Abstract
The objective of this project was to document the early stages of growth of carbonate minerals in the presence of two different organic compounds commonly associated with cell walls or found in biofilms. Organic molecules are believed to influence an environment to be more favorable for carbonate mineral precipitation or serve as a substrate for the initiation of crystal growth. Palmitic and stearic acids are common fatty acids that bind to cell walls and are the most common organic molecules in marine environments. Scanning electron microscope (SEM), transmission electron microscope (TEM), and energy dispersive X-ray spectroscopy (X-EDS) analyses were used to image the interface between the organic molecules and calcite minerals. The SEM and TEM images were used to further understand the interactions between organic compounds and calcite minerals. The palmitic and stearic acid showed a curious formation of spheroidal structures in a spatial relationship with calcite crystal growth. This research is significant because it shows that a spatial relationship exists between organic matter and the mineral calcite. More importantly, the organic material may be acting as a nucleation surface. These experiments represent patterns similar to those observed in nature.
Keywords:
geobiology; carbonates; palmitic acid; stearic acid; SEM; TEM; calcite; precipitation; interface 1. Introduction
The precipitation of carbonate minerals is important in the fields of sedimentology, oceanography, and limnology [1]. Since the 1980s, when the study of travertine deposits in Italy promoted the development of the field of geomicrobiology and initiated interest in the smallest components of rocks, once termed nannobacteria, debate has continued about the influence of microbes and associated organic material on crystal precipitation [2,3,4,5,6,7,8,9,10,11,12,13]. Spherical to subspherical structures 200–300 nm in size were first thought to be simple, nanometer-scale organisms and were suggested as initiators of carbonate mineral growth [14]. Continued research into the early 2000s documented the role of microbes as mediators of initial crystal growth and included crystals previously thought to only be of fully inorganic origin [15,16,17,18,19]. Many sedimentologists now acknowledge that microbes can play a role in carbonate mineral precipitation. Understanding of the role of organic molecules in many processes is increasing [20,21,22,23], but questions remain about detailed aspects and significance of their role in the initiation of mineral precipitation. The objective of this project is to attempt to precipitate the spheroidal structures in a laboratory setting using organic materials and to mimic what has been commonly documented in nature. This paper does not seek to explain the mechanisms but instead introduces evidence that organic molecules can precipitate the same structures that have been identified with microbes, EPS, and viruses.
2. Background Information
2.1. Microbial Importance
Microbes, particularly cyanobacteria, are noted for their role in the formation of microbial carbonate rocks and are the organism primarily responsible for the development of some distinctive carbonate structures [14,18]. Cyanobacteria, also known as cyanophytes, are aerobic phototrophs with a geologic history going as far back as the Archaean Eon [18]. They are capable of photosynthesis or gas fixation and thrive in water columns, especially near the sediment–water threshold. Most bacteria are anaerobic heterotrophs that can occupy dark, anaerobic conditions; for example, sediment pore spaces [24]. Many bacteria obtain their energy by degrading minerals and other materials. This mineral dissolution is a key aspect of the weathering process and makes microbes important to the biogeochemical cycle of elements [18].
2.2. Nucleation Points in Carbonate Mineral Formation
Literature on the nucleation of carbonate minerals is very qualitative and rarely touches on the kinetics of the process, which remain poorly understood [25], especially in the presence of organic matter. Nucleation of calcium carbonate on microbial cell material is considered a dominant mode of carbonate formation throughout Earth’s history. The hypothesis is that nucleation takes place on the cell surface or other extracellular polymeric substances (EPSs) [26]. Previous research has shown that the role of organic matter in the nucleation processes rather than microbial metabolism may control microbial carbonate formation [15].
The majority of the evidence for the microbial and carbonate crystal growth relationship is the close spatial relationship they share [27]. The majority of laboratory work/experiments provide indirect evidence. Microbial cell surfaces, along with other negatively charged functional groups like carboxyl and phosphate, carry a negative electric charge and therefore can bind Ca2+ ions [28]. After EPS is degraded, calcium saturation results in calcium carbonate precipitation, and cell walls have been frequently cited as areas of carbonate nucleation (Figure 1) [27,28,29,30]. If calcium carbonate nucleation does take place on a cell, it would probably lead to cell entombment and eventually death. Microbes, in prior experiments investigating bacterial nanoglobules, only had a lifespan of a few hours [29].
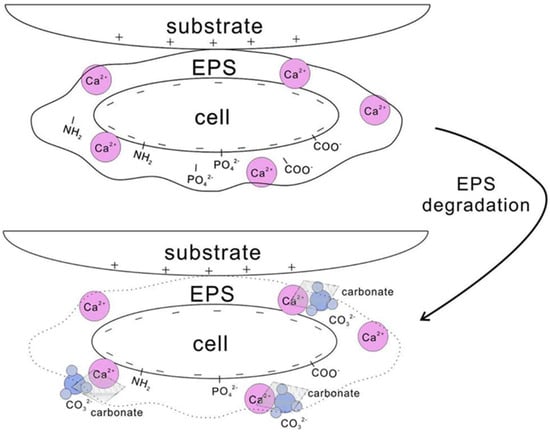
Figure 1.
Nucleation of carbonate crystals when EPS captures Ca2+. Once EPS is degraded, saturation of Ca2+ results in the precipitation of calcium carbonate using the cell wall as a source of nucleation (modified from Zhu and Dittrich [28]).
Previous experiments have shown that the nucleation points on cells or other extracellular organic material were positively charged, but the overall results were inconclusive. In Krause et al. [26], Desulfobulbus mediterraneus, a marine sulfate-reducing bacterium, was introduced into a Mg2+ and Ca2+-saturated solution where dolomite crystals precipitated excessively within or attached to spheroidal extracellular organic material. Krause et al. [26] believed the Ca2+ and Mg2+ ions seemed to effectively bind to the extracellular material.
D. desulfuricans-strain bacteria were used in a bicarbonate medium to determine if the presence of bacteria stimulates calcite precipitation. Unlike D. mediterraneus, D. desulfuricans is not a sulfate-reducing organism, but it is a facultative anaerobe. Therefore, it can survive in an oxygenated environment through aerobic processes; however, it is capable of switching to anaerobic respiration if needed. It is usually found in the human gut and is distantly related to E. coli HB101. The bacterium was introduced to nigericin to collapse its membrane and UV light to prevent cell division. This stops the cell’s metabolic process. The bacterium still modified its medium to precipitate 82% more calcite than the controls with the same medium through organic substrates for nucleation. SEM analysis from their experiment showed large amounts of crystal growth on the bacterium and its spherical extracellular material, which were released when the membrane collapsed [15].
2.3. Observations in Nature
The spheroidal structures have been identified in nature (Figure 2) and discussed thoroughly, with no overall agreed upon understanding of what they are or how they are formed. Over time, terms have evolved to describe these spheroidal structures, like microbilites, microorganisms, biofilm, and nanospheres that vary in size, shape, and form. These spheroidal shapes most recently are explained through EPS and have even been correlated to virus-like particles [31,32]. Past works have also given them potential roles in the precipitation or nucleation of carbonate minerals [2,3,4,5,6,7,8,9,10,11,26,31,32].

Figure 2.
(A) SEM image of spheroidal shapes identified as nanocrystalline structures within extracellular polymeric substance (EPS) matrix in dolomite precipitation experiments (modified from Krause et al. [26]). (B) SEM image of spheroidal structures closely associated with microorganisms in a microbial mat. White arrow spheroidal structures are identified as EPS making a transition to neo-formed carbonate minerals. Black arrow represents bacterial cells (modified from Perri et al. [31]). (C) SEM image of EPS-formed spheroidal shapes (black arrows) classified as nanospheres in a modern microbial mat from Qatar (modified from Tucker [32]).
2.4. Organic Samples Used
Palmitic and stearic acids were chosen as the two organic molecules for this project because they are two of the most common fatty acids in the marine environment [33]. Palmitic and stearic acids are also the most abundant fatty acids found in cell walls in biofilms [34,35]. When palmitic and stearic acid begin to degrade, like all fatty acids, they release energy and interact with the marine environment [36,37]. Palmitic and stearic acid are also found in biofuels produced from marine microalgae [37]. This suggests that both fatty acids are found where bacteria are believed to be producing carbonate structures.
3. Materials and Methods
Precipitation experiments were conducted to further understand the interactions between the organic compounds and calcite minerals. The samples were grown for a set amount of time: 18, 24, and 48 h. Each sample was then prepared and analyzed using SEM and TEM.
3.1. Pre-Precipitation Preparation
The glassware used in the experiments was sterilized using an autoclave for 30 min at 121 °C. The glassware was then allowed to dry under a laminar fume hood. Calcium oxide was created by heating 0.02 g of reagent-grade calcium carbonate to 1000 °C for four hours to combust any organic compounds. The calcium oxide was put into solution with distilled water in three separate beakers with one for each of the organic compounds and one with no organic matter, just calcium oxide and distilled water, as a control following a protocol previously used by Kirkland et al. [23].
3.2. Carbonate Mineral Precipitation
Three sets of experiments were conducted, and in each experiment, each organic compound interacted with calcium oxide for 18, 24, and 48 h. The pairing consisted of palmitic acid and calcium oxide, stearic acid and calcium oxide, and a control of pure calcium oxide in distilled water. Each paring contained 0.02 g of the organic compound along with 0.02 g of heated calcium oxide in 40 mL of sterilized, pyrogen-free water. Each experiment was conducted in a laminar fume hood at room temperature and was covered with Speedy-FoilTM to reduce atmospheric CO2. The experiments were not conducted in a vacuum canister, and atmospheric CO2 was still present. This allows for atmospheric CO2 to dissolve into the samples as well as potential degradation of organic material. Because the experiments were testing the interaction with organic compounds and calcite minerals, a sub-experiment was conducted where palmitic acid was allowed to precipitate for 24 h with silica glass to see if similar substrate growth could be documented.
3.3. Polycarbonate Filtering
Once the organic compounds were allowed to interact for their set amount of time, the samples were filtered. A polycarbonate 0.01 µm, 47 mm diameter membrane filter was used because organic components and precipitates in previous experiments were as small as 0.2–0.3 µm [14]. The membrane filter was mounted to a Nalgene vacuum canister. The top layer of the solution in the beaker was slowly poured into the vacuum canister, allowing the precipitates to adhere to the membrane filter.
3.4. Scanning Electron Microscopy
A 1 mm2 section of carbon tape was cut and mounted onto a stainless steel stub. The stub was then dipped down onto the membrane filter, and the precipitates adhered to the carbon tape. A 5 µm coating of platinum was applied using an EMS 150T ES high-resolution sputter coater to reduce the amount of charging in the SEM. A JEOL JSM-6500F Field Emission SEM was used to analyze the interaction between the precipitants and organic material. Images ranging from 1600× to 33,000× at 5 kV–30 kV were taken.
3.5. Transmission Electron Microscope
The precipitants were carefully transferred from the polycarbonate membrane filter to a 300-count copper grid filter by dipping the filter onto the copper grid. The precipitants were not allowed to fully dry before mounting to attempt to catch images of new precipitants forming. The grid was loaded into a JEOL 1230 120 KV TEM, and the samples were inspected at 10,000× to 300,000× at 120 kV.
Selected area electron diffraction (SAED) was conducted on the carbonate minerals to confirm calcite mineralogy throughout the precipitation experiments.
3.6. Energy-Dispersive X-Ray Spectroscopy
An energy-dispersive X-ray spectrometer (X-EDS) attached to the JEOL 1230 120 KV TEM and Oxford Instruments INCA Energy+ software was used to conduct electron beam-induced X-ray elemental analysis of individual samples. Areas of interest that showed a well-established transition between the precipitants and organic material were analyzed with X-EDS. The elemental maps were used to determine areas of organic concentrations near crystal growth. Point scans were used to verify the elemental maps and to study the transitional areas between the organic material and carbonate mineral growth.
4. Results
4.1. Calcite Identification
Selected area electron diffraction (SAED) was conducted on the carbonate minerals in the precipitation experiments for the control and for each organic molecule. The diffraction patterns showed the structure of the calcite crystal lattice (Figure 3).
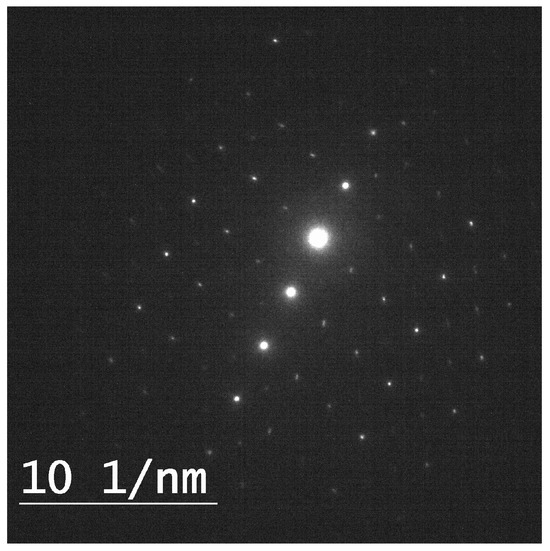
Figure 3.
SAED pattern of calcite along the [01−10] plane obtained at 10 per [1/nm] scale. Taken from palmitic and calcite precipitation experiment at 48 h.
4.2. Scanning Electron Microscopy Analysis
SEM analysis was conducted on the precipitants created from the stearic acid, palmitic acid, and calcium oxide experiments. The control experiment with just calcium oxide at 24 h showed clumps of anhedral calcium carbonate crystals no larger than 1 µm in size (Figure 4A). At 48 h, the control experiment showed well-defined rhombohedral crystals ranging from 0.5 to 7 µm in size with no spheroidal structures (Figure 4B). A control experiment was created where palmitic acid was allowed to precipitate for 24 h with silica glass to see if similar substrate growth to calcium carbonate and palmitic acid could be documented (Figure 4C). No precipitation or relationship was observed between the silica glass and palmitic acid.
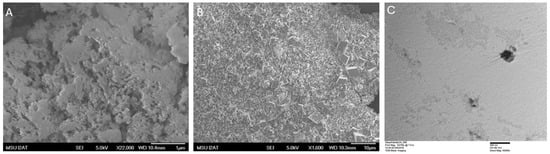
Figure 4.
(A) SEM photomicrograph of control after 24 h showing calcium carbonate precipitate formed from anhedral crystal nuclei. (B) SEM photomicrograph of control sample after 48 h showing only calcium carbonate precipitate, consisting of large clusters of visually identifiable calcite crystals ranging from 0.5 to 7 µm. (C) TEM photomicrograph showing sub-experiment of palmitic acid with silica glass for 24 h. No substrate growth occurred.
Palmitic acid with calcium oxide experiments at 24 h showed well-defined rhombohedral crystal growth ranging between 1 µm and 3 µm in size and an abundance of spheroidal structures (Figure 5A). At 48 h, the size of the rhombohedral crystals size was similar to the size of the crystals in the 24 h experiment, but the number of crystals and spheroidal structures had increased significantly (Figure 5B).
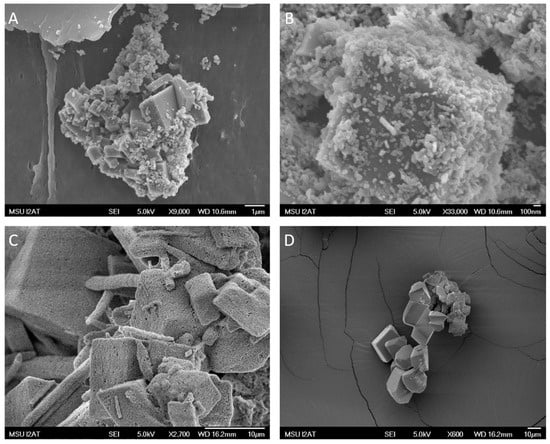
Figure 5.
(A) SEM photomicrograph showing calcium carbonate precipitate formed in a solution containing palmitic acid after 24 h. The image shows organic spherical to subspherical debris of 0.1 to 0.4 µm intermixed with euhedral calcite crystals. (B) SEM photomicrograph showing precipitate formed in a solution containing palmitic acid after 48 h showing organic material attached to large euhedral calcite crystals. (C) SEM photomicrograph showing calcium carbonate precipitate formed in a solution containing stearic acid after 24 h. Crystals seem more textured, and there is a reduction in spheroidal structures. (D) SEM photomicrograph showing calcium carbonate precipitate formed in a solution containing stearic acid after 48 h.
Precipitates created in a solution containing stearic acid showed results similar to the precipitates grown with palmitic acid with regard to crystal size. However, in the stearic acid solution sample pulled after 24 h, the calcite minerals seemed porous and more textural than the crystals grown during the control and palmitic acid experiments (Figure 5C). The sample pulled after 48 h showed a significant decrease in spheroidal structures, and the calcite minerals were more euhedral (Figure 5D).
4.3. Transmission Electron Microscopy Analysis
TEM analysis was conducted on the precipitants created from the stearic acid, palmitic acid, and calcium oxide experiments. The TEM images showed carbonate crystal growth occurring within the solutions containing the palmitic and stearic acid. Rhombohedral calcite crystals were seen growing near or within the organic material in both the palmitic and stearic acid experiments (Figure 6A,B, Figure 7A–C and Figure 8A–D).
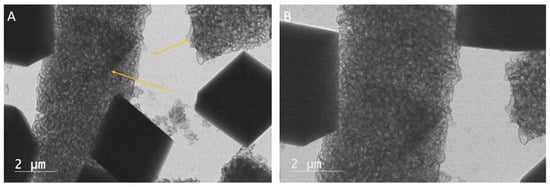
Figure 6.
(A) TEM micrograph of palmitic acid with calcium oxide after 24 h. Calcium carbonate, or in this case, crystals of the mineral termed calcite, appear as dense, therefore dark, black rhombs, and are more apparent. Rhombohedral structures seem to be composed of the organic compounds from the surrounding area, indicated by the arrows. The arrow on the right points to a cluster of palmitic acid in the rhombohedral shape of a calcite crystal. The arrow on the left points to the margin of a calcite crystal that appears to be forming within the palmitic acid. (B) TEM micrograph of palmitic with calcium oxide after 24 h. The magnified view in Figure 5A shows the features indicated by the arrows. The palmitic acid appears both as clumps being organized into rhombohedral crystals and clumps of palmitic acid, appearing to be nucleation sites for calcite crystals.
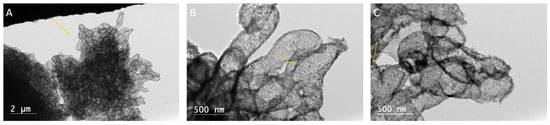
Figure 7.
(A) TEM micrograph of palmitic acid with calcium oxide after 18 h. This image shows dark rhombohedral shapes suggesting calcite/calcium carbonate crystals (arrow) growing within clusters of palmitic acid. (B) TEM micrograph of palmitic acid with calcium oxide after 18 h. Image shows carbonate rhombohedral crystal structures beginning to grow within the palmitic acid, as indicated by arrows. Magnified micrograph from Figure 6A. (C) TEM micrograph of palmitic acid with calcium oxide after 18 h. Incipient carbonate crystal structures are visible, nucleating within the palmitic acid. Magnified micrograph from Figure 6B.
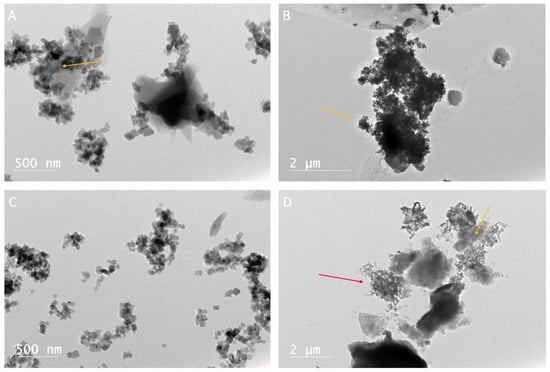
Figure 8.
(A) TEM micrograph of stearic acid with calcium oxide after 18 h. Arrow indicates calcium carbonate crystals as rhombohedrons growing in clusters; the crystal nucleation point must be within the stearic acid. (B) TEM micrograph of stearic acid with calcium oxide after 24 h. Micrograph shows carbonate rhombohedral minerals (arrow) growing intermixed with stearic acid (dark amorphous sections). (C) TEM micrograph of stearic acid with calcium oxide after 48 h. Photomicrograph shows carbonate minerals are more pronounced. (D) TEM photomicrograph of stearic acid with calcium oxide after 48 h. A dense cluster of carbonate crystals is indicated by the yellow arrow. Stearic acid without precipitate is indicated by the red arrow, but it is less abundant at 48 h than it was in samples drawn off at 18 and 24 h.
After 24 h, crystal nucleation in the palmitic acid experiments is seen, with organic compounds caught up within the crystal structure. The photomicrograph shows that the palmitic acid appears as clusters of small, subspherical, amorphous structures (Figure 6A,B). Within the rhombohedral crystal shapes are an abundance of these small, sub-spherical amorphous structures that resemble tiny carbonate proto-crystals within a larger rhombohedral crystal. In some cases, rhombohedral calcite crystals appear to be forming within the clumps of palmitic acid; structures that appear as elongate, irregularly curving, worm-like structures. Low-magnification micrographs show that the calcium carbonate crystals grew in clusters both within and around the palmitic acid (Figure 7A–C).
TEM analysis of the precipitates from the solutions containing stearic acid showed that the organic compounds were commonly found in clumps. The clumps of stearic acid contained clusters of rhombohedral calcium carbonate crystals 0.5 to 2 nm in size (Figure 8A–D). The crystals were consistently found forming within and surrounded by the stearic acid. The nucleation site for the crystals appeared to be within the clumps of stearic acid.
4.4. Energy-Dispersive X-Ray Spectroscopy
X-EDS analysis was used on areas with well-established transition zones between organic molecules, and carbonate mineral growth shows a clear transition from carbon-rich organic molecules and calcium-rich crystals. Carbonate minerals can be seen directly nucleating from a single point of palmitic acid (Figure 9). X-EDS spectral analysis and map analysis were used to verify the difference between carbonate minerals and organic material. The TEM micrograph (Figure 9) showed a larger concentration of carbon at 37.3 wt%; oxygen was at 10.8 wt%; and calcium was at 7.8 wt% (Figure 10). Elemental map analysis showed the concentration of carbon throughout the carbonate minerals and palmitic acid, with concentrations of calcium and oxygen only in the crystal structures (Figure 11).
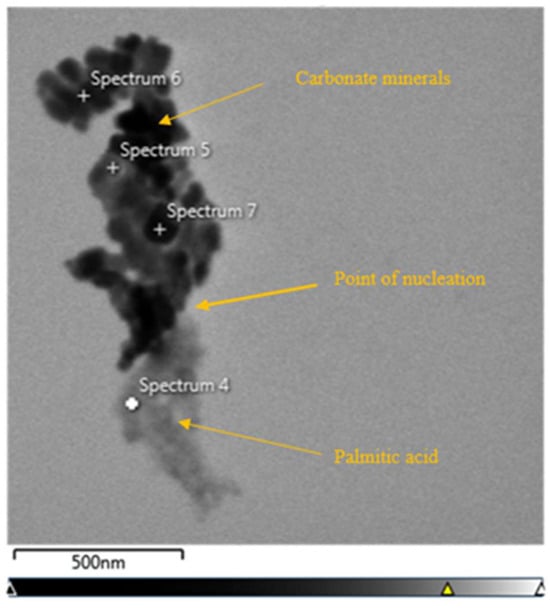
Figure 9.
TEM micrograph of palmitic acid with calcium oxide after 24 h showing a transition from palmitic acid (bottom) and carbonate mineral growth (top).
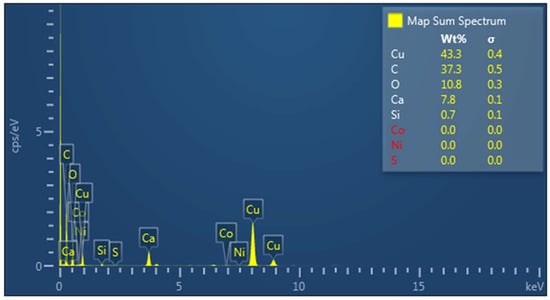
Figure 10.
X-EDS overall elemental spectrum of TEM micrograph (Figure 8) showing a high concentration of carbon and calcium carbonate crystals in the overall micrograph.
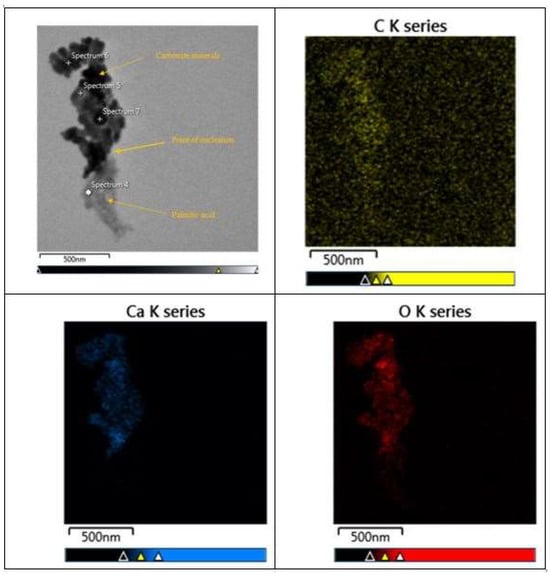
Figure 11.
Elemental map analysis of Figure 8 showing the distribution of elemental concentrations of carbon, oxygen, and calcium.
5. Discussion
5.1. Scanning Electron Microscopy
The SEM analysis of the calcium oxide controls showed that clusters of anhedral calcite crystals, each less than one micron in size, were common in the 18 and 24 h experiments. With increased precipitation time, well-defined rhombohedral crystals of 0.5 to 7 µm in size developed. The palmitic acid and calcium oxide experiments showed well-defined calcite crystals after 18 and 24 h of precipitation. This suggests faster calcite crystal growth in the presence of palmitic acid than in the control experiment. An abundance of spheroidal structures was also noted in the 18 and 24 h experiments with palmitic acid. After 48 h, the rhombohedral carbonate mineral crystal sizes were similar to the 18 and 24 h experiments, but there was a notable decrease in small (<100 nm) spheroidal structures, which appeared to serve as protocrystals.
The stearic acid experiments were unexpectedly different from the experiments using palmitic acid. The crystal sizes were similar in the 18, 24 and 48 h experiments, but fewer spheroidal structures were found when precipitating the calcite with the stearic acid. A spheroidal textured pattern was common on many of the crystal faces as well at the 18 and 24 h experiments.
5.2. Transmission Electron Microscopy
The purpose of this project is to determine the interaction between organic compounds or fatty acids and the initial phases of carbonate precipitation. TEM micrographs of precipitation experiments conducted with calcium oxide and organic molecules show a relationship between organic material and calcite minerals. TEM micrographs of organic compounds show an unordered cluster of low-density organic material with no set shape or structure. When both palmitic and stearic organic compounds were combined with 0.02 g of calcium oxide, crystal growth began to appear as expected, but the organic compounds still maintained an amorphous structure. TEM imaging revealed the progress of crystal formation over a period of 18 to 48 h.
5.3. Palmitic Acid
Solutions of palmitic acid with calcium oxide allowed to precipitate for 18–24 h showed similar results. In both the 18 and 24 h precipitation times, well-established amorphous organic structures could be seen. The precipitants allowed to grow for 18 h showed an abundance of calcite crystals about 2 nm in size growing within the organic structures (Figure 7A–C). The 24 h experiments showed well-established rhombohedral crystals. Some of the carbonate crystals had palmitic acid as their core internal structure (Figure 6A,B). Calcite minerals were also found directly nucleating from singular points on the palmitic acid (Figure 8). X-EDS analysis was conducted as a check to verify the transition from palmitic acid to calcite (Figure 10 and Figure 11). The relationship between calcite growing within the palmitic acid (Figure 7A–C), and inversely, the palmitic acid making up the internal structure of some of the calcite (Figure 6A,B), showed a direct relationship between the two.
5.4. Stearic Acid
Stearic acid showed results similar to the palmitic precipitants. Carbonate minerals appeared to nucleate within clumps of stearic acid. No significant change was noticeable between the precipitants after 18, 24, or 48 h. The growth of calcite crystals is clearly promoted by stearic acid. The majority of the calcite crystals appeared to grow near or intermixed within the stearic acid (Figure 8A–D).
5.5. Relation to Observations in Nature
Spheroidal structures such as the ones described in this paper have been identified in nature and discussed thoroughly, with no set understanding of what they are or how they have formed. Most hypotheses relate their formation to biological activity, as this paper supports.
6. Conclusions
The SEM and TEM captured high-magnification images that showed distinguishable differences in crystal growth, crystal morphology, and spheroidal structures depending on which organic compound was used in the solution. The TEM allowed for a better understanding of the internal working relationship between the initial growth of calcite crystals and organic molecules.
- The calcite crystals formed in association with palmitic acid showed that at short temporal precipitation periods, carbonate minerals, some with rhombohedral shapes, are found as internal structures within clumps of palmitic acid. The crystals appeared to be nucleating within the palmitic acid.
- The crystals formed in association with palmitic acid at 18, 24 and 48 h showed calcium carbonate crystals grown in rhombohedral shapes that were visually identifiable as calcite. The calcite crystals seemed to grow within the palmitic acid and also to organize the palmitic acid into rhombohedral crystals. The carbonate crystals appeared to be primarily composed of palmitic acid in a rhombohedral package. The TEM images in Figure 5A,B show the palmitic acid forming a uniform structure that appears to mimic the surrounding calcite crystals.
- The stearic acid experiments ranging from 18 to 48 h showed a close relationship between the organic compound and carbonate mineral growth. Though no direct incorporation of the stearic acids was imaged in the carbonate minerals, the consistent association and overlapping of the minerals and stearic acid suggest that a chemical relationship does occur.
The palmitic and stearic acid showed a curious spatial relationship with calcite crystal growth. Specifically, palmitic acid was found forming alongside calcite crystal structures. The objective of this project was to visualize the relationship between calcium carbonate precipitation and organic compounds. The specific details of how this process works on the molecular level are open to interpretation, and further investigation is encouraged.
The relationship between organic compounds and carbonate mineral growth has been identified throughout nature. In geology, these patterns are found in a variety of settings, from travertine rocks in Italy to calcified algal mats and potentially as far back as the Precambrian Era [3,4,14,15,18]. In medical research, organic subspherical structures are found with carbonate minerals in arterial pathways and urinary infections [38]. As often as these patterns have been documented, no widely accepted mechanism or understanding has been discovered. Recently published research has investigated similar structures that may be related to the roles viruses play in biomineralization [31,32]. Carbonate precipitation and cementation as a byproduct of organic influences and microbial influences is an important process that requires better understanding [28]. This work achieved its goal of precipitating organic spheroidal structures in spatial relationship with calcite crystals, similar to what has been documented in nature. The purpose of this work was to recreate these structures in a laboratory setting and image and document these relationships. For further imaging of the interfaces of these patterns in ancient rocks and modern rocks, laboratory experiments are the only way to better understand these relationships. The objective of this work was not to investigate the kinetics of carbonate mineral precipitation with organic material. However, these experiments do add to the data showing that similar patterns occur at the interface between organic molecules and carbonate minerals, and the data mimic what has been seen in nature and laboratory settings with EPS, viruses, and direct contact with microbes.
Author Contributions
Conceptualization, M.P.T. and B.L.K.; methodology, M.P.T. and B.L.K.; formal analysis, M.P.T., E.B.L., and B.L.K.; investigation, M.P.T. and B.L.K.; writing—original draft preparation, M.P.T., E.B.L., and B.L.K.; writing—review and editing, M.P.T., E.B.L., and B.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation, grant number: DGE-0947419.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors thank Roohan Venkatesh KG Thirumalai, Amanda Lawrence, and I-wei Chu at Mississippi State University’s Institute for Imaging and Analytical Technologies. The authors also thank Adam Skarke and Brittany Garner for all of their help.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kazmierczak, T.F.; Tomson, M.B.; Nancollas, G.H. Crystal growth of calcium carbonate. A controlled composition kinetic study. J. Phys. Chem. 1982, 86, 103–107. [Google Scholar] [CrossRef]
- Cartwright, J.H.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Meredith, J.C. Recent Travertine Pisoliths (Pisoids) from Southeastern Idaho, U.S.A. In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 450–455. [Google Scholar]
- Chafetz, H.S.; Folk, R.L. Travertines: Depositional morphology and the bacterially constructed constituents. J. Sediment. Res. 1984, 54, 289–316. [Google Scholar]
- Folk, R.L. In Defense of Nannobacteria. Science 1996, 274, 1288. [Google Scholar] [CrossRef]
- Folk, R.L.; Lynch, F.L. Possible role of nannobacteria (dwarf bacteria) in clay mineral diagenesis and the importance of careful sample preparation in high-magnification SEM study. J. Sediment. Res. 1997, 67, 583–589. [Google Scholar]
- Folk, R.L.; Lynch, F.L. Nannobacteria on Earth Are Truly Living Organisms. In Proceedings of the Mars 2001: Integrated Science in Preparation for Sample Return and Human Exploration, Houston, TX, USA, 2–4 October 1999. [Google Scholar]
- Freer, A.; Greenwood, D.; Chung, P.; Pannell, C.L.; Cusack, M. Aragonite Prism-Nacre Interface in Freshwater Mussels Anodonta anatina (Linnaeus, 1758) and Anodonta cygnea (L. 1758). Cryst. Growth Des. 2010, 10, 344–347. [Google Scholar] [CrossRef]
- Hammes, F.; Boon, N.; de Villiers, J.; Verstraete, W.; Siciliano, S.D. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl. Environ. Microbiol. 2003, 69, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Kosamu, I.B.M.; Obst, M. The influence of picocyanobacterial photosynthesis on calcite precipitation. Int. J. Environ. Sci. Technol. 2009, 6, 557–562. [Google Scholar] [CrossRef]
- Pedone, V.A.; Folk, R.L. Formation of aragonite cement by nannobacteria in the Great Salt Lake, Utah. Geology 1996, 24, 763–765. [Google Scholar] [CrossRef]
- Sancho-Tomás, M.; Fermani, S.; Gómez-Morales, J.; Falini, G.; Garcia-Ruiz, J.M. Calcium carbonate bio-precipitation in counter-diffusion systems using the soluble organic matrix from nacre and sea urchin spine. Eur. J. Mineral. 2014, 26, 523–535. [Google Scholar] [CrossRef]
- Schwartz, M.K.; Hunter, L.W.; Huebner, M.; Lieske, J.C.; Miller, V.M. Characterization of biofilm formed by human-derived nanoparticles. Nanomedicine 2009, 4, 931–941. [Google Scholar] [PubMed]
- Folk, R.L. SEM imaging of bacteria and nannobacteria in carbonate rocks. J. Sediment. Petrol. 1993, 63, 990–999. [Google Scholar]
- Bosak, T.; Newman, D.K. Microbial nucleation of calcium carbonate in the Precambrian. Geology 2003, 31, 577–580. [Google Scholar]
- Muralidhar, K.; Mazumdar, A.; Karisiddaiah, S.M.; Borole, D.V.; Rao, B.R. Evidences of methane-derived authigenic carbonates from the sediments of the Krishna–Godavari Basin, eastern continental margin of India. Curr. Sci. 2006, 91, 318–323. [Google Scholar]
- Perry, R.S.; Mcloughlin, N.; Lynne, B.Y.; Sephton, M.A.; Oliver, J.D.; Perry, C.C.; Campbell, K.; Engel, M.H.; Farmer, J.D.; Brasier, M.D.; et al. Defining biominerals and organominerals: Direct and indirect indicators of life. Sedimentol. Geol. 2007, 201, 157–179. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Vissher, P.T.; Bittermann, A.G.; Van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar]
- Chafetz, H.S. Photography of bacterial shrubs in travertine, Idaho and Italy. J. Sedimentol. Petrol. 1981, 51, 1162. [Google Scholar]
- Cui, M.; Ma, A.; Qi, H.; Zhuang, X.; Zhuang, G. Anaerobic oxidation of methane: An “active” microbial process. MicrobiologyOpen 2015, 4, 1–11. [Google Scholar]
- Folk, R.L.; Chafetz, H.S. Quaternary Travertine of Tivoli (Roma), Italy: Bacterially Constructed Carbonate Rock. In Proceedings of the Geological Society of America Annual Meeting, Atlanta, GA, USA, 17–20 November 1980. [Google Scholar]
- Kirkland, B.L.; Lynch, F.L.; Rahnis, M.A.; Folk, R.L.; Molineux, I.J.; McLean, R.J.C. Alternative origins for nannobacteria-like objects in calcite. Geology 1999, 27, 347–350. [Google Scholar]
- Hu, Q.; Nielsen, M.H.; Freeman, C.L.; Hamm, L.M.; Tao, J.; Lee, J.R.I.; Han, T.Y.J.; Becker, U.; Harding, J.H.; Dove, P.M.; et al. The thermodynamics of calcite nucleation at organic interfaces: Classical vs. non-classical pathways. Faraday Discuss. 2012, 159, 509–523. [Google Scholar] [CrossRef]
- Besson, R.F.; Favergeon, L. Atomic-scale study of calcite nucleation in calcium oxide. J. Phys. Chem. 2013, 117, 8813–8821. [Google Scholar] [CrossRef]
- Krause, S.; Liebetrau, V.; Gorb, S.; Sánchez-Román, M.; McKenzie, J.A.; Treude, T. Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma. Geology 2012, 40, 587–590. [Google Scholar]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Microbial fossilization in carbonate sediments: A result of the bacterial surface involvement in carbonate precipitation. Sedimentology 2003, 50, 237–245. [Google Scholar]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Aloisi, G.G. Nucleation of calcium carbonates on bacterial nanoglobules. Geology 2006, 34, 1017–1020. [Google Scholar]
- Dupraz, C.V.; Visscher, P.T.; Baumgartner, L.K.; Reid, R.P. Microbe-mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 2004, 51, 745–765. [Google Scholar]
- Perri, E.; Tucker, M.E.; Slowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar]
- Tucker, M. Fossil viruses. Geol. Today 2020, 36, 156–160. [Google Scholar] [CrossRef]
- Mochida, M.; Kitamori, Y.; Kawamura, K.; Nojiri, Y.; Suzuki, K. Fatty acids in the marine atmosphere: Factors governing their concentrations and evaluation of organic films on sea-salt particles. J. Geophys. Res. Atmos. 2002, 107, AAC1-1–AAC1-10. [Google Scholar]
- Gomes, L.C.; Moreira, J.M.R.; Araújo, J.D.P.; Mergulhão, F.J. Surface conditioning with Escherichia coli cell wall components can reduce biofilm formation by decreasing initial adhesion. AIMS Microbiol. 2017, 3, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Li, M.X.; Lu, L.J.; Yang, S.; Liu, J. Relationship of cell-wall bound fatty acids and the demulsification efficiency of demulsifying bacteria Alcaligenes sp. S-XJ-1 cultured with vegetable oils. Bioresour. Technol. 2012, 104, 5310–5536. [Google Scholar] [CrossRef] [PubMed]
- Palmitic Acid in Cell Culture. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/palmitic-acid (accessed on 13 February 2025).
- Sharmin, T.; Hasan, C.M.M.; Aftabuddin, S.; Rahman, M.A.; Khan, M. Growth, Fatty Acid, and Lipid Composition of Marine Microalgae Skeletonema costatum Available in Bangladesh Coast: Consideration as Biodiesel Feedstock. J. Mar. Biol. 2016, 2016, 6832847. [Google Scholar] [CrossRef]
- Broomfield, R.J.; Morgan, S.D.; Khan, A.; Stickler, D.J. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: A simple method of control. J. Med. Microbiol. 2009, 58, 1367–1375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).