Direct Lithium Extraction from Seawater Brine: An Assessment of Technology and Existing Commercial Systems
Abstract
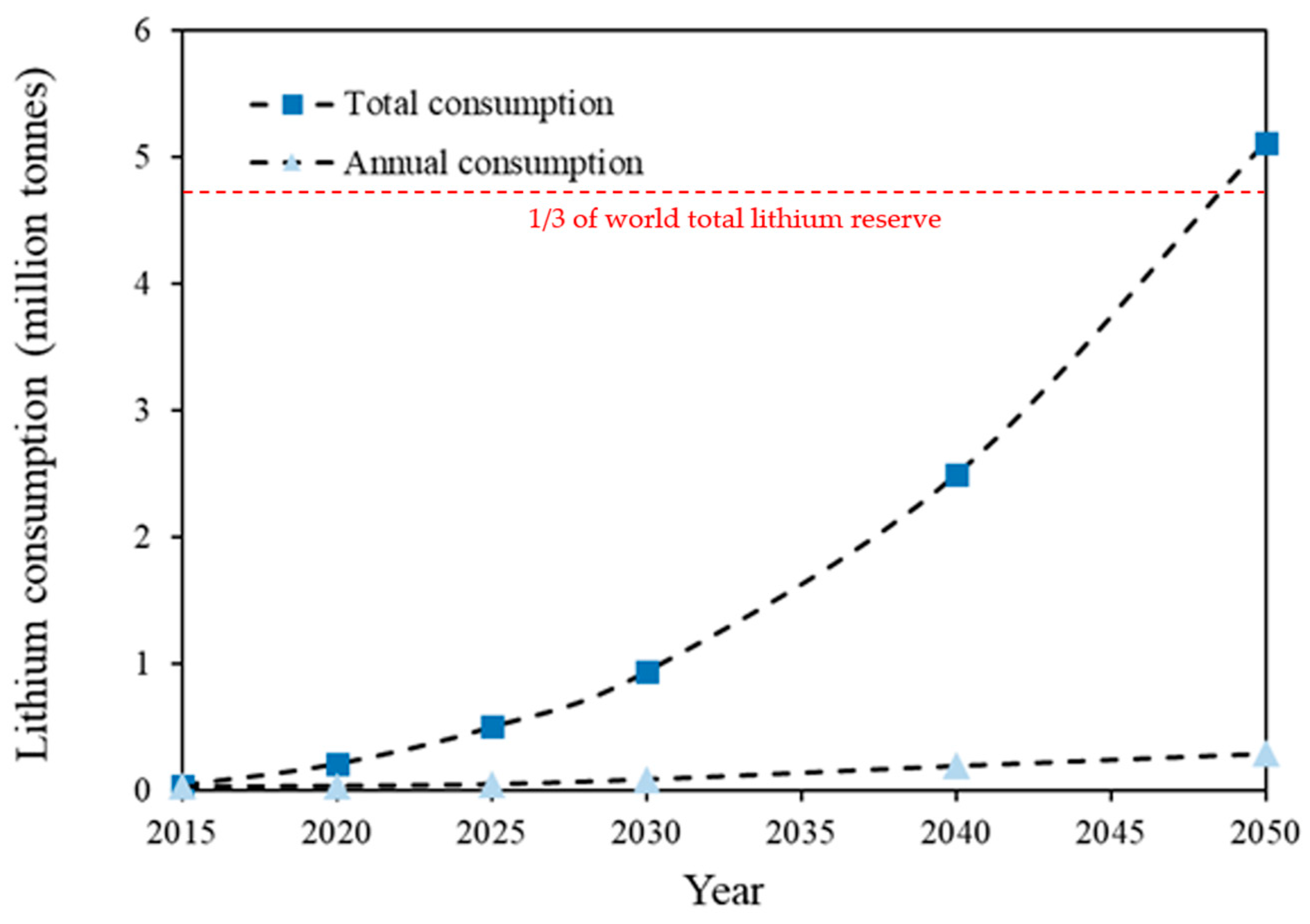
1. Introduction
2. Lithium in Seawater
| Minerals and Metals | Concentration (ppm) | Minerals and Metals | Concentration (ppm) |
|---|---|---|---|
| Chloride | 18,980 | Sodium | 10,561 |
| Magnesium | 1272 | Sulfur | 884 |
| Calcium | 400 | Potassium | 380 |
| Bromine | 65 | Inorganic Carbon | 28 |
| Strontium | 13 | Boron | 4.6 |
| Silicon | 4 | Organic Carbon | 3 |
| Aluminum | 1.9 | Fluorine | 1.4 |
| Nitrate | 0.7 | Organic Nitrogen | 0.2 |
| Rubidium | 0.2 | Lithium | 0.18 |
| Phosphorous | 0.1 | Copper | 0.09 |
| Barium | 0.05 | Iodine | 0.05 |
| Nitrite | 0.05 | Ammonia | 0.05 |
| Arsenic | 0.024 | Iron | 0.02 |
| Organic Phosphorous | 0.016 | Zinc | 0.014 |
| Manganese | 0.01 | Lead | 0.005 |
| Selenium | 0.004 | Tin | 0.003 |
| Cesium | 0.002 | Molybdenum | 0.002 |
| Uranium | 0.0016 | Gallium | 0.0005 |
| Nickel | 0.0005 | Thorium | 0.0005 |
| Cerium | 0.0004 | Vanadium | 0.0003 |
| Lanthanum | 0.0003 | Yttrium | 0.0003 |
| Mercury | 0.0003 | Silver | 0.0003 |
| Bismuth | 0.0002 | Cobalt | 0.0001 |
| Gold | 0.000008 |
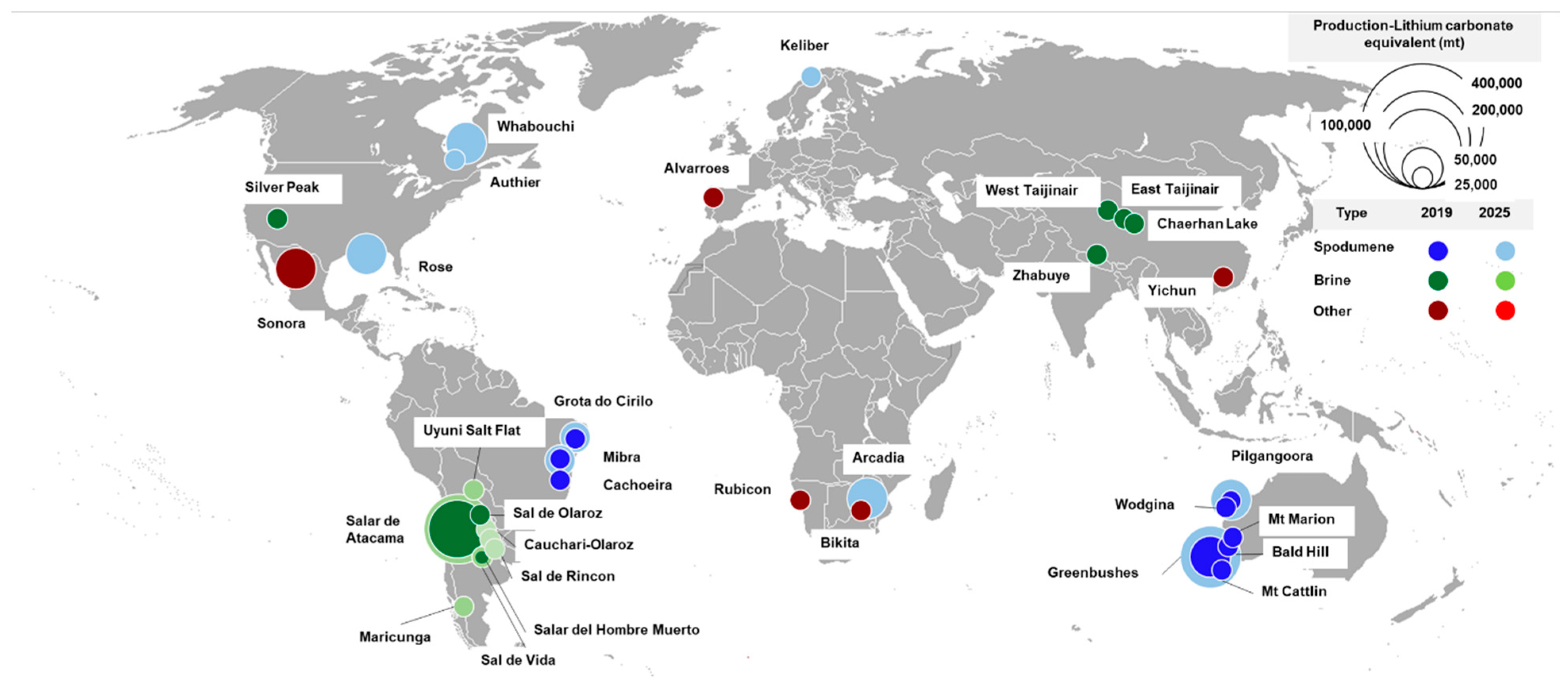
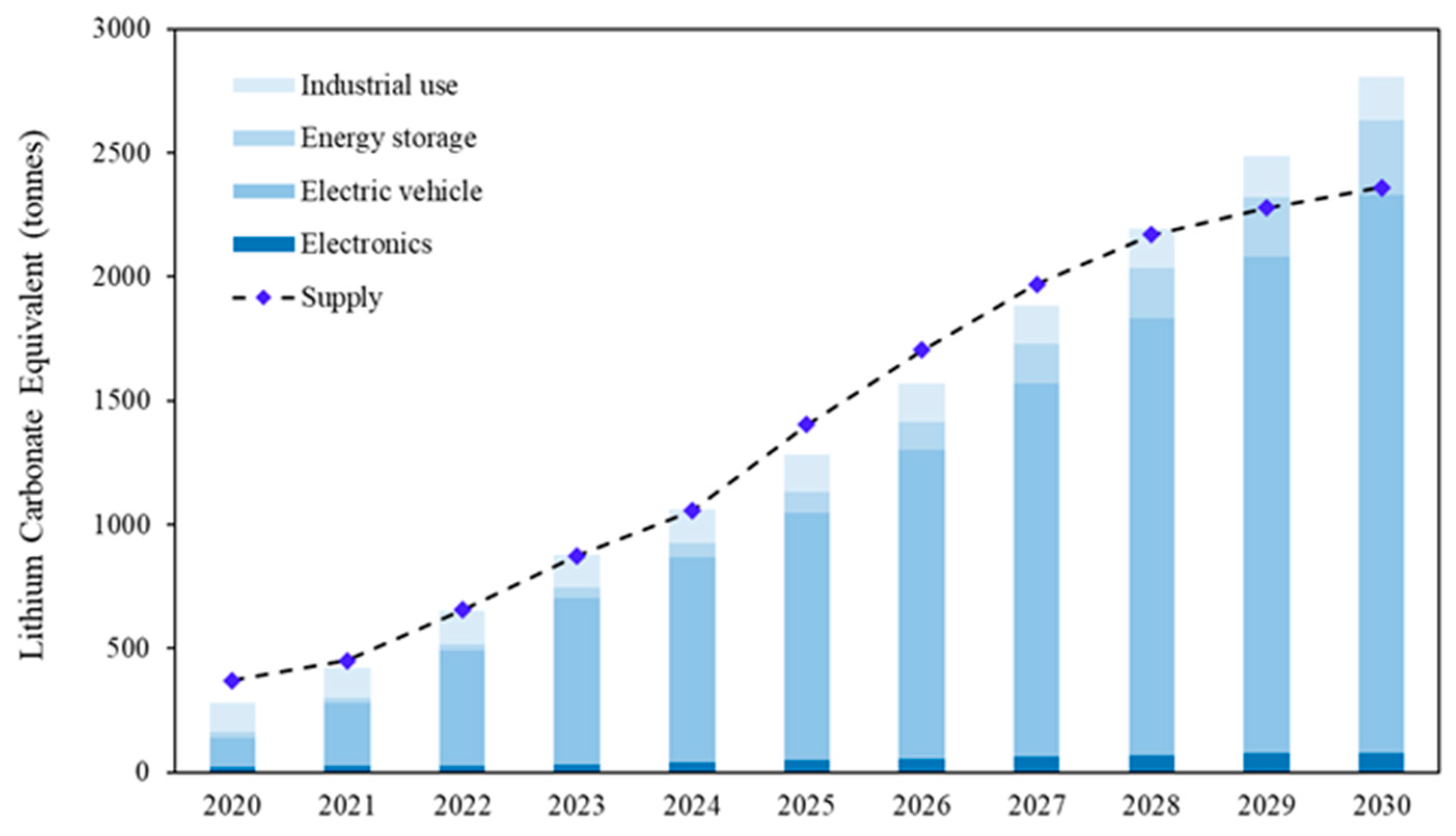
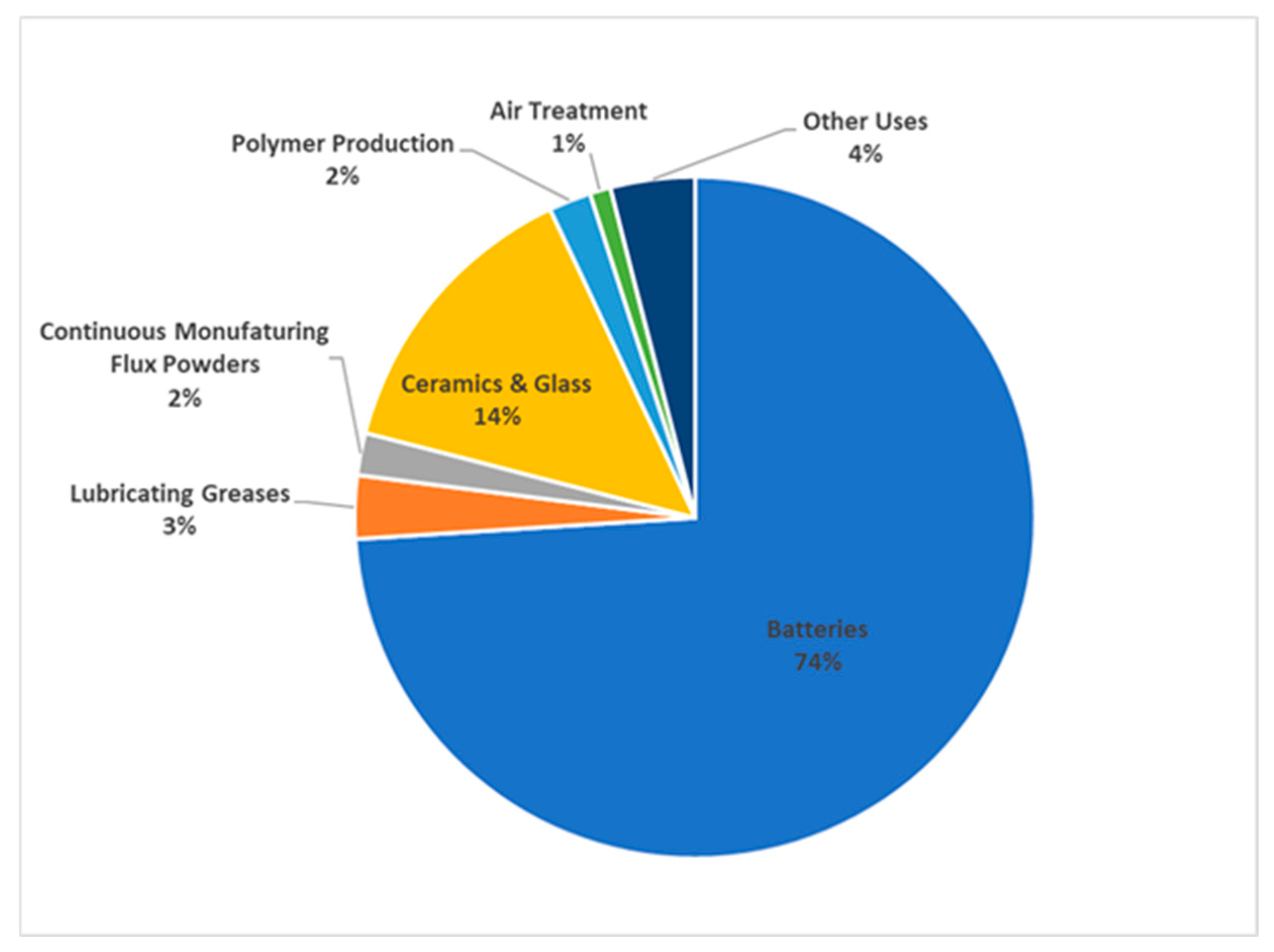
3. Products and Market
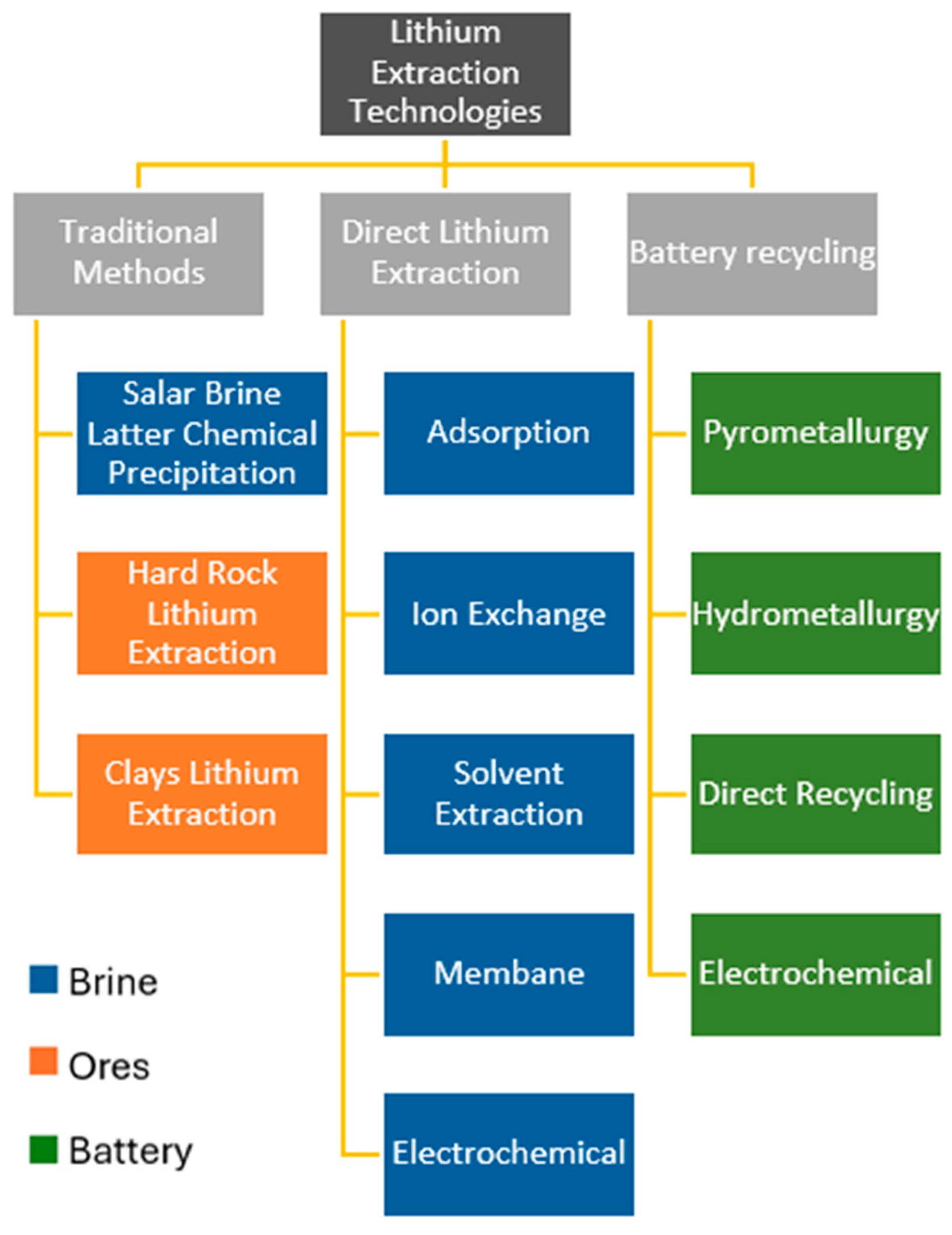
4. Lithium Extraction Technologies
4.1. Traditional Lithium Extraction from Brines
4.2. Direct Lithium Extraction
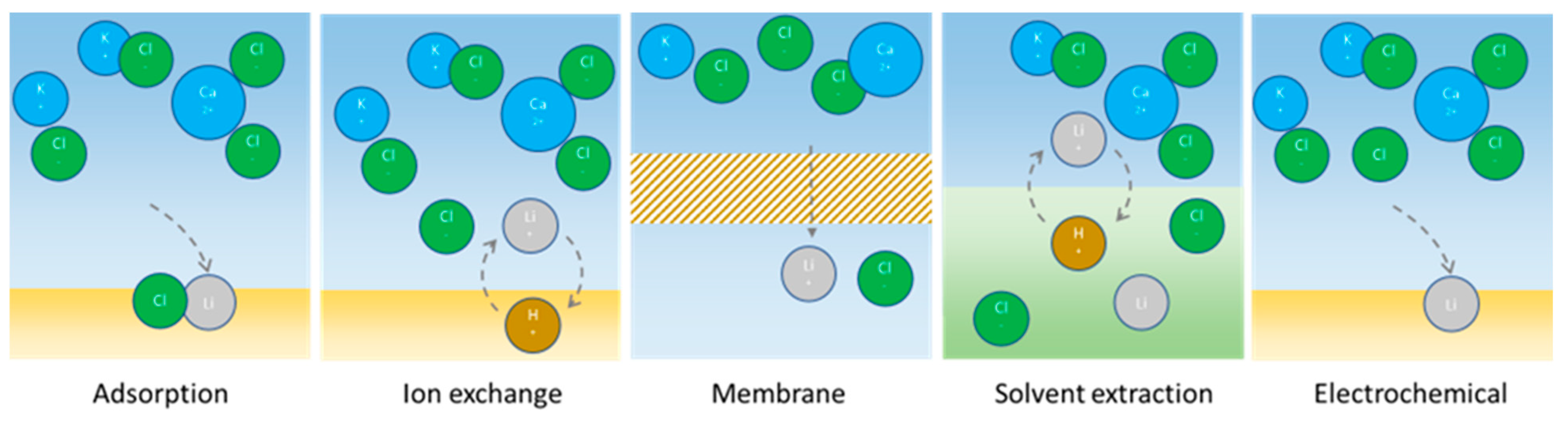
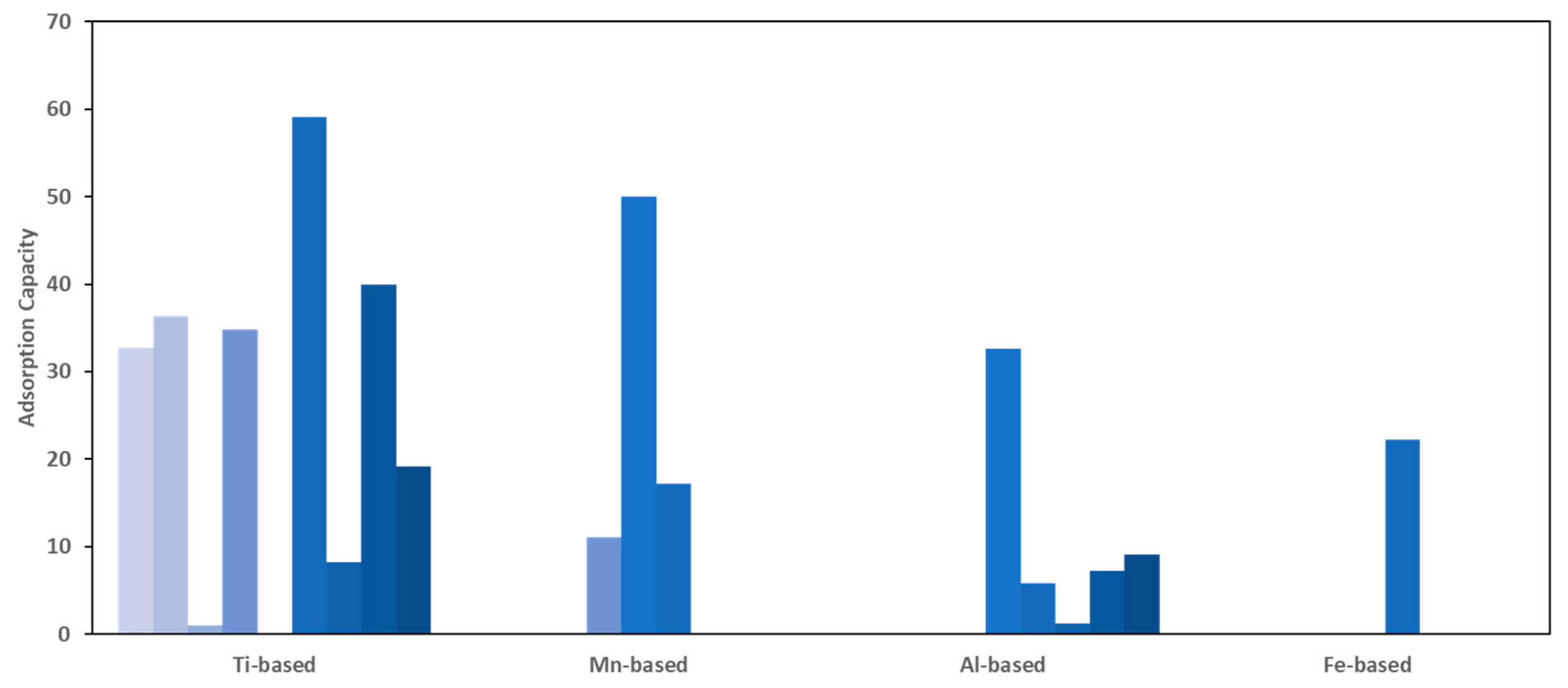
4.2.1. Adsorption
- Aluminum-Based Adsorbents (LiAl-LDHs)
- Commercial Technologies
4.2.2. Ion Exchange
- Manganese-based adsorbents (LMOs)
- Titanium-Based Adsorbents
- Commercial Technologies
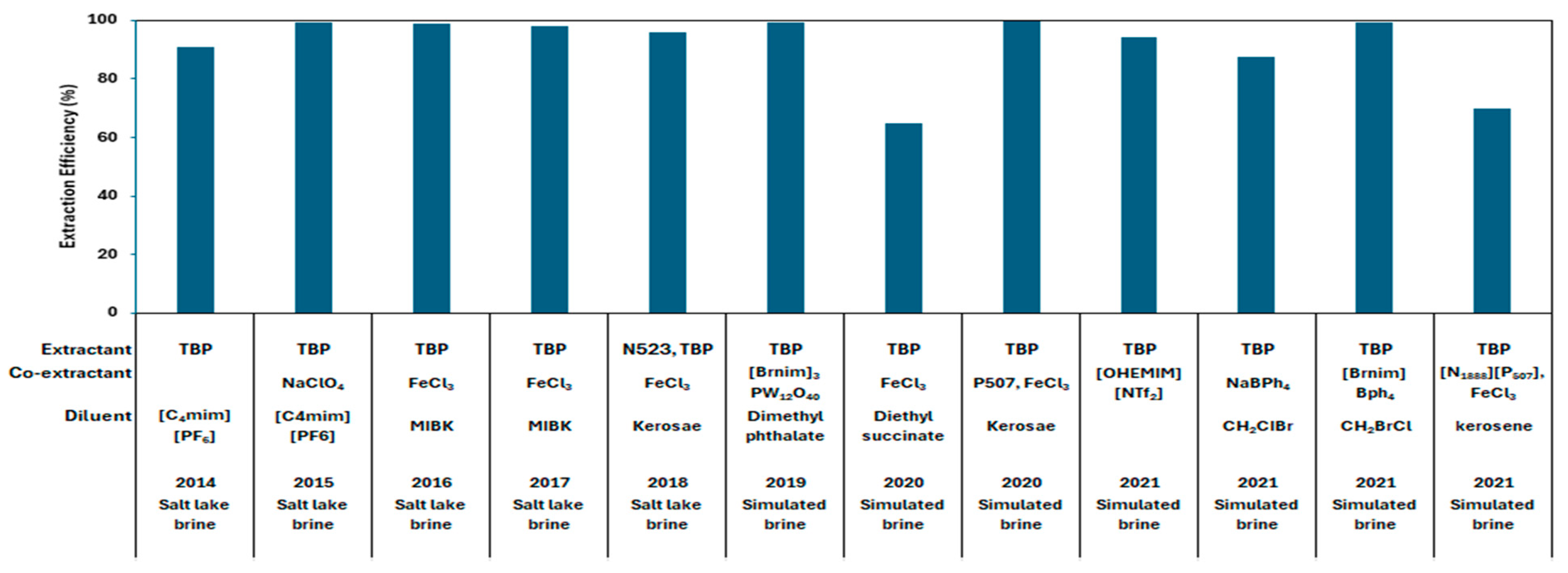
4.2.3. Solvent Extraction
- Commercial Technologies
4.2.4. (Electro-)Membrane Separation
- Nanofiltration (NF)
- Electrodialysis (ED)
- Electrodialysis Bipolar Membranes (EDBM)
- Membrane Capacitive Deionization (CDI)
- Commercial Technologies
4.2.5. Electrochemical Separation or Refining
- Electrocoagulation (EC)
- Commercial Technologies
5. Outlook and Perspective
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Global Water Intelligence. 21 November 2019. Available online: https://www.globalwaterintel.com/articles/is-the-lithium-mining-market-worth-it (accessed on 2 July 2024).
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Shankleman, J.; Biesheuvel, T.; Ryan, J.; Merrill, D. We’re Going to Need More Lithium; Bloomberg: New York, NY, USA, 2017. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; pp. 1–216. [Google Scholar] [CrossRef]
- Lithium-Element Information, Properties and Uses|Periodic Table. Available online: https://www.rsc.org/periodic-table/element/3/lithium (accessed on 2 July 2024).
- Sun, Y.; Wang, Q.; Wang, Y.; Yun, R.; Xiang, X. Recent Advances in Magnesium/Lithium Separation and Lithium Extraction Technologies from Salt Lake Brine. Sep. Purif. Technol. 2021, 256, 117807. [Google Scholar] [CrossRef]
- Grosjeana, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of World Lithium Resources and Consequences of Their Geographic Distribution on the Expected Development of the Electric Vehicle Industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Leece, A.; Jiang, C. A Preliminary Techno-Economic Assessment of Lithium Extraction from Flowback and Produced Water from Unconventional Shale and Tight Hydrocarbon Operations in Western Canada; Geological Survey of Canada: Ottawa, ON, Canada, 2023; p. 8975. [Google Scholar]
- Lopez, J. Direct Lithium Extraction (DLE), the Latest Buzzword in the Climate Tech Space; Medium: San Francisco, CA, USA, 2023. [Google Scholar]
- Altmann, T.; Das, R. Process Improvement of Sea Water Reverse Osmosis (SWRO) and Subsequent Decarbonization. Desalination 2021, 499, 114791. [Google Scholar] [CrossRef]
- Altmann, T.; Buijs, P.J.; Farinha, A.S.F.; Borges, V.R.P.; Farhat, N.M.; Vrouwenvelder, J.S.; Das, R. Seawater Reverse Osmosis Performance Decline Caused by Short-Term Elevated Feed Water Temperature. Membranes 2022, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium Metal Extraction from Seawater. Joule 2018, 2, 1648–1651. [Google Scholar] [CrossRef]
- International Battery Metals-Leading Lithium Extraction. Available online: https://www.ibatterymetals.com (accessed on 7 July 2024).
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Cen, J. Direct Lithium Extraction 2025–2035: Technologies, Players, Markets and Forecasts; IDTechEx: Cambridge, UK, 2024; ISBN 978-1-83570-054-9. [Google Scholar]
- Recent Advances in Lithium Extraction. Available online: https://www.mdpi.com/2673-4591/67/1/52 (accessed on 10 April 2025).
- Dry, M.J. Extraction of Lithium from Brine—Old and New Chemistry; Springer: Cham, Switzerland, 19 August 2018; pp. 2225–2234. [Google Scholar]
- Gangadharan, S. Direct Lithium Extraction: Faster, Cleaner And Cheaper Way To Produce The Metal? StratNews Global, 10 July 2024. [Google Scholar]
- The Best Opportunities in Lithium. Available online: https://www.institutionalinvestor.com/article/2bxrlchbu6gr5g0e5rxmo/innovation/the-best-opportunities-in-lithium (accessed on 4 July 2024).
- Jacoby, M. Can Seawater Give Us the Lithium to Meet Our Battery Needs? Chem. Eng. News 2021, 99, 9–21. [Google Scholar]
- Schmidt, A.; Mestmäcker, F.; Brückner, L.; Elwert, T.; Strube, J. Liquid-Liquid Extraction and Chromatography Process Routes for the Purification of Lithium. Mater. Sci. Forum 2019, 959, 79–99. [Google Scholar] [CrossRef]
- Precise Biofilm Thickness Prediction in SWRO Desalination from Planar Camera Images by DNN Models|Npj Clean Water. Available online: https://www.nature.com/articles/s41545-025-00451-9 (accessed on 11 April 2025).
- Yousry, A.; Ridwan, M.G.; Altmann, T.; Rousseva, A.; Azab, K.; Das, R. Performance Model for Reverse Osmosis. Chem. Eng. Res. Des. 2022, 186, 416–432. [Google Scholar] [CrossRef]
- Campbell, K. Over 40 Minerals and Metals Contained in Seawater, Their Extraction Likely to Increase in the Future. Available online: https://www.miningweekly.com/article/over-40-minerals-and-metals-contained-in-seawater-their-extraction-likely-to-increase-in-the-future-2016-04-01 (accessed on 4 July 2024).
- SAMCO. Is It Possible to Extract Lithium from Seawater? Available online: https://samcotech.com/is-it-possible-to-extract-lithium-from-seawater/#:~:text=While%20technically%2C%20yes%2C%20it%20is,lithium%20recovery%20from%20ocean%20water (accessed on 2 July 2024).
- STT Systems & Solutions Lithium Extraction and Refining Technology. Available online: https://systems.carmeuse.com/en/industries/lithium-extraction/ (accessed on 4 July 2024).
- Reports, V.M. Battery Grade Lithium Salt Market Size, Insights, Industry Growth & Forecast 2032. Available online: https://www.verifiedmarketreports.com/product/battery-grade-lithium-salt-market/ (accessed on 11 March 2025).
- Daily Metal Price: Lithium Price (USD/Kilogram) Chart for the Last Year. Available online: https://www.dailymetalprice.com/metalpricecharts.php?c=li&u=kg&d=240 (accessed on 11 March 2025).
- Perry, C.; Thomson, E.; the Fastmarkets Team. Facing the Tightening Lithium Supply Challenge in 2025; Fastmarkets: London, UK, 2025. [Google Scholar]
- Global Lithium Salts Market Research Report 2023. QYResearch. Available online: https://www.qyresearch.com/reports/868627/lithium-salts (accessed on 11 March 2025).
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Industrial-Grade Lithium Hydroxide Price Today|Historical Industrial-Grade Lithium Hydroxide Price Charts|SMM Metal Market. Available online: https://www.metal.com/Chemical-compound/202005200001 (accessed on 11 March 2025).
- Lithium-Price-Chart-Historical Data-News. Available online: https://tradingeconomics.com/commodity/lithium (accessed on 11 March 2025).
- Alerts, I. Lithium Carbonate Prices|Current and Forecast. Available online: https://www.intratec.us/chemical-markets/lithium-carbonate-price (accessed on 11 March 2025).
- Alerts, I. Lithium Hydroxide Prices|Current and Forecast. Available online: https://www.intratec.us/chemical-markets/lithium-hydroxide-price (accessed on 11 March 2025).
- Lithium Statistics and Information|U.S. Geological Survey. Available online: https://www.usgs.gov/centers/national-minerals-information-center/lithium-statistics-and-information (accessed on 11 March 2025).
- Cycles, T. Text Provides General Information S. Assumes no Liability for the Information Given Being Complete or Correct D. to Varying Update; Text, S.C.D.M. Up-to-D.D.T.R. in the Topic: Lithium Industry Worldwide. Available online: https://www.statista.com/topics/3217/lithium/ (accessed on 11 March 2025).
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- U.S. Geological Survey Lithium. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-lithium.pdf (accessed on 11 March 2025).
- Roberts, J.; Zhao, V. Lithium: 10-Year Forecast 2024. Available online: https://www.fastmarkets.com/uploads/2024/05/Lithium_LTF_Q1_2024_Sample_final.pdf (accessed on 2 July 2024).
- Natural Resources Canada Lithium Facts. Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/mining-data-statistics-and-analysis/minerals-metals-facts/lithium-facts/24009 (accessed on 4 July 2024).
- Boroumand, Y.; Razmjou, A. Adsorption-Type Aluminium-Based Direct Lithium Extraction: The Effect of Heat, Salinity and Lithium Content. Desalination 2024, 577, 117406. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A Review of Technologies for Direct Lithium Extraction from Low Li+ Concentration Aqueous Solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Marazuela, M.A.; Vázquez-Suñé, E.; Ayora, C.; García-Gil, A. Towards More Sustainable Brine Extraction in Salt Flats: Learning from the Salar de Atacama. Sci. Total Environ. 2020, 703, 135605. [Google Scholar] [CrossRef]
- Water Mining And Extractivism Of The Salar De Atacama, Chile. WIT Trans. Ecol. Environ. 2020, 245, 189–199.
- Stower, H. Direct Lithium Extraction: New Technologies to Disrupt Traditional Refining and Mining. Available online: https://www.cleantech.com/direct-lithium-extraction/ (accessed on 2 July 2024).
- Werny, M.; Reem, Y.; ten Have, I.; Bartels, F. Does Lithium Have a Green Future? Building Sustainable and Resilient Supply Chains with Direct Lithium Extraction and Battery Recycling; Extantia Capital: Berlin, Germany, 2024. [Google Scholar]
- Werny, M.; Reem, Y.; ten Have, I.; Bartels, F. The Technology Overview: Closing the Lithium Supply Gap with Direct Lithium Extraction (DLE) and Battery Recycling; Extantia Capital: Berlin, Germany, 2023. [Google Scholar]
- Zhang, Y.; Sun, W.; Xu, R.; Wang, L.; Tang, H. Lithium Extraction from Water Lithium Resources through Green Electrochemical-Battery Approaches: A Comprehensive Review. J. Clean. Prod. 2021, 285, 124905. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Arshadi, F.; Mofidi, Z.; Mohseni-Dargah, M.; Kök, C.; Assefi, M.; Soozanipour, A.; Zargar, M.; Asadnia, M.; Boroumand, Y.; et al. Direct Lithium Extraction: A New Paradigm for Lithium Production and Resource Utilization. Desalination 2024, 575, 117249. [Google Scholar] [CrossRef]
- Livent Invests in ILiAD Technologies to Strengthen Leadership in Direct Lithium Extraction Production Processes. Available online: https://www.iliadtech.com/livent-invest (accessed on 4 July 2024).
- ILiAD Technologies|Lithium. Available online: https://www.iliadtech.com (accessed on 2 July 2024).
- Harvest, L. Innovative Lithium Extraction Technology. Available online: https://lithiumharvest.com/services/our-technology/ (accessed on 21 August 2024).
- Direct Lithium Extraction (DLE) from Salar Brine and Geothermal Brine. Available online: https://www.seplite.com/direct-lithium-extraction/ (accessed on 2 July 2024).
- Revolutionizing Green Lithium Extraction. Available online: https://sorciaminerals.ensorciametals.com/ (accessed on 4 July 2024).
- Eramet: Lithium Project in Argentina-BofA Lithium (DLE)/Battery Materials Fieldtrip. Available online: https://www.eramet.com/en/news/2022/09/eramet-lithium-project-in-argentina-bofa-lithium-dle-battery-materials-fieldtrip/ (accessed on 2 July 2024).
- Eramet in Argentina: Centenario-Ratones Lithium Project. Available online: https://www.eramet.com/en/news/2023/03/eramet-in-argentina-centenario-ratones-lithium-project/ (accessed on 2 July 2024).
- Commercial Scale Modular Direct Lithium Extraction Technology by IBAT. Available online: https://www.ibatterymetals.com/direct-lithium-extraction (accessed on 2 July 2024).
- Li-ProTM Direct Lithium Extraction Technology. Available online: https://www.kochtechsolutions.com/li-pro-direct-lithium-extraction-technology/ (accessed on 7 July 2024).
- Launching a Lithium Extraction Process with Li-PROTM. Available online: https://news.kochind.com//news/2022/Launching-a-Lithium-Extraction-Process-with-Li-PRO (accessed on 2 July 2024).
- Our Technology|Summit Nanotech. Available online: https://www.summitnanotech.com/our-technology (accessed on 2 July 2024).
- ATLiS. Available online: https://www.esminerals.com/atlis (accessed on 4 July 2024).
- DLEC Process. Available online: https://www.watercycletechnologies.com/technology (accessed on 4 July 2024).
- Zero Carbon LithiumTM Project. Available online: https://v-er.eu/ (accessed on 4 July 2024).
- ExSorbtion’s Advantages. Available online: https://exsorbtion.com/technology/ (accessed on 2 July 2024).
- We Are Builders of Future. Available online: https://eonminerals.com/ (accessed on 4 July 2024).
- Amanecer Lithium Project. Available online: https://www.eonlithiumcorp.com/projects/ (accessed on 2 July 2024).
- BHE Renewables. Available online: https://www.brkenergy.com/our-businesses/bhe-renewables (accessed on 2 July 2024).
- Scheyder, E. Insight: U.S. Steps Away from Flagship Lithium Project with Buffett’s Berkshire|Reuters. Reuters, 5 October 2022. [Google Scholar]
- Richter, A. BHE Renewables Receives m in U.S. DOE Grant Funding for Geothermal Lithium Demonstration Project|ThinkGeoEnergy-Geothermal Energy News. Available online: https://www.thinkgeoenergy.com/bhe-renewables-receives-15m-in-u-s-doe-grant-funding-for-geothermal-lithium-demonstration-project/ (accessed on 21 August 2024).
- Rincon Lithium Project. Available online: https://www.riotinto.com/en/operations/projects/rincon (accessed on 4 July 2024).
- Rincon Lithium Project-Argentina. Available online: https://www.argosyminerals.com.au/rincon-lithium-project-argentina (accessed on 21 August 2024).
- Lithium Extraction Technology for an Electrified Future. Available online: https://www.terralithium.com (accessed on 4 July 2024).
- EnergyX-Next Generation Lithium Extraction & Battery Technology. Available online: https://energyx.com/ (accessed on 4 July 2024).
- Next Generation Filtration Media Mining, Refining, & Wastewater Treatment. Available online: https://www.precisionperiodic.com (accessed on 4 July 2024).
- About Olokun Minerals. Available online: https://olokunminerals.com/about (accessed on 2 July 2024).
- Watkins-Curry, P. Olokun Minerals. Available online: https://ondemand.ceraweek.com/detail/video/6322943945112/olokun-minerals (accessed on 4 July 2024).
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium Recovery from Salt Lake Brine by H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Zhang, X.; Zheng, S.; Zhang, Y. Selective Adsorption of Lithium from High Mg-Containing Brines Using HxTiO3 Ion Sieve. Hydrometallurgy 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S. Lithium Adsorption from Brine by Iron-Doped Titanium Lithium Ion Sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Wei, S.; Wei, Y.; Chen, T.; Liu, C.; Tang, Y. Porous Lithium Ion Sieves Nanofibers: General Synthesis Strategy and Highly Selective Recovery of Lithium from Brine Water. Chem. Eng. J. 2020, 379, 122407. [Google Scholar] [CrossRef]
- Marthi, R.; Asgar, H.; Gadikota, G.; Smith, Y.R. On the Structure and Lithium Adsorption Mechanism of Layered H2TiO3. ACS Appl. Mater. Interfaces 2021, 13, 8361–8369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yue, H.; Sun, W.; Zhang, L.; Cui, Q.; Wang, H. Study on Adsorption Extraction Process of Lithium Ion from West Taijinar Brine by Shaped Titanium-Based Lithium Ion Sieves. Sep. Purif. Technol. 2021, 274, 119099. [Google Scholar] [CrossRef]
- Zandvakili, S.; Ranjbar, M. Preparation and Characterisation of Lithium Ion Exchange Composite for the Recovery of Lithium from Brine. Miner. Process. Extr. Metall. 2018, 127, 176–181. [Google Scholar] [CrossRef]
- Ohashi, F.; Tai, Y. Lithium Adsorption from Natural Brine Using Surface-Modified Manganese Oxide Adsorbents. Mater. Lett. 2019, 251, 214–217. [Google Scholar] [CrossRef]
- Marthi, R.; Smith, Y.R. Selective Recovery of Lithium from the Great Salt Lake Using Lithium Manganese Oxide-Diatomaceous Earth Composite. Hydrometallurgy 2019, 186, 115–125. [Google Scholar] [CrossRef]
- Lai, X.; Yuan, Y.; Chen, Z.; Peng, J.; Sun, H.; Zhong, H. Adsorption–Desorption Properties of Granular EP/HMO Composite and Its Application in Lithium Recovery from Brine. Ind. Eng. Chem. Res. 2020, 59, 7913–7925. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, E.; Yan, G.; Yang, Y.; Liu, W.; Liu, X. A Lithium Ion-Imprinted Adsorbent Using Magnetic Carbon Nanospheres as a Support for the Selective Recovery of Lithium Ions. New Carbon Mater. 2020, 35, 696–706. [Google Scholar] [CrossRef]
- Lilac Solutions-Modern Lithium Extraction Technology. Available online: https://lilacsolutions.com/ (accessed on 4 July 2024).
- Chemionex|Chemionex Developing Innovative Aqueous Chemical Separation Solutions. Available online: https://chemionex.com/ (accessed on 2 July 2024).
- Geolith-Global Leader of Direct Lithium Extraction (DLE). Available online: https://geolith.fr/ (accessed on 2 July 2024).
- America’s 21st Century Lithium Company. Available online: https://www.standardlithium.com (accessed on 2 July 2024).
- Transforming Oilfield Brine into High-Quality Lithium Carbonate. Available online: https://voltlithium.com/ (accessed on 4 July 2024).
- Unlocking a New Source of Canadian Lithium. Available online: https://www.e3lithium.ca/ (accessed on 4 July 2024).
- Creating a Sustainable Energy Future Through Lithium Technology Development. Available online: https://conductive.energy/ (accessed on 2 July 2024).
- XtraLit—Revolutionary Technology of Direct Lithium Extraction. Available online: https://xtralit.com/ (accessed on 4 July 2024).
- The Imperative for Sustainable Direct Lithium Recovery. Available online: https://geo40.com/geothermallithium/ (accessed on 4 July 2024).
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Shi, C.; Duan, D.; Jia, Y.; Jing, Y. A Highly Efficient Solvent System Containing Ionic Liquid in Tributyl Phosphate for Lithium Ion Extraction. J. Mol. Liq. 2014, 200, 191–195. [Google Scholar] [CrossRef]
- Shi, C.; Jia, Y.; Zhang, C.; Liu, H.; Jing, Y. Extraction of Lithium from Salt Lake Brine Using Room Temperature Ionic Liquid in Tributyl Phosphate. Fusion Eng. Des. 2015, 90, 1–6. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Extraction of Lithium from Salt Lake Brine Containing Borate Anion and High Concentration of Magnesium. Hydrometallurgy 2016, 166, 9–15. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Lithium Recovery from Salt Lake Brine by Counter-Current Extraction Using Tributyl Phosphate/FeCl3 in Methyl Isobutyl Ketone. Hydrometallurgy 2017, 171, 27–32. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Peng, X.; Li, L.; Song, F.; Nie, F.; Ji, L.; Zhang, Y. Extraction of Lithium from Salt Lake Brine Containing Boron Using Multistage Centrifuge Extractors. Desalination 2018, 441, 44–51. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Fan, J.; Liu, X.; Hu, Y.; Hu, Y.; Zhou, Z.; Ren, Z. Recovery of Lithium Ions from Salt Lake Brine with a High Magnesium/Lithium Ratio Using Heteropolyacid Ionic Liquid. ACS Sustain. Chem. Eng. 2019, 7, 3062–3072. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, J.; Liu, X.; Hu, Y.; Wei, X.; Hu, Y.; Wang, W.; Ren, Z. Recovery of Lithium from Salt-Lake Brines Using Solvent Extraction with TBP as Extractant and FeCl3 as Co-Extraction Agent. Hydrometallurgy 2020, 191, 105244. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Zhu, Z.; Wang, L.; Qi, T. Recovery of Lithium from Salt Lake Brine Using a Mixed Ternary Solvent Extraction System Consisting of TBP, FeCl3 and P507. Hydrometallurgy 2020, 197, 105487. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, S.; Li, Z. Recovery of Lithium from Brine with a High Mg/Li Ratio Using Hydroxyl-Functionalized Ionic Liquid and Tri-n-Butyl Phosphate. J. Sustain. Metall. 2021, 7, 256–265. [Google Scholar] [CrossRef]
- Ren, Z.; Wei, X.; Li, R.; Wang, W.; Wang, Y.; Zhou, Z. Highly Selective Extraction of Lithium Ions from Salt Lake Brines with Sodium Tetraphenylborate as Co-Extractant. Sep. Purif. Technol. 2021, 269, 118756. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.; Wang, Y.; Wei, X.; Cai, Z.; Zhou, Z. Novel Ionic Liquid as Co-Extractant for Selective Extraction of Lithium Ions from Salt Lake Brines with High Mg/Li Ratio. Sep. Purif. Technol. 2021, 277, 119471. [Google Scholar] [CrossRef]
- Bai, R.; Wang, J.; Wang, D.; Zhang, Y.; Cui, J. Selective Separation of Lithium from the High Magnesium Brine by the Extraction System Containing Phosphate-Based Ionic Liquids. Sep. Purif. Technol. 2021, 274, 119051. [Google Scholar] [CrossRef]
- Solvay to Showcase Latest Innovations in Solvent Extraction Technology and New Digital Offering at Hydroprocess 2018. Available online: https://www.solvay.com/en/press-release/solvay-showcase-latest-innovations-solvent-extraction-technology-and-new-digital (accessed on 4 July 2024).
- CYANEX® 936P EXTRACTANT. Available online: https://www.syensqo.com/en/product/cyanex-936p-extractant (accessed on 2 July 2024).
- Greener Lithium to Meet Growing Demand. Available online: https://www.adionics.com/ (accessed on 2 July 2024).
- He, K.; Achyuta, A. Why We Invested in Novalith. Available online: https://tdk-ventures.com/why-we-invested-in-novalith/ (accessed on 4 July 2024).
- Novalith-Clean Lithium Chemicals Production. Available online: https://www.novalith.com.au/ (accessed on 4 July 2024).
- Vassiloudis, S. The Future of Lithium: Part 1; Medium: San Francisco, CA, USA, 2023. [Google Scholar]
- Foo, Z.H.; Liu, S.; Kanias, L.; Lee, T.R.; Heath, S.M.; Tomi, Y.; Miyabe, T.; Keten, S.; Lueptow, R.M.; Lienhard, J.H. Positively-Coated Nanofiltration Membranes for Lithium Recovery from Battery Leachates and Salt-Lakes: Ion Transport Fundamentals and Module Performance. Adv. Funct. Mater. 2024, 34, 2408685. [Google Scholar] [CrossRef]
- Water Treatment and Purification. Available online: https://www.lenntech.com/index.htm (accessed on 4 July 2024).
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-Fractionation of Lithium Ions from Magnesium Ions by Electrodialysis Using Monovalent Selective Ion-Exchange Membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Patel, S.K.; Iddya, A.; Pan, W.; Qian, J.; Elimelech, M. Approaching Infinite Selectivity in Membrane-Based Aqueous Lithium Extraction via Solid-State Ion Transport. Sci. Adv. 2025, 11, eadq9823. [Google Scholar] [CrossRef]
- Zhao, X.; He, Z.; Li, M.; He, C.; Xu, Y.; Wang, Y.; Ma, J.; Yang, H.Y. Advanced Strategies for Improving the Energy Efficiency of Capacitive Deionization Technologies. Desalination 2025, 597, 118377. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Xu, Y.; Yan, Z.C.; Wei, H.; Wang, Y.; Yang, H.Y. A Mini-Review about Overcoming Challenges in Hydrophilicity: Towards Efficient Capacitive Deionization Electrodes. Sep. Purif. Technol. 2025, 354, 129211. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, L.; Hou, Y.; Wang, Y.; Zhu, L. Pulsed Electric Field Controlled Lithium Extraction Process by LMO/MXene Composite Electrode from Brines. Chem. Eng. J. 2022, 450, 138454. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.; Zhang, L.; Fang, R.; Wang, J. 3D Heterostructure Constructed by Few-Layered MXenes with a MoS2 Layer as the Shielding Shell for Excellent Hybrid Capacitive Deionization and Enhanced Structural Stability. ACS Appl. Mater. Interfaces 2022, 14, 2833–2847. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, S.; Song, X.; Wang, Y.; Zhang, H.; Li, M.; Wang, Y. Enhanced Lithium Extraction from Brines: Prelithiation Effect of FePO4 with Size and Morphology Control. Adv. Sci. 2024, 11, 2405176. [Google Scholar] [CrossRef] [PubMed]
- Lithium Processing Solutions & Services|Veolia. Available online: https://www.watertechnologies.com/applications/lithium-processing (accessed on 4 July 2024).
- Lithium Extraction and Refining. Available online: https://www.saltworkstech.com/applications/lithium-extraction-and-refining/ (accessed on 4 July 2024).
- FilmTecTM LiNE-XD. Available online: https://www.dupont.com/products/filmtec-line-xd.html (accessed on 21 August 2024).
- Direct Lithium Extraction|DLE Technology. Available online: https://www.dle.technology/ (accessed on 2 July 2024).
- DLE-R. Available online: https://www.electralith.com/dle-r (accessed on 2 July 2024).
- SiTration’s Silicon Membrane Technology Enables Ultra-Efficient Separations. Available online: https://sitration.com/ (accessed on 4 July 2024).
- Clean Tech Is the Future. Available online: https://www.almaenergy.net/ (accessed on 2 July 2024).
- Al-Ajmi, F.; Al-Marri, M.; Almomani, F. Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water 2025, 17, 23. [Google Scholar] [CrossRef]
- Khan, S.U.; Khalid, M.; Hashim, K.; Jamadi, M.H.; Mousazadeh, M.; Basheer, F.; Farooqi, I.H. Efficacy of Electrocoagulation Treatment for the Abatement of Heavy Metals: An Overview of Critical Processing Factors, Kinetic Models and Cost Analysis. Sustainability 2023, 15, 1708. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Sun, W.; Wang, L.; Tang, H. Li Extraction from Model Brine via Electrocoagulation: Processing, Kinetics, and Mechanism. Sep. Purif. Technol. 2020, 250, 117234. [Google Scholar] [CrossRef]
- Lithium. Available online: https://tenova.com/technologies/hydrometallurgy/lithium (accessed on 2 July 2024).
- Van Alphen, H.; Ritchie, M. Wealth Receives Positive Results from Tenova Advanced Technologies Process Study for the Laguna Verde Project in Chile; Wealth Minerals: Vancouver, BC, Canada, 2017. [Google Scholar]
- Mangrove Lithium-Battery Grade Lithium Refining. Available online: https://www.mangrovelithium.com/ (accessed on 2 July 2024).
- A New Breed Of Electrolysis Technology. Available online: https://aepnus.com/technology (accessed on 2 July 2024).
- Broos, K. Technology from VITO Helps Extract and Recover Materials and Raw Materials. Available online: https://vito.be/en/news/technology-vito-helps-extract-and-recover-materials-and-raw-materials (accessed on 2 July 2024).
- Chemical Free Lithium Extraction. Available online: https://www.ellex.co (accessed on 2 July 2024).
- Sustainable Lithium Made in America. Available online: https://www.eflowtech.com (accessed on 4 July 2024).
- Global Supply Chains of EV Batteries—Analysis. Available online: https://www.iea.org/reports/global-supply-chains-of-ev-batteries (accessed on 27 August 2024).
- About Us. Fastmarkets. Available online: https://www.fastmarkets.com/insights/facing-the-tightening-lithium-supply-challenge-in-2025/ (accessed on 2 July 2024).
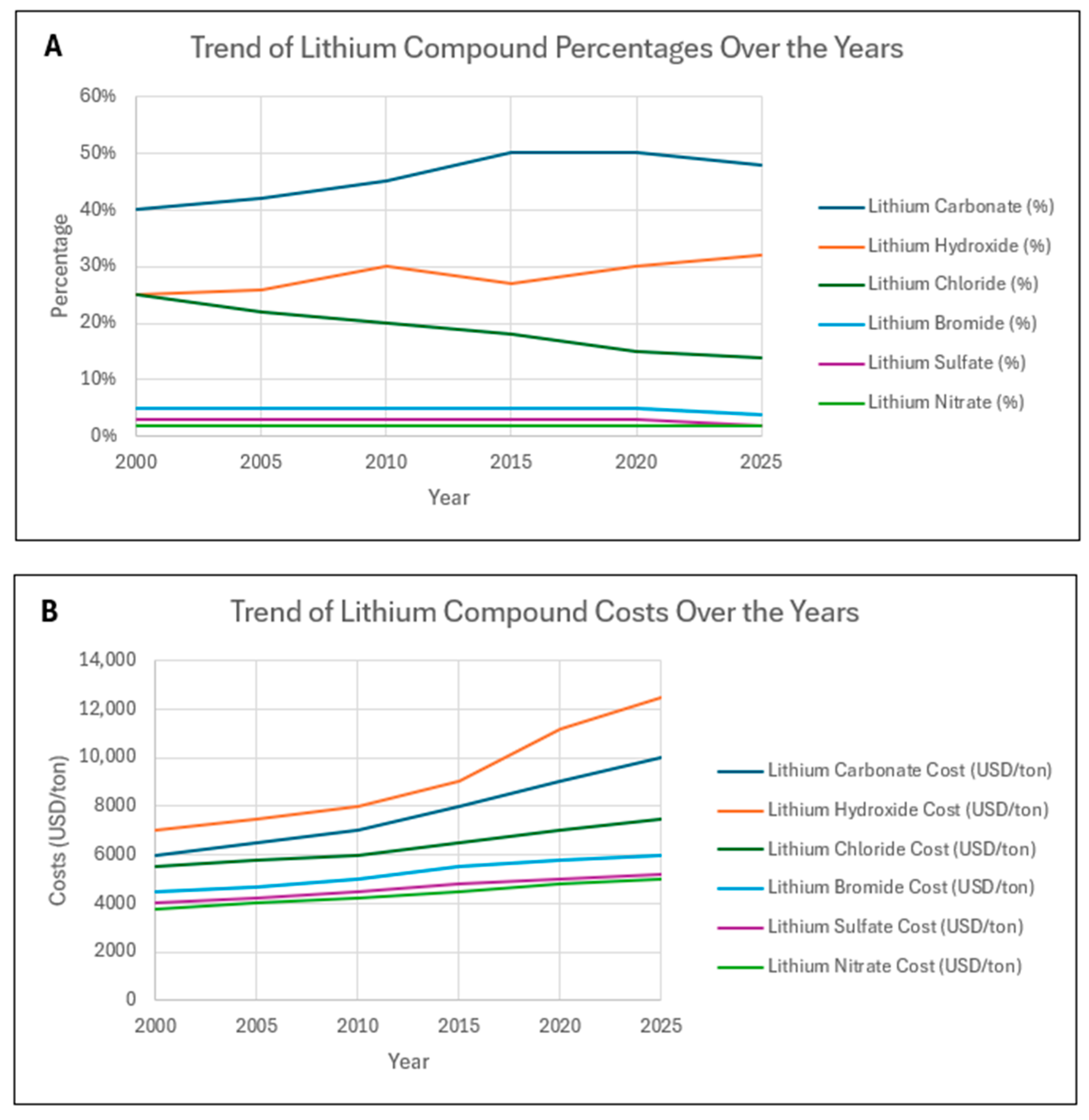

| Year | Lithium Carbonate (%) | Lithium Carbonate Cost (USD/ton) | Lithium Hydroxide (%) | Lithium Hydroxide Cost (USD/ton) | Lithium Chloride (%) | Lithium Chloride Cost (USD/ton) | Lithium Bromide (%) | Lithium Bromide Cost (USD/ton) | Lithium Sulfate (%) | Lithium Sulfate Cost (USD/ton) | Lithium Nitrate (%) | Lithium Nitrate Cost (USD/ton) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 40% | 6000 | 25% | 7000 | 25% | 5500 | 5% | 4500 | 3% | 4000 | 2% | 3800 |
| 2005 | 42% | 6500 | 26% | 7500 | 22% | 5800 | 5% | 4700 | 3% | 4200 | 2% | 4000 |
| 2010 | 45% | 7000 | 30% | 8000 | 20% | 6000 | 5% | 5000 | 3% | 4500 | 2% | 4200 |
| 2015 | 50% | 8000 | 27% | 9000 | 18% | 6500 | 5% | 5500 | 3% | 4800 | 2% | 4500 |
| 2020 | 50% | 9000 | 30% | 11,200 | 15% | 7000 | 5% | 5800 | 3% | 5000 | 2% | 4800 |
| 2025 | 48% | 10,000 | 32% | 12,500 | 14% | 7500 | 4% | 6000 | 2% | 5200 | 2% | 5000 |
| Direct Lithium Extraction | Traditional Lithium Extraction | |
|---|---|---|
| Process duration | Hours to days, which can be done in a single-stage operation | 12 to 18 months, which requires a multi-stage operation |
| Land footprint | Compact operation allows for a larger scale with a small footprint. | Massive footprint due to the need for large open areas that are suitable for pond evaporation |
| Geographical dependent | Weather-dependent and less land-dependent due to its small footprint | Heavily reliant on weather and land suitability |
| Water consumption | DLE elimination of evaporative ponds significantly reduces water consumption | Massive water consumption and large amounts of water lost during the evaporation process |
| Energy consumption | DLE tends to have higher energy consumption. However, the introduction of renewable energy sources within the plant can help reduce both energy costs and environmental impact. DLE also reduces the intensity and requirement of the post-treatment and purification processes, making it potentially less energy-intensive | Low energy consumption is primarily attributed to the use of solar power for brine evaporation. Nevertheless, if post-treatment and purification processes are included, the energy consumption would increase significantly |
| Environmental impact | Smaller ecological footprint due to compact size, utilization of more efficient lithium-selective techniques, and reduction in water and chemical usage. Additionally, the selectivity of lithium and low chemical usage means that natural brine can be recycled back to its reservoir after lithium extraction, preventing the destruction of the existing ecosystem | Pond evaporation results in soil degradation, water pollution, and consumption of high natural brines, leading to habitat and biodiversity destruction |
| Carbon intensity | Consuming less energy per product, using efficient techniques, and implementing renewable, clean energy will reduce energy consumption and greenhouse gas emissions. In addition, increasing lithium production aids the speed of transaction to clean renewable energy sources | The pond evaporation process requires low energy. However, its low efficiency requires energy-intensive post-treatment to meet market demand |
| Cost-effectiveness | Efficient and selective extraction reduces process duration, water consumption, and purification processes, offering long-term cost advantages. However, the initial investment costs for implementing it can be high. Additionally, DLE has higher energy consumption. Nevertheless, DLE is still evolving, and its evaluation at a larger industrial scale remains uncertain | Longer process duration and high water consumption contribute to higher costs. In addition, the lower process efficiency leads to the need for extensive purification and pretreatment steps, increasing chemical consumption. Regardless, traditional extraction exhibits low energy consumption |
| Complexity and scalability | DLE processes tend to be more complex and compact than traditional methods. This may offer many advantages, but it also has its challenges. These challenges include safety and maintenance concerns and scalability issues. Most DLE technologies are still in their initial stages, and their scalability to meet industrial production demands remains to be proven | Less complexity than DLE means lower maintenance and safety concerns. It is also scalable and has been used for years in industrial lithium production |
| Product purity | DLE’s high efficiency and selectivity of lithium lead to higher product purity and lithium recovery | Low efficiency and selectivity of the process means that extensive purification processes are needed to meet the market requirements |
| Resource availability | The availability of suitable adsorbents, resins, or membranes limits the widespread adoption of DLE methods | The long-standing industrial establishment of traditional methods means finding resources and equipment is much easier and cheaper |
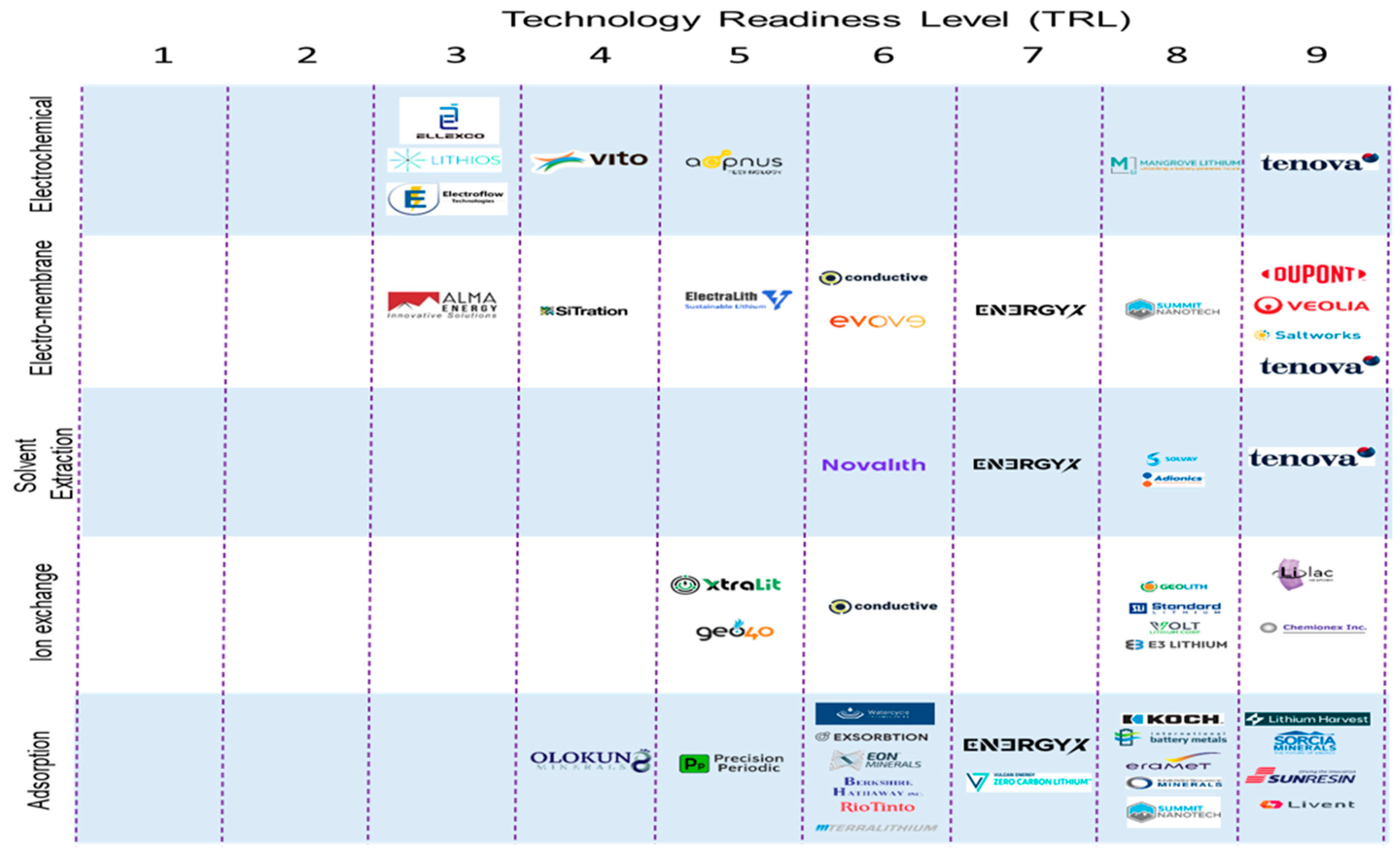
| DLE Technologies | Advantages | Disadvantages | Seawater Brine Readiness |
|---|---|---|---|
| Adsorption (TRL: 7–9) |
|
| Not ready as it requires a higher lithium concentration to be economically viable |
| Ion Exchange (TRL: 7–9) |
|
| Not ready as it requires a higher lithium concentration to be economically viable |
| Solvent Extraction (TRL: 7–8) |
|
| Not ready, but it has the highest readiness of all DLE (depending on the solvent) |
| (Electro-)Membrane Separation (TRL: 4–8) |
|
| The CDI membrane shows great promise for the future but is not yet ready. NF also shows potential, primarily as pre-treatment for removing multivalent ions. ED systems are also promising. All membrane technologies, except for NF for pre-treatment purposes, are not yet ready for use with low-concentration lithium brines |
| Electrochemical Separation (TRL: 3–8) |
|
| This category shows the most futuristic potential, especially EIPS and CDI. Nevertheless, it is not yet ready |
| Company | Technology/Project Name | Technology Category | Development Stage | Technology Readiness Level | Lithium Based-Products | Company’s Claims | Logo |
|---|---|---|---|---|---|---|---|
| Livent | ILiAD | Adsorption | Commercial | TRL 9 | Lithium chloride | 99.9% impurity rejection >90% Li recovery Li feed concentration of 50–2000 ppm |  |
| SUNRESIN | DLE System | Adsorption | Commercial | TRL 9 | Lithium carbonate and lithium hydroxide | Production capacity as of 2022 is 4000–25,000 tons/year |  |
| Eramet | BofA Li DLE | Adsorption | Demo | TRL 8 | Lithium carbonate | 90% Li recovery |  |
| International Battery Metals | IBAT DLE | Adsorption | Demo | TRL 8 | Lithium chloride | 95% Li recovery >50 °C operation requirement |  |
| KOCH | Li-PRO™ | Adsorption | Demo | TRL 8 | Lithium chloride | 95% Li recovery |  |
| Summit Nanotech | denaLi™ | Adsorption | Demo | TRL 8 | Lithium carbonate | >90 Li recovery |  |
| Membrane | |||||||
| ENERGYSOURCE MINERALS | ATLiS | Adsorption | Demo | TRL 8 | Lithium chloride | 7 to 80 times less footprint (than conventional) 3 to 9 times less water consumption (than conventional) 2 to 8 times less CO2 emission (than conventional) |  |
| Watercycle Technologies | DLEC | Adsorption | Pilot | TRL 6 | Lithium hydroxide | >95% Li recovery |  |
| Vulcan Energy | Zero Carbon Lithium | Adsorption | Demo | TRL 7 | Lithium chloride and lithium hydroxide | >90% Li recovery >50 °C operation requirement |  |
| ExSorbtion | CO2 generate DLE | Adsorption | Pilot | TRL 6 | Lithium carbonate | Feed concentration as low as 40 ppm 3 tons of water per ton of product |  |
| EON Minerals | Amanecer | Adsorption | Pilot | TRL 6 | Lithium carbonate | Hybrid system of DLE and advanced evaporation processes |  |
| Berkshire Hathaway Inc. | Salton Sea | Adsorption | Pilot | TRL 6 | Lithium carbonate and lithium chloride | Capacity of 100 gpm Energy is provided through 100% renewable sources |  |
| Rio Tinto | Rincon | Adsorption | Pilot | TRL 6 | Lithium carbonate | Capacity of 3000 tons/year |  |
| TerraLithium | Proprietary DLE | Adsorption | Pilot | TRL 6 | Lithium carbonate and lithium hydroxide | Minimal footprint and environmental impact Replicable design for scale-up and cost efficiency |  |
| EnergyX | LiTAS™ | Adsorption | Demo | TRL7 | Lithium chloride | No chemical usage, 90% Li recovery Process time 1–2 days. Production cost as low as $100/ton |  |
| Solvent Extraction | |||||||
| Membrane | |||||||
| Precision Periodic | Nano Beads™ | Adsorption | Pilot | TRL 5 | Lithium chloride | >95% Li recovery, maximum 200 mg/g adsorption capacity |  |
| Olokun Minerals | zSMB | Adsorption | Lab | TRL 4 | Lithium chloride, lithium carbonate, and lithium hydroxide | >95% Li recovery >99% product purity |  |
| Lilac Solutions | Lilac IEX | Ion Exchange | Commercial | TRL 9 | Lithium chloride | 10 acres footprint Water consumption of 2–20 m3/ton 99% Li recovery |  |
| Chemionex | DLE System | Ion Exchange | Commercial | TRL 9 | Lithium chloride and lithium carbonate | Focus on low lithium levels from concentrated brine |  |
| GeoLith | Li-Capt® | Ion Exchange | Demo | TRL 8 | Lithium concentrate (LiCl, Li2SO4) | <90% Li recovery Ability to treat a wide range of feed brine with Li concentration as low as 5 mg/L |  |
| Standard Lithium | SLi DLE | Adsorption | Demo | TRL 8 | Lithium carbonate | >95% Li recovery 94%–99% impurity rejection |  |
| Volt Lithium Corp | Proprietary DLE | Ion Exchange | Demo | TRL 8 | Lithium carbonate | 98% Li recovery |  |
| E3 Lithium | E3 DLE | Ion Exchange | Demo | TRL 8 | Lithium concentrate | 90% Li recovery 98% impurity removal |  |
| Conductive Energy Inc. | Conductive’s IEX | Ion Exchange | Pilot | TRL 6 | Lithium chloride and lithium carbonate | 95% Li recovery 99.5% product purity |  |
| Conductive’s ED | Membrane | ||||||
| XtraLit | XL-DLE | Ion Exchange | Pilot | TRL 5 | Lithium hydroxide | Feed Li concentration 5–300 ppm 95% Li recovery Operation pH 3 to 12 |  |
| Geo40 | Geoseive | Ion Exchange | Pilot | TRL 5 | Lithium concentrate | 90% Li recovery |  |
| Solvay | CYANEX® 936P | Solvent Extraction | Demo | TRL 8 | Lithium concentrate | 99% Li recovery and product purity High Li/Na selectivity |  |
| Adionics | Proprietary DLE | Solvent Extraction | Demo | TRL 8 | Lithium chloride and lithium carbonate | Feed Li concentration of 50 mg/L to 50 g/L >90% Li recovery 86%–97% purity of LiCl and >99% purity of Li2CO3 |  |
| Tenova | LiSX™ | Solvent Extraction | Commercial | TRL 9 | Lithium sulfate and lithium hydroxide | 99.9% Li recovery 88% Ca removal 97% Mg removal 99.9% product purity |  |
| LiP™ | Membrane | ||||||
| LiEL™ | Electrochemical | ||||||
| Novalith | LiCAL® | Solvent Extraction | Pilot | TRL 6 | Lithium carbonate | Compared to conventional, production cost, plant cost, plant footprint, and water consumption of 65%, 50%, 25%, and 90% less, respectively |  |
| Veolia | Proprietary DLE | Membrane | Commercial | TRL 9 | Lithium carbonate and lithium hydroxide | 90% less water consumption |  |
| Saltworks | Li Refining | Membrane | Commercial | TRL 9 | Lithium carbonate and lithium hydroxide Concentrated brine stream | 99.9 product purity RO membrane can concentrate brine up to 120 bar and 130,000 mg/L feed TDS |  |
| Evove | Proprietary DLE | Membrane | Pilot | TRL 6 | Lithium chloride and lithium hydroxide | >90% Li recovery 99.5% product purity Capacity of 4 tons LCE/LHM per year |  |
| ElectraLith | DLE-R | Membrane | Pilot | TRL 5 | Lithium hydroxide | Single-step DLE process |  |
| SiTration | Proprietary DLE | Membrane | Lab | TRL 4 | Lithium carbonate or lithium hydroxide | Chemical-free process Mainly focus on recycling Li battery |  |
| Mangrove Lithium | Clear-Li™ | Electrochemical | Demo | TRL 8 | Lithium carbonate and lithium hydroxide | Refine LiCl or Li2SO4 to Li2CO3 or LiOH Reduce refinement cost by 40% compared to conventional |  |
| Aepnus Technology | Proprietary DLE | Electrochemical | Pilot | TRL 5 | Lithium carbonate or lithium hydroxide | Extraction of various cations No chemicals |  |
| Lithios | ALE | Electrochemical | Lab | TRL 3 | Lithium carbonate and lithium hydroxide | Uses minimal power and freshwater Chemical-free Wide range of brine and concentrations |  |
| VITO NV | GDEx | Electrochemical | Lab | TRL 4 | Lithium carbonate | Gas-diffusion electrode Low carbon |  |
| Ellexco | Proprietary DLE | Electrochemical | Lab | TRL 3 | Lithium chloride and lithium hydroxide | Chemical-free |  |
| Electroflow Technologies | Proprietary DLE | Electrochemical | Lab | TRL 3 | Lithium concentrate | Treat brine with 10–200 ppm Li concentration 90% Li recovery |  |
| Sorcia Minerals | Proprietary DLE | Adsorption | Commercial | TRL 9 | Lithium chloride, lithium carbonate, and lithium hydroxide | Li extraction efficiency of 75%–90% Recycle > 98% of process waters |  |
| Alma Energy | Proprietary DLE | Membrane | Lab | TRL 3 | Lithium carbonate and lithium hydroxide | No chemicals Emission-free |  |
| Lithium Harvest | Proprietary DLE | Adsorption | Commercial | TRL 9 | Lithium carbonate | 96% less water consumption of 20 million gal per 1000 mt LCE consumption (than conventional) 70% lower CAPEX (than conventional) and 35% lower OPEX (than conventional) >95% Li recovery footprint of 1.4 acres per 1000 mt LCE (99% lower than the conventional) |  |
| DuPont | FilmTec™ LiNE-XD | Membrane | Commercial | TRL 9 | Monovalent rich brine | 99.2 salt rejection high monovalent-divalent ion selectivity |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, M.; Altmann, T.; Das, R. Direct Lithium Extraction from Seawater Brine: An Assessment of Technology and Existing Commercial Systems. Minerals 2025, 15, 512. https://doi.org/10.3390/min15050512
Alghamdi M, Altmann T, Das R. Direct Lithium Extraction from Seawater Brine: An Assessment of Technology and Existing Commercial Systems. Minerals. 2025; 15(5):512. https://doi.org/10.3390/min15050512
Chicago/Turabian StyleAlghamdi, Mosaab, Thomas Altmann, and Ratul Das. 2025. "Direct Lithium Extraction from Seawater Brine: An Assessment of Technology and Existing Commercial Systems" Minerals 15, no. 5: 512. https://doi.org/10.3390/min15050512
APA StyleAlghamdi, M., Altmann, T., & Das, R. (2025). Direct Lithium Extraction from Seawater Brine: An Assessment of Technology and Existing Commercial Systems. Minerals, 15(5), 512. https://doi.org/10.3390/min15050512










