Supported Hybrid Amines Within Porous Aluminosilicate Clays with Natural Different Morphologies for Efficient CO2 Capture
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
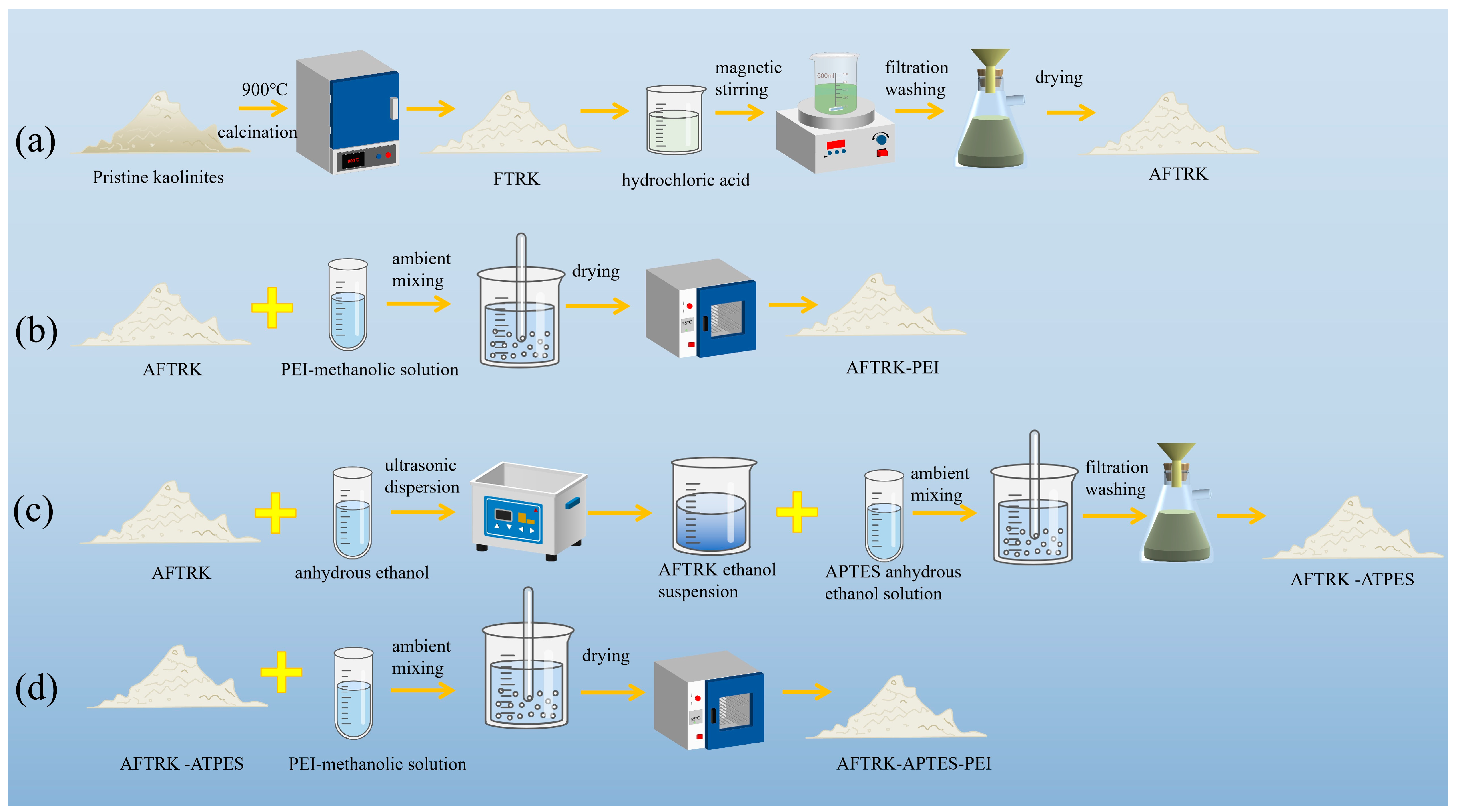
2.2. Adsorbent Preparation
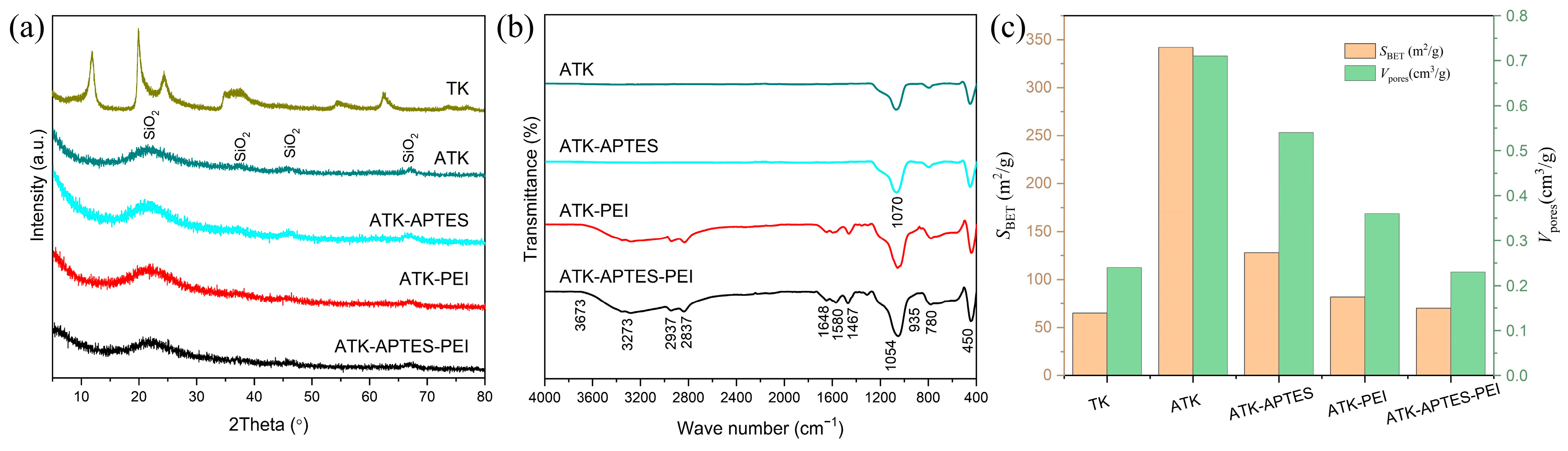
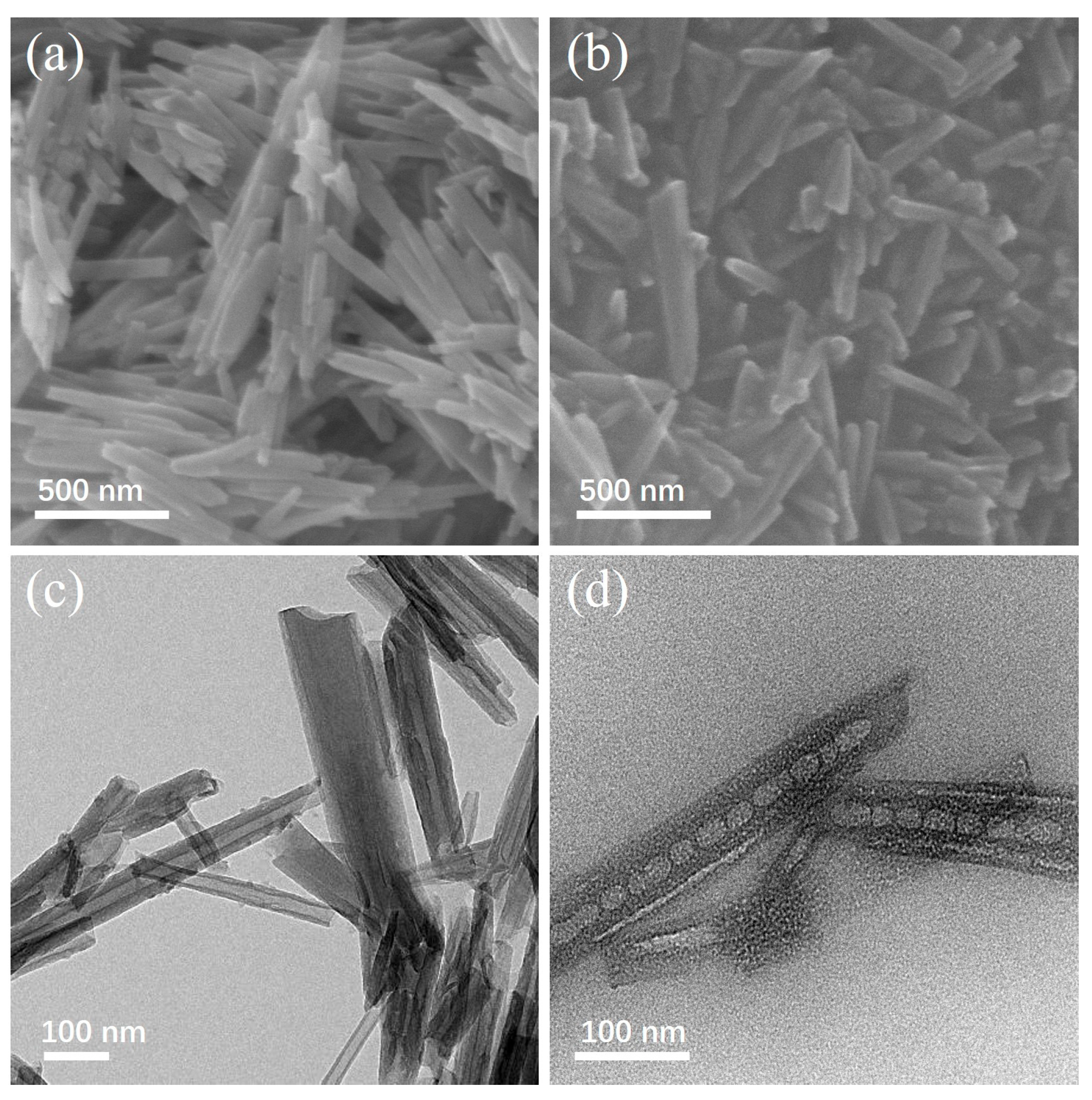
2.3. Sample Characterization
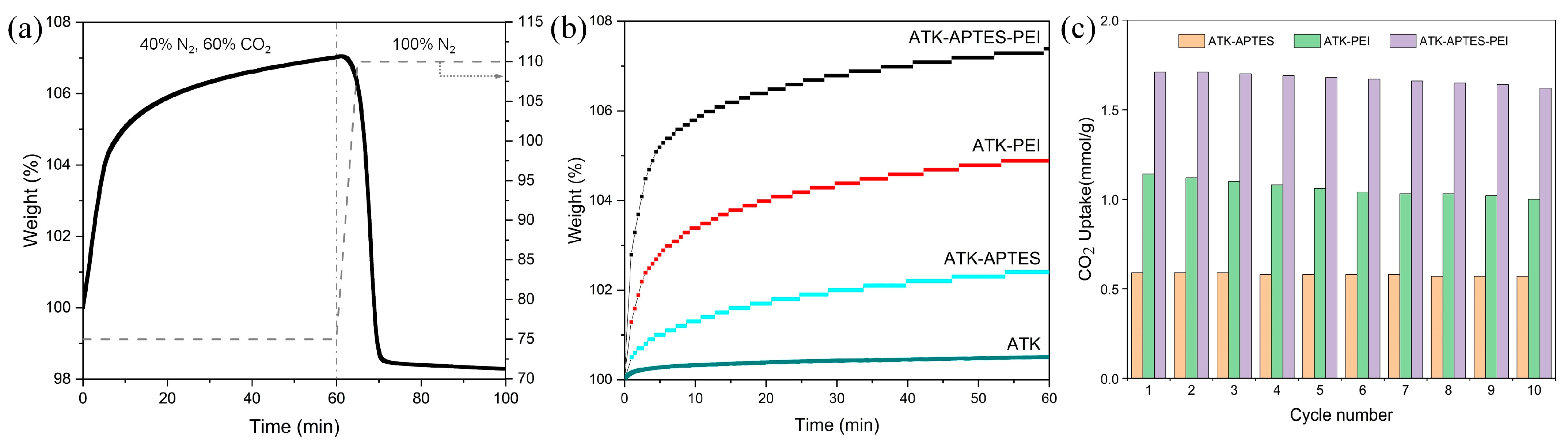
2.4. CO2 Sorption/Desorption Performances
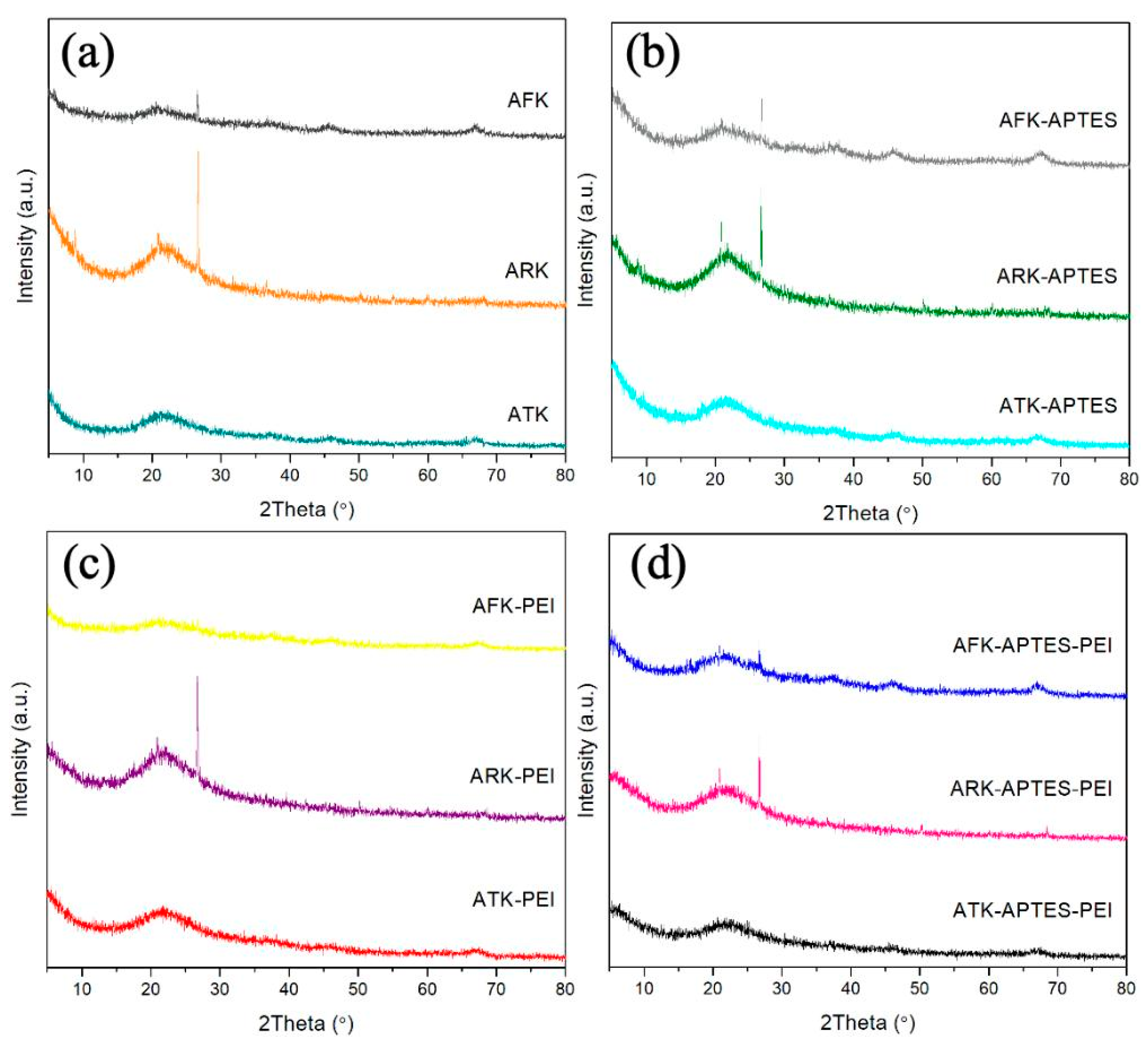
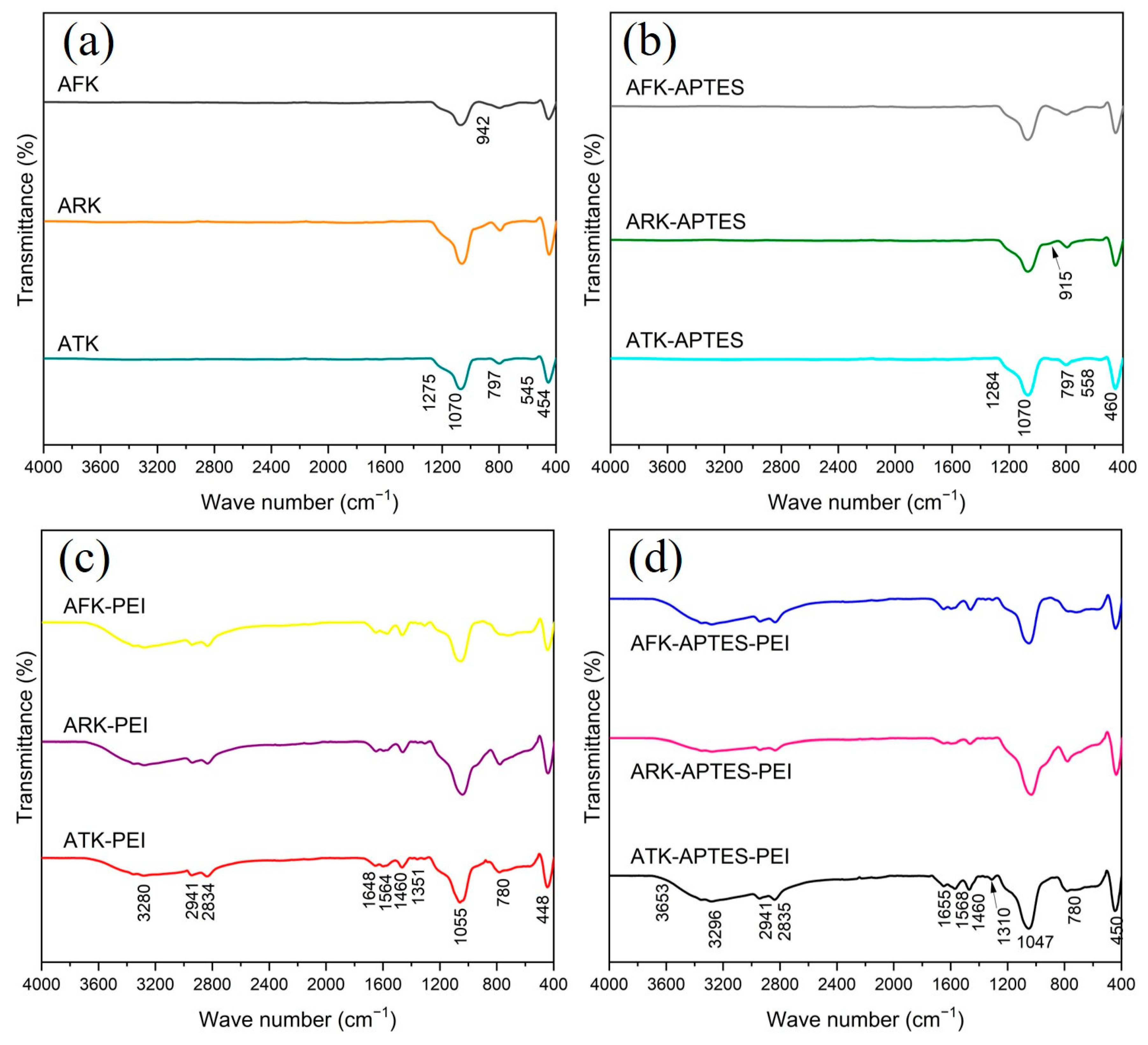
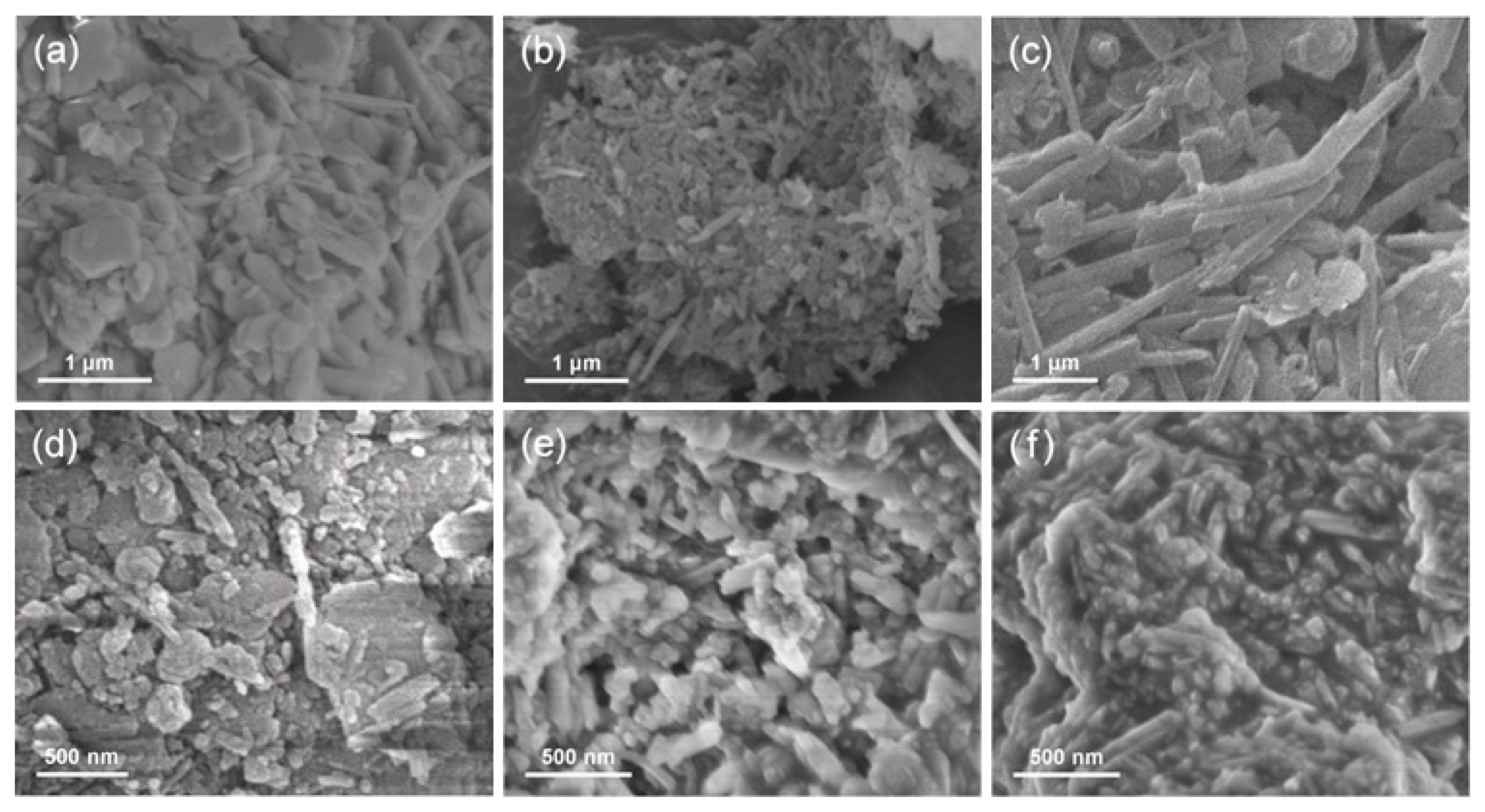
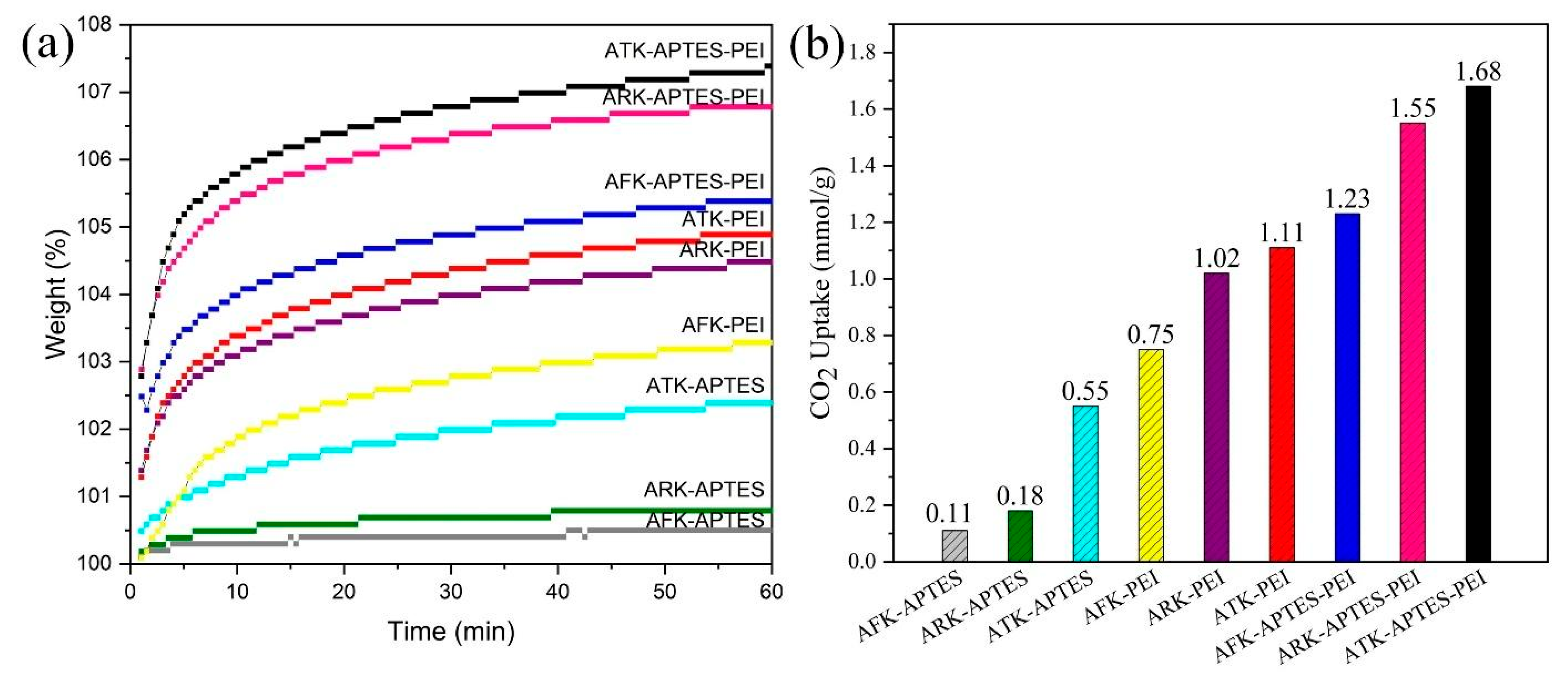
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCS | Carbon capture and storage |
| MOFs | Metal–organic frameworks |
| FK | Flake-like kaolinite |
| TK | Tubular halloysite |
| RK | Rod-like kaolinite |
| APTES | 3-aminopropyltriethoxysilane |
| AFTRK | Acid-activated kaolin |
| AFTRK-APTES | APTES-grafted acid activated kaolin |
| AFTRK-PEI | PEI-impregnated acid activated kaolin |
| AFTRK-APTES-PEI | Hybrid amine-modified sample |
| XRD | Powder X-ray diffraction |
| FT-IR | Fourier transform infrared |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| TGA-MS | Thermogravimetric analysis coupled with mass spectrometry |
| SBA-15 | Santa Barbara Amorphous-15 |
References
- Armstrong, R.C.; Wolfram, C.; de Jong, K.P.; Gross, R.; Lewis, N.S.; Boardman, B.; Ragauskas, A.J.; Ehrhardt-Martinez, K.; Crabtree, G.; Ramana, M.V. The frontiers of energy. Nat. Energy 2016, 1, 15020. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Peng, K.; Zhao, K.; Bai, M.; Li, H.; Gao, W.; Gong, Z. Amine-impregnated porous carbon–silica sheets derived from vermiculite with superior adsorption capability and cyclic stability for CO2 capture. Chem. Eng. J. 2023, 464, 142662. [Google Scholar] [CrossRef]
- Peng, K.; Ye, J.; Wang, H.; Song, H.; Deng, B.; Song, S.; Wang, Y.; Zuo, L.; Ye, J. Natural halloysite nanotubes supported Ru as highly active catalyst for photothermal catalytic CO2 reduction. Appl. Catal. B-Environ. 2023, 324, 122262. [Google Scholar] [CrossRef]
- Hunka, N.; Duncanson, L.; Armston, J.; Dubayah, R.; Healey, S.P.; Santoro, M.; May, P.; Araza, A.; Bourgoin, C.; Montesano, P.M.; et al. Intergovernmental Panel on Climate Change (IPCC) Tier 1 forest biomass estimates from Earth Observation. Sci. Data 2024, 11, 1127. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Shao, B.; Wang, Z.Q.; Gong, X.Q.; Liu, H.; Qian, F.; Hu, P.; Hu, J. Synergistic promotions between CO2 capture and in-situ conversion on Ni-CaO composite catalyst. Nat. Commun. 2023, 14, 996. [Google Scholar] [CrossRef]
- Wang, J.Y.; Huang, L.; Yang, R.Y.; Zhang, Z.; Wu, J.W.; Gao, Y.S.; Wang, Q.; O’Hare, D.; Zhong, Z.Y. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Loganathan, S.; Ghoshal, A.K. Amine tethered pore-expanded MCM-41: A promising adsorbent for CO2 capture. Chem. Eng. J. 2017, 308, 827–839. [Google Scholar] [CrossRef]
- Ahsan, S.; Ayub, A.; Meeroff, D.; Lashak, M.J. A comprehensive comparison of zeolite-5A molecular sieves and amine-grafted SBA-15 silica for cyclic adsorption-desorption of carbon dioxide in enclosed environments. Chem. Eng. J. 2022, 437, 135139. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Meng, Y.; Xue, H.; Chen, J.; Peng, K. Additive promoted supported mixed amines on mesoporous silica for cyclic capture of carbon dioxide. Sep. Purif. Technol. 2025, 362, 131824. [Google Scholar] [CrossRef]
- Kim, J.; Lin, L.-C.; Swisher, J.A.; Haranczyk, M.; Smit, B. Predicting large CO2 adsorption in aluminosilicate zeolites for postcombustion carbon dioxide capture. J. Am. Chem. Soc. 2012, 134, 18881–19308. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Zhu, J.; Liu, X.Q.; Geng, J.C.; Sun, L.B. Molecular template-directed synthesis of microporous polymer networks for highly selective CO2 capture. ACS. Appl. Mater. Interfaces 2014, 6, 20340–20349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Yao, Z.Z.; Xiang, S.C.; Chen, B.L. Perspective of microporous metal-organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef]
- Qin, C.L.; Yin, J.J.; Ran, J.Y.; Zhang, L.; Feng, B. Effect of support material on the performance of K2CO3 based pellets for cyclic CO2 capture. Appl. Energy 2014, 136, 280–288. [Google Scholar] [CrossRef]
- Zhao, T.X.; Guo, B.; Zhang, F.; Sha, F.; Li, Q.; Zhang, J.B. Morphology control in the synthesis of CaCO3 microspheres with a novel CO2-storage material. ACS Appl. Mater. Interfaces 2015, 7, 15918–15927. [Google Scholar] [CrossRef]
- Wang, W.L.; Xiao, J.; Wei, X.L.; Ding, J.; Wang, X.X.; Song, C.S. Development of a new clay supported polyethylenimine composite for CO2 capture. Appl. Energy 2014, 113, 334–341. [Google Scholar] [CrossRef]
- Irani, M.; Fan, M.H.; Ismail, H.; Tuwati, A.; Dutcher, B.; Russell, A.G. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy 2015, 11, 235–246. [Google Scholar] [CrossRef]
- Sánchez-Zambrano, K.S.; Lima Duarte, L.; Maia, D.A.S.; Vilarrasa-García, E.; Bastos-Neto, M.; Rodríguez-Castellón, E.; de Azevedo, D.C.S. CO2 capture with mesoporous silicas modified with amines by double functionalization: Assessment of adsorption/desorption cycles. Materials 2018, 11, 887. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, D.-L. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Z.; Cao, Z.; Han, F.; Wang, Y.; Cheng, H. Molecular simulation of CO2 adsorption on kaolinite: Insights into geological storage of CO2. Appl. Clay Sci. 2024, 258, 107495. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, D.L. Amine modification on kaolinites to enhance CO2 adsorption. J. Colloid Interfaces Sci. 2014, 436, 47–51. [Google Scholar] [CrossRef]
- Ran, Y.; Peng, K.; Li, Z.; Guo, X.; Chen, H.; Cai, S.; Su, L.; Niu, M.; Lu, D.; Wang, H. Amine-Functionalized SiC nanowires aerogel synchronizes high capacity and ultra-rapid kinetics for CO2 capture. Chem. Eng. J. 2025, 514, 163149. [Google Scholar] [CrossRef]
- Kole, K.; Das, S.; Samanta, A.; Jana, S. Parametric study and detailed kinetic understanding of CO2 adsorption over high-surface-area flowery silica nanomaterials. Ind. Eng. Chem. Res. 2020, 59, 21393–21402. [Google Scholar] [CrossRef]
- de Sousa, J.A.R.; Amâncio, R.; Morales-Ospino, R.; de Oliveira, J.L.B.; Cecilia, J.A.; Vilarrasa-García, E.; Bastos-Neto, M.; Rodríguez-Castellón, E.; de Azevedo, D.C.S. H2S and H2O combined effect on CO2 capture by amino functionalized hollow microsphere silicas. Ind. Eng. Chem. Res. 2021, 60, 10139–10154. [Google Scholar] [CrossRef]
- Jana, S.; Das, S.; Ghosh, C.; Maity, A.; Pradhan, M. Halloysite nanotubes capturing isotope selective atmospheric CO2. Sci. Rep. 2015, 5, 8711. [Google Scholar] [CrossRef]
- Ko, Y.G.; Lee, H.J.; Oh, H.C.; Choi, U.S. Amines immobilized double-walled silica nanotubes for CO2 capture. J. Hazard Mater. 2013, 250, 53–60. [Google Scholar] [CrossRef]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 adsorption on amine-functionalized periodic mesoporous benzenesilicas. ACS. Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef]
- Chai, S.H.; Liu, Z.M.; Huang, K.; Tan, S.; Dai, S. Amine functionalization of microsized and nanosized mesoporous carbons for carbon dioxide capture. Ind. Eng. Chem. Res. 2016, 55, 7355–7361. [Google Scholar] [CrossRef]
- Builes, S.; Vega, L.F. Effect of Immobilized Amines on the Sorption Properties of Solid Materials: Impregnation versus Grafting. Langmuir 2013, 29, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, J.A.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.M.A.; Azevedo, D.C.S.; Cavalcante, C.L.; Rodríguez-Castellón, E. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO2 capture. Int. J. Greenh. Gas Control 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, E.R.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Xue, H.; Dong, X.; Fan, Y.; Ma, X.; Yao, S. Study of structural transformation and chemical reactivity of kaolinite-based high ash slime during calcination. Minerals 2023, 13, 466. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; García-Garzón, V.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Sánchez-Garrido, J.A.; Garzón, E. Influence of features and firing temperature on the ceramic properties and phase evolution of raw kaolins. Constr. Build. Mater. 2025, 466, 140215. [Google Scholar] [CrossRef]
- Swasy, M.I.; Campbell, M.L.; Brummel, B.R.; Guerra, F.D.; Attia, M.F.; Smith, G.D.; Alexis, F.; Whitehead, D.C. Poly(amine) modified kaolinite clay for VOC capture. Chemosphere 2018, 213, 19–24. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Zhao, X.; Zhao, Y.; Zhang, B.; Zhao, K.; Peng, K. Synergy tetraethylenepentamine and diethanolamine impregnated within silica nanotubes derived from natural halloysite for efficient CO2 capture. J. Clean. Prod. 2024, 434, 140405. [Google Scholar] [CrossRef]
- Du, C.; Yang, H. Investigation of the physicochemical aspects from natural kaolin to Al-MCM-41 mesoporous materials. J. Colloid Interfaces Sci. 2012, 369, 216–222. [Google Scholar] [CrossRef]
- Liu, L.; Jin, S.; Ko, K.; Kim, H.; Ahn, I.S.; Lee, C.H. Alkyl-functionalization of (3-Aminopropyl) triethoxysilane-grafted zeolite beta for carbon dioxide capture in temperature swing adsorption. Chem. Eng. J. 2020, 382, 122834. [Google Scholar] [CrossRef]
- Yang, F.; Xing, L.A.; Opoku, K.N.; Zhao, H.Y.; Wang, Z.X.; Ni, R.T.; Gao, Q.; Guo, Z.J.; Zeng, F.; Yuan, A.H.; et al. Wastes against wastes treatment: Industrial silica fume derived porous solid amine adsorbent for efficient and reversible ultralow-pressure CO2 adsorption. Sep. Purif. Technol. 2025, 352, 128257. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, H.R.; Zhao, X.Q.; Peng, K. Amine-impregnated natural inorganic nanotubes via layer-by-layer electrostatically self-assembly approach for efficient CO2 adsorption. Sep. Purif. Technol. 2024, 349, 127864. [Google Scholar] [CrossRef]
- Wang, Z.L.; Pang, Y.H.; Guo, H.X.; Wang, H.; Liu, L.; Wang, X.; Zhang, S.; Cui, W.Q. Increased CO2 capture capacity via amino-bifunctionalized halloysite nanotubes adsorbents. Fuel 2024, 364, 131036. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.W.; Baltrus, J.P.; Stevens, R.W.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Abstr. Pap. Am. Chem. Soc. 2004, 86, 1449–1455. [Google Scholar] [CrossRef]
| Supports | Amine Type | Amine Loading (wt.%) | Adsorption Conditions | CO2 Uptake (mmol/g) | Ref. | |
|---|---|---|---|---|---|---|
| T (°C) | Atmosphere | |||||
| ATK | APTES | 32.11 | 75 | 60%CO2/40%N2 | 0.55 | This study |
| ATK | PEI | 40 | 75 | 60%CO2/40%N2 | 1.11 | This study |
| ATK | APTES-PEI | 60 | 75 | 60%CO2/40%N2 | 1.68 | This study |
| AFK | APTES-PEI | 60 | 75 | 60%CO2/40%N2 | 1.23 | This study |
| ARK | APTES-PEI | 60 | 75 | 60%CO2/40%N2 | 1.55 | This study |
| Zeolite Beta | Alkyl-APTES | - | 90 | 15%CO2/85%N2 | 1.44 | [39] |
| Industrial Waste Silica Fume | TEPA | 30 | 50 | 99.999% CO2 | 2.22 | [40] |
| MSU-1 Halloysite | APTES | 48 | 25 | 100% air | 0.13 | [27] |
| Halloysites | PEI | 20 | 80 | 60%CO2/40%N2 | 0.87 | [41] |
| Halloysites | AP-PEI | 20/40 | 80 | 50%CO2/50%N2 | 1.51 | [42] |
| SBA-15 | APTS | 15 | 25 | 10%CO2 | 2.01 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, J.; Zhang, W.; Wang, C.; Ma, H.; Peng, K.; Zhou, Z. Supported Hybrid Amines Within Porous Aluminosilicate Clays with Natural Different Morphologies for Efficient CO2 Capture. Minerals 2025, 15, 506. https://doi.org/10.3390/min15050506
Li X, Chen J, Zhang W, Wang C, Ma H, Peng K, Zhou Z. Supported Hybrid Amines Within Porous Aluminosilicate Clays with Natural Different Morphologies for Efficient CO2 Capture. Minerals. 2025; 15(5):506. https://doi.org/10.3390/min15050506
Chicago/Turabian StyleLi, Xiaoyu, Jie Chen, Wenqing Zhang, Chenyu Wang, Hui Ma, Kang Peng, and Zheng Zhou. 2025. "Supported Hybrid Amines Within Porous Aluminosilicate Clays with Natural Different Morphologies for Efficient CO2 Capture" Minerals 15, no. 5: 506. https://doi.org/10.3390/min15050506
APA StyleLi, X., Chen, J., Zhang, W., Wang, C., Ma, H., Peng, K., & Zhou, Z. (2025). Supported Hybrid Amines Within Porous Aluminosilicate Clays with Natural Different Morphologies for Efficient CO2 Capture. Minerals, 15(5), 506. https://doi.org/10.3390/min15050506








