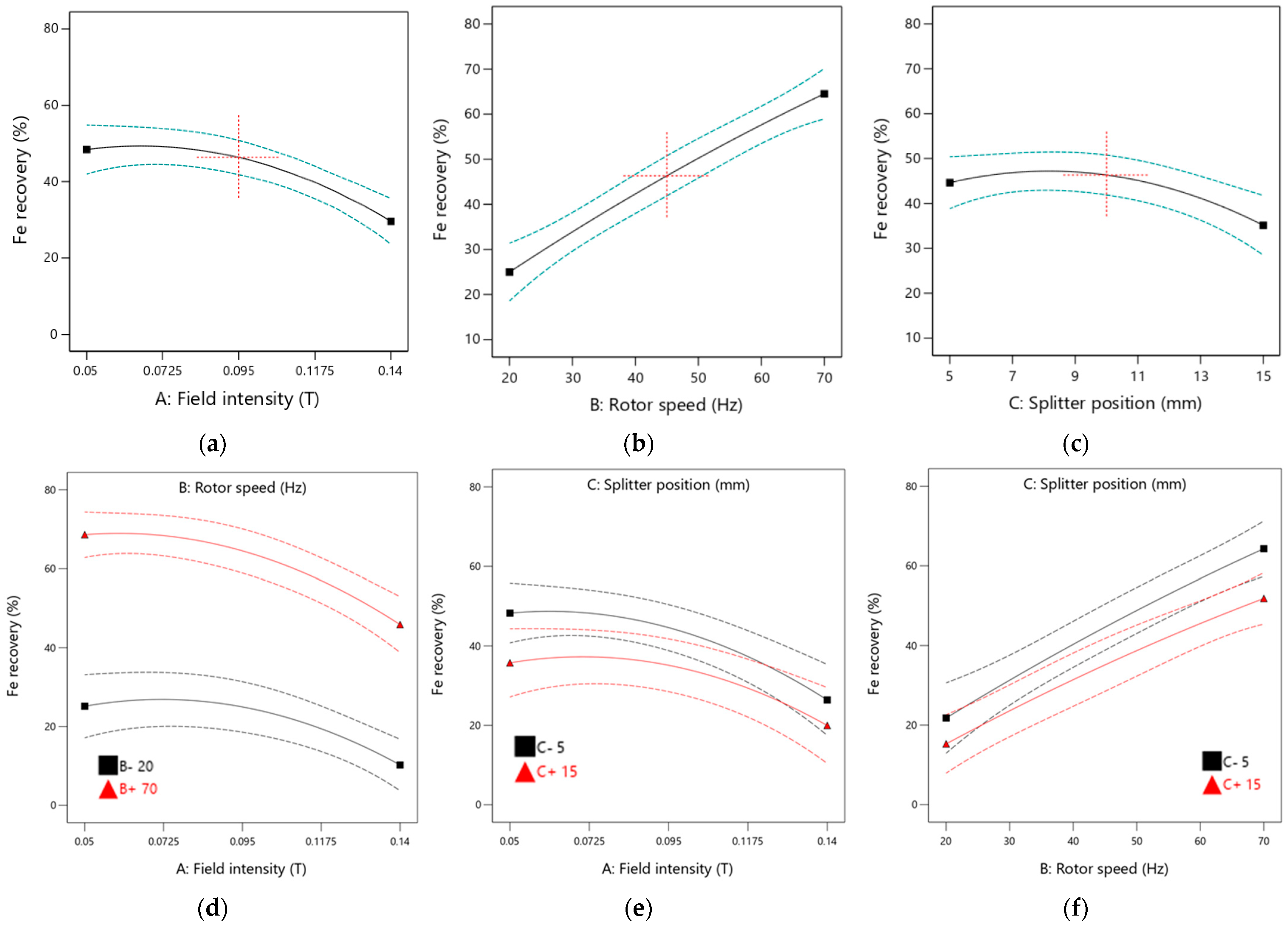
Based on
Figure 13, field intensity and rotor speed positively influence Fe recovery, as indicated by the steep slopes in the main effect plots. The Fe recovery increases with higher rotor speeds, while an increase in field intensity has a negative impact on recovery, suggesting that optimal results are achieved at lower field intensities. The main plot for the splitter position remains nearly parallel to the horizontal axis, indicating that adjustments to the splitter position have minimal effect on Fe recovery. This observation is consistent with the ANOVA analysis, which reveals a low F-value for the splitter position.
Interaction effect plots for field intensity and rotor speed, field intensity and splitter position, and rotor speed and splitter position on Fe recovery are shown in
Figure 13. It is noted that increasing field intensity adversely affects Fe recovery. However, operating the equipment at lower field intensity and rotor speed produces better Fe recoveries. Operating the equipment at a lower rotor speed is not advisable, resulting in significantly lower Fe recovery. The interaction between field intensity and splitter position has a moderate effect on Fe recovery, with optimal conditions identified as low field intensity and low splitter position. Increasing rotor speed while maintaining a lower splitter position enhances Fe recovery values. Nonetheless, all interaction effects have minimal influence on Fe recovery, as indicated by low F-values, with all corresponding
p-values exceeding 0.05, which aligns with the ANOVA analysis data.
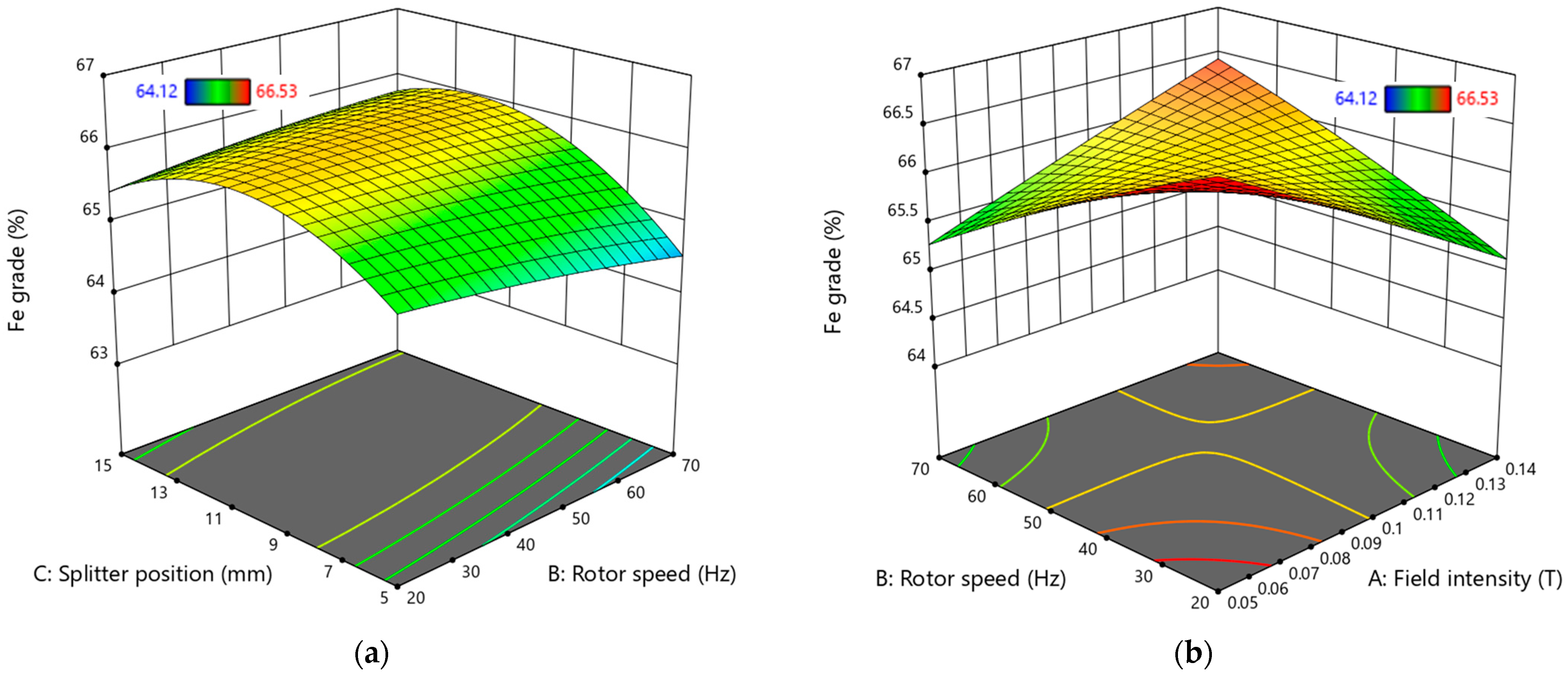
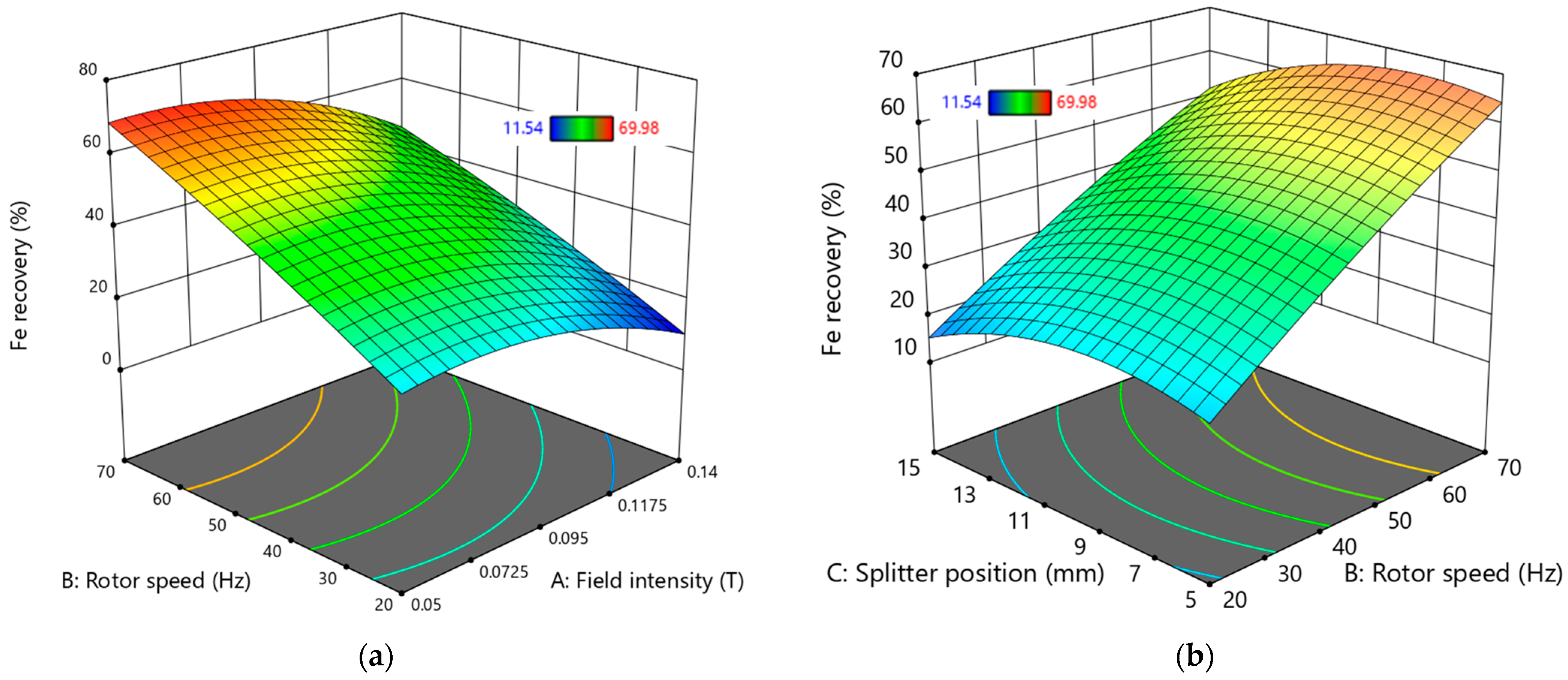
The 3D surface plots illustrating the interactions of variables affecting Fe grade and Fe recovery are presented in
Figure 14 and
Figure 15.
According to
Figure 14a, the range that promotes a higher Fe grade is found with a splitter position between 10 mm and 11.5 mm at any rotor speed. However, selecting a lower rotor speed is advisable for economic reasons, as it can reduce energy costs. This is further supported by
Figure 14b, which indicates that operating the equipment at a low rotor speed and in a lower field intensity region yields optimal Fe grade values.
In
Figure 15a, the conditions for improved Fe recovery are highlighted, showing that a high rotor speed above 65 Hz is necessary while maintaining a field intensity of less than 0.09 T.
Figure 15b reveals that, for higher Fe recovery values, the product splitter position should be set below 11 mm at rotor speeds exceeding 65 Hz.
Table 14 presents the best solutions for the variables necessary to achieve the desired Fe grade and recovery. Based on
Table 14, the optimum working conditions include a magnetic field intensity of 0.105 T, a rotor speed of 70 Hz, and a product splitter position of 11 mm, resulting in an Fe grade of 66.2% and a recovery rate of 60.2%.
To validate these optimal conditions, an experiment was conducted, resulting in an Fe recovery rate of 58.29% and an Fe grade of 65.33%. The experimental response values aligned well with the predicted values, as shown in
Table 14.