The Contribution of Evaporite Layers in the Formation of the Subvolcanic Type Fe Deposit in the Emeishan Large Igneous Province, Southwestern China: Insights from the S and O Isotopic Characteristics of the Kuangshanliangzi Deposit
Abstract
1. Introduction
2. Regional Geology
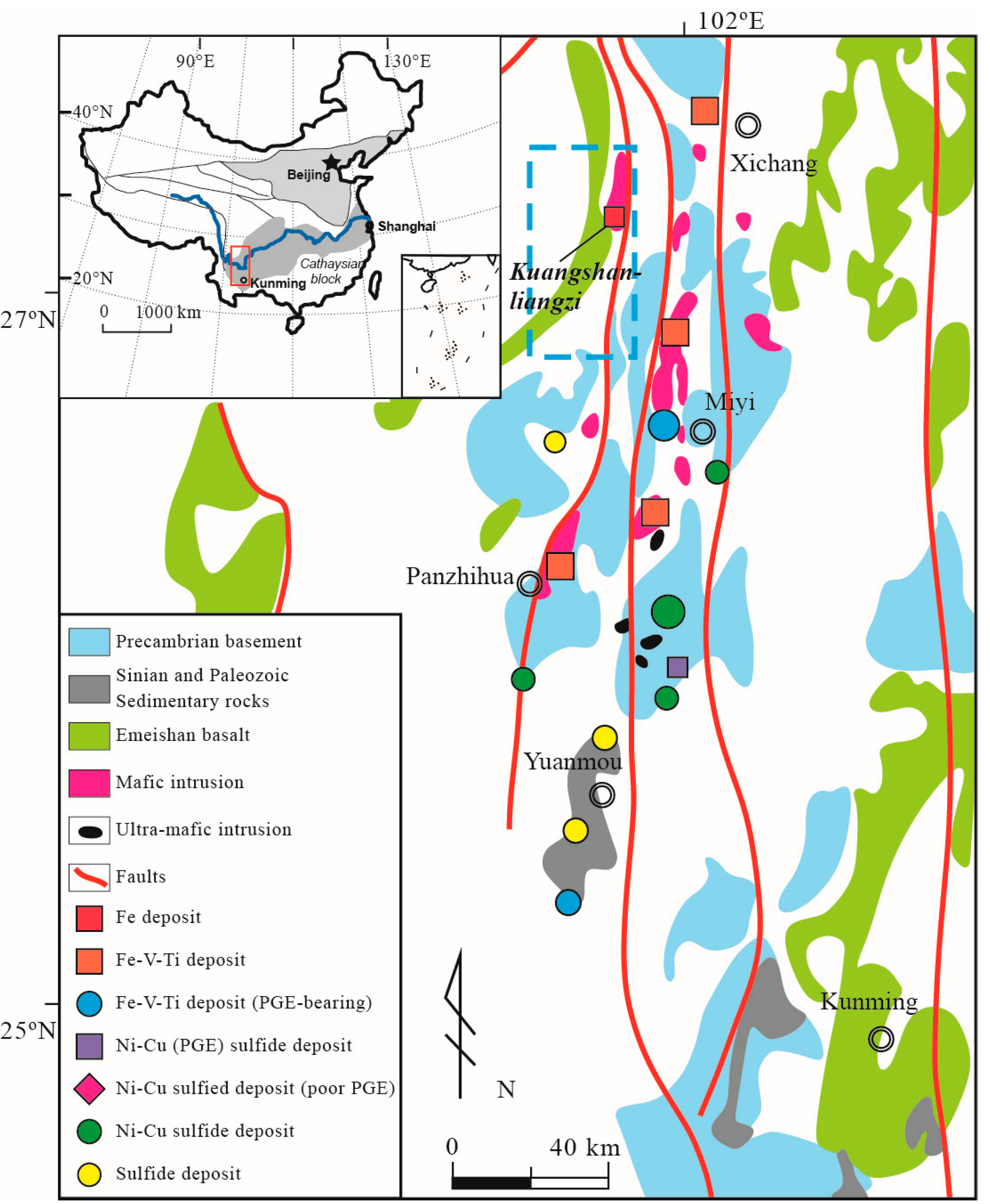

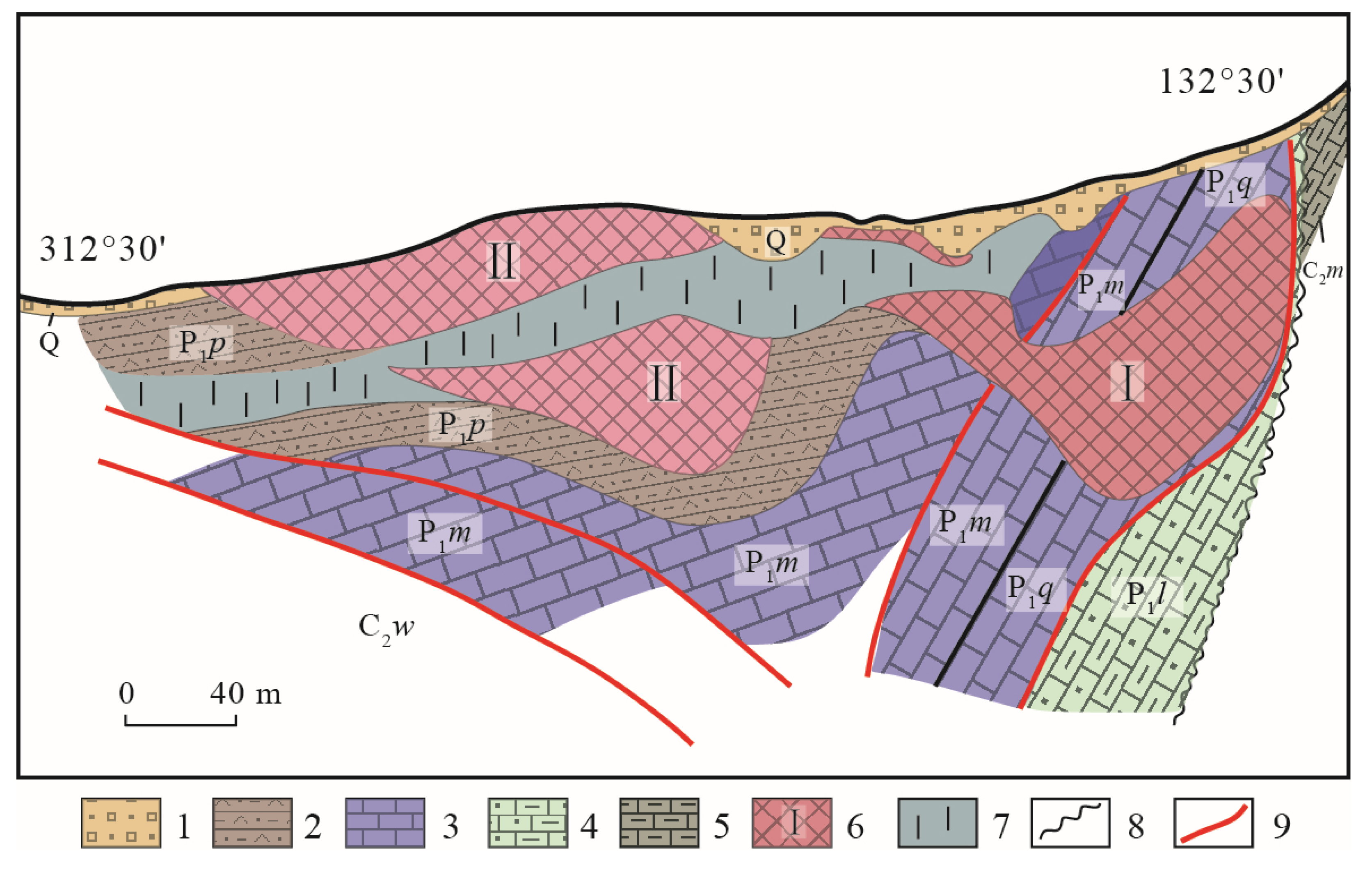
3. Deposit Geology
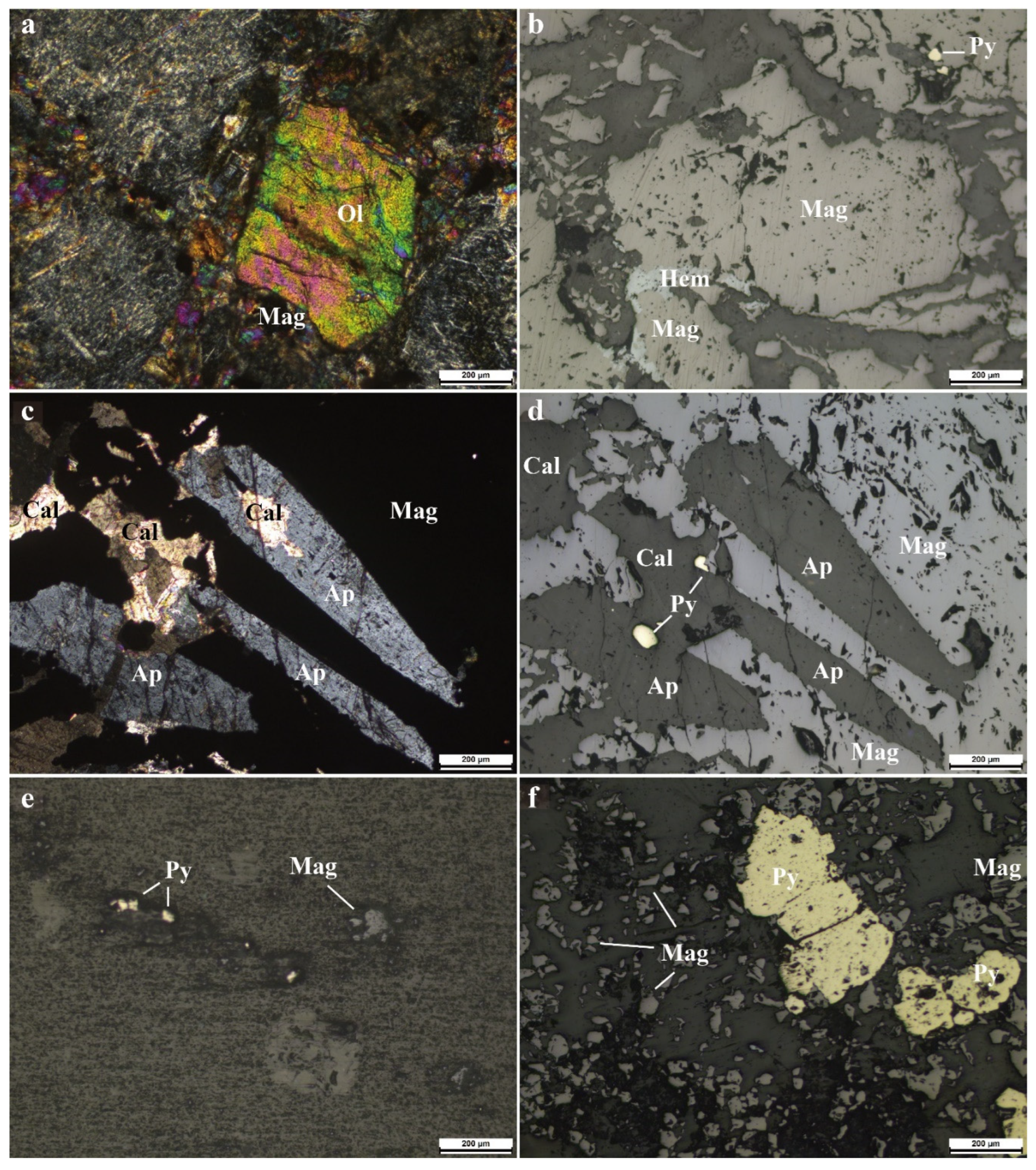
4. Analytical Methods and Results
4.1. Analytical Methods
4.2. Results
5. Discussion
5.1. Involvement of Sedimentary Rocks in Fe Mineralization
5.1.1. Sources of Sulfur
5.1.2. Oxygen Isotopes
5.2. Contribution of Evaporite Layers in Fe Mineralization
5.3. Implications for Metallogeny of Fe and Ni-Cu Sulfide in Pingchuan Area
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Ji, X.T.; Li, J.C.; Huang, M.; Kan, Z.Z. Mineral Resources of the Panzhihua Region; Sichuan Science and Technology Press: Chengdu, China, 2003; pp. 1–275. (In Chinese) [Google Scholar]
- Zhou, M.F.; Robinson, P.T.; Lesher, C.M.; Keays, R.R.; Zhang, C.J.; Malpas, J. Geochemistry, petrogenesis and metallogenesis of the Panzhihua gabbroic layered intrusion and associated V–Ti–Fe oxide deposits, Sichuan Province, SW China. J. Petrol. 2005, 46, 2253–2280. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Hou, T.; Santosh, M.; Li, H.M.; Li, J.W.; Zhang, Z.H.; Song, X.Y.; Wang, M. Spatio-temporal distribution and tectonic settings of the major iron deposits in China: An overview. Ore Geol. Rev. 2014, 57, 247–263. [Google Scholar] [CrossRef]
- Xu, Y.G.; Chung, S.L.; Jahn, B.M.; Wu, G.Y. Petrologic and geochemical constraints on the petrogenesis of Permian–Triassic Emeishan flood basalts in southwestern China. Lithos 2001, 58, 145–168. [Google Scholar] [CrossRef]
- Zhou, M.F.; Arndt, N.T.; Malpas, J.; Wang, C.Y.; Kennedy, A.K. Two magma series and associated ore deposit types in the Permian Emeishan large igneous province, SW China. Lithos 2008, 103, 352–368. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Mao, J.W.; Saunders, A.D.; Ai, Y.; Li, Y.; Zhao, L. Petrogenetic modeling of three mafic–ultramafic layered intrusions in the Emeishan large igneous province, SW China, based on isotopic and bulk chemical constraints. Lithos 2009, 113, 369–392. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Song, X.Y.; Chen, L.M.; Yu, S.Y.; Xie, W.; Deng, Y.F.; Zhang, J.F.; Gui, S.G. Chalcophile element geochemistry of the Baima layered intrusion, Emeishan Large Igneous Province, SW China: Implications for sulfur saturation history and genetic relationship with high-Ti basalts. Contrib. Mineral. Petrol. 2013, 166, 193–209. [Google Scholar] [CrossRef]
- Liu, W.H.; Zhang, J.; Sun, T.; Zhou, L.; Liu, A.L. Low-Ti iron oxide deposits in the Emeishan large igneous province related to low-Ti basalts and gabbroic intrusions. Ore Geol. Rev. 2015, 65, 180–197. [Google Scholar] [CrossRef]
- You, M.X.; Zhang, Z.W.; Liu, J.M.; Zhang, J.W.; Wang, Y.L.; Qian, B. Geochemistry of mafic-ultramafic intrusion and Cu-Ni-(PGE) sulfide deposits in Panxi region, China. Northwest. Geol. 2017, 50, 146–161, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y. Origin of the Permian Baimazhai magmatic Ni–Cu–(PGE) sulfide deposits, Yunnan: Implication for the relationship of crustal contamination and mineralization. Bull. Mineral. Petrol. Geochem. 2008, 27, 332–343, (In Chinese with English Abstract). [Google Scholar]
- Tang, Q.Y.; Ma, Y.S.; Zhang, M.J. The Origin of Ni-Cu-PGE Sulfide Mineralization in the Margin of the Zhubu Mafic-Ultramafic Intrusion in the Emeishan Large Igneous Province, Southwestern China. Econ. Geol. 2013, 108, 1889–1901. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.C.; Santosh, M.; Hou, T. Geochemistry of Late Permian picritic porphyries and associated Pingchuan iron ores, Emeishan Large Igneous Province, Southwest China: Constraints on petrogenesis and iron sources. Ore Geol. Rev. 2014, 57, 602–617. [Google Scholar] [CrossRef]
- Li, Y.H.; Duan, C.; Han, D.; Chen, X.W.; Wang, C.L.; Yang, B.Y.; Zhang, C.; Liu, F. Effect of sulfate evaporate salt layer for formation of porphyrite iron ores in the Middle-Lower Yangtze River area. Acta Petrol. Sin. 2014, 30, 1355–1368, (In Chinese with English Abstract). [Google Scholar]
- Duan, C.; Li, Y.H.; Mao, J.W.; Zhu, Q.Q.; Xie, G.Q.; Wan, Q.; Jian, W.; Hou, K.J. The role of evaporite layers in the ore-forming processes of iron oxide-apatite and skarn Fe deposits: Examples from the middle-lower Yangtze River metallogenic Belt, East China. Ore Geol. Rev. 2021, 138, 104352. [Google Scholar] [CrossRef]
- Xu, X.Y.; Bain, W.M.; Tornos, F.; Hanchar, J.M.; Lamadrid, H.M.; Lehmann, B.; Xu, X.C.; Steadman, J.A.; Bottrill, R.S.; Soleymani, M.; et al. Magnetite-apatite ores record widespread involvement of molten salts. Geology 2024, 52, 417–422. [Google Scholar] [CrossRef]
- Li, C.; Ripley, E.M.; Naldrett, A.J. Compositional variations of olivine and sulfur isotopes in the Noril’sk and Talnakh intrusions, Siberia: Implications for ore forming processes in dynamic conduits. Econ. Geol. 2003, 98, 69–86. [Google Scholar] [CrossRef]
- Li, C.; Ripley, E.M.; Naldrett, A.J.; Schnitt, A.K.; Moore, C.H. Magmatic anhydrite–sulfide assemblages in the plumbing system of the Siberian Traps. Geology 2009, 37, 259–262. [Google Scholar] [CrossRef]
- Ripley, E.M.; Li, C.; Moore, C.H.; Schmitt, A.K. Micro-scale S isotope studies of the Kharaelakh intrusion, Noril’sk region, Siberia: Constraints on the genesis of coexisting anhydrite and sulfide minerals. Geochim. Cosmochim. Acta 2010, 74, 634–644. [Google Scholar] [CrossRef]
- Ripley, E.M.; Li, C.S. Sulfide Saturation in Mafic Magmas: Is External Sulfur Required for Magmatic Ni-Cu-(PGE) Ore Genesis? Econ. Geol. 2013, 108, 45–58. [Google Scholar] [CrossRef]
- Li, Y.H.; Xie, G.Q.; Duan, C.; Han, D. Effect of sulfate evaporate salt layer over the formation of skarn-type iron ores. Acta Geol. Sin. 2013, 87, 1324–1334, (In Chinese with English Abstract). [Google Scholar]
- Li, Y.H.; Duan, C.; Fan, C.F.; Hu, B.; Wu, X.P. Effect of gypsum layer for formation of Ni-Cu-PGE sulfide deposits: A case of Noril’sk ores, Russia. Miner. Depos. 2020, 39, 619–630, (In Chinese with English Abstract). [Google Scholar]
- Malitch, K.N.; Latypov, R.M.; Badanina, I.Y.; Sluzhenikin, S.F. Insights into ore genesis of Ni- Cu- PGE sulfide deposits of the Noril’sk Province (Russia): Evidence from copper and sulfur isotopes. Lithos 2014, 204, 172–187. [Google Scholar] [CrossRef]
- Yang, S.H.; Que, M.Y. Magnetite Characteristics of Magnetite from Iron Deposits in the Xichang-Central Yunnan Region and the Genesis of the Deposits; Chongqing Press: Chongqing, China, 1987; pp. 1–105, (In Chinese with English Abstract). [Google Scholar]
- Yao, Z.D.; Yan, Y.Q. Re-understanding metallogenesis of the Kuangshan Liangzi-Niuchang magnitite ore deposits, Yanyuan, Sichuan. Acta Sichuan Geol. 1991, 11, 117–126, (In Chinese with English Abstract). [Google Scholar]
- Wang, M.; Zhang, Z.C.; Hou, T.; Luo, W.J. Geochronology and geochemistry of the Dabanshan intrusion in Panxi district and its constraints on the metallogenesis of Cu-Ni sulfide deposits. Acta Petrol. Sin. 2011, 27, 2665–2678, (In Chinese with English Abstract). [Google Scholar]
- Li, B.; Wang, S.Z.; Jing, W.C. The relationship between causing intrusions and Dabanshan mass of rock of the Pingchuan iron deposit in the Liangshan prefecture, Sichuan province. China Min. Mag. 2015, 24, 158–161, (In Chinese with English Abstract). [Google Scholar]
- Zeng, L.G.; Zhang, J.; Sun, T.; Li, B.; Zhu, G.H.; Jia, Z.C.; Fang, Q.; Chen, G.H. Geological characteristics, genesis, and its prospecting exploration enlightenment of Lanzhichang iron deposit in the Emeishan Large Igneous Province. J. Jilin Univ. 2016, 46, 412–424, (In Chinese with English Abstract). [Google Scholar]
- Liu, S.; Wang, S.Z.; Gao, L.F.; Jia, Z.W.; Ren, K.K.; Zhu, Y. The characteristics of ore fabric and its geological significance of Pingchuan iron deposit in the South of Sichuan province. China Min. Mag. 2018, 27, 182–184, (In Chinese with English Abstract). [Google Scholar]
- Sun, T.; Lu, X.S.; Zhou, X.N.; Liu, W.H.; Wang, J.; Zeng, L.G.; Fan, C. Geochemical characteristics and genesis of Daopingzi iron deposit in Panzhihuan-Xichang Region. Resour. Environ. Eng. 2021, 35, 408–417, (In Chinese with English Abstract). [Google Scholar]
- Li, H.M.; Chen, Y.C.; Li, L.X.; Wang, D.H. Metallogeny of the Iron Deposits in China; Geological Publishing House: Beijing, China, 2012; pp. 136–140, (In Chinese with English Abstract). [Google Scholar]
- Zeng, L.G.; Zhang, J.; Sun, T.; Guo, D.H. Zircon U-Pb age of mafic-ulreamafic rock from Pingchuan region in southern Sichuan and its geological implications. Earth Sci.-J. China Univ. Geosci. 2013, 38, 1197–1213, (In Chinese with English Abstract). [Google Scholar]
- Zeng, L.G. A Study on Pingchuan Iron Deposit in Metallogenic Regularity of Yanyuan County, Sichuan Province. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2011. [Google Scholar]
- Yu, S.Y.; Song, X.Y.; Ripley, E.M.; Li, C.S.; Chen, L.M.; She, Y.W.; Luan, Y. Integrated O-Sr-Nd isotope constraints on the evolution of four important Fe-Ti oxide ore-bearing mafic-ultramafic intrusions in the Emeishan large igneous province, SW China. Chem. Geol. 2015, 401, 28–42. [Google Scholar] [CrossRef]
- Jiang, X.D.; Wei, G.F.; Nie, J.T. Jianchaling nickel deposit: Magmatic or hydrothermal origin. Miner. Depos. 2010, 38, 1197–1213, (In Chinese with English Abstract). [Google Scholar]
- Tornos, F.; Velasco, F.; Hanchar, J.M. Iron-rich melts, magmatic magnetite, and superheated hydrothermal systems: The El Laco deposit, Chile. Geology 2016, 44, 427–430. [Google Scholar] [CrossRef]
- Li, Y.H.; Duan, C.; Han, D.; Liu, F.; Wan, D.F.; Wang, C.Y. Oxygen isotopic discriminant marker of magmatic iron deposits: Ningwu porphyrite iron ore as an example. Acta Petrol. Sin. 2017, 33, 3411–3421, (In Chinese with English Abstract). [Google Scholar]
- Zhu, G.Y.; Zhang, S.C.; Liang, Y.B.; Li, Q.R. Genesis and evidence of hydrogen sulfide in Weiyuan gas field, Sichuan Basin. Chin. Sci. Bull. 2006, 51, 2780–2788, (In Chinese with English Abstract). [Google Scholar]
- Xing, C.M.; Wang, C.Y.; Zhang, M.J. Volatile and C-H-O isotopic compositions of giant Fe-Ti-V oxide deposits in the Panxi region and their implications for the sources of volatiles and the origin of Fe-Ti oxide ores. Sci. China Earth Sci. 2012, 55, 1782–1795. [Google Scholar] [CrossRef]
- Wu, X.P.; Fan, C.F.; Hu, B.; Gao, J.F.; Li, Y.H. Measurements of sulfur isotope composition on ultrasmall (80 nanomole) sulfide and sulfate samples by a modified EA-IRMS. Acta Geol. Sin. Engl. Ed. 2022, 96, 1792–1799. [Google Scholar] [CrossRef]
- Li, Y.H.; Wan, D.F.; Zhao, X.Y. Geological and Mineral Industry Standards of the People’s Republic of China (DZ/T 0184.23-2024)—Isotope Analysis Methods for Geological Samples, Part 27: Determination of Oxygen Isotope Composition of Silicate and Oxide Minerals Using Bromine Pentafluoride Method; Geological Publishing House: Beijing, China, 2024. (In Chinese) [Google Scholar]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–45. [Google Scholar] [CrossRef]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Zhu, Q.Q.; Xie, G.Q. Sulfur isotopic character and geological implications for the Jinshandian Fe skarn ore field, Hubei Province. Acta Petrol. Sin. 2018, 34, 2518–2534, (In Chinese with English Abstract). [Google Scholar]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in metal oxides. Geochim. Cosmochim. Acta 1991, 55, 2299–2307. [Google Scholar]
- Taylor, H.P. The oxygen isotope geochemistry of igneous rocks. Contrib. Mineral. Petrol. 1968, 19, 1–71. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Valley, J.W. Oxygen isotope study of the long Valley-Glass Mountain magmatic system, California: Isotope thermometry, and t convection in large silicic magma bodies. Contrib. Mineral. Petrol. 2002, 144, 185–205. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Valley, J.W. Rapid generation of both high- and low-δ18O, large volume silicic magmas at Timber Mountain/Oasis Valley caldera complex, Nevada. Bull. Geol. Soc. Am. 2003, 115, 581–595. [Google Scholar] [CrossRef]
- Taylor, H.P. Oxygen isotope studies of hydrothermal mineral deposits. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Holt, Rinehart & Winston Inc.: New York, NY, USA, 1967; pp. 109–142. [Google Scholar]
- Zhao, Z.F.; Zheng, Y.F. Calculation of oxygen isotope fractionation in magmatic rocks. Chem. Geol. 2003, 193, 59–80. [Google Scholar] [CrossRef]
- Canil, D.; Lacourse, T. Geothermometry using minor and trace elements in igneous and hydrothermal magnetite. Chem. Geol. 2020, 541, 119576. [Google Scholar] [CrossRef]
- Hou, T.; Charlier, B.; Holtz, F.; Veksler, I.; Zhang, Z.C.; Thomas, R.; Namur, O. Immiscible hydrous Fe–Ca–P melt and the origin of iron oxide-apatite ore deposits. Nat. Commun. 2018, 9, 1415–1423. [Google Scholar] [CrossRef]
- Whitney, J.A.; Hemley, J.; Simon, F.O. The concentration of iron in chloride solutions equilibrated with synthetic granitic compositions: The sulfur-free system. Econ. Geol. 1985, 80, 444–460. [Google Scholar] [CrossRef]
- Hemley, J.J.; Cygan, G.L.; Fein, J.B.; Robinson, G.R.; d’Angelo, W.M. Hydrothermal ore-forming processes in the light of studies in rock-buffered systems; I, iron-copper-zinc-lead sulfde solubility relations. Econ. Geol. 1992, 87, 23–43. [Google Scholar] [CrossRef]
- Borisov, A.; Behrens, H.; Holtz, F. Effects of strong network modifiers on Fe3+/Fe2+ in silicate melts: An experimental study. Contrib. Mineral. Petrol. 2017, 172, 34. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Burrows, D.R. New field evidence bearing on the origin of the El Laco magnetite deposit, Northern Chile. Econ. Geol. 2002, 75, 1101–1109. [Google Scholar]
- Tornos, F.; Velasco, F.; Hanchar, J.M. The magmatic to magmatic-hydrothermal evolution of the El Laco deposit (Chile) and its implications for the genesis of magnetite-apatite deposits. Econ. Geol. 2017, 112, 1595–1628. [Google Scholar] [CrossRef]
- Mungall, J.E.; Long, K.; Brenan, J.M.; Smythe, D.; Naslund, H.R. Immiscible shoshonitic and Fe-P-oxide melts preserved in unconsolidated tephra at El Laco volcano, Chile. Geology 2018, 46, 255–258. [Google Scholar] [CrossRef]
- Xie, Q.H.; Zhang, Z.C.; Hou, T.; Cheng, Z.G.; Eduardo, C.; Wang, Z.C.; Fei, X.H. New insights for the formation of Kiruna-type iron deposits by immiscible hydrous Fe-P Melt and high-temperature hydrothermal processes: Evidence from El Laco deposit. Econ. Geol. 2019, 114, 35–46. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Duan, C.; Fan, C. Involvement of Evaporite Layers in the Formation of Iron Oxide-Apatite Ore Deposits: Examples from the Luohe Deposit in China and the El Laco Deposit in Chile. Minerals 2022, 12, 1043. [Google Scholar] [CrossRef]
- Ripley, E.M.; Taib, N.I.; Li, C.; Moore, C.H. Chemical and mineralogical heterogeneity in the basal zone of the Partridge River intrusion: Implications for the origin of Cu-Ni sulfide mineralization in the Duluth Complex, Midcontinent Rift System. Contrib. Mineral. Petrol. 2007, 154, 35–54. [Google Scholar] [CrossRef]
- Keays, R.R. The role of komatiitic and picritic magmatism and S-saturation in the formation of ore deposits. Lithos 1995, 34, 1–18. [Google Scholar] [CrossRef]
- Naldrett, A.J.; Fedorenko, V.A.; Asif, M.; Lin, S.; Kunilov, V.A.; Stekhin, A.I.; Lightfoot, P.C.; Gorbachev, N.S. Controls on the compositions of Ni-Cu sulfide deposits as illustrated by those at Noril’sk, Siberia. Econ. Geol. 1996, 91, 751–773. [Google Scholar] [CrossRef]
- Naldrett, A.J. Magmatic Sulfide Deposits: Geology, Geochemistry, and Exploration; Springer: Berlin, Germany, 2004; pp. 1–727. [Google Scholar]
- Keays, R.R.; Lightfoot, P.C. Crustal sulfur is required to form magmatic Ni-Cu sulfide deposits: Evidence from chalcophile element signatures of Siberian and Deccan Trap basalts. Miner. Depos. 2010, 45, 241–257. [Google Scholar] [CrossRef]
- Godel, B.; Seat, Z.; Maier, W.D.; Barne, S.J. The Nebo-BabelNiCu-PGE sulfide deposit (West Musgrave block, Australia): Pt. 2. Constraintson parental magma and processes, with implications for mineral exploration. Econ. Geol. 2011, 106, 557–584. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhu, D.; Xiao, J.F.; Zhu, W.G.; Chen, L.M. New insights on the formation of magmatic sulfide deposits in magma conduit system. Earth Sci. Front. 2010, 17, 153–163, (In Chinese with English Abstract). [Google Scholar]
- Song, X.Y. Current research status and important issues of magmatic sulfide deposits. Miner. Depos. 2019, 38, 699–712, (In Chinese with English Abstract). [Google Scholar]
- Mapiloko, M.; Yudovskaya, M.; McCreesh, M.; Velivetskaya, T.; Nex, P.; Kinnaird, J.; Montjoie, R.; Bekker, A. Contribution of the oldest Paleoproterozoic marine sulfate evaporites to Bushveld Complex Lower Zone mineralization. Geology 2025, 53, 355–359. [Google Scholar] [CrossRef]


| Deposit/Formation | Samples | Lithology | Minerals | δ18OV-SMOW (‰) | δ13CV-PDB (‰) | δ34SV-CDT (‰) | References |
|---|---|---|---|---|---|---|---|
| Kuangshanliangzi | KSLZ13-1 | Sulfide-bearing magnetite ore | Pyrite | 19.9 | This study | ||
| Kuangshanliangzi | KSLZ13-2 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 7.9 | 3.3 | This study | |
| Kuangshanliangzi | KSLZ13-3 | Sulfide-bearing magnetite ore | Magnetite | 8.6 | This study | ||
| Kuangshanliangzi | KSLZ13-4 | Picrite porphyrite with Fe mineralization | Pyrite | 20.6 | This study | ||
| Kuangshanliangzi | KSLZ13-5 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 6.5 | 23.6 | This study | |
| Kuangshanliangzi | KSLZ13-7 | Near ore country rock | Pyrite | 14.0 | This study | ||
| Kuangshanliangzi | KSLZ13-14 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 3.7 | 21.2 | This study | |
| Kuangshanliangzi | KSLZ13-16 | Sulfide-bearing magnetite ore | Pyrite | 20.1 | This study | ||
| Kuangshanliangzi | KSLZ13-17 | Picrite porphyrite with actinolite alteration | Magnetite | 6.6 | This study | ||
| Kuangshanliangzi | KSLZ13-19 | Pyrite vein | Pyrite | 11.8 | This study | ||
| Kuangshanliangzi | KSLZ13-21 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 5.2 | 21.3 | This study | |
| Kuangshanliangzi | DF-4(1) | Breccia magnetite ore | Magnetite | 8.6 | [23] | ||
| Kuangshanliangzi | DF-4(2) | Breccia magnetite ore | Magnetite | 8.8 | [23] | ||
| Kuangshanliangzi | DF-12(1) | Magnetite ore | Magnetite | 8.5 | [23] | ||
| Kuangshanliangzi | DF12-(2) | Magnetite ore | Magnetite | 10.3 | [23] | ||
| Kuangshanliangzi | DF-22 | Magnetite ore | Magnetite | 5.6 | [23] | ||
| Kuangshanliangzi | DF-3 | Siderite–magnetite ore | Pyrrhotite | 11.7 | [23] | ||
| Kuangshanliangzi | DF-6 | Massive pyrite ore | Pyrite | 5.1 | [23] | ||
| Kuangshanliangzi | DF-7(a) | Siderite–magnetite ore | Pyrite | 14.8 | [23] | ||
| Kuangshanliangzi | DF-7(b) | Siderite–magnetite ore | Pyrrhotite | 16.0 | [23] | ||
| Kuangshanliangzi | DF-8 | Siderite–magnetite ore | Pyrite | 18.0 | [23] | ||
| Kuangshanliangzi | DF-9 | Breccia pyrite ore | Pyrite | 16.1 | [23] | ||
| Kuangshanliangzi | DF-11 | Breccia pyrite ore | Pyrite | 20.6 | [23] | ||
| Kuangshanliangzi | DF-14 | Near ore country rock | Pyrite | 15.0 | [23] | ||
| Kuangshanliangzi | DF-16 | Pyrite–magnetite ore | Pyrite | 10.6 | [23] | ||
| Kuangshanliangzi | DF-17(a) | Breccia pyrite ore | Pyrite | 5.5 | [23] | ||
| Kuangshanliangzi | DF-17(b) | Breccia pyrite ore | Pyrrhotite | 9.0 | [23] | ||
| Kuangshanliangzi | DF-19(1) | Magnetite ore | Siderite | 18.4 | −1.6 | [23] | |
| Kuangshanliangzi | DF-19(2) | Magnetite ore | Siderite | 15.5 | −1.8 | [23] | |
| Kuangshanliangzi | DF-23 | Magnetite ore | Siderite | 19.5 | −2.5 | [23] | |
| Kuangshanliangzi | DF-24 | Magnetite ore | Siderite | 19.0 | −2.5 | [23] | |
| Kuangshanliangzi | DF-25 | Magnetite ore | Siderite | 19.3 | −2.6 | [23] | |
| Kuangshanliangzi | DF-26 | Magnetite ore | Siderite | 19.7 | −1.7 | [23] | |
| Panzhihua | PZH13-15 | Magnetite ore | Magnetite | 2.3 | This study | ||
| Panzhihua | PZH13-16 | Magnetite ore | Magnetite | 0.9 | This study | ||
| Panzhihua | PZH13-17 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.4 | 5.5 | This study | |
| Panzhihua | PZH13-18 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 2.0 | 1.1 | This study | |
| Panzhihua | PZH13-19 | Magnetite ore | Magnetite | 0.3 | This study | ||
| Panzhihua | PZH13-24 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.3 | 2.3 | This study | |
| Panzhihua | PZH13-29 | Magnetite ore | Magnetite | 0.4 | This study | ||
| Panzhihua | PZH13-39 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.5 | 0.4 | This study | |
| Panzhihua | PZH13-40 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.6 | −0.4 | This study | |
| Panzhihua | PZH13-43 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.1 | 0.1 | This study | |
| Panzhihua | PZH13-45 | Sulfide-bearing magnetite ore | Magnetite–pyrite | 1.7 | 0.6 | This study | |
| Panzhihua | Magnetite ore | Magnetite | 1.2~5.0 (2.7) | [33] | |||
| Jianchaling | Cu-Ni ore | Pyrite | 9.9~13.3 (11.68) | [34] | |||
| El Laco, Chile | Magnetite ore | Magnetite | 4.3~5.0 | [35] | |||
| Gushan | Magnetite–hematite ore | Magnetite–hematite | −1.2~5.2 (1.81) | [36] | |||
| Dengying Formation | Evaporite layer | Gypsum | 20.8~22.5 (21.8) | [37] | |||
| Dengying Formation | Carbonate | Limestone; dolomite | 20~29 | −2~4 | [38] |
| Sample | δ34S | 2SE | Sample | δ34S | 2SE |
|---|---|---|---|---|---|
| KSLZ13-6-1 | 19.68 | 0.13 | KSLZ13-13-3 | 14.15 | 0.08 |
| KSLZ13-6-2 | 20.31 | 0.18 | KSLZ13-13-4 | 22.39 | 0.10 |
| KSLZ13-6-3 | 13.67 | 0.10 | KSLZ13-13-5 | 21.49 | 0.09 |
| KSLZ13-6-4 | 20.10 | 0.06 | KSLZ13-1-1 | 15.93 | 0.21 |
| KSLZ13-6-5 | 19.82 | 0.10 | KSLZ13-1-2 | 15.44 | 0.29 |
| KSLZ13-3-1 | 18.78 | 0.07 | KSLZ13-1-3 | 16.13 | 0.13 |
| KSLZ13-3-2 | 21.74 | 0.08 | KSLZ13-1-4 | 16.17 | 0.17 |
| KSLZ13-3-3 | 22.25 | 0.09 | KSLZ13-1-5 | 17.05 | 0.11 |
| KSLZ13-16-1 | 13.93 | 0.08 | KSLZ13-21-1 | 15.20 | 0.24 |
| KSLZ13-16-2 | 18.95 | 0.10 | KSLZ13-21-2 | 13.39 | 0.31 |
| KSLZ13-16-3 | 20.72 | 0.09 | KSLZ13-21-3 | 15.42 | 0.26 |
| KSLZ13-13-1 | 22.68 | 0.07 | KSLZ13-21-4 | 16.27 | 0.10 |
| KSLZ13-13-2 | 21.37 | 0.09 | KSLZ13-21-5 | 15.43 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Q.; Duan, C.; Li, Y.; Hu, B.; Hou, K.; Wang, T. The Contribution of Evaporite Layers in the Formation of the Subvolcanic Type Fe Deposit in the Emeishan Large Igneous Province, Southwestern China: Insights from the S and O Isotopic Characteristics of the Kuangshanliangzi Deposit. Minerals 2025, 15, 456. https://doi.org/10.3390/min15050456
Wan Q, Duan C, Li Y, Hu B, Hou K, Wang T. The Contribution of Evaporite Layers in the Formation of the Subvolcanic Type Fe Deposit in the Emeishan Large Igneous Province, Southwestern China: Insights from the S and O Isotopic Characteristics of the Kuangshanliangzi Deposit. Minerals. 2025; 15(5):456. https://doi.org/10.3390/min15050456
Chicago/Turabian StyleWan, Qiu, Chao Duan, Yanhe Li, Bin Hu, Kejun Hou, and Tianshun Wang. 2025. "The Contribution of Evaporite Layers in the Formation of the Subvolcanic Type Fe Deposit in the Emeishan Large Igneous Province, Southwestern China: Insights from the S and O Isotopic Characteristics of the Kuangshanliangzi Deposit" Minerals 15, no. 5: 456. https://doi.org/10.3390/min15050456
APA StyleWan, Q., Duan, C., Li, Y., Hu, B., Hou, K., & Wang, T. (2025). The Contribution of Evaporite Layers in the Formation of the Subvolcanic Type Fe Deposit in the Emeishan Large Igneous Province, Southwestern China: Insights from the S and O Isotopic Characteristics of the Kuangshanliangzi Deposit. Minerals, 15(5), 456. https://doi.org/10.3390/min15050456








