HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Receipt and Preparation
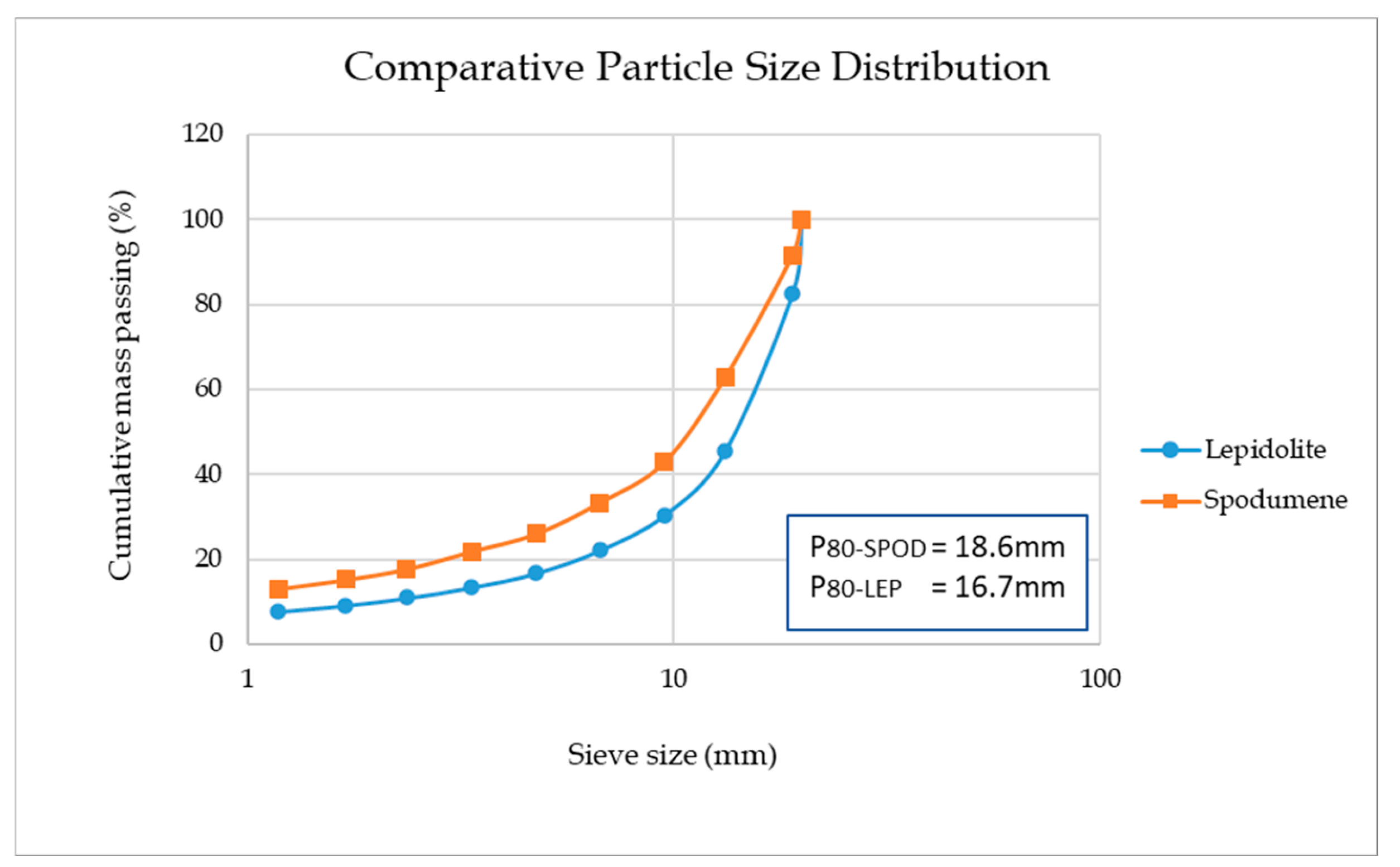
2.2. Head Assay, Particle Size Distribution and Size by Assay
2.3. Gravity Separation Testwork
Heavy Liquid Separation (HLS) Testwork
3. Results and Discussion
3.1. Head Grade Analysis
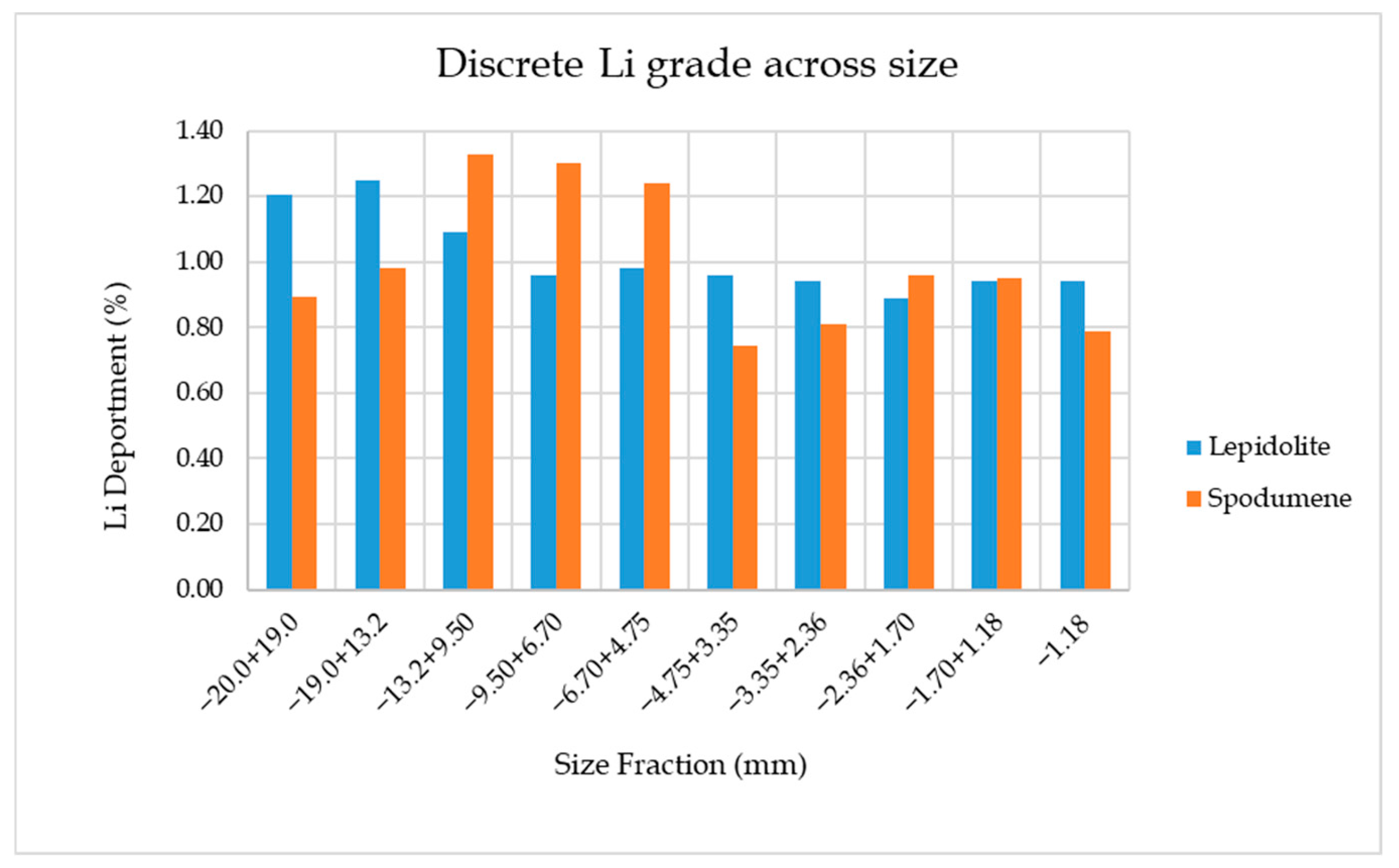
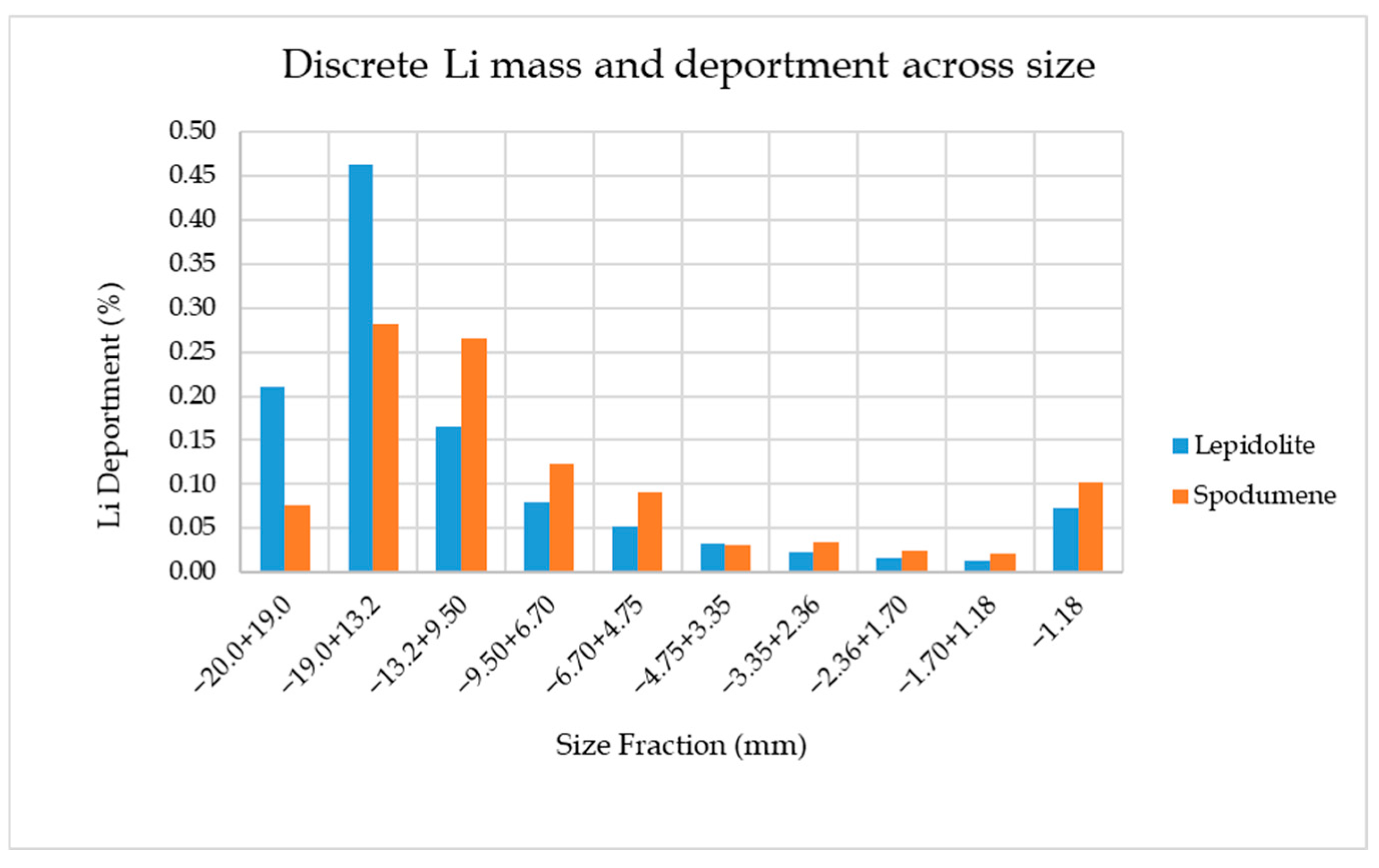
3.2. Size by Assay Analysis
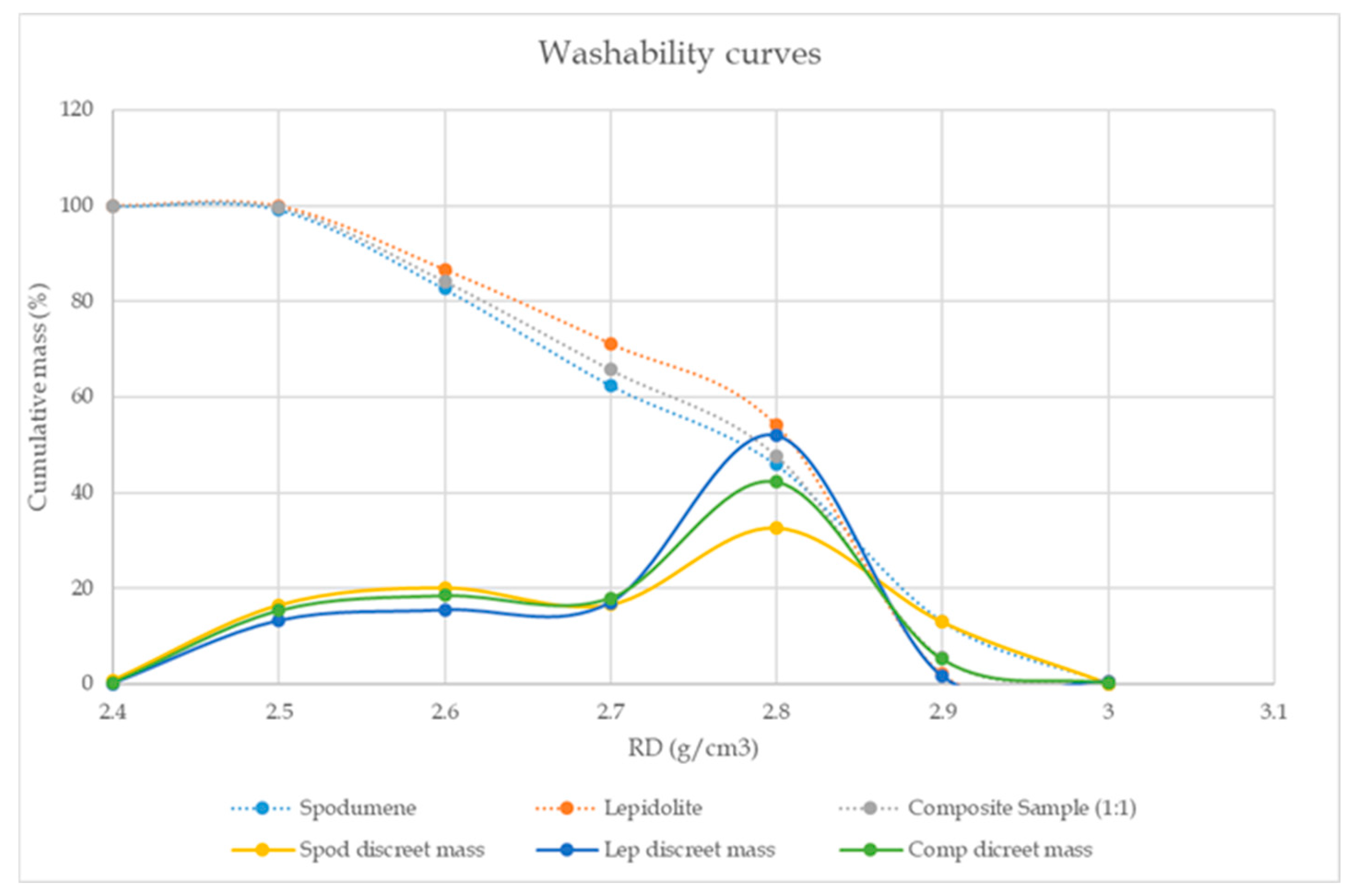
3.3. Gravity Separation: Laboratory Heavy Liquid Separation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2018, 131, 170–184. [Google Scholar] [CrossRef]
- Chen, P.; Lin, Z.; Tan, T.; Zhang, Y. Lithium-Ion Battery Development with High Energy Density. Highlights Sci. Eng. Technol. 2022, 27, 806–813. [Google Scholar] [CrossRef]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T.; Smith, D.L.; Bottomer, L. The recovery and concentration of spodumene using dense media separation. Minerals 2021, 11, 649. [Google Scholar] [CrossRef]
- Garside, M. Lithium—Statistics & Facts. In Mining, Metals & Minerals; Statista: Hamburg, Germany, 2022; Available online: https://www.statista.com/topics/3217/lithium/#topicheader_wrapper (accessed on 4 May 2024).
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium extraction from hard rock lithium ores (spodumene, lepidolite, zinnwaldite, petalite): Technology, resources, environment and cost. China Geol. 2022, 6, 137–153. [Google Scholar]
- Abaka-Wood, G.B.; Acquah, G.; Owusu, C.K.; Addai-Mensah, J. A Review of Characterization Techniques and Processing Methods for Lithium Extraction. In Proceedings of the 7th UMaT Biennial International Mining and Minerals Conference, Tarkwa, Ghana, 30 July–1 August 2022; pp. 1–10. [Google Scholar]
- Gibson, C.; Aghamirian, M.; Grammatikopoulos, T. A Review: The Beneficiation of Lithium Minerals from Hard Rock Deposits; Society for Mining, Metallurgy & Exploration: Denver, CO, USA, 2017. [Google Scholar]
- Garret, D. Handbook of Lithium and Natural Calcium Chloride, Part 1; Elsevier: New York, NY, USA, 2004; pp. 1–235. [Google Scholar]
- Chesnutt, J.; Qafoku, N.; Kreuzer, R. Lithium Occurrence and Concentration Grade Around the Nation. 2023. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-35152.pdf (accessed on 3 April 2024).
- Maistry, N.; Singh, A. HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade. In Proceeding of the MEI’s 8th Physical Separation Conference, Cape Town, South Africa, 10–12 June 2024; MEI Conferences. Available online: https://www.min-eng.com/physicalseparation24/drafts/session2/maistry.pdf (accessed on 15 November 2024).
- Morgan, C. Theoretical and Practical Aspects of Variography: In Particular, Estimation and Modelling of Semi-Variograms over Areas of Limited and Clustered or Widely Spaced Data in a Two-Dimensional South African Gold Mining Context. 2012. Available online: https://wiredspace.wits.ac.za/items/6fc3aadc-75d3-462f-9d04-edc500885e2c (accessed on 21 April 2024).
- Shanghai Metals Market. Galaxy Resources: Q1 Produces 41874 Metric Tons of Spodumene Concentrate. 2023. Available online: https://news.metal.com/newscontent/100921168/[SMM-News]-galaxy-resources:-q1-produces-41874-metric-tons-of-spodumene-concentrate (accessed on 11 May 2024).

| Grade % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Al | Ca | Co | Cr | Cu | Fe | Li | Mg | Mn | Ni | Pb | Si | Ti | V | Zn |
| Spodumene | 8.89 | 0.16 | 0.05 | 0.05 | 0.06 | 0.11 | 1.14 | 0.05 | 0.06 | 0.05 | 0.05 | 34.2 | 0.05 | 0.05 | 0.06 |
| Lepidolite | 12.1 | 0.12 | 0.05 | 0.05 | 0.05 | 0.05 | 1.18 | 0.05 | 0.12 | 0.05 | 0.05 | 27.0 | 0.05 | 0.05 | 0.05 |
| Sample | Density (g/cm3) | Relative Density (g/cm3) | % Mass | %Cum Mass | % Grade | Recovery % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discrete | Cumulative | Discrete | Cumulative | |||||||||
| Li | Li2O | Li | Li2O | Li | Li2O | Li | Li2O | |||||
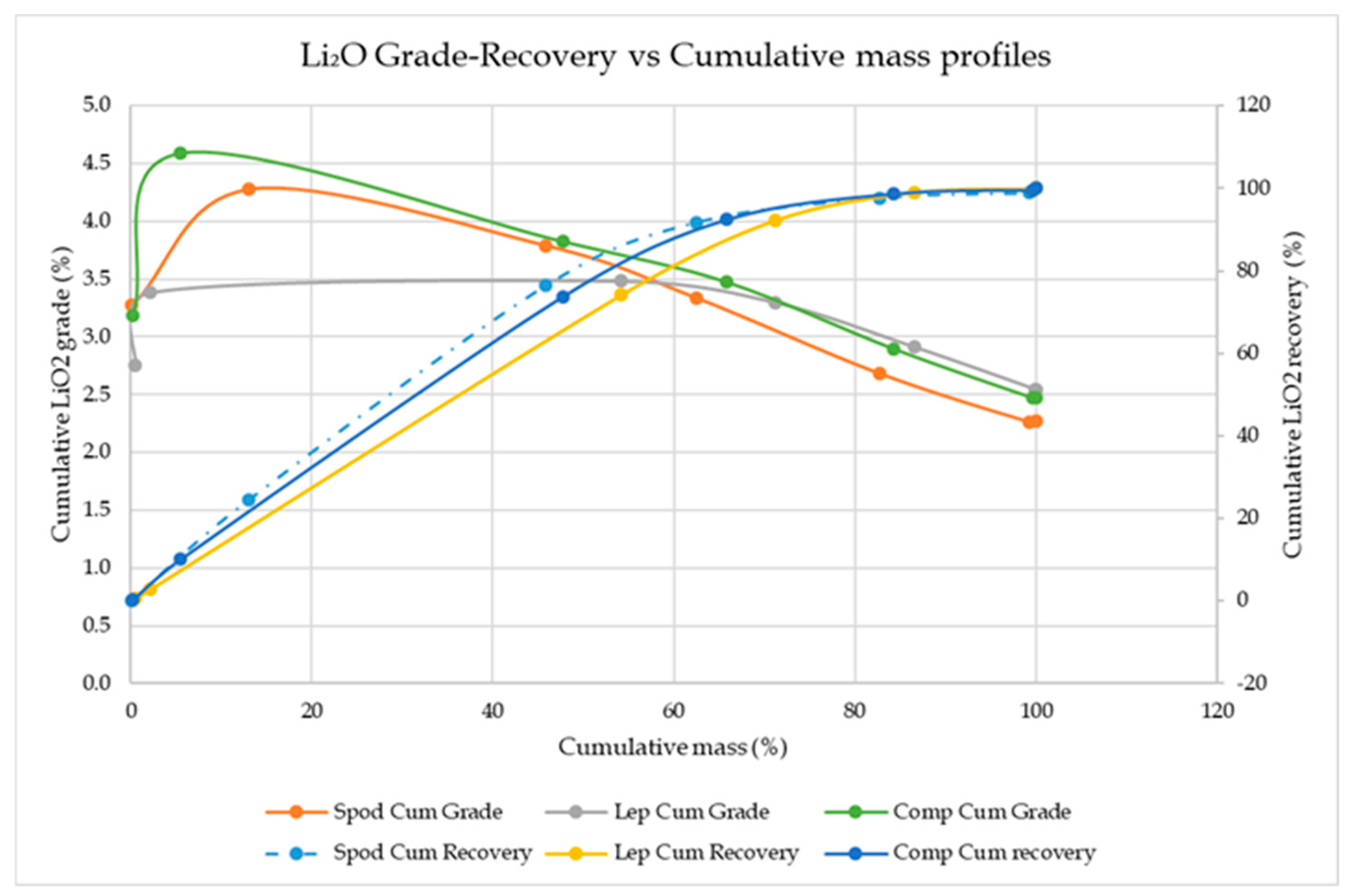
| Spodumene | +3.00 | 3.00 | 0.10 | 0.10 | 1.52 | 3.27 | 1.52 | 3.27 | 0.14 | 0.14 | 0.14 | 0.14 |
| −3.00 + 2.90 | 2.90 | 13.0 | 13.1 | 1.99 | 4.28 | 1.99 | 4.28 | 24.5 | 24.5 | 24.6 | 24.6 | |
| −2.90 + 2.80 | 2.80 | 32.8 | 45.9 | 1.67 | 3.60 | 1.76 | 3.79 | 51.8 | 51.8 | 76.5 | 76.5 | |
| −2.80 + 2.70 | 2.70 | 16.7 | 62.5 | 0.97 | 2.09 | 1.55 | 3.34 | 15.3 | 15.3 | 91.8 | 91.8 | |
| −2.70 + 2.60 | 2.60 | 20.2 | 82.7 | 0.31 | 0.67 | 1.25 | 2.69 | 5.93 | 5.93 | 97.7 | 97.7 | |
| −2.60 + 2.50 | 2.50 | 16.5 | 99.2 | 0.08 | 0.17 | 1.05 | 2.27 | 1.25 | 1.25 | 99.0 | 99.0 | |
| −2.50 | 2.40 | 0.78 | 100 | 1.42 | 3.05 | 1.06 | 2.27 | 1.05 | 1.05 | 100 | 100 | |
| Lepidolite | +3.00 | 3.00 | 0.49 | 0.49 | 1.28 | 2.76 | 1.28 | 2.75 | 0.53 | 0.53 | 0.53 | 0.53 |
| −3.00 + 2.90 | 2.90 | 1.60 | 2.08 | 1.66 | 3.57 | 1.57 | 3.38 | 2.24 | 2.24 | 2.77 | 2.77 | |
| −2.90 + 2.80 | 2.80 | 52.0 | 54.1 | 1.62 | 3.49 | 1.62 | 3.48 | 71.3 | 71.3 | 74.1 | 74.1 | |
| −2.80 + 2.70 | 2.70 | 17.0 | 71.2 | 1.26 | 2.71 | 1.53 | 3.3 | 18.2 | 18.2 | 92.3 | 92.3 | |
| −2.70 + 2.60 | 2.60 | 15.5 | 86.6 | 0.52 | 1.12 | 1.35 | 2.91 | 6.82 | 6.82 | 99.1 | 99.1 | |
| −2.60 + 2.50 | 2.50 | 13.3 | 99.9 | 0.08 | 0.16 | 1.18 | 2.54 | 0.84 | 0.84 | 99.9 | 99.9 | |
| −2.50 | 2.40 | 0.09 | 100 | 0.94 | 2.02 | 1.18 | 2.54 | 0.07 | 0.07 | 100 | 100 | |
| Composite | +3.00 | 3.00 | 0.23 | 0.23 | 1.48 | 3.19 | 1.48 | 3.19 | 0.30 | 0.30 | 0.30 | 0.30 |
| −3.00 + 2.90 | 2.90 | 5.23 | 5.46 | 2.16 | 4.65 | 2.13 | 4.59 | 9.83 | 9.83 | 10.1 | 10.1 | |
| −2.90 + 2.80 | 2.80 | 42.3 | 47.8 | 1.73 | 3.72 | 1.78 | 3.82 | 63.7 | 63.7 | 73.8 | 73.8 | |
| −2.80 + 2.70 | 2.70 | 18.0 | 65.8 | 1.19 | 2.55 | 1.61 | 3.48 | 18.6 | 18.6 | 92.4 | 92.4 | |
| −2.70 + 2.60 | 2.60 | 18.5 | 84.3 | 0.39 | 0.84 | 1.35 | 2.90 | 6.28 | 6.28 | 98.7 | 98.7 | |
| −2.60 + 2.50 | 2.50 | 15.4 | 99.7 | 0.07 | 0.15 | 1.15 | 2.47 | 0.92 | 0.92 | 99.6 | 99.6 | |
| −2.50 | 2.40 | 0.30 | 100 | 1.51 | 3.25 | 1.15 | 2.47 | 0.40 | 0.40 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maistry, N.; Singh, A. HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade. Minerals 2025, 15, 396. https://doi.org/10.3390/min15040396
Maistry N, Singh A. HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade. Minerals. 2025; 15(4):396. https://doi.org/10.3390/min15040396
Chicago/Turabian StyleMaistry, Nichole, and Ashma Singh. 2025. "HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade" Minerals 15, no. 4: 396. https://doi.org/10.3390/min15040396
APA StyleMaistry, N., & Singh, A. (2025). HLS Testwork on Spodumene and Lepidolite Samples to Determine Maximum Achievable Lithium Upgrade. Minerals, 15(4), 396. https://doi.org/10.3390/min15040396







