Controls on the Transformation of Clay Minerals in the Miocene Evaporite Deposits of the Ukrainian Carpathian Foredeep
Abstract
1. Introduction
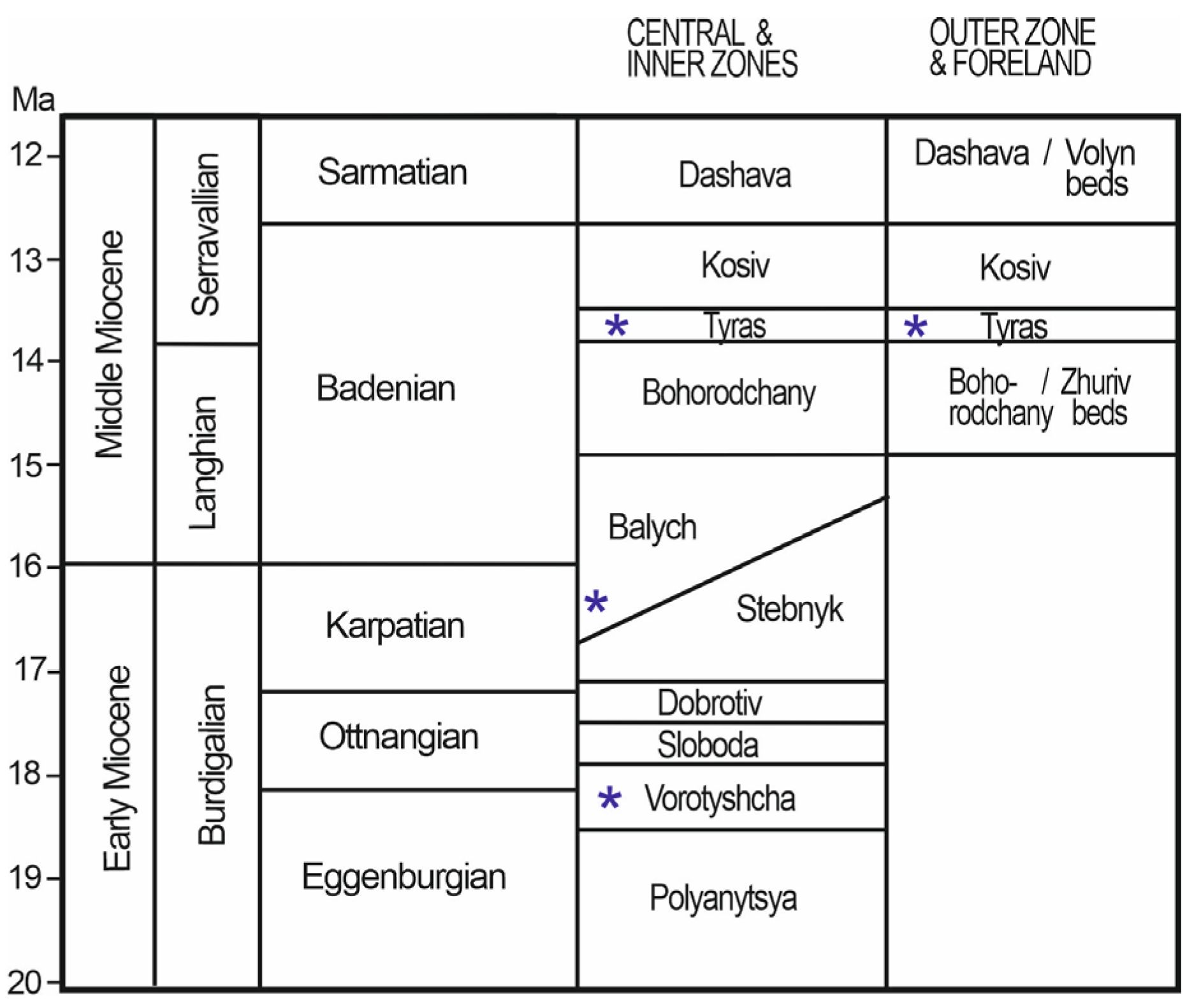
Geological Setting
2. Materials and Methods
2.1. Materials
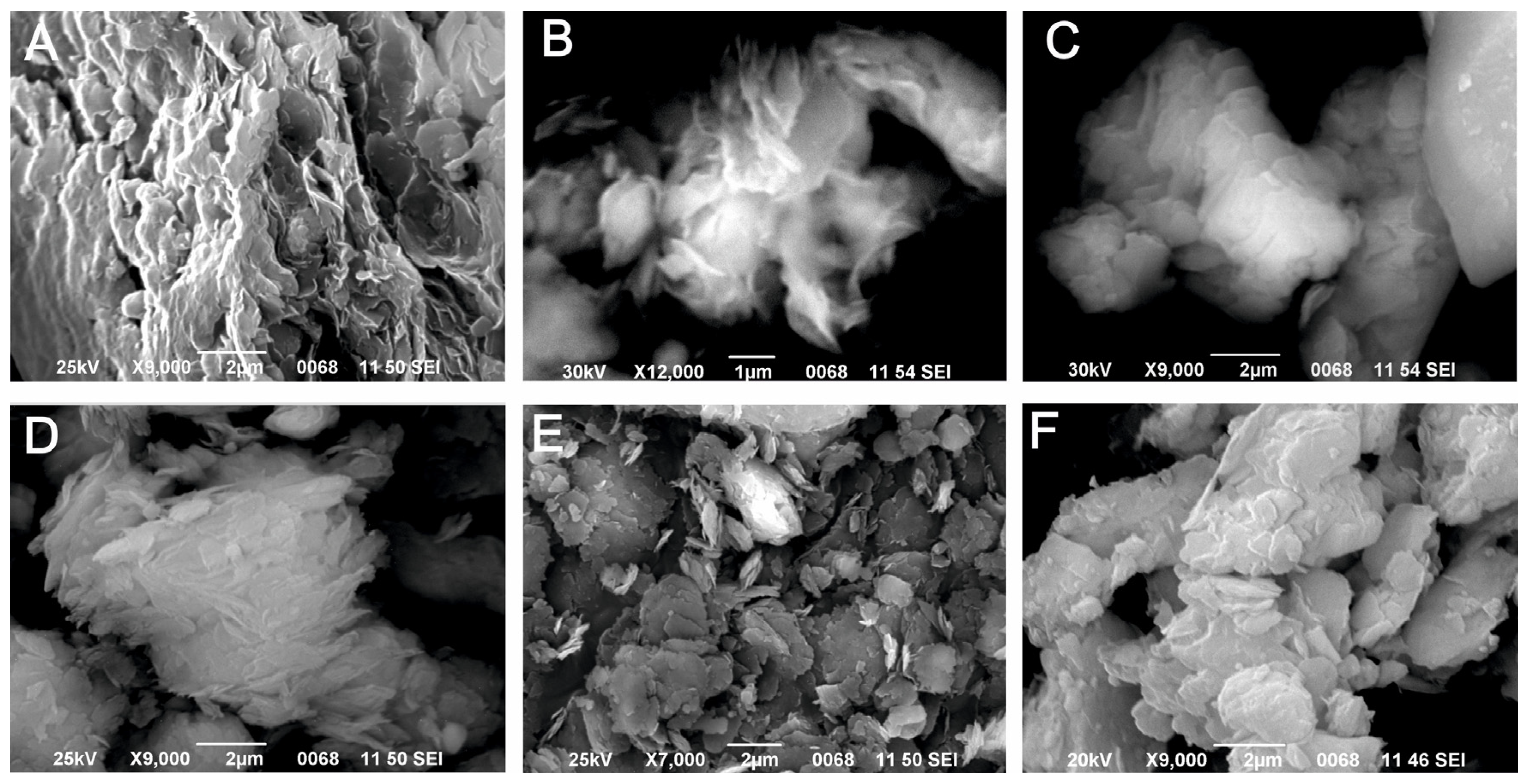
2.2. Methods
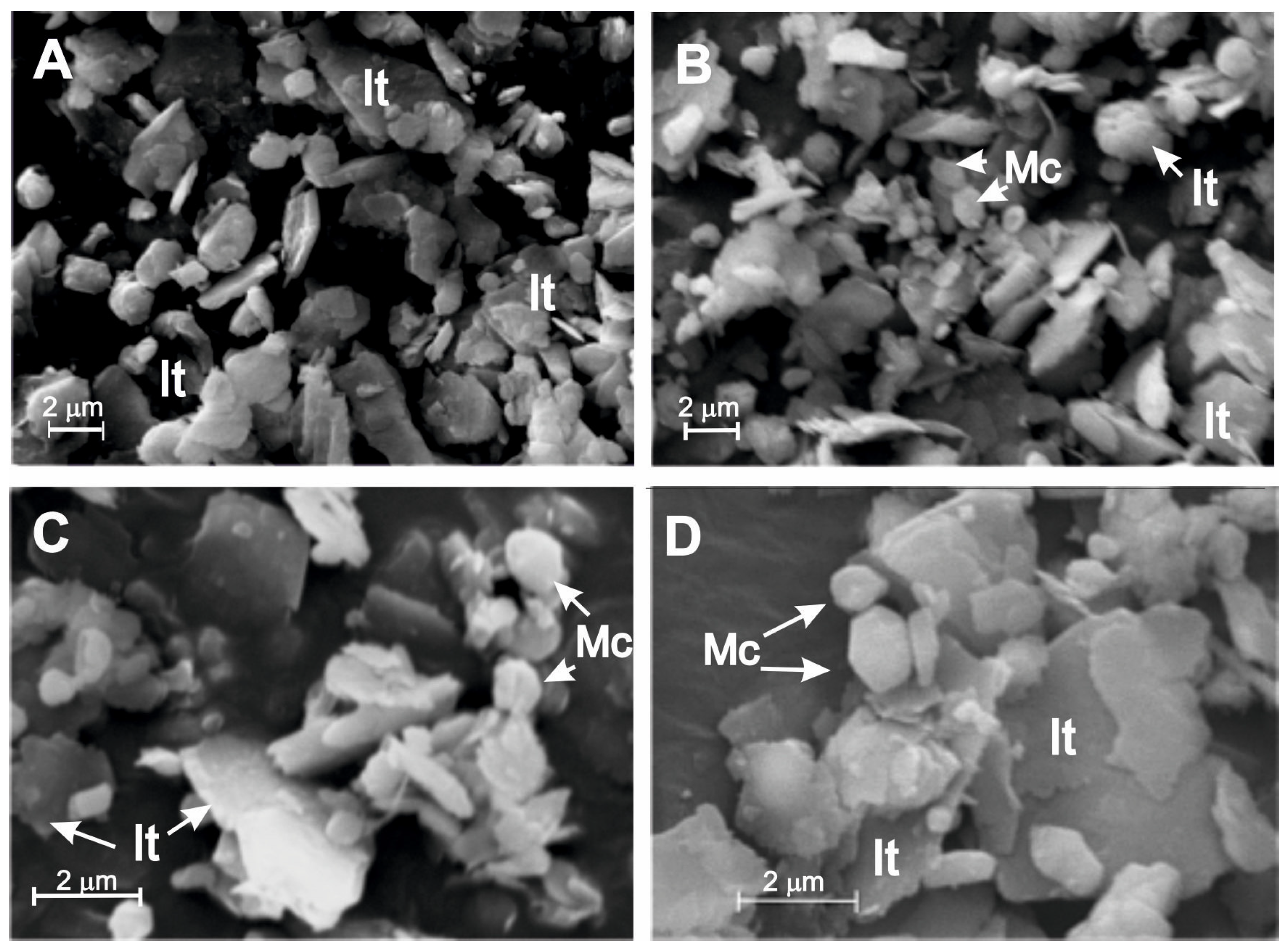
3. Results
3.1. Gypsum Facies
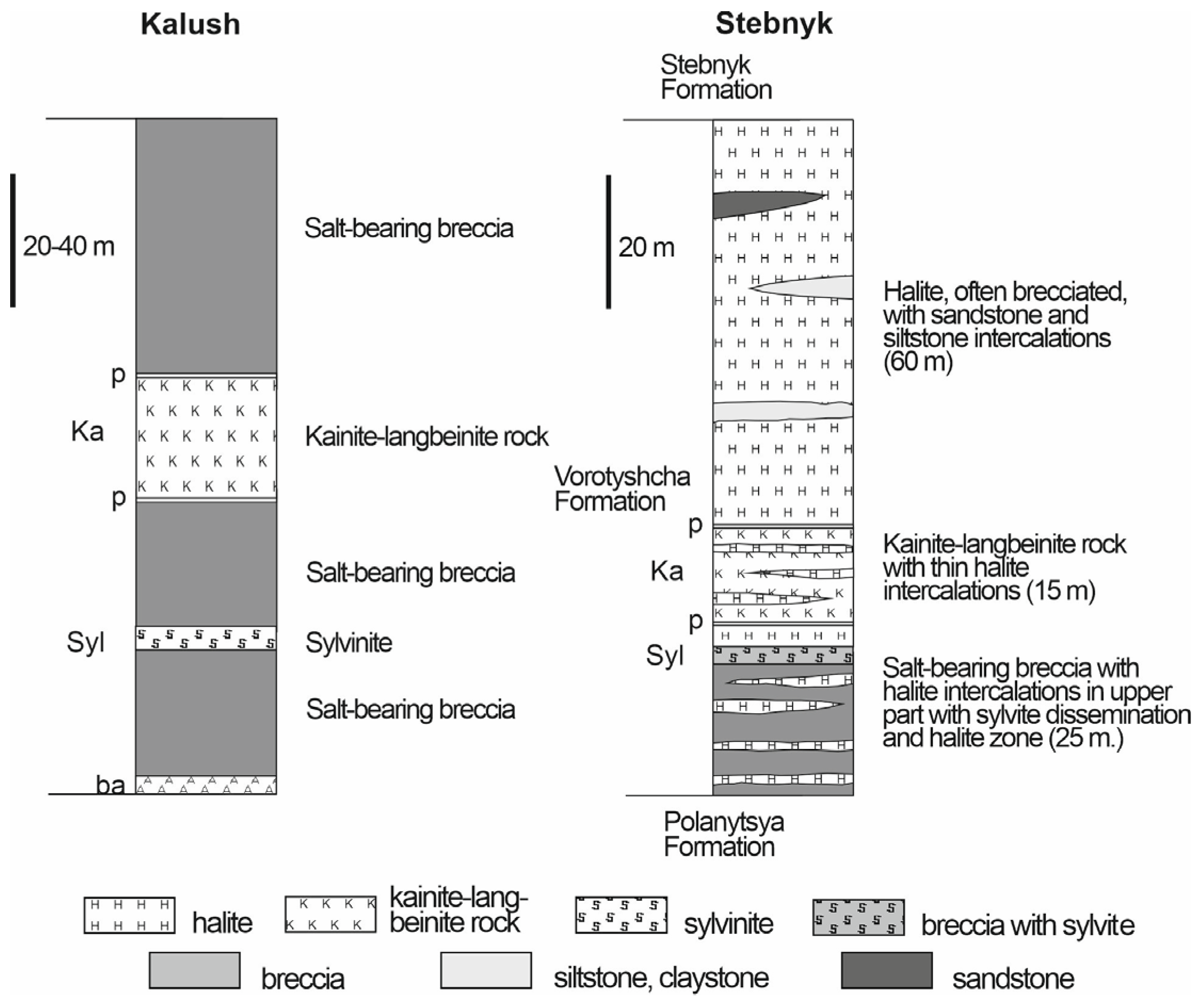
3.2. Halite Facies
3.3. Potash Facies
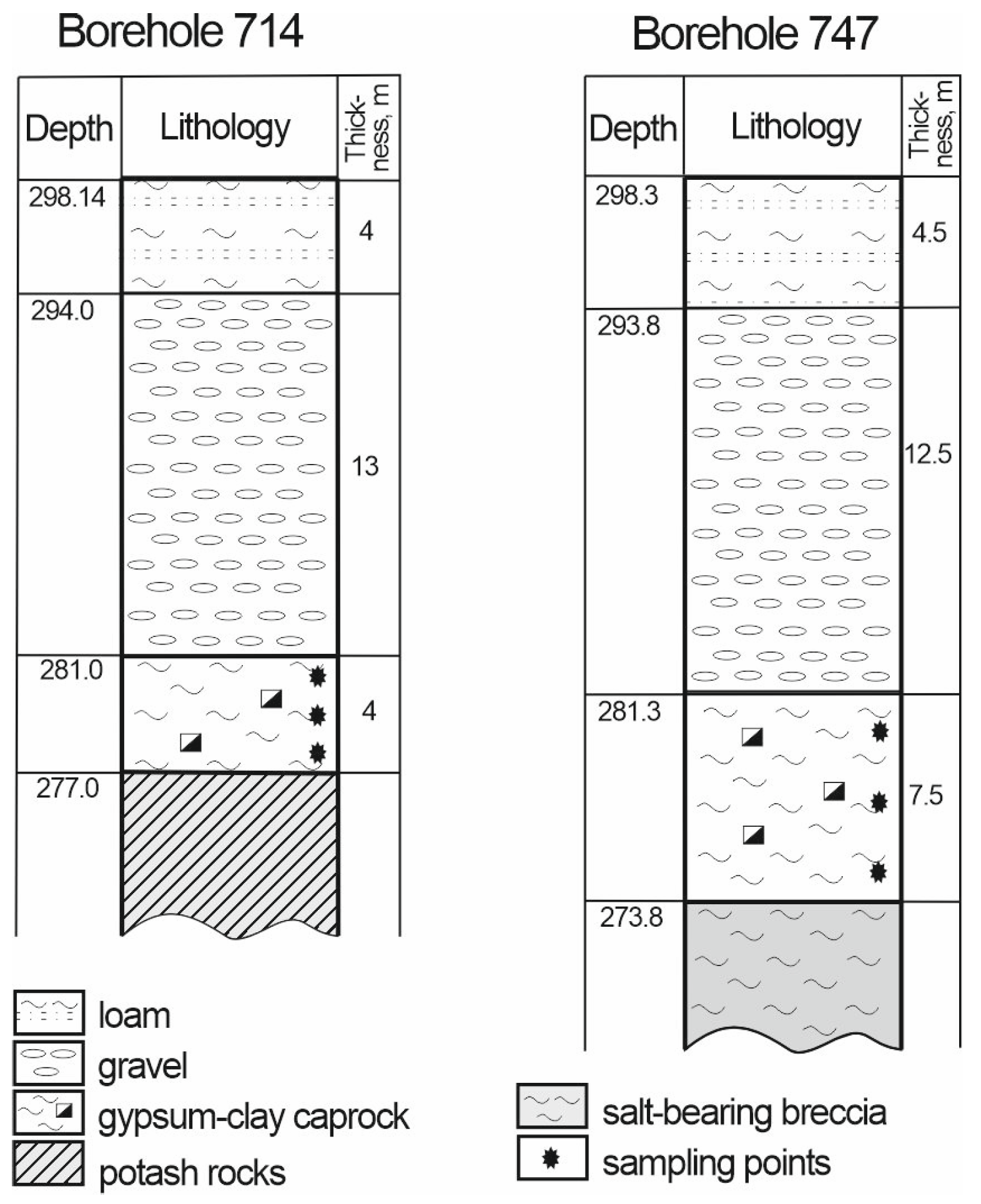
3.4. Weathering Zone of the Kalush-Holyn Deposit
4. Interpretation
4.1. Brine Concentration Control
4.2. Organic Matter Control
4.3. Fresh Water Control During Hypergenesis
4.4. Burial Depth and Geothermal Regime Control
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millot, G. Geology of Clays: Weathering, Sedimentology, Geochemistry; Springer: New York, NY, USA; Berlin, Germany, 1970; p. 429. [Google Scholar]
- Eberl, D.D.; Farmer, V.C.; Barrer, R.M. Clay Mineral Formation and Transformation in Rocks and Soils [and Discussion]. Philos. Trans. R. Soc. Lond. 1984, 311, 241–257. [Google Scholar]
- Lucas, J. La transformation des minéraux argileux dans la sédimentation. Etudes sur les argiles du Trias. Mém. Serv. Carte Géol. d’Alsace Lorraine 1962, 23, 1–202. [Google Scholar]
- Millot, G.; Lucas, J.; Paquet, H. Evolution géochimique par dégradation et agradation des minéraux argileux dans l’hydrosphère. Geol. Rundsch. 1966, 55, 1–20. [Google Scholar] [CrossRef]
- Dunoyer de Segonzac, G. The transformation of clay minerals during diagenesis and low-grade metamorphism: A review. Sedimentology 1970, 15, 281–346. [Google Scholar] [CrossRef]
- Lanson, B.; Sakharov, B.A.; Claret, F.; Drits, V.A. Diagenetic smectite-to-illite transition in clay-rich sediments: A reappraisal of X-ray diffraction results using the multi-specimen method. Am. J. Sci. 2009, 309, 476–516. [Google Scholar] [CrossRef]
- Bodine, M.W., Jr. Trioctahedral Clay Mineral Assemblages in Paleozoic Marine Evaporite Rocks. In Sixth International Symposium on Salt; Salt Institute: Alexandria, VA, USA, 1985; Volume 1, pp. 267–284. [Google Scholar]
- Warren, J.K. Evaporites: A Compendium; Springer: Berlin/Heidelberg, Germany, 2016; p. 1854. [Google Scholar]
- Kossovskaya, A.G.; Drits, V.A. Kristallokhimiya dioktaedricheskikh slyud, khloritov i korrensitov kak indikatorov geologicheskikh obstanovok. In Problemy Litologii i Geokhimii Osadochnykh Porod i Rud; Nauka: Moskva, Russia, 1975; pp. 60–69. (In Russian) [Google Scholar]
- Sokolova, T.N. Autigennoe silikatnoe mineraloobrazovanie raznykh stadiy osolonennya. Tr. GIN 1982, 361, 1–164. (In Russian) [Google Scholar]
- Bilonizhka, P.M. The clay minerals as indicators of salt deposition conditions in Precarpathian Foredeep. Geol. Geochem. Comb. Min. 1992, 78, 95–102, (In Ukrainian with English abstract). [Google Scholar]
- Bilonizhka, P.M. The transformation of terrigenous clay minerals in the saliferous process. Miner. Zb. 1992, 45, 51–56, (In Ukrainian with English abstract). [Google Scholar]
- Calvo, J.P.; Blanc-Valleron, M.M.; Rodriguez Arandia, J.P.; Rouchy, J.M.; Sanz, M.E. Authigenic clay minerals in continental evaporitic environments. Palaeoweathering Palaeosurfaces Relat. Cont. Depos. 1999, 27, 129–151. [Google Scholar]
- Turner, C.E.; Fishman, N.S. Jurassic Lake T’oo’dichi: A large alkaline, saline lake, Morison Formation, eastern Colorado Plateau. Geol. Soc. Am. Bull. 1991, 103, 538–558. [Google Scholar] [CrossRef]
- Uhlík, P.; Honty, M.; Šucha, V.; Franců, J.; Biroň, A.; Clauer, N.; Hanzelyová, Z.; Majzlan, J. Influence of salt-bearing environment to illitization. In Proceedings of the XVII Congress of CBGA, Bratislava, Slovakia, 1–4 September 2002. [Google Scholar]
- Honty, M.; Uhlík, P.; Šucha, V.; Čaplovičova, M.; Franců, J.; Clauer, N.; Biroň, A. Smectite-to-illite alteration in salt-bearing bentonites (East Slovak Basin). Clay Clay Miner. 2004, 52, 533–551. [Google Scholar] [CrossRef]
- Bilonizhka, P.; Iaremchuk, I.; Hryniv, S.; Vovnyuk, S. Clay minerals of Miocene evaporites of the Carpathian Region, Ukraine. Biul. Państwowego Inst. Geol. 2012, 449, 137–146. [Google Scholar]
- Velde, B. Clay Minerals. A Physico-Chemical Explanation of Their Occurrence; Elsevier: Amsterdam, The Netherlands, 1985; p. 427. [Google Scholar]
- Oszczypko, N.; Krzywiec, P.; Popadyuk, I.; Peryt, T. Carpathian Foredeep Basin (Poland and Ukraine): Its sedimentary, structural, and geodynamic evolution. AAPG Mem. 2006, 84, 293–350. [Google Scholar]
- Oszczypko, N.; Uchman, A.; Bubniak, I. The Dobrotiv Formation (Miocene) in the Boryslav-Pokuttya and Sambir nappes of the Ukrainian Carpathians: A record of sedimentary environmental change in the development of the Carpathian Foredeep Basin. Geol. Quart. 2014, 58, 393–408. [Google Scholar] [CrossRef][Green Version]
- Hnylko, O.M. Tectonic zoning of the Carpathians in terms of the terrane tectonics Article 2. The Flysch Carpathian—Ancient accretionary prism. Geodynamics 2012, 12, 67–78, (In Ukrainian with English summary). [Google Scholar] [CrossRef]
- Peryt, T.M. The beginning, development and termination of the Middle Miocene Badenian salinity crisis in Central Paratethys. Sediment. Geol. 2006, 188–189, 379–396. [Google Scholar] [CrossRef]
- Rudko, H.I.; Petryshyn, V.Y. Soliani Resursy Peredkarpattia ta Perspektyvy Yikh Vykorystannia; Bukrek: Kyiv, Chernivtsi, Ukraine, 2017. (In Ukrainian) [Google Scholar]
- Andreyeva-Grigorovich, A.S.; Oszczypko, N.; Ślączka, A.; Oszczypko-Clowes, M.; Savitskaya, N.; Trofimovicz, N. New data on the stratigraphy of the folded Miocene Zone at the front of the Ukrainian Outer Carpathians. Acta Geol. Pol. 2008, 58, 325–353. [Google Scholar]
- Vashchenko, V.O.; Hnylko, O.M. About stratigraphy and sedimentary features of the Neogene molasse of the Boryslav-Pokuttya and Sambir Nappes of the Ukrainian Fore-Carpathians. Heol. Heokh. Hor. Kop. 2003, 106, 87–101, (In Ukrainian with English summary). [Google Scholar]
- Vashchenko, V.O.; Hnylko, O.M. About stratigraphy of the salt-bearing molasse of the Ukrainian Fore-Carpathians Zbirn. Nauk. Prats UkrDGRI 2013, 2, 71–77, (In Ukrainian with English summary). [Google Scholar]
- Piller, W.E.; Harzhauser, M.; Mandic, O. Miocene Central Paratethys stratigraphy—Current status and future directions. Stratigraphy 2007, 4, 151–168. [Google Scholar] [CrossRef]
- Vyalov, O.S. Stratigrafiya Neogenovykh Molass Predkarpatskogo Progiba; Naukova Dumka: Kiev, Ukraine, 1965; p. 192. (In Russian) [Google Scholar]
- Dzhinoridze, N.M. Tertiary potassium basins. In Deposits of Potassium Salts of the USSR; Raievskiy, V.I., Fiveg, M.P., Eds.; Nedra: Leningrad, Russia, 1973; pp. 183–234. (In Russian) [Google Scholar]
- Dzhinoridze, N.M. Karpatskiy kalienosnyy region. In Zakonomernosti Razmeshchenia i Kriterii Poiskov Kaliynykh Soley SSSR; Izdatelstvo “Mecniereba”: Tbilisi, Russia, 1980; pp. 73–159. (In Russian) [Google Scholar]
- Koriń, S.S. Geology of the Miocene salt-bearing formations of the Ukrainian Fore-Carpathians). Przegląd Geol. 1994, 42, 744–747. (In Polish) [Google Scholar]
- Vashchenko, V.O.; Turchynova, S.M.; Turchynov, I.I.; Polikha, G.G. Derzhavna Geologichna Karta Ukrainy 1:200,000. Karpatska Seriya. Arkush M-35-XXV (Ivano-Frankivsk); Poyasnyu valna zapiska; UkrDGI: Kyiv, Ukraine, 2007. (In Ukrainian) [Google Scholar]
- Andreyeva-Grigorovich, A.S.; Vashchenko, V.O.; Hnylko, O.M.; Trofimovich, N.A. Stratigraphy of Neogene deposits of the Ukrainian Carpathians and Carpathian Foreland. Tekton. Strat. 2011, 28, 67–77, (In Ukrainian with English summary). [Google Scholar] [CrossRef]
- Hnylko, O. Olistostromes in the Miocene salt-bearing folded deposits at the front of the Ukrainian Carpathian orogen. Geol. Quart. 2014, 58, 381–392. [Google Scholar] [CrossRef]
- Korin, S.S. Tektonicheskiye usloviya formirovaniya struktury kaliynykh mestorozhdeniy v Boryslavsko-Pokutskom pokrove. Otech. Geol. 1992, 20–26. (In Russian) [Google Scholar]
- Peryt, T.M.; Kovalevich, V.M. Association of redeposited salt breccias and potash evaporites in the Lower Miocene of Stebnyk (Carpathian Foredeep, West Ukraine). J. Sediment. Res. 1997, 67, 913–922. [Google Scholar]
- Korenevskiy, S.M.; Donchenko, K.B. Geologiya i usloviya formirovaniya kaliynykh otlozheniy Sovetskogo Predkarpatia. Tr. VSEGEI N.S. 1963, 99, 3–153. (In Russian) [Google Scholar]
- Czapowski, G.; Bukowski, K.; Tomassi-Morawiec, H.; Toboła, T. Geochemistry and mineralogy of Miocene Zuber rocks in the Carpathian Foredeep (southern Poland). Przegląd Solny 2023, 17, 39–50. [Google Scholar]
- Shestopalov, M.; Liutyi, H.; Sanina, I. Suchasni pidkhody do hidroheolohichnoho raionuvannia Ukrainy. Miner. Resur. Ukr. 2019, 2, 3–12. (In Ukrainian) [Google Scholar]
- Wójtowicz, A.; Hryniv, S.P.; Peryt, T.M.; Bubniak, A.; Bubniak, I.; Bilonizhka, P.M. K-Ar dating of the Miocene potash salts of the Carpathian Foredeep (West Ukraine): Application to dating of tectonic events. Geol. Carp. 2003, 54, 243–249. [Google Scholar]
- de Leeuw, A.; Bukowski, K.; Krijgsman, W.; Kuiper, K.F. Age of the Badenian salinity crisis; impact of Miocene climate variability on the circum-Mediterranean region. Geology 2010, 38, 715–718. [Google Scholar] [CrossRef]
- Peryt, T.M. Sedimentology of Badenian (middle Miocene) gypsum in eastern Galicia, Podolia and Bukovina (West Ukraine). Sediment 1996, 43, 571–588. [Google Scholar] [CrossRef]
- Bąbel, M. Badenian evaporite basin of the northern Carpathian Foredeep as a drawdown salina basin. Acta Geol. Pol. 2004, 54, 317–337. [Google Scholar]
- Bąbel, M. Selenite-gypsum microbialite facies and sedimentary evolution of the Badenian evaporitic basin of the northern Carpathian Foredeep. Acta Geol. Pol. 2005, 55, 187–210. [Google Scholar]
- Kasprzyk, A.; Orti, F. Paleogeographic and Burial Controls on Anhydrite Genesis: The Badenian Basin in the Carpthian Foredeep (Southern Poland, Western Ukraine). Sediment 1998, 45, 889–907. [Google Scholar] [CrossRef]
- Hryniv, S.P.; Dolishniy, B.V.; Khmelevska, O.V.; Poberezhskyy, A.V.; Vovnyuk, S.V. Evaporites of Ukraine: A review. Geol. Soc. Spec. Publ. 2007, 285, 309–334. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Zinchuk, I.M.; Meng, F. The Temperature of Halite Crystallization in the Badenian Saline Basins, in the Context of Paleoclimate Reconstruction of the Carpathian Area. Minerals 2021, 11, 831. [Google Scholar] [CrossRef]
- Bukowski, K. Badenian Saline Sedimentation Between Rybnik and Dębica Based on Geochemical, Isotopic, and Radiometric Research; Dissertation Monographs 236; AGH: Kraków, Poland, 2011; p. 184, (In Polish with English summary). [Google Scholar]
- Palcu, D.V.; Mariș, I.; de Leeuw, A.; Melinte-Dobrinescu, M.; Anton, E.; Frunzescu, D.; Popov, S.; Stoica, M.; Jovane, L.; Krijgsman, W. The legacy of the Tethys Ocean: Anoxic seas, evaporitic basins, and megalakes in the Cenozoic of Central Europe. Earth-Sci. Rev. 2023, 246, 104594. [Google Scholar] [CrossRef]
- Iaremchuk, I.; Galamay, A. Mineral composition of the water insoluble residue from rock salt Hrynivka area of the Carpathian Foredeep. Heol. Heokh. Hor. Kop. 2009, 146, 79–90, (In Ukrainian with English abstract). [Google Scholar]
- Iaremchuk, I.; Poberezhsky, A. Clay mineral composition of Badenian gypsum of the Dnister-River region. Miner. Zb. 2009, 59, 116–127, (In Ukrainian with English summary). [Google Scholar]
- Iaremchuk, I. Clay Minerals of Phanerozoic Evaporates and Their Dependence Upon Brine Concentration Stage and Seawater Chemical Type; Avtoref. Dis. Kand. Heol. Nauk: Lviv, Ukraine, 2010; p. 24, (In Ukrainian with English summary). [Google Scholar]
- Iaremchuk, I. Dependence of clay mineral associations of the Neogene evaporates from the Carpathian region upon the salt brines concentration in salt-producing basins. Heol. Heokh. Hor. Kop. 2012, 160–161, 119–130, (In Ukrainian with English summary). [Google Scholar]
- Iaremchuk, I.; Hryniv, S. Organic matter effect on composition and genesis of clay minerals of rock salt deposits of the Carpathian Foredeep. Heol. Heokh. Hor. Kop. 2013, 162–163, 60–70, (In Ukrainian with English abstract). [Google Scholar]
- Hryniv, S.; Yaremchuk, Y.; Radkovets, N. The influence of marine and continental waters on the clay minerals transformation processes of evaporite deposits (on the example of the Kalush-Holin’ deposit, Carpathian Foredeep). Geol. Geochem. Comb. Min. 2023, 191–192, 122–134, (In Ukrainian with English summary). [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: New York, NY, USA, 1997; p. 376. [Google Scholar]
- Brown, G.; Brindley, G.W. X-Ray Diffraction Procedures for Clay Mineral Identification. Mineral. Soc. Monogr. 1980, 5, 305–356. [Google Scholar]
- Bish, D.L.; Duffy, C.J. Thermometric analysis of minerals. Clay Miner. Soc. Workshop Lect. 1990, 3, 96–157. [Google Scholar]
- Drits, V.A.; Kossovskaya, A.G. Glinistyye Mineraly: Smektity, Smeshanosloynyye Obrazovaniya; Nauka: Moskva, Russia, 1990; p. 214. (In Russian) [Google Scholar]
- Bilonizhka, P.M. Pryroda mizhsharovoi vody v hidrosliudakh. Miner. Zb. 2001, 51, 142–148. (In Ukrainian) [Google Scholar]
- Drits, V.A.; Sakharov, B.A. X-Ray Structure Analysis of Interstratified Minerals; Nauka: Moskva, Russia, 1976; p. 256. (In Russian) [Google Scholar]
- Galán, E. Genesis of Clay Minerals. In Developments in Clay Science: Vol. 1. Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 14; pp. 1129–1162. [Google Scholar]
- Bąbel, M.; Schreiber, B.C. Geochemistry of Evaporites and Evolution of Seawater. Treatise Geochem. 2014, 9, 483–560. [Google Scholar]
- Yaremchuk, Y.; Hryniv, S.; Peryt, T.; Vovnyuk, S.; Meng, F. Controls on Associations of Clay Minerals in Phanerozoic Evaporite Formations: An Overview. Minerals 2020, 10, 974. [Google Scholar] [CrossRef]
- Lanson, B.; Beaufort, D.; Berger, G.; Bauer, A.; Cassagnabere, A.; Meunier, A. Authigenic kaolin and illitic minerals during burial diagenesis of sandstones: A review. Clay Miner. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- McCaffrey, M.A.; Lazar, B.; Holland, H.D. The evaporation path of seawater and the coprecipitation of Br and K with halite. J. Sediment. Res. 1987, 57, 928–937. [Google Scholar]
- Dopieralska, J.; Belka, Z.; Zieliński, M.; Górka, M.; Poberezhskyy, A.; Stupka, O.; Walczak, A.; Wysocka, A. Neodymium and strontium isotopes track the origin of parent brines of primary gypsum deposits (Miocene, Fore-Carpathian Basin). Chem. Geol. 2024, 648, 121963. [Google Scholar] [CrossRef]
- Petrichenko, O.I.; Peryt, T.M.; Poberegsky, A.V. Pecularities of gypsum sedimentation in the Middle Miocene Badenian evaporite basin of Carpathian Foredeep. Slovak Geol. Mag. 1997, 3, 91–104. [Google Scholar]
- Kovalevich, V.M.; Petrichenko, O.I. Chemical composition of brines in Miocene evaporite basins of Carpathian region. Slovak Geol. Mag. 1997, 3, 173–180. [Google Scholar]
- Peryt, T.M.; Szaran, J.; Jasionowski, M.; Halas, S.; Peryt, D.; Poberezhskyy, A.; Karoli, S.; Wójtowicz, A. S and O isotope composition of the Badenian (Middle Miocene) sulphates in the Carpathian Foredeep. Geol. Carp. 2002, 53, 391–398. [Google Scholar]
- Peryt, T.M.; Hryniv, S.P.; Anczkiewicz, R. Strontium isotope composition of Badenian (Middle Miocene) Ca-sulphate deposits in West Ukraine: A preliminary study. Geol. Quart. 2010, 54, 465–476. [Google Scholar]
- Galamay, A.R. Physico-chemical conditions of the formation of the Badenian salt deposits of the Ukrainian Forecarpathians (Grynivka area). Heol. Heokh. Hor. Kop. 2010, 151, 64–77, (In Ukrainian with English summary). [Google Scholar]
- Galamay, A.R. Influence of continental run-off on the composition of marine brines of Badenian salt basin central part (Ukrainian Carpathian Foredeep). Miner. Zb. 2012, 62, 228–235, (In Ukrainian with English summary). [Google Scholar]
- Środoń, J. Illite group clay minerals. In Encyclopedia of Sediments and Sedimentary Rocks; Middleton, G.V., Church, M.J., Coniglio, M., Hardie, L.A., Eds.; Springer: Dordrecht, The Netherlands, 1978; p. 115. [Google Scholar]
- Meunier, A. Clays; Springer: Berlin/Heidelberg, Germany, 2005; 472p. [Google Scholar]
- Zhao, G.; Peacor, D.R.; McDowell, S.D. “Retrograde diagenesis” of clay minerals in the Precambrian Freda Sandstone, Wisconsin. Clays Clay Miner. 1999, 47, 119–130. [Google Scholar] [CrossRef]
- Rosenberg, P.E. The nature, formation, and stability of end-member illite: A hypothesis. Am. Miner. 2002, 87, 103–107. [Google Scholar] [CrossRef]
- Korenevskiy, S.M. Miotsenovyye vulkanicheskiye tufy Predkarpatia. Tr. VNIIGalurgii 1954, 29, 176–196. (In Russian) [Google Scholar]
- Dzhinoridze, N.M.; Rogova, M.S.; Telegin, V.P. Vulkanogennyye porody Kalush-Golynskogo mestorozhdeniya kaliynykh soley. Tr. VNIIGalurgii 1974, 71, 36–56. (In Russian) [Google Scholar]
- Turchinov, I.I. The Badenian (Middle Miocene) gypsum section in Kryvche (Podolia, West Ukraine). Biul. Państw. Inst. Geol. 1999, 387, 70–74. [Google Scholar]
- Claret, F.; Bauer, A.; Schäfer, T.; Griffault, L.; Lanson, B. Experimental investigation of the interaction of clays with high pH solutions: A case study from the Callovo-Oxfordian formation, Meuse-Haute Marne underground laboratory (France). Clays Clay Miner. 2002, 50, 633–646. [Google Scholar] [CrossRef]
- Claret, F.; Sakharov, B.A.; Drits, V.A.; Velde, B.; Meunier, A.; Griffault, L.; Lanson, B. Clay minerals in the Meuse-Haute Marne underground laboratory (France): Possible influence of organic matter on clay mineral evolution. Clays Clay Miner. 2004, 52, 515–532. [Google Scholar] [CrossRef]
- Klubova, T.T. Glinistye Mineraly i Ikh Rol v Genezise, Migratsii i Akkumulatsii Nefti; Nedra: Moskva, Russia, 1973; p. 255. (In Russian) [Google Scholar]
- Rowe, M.C.; Brewer, B.J. AMORPH: A statistical program for characterizing amorphous materials by X-ray diffraction. Comput. Geosci. 2018, 230, 21–31. [Google Scholar] [CrossRef]
- Korchagina, Y.I.; Chetverikova, O.P. Metody Issledovaniya Rasseyannogo Organicheskogo Veshchestva Osadochnych Porod; Nedra: Moskva, Russia, 1976; p. 229. (In Russian) [Google Scholar]
- Grim, R.E. Clay Mineralogy; McGraw-Hill Book Company: New York, NY, USA, 1969; p. 422. [Google Scholar]
- Lagaly, G.; Ogawa, M.; De’ka’ny, I. Clay Mineral Organic Interactions. In Developments in Clay Science: Vol. 1. Handbook of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 309–378. [Google Scholar]
- Więcław, D.; Lytvyniuk, S.; Kovalevych, V.; Peryt, T.M. Fluid inclusions in halite and bitumens in rock salt from Miocene evaporites in the Ukrainian Fore-Carpathian region: Evidence for hydrocarbon accumulations in the underlying strata. Prz. Geol. 2008, 56, 837–841, (In Polish with English summary). [Google Scholar]
- Semchuk, Y.M. Naukovi ta Metodychni Osnovy Okhorony Heolohichnoho Seredovyshcha v Raionakh Rozrobky Kaliinykh Rodovyshch (na Prykladi Peredkarpattia); Vasyl Stefanyk Precarpathian National University: Ivano-Frankivsk, Ukraine, 1995. (In Ukrainian) [Google Scholar]
- Lipnitskiy, V.K. Litologicheskiye osobennosti i solevoy kompleks chetvertichnykh otlozheniy i porod gipsovo-glinistoy shlyapy Stebnikskogo mestorozhdeniya kaliynykh soley. In Materialy po Gidrogeologii i Geologicheskoy Roli Podzemnykh Vod; Izdatelstvo Leningradskogo Universiteta: Leningrad, Russia, 1971; pp. 98–108. (In Russian) [Google Scholar]
- Środoń, J.; Elsass, F.; McHardy, W.J.; Morgan, D.J. Chemistry of illite-smectite inferred from TEM measurements of fundamental particles. Clay Miner. 1992, 27, 137–158. [Google Scholar] [CrossRef]
- Petrichenko, O.Y. Fiziko-Khimicheskiye Usloviya Osadkoobrazovaniya v Drevnikh Solerodnykh Basseynakh; Naukova Dumka: Kiev, Russia, 1988; p. 128. (In Russian) [Google Scholar]
- Kovalevich, V.M. Fiziko-Khimicheskie Uslovia Formirovania Soley Stebnikskogo Kaliynogo Mestorozhdenia; Naukova Dumka: Kiev, Russia, 1978; p. 100. (In Russian) [Google Scholar]
- Petrichenko, O.I. Epigenez Evaporitov; Naukova Dumka: Kiev, Russia, 1989; p. 64. (In Russian) [Google Scholar]
- Kutas, R.I. Heat flow and geothermal crustal model of the Ukrainian Carpathians. Geophys. J. 2014, 6, 3–25. [Google Scholar]
- Kotarba, M.J.; Koltun, Y.V. The origin and habitat of hydrocarbons of the Polish and Ukrainian parts of the Carpathian Province. Am. Ass. Petrol. Geol. Mem. 2006, 84, 395–442. [Google Scholar]
- Andreucci, B.; Castelluccio, A.; Corrado, S.; Jankowski, L.; Mazzoli, S.; Szaniawski, R.; Zattin, M. Interplay between the thermal evolution of an orogenic wedge and its retro-wedge basin: An example from the Ukrainian Carpathians. Geol. Soc. Am. Bull. 2015, 127, 410–427. [Google Scholar] [CrossRef]
- Vityk, M.O.; Bodnar, R.J.; Dudok, I.V. Fluid inclusions in “Marmarosh Diamonds”: Evidence for tectonic history of the Folded Carpathian Mountains, Ukraine. Tectonophysics 1996, 255, 163–174. [Google Scholar] [CrossRef]


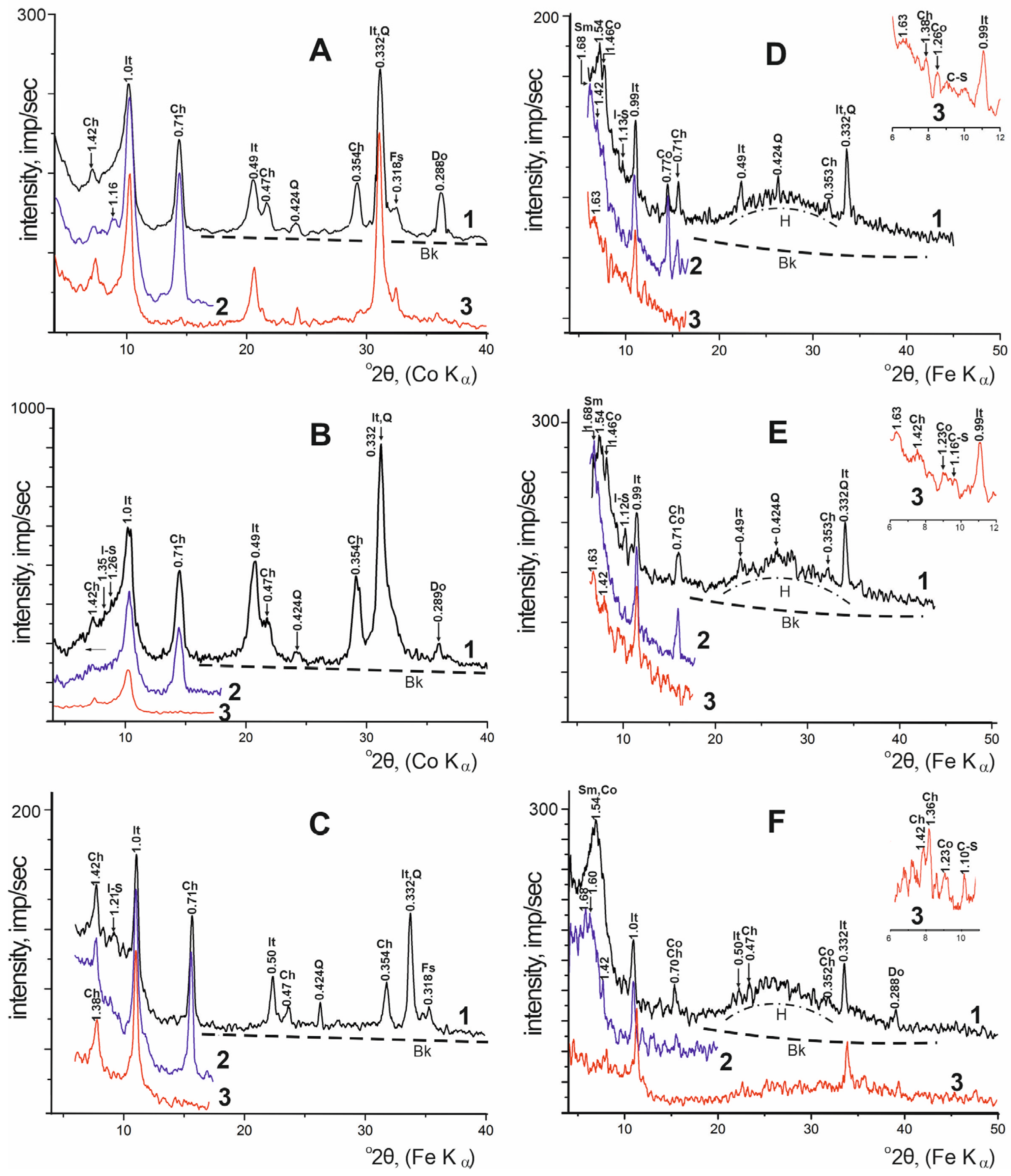
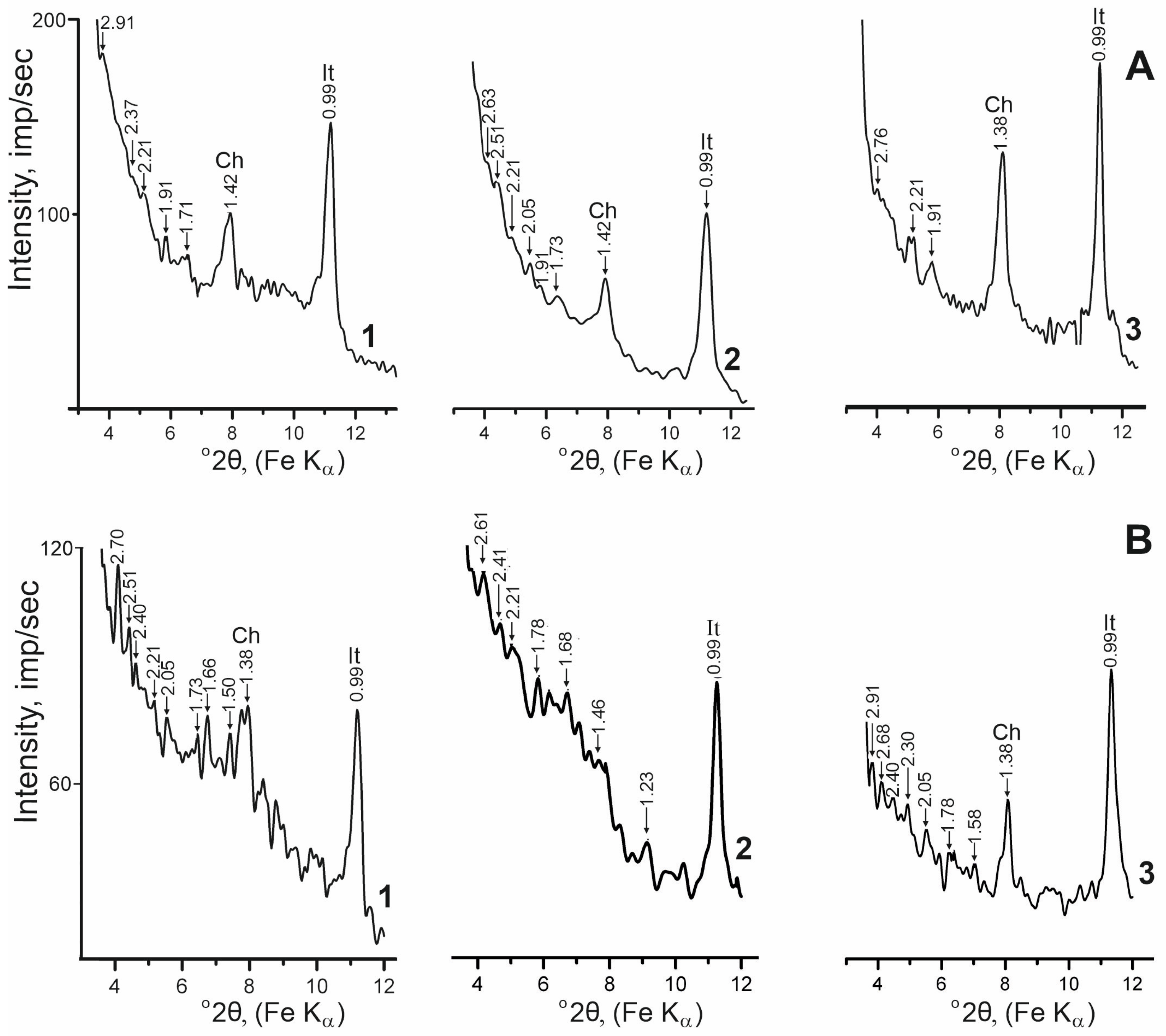

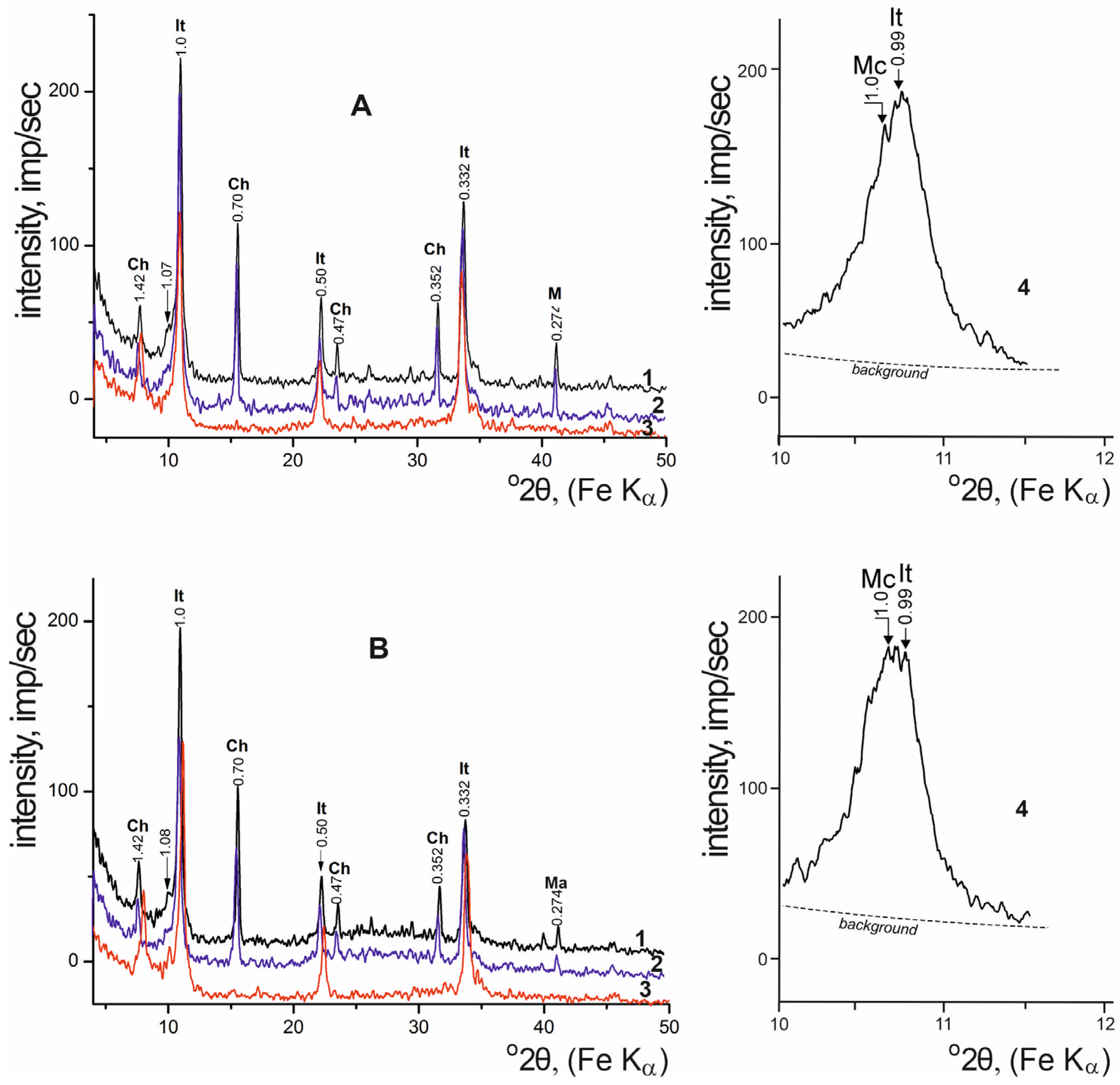
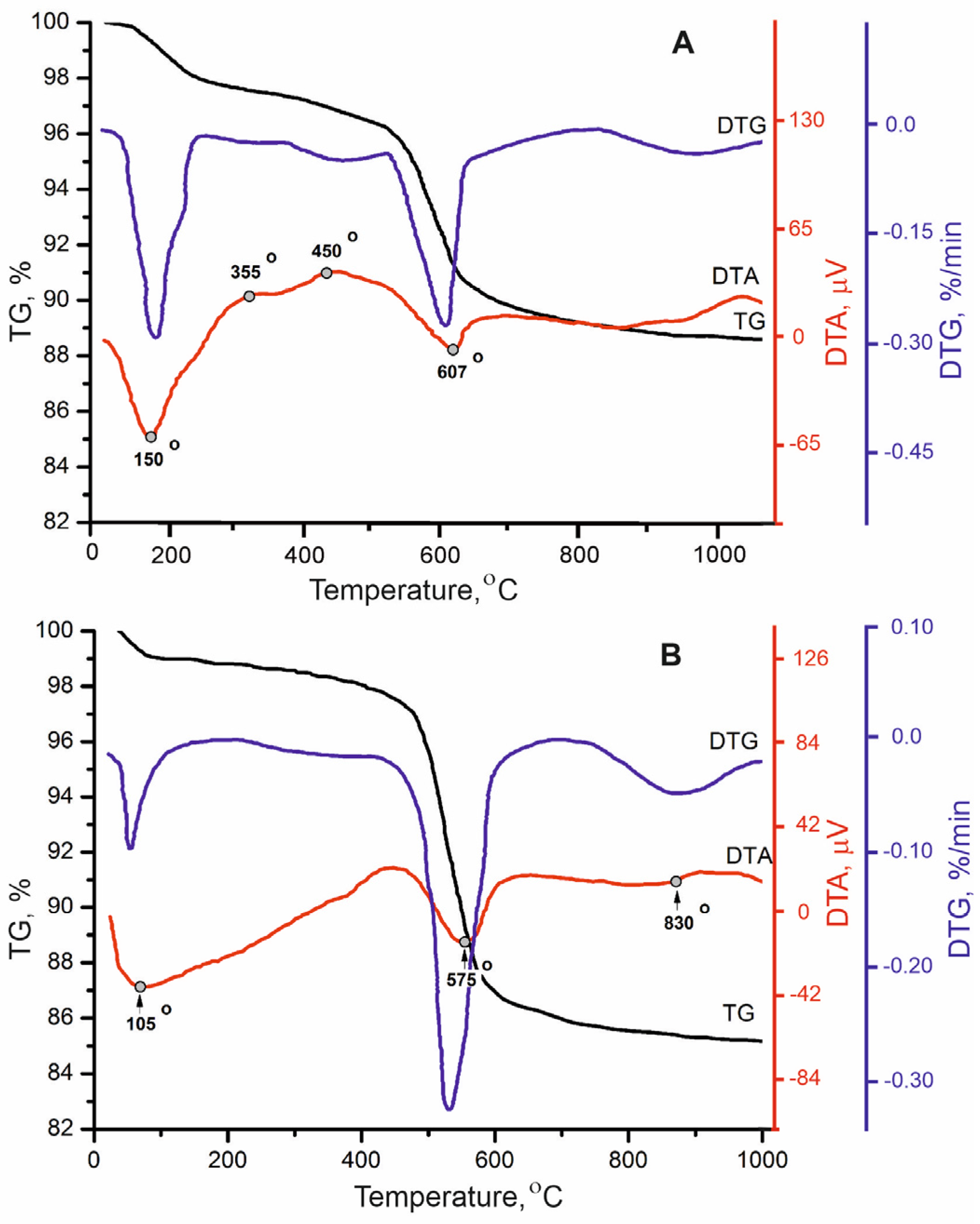
| Locality | Sample Number | Gypsum Type | Clay Minerals | Other Minerals | ||||
|---|---|---|---|---|---|---|---|---|
| Smectite | Chlorite- Smectite | Illite- Smectite | Chlorite | Illite | ||||
| Shchyrets | 2313 | Stromatolitic gypsum | ++ | – | + | – | + | Ca + |
| 2316 | Bedded gypsum | ++ | (+) | – | (+) | ++ | Q + | |
| 2317 | Gypsum breccia | ++ | – | + | – | + | Ca ++ | |
| Pisky | 2319 | Sabre gypsum | ++ | + | + | – | + | Q (+) |
| 2320 | Grass-like gypsum | ++ | – | (+) | + | + | Q +, | |
| 2321 | Laminated gypsum with salin spar | + | ++ | + | – | + | Q (+), Fs (+) | |
| Verenchanka (borehole 20) | 822 | Grass-like gypsum | ++ | + | (+) | – | + | Fs (+), Ca + |
| 20 | Sabre gypsum | ++ | + | + | – | + | Fs (+), Do ++, Ca + | |
| Nagoryany | 818 | Sabre gypsum | ++ | – | – | + | + | Q (+), Fs (+) |
| Criva (borehole 84) | 102 | Massive gypsum | ++ | (+) | + | – | +? | – |
| 113 | Massive gypsum | + | + | + | – | – | Ca ++ | |
| Sample Number | Depth [m] | Clay Minerals | Other Minerals | |||||
|---|---|---|---|---|---|---|---|---|
| Smectite | Corrensite | Chlorite-Smectite | Illite-Smectite | Chlorite | Illite | |||
| Borehole 29, Verkhniy Strutyn, Vorotyshcha Suite | ||||||||
| 2573 | 562 | – | – | – | + | ++ | ++ | Q (+), Fs –, Do –, Ca –, Ma – |
| 2574 | 626 | – | – | – | + | ++ | ++ | Q (+), Fs (+), Do +, Ca –, Ma – |
| 2575 | 698 | – | – | – | ++ | ++ | ++ | Q (+), Fs +, Do –, Ca –, Ma – |
| Borehole 17Dr, Boryslav, Vorotyshcha Suite | ||||||||
| 2567 | 352 | – | – | – | – | ++ | ++ | Q +, Fs +, Do –, Ca –, Ma ++ |
| 2566 | 353 | – | – | – | – | ++ | ++ | Q ++, Fs +, Do –, Ca –, Ma + |
| 2569 | 377 | – | – | (+) | + | ++ | ++ | Q +, Fs (+), Do (+), Ca –, Ma – |
| 2570 | 409 | – | – | – | + | ++ | ++ | Q (+), Fs –, Do (+), Ca –, Ma – |
| Borehole 9MD, Dolyna, Vorotyshcha Suite | ||||||||
| 858 | 38 | – | – | +? | + | ++ | ++ | Q –, Fs (+), Do (+), Ca –, Ma – |
| 859 | 73 | – | – | – | + | ++ | ++ | Q +, Fs (+), Do –, Ca –, Ma – |
| 863 | 152 | – | – | – | + | ++ | ++ | Q (+), Fs (+), Do –, Ca –, Ma – |
| Boreholes 348 and 671, Silets-Stupnytsya, Tyras Suite | ||||||||
| 902 | 132 | + | + | + | + | + | + | Q (+), Fs–, Do –, Ca –, Ma – |
| 907 | 172.5 | – | – | ++ | + | + | + | Q +, Fs –, Do +, Ca –, Ma – |
| 58 | 272 | + | – | ++ | + | (+) | + | Q +, Fs –, Do +, Ca –, Ma – |
| 54 | 302 | – | – | ++ | (+) | + | + | Q (+), Pt –, Do +, Ca –, Ma – |
| 47 | 449 | +? | – | ++ | + | (+) | + | Q (+), Pt –, Do +, Ca –, Ma – |
| 48 | 464 | + | + | + | (+) | (+) | + | Q +, Fs Do –, Ca –, Ma – |
| Borehole 525, Hrynivka, Tyras Suite | ||||||||
| 1342 | 304 | + | ++ | + | (+) | + | + | Q +, Fs (+), Do –, Ca –, Ma – |
| 1344 | 310.1 | + | + | + | + | + | + | Q +, Fs (+), Do –, Ca –, Ma – |
| 1348 | 330 | +? | – | ++ | (+) | (+) | + | Q +, Fs –, Do –, Ca –, Ma – |
| 1350 | 342 | + | +? | ++ | + | (+) | + | Q (+), Fs (+), Do +, Ca +, Ma – |
| 1352 | 362 | + | +? | ++ | (+) | (+) | + | Q (+), Fs –, Do ++, Ca –, Ma – |
| 1354 | 376 | – | – | ++ | + | + | ++ | Q +, Fs (+), Do +, Ca +, Ma – |
| 1356 | 395–399 | + | ++ | (+) | – | (+) | ++ | Q +, Fs –, Do +, Ca –, Ma – |
| 1357 | 410–411 | + | ++ | + | + | + | Q–, Fs–, Do +, Ca –, Ma – | |
| 1361 | 444–448 | + | ++ | (+) | (+) | (+) | + | Q (+), Fs (+), Do +, Ca –, Ma – |
| 1362 | 454–456 | ++ | +? | (+) | + | + | + | Q (+), Fs –, Do +, Ca –, Ma – |
| 1364 | 474 | + | ++ | + | – | (+) | + | Q (+), Fs –, Do +, Ca –, Ma – |
| 1365 | 480 | +? | ++ | + | + | (+) | + | Q –, Fs –, Do +, Ca –, Ma – |
| 1366 | 489 | – | +? | ++ | – | + | ++ | Q (+), Fs –, Do +, Ca –, Ma – |
| 1369 | 518 | ++ | ++ | (+) | – | (+) | + | Q (+), Fs (+), Do +, Ca –, Ma – |
| 1370 | 521–528 | + | – | ++ | + | (+) | ++ | Q +, Fs (+), Do +, Ca +, Ma – |
| 1371 | 532 | + | +? | ++ | (+) | + | + | Q +, Fs (+), Do –, Ca –, Ma – |
| 1372 | 536 | + | – | ++ | + | + | ++ | Q +, Fs (+), Do –, Ca –, Ma – |
| Locality | Sample Number | Lithology | Clay Minerals | Other Minerals | |
|---|---|---|---|---|---|
| Illite | Chlorite | ||||
| Dombrovo | 3 | Clayey kainite rock | ++ | ++ | – |
| Northern Kainite field | 1006b | Kainite rock | ++ | ++ | – |
| Northern Kainite field | 1029 | Kainite rock | ++ | ++ | – |
| Dombrovo | 2251 | Kainite rock | ++ | ++ | Ma + |
| Dombrovo | 2 | Langbeinite rock | ++ | ++ | * |
| Dombrovo | 1105 | Langbeinite rock | ++ | ++ | * |
| Dombrovo | 2250 | Langbeinite rock | ++ | ++ | Ma + |
| Dombrovo | 1 | Kainite–langbeinite rock | ++ | ++ | Ma + |
| Dombrovo | 1097a | Kainite–langbeinite rock | ++ | ++ | Ma + |
| Dombrovo | 2249 | Kainite–langbeinite rock | ++ | ++ | Ma + |
| Khotyn field | 1017 | Sylvinite | ++ | ++ | Ma + |
| Dombrovo | 5 | Clayey polyhalite rock | ++ | ++ | – |
| Northern Kainite field | 1006a | Salt-bearing breccia | ++ | ++ | Ma + |
| Khotyn field | 1019 | Salt-bearing breccia | ++ | ++ | – |
| Dombrovo | 1117 | Salt-bearing breccia | ++ | ++ | – |
| Holyn | 2219 | Halite from salt-bearing breccia | ++ | ++ | Ma + |
| Holyn | 1035 | Greenish-grey halite from the potash rocks | ++ | + | – |
| Holyn | 1039 | Greenish-grey halite from the potash rocks | ++ | + | Ma + |
| Dombrovo | 1097 | Greenish-grey halite from the potash rocks | ++ | + | Ma + |
| Dombrovo | 14 | Halite from alternated halite and halopelite layers | ++ | + | Ma + |
| Dombrovo | 15 | Halite from alternation of halite and halopelite layers | ++ | + | Ma + |
| Holyn | 2185 | Halite from alternated halite and halopelite layers | ++ | ++ | Ma + |
| Northern Kainite field | 1026 | Halite from alternated halite and halopelite layers | ++ | + | – |
| Holyn | 1060 | Greenish-grey halite from alternated halite and halopelite layers | ++ | + | – |
| Holyn | 1062 | Halite from alternated halite and halopelite layers | ++ | + | * |
| Dombrovo | 25 | Halopelite from alternated halite and halopelite layers | ++ | + | Ma + |
| Dombrovo | 1096 | Halopelite from alternated halite and halopelite layers | ++ | + | * |
| Dombrovo | 1104 | Halopelite from alternated halite and halopelite layers | ++ | + | – |
| Sample Number | Lithology | Clay Minerals | Other Minerals | |||
|---|---|---|---|---|---|---|
| Illite | Chlorite | Illite- Smectite | Kaolinite | |||
| Clays from the gypsum-clay caprock above potash rocks | ||||||
| 2253 | Clay with gypsum | ++ | ++ | + | – | Ma + |
| 2255 | Clay with syngenite | ++ | ++ | + | – | – |
| 2257 | Clay with mirabilite | ++ | ++ | + | – | Ma + |
| 2300 | Clay with syngenite | ++ | ++ | + | – | Ma + |
| 2308 | Grey clay, upper part of the gypsum–clay caprock | ++ | ++ | + | + | – |
| 2309 | Grey clay, middle part of the gypsum–clay caprock | ++ | ++ | + | + | – |
| 2310 | Grey clay, lower part of the gypsum–clay caprock | ++ | ++ | + | + | – |
| Clays from the gypsum–clay caprock above the salt-bearing breccia | ||||||
| 2305 | Grey clay, upper part of the gypsum–clay caprock | ++ | ++ | + | + | – |
| 2306 | Grey clay with gypsum and syngenite, middle part of the gypsum–clay caprock | ++ | ++ | + | – | – |
| 2307 | Grey clay, lower part of the gypsum–clay caprock | ++ | ++ | + | + | Ma + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaremchuk, Y.; Hryniv, S.; Peryt, T. Controls on the Transformation of Clay Minerals in the Miocene Evaporite Deposits of the Ukrainian Carpathian Foredeep. Minerals 2025, 15, 395. https://doi.org/10.3390/min15040395
Yaremchuk Y, Hryniv S, Peryt T. Controls on the Transformation of Clay Minerals in the Miocene Evaporite Deposits of the Ukrainian Carpathian Foredeep. Minerals. 2025; 15(4):395. https://doi.org/10.3390/min15040395
Chicago/Turabian StyleYaremchuk, Yaroslava, Sofiya Hryniv, and Tadeusz Peryt. 2025. "Controls on the Transformation of Clay Minerals in the Miocene Evaporite Deposits of the Ukrainian Carpathian Foredeep" Minerals 15, no. 4: 395. https://doi.org/10.3390/min15040395
APA StyleYaremchuk, Y., Hryniv, S., & Peryt, T. (2025). Controls on the Transformation of Clay Minerals in the Miocene Evaporite Deposits of the Ukrainian Carpathian Foredeep. Minerals, 15(4), 395. https://doi.org/10.3390/min15040395







