Abstract
Zinc processing tailings (ZPTs) of the Kharzet Youcef processing complex, Setif, Algeria, are mainly stockpiled in tailing dumps without use, occupying significant surfaces and negatively influencing the human environment and health. Incorporating ZPTs into building materials manufacturing is an effective solution to meet the dual objectives of environmental protection and economic development. This study investigates the influence of firing temperature and integrating ZPTs as a partial replacement for clay on the physic-mechanical properties of fired clay bricks (FCBs). Microstructural, chemical, and mineralogical analyses of ZPTs and clay were carried out by SEM-EDS, XRF, and XRD, respectively. Seven mixtures were produced with various percentages of ZPTs added to clay (0%, 5%, 10%, 15%, 20%, 25%, and 30%) and were fired at two different temperatures (900 and 1000 °C) at a ramp rate of 5 °C. Physic-mechanical tests were carried out on different brick specimens, and the results obtained showed that the FCBs incorporated with 10% of ZPTs produced the highest flexural strength of 6.24 MPa, compressive resistance of 29.78 MPa, bulk density of 1.37 g/cm3, and water absorption of 15.1% at 900 °C. Therefore, the recycling of ZPTs for FCBs manufacturing is feasible and an effective alternative waste disposal solution for sustainable development while reducing negative environmental impacts.
1. Introduction
Non-renewable mineral resources are the basic building blocks supporting global economic and social growth [1]. The exponential growth in mining activities has generated large amounts and various types of wastes. These wastes are mainly in the form of tailings that are disposed in dumps without proper management, inevitably affecting the environment [2,3,4]. In this context, zinc mining and processing tailings are a primary source of toxic heavy metal discharge into the environment [1,2,5].
In this regard, the recycling and utilisation of mining wastes and tailings as alternative raw materials is regarded as an environmentally friendly approach to reduce their negative impact on the environment on the one hand and to prevent the depletion of non-renewable resources and improve the population’s health through the proper and effective disposal of wastes on the other hand [6,7,8].
Furthermore, zinc wastes have a chemical composition similar to that of the natural raw materials used in the building and construction industries [9], so they can be used as a partial replacement for clay in bricks [10,11,12], cement [13,14,15,16], concrete admixture [17,18,19], mortar [20,21,22], the production of ceramics [23,24,25,26], and cementitious paste [27,28].
In other research [11], lead–zinc tailings were used as the primary raw material and combined with clay and fly ash as supplementary components to produce fired bricks. The optimal manufacturing conditions included a ratio of lead–zinc tailings to clay to fly ash equal to 6:3:1, a forming pressure of 20 MPa, a firing temperature of 1080 °C, and a holding time of 60 min. Under these conditions, the bricks attained a compressive strength of 34.94 MPa and a water absorption rate of 16.02%, meeting the requirements of the Chinese sintered ordinary bricks standard.
Lead–zinc tailings have also been utilised in the production of unfired bricks [29]. Lightweight unfired bricks were developed using lead–zinc tailings as fine aggregates, achieving a compressive strength of 9.3 MPa, making them suitable as building filler blocks. Similarly [30], unfired bricks incorporating lead–zinc tailings as a pozzolanic material were manufactured, with a compressive strength meeting the MU20 standard, reaching an average of 20 MPa. These studies demonstrate that unfired bricks made from lead–zinc tailings can attain acceptable mechanical properties.
In Algeria, Chaabet El Hamra deposit is the only zinc deposit in the country; it is operated by the National Company of Non-Ferrous Mining Products (ENOF). Its raw ore is extracted using the room and pillar method and directly conveyed to the mineral processing plant in order to increase its quality. Froth flotation is the method used to produce a final high-grade concentrate, while rejects are deposited in a dump. This dump occupies an area of 7 ha, having received residue issues from the treatment of Chaabet El Hamra ore since 1994.
These tailings have primarily been studied from an environmental perspective, focusing on heavy metal accumulation in species [31,32,33]. However, rejects have not been specifically examined for incorporation into fired clay bricks. This study follows a circular economy approach by evaluating the suitability of ZPTs as a partial replacement for clay in fired brick manufacturing in Algeria, aiming to explore their potential as a sustainable raw material. The novelty of this research will contribute to waste reduction and environmental sustainability. Through physical–mechanical testing and microstructural analysis of the produced brick specimens, the research aims to transform these industrial residues into a valuable resource. In addition to reducing environmental impact, this approach offers a sustainable alternative for the construction industry, with findings that will help to assess the potential of these materials and optimise their performance for large-scale applications.
2. Study Area Description
Geographic Location
The mineral processing plant of Kherzet Youcef ore (Chaabet El Hamra Mine) is located in eastern Algeria, 5 km northwest of Ain Azel, approximately 50 km southeast of the municipality of Setif (Figure 1).
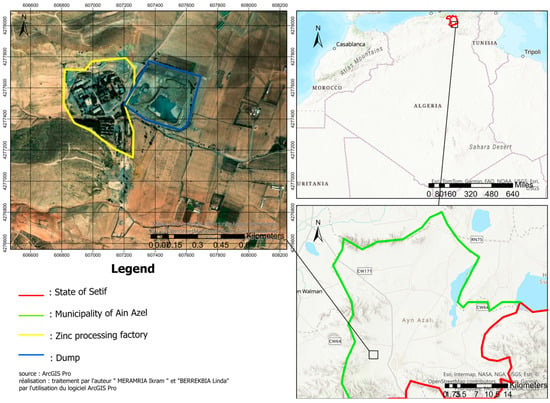
Figure 1.
Geographic location of the study area. Red colour present the borders of Setif state.
3. Materials and Methods
3.1. Materials
The clay used in this study was obtained from the El Amel brick factory, El Taref, Algeria. The sampling procedure was applied in the discharge of a rolling mill (<1 mm). The sampling was performed using a steel shovel, avoiding any contamination of the sample on the one hand and ensuring that the sample taken reflected the composition and characteristics of the material used in the brick manufacturing process on the other hand.
The ZPT samples were obtained from five distinctive dump locations of the Kharzet Youcef zinc processing plant. Then, subsamples from each zone were homogenised and five samples were finally obtained. After that, the materials (clay and ZPTs) were dried at 105 °C for 24 h. ZPTs were ground using a planetary ball mill type Welte WTR 950 W/R-V and then sieved to select particles with an average diameter of less than 0.074 mm.
Five samples of ZPTs collected from the Kharzet Youcef dumps were thoroughly mixed and prepared by conning and quartering to prepare a single representative sample.
3.2. Moulding and Sintering of Brick Specimens
To manufacture bricks, ZPTs were properly mixed with clay in varying portions, as presented in Table 1. The different mixtures were first blended for 10 min in a blender, with 10% by mass of water in order to obtain semi-dry homogenous mixes. Homogenised mixtures were manually pressed using a mould with dimensions of 40 × 40 × 160 mm to produce nine (9) brick specimens for each combination. The obtained specimens were left in ambient air for 24 h, and then dried in an oven at 105 °C for 24 h to decrease their moisture content. The dried specimens were fired in a laboratory electrical furnace at a rate of 5 °C/min from room temperature to the desired firing temperature, where two different temperatures were targeted (900 °C and 1000 °C), and held for two hours at the desired firing temperature. After firing, the bricks were cooled to room temperature by natural convection inside the laboratory electrical furnace.

Table 1.
Mass ratios of different raw materials.
The procedure followed for brick manufacturing at the laboratory is shown in Figure 2.
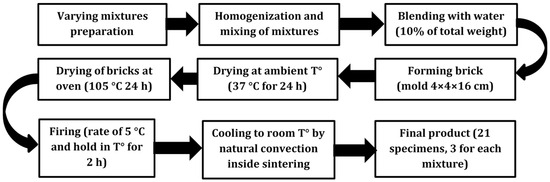
Figure 2.
Schematic representation of brick specimens manufacturing, own elaboration.
3.3. Characterisation
The main chemical composition of the raw materials was determined by X-Ray Fluorescence spectroscopy (XRF PANalytical AXIOS minerals, Malvern Panalytical, Almelo, Netherlands) and Inductively Coupled Plasma Optical Emission spectroscopy (Agilent technologies 5900, Agilent Technologies, Santa Clara, CA, USA). The loss on ignition (LOI) was measured by heating the samples at 1000 °C for 2 h in a muffle furnace, followed by weighing the residue to determine the mass loss.
The mineralogical composition was investigated with X-Ray Diffractions using a device type X’pert Pro MRD (Malvern Panalytical, Almelo, The Netherlands).
The surface morphology and pore structure of the raw materials and sintered brick specimens were investigated by SEM model FEI Quanta 250 (FEI, Eindhoven, The Netherlands) equipped with an energy-dispersive X-ray spectroscopy (EDS) detector.
The plasticity and liquidity limits of the mixtures were determined due to ASTM D4318-17e1 [34], while linear shrinkage was measured according to ASTM D4943-18 [35]. The bulk density and water absorption values of the sintered samples were calculated using the Archimedes method according to ASTM C29/C29M-17a [36] and ASTM ASTM C373-18 [37], respectively. These parameters were determined using the following equations:
Mdry is the dry mass of the sample (g),
V is the volume of the sample (cm3).
Mimmersed is the mass of the sample after immersion (g),
Mdry is the dry mass of the sample (g).
Linear shrinkage (%) was determined from the lengths of the brick specimens after drying and after sintering treatment by a calliper with a precision of ±0.01 mm according to ASTM C326-09 [38].
Linitial is the initial length of the sample (mm),
Lfinal is the length after thermal treatment (mm).
Compressive resistance was assessed using a mechanical testing machine ‘Bera Test’ (MaTest, Triviolo, Italy) with a capacity of 2000 kN; it can apply a maximum pressure equal to 618.17 bars. The surface area of the piston is 323.65 cm2, according to ASTM C67-22 [39]. Flexural strength was measured using the mechanical testing machine Bera Test’ with a capacity of 150 kN, which can apply a maximum pressure equal to 122.28 bars. The surface area of the piston is 122.71 cm2, according to ASTM C674-88 [40].
where:
F is the maximum applied force (N),
A is the loaded surface area (mm2).
where:
F is the maximum applied load (N),
L is the span between supports (mm),
b is the width of the sample (mm),
h is the thickness of the sample (mm).
4. Results and Discussions
4.1. ZPTs and Clay Characterisation
4.1.1. Phases Identification
The results of the XRD analysis of clay (Figure 3) confirm the presence of kaolinite (Al2Si2O5(OH)4), quartz (SiO2), dolomite (CaMg(CO3)2), hematite (Fe2O3), and fluorite (CaF2). The dominant peak of quartz is consistent with the high SiO2 content (42.9%), indicating its abundance in the sample. The presence of kaolinite aligns with the significant Al2O3 content (23.3%), which plays a crucial role in ceramic phase formation during firing. Hematite is supported by the total iron (FeT) content (8.25%), contributing to fired bricks’ colouration changes. Dolomite, identified by its peaks, corresponds to the CaO (8.9%) and MgO (4.1%) content, and its decomposition during heating can influence porosity and densification.
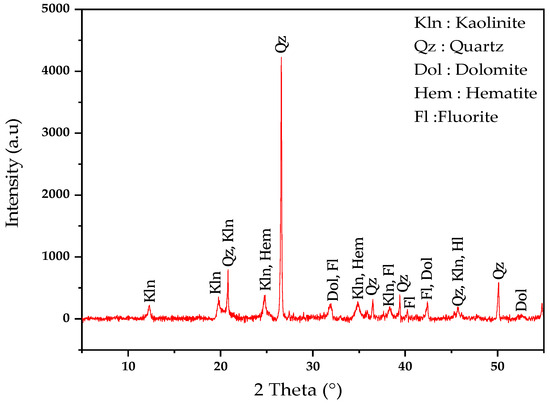
Figure 3.
X-ray diffraction patterns of clay.
The X-ray diffraction (XRD) results of the ZPTs presented in Figure 4 indicate the presence of dolomite (CaMg(CO3)2), quartz (SiO2), gypsum (CaSO4∙2H2O), and pyrite (FeS2).
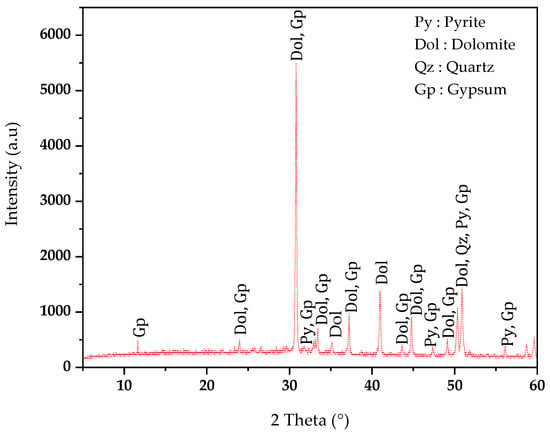
Figure 4.
XRD phase’s identification of Kharzet Youcef ZPTs.
4.1.2. Chemical Composition
Chemical analysis of the clay used in this study (Table 2) reveals a composition with a high content of SiO2 (42.9%) and Al2O3 (23.3%), accompanied by significant levels of total iron (FeT = 8.25%) and CaO (8.9%).

Table 2.
Chemical composition of clay.
The percentage of zinc in the five collected samples was quantified and the results are presented in Table 3. A variation in zinc concentrations is shown between the different areas of the site. Zone 4 has the highest concentration of zinc (1.01%), while zone 3 has the lowest concentration (0.23%). This variation may be due to differences in the treatment process or in local geology.

Table 3.
Zinc content in collected samples of ZPTs.
The main components of the ZPTs of the composite sample are presented in Table 4. The sample is primarily composed of CaO (31.62%) and exhibits a high loss on ignition (LOI) of 35.46%, which suggests the presence of significant volatile components and/or decomposable phases. This elevated LOI value can be attributed to the thermal decomposition of carbonates (such as calcium carbonate, CaCO3, and magnesium carbonate, MgCO3), leading to the release of CO2. SiO2 and MgO are also found in significant quantities, representing 9.45% and 10.74%, respectively.

Table 4.
Chemical composition of Kharzet Youcef ZPTs.
The percentages of heavy metals are shown in Table 5. Zinc, lead, and strontium are present in notable quantities, indicating potential environmental and health risks linked to the dispersion of these rejects.

Table 5.
Heavy metals content of Kharzet Youcef ZPTs.
In conclusion, the silica and alumina content in ZPTs can qualify them to partially replace clay in bricks manufacturing.
4.1.3. Microstructure
- -
- Clay:
The SEM image (Figure 5) shows a heterogeneous microstructure mixed with compact and porous regions. The appearance of visible particles of varying sizes (from a few micrometres to around 100 μm) and irregular shapes indicates a complex mixture of different mineral phases. Angular particles corresponding to quartz are identified in the XRD results.
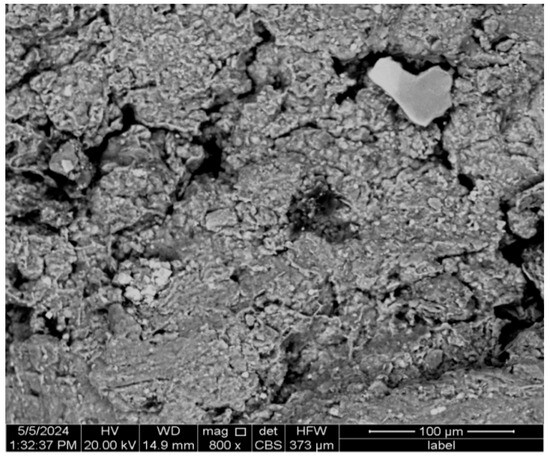
Figure 5.
SEM-EDS results of clay.
There are several visible pores and voids within the clay matrix. Their distribution and size suggest that the clay has a certain porosity level, which can affect its behaviour during the drying and firing processes in brick manufacturing.
EDS analysis (Table 6) confirms the presence of oxygen (O), magnesium (Mg), aluminium (Al), silicon (Si), chlorine (Cl), potassium (K), calcium (Ca), titanium (Ti), and iron (Fe). These results are consistent with the XRD findings, which identified quartz (SiO2), kaolinite (Al2Si2O5(OH)4), siderite (FeCO3), and halite (NaCl). Oxygen, silicon, and aluminium are fundamental to the clay structure, forming part of quartz and kaolinite. Magnesium and calcium likely originate from minor amounts of carbonate minerals. Iron, present in siderite, is key in influencing the brick’s colour, imparting a reddish hue while enhancing its strength and hardness. Notably, potassium (K) and sodium (Na), also identified in the sample, act as fluxing agents during the sintering process, lowering the firing temperature, promoting densification, and contributing to improved mechanical properties. The minor presence of titanium and chlorine suggests impurities that must be managed.

Table 6.
EDS results of clay-scanned zone.
- -
- ZPTs:
The image obtained by SEM of the ZPTs (Figure 6) shows a heterogeneous granular structure composed of various mineral phases. The EDS analysis (Table 7) reveals a composition mainly dominated by oxides, carbonates, sulphates, and silicates, reflecting the mineralogical diversity of zinc processing discharges. The presence of sulphur and iron could indicate phases such as pyrite, while calcium and magnesium are consistent with dolomite and gypsum.

Figure 6.
SEM-EDS results of ZPTs.

Table 7.
EDS results of ZPTs.
- -
- Bricks:
SEM-EDS was carried out on bricks made with clay and ZPTs with the best mechanical resistance values. The analysis of the microstructure of the briquette prepared with 100% clay and fired at 950 °C, as presented in Figure 7, shows a porous and heterogeneous texture, characterised by dense zones and cavities. The EDS analysis (Table 8) indicates a majority composition of oxygen (56.13%), silicon (18.50%), aluminium (10.84%), and iron (11.26%). The significant presence of iron may indicate a contribution to the typical reddish colouration of fired bricks. These results agree with the XRD analysis, which identified dominant crystalline phases such as quartz, kaolinite, and siderite, while XRF confirmed their elemental composition.

Figure 7.
SEM results of brick prepared with 100% clay and fired at 900 °C.

Table 8.
EDS results of brick prepared with 100% clay and fired at 900 °C.
According to [41], kaolinite dehydroxylates around 500–600 °C, leading to the formation of an amorphous metakaolinite phase, which subsequently transforms into mullite (~950–1000 °C), a key contributor to the mechanical strength of bricks. Similarly, siderite decomposes at approximately 600 °C, releasing CO2 and forming hematite (Fe2O3), which enhances the reddish colouration of bricks and contributes to densification. Improvements in mechanical strength and are is primarily attributed to the reduction in porosity due to sintering and the presence of quartz and mullite, which provide a rigid structural framework embedded in a glassy matrix. These findings are consistent with previous research on the mineralogical evolution of fired clay bricks.
These phases are typical of bricks fired at high temperatures, giving the briquettes their mechanical and durability properties.
The SEM image of the clay briquette with 30% ZPTs (Figure 8) shows a heterogeneous structure, with grains and pores of varying sizes, which can influence the mechanical properties and durability of briquettes. The EDS results (Table 9) confirm the presence of dominant elements (O, Mg, Al, Si, Ca, and Fe) characteristic of clay and ZPTs. The appearance of zinc (1.51%) and the augmentation of Mg, K, Ti, and Ca in the analysis of this briquette confirm the good incorporation of ZPTs into the matrix.

Figure 8.
SEM results of brick prepared with 10% of ZPTs and fired at 900 °C.

Table 9.
EDS results of bricks made with 30% ZPTs and fired at 900 °C.
According to [2,12], the heavy metals in waste residues and sludge become stable in raw materials after irreversible transformation into mineral phases by thermal processes (brick sintering), and metal leaching is inhibited dramatically. These thermal treatments not only offer a cost-effective method for producing construction materials incorporating tailings, but also contribute to the stabilisation of heavy metals within the newly fabricated materials. Similar to the adsorption mechanisms observed in zeolite-based materials, the thermal treatment of ZPT-containing bricks may facilitate the immobilization of heavy metals by incorporating them into stable mineral phases, thus reducing their leaching potential [42].
4.1.4. Mapping
Mapping was carried out on bricks made with clay and ZPTs with the best mechanical resistance values. The results are shown in Figure 9 and Figure 10.
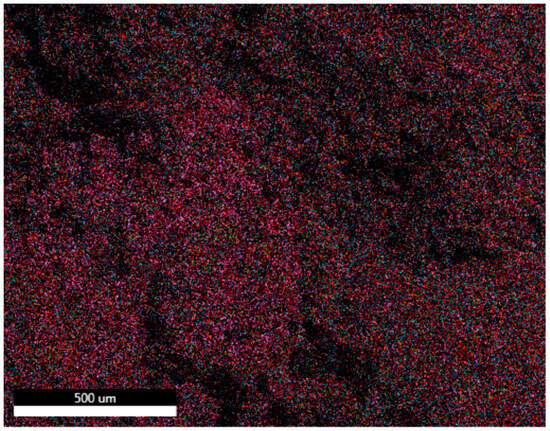
Figure 9.
SEM cartography of briquette made with 100% clay and fired at 900 °C.
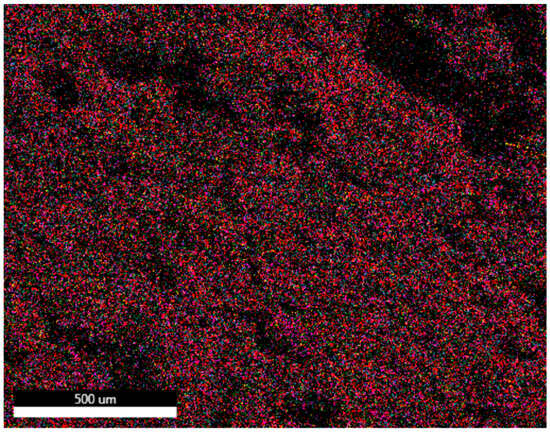
Figure 10.
SEM cartography of briquette made with 10% ZPTs and fired at 900 °C.
- -
- Briquette of 100% clay:
SEM mapping (Figure 9) shows a homogeneous structure of the briquette with specific inclusions detectable by the localised distribution of elements like silicon, oxygen, and aluminium, corresponding to their high percentages in the chemical composition of the clay. Silicon (purple) and oxygen (red) cover the surface evenly, while aluminium (sky blue) is also well distributed. Iron (in blue), on the other hand, is present in a more localised manner, indicating specific iron-rich inclusions or phases. Magnesium (in orange) and potassium (in mustard green) appear in lower quantities, reflecting their lower proportion in the clay.
- -
- Briquette of 10% Zn-900 °C:
The SEM mapping image shows the distribution of elements in a briquette manufactured by integrating 10% of zinc waste with clay and firing at 900 °C. The result shows that the integration of ZPTs did not disturb the uniform distribution of essential elements, including aluminium, silica, iron, and oxygen, showing a good compatibility between the two components (ZPTs and clay) and the good integration of ZPTs into the clay matrix. The presence of sulphur indicates its introduction via ZPTs.
Magnesium and calcium, present in high concentrations in zinc processing tailings (CaO = 31.62% and MgO = 10.74%), play a crucial role in the sintering process. These elements act as fluxing agents, promoting vitrification at lower firing temperatures, enhancing the bricks’ density and mechanical strength while reducing overall porosity. This can lead to improved water and frost resistance, reduced drying shrinkage, and minimised warping during firing. Additionally, magnesium contributes to high-temperature strength, making it particularly beneficial for refractory applications. However, despite these advantages, the decomposition of magnesium- and calcium-bearing phases can also generate significant gas release during firing, increasing brick porosity. This gas-induced porosity can compromise mechanical properties and water resistance, counteracting the densification effects of sintering. The observed increase in water absorption and reduction in mechanical strength after ZPT addition suggest that gas release plays a dominant role in determining the final properties of the bricks. Therefore, careful control of the firing temperature and atmosphere is essential to balance these competing effects and optimise brick performance.
4.1.5. Profilometry 3D
The surface roughness of bricks made with clay and ZPTs with the best mechanical resistance values was measured. The results are shown in Figure 11, Figure 12, Figure 13 and Figure 14.
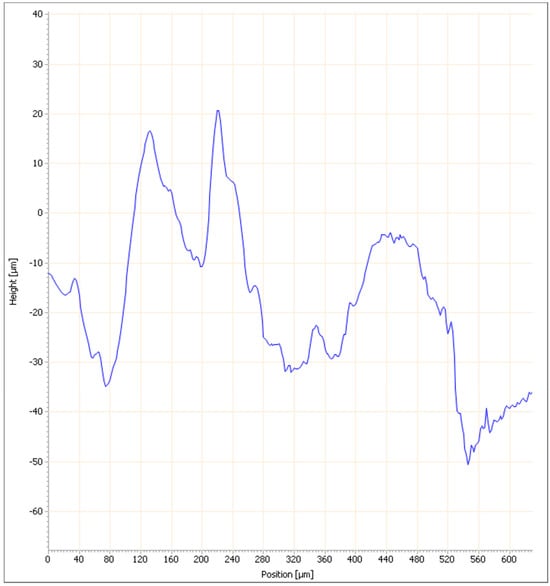
Figure 11.
Surface roughness measurement results of briquette made with 100% clay: Cross-section profile curve.
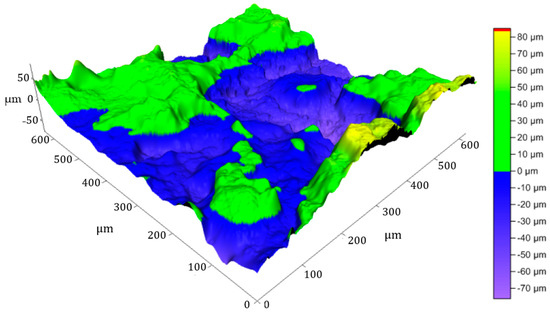
Figure 12.
Surface roughness measurement results of briquette made with 100% clay: Surface topography.
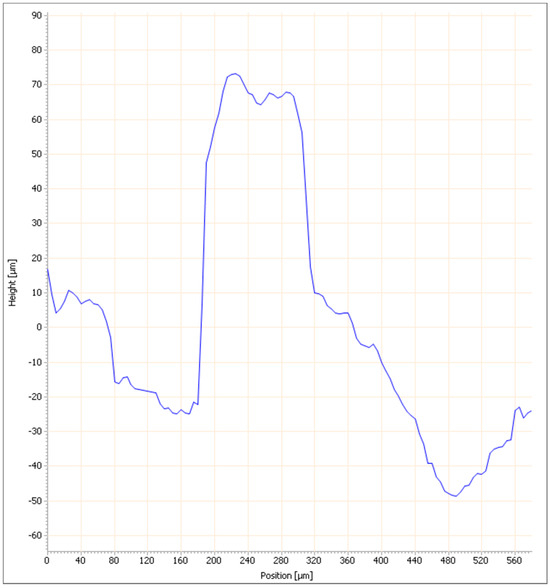
Figure 13.
Surface roughness results of bricks made with 10% ZPTs and fired at 900 °C: Cross-section profile curve.
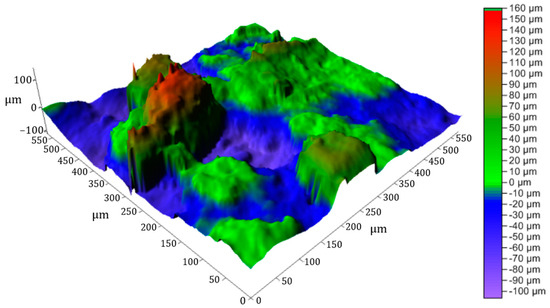
Figure 14.
Surface roughness results of bricks made with 10% ZPTs and fired at 900 °C: Surface topography.
- -
- Briquette of 100% clay:
The curve representing the height variation as a function of position for a profile line of the briquette (Figure 11) shows significant fluctuations in this line, with variations ranging from −60 µm to approximately 30 µm. This line’s average roughness (Ra) is 4.42 µm, while Rq = 5.525 µm and Rz = 17.68 µm, corresponding to a moderate roughness.
The 3D image of the clay briquette surface (Figure 12) reveals more detailed height variations, confirming the irregularities observed in the profile line curve on the same surface and that the briquette is specified by a moderate roughness, with peaks and relatively pronounced troughs.
- -
- Briquette of 10% ZPTs fired at 900 °C:
The results of measuring the roughness of the briquette with the best physic-mechanical properties (10% of ZPTs added to the clay and fired at 900 °C) are presented in Figure 13 and Figure 14.
The linear profile curve representing the height variation as a function of position for the clay briquette with 10% ZPTs fired at 900 °C (Figure 13) shows marked fluctuations and significant irregularities, ranging from −60 µm to approximately 80 µm. The peak and valley values are more pronounced than those observed in the clay briquette alone, suggesting that adding 10% ZPTs increases the surface roughness.
The 3D image of the surface of the clay brick with 10% ZPTs (Figure 14) confirms the irregularities observed in the previous curve. The specific roughness values processed by ‘cyber TECHNOLOGIES scan 8’ software version 8.5 for this briquette are as follows: Ra = 6.48 µm, Rq = 8.55 µm, and Rz = 26.56 µm (rectangular raster) and Ra = 4.95 µm, Rq = 6.36 µm, and Rz = 17.84 µm (linear profile). These values show that the surface of the clay briquette with 30% ZPTs has a higher roughness than that of the clay briquette alone (Ra = 4.42 µm, Rq = 5.525 µm, and Rz = 17.68 µm). The 3D image highlights significant height variations with marked peaks and valleys, confirming that adding 10% ZPTs results in an increase in surface roughness. Compared to the briquette made with 100% clay, adding ZPTs appears to significantly alter the surface texture, making the briquette less homogeneous and rougher.
4.2. Physic-Mechanical Tests
4.2.1. Bulk Density of Dry Mixtures
The mass and volume of the mixtures prepared for briquetting reveal interesting trends in density. The mass of the briquettes gradually increases with an increase in the added percentage of ZPTs, showing a trend toward a greater material compactness with a greater amount of added ZPTs. This increase in mass is also reflected in the density, which increases linearly with the percentage of ZPTs. As shown in Figure 15, the bulk density of the samples without ZPTs is above 1.03 g/cm3. The addition of ZPTs increases the bulk density of mixtures up to 1.37 g/cm3, which is due to the relatively high unit weight of ZPTs. These results suggest that adding ZPTs to clay leads to denser briquettes, which may impact their strength and ability to withstand loads and stresses during use.
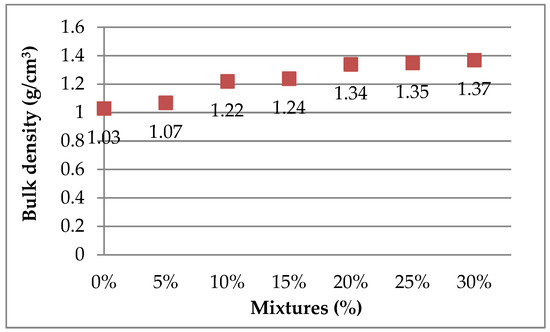
Figure 15.
Variation in bulk density of different clay mixtures.
4.2.2. Plasticity Limits
The plasticity limit results of the mixtures, illustrated in Figure 16, show a clear inverse correlation between the addition of ZPTs and plasticity. Specifically, the plasticity limit decreases from 25.7% for pure clay to 17.09% at 30% ZPTs, indicating a significant reduction in the workability of the clay paste. This trend suggests that ZPTs acts as a de-plasticiser, reducing the clay matrix’s cohesion and water retention capacity.
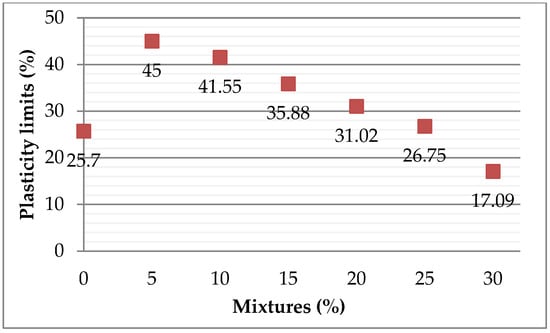
Figure 16.
Results of plasticity limits.
Higher ZPT percentages may require additional processing adjustments, such as an increased water content or the use of plasticising additives, to maintain the necessary shaping properties. Based on the observed plasticity values, formulations with lower ZPT percentages (5%–15%) may offer the best compromise, maintaining a sufficient plasticity for shaping while benefiting from the influence of ZPTs in modifying the final ceramic properties.
4.2.3. Water Absorption
Figure 17 represents the results of water absorption tests in the following two cases: 900 °C and 1000 °C. At 900 °C, a general increase in water absorption is clear, suggesting an increased porosity of the briquettes. At 1000 °C, variations in water absorption are less pronounced.
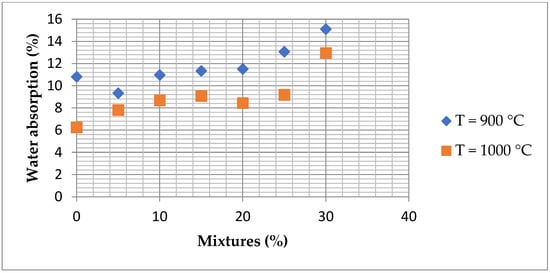
Figure 17.
Water absorption as a function of mixtures fired at 900 °C and 1000 °C.
The increased water absorption observed in bricks containing ZPTs can be primarily attributed to these residues’ high loss on ignition (LOI). The elevated LOI indicates a significant volatile matter content, leading to substantial gas release during firing. This gas evolution creates additional porosity within the ceramic matrix, increasing the capacity of the bricks to absorb water.
Despite the presence of fluxing agents (Ca and Mg), which generally promote sintering and densification, the dominant effect of gas release disrupts matrix consolidation, resulting in a porous structure.
4.2.4. Bulk Density of Fired Bricks
The bulk densities of the produced bricks with different proportions of ZPTs fired at different temperatures (900–1000 °C) are presented in Figure 18. The results reveal significant variations in the density depending on the added quantity of ZPTs and the firing temperature. As presented, the bulk densities of the samples without ZPTs, which were fired at 900 °C and 1000 °C, are above 1.18 and 1.40 g/cm3, respectively. The addition of the ZPTs influences the bulk density of the bricks. At 900 °C, the density varies irregularly, with peaks observed at 5% and 10% of added ZPTs. On the other hand, at 1000 °C, ZPTs’ density augmentation increases with an increase in ZPTs, reaching maximum values at 15% and 20% additions.
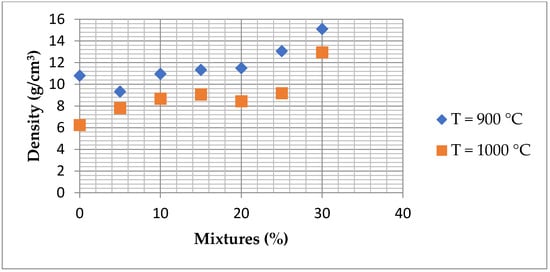
Figure 18.
Graphical representation of density variation as function of mixtures and firing temperature.
The variation in bulk density reflects the interplay between densification and gas release during firing. At lower ZPT contents, fluxing agents (Ca and Mg) facilitate partial sintering, improving densification and leading to an initial increase in bulk density. However, as the ZPT proportion rises, the high loss on ignition (LOI) causes substantial gas evolution during thermal decomposition, generating an internal porosity that counteracts the densification process. This effect is more pronounced at 900 °C, where sintering remains incomplete, resulting in lower density values. In contrast, at 1000 °C, the densification effect becomes more pronounced due to enhanced sintering and vitrification promoted by CaO and MgO from ZPTs. This leads to a more compact structure, counteracting the porosity increase and resulting in a gradual bulk density increase of up to 15%–20% ZPT addition.
4.2.5. Linear Shrinkage
In Figure 19, a general reduction trend in linear shrinkage is observed with an increasing ZPT addition, suggesting less contraction of the briquettes at higher concentrations. This can be attributed to a decrease in clay minerals, particularly kaolinite, primarily responsible for shrinkage during drying. The reduction in these minerals limits the extent of water loss-induced contraction. Additionally, better adhesion and a more homogeneous structure of the materials at these concentrations reduce the internal stresses responsible for shrinkage. These findings highlight the influence of ZPT content on the physical properties of the briquettes, particularly their drying behaviour, which may affect their final dimensions and compatibility with intended applications.
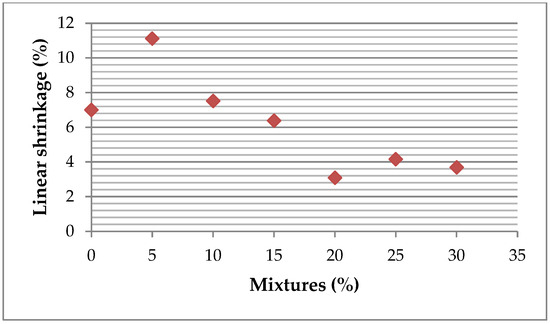
Figure 19.
Linear shrinkage of bricks after drying.
Figure 20 presents the linear shrinkage results of bricks fired at 900 °C and 1000 °C as a function of ZPT content. The data indicate a general decreasing trend in shrinkage with an increasing ZPT percentage at both firing temperatures. At 900 °C, shrinkage starts at approximately 7% for the control sample (0% ZPTs) and gradually decreases with an increasing ZPT content. Similarly, at 1000 °C, shrinkage follows a comparable decreasing trend, with slightly higher values than 900 °C. This behaviour can be attributed to reducing clay minerals, particularly kaolinite, which limits contraction during drying and firing. The presence of ZPTs also influences the sintering process, potentially modifying densification mechanisms and reducing the internal stresses responsible for shrinkage.
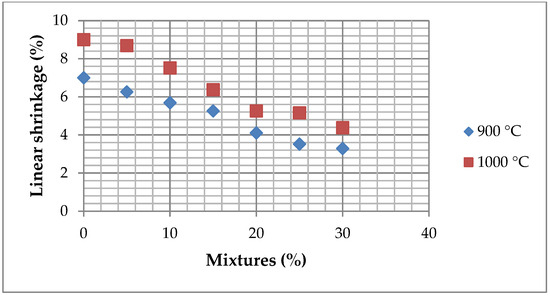
Figure 20.
Linear shrinkage of bricks fired at 900 and 1000 °C with 0%–30% of added ZPTs.
4.2.6. Mechanical Resistance
The mechanical resistance tests conducted on fired clay bricks incorporating from 0 to 30 wt% of ZPTs at firing temperatures of 900 °C and 1000 °C, as presented in Figure 21, reveal significant variations in mechanical performance, strongly influenced by the mineralogical and chemical composition of ZPTs.
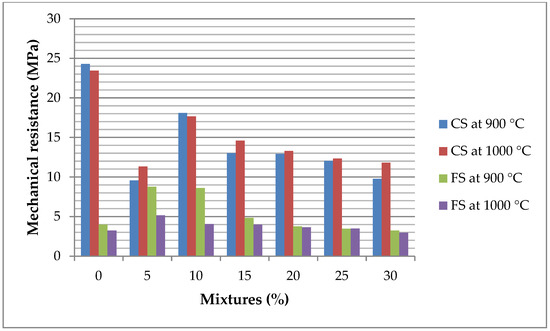
Figure 21.
Mechanical resistance of fired clay bricks at 900–1000 °C with from 0 to 30 wt% of ZPT additions (CS: compressive strength and FS: flexural strength).
At 900 °C, the flexural strength (FS) decreases with increasing of ZPTs content. This decline suggests a disruption in the formation of a cohesive ceramic matrix, likely due to the presence of volatile compounds and organic residues in ZPTs, which decompose during firing. The high loss on ignition (LOI) of ZPTs leads to the release of gases (such as CO2 from carbonates and SO2 from sulphates), increasing porosity and reducing the ability of the bricks to withstand flexural stresses. Additionally, the lower sintering activity of ZPT-containing mixtures at this temperature may result in weaker particle bonding.
In contrast, the compressive strength (CS) at 900 °C does not follow a linear trend, but reaches a peak at 10% ZPTs before declining at higher concentrations. This behaviour can be attributed to the presence of iron oxides (Fe2O3) and calcium compounds (CaO, CaCO3) in ZPTs, which can act as fluxing agents, promoting partial vitrification and enhancing densification at moderate ZPT levels. However, beyond 10% ZPTs, the increase in porosity due to excessive gas release counteracts these benefits, leading to a progressive decrease in compressive strength.
At 1000 °C, the flexural strength continues to decrease, confirming the negative effect of ZPT addition on the ability of the bricks to withstand bending stresses. The persistence of this trend suggests that, even at higher temperatures, the porosity induced by ZPTs prevents the formation of a dense and mechanically resistant microstructure. Meanwhile, the compressive strength exhibits more pronounced fluctuations, with peaks at 10% and 15% ZPTs. This non-linear behaviour is likely due to phase transformations occurring at high temperatures. At 1000 °C, iron oxides may contribute to the formation of liquid phases that enhance densification at intermediate ZPT levels. Still, excessive amounts of ZPTs could form fragile crystalline phases (such as excessive hematite Fe2O3 or anorthite CaAl2Si2O8), which may compromise mechanical integrity.
These findings highlight the crucial roles of ZPT content and firing temperature in determining the mechanical properties of bricks. While small additions of ZPTs (up to 10%–15%) may improve compressive strength due to densification effects, excessive incorporation leads to an increased porosity and the potential formation of brittle phases, ultimately limiting the mechanical performance of the bricks. Therefore, optimising the mixture design requires a careful balance between ZPT content and firing conditions to achieve the desired mechanical properties for construction applications.
5. Conclusions
This study explored the feasibility of incorporating zinc processing tailings (ZPTs) as a partial replacement for clay in fired brick production. The experimental findings demonstrated that ZPTs affect the plasticity, sintering behaviour, and final properties of bricks, leading to challenges and opportunities in optimising formulations.
The plasticity tests showed a progressive decrease in plasticity with an increasing ZPT content, confirming its role as a de-plasticiser. This reduction in workability can affect the shaping and forming process, necessitating adjustments such as an increased water content or the use of plasticising agents.
A key finding was the influence of ZPTs’ high loss on ignition (LOI = 35.46%), which induces gas release during firing. Despite the presence of fluxing agents (Ca and Mg) that generally promote sintering and densification, the dominant effect of volatile release led to an increased porosity, as evidenced by the higher water absorption values observed at 900 °C. The microstructural impact of this gas release resulted in a lower bulk density, indicating a need to optimise firing parameters to counterbalance porosity.
In terms of mechanical performance, an increasing ZPT content weakened compressive strength due to a higher porosity. However, at 10% ZPTs and 900 °C, a balance was achieved between mechanical integrity and durability, making this formulation the most suitable for practical application in brick manufacturing.
Compared to previous research on industrial waste incorporation in ceramics, this study provides a new perspective on the trade-off between sintering enhancement and porosity increase due to gas evolution. It highlights the need for process control strategies to mitigate the adverse effects of ZP41T decomposition. Future research should focus on optimising the thermal profile, exploring stabilising additives, or implementing alternative firing atmospheres to improve the densification and strength of ZPT-containing bricks.
Overall, this study advances the field by demonstrating that ZPTs can be effectively reused in brick production when carefully optimised, contributing to both sustainable waste management and innovative material development in the ceramics industry.
6. Prospects
Therefore, further in-depth research is essential to develop ZPT utilisation as a partial replacement for clay in fired bricks, with a focus on the following aspects:
- The thermal characteristics of ZPTs should be studied;
- The effect of ZPTs on the performance of fired bricks as a building material needs to be further explored;
- Heavy metal leaching in bricks made from ZPTs could be considered to precisely quantify the mobility of heavy metals after firing and to ensure the security and workability of ZPT bricks.
Author Contributions
Conceptualisation, A.M., B.F.P., V.N. and A.B.; methodology, A.M.; formal analysis, A.M., J.M.M.-A., L.B., I.M. and M.T.; investigation, J.M.M.-A., L.B., I.M. and M.T.; resources, A.M., B.F.P. and A.B.; writing—original draft preparation, A.M. and V.N.; writing—review and editing, A.M., J.M.M.-A., V.N. and M.T.; All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this paper had the financial support of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, within the funding of the scientific research work at the University of Belgrade, Technical Faculty in Bor, according to the contract with registration number 451-03-137/2025-03/200131.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, R.; Gao, Y.; Yang, Y. Leaching of heavy metals from lead-zinc mine tailings and the subsequent migration and transformation characteristics in paddy soil. Chemosphere 2022, 291, 132792. [Google Scholar] [CrossRef]
- Li, C.; Wen, Q.; Hong, M.; Liang, Z.; Zhuang, Z.; Yu, Y. Heavy metals leaching in bricks made from lead and zinc mine tailings with varied chemical components. Constr. Build. Mater. 2017, 134, 443–451. [Google Scholar] [CrossRef]
- Ngayakamo, B.H.; Bello, A.; Onwualu, A.P. Development of eco-friendly fired clay bricks incorporated with granite and eggshell wastes. Environ. Chall. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, X.; Chen, T.; Yan, B.; Li, L. Mechanical properties and toxicity risks of lead-zinc sulfide tailing-based construction materials. Materials 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, O.; Rhoujjati, A.; Moreno-Jimenez, E.; Hachimi, M.L.E. Environmental and geochemical characteristics of heavy metals in soils around the former mining area of zeïda (high moulouya, Morocco). Water Air Soil Pollut. 2023, 234, 110. [Google Scholar] [CrossRef]
- Wiemes, L.; Pawlowsky, U.; Mymrin, V. Incorporation of industrial wastes as raw materials in brick’s formulation. J. Clean. Prod. 2017, 142, 69–77. [Google Scholar] [CrossRef]
- Behera, S.K.; Ghosh, C.N.; Mishra, K.; Mishra, D.P.; Singh, P.; Mandal, P.K.; Buragohain, J.; Sethi, M.K. Utilisation of lead-zinc mill tailings and slag as paste backfill materials. Environ. Earth Sci. 2020, 79, 1–18. [Google Scholar] [CrossRef]
- Doğan-Sağlamtimur, N.; Bilgil, A.; Szechyńska-Hebda, M.; Parzych, S.; Hebda, M. Eco-friendly fired brick produced from industrial ash and natural clay: A study of waste reuse. Materials 2021, 14, 877. [Google Scholar] [CrossRef]
- Li, R.; Yin, Z.; Lin, H. Research status and prospects for the utilisation of lead–zinc tailings as building materials. Buildings 2023, 13, 150. [Google Scholar] [CrossRef]
- Yaras, A.; Sutcu, M.; Erdogmus, E.; Gencel, O. Recycling and immobilisation of zinc extraction residue in clay-based brick manufacturing. J. Build. Eng. 2021, 41, 102421. [Google Scholar] [CrossRef]
- Lin, H.; Li, R.; Li, S. Fabrication of Lead–Zinc Tailings Sintered Brick and Its Effect Factors Based on an Orthogonal Experiment. Materials 2024, 17, 2352. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ning, X.A.; Lu, X.; Lai, X.; Cai, H.; Liu, Y.; Zhang, T. Effect of sintering temperature on mineral composition and heavy metals mobility in tailings bricks. Waste Manag. 2019, 93, 112–121. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Qi, Y.Y.; Deng, L. Effect of ZnO on the Mineral Composition of Portland cement clinker. Bull. Chin. Ceram. Soc. 2017, 36, 1567–1572. [Google Scholar]
- Nouairi, J.; Hajjaji, W.; Costa, C.S.; Senff, L.; Patinha, C.; da Silva, E.F.; Labrincha, J.A.; Rocha, F.; Medhioub, M. Study of Zn-Pb ore tailings and their potential in cement technology. J. Afr. Earth Sci. 2018, 139, 165–172. [Google Scholar] [CrossRef]
- Luo, Z.; Tang, C.; Mu, Y.; Liu, X. Recycle of lead-zinc tailings in blended cement: Mechanical property and stabilisation/solidification of heavy metals. Prepr. 2022 Miner. 2019, 9, 710. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, S.; Yang, H.; Ni, W.; Li, J.; Zhang, G.; Teng, G. Influence on fine lead-zinc tailings solidified/stabilised by clinker-free slag-based binder. J. Environ. Chem. Eng. 2022, 10, 108692. [Google Scholar] [CrossRef]
- Gou, M.; Zhou, L.; Then, N.W.Y. Utilisation of tailings in cement and concrete: A review. Sci. Eng. Compos. Mater. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Darban, A.K.; Mohseni, M.; Nejati, H. Utilization of lead–zinc mine tailings as cement substitutes in concrete construction: Effect of sulfide content. J. Build. Eng. 2022, 57, 104865. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Mohseni, M.; Darban, A.K.; Nejati, H. Mechanical activation of lead–zinc mine tailings as a substitution for cement in concrete construction. Constr. Build. Mater. 2023, 364, 129973. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, L.; Tao, Q.; Xie, L. Study on preparation and shielding effect of lead-zinc tailings sand mortar. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 022152. [Google Scholar] [CrossRef]
- Wang, H.; Ju, C.; Zhou, M.; Dong, Y.; Hou, H.; Liu, S. Grinding kinetics of lead–zinc tailing powders and its optimal particle size as a pozzolanic admixture in cement mortar. Adv. Powder Technol. 2022, 33, 103730. [Google Scholar] [CrossRef]
- Qu, G.; Ge, R.; Li, J.; Zhang, Y. Lead-Zinc Tailings-Based Construction Mortars/Preparation, Performance and Stabilization Mechanism of Heavy Metals. Prep. Perform. Stab. Mech. Heavy Met. 2022. [Google Scholar] [CrossRef]
- Peng, H.; Wang, D.; Sofi, M.; Mendis, P.; Zhou, Z.; Liu, J. Feasibility of Using Lead–Zinc Tailings to Produce Environmentally Friendly Ceramisite. J. Mater. Civ. Eng. 2021, 402, 331298. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Li, D. Study on low-cost preparation of glass–ceramic from municipal solid waste incineration (MSWI) fly ash and lead-zinc tailings. Constr. Build. Mater. 2022, 356, 129231. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Hassan, Q.U.; Pan, D.; Zhu, G. Preparation and characterisation of glass ceramics synthesised from lead slag and lead-zinc tailings. Ceram. Int. 2023, 49, 16164–16173. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, J.; Liu, X.; Mu, Y.; Zhang, M.; Zhang, M.; Tian, C.; Ou, J.; Mi, J. Preparation of ceramsite from lead-zinc tailings and coal gangue: Physical properties and solidification of heavy metals. Constr. Build. Mater. 2023, 368, 130426. [Google Scholar] [CrossRef]
- Akkaya, U.G.; Cinku, K.; Yilmaz, E. Characterization of strength and quality of cemented mine backfill made up of lead-zinc processing tailings. Front. Mater. 2021, 8, 740116. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Zhang, J.; Gui, X.; Zhu, X.; Zhao, C. Effects of slag-based cementitious material on the mechanical behavior and heavy metal immobilisation of mine tailings based cemented paste backfill. Heliyon 2022, 8, e10695. [Google Scholar] [CrossRef]
- Feng, Q.M.; Wang, W.Q.; Zhang, B.L.; Huang, Y. Research on technics of lightweight baking-free brick made of lead-zinc ore tailings from Qinghai Province. Non-Met. Mines 2011, 34, 6–8. [Google Scholar]
- Li, C.; Xu, Y.L.; Yu, Y.; Lin, Y.B.; Lin, J. The preparation and research of unburned and absorptive bricks of Pb-Zn mine tailings. Mater. Sci. Technol. 2016, 24, 46–51. [Google Scholar]
- Lograda, T.; Harkati, Z.; Adel, K.; Ramdani, M. Heavy metals accumulation in species from mine Karzet Youcef (Algeria). Word. J. Pharmaceut. Res. 2016, 5, 250–260. [Google Scholar]
- Belguidoum, A.; Lograda, T.; Ramdani, M. Heavy metals accumulation in Hertia cheirifolia along the highway in Setif region, Algeria. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Omara, R. Ecological and Geochemical Assessment of the Environment in the Zinc Ore Recovery Zone; Case of CHAABET EL-HAMRA Mining Complex (Algeria). In Advances in Green Energies and Materials Technology: Selected Articles from the Algerian Symposium on Renewable Energy and Materials (ASREM-2020); Springer: Singapore, 2021. [Google Scholar]
- ASTM D4318-17e1; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Concord, PA, USA, 2017.
- ASTM D4943-18; Standard Test Method for Shrinkage Factors of Soils by the Wax Method. ASTM International: West Concord, PA, USA, 2018.
- ASTM C29/C29M-17a; Standard Test Method for Bulk Density (“Unit Weight”) and Voids in Aggregate. ASTM International: West Concord, PA, USA, 2017.
- ASTM C373-18; Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired White ware Products. ASTM International: West Concord, PA, USA, 2018.
- ASTM C326-09; Standard Test Method for Drying and Firing Shrinkages of Ceramic White ware Clays. ASTM International: West Concord, PA, USA, 2016.
- ASTM C67-22; Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile. ASTM International: West Concord, PA, USA, 2022.
- ASTM C674-88; Standard Test Method for Flexural Properties of Ceramic White ware Materials. ASTM International: West Concord, PA, USA, 2019.
- Wang, S.; Gainey, L.; Baxter, D.; Wang, X.; Mackinnon, I.D.; Xi, Y. Thermal behaviours of clay mixtures during brick firing: A combined study of in-situ XRD, TGA and thermal dilatometry. Constr. Build. Mater. 2021, 299, 124319. [Google Scholar] [CrossRef]
- Milićević, S.; Milošević, V.; Povrenović, D.; Stojanović, J.; Martinović, S.; Babić, B. Removal of Heavy Metals from Aqueous Solution Using Natural and Fe(III) Oxyhydroxide Clinoptilolite. Clays Clay Miner. 2013, 61, 508–517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).