Barian Micas and Exotic Ba-Cr and Ba-V Micas Associated with Metamorphosed Sedimentary Exhalative Baryte Deposits near Aberfeldy, Scotland, UK
Abstract
1. Introduction
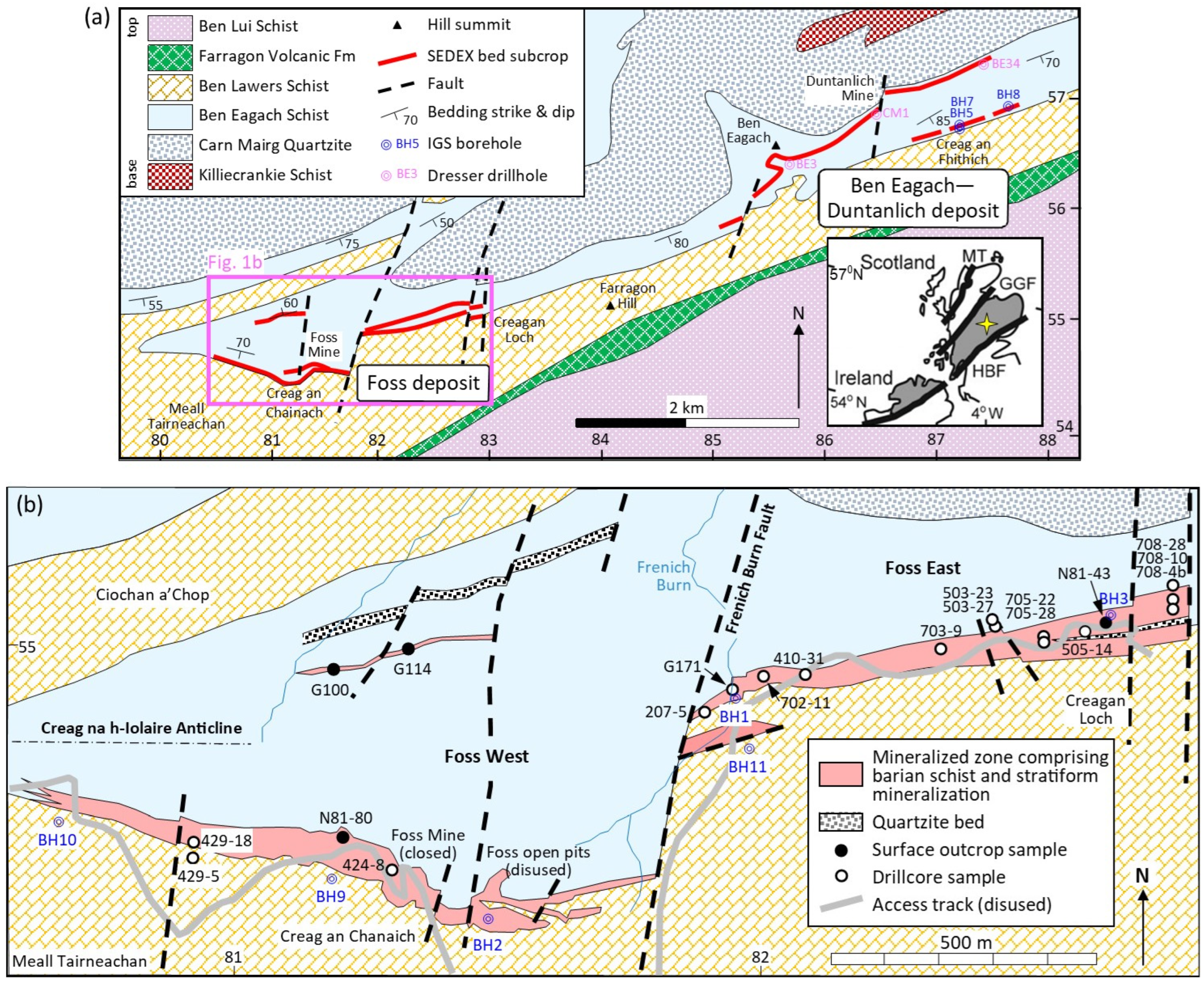
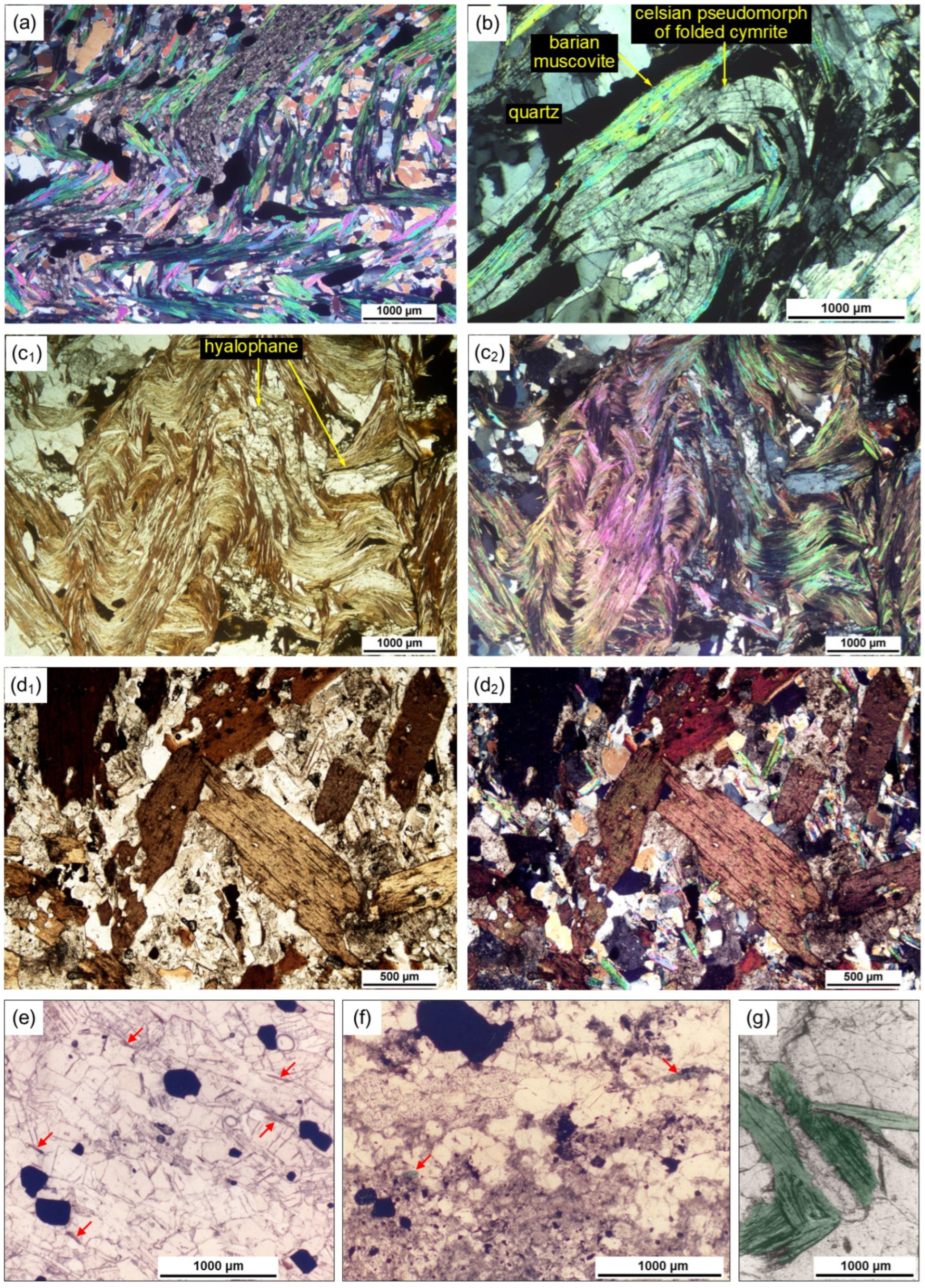
2. Occurrence of Micas Associated with the Aberfeldy SEDEX Mineralization
| Sample Name | Type | Rock Description | Mineralogy † |
|---|---|---|---|
| G100d | outcrop | Quartz celsian chert with sulphate pseudomorphs | CLS, QZ, ms, bt, py, rt, (sp) |
| G114a | outcrop | Quartz chert with pyrite laminae | QZ, PY, cls, hyal, ms, sp, pyh |
| 429-5 | core | Garnet hornblende mica schist (BLS) | QZ, PL, MS, GT, HB, DOL, bt, chl, cal |
| 429-18 | core | Baryte rock | BRT, py, cls, qz, bt, hyal, (sp, rt, cal) |
| BH9-28.6 | core * (4330) | Quartz muscovite hyalophane schist | QZ, MS, HYAL, cls, py, sp, (rt, brt) |
| 09-05 [BH9] | core | Muscovite dolomite schist (MB) | DOL, MS, QZ, PYH, (rt, ap) |
| N81-80 | outcrop | Hyalophane chert | HYAL, QZ, bt, ms, py, rt, brt, sp, (ap) |
| 424-8 | core | Hyalophane biotite schist | HYAL, DOL, BT, QZ, PY, ms, pyh, rt, (sp) |
| BH3-27.8 | core * (3963) | Banded baryte rock | BRT, QZ, PY, cal, mag, bt, dol |
| G171 | outcrop | Baryte rock | BRT, qz, bt, cls, py, dol |
| 702-11 | core | Banded baryte–dolomite rock | BRT, DOL, py, qz, sp, bt, mag, cls, gn, (phy, ap) |
| 207-5 | core | Biotite calcite schist (MB) | BT, QZ, CAL, PL, chl, dol, py, rt, ms, ap, (ccp) |
| 410-31 | core | Sulphidic baryte rock | BRT, PY, QZ, sp, dol, ms, (gn, cal, ap, ccp) |
| 703-9 | core | Quartz chert and sulphide breccia | QZ, PY, CLS, SP, gn, ms, (ccp, ap) |
| 503-23 | core | Graphitic dolostone | DOL, sp, phy, qz, bt, ms, py, gn, (chl, rt, ccp, ap) |
| 503-27 | core | Graphitic garnet mica schist | QZ, MS, GT, pl, bt, chl, cal, ap, py, rt |
| 705-22 | core | Muscovite dolomite schist (metabasite) | DOL, MS, QZ, pyh, (rt, ap, ccp) |
| 705-28 | core | Calcareous sulphidic baryte rock | BRT, CAL, PY, qz, sp, dol, (gal, ms, ccp) |
| 505-14 | core | Calcareous sulphidic baryte rock | CAL, QZ, BRT, PY SP, dol, gal, (ms, ccp, ap) |
| N81-43 | outcrop | Biotite calcite schist (MB) | BT, QZ, CAL, MS, (pyh, ap, ccp) |
| 708-4b | core | Quartz muscovite hyalophane schist | QZ, MS, HYAL, py, sp, cls, chl, rt, gn |
| 708-10 | core | Sulphidic quartz chert | QZ, PY, sp, hyal, dol, cls, gn, ccp, bt, ms, ap, (rt) |
| 708-28 | core | Calcareous sulphidic baryte rock | BRT, CAL, PY, qz, sp, dol, (gn, ms, ccp) |
3. Materials and Methods
4. Results
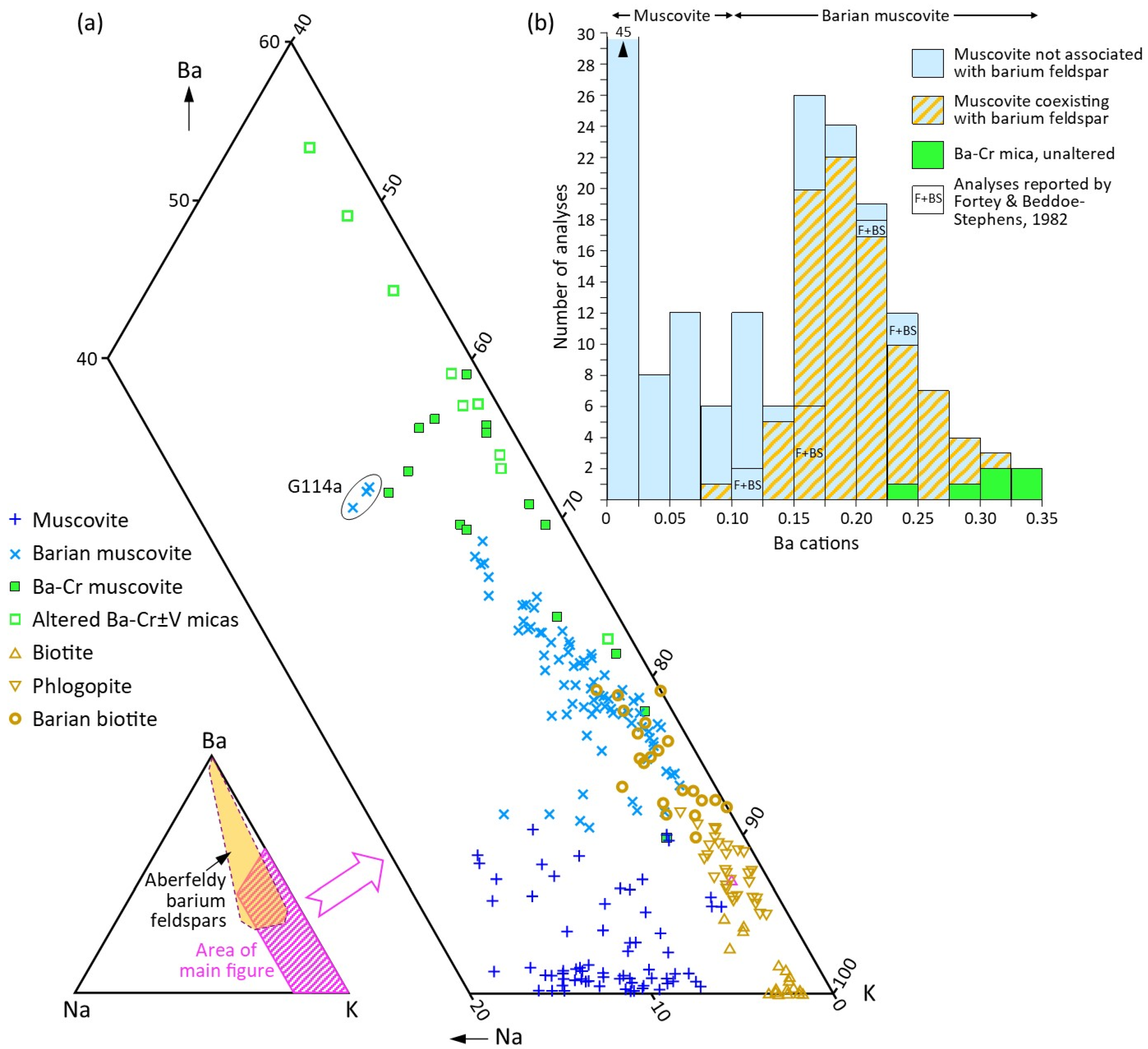
4.1. Muscovite and Barian Muscovite
4.2. Chromium- and Vanadium-Bearing Barian Micas
4.3. Biotite, Phlogopite, and Barian Biotite

4.4. Altered (Interlayer-Deficient) Biotite
5. Discussion
5.1. Di-Octahedral Mica Compositions and Substitution Mechanisms
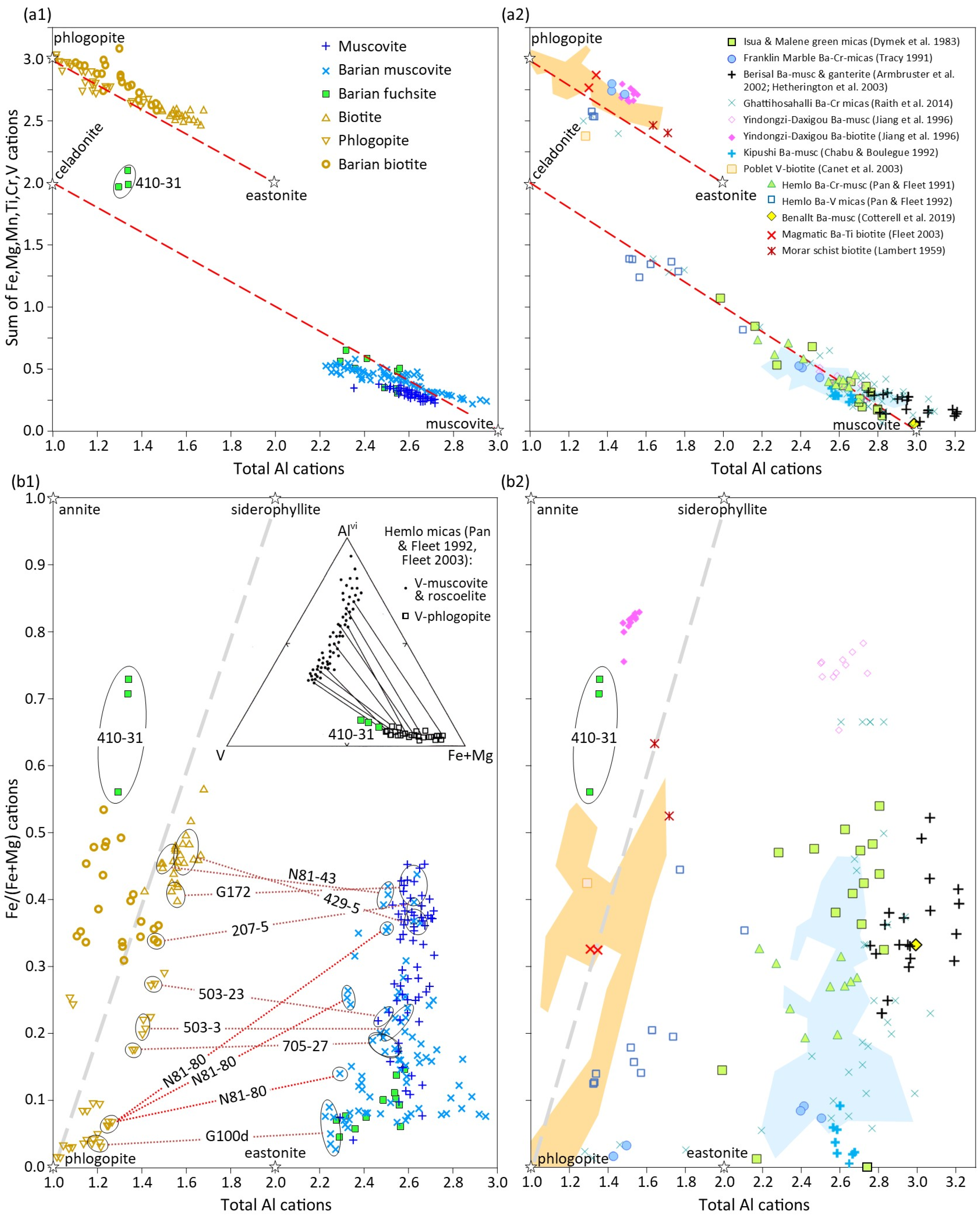
5.2. Tri-Octahedral Mica Compositions and Substitution Mechanisms
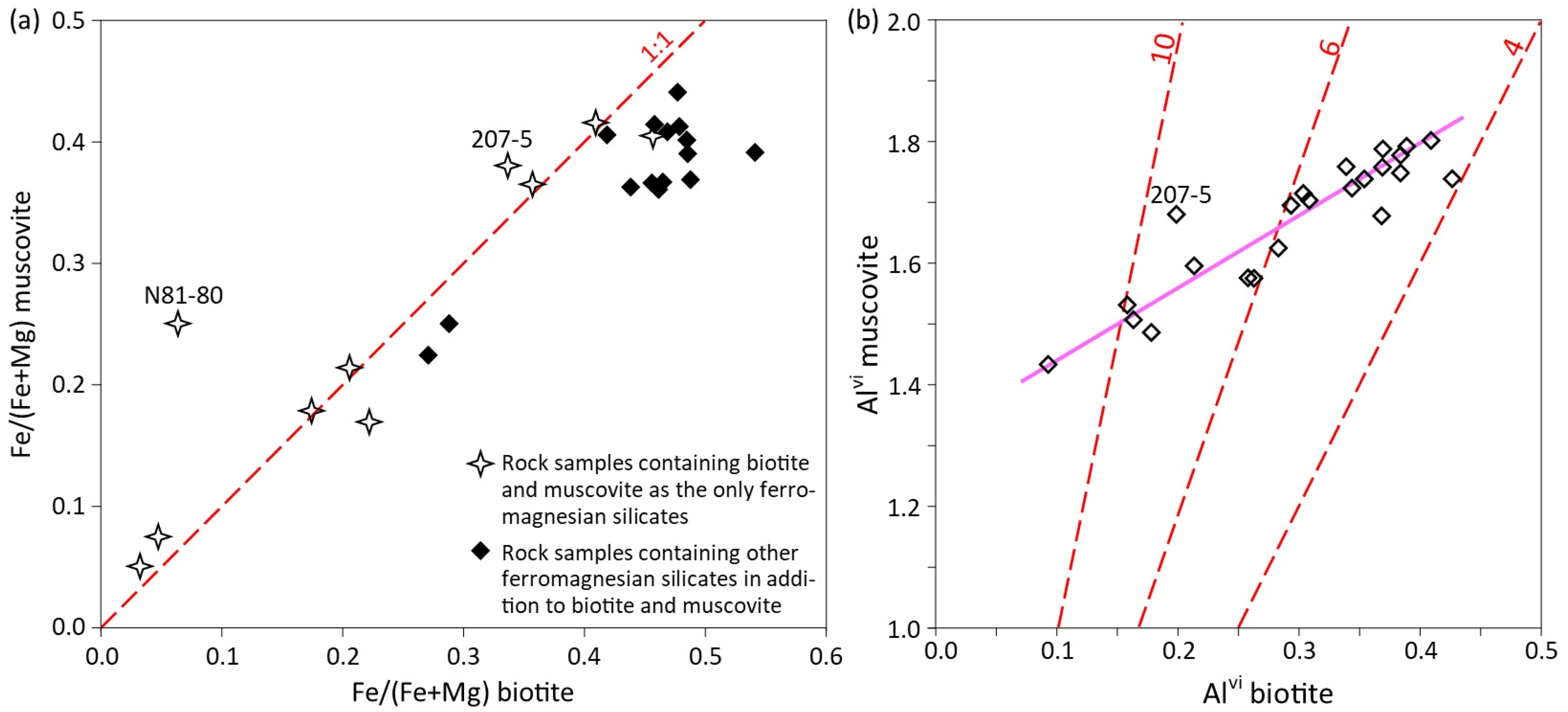
5.3. Compositional Variations in Micas Coexisting in Rock Samples
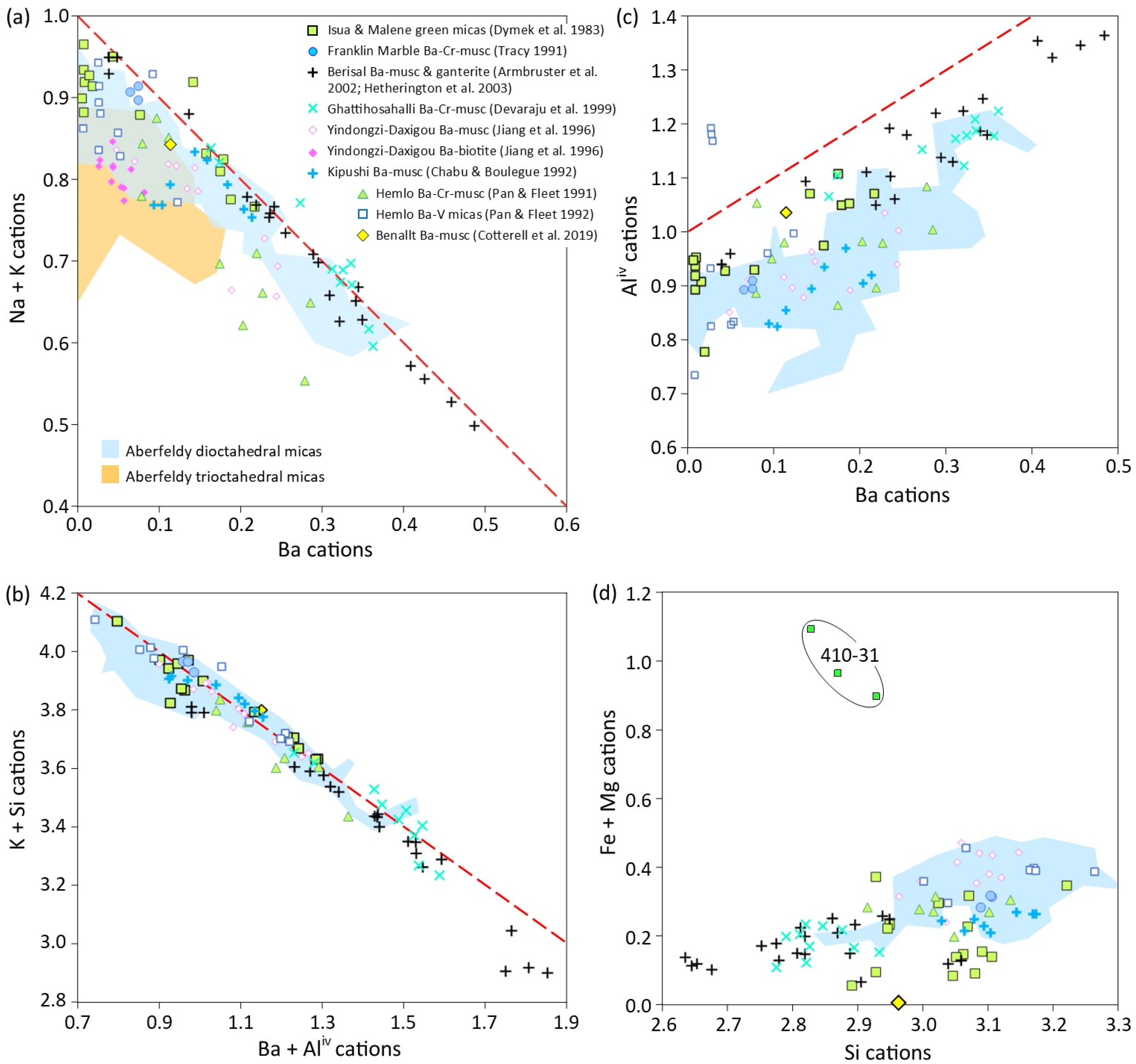
5.4. Other SEDEX Ba- (±Cr ±V) Mica Occurrences and Comparisons with Aberfeldy Barian Micas
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleet, M.E. Deer, Howie and Zussman: Rock-Forming Minerals, Vol. 3A Micas, 2nd ed.; The Geological Society: London, UK, 2003; 758p. [Google Scholar]
- Rieder, M.; Cavazzini, G.; D’Yakonov, Y.S.; Frank-Kamenetskii, V.A.; Gottardi, G.; Guggenheim, S.; Koval, P.V.; Müller, G.; Neiva, A.M.R.; Radoslovich, E.W.; et al. Nomenclature of the micas. Mineral. Mag. 1999, 63, 267–279. [Google Scholar] [CrossRef]
- Foster, M.D. Interpretation of the Composition of Trioctahedral Micas; U.S. Geological Survey Professional Paper; USGS: Reston, VA, USA, 1960; Volume 354-B, pp. 11–49. [Google Scholar]
- Tischendorf, G.; Förster, H.-J.; Gottesmann, B.; Rieder, M. True and brittle micas: Composition and solid solution series. Mineral. Mag. 2007, 71, 285–320. [Google Scholar] [CrossRef]
- Shaw, C.S.J.; Penczak, R.S. Barium- and titanium-rich biotite and phlogopite from the Western and Eastern Gabbro, Coldwell alkaline complex, Northwestern Ontario. Can. Mineral. 1996, 34, 967–975. [Google Scholar]
- Majka, J.; Kruszewski, Ł.; Rosén, Å.; Klonowska, I. Ba- and Ti-enriched dark mica from the UHP metasediments of the Seve Nappe Complex, Swedish Caledonides. Mineralogia 2015, 46, 41–50. [Google Scholar] [CrossRef]
- Henderson, C.M.B. Ti- and Ba-rich phlogopitic micas in alkaline basic and upper mantle igneous rocks; stoichiometry, stability, and Fe valence estimation reassessed and rationalised. Geochim. Cosmochim. Acta 2024, in press. [Google Scholar] [CrossRef]
- Bol, L.C.G.M.; Bos, A.; Sauter, P.C.C.; Jansen, J.B.H. Barium-titanium-rich phlogopites in marbles from Rogaland, southwest Norway. Am. Mineral. 1989, 74, 439–447. [Google Scholar]
- Jiang, S.-Y.; Palmer, M.R.; Li, Y.-H.; Xue, C.-J. Ba-rich micas from the Yindongzi-Daxigou Pb-Zn-Ag and Fe deposits, Qinling, northwestern China. Mineral. Mag. 1996, 60, 433–445. [Google Scholar] [CrossRef]
- Dymek, R.F.; Boak, J.L.; Kerr, M.T. Green micas in the Archaean Isua and Malene supracrustral rocks, southern West Greenland, and the occurrence of barian-chromian muscovite. Rapp. Grønlands Geol. Unders 1983, 112, 71–82. [Google Scholar]
- Pan, Y.; Fleet, M.E. Barium feldspar and barian-chromian muscovite from the Hemlo area, Ontario. Can. Minerol. 1991, 29, 481–498. [Google Scholar]
- Snetsinger, K.G. Barium-vanadium muscovite and vanadium tourmaline from Mariposa County, California. Am. Mineral. 1966, 51, 1623–1639. [Google Scholar]
- Pan, Y.; Fleet, M.E. Mineral chemistry and geochemistry of vanadian silicates in the Hemlo gold deposit, Ontario, Canada. Contr. Min. Petr. 1992, 109, 511–525. [Google Scholar] [CrossRef]
- Coats, J.S.; Smith, C.G.; Fortey, N.J.; Gallagher, M.J.; May, F.; McCourt, W.J. Stratabound barium-zinc mineralization in Dalradian Schist near Aberfeldy, Scotland. Trans. Inst. Min. Metall. 1980, 89, B110–B129. [Google Scholar]
- Coats, J.S.; Smith, C.G.; Gallagher, M.J.; May, F.; McCourt, W.J.; Parker, M.E.; Fortey, N.J. Stratabound Barium-Zinc Mineralization in Dalradian Schist Near Aberfeldy, Scotland. Final Report; Mineral Reconnaissance Programme, Report No. 40; Institute of Geological Sciences: London, UK, 1981. [Google Scholar]
- Smith, M.; Butler, P.; Gillespie, M.R.; Haszeldine, R.S.; Jones, D.; Monaghan, A.; Rice, C.M. Scotland’s mineral, water and energy resources: Building a low-carbon future. In The Geology of Scotland; Smith, M., Strachan, R.A., Eds.; Geological Society: London, UK, 2024; Chapter 17; pp. 563–608. [Google Scholar]
- Prave, A.; Fallick, A.E.; Strachan, R.A.; Krabbendam, M.; Leslie, A.G. Middle Proterozoic-Early Ordovician: Foreland basins, climatic extremes and rift-to-drift margins. In The Geology of Scotland; Smith, M., Strachan, R.A., Eds.; Geological Society: London, UK, 2024; Chapter 5; pp. 111–138. [Google Scholar]
- Stephenson, D.; Gould, D. The Grampian Highlands, 4th ed.; British Regional Geology; HMSO for the British Geological Survey: London, UK, 1995. [Google Scholar]
- Hall, A.J. Stratiform mineralization in the Dalradian of Scotland. In Mineralization in the British Isles; Pattrick, R.A.D., Polya, D.A., Eds.; Chapman & Hall: London, UK, 1993; pp. 38–101. [Google Scholar]
- Moles, N.R. Metamorphic conditions and uplift history in central Perthshire: Evidence from mineral equilibria in the Foss celsian-baryte-sulphide deposit, Aberfeldy. J. Geol. Soc. 1985, 142, 39–52. [Google Scholar] [CrossRef]
- Moles, N.R. Geology, geochemistry and petrology of the Foss stratiform baryte—Base metal deposit and adjacent Dalradian metasediments, near Aberfeldy, Scotland. Unpublished. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1985. [Google Scholar]
- Moles, N.R.; Boyce, A.J.; Fallick, A.E. Abundant sulphate in the Neoproterozoic ocean: Implications of constant δ34S of barite in the Aberfeldy SEDEX deposits, Scottish Dalradian. In Ore Deposits in an Evolving Earth; Jenkin, G.R.T., Lusty, P.A.J., McDonald, I., Smith, M.P., Boyce, A.J., Wilkinson, J.J., Eds.; Special Publication of the Geological Society of London: London, UK, 2015; Volume 393, pp. 189–212. [Google Scholar]
- Moles, N.R.; Selby, D. Implications of new geochronological constraints on the Aberfeldy stratiform baryte deposits, Scotland, for the depositional continuity and global correlation of the Neoproterozoic Dalradian Supergroup. Precambrian Res. 2023, 384, 106925. [Google Scholar] [CrossRef]
- Moles, N.R.; Boyce, A.J.; Warke, M.R.; Claire, M.W. Syn-sedimentary exhalative or diagenetic replacement? Multi-proxy evidence for origin of metamorphosed stratiform baryte–sulfide deposits near Aberfeldy, Scottish Highlands. Minerals 2024, 14, 865. [Google Scholar] [CrossRef]
- Leslie, A.G.; Stone, P.; Strachan, R.A. Early-Middle Ordovician Grampian orogenesis: Ophiolite obduction and arc-continent collision. In The Geology of Scotland; Smith, M., Strachan, R.A., Eds.; Geological Society: London, UK, 2024; Chapter 6; pp. 139–170. [Google Scholar]
- Fortey, N.J.; Beddoe-Stephens, B. Barium silicates in stratabound Ba-Zn mineralization in the Scottish Dalradian. Mineral. Mag. 1982, 46, 63–72. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Sweatman, T.R.; Long, J.V.P. Quantitative electron-probe-microanalysis of rock-forming minerals. J. Petrol. 1969, 10, 332–379. [Google Scholar] [CrossRef]
- Heinrich, K.F.J. X-ray absorption uncertainty. In The Electron Microprobe; McKinley, T.D., Heinrich, K.F.J., Wittey, D.B., Eds.; Wiley: London, UK, 1966; pp. 296–377. [Google Scholar]
- Norrish, K.; Chappell, B.W. X-ray fluorescence spectrometry. In Physical Methods in Determinative Mineralogy, 2nd ed.; Zussman, J., Ed.; Academic Press: London, UK, 1977; pp. 201–272. [Google Scholar]
- Grapes, R.H. Barian mica and distribution of barium in metacherts and quartzofeldspathic schists, Southern Alps, New Zealand. Mineral. Mag. 1993, 57, 265–272. [Google Scholar] [CrossRef]
- Chopin, C.; Maluski, H. 40Ar-39Ar dating of high pressure metamorphic micas from the Paradisco Area (Western Alps): Evidence against the blocking temperature concept. Contrib. Mineral. Petrol. 1980, 74, 109–122. [Google Scholar] [CrossRef]
- Raith, M.M.; Devaraju, T.C.; Spiering, B. Paragenesis and chemical characteristics of the celsian−hyalophane−K-feldspar series and associated Ba-Cr micas in baryte-bearing strata of the Mesoarchaean Ghattihosahalli Belt, Western Dharwar Craton, South India. Mineral. Petrol. 2014, 108, 153–176. [Google Scholar] [CrossRef]
- Chatterjee, N.D.; Froese, E. A thermodynamic study of the pseudo-binary join margarite-paragonite in the system KAlSi3O8-Al2O3-SiO2-H2O. Am. Mineral. 1975, 60, 985–993. [Google Scholar]
- Blencoe, J.G.; Guidotti, C.V.; Sassi, F.P. The paragonite-muscovite solvus: II. Numerical geothermometers for natural, quasibinary paragonite-muscovite pairs. Geochim. Cosmochim. Acta 1994, 58, 2277–2288. [Google Scholar] [CrossRef]
- Green, D.I.; Cotterell, T.F.; Tindle, A.G. Barium substitution in muscovite with a comment on ganterite. J. Russell Soc. 2019, 22, 48–58. [Google Scholar]
- Gessmann, C.L.; Spiering, B.; Raith, M.M. Experimental study of the Fe-Mg exchange between garnet and biotite: Constraints on the mixing behavior and analysis of the cation-exchange mechanisms. Am. Mineral. 1977, 82, 1225–1240. [Google Scholar] [CrossRef]
- Guo, J.; Green, T.H. Experimental study of Ba partitioning between phlogopite and silicate liquid at upper mantle pressure and temperature. Lithos 1990, 24, 83–95. [Google Scholar] [CrossRef]
- Tracy, R.J. Ba-rich micas from the Franklin Marble, Lime Crest and Sterling Hill, New Jersey. Am. Mineral. 1991, 76, 1683–1693. [Google Scholar]
- Munoz, J.L. F-OF and Cl-OH exchange in micas with applications to hydrothermal ore deposits. In Mineralogical Society of America, Reviews in Mineralogy 13, ‘Micas’; Bailey, S.W., Ed.; Mineralogical Society of America: Washington, DC, USA, 1984; p. xii + 584. [Google Scholar]
- Sanz, J. NMR study of micas, II. Distribution of Fe2+, F−, and OH− in the octahedral sheet of phlogopites. Am. Mineral. 1979, 64, 119–126. [Google Scholar]
- Dempster, T.J. Uplift patterns and orogenic evolution in the Scottish Dalradian. J. Geol. Soc. 1985, 142, 111–128. [Google Scholar] [CrossRef]
- Wendlandt, R.F. Barium-phlogopite from Haystack Butte, Highwood Mountains, Montana. Carnegie Inst. Geophys. Lab. Yearb. 1977, 76, 534–539. [Google Scholar]
- Mitchell, R.H. Kimberlites, Orangeites, and Related Rocks; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Armbruster, T.; Berlepsch, P.; Gnos, E.; Hetherington, C.J. Crystal chemistry and structure refinements of barian muscovites from the Berisal Complex, Simplon region, Switzerland. Schweiz. Mineral. Petrogr. Mitt. 2002, 82, 537–547. [Google Scholar]
- Hetherington, C.J.; Gieré, R.; Graeser, S. Composition of barium-rich white micas from the Berisal Complex, Simplon Region, Switzerland. Can. Mineral. 2003, 41, 1281–1291. [Google Scholar] [CrossRef]
- Chabu, M.; Boulègue, J. Barian feldspar and muscovite from the Kipushi Zn-Pb-Cu deposit, Shaba, Zaire. Can. Mineral. 1992, 30, 1143–1152. [Google Scholar]
- Canet, C.; Alfonso, P.; Melgarejo, J.-C.; Jorge, S. V-rich minerals in contact-metamorphosed Silurian SEDEX deposits in the Poblet area, Southwestern Catalonia, Spain. Can. Mineral. 2003, 41, 561–579. [Google Scholar] [CrossRef]
- Cotterell, T.F.; Green, D.I.; Tindle, A.G.; Kerbey, H.; Dossett, I. Barium-rich muscovite and hyalophane from Benallt Mine, Rhiw, Pen Llŷn, Gwynedd. J. Russell Soc. 2019, 22, 43–47. [Google Scholar]
- Lambert, R.S.J. The mineralogy and metamorphism of the Moine schists of the Morar and Knoydart districts of Inverness-shire. Trans. R. Soc. Edinb. 1959, 63, 553–588. [Google Scholar] [CrossRef]
- Mansker, W.L.; Ewing, R.C.; Keil, K. Barian-titanian biotites in nephelinites from Oahu, Hawaii. Am. Mineral. 1979, 64, 156–159. [Google Scholar]
- Gaspar, J.C.; Wyllie, P.J. Barium phlogopite from the Jacupiranga carbonatite, Brazil. Am. Mineral. 1982, 67, 997–1000. [Google Scholar]
- Robert, J.L. Titanium solubility in synthetic phlogopite solid solutions. Chem. Geol. 1976, 17, 213–227. [Google Scholar] [CrossRef]
- Righter, K.; Dyar, M.D.; Delaney, J.S.; Vennemann, T.W.; Hervig, R.L.; King, P.L. Correlations of octahedral cations with OH−, O2−, Cl− and F− in biotite from volcanic rocks and xenoliths. Am. Mineral. 2002, 87, 142–153. [Google Scholar] [CrossRef]
- Arima, M.; Edgar, A.D. Substitution mechanisms and solubility of titanium in phlogopites from rocks of probable mantle origin. Contrib. Mineral. Petrol. 1981, 77, 288–295. [Google Scholar] [CrossRef]
- Ventruti, G.; Caggianelli, A.; Festa, V.; Langone, A. Crystal chemistry of barian titanian phlogopite from a lamprophyre of the Gargano Promontory (Apulia, Southern Italy). Minerals 2020, 10, 766. [Google Scholar] [CrossRef]
- Zelinková, T.; Racek, M.; Abart, R. Compositional trends in Ba-, Ti-, and Cl-rich micas from metasomatized mantle rocks of the Gföhl Unit, Bohemian Massif, Austria. Am. Mineral. 2023, 108, 1840–1851. [Google Scholar] [CrossRef]
- Mohr, D.W.; Newton, R.C. Kyanite-staurolite metamorphism in sulfidic schists of the Anokeesta Formation, Great Smoky Mountains, North Carolina. Am. J. Sci. 1983, 283, 97–134. [Google Scholar] [CrossRef]
- Ferry, J.M. Petrology of graphitic sulphide-rich schists from south-central Maine: An example of desulphidation during regional metamorphism. Am. Mineral. 1981, 66, 908–930. [Google Scholar]
- Wenke, E. Distribution of Al between coexisting micas in metamorphic rocks from the central Alps. Contrib. Mineral. Petrol. 1970, 26, 50–61. [Google Scholar] [CrossRef]
- Mevel, C.; Kienast, J.R. Chromian jadeite, phengite, pumpellyite, and lawsonite in a high-pressure metamorphosed gabbro. Mineral. Mag. 1980, 43, 979–984. [Google Scholar] [CrossRef]
- Matthews, D.W. Zoned ultramafic bodies in the Lewisian of the Moine nappe in Skye. Scott. J. Geol. 1967, 3, 17–33. [Google Scholar] [CrossRef]
- Neiva, A.M.R. Barian chromium-bearing hydromuscovite from Mozambique. Mineral. Mag. 1978, 42, 292–293. [Google Scholar] [CrossRef]
- Devaraju, T.C.; Raith, M.M.; Spiering, B. Mineralogy of the Archean baryte deposit of Ghattihosahalli, Karnataka, India. Can. Mineral. 1999, 37, 603–617. [Google Scholar]
- Kazachenko, V.T.; Butsik, L.A.; Sapin, V.I.; Kitaev, I.V.; Barinov, N.N.; Narnov, G.A. Vanadian-chromian tourmaline and vanadian muscovite in contact-metamorphosed carbonaceous rocks, Primorye, Russia. Can. Mineral. 1993, 31, 347–356. [Google Scholar] [CrossRef]
- Heinrich, E.W.; Levinson, A.A. Studies in the mica group; X-ray data on roscoelite and barium-muscovite. Am. J. Sci. 1955, 253, 39–43. [Google Scholar] [CrossRef]


| Barian Muscovite (Sample 504-5) | Biotite (Sample 429-5) | ||||||
|---|---|---|---|---|---|---|---|
| wt% | 2σ | det | wt% | 2σ | det | ||
| SiO2 | 42.76 | 0.27 | 0.06 | SiO2 | 37.29 | 0.25 | 0.03 |
| TiO2 | 0.59 | 0.06 | 0.04 | TiO2 | 1.76 | 0.08 | 0.04 |
| Al2O3 | 31.87 | 0.17 | 0.02 | Al2O3 | 17.57 | 0.14 | 0.02 |
| FeO | 0.93 | 0.06 | 0.05 | FeO | 17.71 | 0.21 | 0.05 |
| MnO | 0.01 | 0.03 | 0.04 | MnO | 0.05 | 0.04 | 0.04 |
| MgO | 2.33 | 0.06 | 0.02 | MgO | 11.52 | 0.11 | 0.02 |
| Na2O | 0.34 | 0.02 | 0.02 | Na2O | 0.20 | 0.01 | 0.02 |
| K2O | 7.70 | 0.14 | 0.03 | K2O | 8.33 | 0.14 | 0.03 |
| BaO | 8.71 | 0.22 | 0.07 | ||||
| F | 0.20 | 0.10 | 0.07 | ||||
| Total | 95.44 | Total | 94.43 | ||||
| Sample | 429-5 | 503-27 | 09-05 | 705-22 | N81-43 | G100d | N81-80 | 705-22 | G100d | G114a |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition expressed as weight % oxides | ||||||||||
| SiO2 | 46.86 | 46.40 | 47.40 | 46.54 | 45.15 | 48.09 | 44.50 | 43.35 | 44.76 | 38.38 |
| Al2O3 | 32.23 | 33.36 | 32.52 | 32.09 | 30.54 | 28.22 | 30.75 | 29.63 | 31.06 | 33.41 |
| TiO2 | 0.39 | 0.44 | 0.53 | 0.62 | 0.82 | 1.56 | 1.50 | 1.40 | 1.44 | 0.69 |
| V2O3 | ||||||||||
| Cr2O3 | 0.09 | |||||||||
| MgO | 1.78 | 1.42 | 2.35 | 3.07 | 2.35 | 3.97 | 2.16 | 3.11 | 3.22 | 1.64 |
| MnO | 0.02 | 0.02 | 0.02 | 0.12 | 0.02 | 0.03 | 0.10 | 0.02 | ||
| FeO * | 1.68 | 1.62 | 1.17 | 0.85 | 2.70 | 0.69 | 2.14 | 1.38 | 0.21 | 0.25 |
| CaO | 0.01 | |||||||||
| Na2O | 0.77 | 0.63 | 0.64 | 0.72 | 0.54 | 0.15 | 0.30 | 0.25 | 0.30 | 0.69 |
| K2O | 9.54 | 9.83 | 9.21 | 9.22 | 8.73 | 8.89 | 8.64 | 7.87 | 7.75 | 6.16 |
| BaO | 0.23 | 1.13 | 3.19 | 4.47 | 5.70 | 6.46 | 7.97 | 9.10 | 10.86 | |
| Total | 93.27 | 93.95 | 95.06 | 96.30 | 95.30 | 97.39 | 96.48 | 94.99 | 97.94 | 92.10 |
| F | 0.14 | 0.16 | 0.25 | 0.32 | 0.18 | 0.23 | 0.40 | 0.12 | ||
| Atoms per formula unit on the basis of 22 positive charges | ||||||||||
| Si | 3.180 | 3.135 | 3.165 | 3.125 | 3.120 | 3.245 | 3.080 | 3.070 | 3.070 | 2.845 |
| Aliv † | 0.820 | 0.865 | 0.835 | 0.875 | 0.880 | 0.755 | 0.920 | 0.930 | 0.930 | 1.155 |
| Alvi | 1.755 | 1.790 | 1.725 | 1.665 | 1.610 | 1.490 | 1.585 | 1.540 | 1.580 | 1.765 |
| Al total | 2.575 | 2.655 | 2.560 | 2.540 | 2.490 | 2.245 | 2.505 | 2.470 | 2.510 | 2.920 |
| Ti | 0.020 | 0.020 | 0.025 | 0.030 | 0.045 | 0.080 | 0.080 | 0.075 | 0.075 | 0.040 |
| V | ||||||||||
| Cr | ||||||||||
| Mg | 0.180 | 0.145 | 0.235 | 0.305 | 0.240 | 0.400 | 0.225 | 0.330 | 0.330 | 0.180 |
| Mn | ||||||||||
| Fe * | 0.095 | 0.090 | 0.065 | 0.050 | 0.155 | 0.040 | 0.125 | 0.080 | 0.010 | 0.015 |
| Na | 0.100 | 0.080 | 0.085 | 0.095 | 0.070 | 0.020 | 0.040 | 0.035 | 0.040 | 0.100 |
| K | 0.825 | 0.845 | 0.785 | 0.790 | 0.770 | 0.765 | 0.765 | 0.710 | 0.680 | 0.585 |
| Ba | 0.005 | 0.030 | 0.085 | 0.120 | 0.150 | 0.175 | 0.220 | 0.245 | 0.315 | |
| Total | 6.975 | 6.975 | 6.950 | 7.020 | 7.010 | 6.945 | 6.995 | 6.990 | 6.960 | 7.000 |
| ∑I interlayer site | 0.927 | 0.936 | 0.898 | 0.967 | 0.963 | 0.935 | 0.978 | 0.966 | 0.962 | 0.997 |
| ∑M octahedral site | 3.806 | 3.836 | 3.785 | 3.715 | 3.660 | 3.502 | 3.597 | 3.567 | 3.580 | 3.766 |
| Fe/(Fe + Mg) | 0.346 | 0.390 | 0.218 | 0.134 | 0.392 | 0.089 | 0.357 | 0.199 | 0.035 | 0.079 |
| Sample | 708-10 | G114a | G100e a | G100e b | 505-14 | 705-28 | 410-31 a | 410-31 b |
|---|---|---|---|---|---|---|---|---|
| Composition expressed as weight % oxides | ||||||||
| SiO2 | 46.39 | 40.53 | 42.42 | 33.94 | 37.29 | 36.66 | 36.40 | 34.77 |
| Al2O3 | 27.95 | 26.30 | 27.21 | 18.42 | 16.41 | 16.59 | 13.67 | 13.77 |
| TiO2 | 1.10 | 1.28 | 1.45 | 1.32 | 0.75 | 0.70 | 1.49 | 1.80 |
| V2O3 | 10.82 | 10.07 | ||||||
| Cr2O3 | 0.98 | 4.19 | 0.45 | 4.60 | 9.27 | 5.15 | 4.25 | 3.48 |
| MgO | 3.82 | 2.62 | 3.31 | 2.14 | 1.44 | 1.55 | 3.30 | 2.13 |
| MnO | 0.02 | 0.07 | 0.04 | 0.08 | 0.16 | 0.04 | 0.04 | 0.08 |
| FeO * | 0.51 | 0.38 | 0.35 | 0.25 | 3.09 | 2.30 | 7.52 | 10.25 |
| ZnO | 0.06 | 0.03 | ||||||
| Na2O | 0.11 | 0.25 | 0.24 | 0.15 | 0.09 | 0.10 | 0.09 | 0.05 |
| K2O | 8.73 | 5.82 | 7.86 | 4.78 | 6.42 | 6.10 | 6.64 | 5.83 |
| BaO | 6.27 | 11.48 | 8.34 | 15.70 | 11.02 | 11.13 | 9.86 | 12.33 |
| Total | 95.88 | 92.92 | 91.67 | 81.38 | 85.94 | 80.32 | 94.14 | 94.59 |
| F | 0.22 | 0.05 | 0.23 | 0.07 | 0.10 | |||
| Atoms per formula unit on the basis of 22 positive charges | ||||||||
| Si | 3.205 | 3.030 | 3.125 | 3.095 | 3.180 | 3.295 | 2.930 | 2.870 |
| Aliv † | 0.795 | 0.970 | 0.875 | 0.905 | 0.820 | 0.705 | 1.070 | 1.130 |
| Alvi | 1.480 | 1.345 | 1.485 | 1.075 | 0.830 | 1.050 | 0.225 | 0.210 |
| Al total | 2.275 | 2.315 | 2.360 | 1.980 | 1.650 | 1.755 | 1.295 | 1.340 |
| Ti | 0.055 | 0.070 | 0.080 | 0.090 | 0.050 | 0.045 | 0.090 | 0.110 |
| V | 0.700 | 0.665 | ||||||
| Cr | 0.055 | 0.250 | 0.025 | 0.330 | 0.625 | 0.365 | 0.270 | 0.225 |
| Mg | 0.395 | 0.290 | 0.365 | 0.290 | 0.185 | 0.210 | 0.395 | 0.260 |
| Mn | 0.010 | 0.005 | ||||||
| Fe * | 0.030 | 0.025 | 0.020 | 0.020 | 0.220 | 0.175 | 0.505 | 0.710 |
| Na | 0.015 | 0.035 | 0.035 | 0.025 | 0.015 | 0.015 | 0.015 | 0.010 |
| K | 0.770 | 0.555 | 0.740 | 0.555 | 0.700 | 0.700 | 0.680 | 0.615 |
| Ba | 0.170 | 0.335 | 0.240 | 0.560 | 0.370 | 0.390 | 0.310 | 0.400 |
| Total | 6.970 | 6.905 | 6.990 | 6.945 | 7.005 | 6.950 | 7.190 | 7.210 |
| ∑I interlayer site | 0.954 | 0.932 | 1.013 | 1.143 | 1.081 | 1.108 | 1.006 | 1.021 |
| ∑M octahedral site | 3.494 | 3.331 | 3.462 | 2.885 | 2.744 | 2.898 | 1.715 | 1.734 |
| Fe/(Fe + Mg) | 0.070 | 0.075 | 0.056 | 0.062 | 0.546 | 0.454 | 0.561 | 0.730 |
| Sample | 429-5 | 503-27 | 503-23 | N81-43 | G100d | 708-10 | 207-5 | BH3-27.8 | 424-8 | 702-11 |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition expressed as weight % oxides | ||||||||||
| SiO2 | 37.15 | 34.68 | 38.03 | 37.01 | 42.01 | 40.44 | 36.47 | 37.56 | 34.87 | 35.99 |
| Al2O3 | 17.49 | 18.25 | 16.46 | 16.77 | 14.56 | 14.16 | 16.46 | 12.56 | 14.56 | 12.89 |
| TiO2 | 1.84 | 1.46 | 1.10 | 2.64 | 0.54 | 0.82 | 2.36 | 0.15 | 3.36 | 1.75 |
| Cr2O3 | 0.34 | |||||||||
| MgO | 11.95 | 9.22 | 16.67 | 11.81 | 24.75 | 24.61 | 14.66 | 16.81 | 13.24 | 14.37 |
| MnO | 0.03 | 0.26 | 0.11 | 0.09 | 0.29 | 0.07 | 0.12 | 0.05 | 0.02 | |
| FeO * | 17.28 | 21.34 | 11.11 | 17.56 | 1.29 | 1.54 | 13.39 | 15.11 | 15.56 | 16.05 |
| CaO | ||||||||||
| Na2O | 0.18 | 0.13 | 0.10 | 0.17 | 0.19 | 0.08 | 0.17 | 0.12 | 0.16 | 0.14 |
| K2O | 8.21 | 7.67 | 8.78 | 8.75 | 8.91 | 8.80 | 8.40 | 7.51 | 7.70 | 6.99 |
| BaO | 0.06 | 0.07 | 1.33 | 2.81 | 2.99 | 3.08 | 4.55 | 4.54 | 5.46 | |
| Total | 94.13 | 93.07 | 92.43 | 96.13 | 95.35 | 93.78 | 95.06 | 94.49 | 94.04 | 93.66 |
| F | 0.24 | 0.68 | 0.09 | 2.71 | 3.08 | 0.63 | 1.62 | 0.90 | 0.97 | |
| Atoms per formula unit on the basis of 22 positive charges | ||||||||||
| Si | 2.805 | 2.710 | 2.850 | 2.785 | 2.965 | 2.915 | 2.735 | 2.910 | 2.735 | 2.850 |
| Aliv † | 1.195 | 1.290 | 1.150 | 1.215 | 1.035 | 1.085 | 1.265 | 1.090 | 1.265 | 1.150 |
| Alvi | 0.360 | 0.390 | 0.305 | 0.270 | 0.175 | 0.120 | 0.190 | 0.055 | 0.080 | 0.050 |
| Al total | 1.555 | 1.680 | 1.455 | 1.485 | 1.210 | 1.205 | 1.455 | 1.145 | 1.345 | 1.200 |
| Ti | 0.105 | 0.085 | 0.060 | 0.150 | 0.030 | 0.045 | 0.135 | 0.010 | 0.200 | 0.105 |
| Cr | 0.020 | |||||||||
| Mg | 1.345 | 1.075 | 1.860 | 1.325 | 2.605 | 2.645 | 1.640 | 1.940 | 1.550 | 1.695 |
| Mn | 0.005 | 0.015 | 0.010 | |||||||
| Fe * | 1.090 | 1.395 | 0.695 | 1.105 | 0.075 | 0.095 | 0.840 | 0.980 | 1.020 | 1.060 |
| Na | 0.025 | 0.020 | 0.015 | 0.025 | 0.025 | 0.010 | 0.025 | 0.020 | 0.025 | 0.020 |
| K | 0.790 | 0.765 | 0.840 | 0.840 | 0.805 | 0.810 | 0.805 | 0.740 | 0.770 | 0.705 |
| Ba | 0.040 | 0.080 | 0.085 | 0.090 | 0.140 | 0.140 | 0.170 | |||
| Total | 7.715 | 7.730 | 7.780 | 7.755 | 7.810 | 7.830 | 7.725 | 7.895 | 7.785 | 7.805 |
| ∑I interlayer site | 0.817 | 0.787 | 0.856 | 0.904 | 0.907 | 0.906 | 0.918 | 0.898 | 0.935 | 0.897 |
| ∑M octahedral site | 3.264 | 3.357 | 3.237 | 3.123 | 3.082 | 3.043 | 2.992 | 3.046 | 2.935 | 2.964 |
| Fe/(Fe + Mg) | 0.448 | 0.565 | 0.272 | 0.455 | 0.028 | 0.034 | 0.339 | 0.335 | 0.397 | 0.385 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moles, N.R. Barian Micas and Exotic Ba-Cr and Ba-V Micas Associated with Metamorphosed Sedimentary Exhalative Baryte Deposits near Aberfeldy, Scotland, UK. Minerals 2025, 15, 511. https://doi.org/10.3390/min15050511
Moles NR. Barian Micas and Exotic Ba-Cr and Ba-V Micas Associated with Metamorphosed Sedimentary Exhalative Baryte Deposits near Aberfeldy, Scotland, UK. Minerals. 2025; 15(5):511. https://doi.org/10.3390/min15050511
Chicago/Turabian StyleMoles, Norman R. 2025. "Barian Micas and Exotic Ba-Cr and Ba-V Micas Associated with Metamorphosed Sedimentary Exhalative Baryte Deposits near Aberfeldy, Scotland, UK" Minerals 15, no. 5: 511. https://doi.org/10.3390/min15050511
APA StyleMoles, N. R. (2025). Barian Micas and Exotic Ba-Cr and Ba-V Micas Associated with Metamorphosed Sedimentary Exhalative Baryte Deposits near Aberfeldy, Scotland, UK. Minerals, 15(5), 511. https://doi.org/10.3390/min15050511






