Utilizing Magnesium Carbonate Induced by CO2 to Modify the Performance of Plastic Clay
Abstract
1. Introduction
2. Materials and Methods
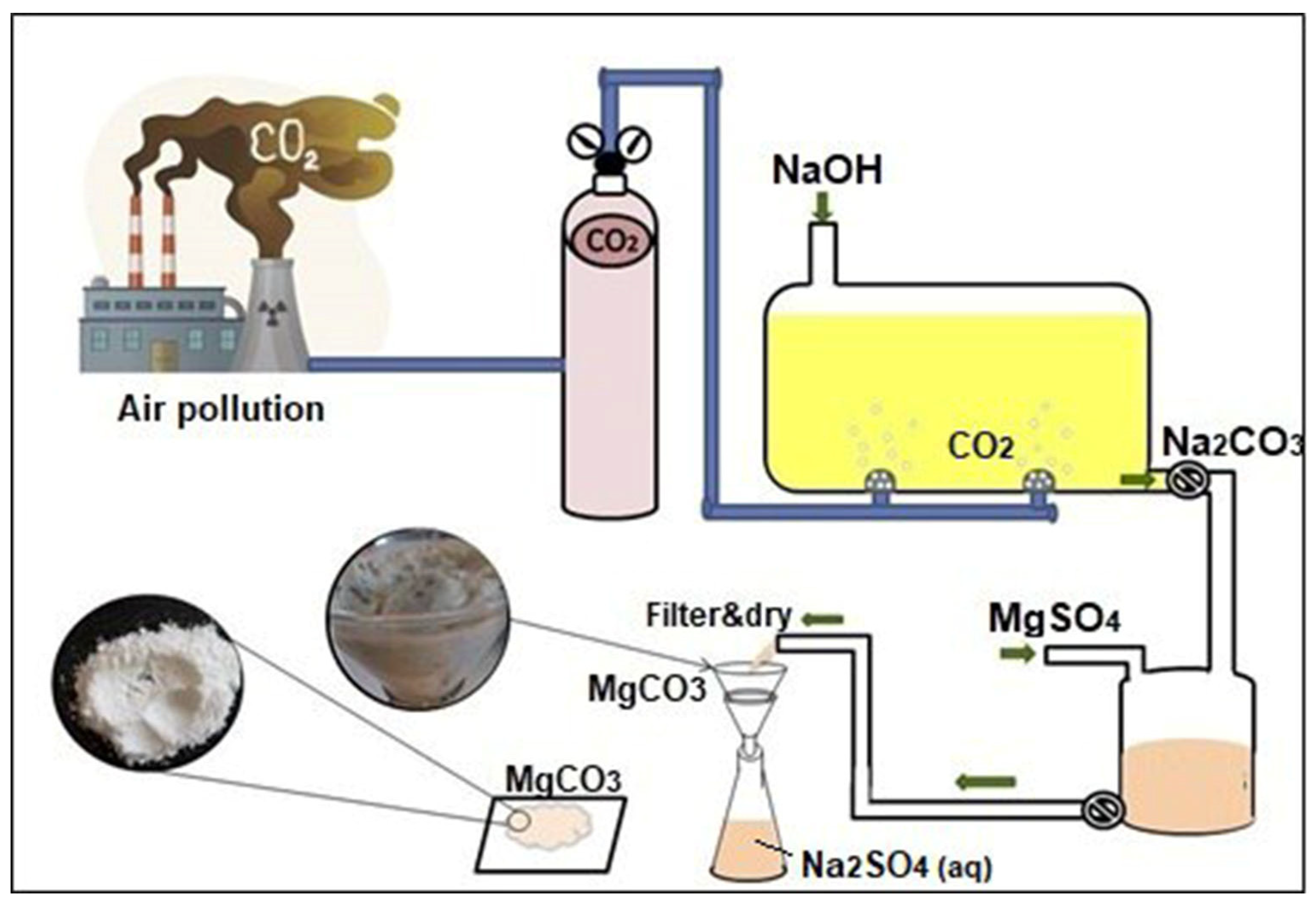
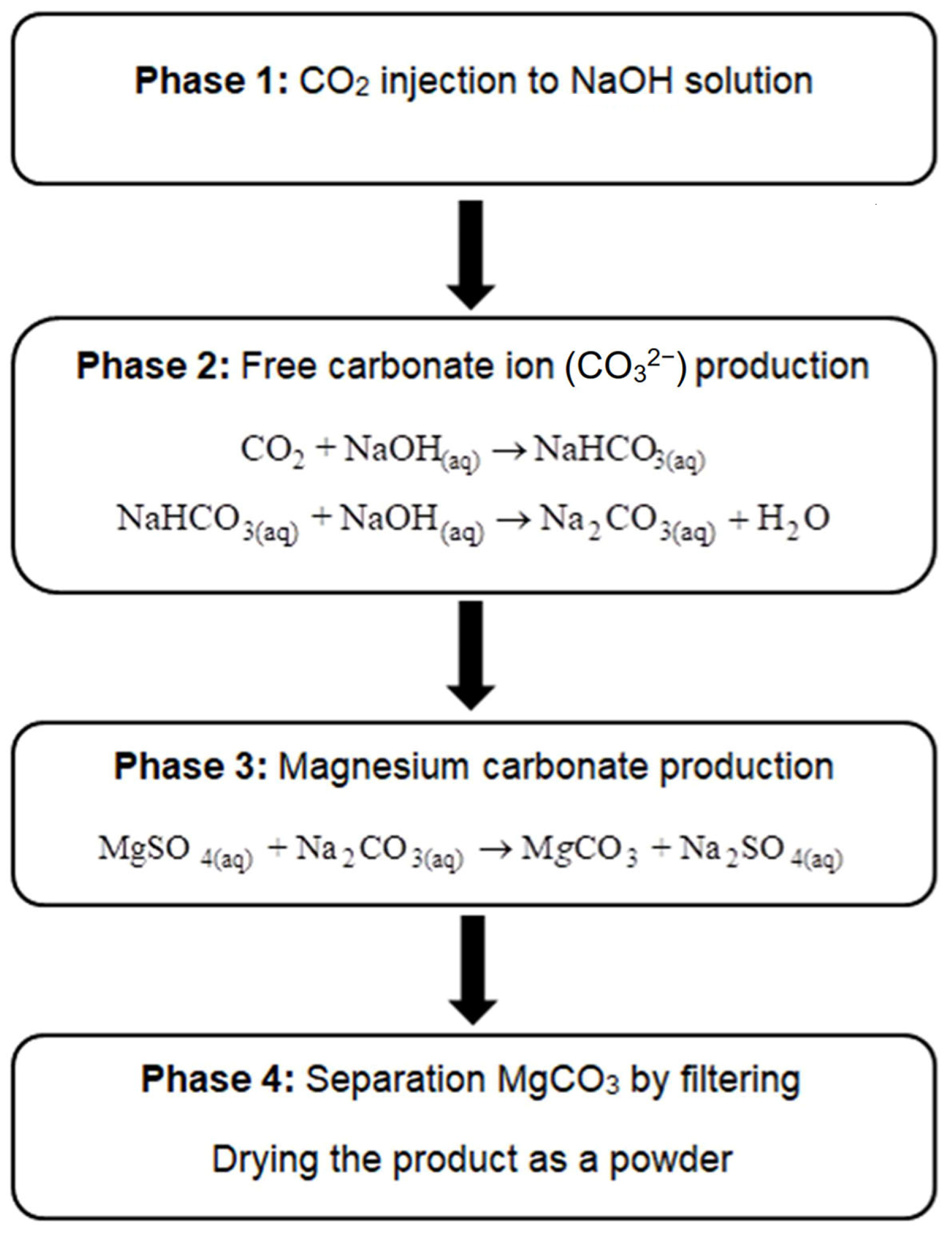
2.1. CO2-Induced Magnesium Carbonate Production
2.2. Soil Type
2.3. Specimen Preparation
2.4. Scanning Electron Microscopy and XRD Analysis
2.5. Evaluation Tests
3. Results
3.1. Soil Index Properties
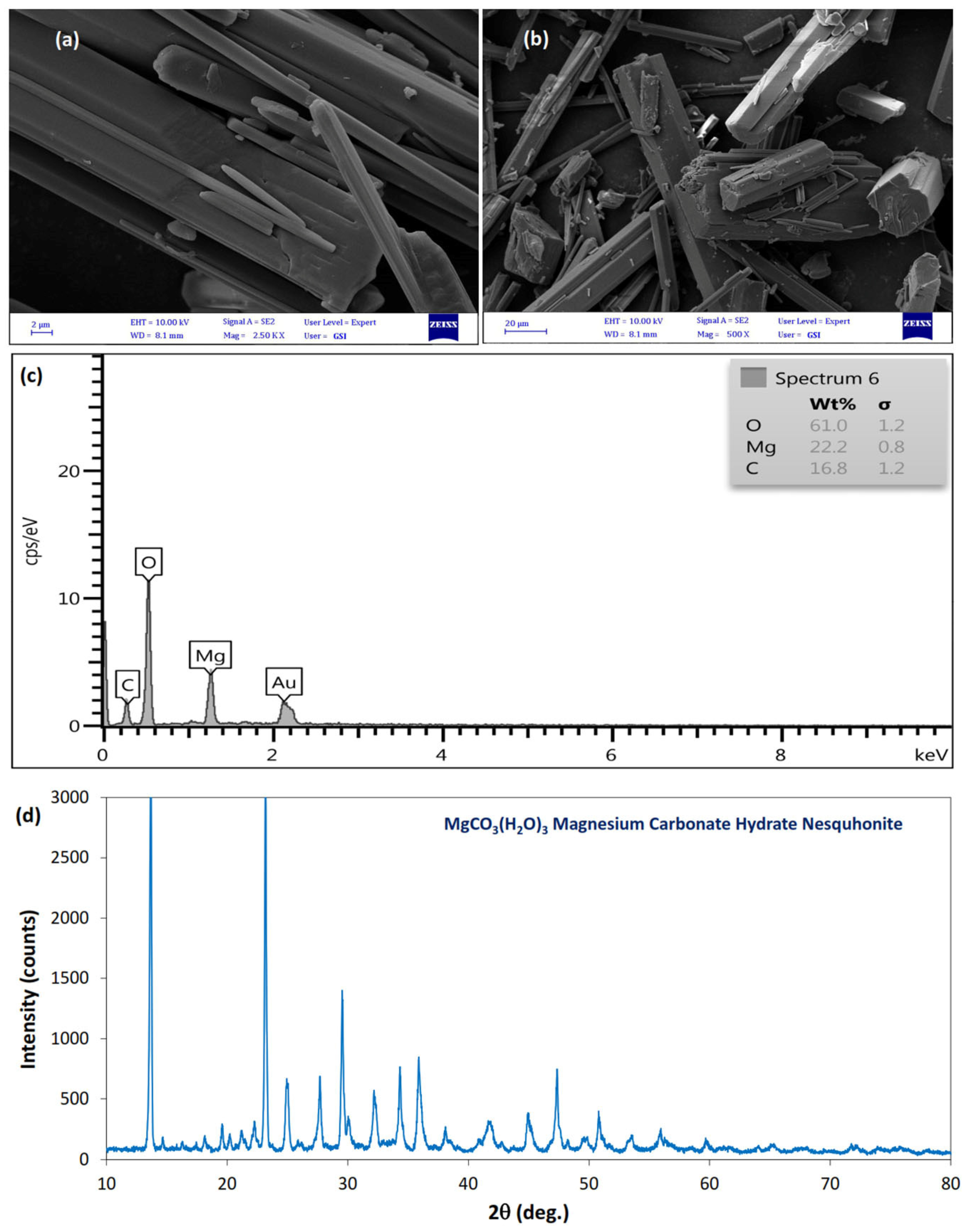
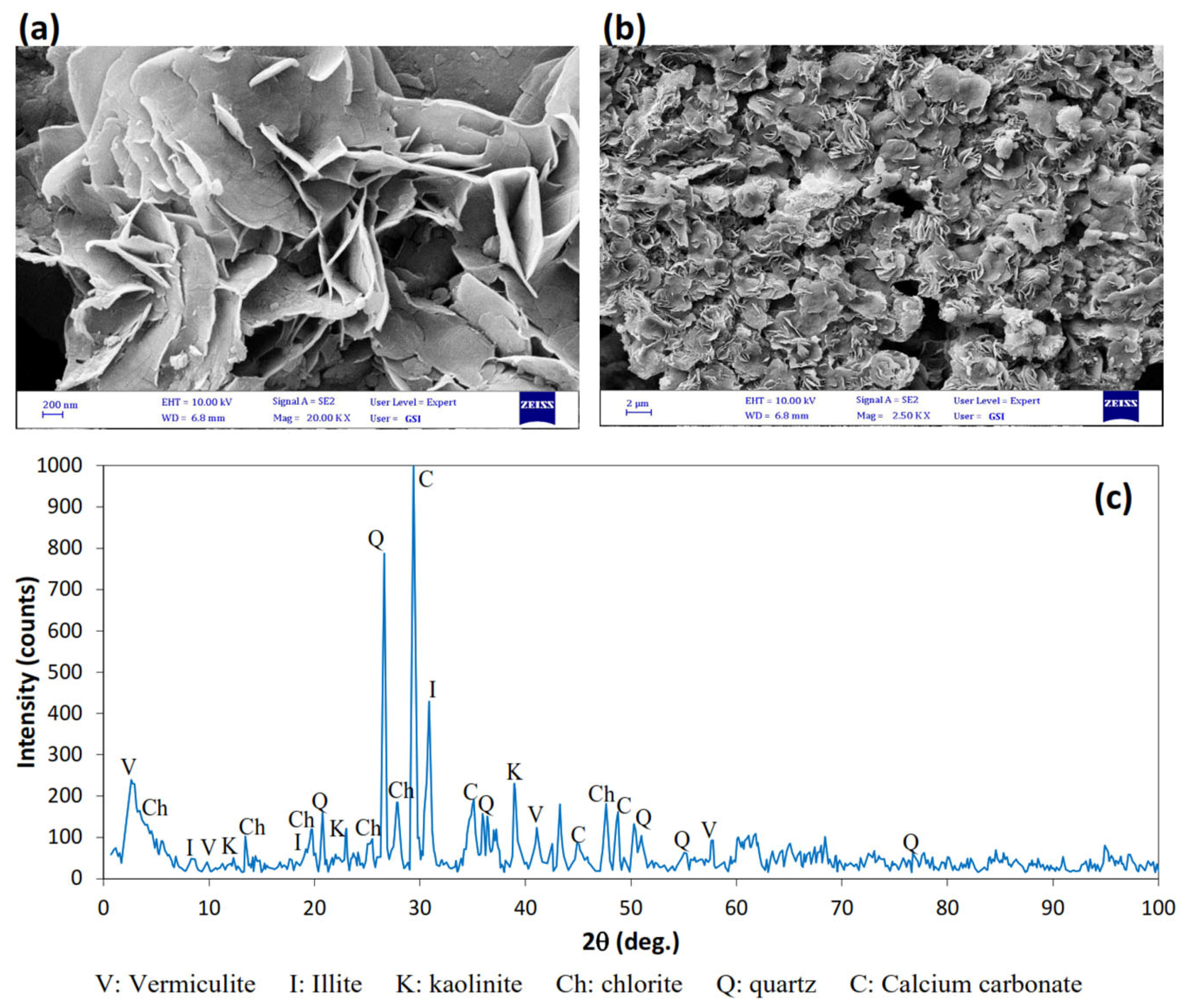
3.2. SEM and XRD Analysis
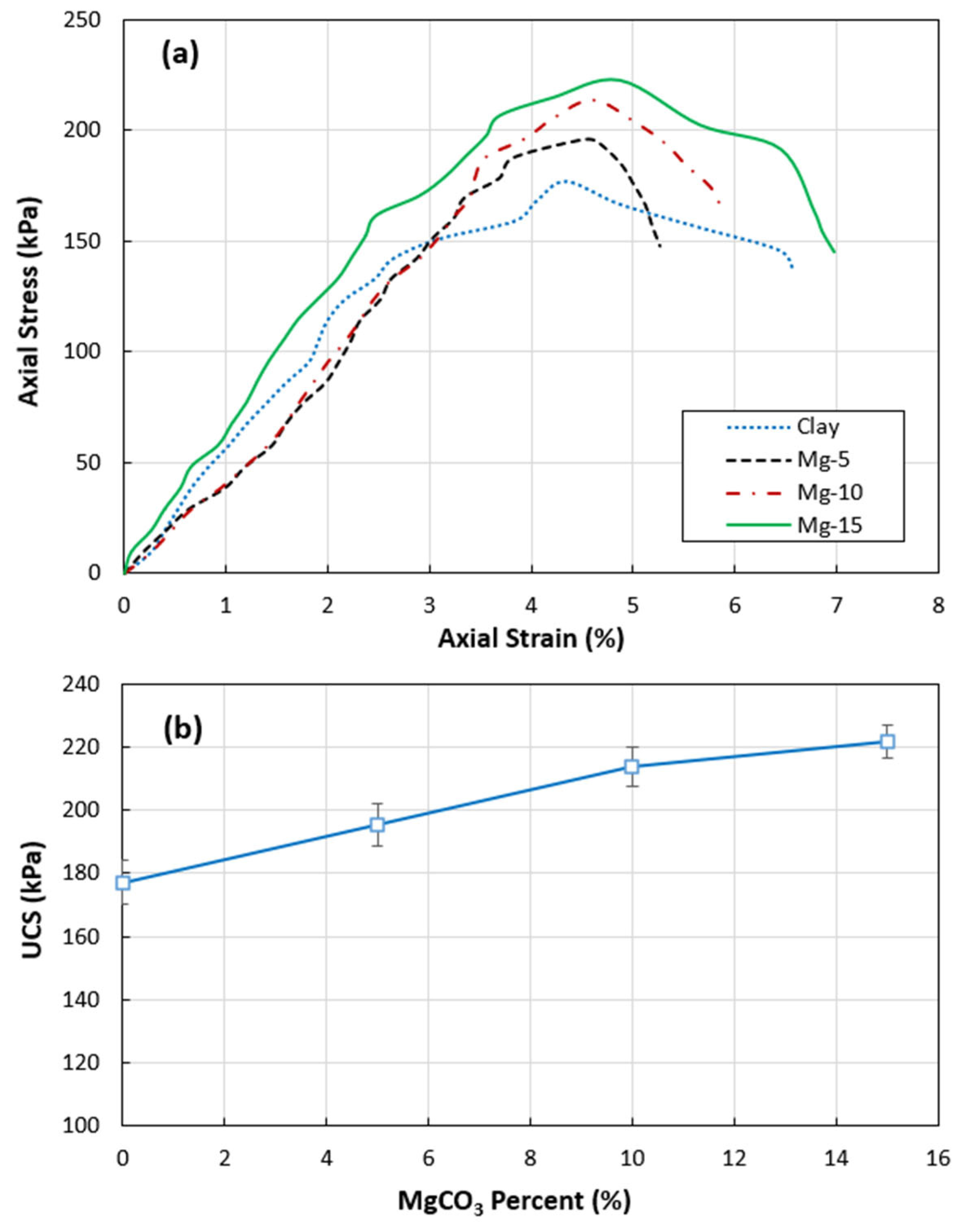
3.3. Unconfined Compression Test
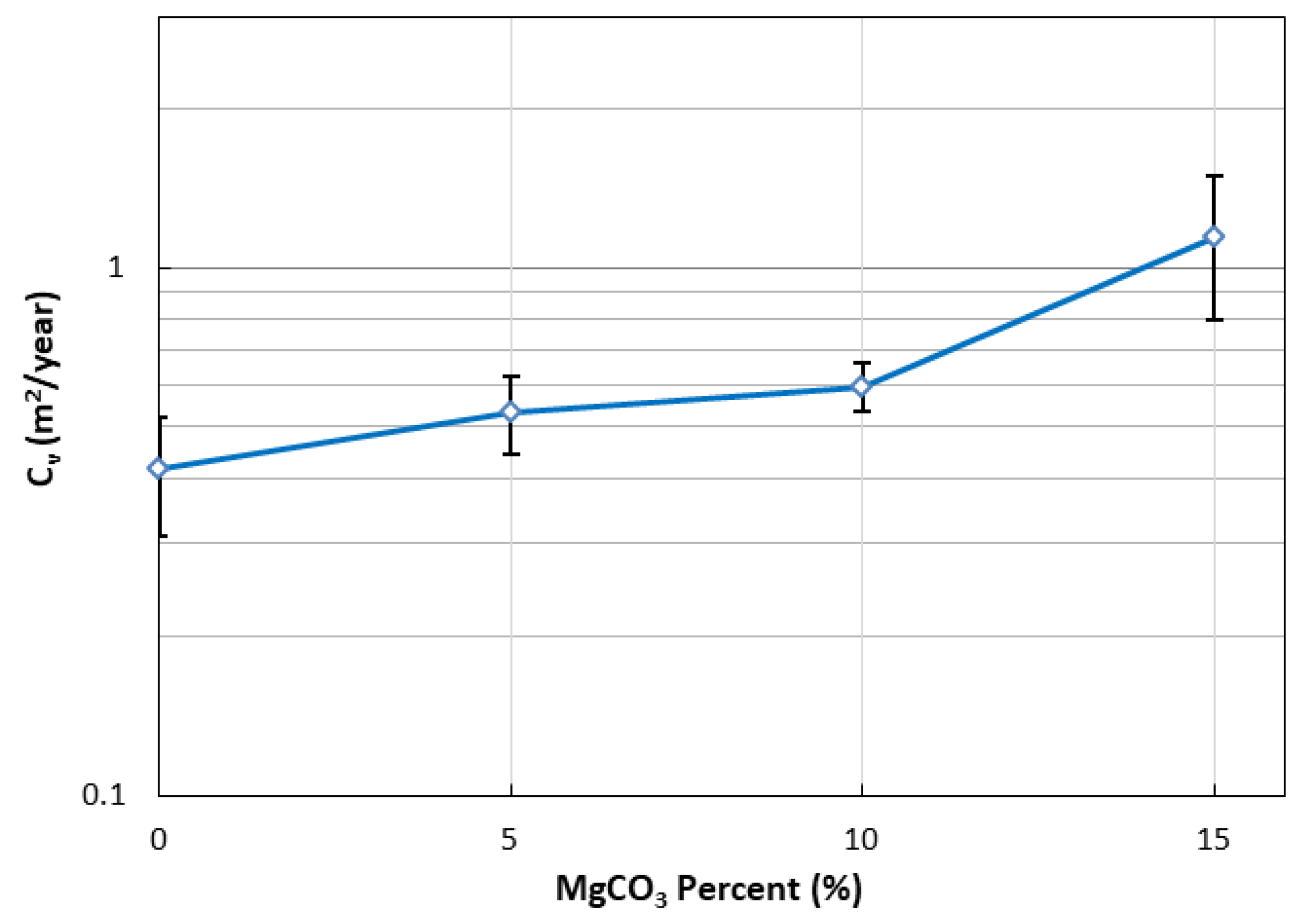
3.4. One-Dimensional Consolidation Test Results
3.5. One-Dimensional Swell Test Results
3.6. New Insights and Future Direction
4. Conclusions
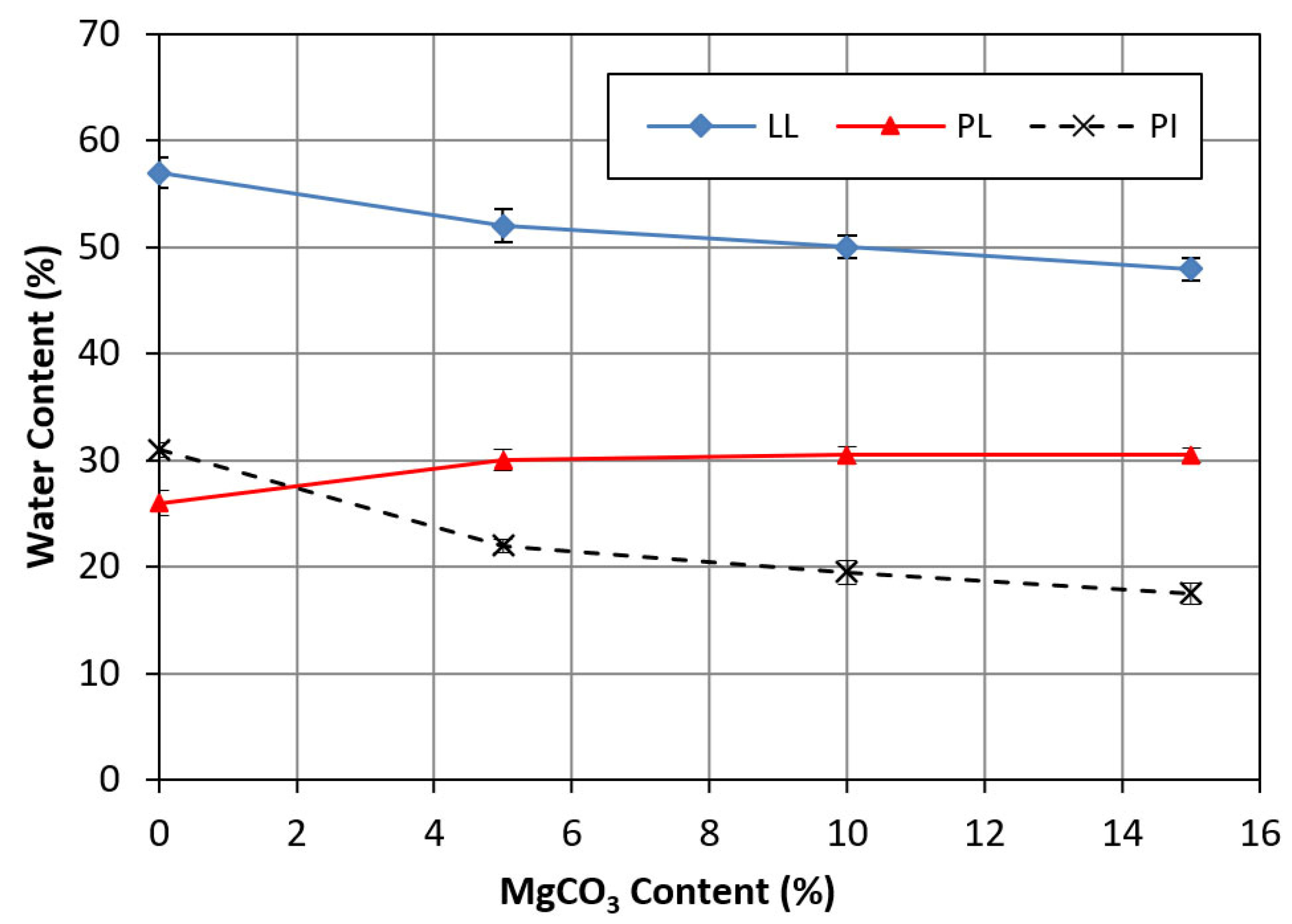
- Atterberg Tests: The liquid limit of the clay decreased by 16% after treatment with 15% magnesium carbonate, and the plasticity index decreased by 43%. Adding magnesium carbonate altered the soil classification from CH to ML, indicating that the magnesium carbonate reduced the plasticity index of the treated clay, making it less sensitive to moisture content changes.
- EM Analysis: The SEM images of the treated soil showed a uniform distribution of needle-shaped magnesium carbonate crystals. This distribution resulted in interlocking between particles and increased granular behavior in the treated clay.
- Unconfined Compression Strength: The unconfined compression strength of the clay increased by 25% when treated with 15% CO2-induced magnesium carbonate, attributed to the reinforcement provided by the needle-like particles within the clay matrix.
- Compression Index and Cs Values: Significant reductions were observed, with up to 33% in the compression index and 30% in Cs values, with increasing magnesium carbonate content up to 15%. These findings suggest that the treated clay has a lower susceptibility to settlement compared to untreated clay. Consolidation tests confirmed that both the consolidation rate and compressibility behavior of the highly plastic clay improved with CO2-induced magnesium carbonate treatment.
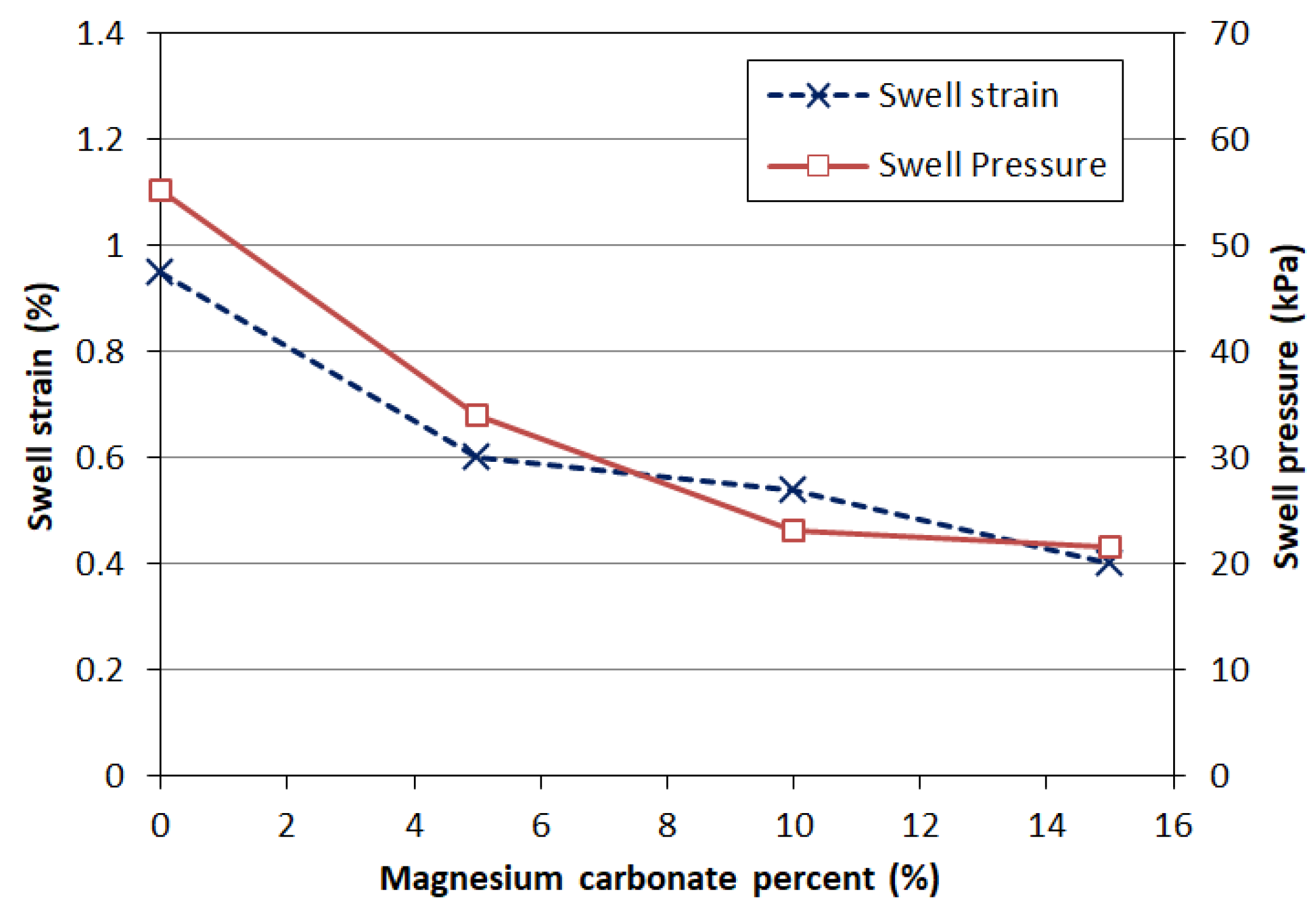
- Swelling Strain: The swelling strain of the highly plastic clay decreased from 1.25% to 0.4% after treatment with 15% magnesium carbonate, demonstrating the significant potential for mitigating swelling soil problems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global carbon budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Liu, S.; Du, K.; Wen, K.; Huang, W.; Amini, F.; Li, L. Sandy soil improvement through microbially induced calcite precipitation (MICP) by immersion. JoVE (J. Vis. Exp.) 2019, 151, e60059. [Google Scholar] [CrossRef]
- Keykha, H.A.; Asadi, A.; Huat, B.B.; Kawasaki, S. Microbial induced calcite precipitation by Sporosarcina pasteurii and Sporosarcina aquimarina. Environ. Geotech. 2018, 6, 562–566. [Google Scholar] [CrossRef]
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Keykha, H.A.; Mohamadzadeh, H.; Asadi, A.; Kawasaki, S. Ammonium-free carbonate- producing bacteria as an ecofriendly soil biostabilizer. Geotech. Test. J. 2019, 42, 19–29. [Google Scholar] [CrossRef]
- Keykha, H.A.; Romiani, H.M.; Zebardast, E.; Asadi, A.; Kawasaki, S. CO2-induced carbonate minerals as soil stabilizing agents for dust suppression. Aeolian Res. 2021, 52, 100731. [Google Scholar] [CrossRef]
- Haldar, S.K. Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Renard, F.; Putnis, C.V.; Montes-Hernandez, G.; King, H.E. Siderite dissolution coupled to iron oxyhydroxide precipitation in the presence of arsenic revealed by nanoscale imaging. Chem. Geol. 2017, 449, 123–134. [Google Scholar] [CrossRef]
- Romiani, H.M.; Keykha, H.A.; Talebi, M.; Asadi, A.; Kawasaki, S. Green soil improvement: Using carbon dioxide to enhance the behaviour of clay. Proc. Inst. Civ. Ground Improv. 2021, 176, 301–309. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int. J. Greenh. Gas Control. 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Santos, H.S.; Nguyen, H.; Venâncio, F.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Correction: Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage. Inorg. Chem. Front. 2023, 10, 2493. [Google Scholar] [CrossRef]
- Chegini, S.; Mohamadzadeh Romiani, H.; Abdeh Keykha, H. Effect of CO2-Induced Magnesium Carbonate on Improving the Behavior of Genaveh Clay. Civ. Infrastruct. Res. 2024, 10, 49–66. [Google Scholar] [CrossRef]
- Islam, M.T.; Chittoori, B.C.; Burbank, M. Evaluating the applicability of biostimulated calcium carbonate precipitation to stabilize clayey soils. J. Mater. Civ. Eng. 2020, 32, 04019369. [Google Scholar] [CrossRef]
- Kannan, K.; Bindu, J.; Vinod, P. Engineering behaviour of MICP treated marine clays. Mar. Georesources Geotechnol. 2020, 38, 761–769. [Google Scholar] [CrossRef]
- Li, B. Geotechnical Properties of Biocement Treated Sand and Clay. Ph.D. Thesis, Nanyang Technological University, Singapore, 2015. [Google Scholar]
- Ivanov, V.; Stabnikov, V. Biocoating of surfaces. In Construction Biotechnology: Biogeochemistry, Microbiology and Biotechnology of Construction Materials and Processes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 199–221. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Cao, Z.; Wu, L.; Chu, J. Study on the influence of magnesium/calcium ratios on bio-cemented sandy soils. Acta Geotech. 2024, 19, 5449–5464. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Wang, H.; Miao, L.; Cao, Z.; Wu, L. Bio-cementation for tidal erosion resistance improvement of foreshore slopes based on microbially induced magnesium and calcium precipitation. J. Rock Mech. Geotech. Eng. 2024, 16, 1696–1708. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, Z.; Wang, H.; Song, W.; Wei, R. Mechanical characteristics and water stability of silt solidified by incorporating lime, lime and cement mixture, and SEU-2 binder. Constr. Build. Mater. 2019, 214, 111–120. [Google Scholar] [CrossRef]
- Saberian, M.; Rahgozar, M.A. Geotechnical properties of peat soil stabilised with shredded waste tyre chips in combination with gypsum, lime or cement. Mires Peat 2016, 18, 1–16. [Google Scholar] [CrossRef]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-shrink and strength behaviors of lime and cement stabilized expansive organic clays. Appl. Clay Sci. 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Feng, R.; Wu, L.; Liu, D.; Wang, Y.; Peng, B. Lime-and Cement-Treated sandy lean clay for highway subgrade in China. J. Mater. Civ. Eng. 2020, 32, 04019335. [Google Scholar] [CrossRef]
- Miller, S.A.; Habert, G.; Myers, R.J.; Harvey, J.T. Achieving net zero greenhouse gas emissions in the cement industry via value chain mitigation strategies. One Earth 2021, 4, 1398–1411. [Google Scholar] [CrossRef]
- Jones, L.D.; Jefferson, I. Expansive soils. In ICE Manual of Geotechnical Engineering; ICE Publishing: London, UK, 2012. [Google Scholar]
- Driscoll, R.M.; Crilly, M.S. Subsidence Damage to Domestic Buildings: Lessons Learned and Questions Remaining; BRE: Bracknell, UK, 2000. [Google Scholar]
- ASTM D854; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D698; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D4318; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2487; Standard Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2000.
- Ladd, R.S. Preparing test specimens using undercompaction. Geotech. Test. J. 1978, 1, 16–23. [Google Scholar] [CrossRef]
- ASTM D2166; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2435/D2435M; Standard Test Methods for One-Dimensional Consolidation Properties of Soils Using Incremental Loading. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4546; Standard Test Methods for One-Dimensional Swell or Collapse of Soils. ASTM International: West Conshohocken, PA, USA, 2018.
- Osinubi, K.J.; Eberemu, A.O.; Gadzama, E.W.; Ijimdiya, T.S. Plasticity characteristics of lateritic soil treated with Sporosarcina pasteurii in microbial-induced calcite precipitation application. SN Appl. Sci. 2019, 1, 829. [Google Scholar] [CrossRef]
- Keykha, H.A.; Zangani, A.; Romiani, H.M.; Asadi, A.; Kawasaki, S.; Radmanesh, N. Characterizing microbial and CO2-induced carbonate minerals: Implications for soil stabilization in sandy environments. Minerals 2023, 13, 976. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Yoo, Y.; Kang, D.; Choi, E.; Park, J.; Huh, I.S. Morphology control of magnesium carbonate for CO2 utilization using Mg2+ ions in industrial wastewater depending on length of alkyl chain of primary alkanolamine, reaction temperature, CO2 concentration, and Mg2+/Na+ ratio. Chem. Eng. J. 2019, 370, 237–250. [Google Scholar] [CrossRef]
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]



| Properties | Values | Method |
|---|---|---|
| Specific gravity | 2.7 | ASTM D-854 [26] |
| Maximum dry density (g/cm3) | 1.49 | ASTM D-698 [27] |
| Optimum moisture content (%) | 26.5 | ASTM D-698 [27] |
| Liquid limit (%) | 57 | ASTM D-4318 [28] |
| Plastic limit (%) | 26 | ASTM D-4318 [28] |
| Plasticity index (%) | 31 | ASTM D-4318 [28] |
| Unified soil classification | CH | ASTM D-2487 [29] |
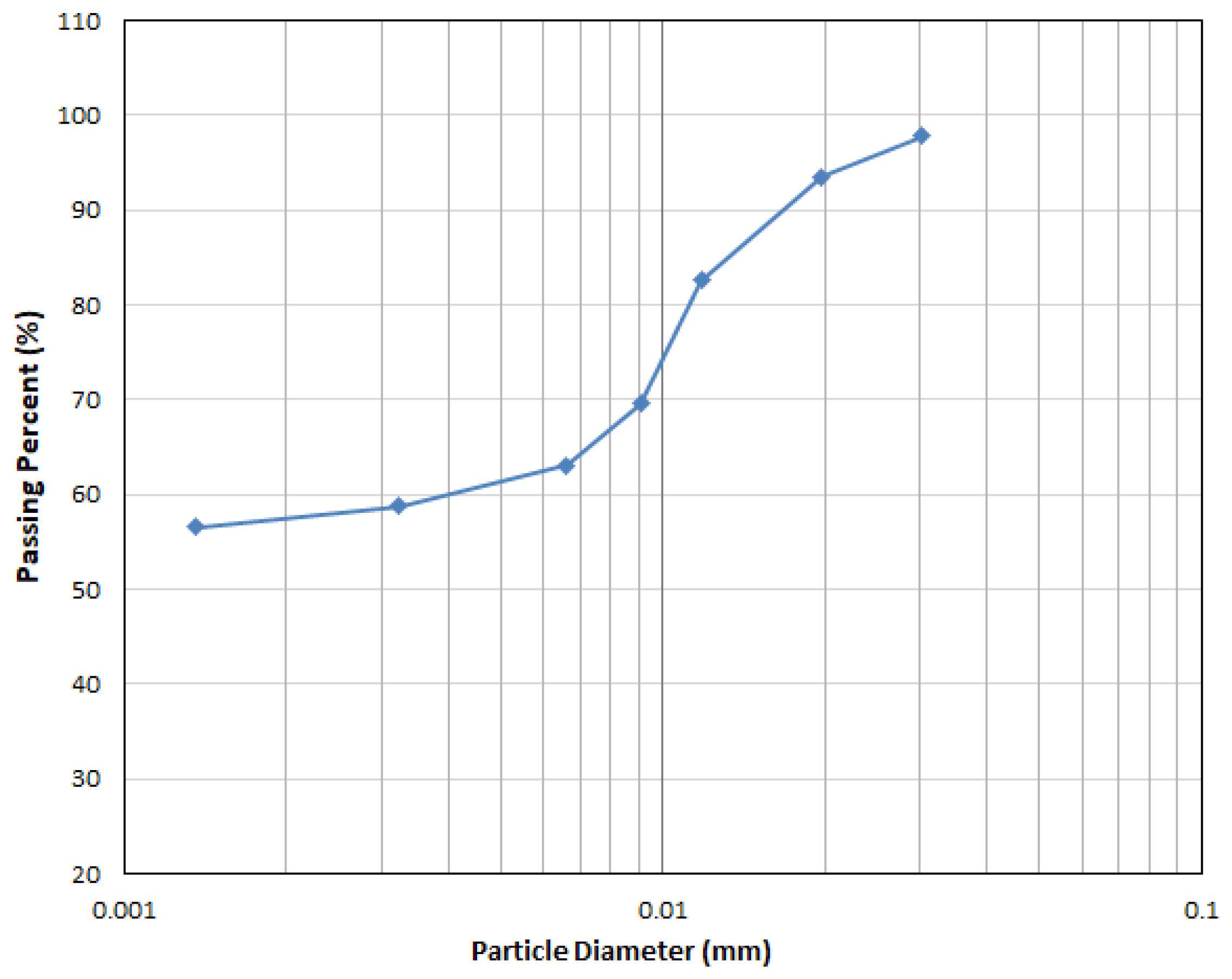
| Silt percent (%) (0.075 to 0.002 mm) | 43 | |
| Clay percent (%) (Less than 0.002 mm) | 57 |
| Mineral | Percent by Weight (%) |
|---|---|
| Calcium Carbonate (Calcite) | 28.89 |
| Silicon Oxide (Quartz) | 22.64 |
| Illite | 19.62 |
| Kaolinite | 5.24 |
| Vermiculite | 1.65 |
| Chlorite | 13.40 |
| Test | Standard | Number of Tests (No.) | Parallel Test (No.) | Carbonate Mineral (%) | Specimen Size (mm) |
|---|---|---|---|---|---|
| Atterberg limits | ASTM D4318 [28] | 4 | 3 | 0, 5, 10, 15 | - |
| Unconfined compression test | ASTM D2166 [31] | 4 | 3 | 0, 5, 10, 15 | Diameter = 37.5 Height = 75 |
| One-dimensional consolidation (Oedometer) | ASTM D2435 [32] | 4 | - | 0, 5, 10, 15 | Diameter = 50 Thickness = 20 |
| One-dimensional swelling test | ASTM D4546 [33] | 4 | - | 0, 5, 10, 15 | Diameter = 50 Thickness = 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamadzadeh Romiani, H.; Keykha, H.A.; Chegini, S.; Asadi, A.; Kawasaki, S. Utilizing Magnesium Carbonate Induced by CO2 to Modify the Performance of Plastic Clay. Minerals 2024, 14, 876. https://doi.org/10.3390/min14090876
Mohamadzadeh Romiani H, Keykha HA, Chegini S, Asadi A, Kawasaki S. Utilizing Magnesium Carbonate Induced by CO2 to Modify the Performance of Plastic Clay. Minerals. 2024; 14(9):876. https://doi.org/10.3390/min14090876
Chicago/Turabian StyleMohamadzadeh Romiani, Hadi, Hamed Abdeh Keykha, Saeed Chegini, Afshin Asadi, and Satoru Kawasaki. 2024. "Utilizing Magnesium Carbonate Induced by CO2 to Modify the Performance of Plastic Clay" Minerals 14, no. 9: 876. https://doi.org/10.3390/min14090876
APA StyleMohamadzadeh Romiani, H., Keykha, H. A., Chegini, S., Asadi, A., & Kawasaki, S. (2024). Utilizing Magnesium Carbonate Induced by CO2 to Modify the Performance of Plastic Clay. Minerals, 14(9), 876. https://doi.org/10.3390/min14090876








