Comparative Analysis of Potentially Toxic Elements (PTEs) in Waste Rock and Tailings: A Case Study from the Recsk Mining Area, Hungary
Abstract
1. Introduction
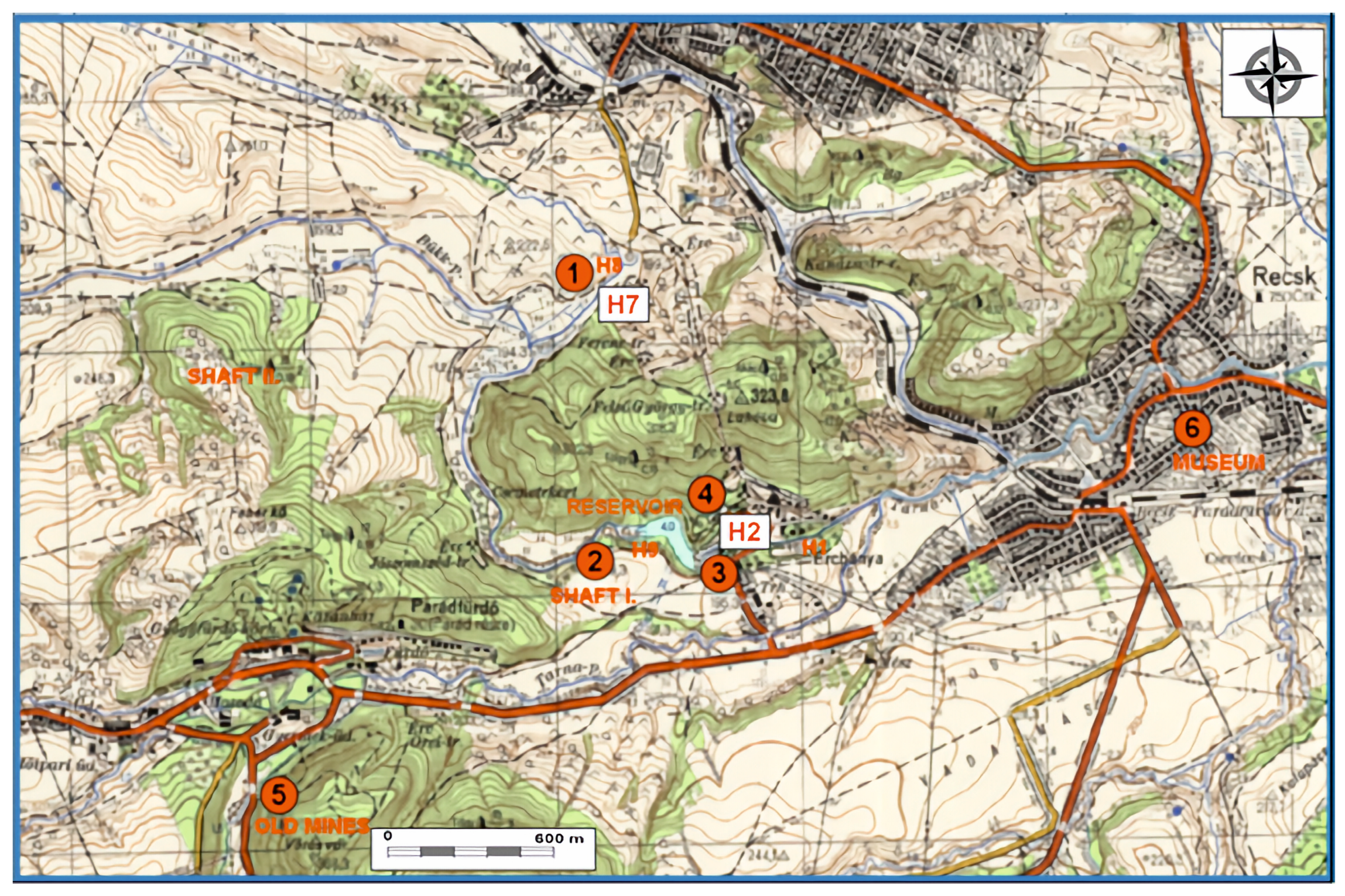
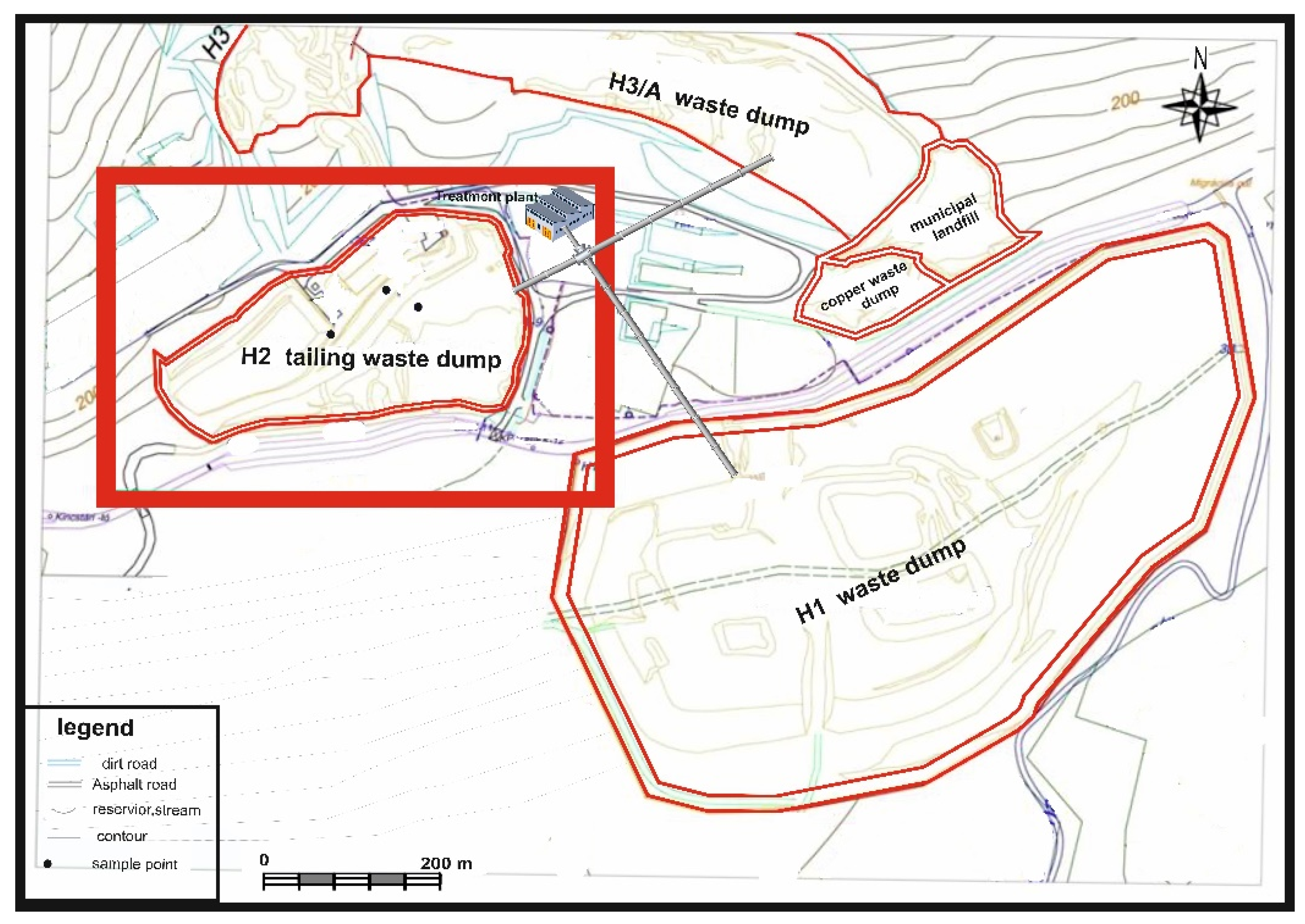
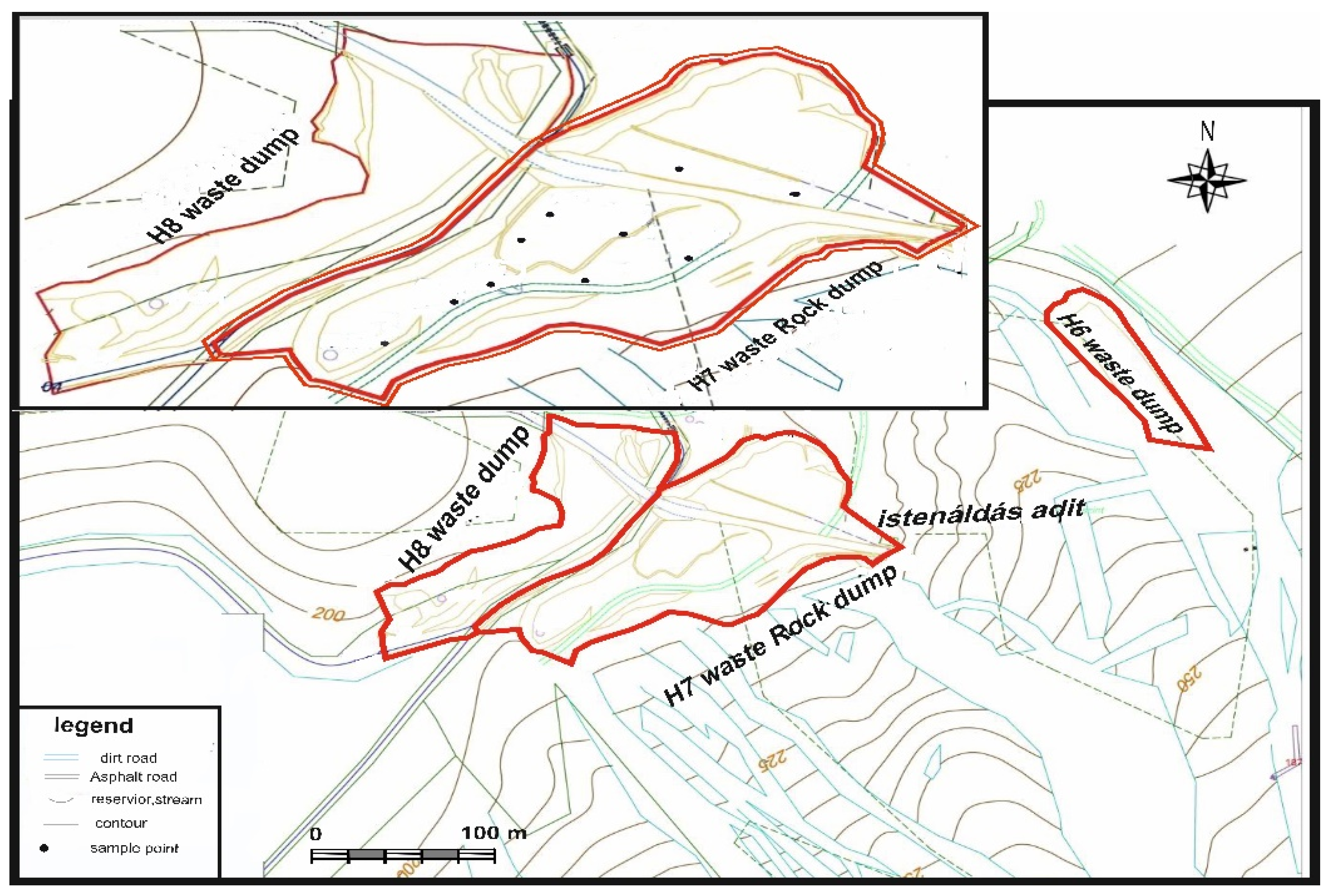
2. Study Site
3. Materials and Methods
3.1. Sampling
3.2. Mineralogical Analysis of Mine Waste Samples
3.3. Chemical Compounds and Extraction Methods
3.4. BCR Three-Step Sequential Extraction
3.5. Assessment of Acid-Generation Potential
3.6. Data Analysis
4. Results
4.1. Mineralogy and Chemical Composition
4.2. Bulk Chemical Composition
4.3. Element Geochemistry
4.3.1. Major Element Geochemistry
4.3.2. Trace Element Geochemistry
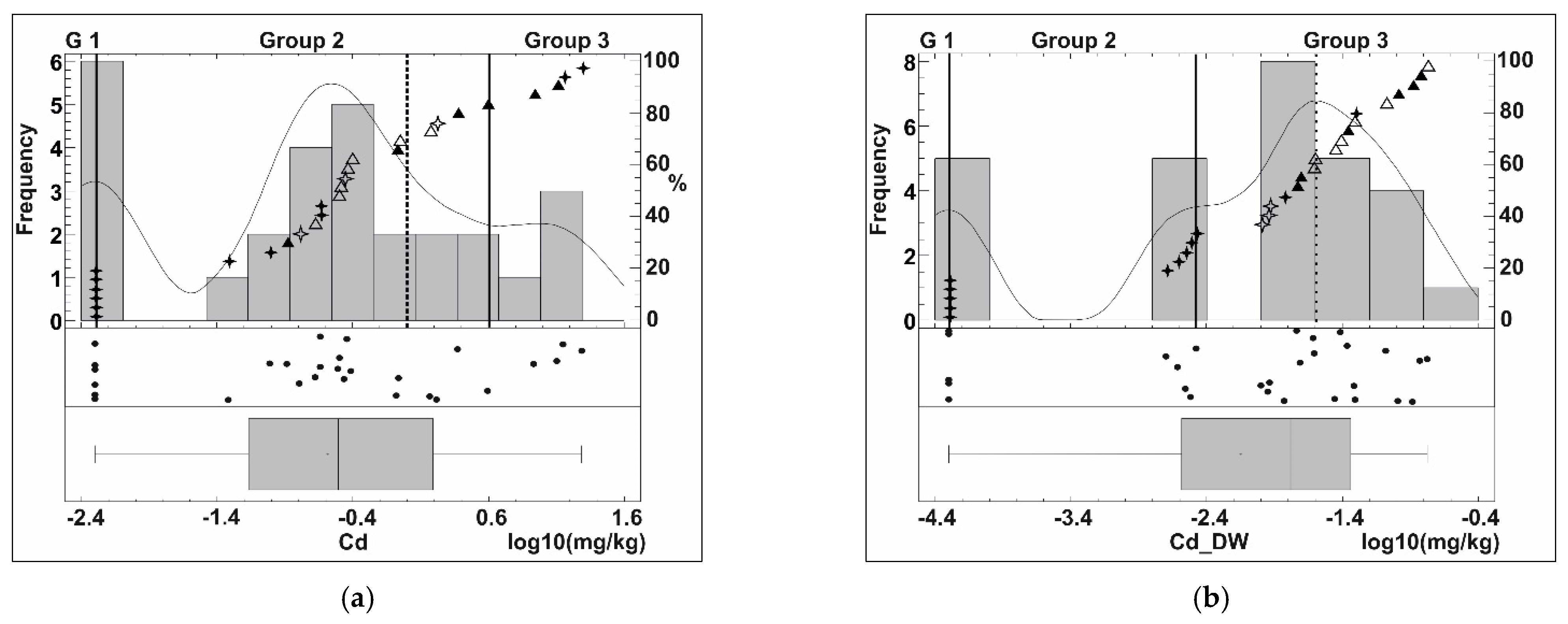
Univariate Analysis
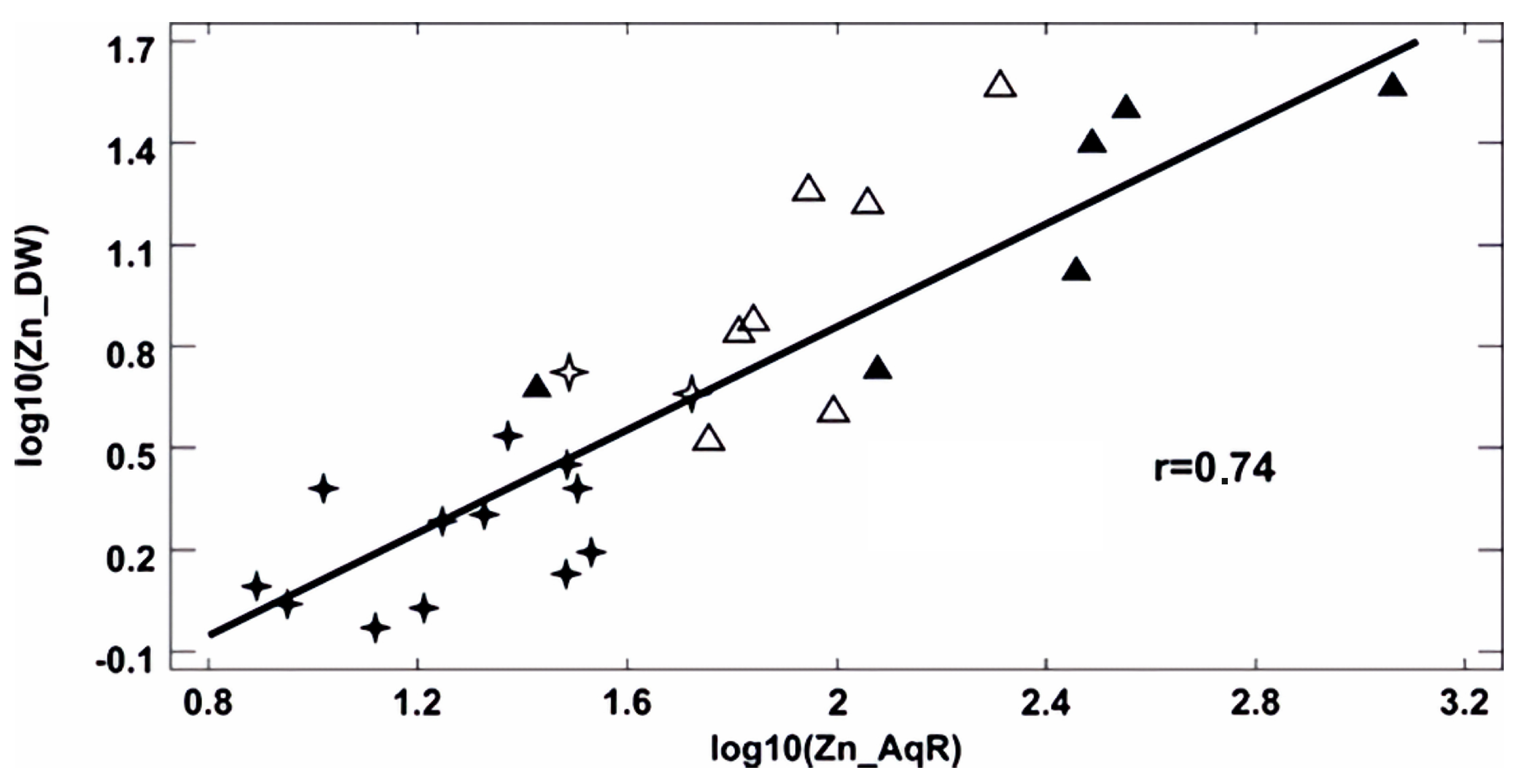
Bivariate Analysis
4.4. Element Mobility Assessment in Mine Waste
BCR Sequential Extraction Analysis: Metal Mobility in Mine Waste
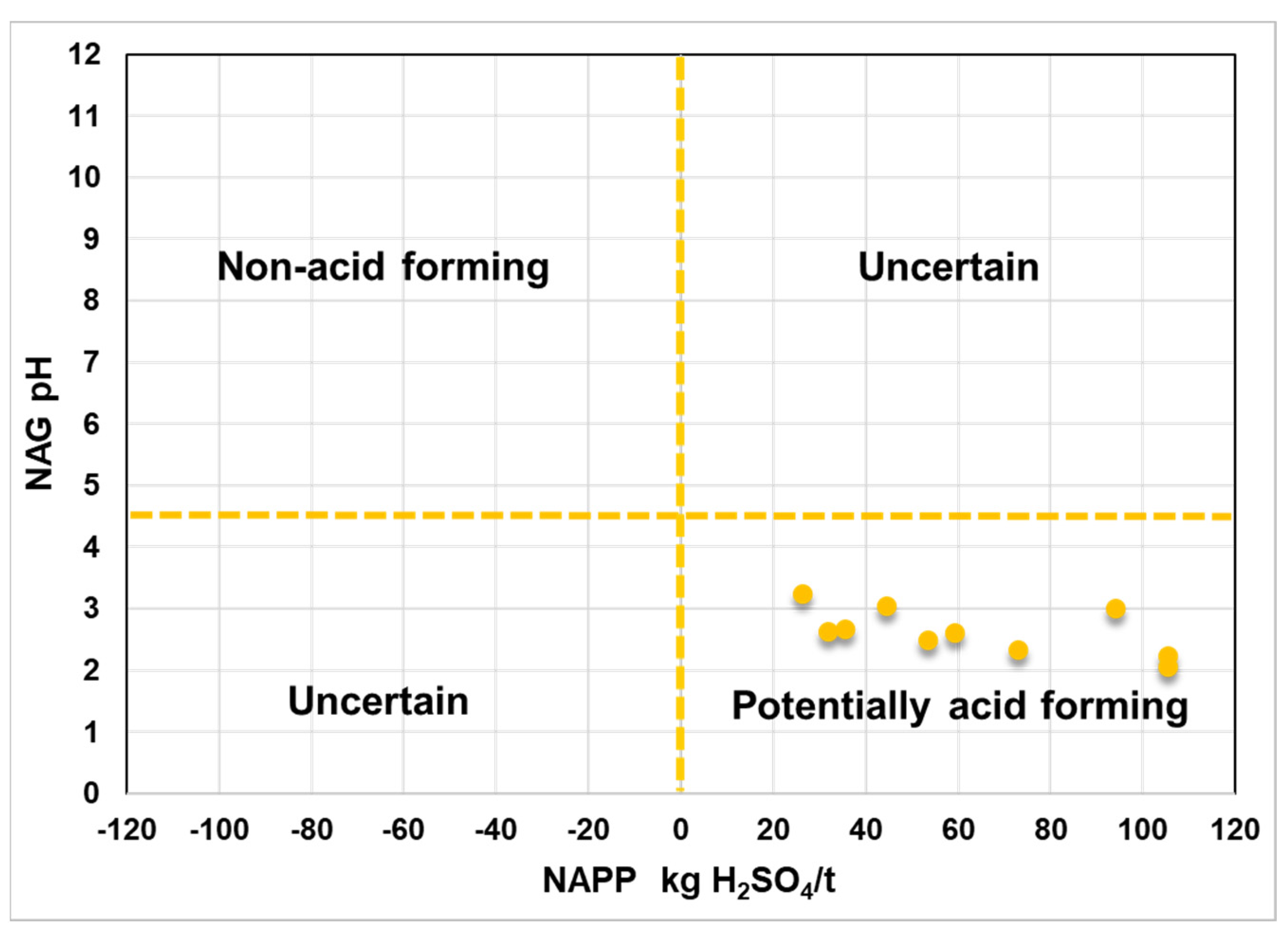
4.5. Evaluation of Acid Mine Drainage Potential in the Recsk Mine Area
5. Discussion
5.1. Mineralogy and Waste Chemcial Composition
5.2. Relationships Between Elements and Redox Conditions
5.3. Element Mobility and Release Mechanisms
5.4. Acid Generation Mechanisms and Environmental Implications
5.5. Implications for Environmental Risk Assessment and Remediation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

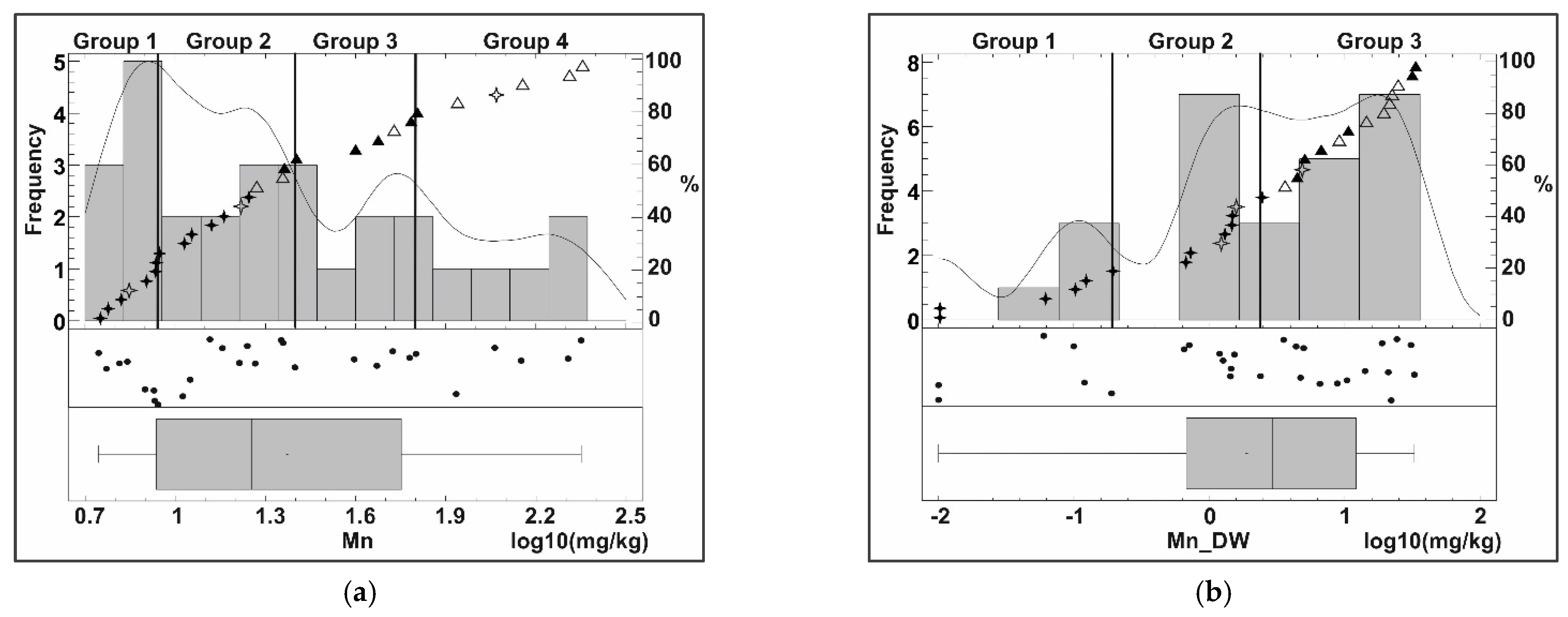
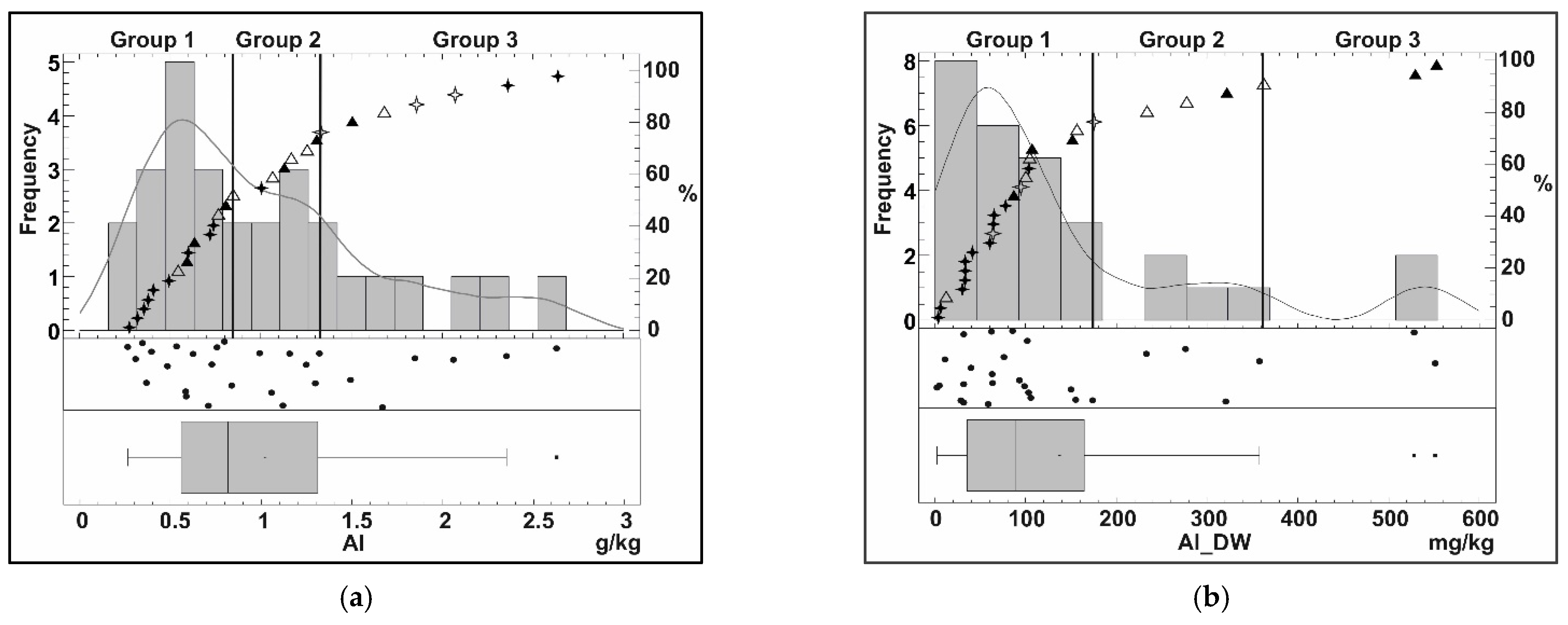

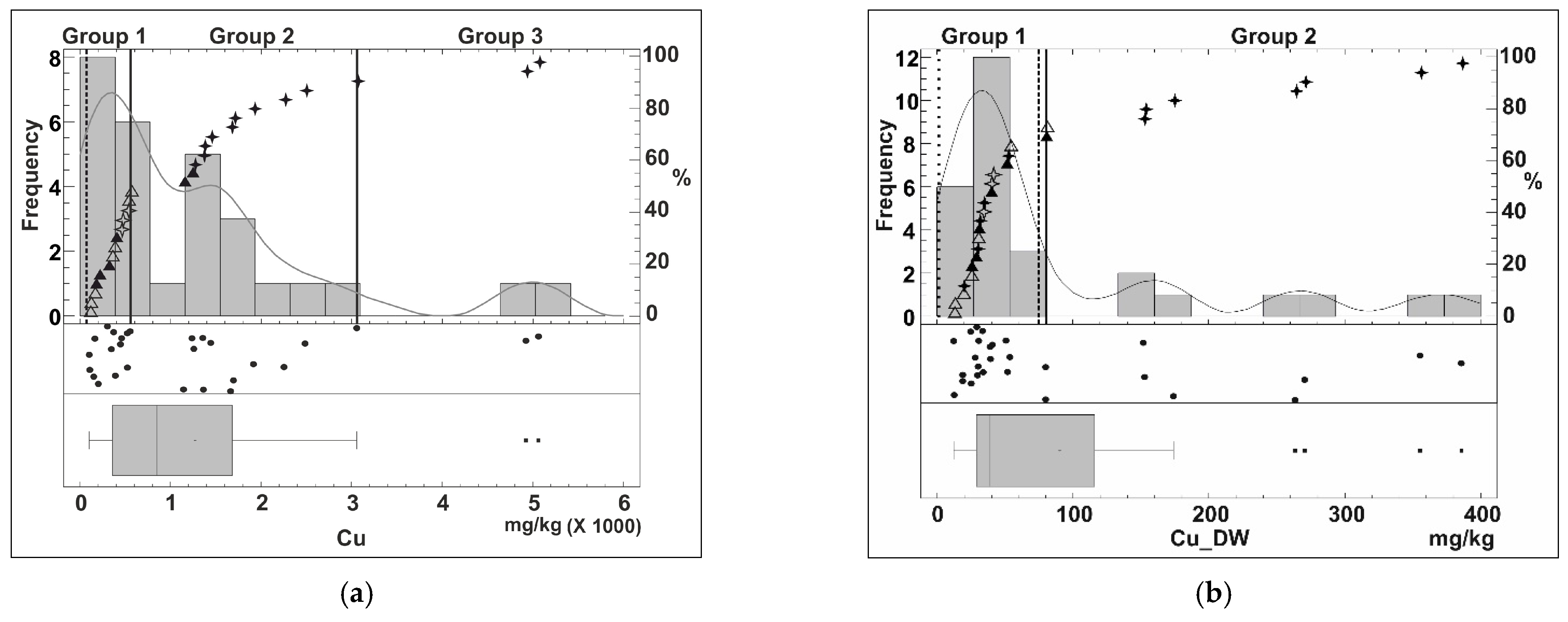
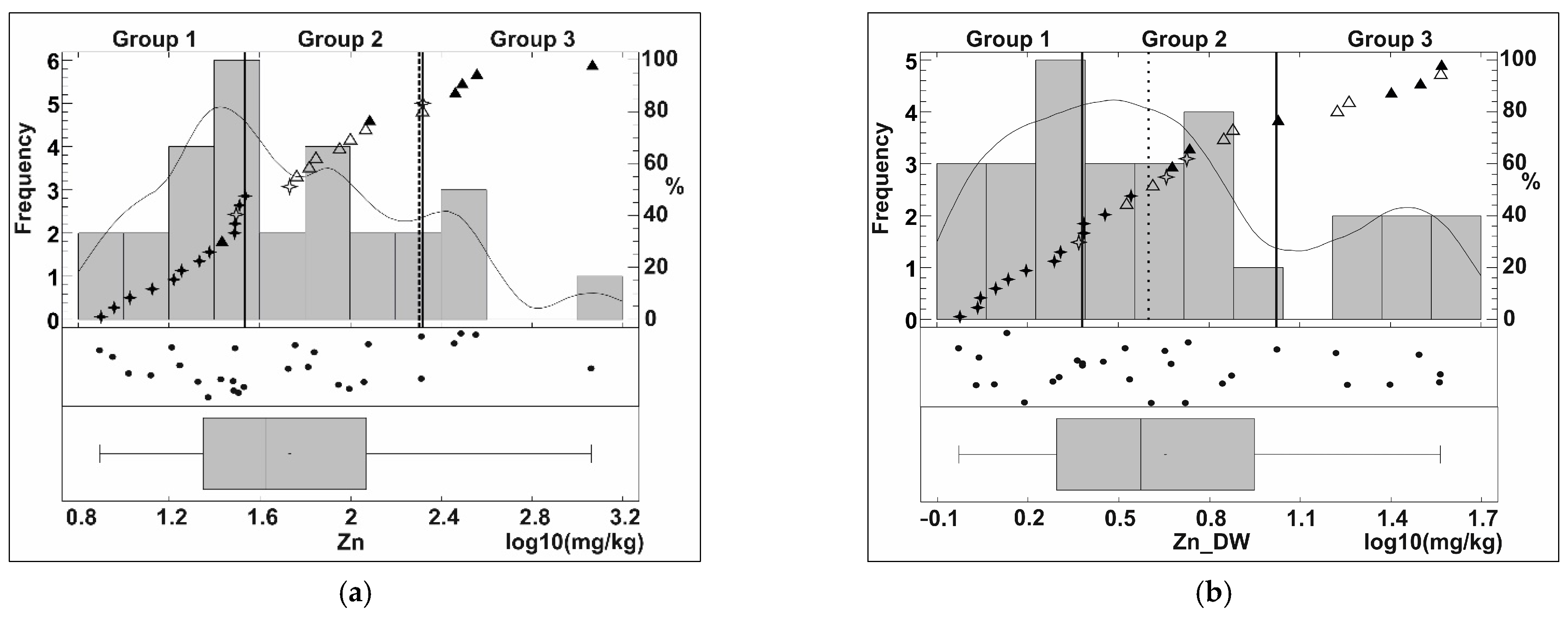




Appendix B
| Sample_ID | pH_DW | EC µS/cm | TDS mg/kg |
|---|---|---|---|
| RECSK-H2-01/R | 3.0 | 850 | 5200 |
| RECSK-H2-03/R | 3.6 | 310 | 2000 |
| RECSK-H2-04/R | 4.0 | 250 | 2650 |
| RECSK-H2-05/R | 4.3 | 188.8 | 1900 |
| RECSK-H2-07/R | 5.4 | 114.8 | 1100 |
| RECSK-H2-09/R | 5.2 | 167.7 | 1450 |
| RECSK-H2-10/R | 4.6 | 500 | 4900 |
| RECSK-H2-13/O | 3.3 | 1150 | 9350 |
| RECSK-H2-13/R | 4.1 | 250 | 2950 |
| RECSK-H2-14/O | 3.8 | 940 | 7950 |
| RECSK-H2-14/R | 3.9 | 240 | 1450 |
| RECSK-H2-15/O | 3.7 | 630 | 4350 |
| RECSK-H2-15/R | 4.2 | 220 | 2650 |
| RECSK-H2-16/R | 3.9 | 350 | 3700 |
| RECSK-H2-17/R | 3.9 | 380 | 3650 |
| RECSK-H7-02/O | 4.1 | 1300 | 11,950 |
| RECSK-H7-02/R | 2.9 | 1350 | 9900 |
| RECSK-H7-04/O | 3.4 | 2510 | 24,700 |
| RECSK-H7-04/R | 3.0 | 1210 | 8300 |
| RECSK-H7-06/O | 3.7 | 2530 | 27,600 |
| RECSK-H7-06/R | 3.0 | 1570 | 13,950 |
| RECSK-H7-09/O | 3.2 | 2620 | 26,900 |
| RECSK-H7-09/R | 2.5 | 3050 | 31,200 |
| RECSK-H7-10/O | 3.2 | 2370 | 22,650 |
| RECSK-H7-10/R | 3.1 | 1010 | 7050 |
| RECSK-H7-13/O | 5.1 | 1670 | 15,550 |
| RECSK-H7-15/O | 2.7 | 3180 | 31,400 |
| RECSK-H7-15/R | 2.5 | 2900 | 29,050 |
References
- Blight, G. Mine Waste: A Brief Overview of Origins, Quantities, and Methods of Storage. In Waste; Elsevier: Amsterdam, The Netherlands, 2011; pp. 77–88. [Google Scholar]
- Mohapatra, D.P.; Kirpalani, D.M. Process Effluents and Mine Tailings: Sources, Effects and Management and Role of Nanotechnology. Nanotechnol. Environ. Eng. 2017, 2, 1. [Google Scholar] [CrossRef]
- Bell, F.G.; Donnelly, L.J. Mining and Its Impact on the Environment; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- IEA. Global Critical Minerals Outlook 2024; IEA: Paris, France, 2024. [Google Scholar]
- Vitalii, L.; Tien, L.K.; Alena, L.; Christian, B.; Lundaev, V.; Solomon, A.A.; Le, T.; Lohrmann, A.; Breyer, C. Review of Critical Materials for the Energy Transition, an Analysis of Global Resources and Production Databases and the State of Material Circularity. Miner. Eng. 2023, 203, 108282. [Google Scholar]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Development of Porous Asphalt with Bitumen Emulsion, Electric Arc Furnace Slag and Cellulose Fibers for Medium Traffic Roads. Minerals 2020, 10, 872. [Google Scholar] [CrossRef]
- Kuhn, K.; Meima, J.A. Characterization and Economic Potential of Historic Tailings from Gravity Separation: Implications from a Mine Waste Dump (Pb-Ag) in the Harz Mountains Mining District, Germany. Minerals 2019, 9, 303. [Google Scholar] [CrossRef]
- Bevandić, S.; Blannin, R.; Auwera, J.V.; Delmelle, N.; Caterina, D.; Nguyen, F.; Muchez, P. Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium. Minerals 2021, 11, 28. [Google Scholar] [CrossRef]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; de la Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of Mining Tailings in México for the Possible Recovery of Strategic Elements. J. S. Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New Approaches for Extracting and Recovering Metals from Mine Tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Nordstrom, D.K.; Beckie, R.D.; Cicerone, D.S.; Elliot, T.; Edraki, M.; Valente, T.; França, S.C.A.; Kumar, P.; Lucero, R.A.O. Guidance for the Integrated Use of Hydrological, Geochemical, and Isotopic Tools in Mining Operations. Mine Water Environ. 2020, 39, 204–228. [Google Scholar]
- Lemos, M.; Valente, T.; Marinho Reis, P.; Fonseca, R.; Delbem, I.; Ventura, J.; Magalhães, M. Mineralogical and Geochemical Characterization of Gold Mining Tailings and Their Potential to Generate Acid Mine Drainage (Minas Gerais, Brazil). Minerals 2021, 11, 39. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing Mine Tailings for Better Environmental, Social and Economic Outcomes: A Review of Alternative Approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine Wastes: Past, Present, Future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Ettler, V. Soil Contamination near Non-Ferrous Metal Smelters: A Review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Condon, P.D.; Jambor, J.L.; Lear, K.G.; Blowes, D.W.; Ptacek, C.J. Mineralogical, Geochemical, and Microbial Investigation of a Sulfide-Rich Tailings Deposit Characterized by Neutral Drainage. Appl. Geochem. 2009, 24, 2212–2221. [Google Scholar] [CrossRef]
- Helser, J.; Cappuyns, V. Trace Elements Leaching from PbZn Mine Waste (Plombières, Belgium) and Environmental Implications. J. Geochem. Explor. 2021, 220, 106659. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally Sustainable Acid Mine Drainage Remediation: Research Developments with a Focus on Waste/by-Products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Dutta, M.; Islam, N.; Rabha, S.; Narzary, B.; Bordoloi, M.; Saikia, D.; Silva, L.F.O.; Saikia, B.K. Acid Mine Drainage in an Indian High-Sulfur Coal Mining Area: Cytotoxicity Assay and Remediation Study. J. Hazard. Mater. 2020, 389, 121851. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The Two-Step Neutralization Ferrite-Formation Process for Sustainable Acid Mine Drainage Treatment: Removal of Copper, Zinc and Arsenic, and the Influence of Coexisting Ions on Ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Hudson-Edwards, K. Tackling Mine Wastes. Science 2016, 352, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Simate, G.S.; Ndlovu, S. Acid Mine Drainage: Challenges and Opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Nguegang, B.; Ambushe, A.A. Sustainable Acid Mine Drainage Treatment: A Comprehensive Review of Passive, Combined, and Emerging Technologies. Environ. Eng. Res. 2024, 30, 240592. [Google Scholar] [CrossRef]
- Bezuidenhout, N.; Verburg, R.; Chatwin, T.; Ferguson, K. INAP’s Global Acid Rock Drainage Guide and the Current State of Acid Rock Drainage Assessment & Management in South Africa. In International Mine Water Conference Proceedings; Water Institute of South Africa: Pretoria, South Africa, 2009; pp. 29–37. [Google Scholar]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and Mineralogical Aspects of Sulfide Mine Tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A Framework for a Sustainable Approach to Mine Tailings Management: Disposal Strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical Characterization of Mine Waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Lottermoser, B.G. A Critical Review of Acid Rock Drainage Prediction Methods and Practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Hällström, L. Mobility of Be, Bi, F, Ga, Ge and W in Surface Water and the Water Quality Impact on Epilithic Diatoms Downstream of the Historical Yxsjöberg Mine Site, Sweden. Mine Water Environ. 2022, 41, 731–747. [Google Scholar] [CrossRef]
- Wade, C.T.; Dold, B.; Fontboté, L. Geochemistry and Mineralogy of The Quiulacocha Tailings Impoundment from The Polymetallic Zn-Pb-(Ag-Bi-Cu) Deposit Cerro De Pasco, Peru. J. Am. Soc. Min. Reclam. 2006, 2006, 2198–2206. [Google Scholar] [CrossRef]
- Piatak, N.M.; Hammarstrom, J.M.; Seal, R.R. Geochemical Characterization of Mine Waste From the Pike Hill Superfund Site in Vermont, Usa. J. Am. Soc. Min. Reclam. 2006, 2006, 1583–1602. [Google Scholar] [CrossRef]
- Molnár, F.; Jung, P.; Kupi, L.; Pogány, A.; Vágó, E.; Viktorik, O.; Pécskay, Z.; Hurai, V. Epithermal Zones of the Porphyry-Skarn-Epithermal Ore Complex at Recsk. Recsk Lahóca. Geol. Paleogene Ore Complex 2008, 73, 99–128. [Google Scholar]
- Földessy, J.; Szebényi, G. The Mineralizations of the Recsk Deeps and Lahóca—Short Geological Overview. Recsk Lahóca. Geol. Paleogene Ore Complex. Univ. Miskolc Ser. A Min. 2008, 73, 85–98. [Google Scholar]
- Jordán, G.; Rukezo, G.; Fügedi, U.; Carranza, E.J.; Somody, A.; Vekerdy, Z.; Szebényi, G.; Lois, L. Environmental Impact of Metal Mining on Cathcment Drainage in the Historic Mining Area of Recsk-Lahóca Mines, Hungary. In Proceedings of the 4th European congress on Regional Scientific Cartography and Informations Systems 2003, Bologna, Italy, 17–20 June 2003. [Google Scholar]
- Baksa, C.; Csillag, J.; Foldessy, J.; Zelenka, T. The Recsk Porphyry and Skarn Copper Deposit, Hungary. In Proceedings of the International Symposium, Bor, Yugoslavia, 18–22 September 1979. [Google Scholar]
- Farkas, I.M.; Weiszburg, T.G.; Pekker, P.; Kuzmann, E. A Half-Century of Environmental Mineral Formation on a Pyrite-Bearing Waste Dump in the Mátra Mountains, Hungary. Can. Miner. 2009, 47, 509–524. [Google Scholar] [CrossRef]
- Tóth, A.; Bobok, E. A Prospect Geothermal Potential of an Abandoned Copper Mine. In Proceedings of the 32nd Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 22–24 January 2007; Volume 2224, p. 13. [Google Scholar]
- Seres-Hartai, É.; Földessy, J.; Kovács, Á. Mineralogy and Genetic Aspects of Gold in the Lahóca (Recsk, Hungary) High Sulfidation Type Epithermal Deposit. Acta Montan. Slovaca 2001, 6, 19–26. [Google Scholar]
- Sas, J.; Osvald, M.; Ramalho, E.; Matos, J.X. Combined Study of Mineral Deposits and Deep Geothermal for Energy Production or Urban Heating--Comparison between the Portuguese (Neves-Corvo) and the Hungarian (Recsk) Case Studies. Cent. Eur. Geol. 2018, 61, 118–135. [Google Scholar] [CrossRef]
- Rt, V.C. Environmental Re-Examination of Recsk Deep-Level Mine; Report for the Recski Ércbányák Ltd.: Miskolc, Hungary, 1996. (In Hungarian) [Google Scholar]
- Rukezo, G. Drainage Geochemistry of the Recsk-Lahoca Mining Area, Matra Mountains, Hungary; ITC: Kolkata, India, 2003. [Google Scholar]
- Wavrer, P.; Morvanb, B.; Louis-Rosec, S. A European Standard for Sampling of Waste Material: EN 14899. In TOS Forum; IM Publications Open: Chichester, UK, 2015. [Google Scholar]
- Davis, B.L. The Estimation of Limits of Detection in RIM Quantitative X-Ray Diffraction Analysis. Adv. X-Ray Anal. 1987, 31, 317–323. [Google Scholar]
- Ure, A.M. Single Extraction Schemes for Soil Analysis and Related Applications. Sci. Total Environ. 1996, 178, 3–10. [Google Scholar]
- EN 13657:2002; Characterization of Waste—Digestion for Subsequent Determination of Aqua Regia Soluble Portion of Elements. CEN: Brussels, Belgium, 2002.
- EN 12457-2:2002; Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. Bsi Group: New Delhi, India, 2002.
- Al-Abed, S.R.; Hageman, P.L.; Jegadeesan, G.; Madhavan, N.; Allen, D. Comparative Evaluation of Short-Term Leach Tests for Heavy Metal Release from Mineral Processing Waste. Sci. Total Environ. 2006, 364, 14–23. [Google Scholar]
- Hageman, P.L. US Geological Survey Field Leach Test for Assessing Water Reactivity and Leaching Potential of Mine Wastes, Soils, and Other Geologic and Environmental Materials; USGS: Reston, VA, USA, 2007.
- Miguel, A.L.R.; Moreiraa, R.P.L.; Oliveira, A.F.D. ISO/IEC 17025: History and Introduction of Concepts. Quim Nova 2021, 44, 792–796. [Google Scholar] [CrossRef]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the Modified BCR Three-Step Sequential Extraction Procedure for the Study of Trace Element Dynamics in Contaminated Soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar]
- National Accreditation Authority (NAH). Accreditation Certificate NAH-1-1798/2016 (Accreditation of testing laboratory: DUNAFERR LABOR Nonprofit Kft.); NAH: Budapest, Hungary, 2016.
- Gazulla, M.F.; Rodrigo, M.; Orduña, M.; Gómez, C.M. Determination of Carbon, Hydrogen, Nitrogen and Sulfur in Geological Materials Using Elemental Analysers. Geostand. Geoanal. Res. 2012, 36, 201–217. [Google Scholar] [CrossRef]
- Sobek, A.A. Field and Laboratory Methods Applicable to Overburdens and Minesoils; Industrial Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1978.
- International, A. ARD Test Handbook: Prediction & Kinetic Control of Acid Mine Drainage, AMIRA P387A; Ian Wark Research Institute and Environmental Geochemistry International Ltd.: Melbourne, Austrilia, 2002. [Google Scholar]
- No, J.D. 6/2009.(IV. 14) KvVM-EüM-FVM of the Ministers of Environmental Protection and Water Management. Public Health, Agriculture and Regional Development on the limit values necessary to protect the quality of geological medium and the groundwater and on measurement of pollution.
- Ministry of Environmental Protection; Management, W. Decree No. 20 of 2006 (IV. 5.) KvVM of the Ministry of Environmental Protection and Water Management on Certain Rules and Conditions Concerning Waste Tipping and Landfills. 2006.
- Kürzl, H. Exploratory Data Analysis: Recent Advances for the Interpretation of Geochemical Data. J. Geochem. Explor. 1988, 30, 309–322. [Google Scholar]
- Data, M.C.; Komorowski, M.; Marshall, D.C.; Salciccioli, J.D.; Crutain, Y. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977; Volume 2. [Google Scholar]
- Ch’ng, C.K.; Mahat, N.I. Winsorize Tree Algorithm for Handling Outlier in Classification Problem. Int. J. Oper. Res. 2020, 38, 278–293. [Google Scholar]
- Morales, C.; Giraldo, R.; Torres, M. Boxplot Fences in Proficiency Testing. Accredit. Qual. Assur. 2021, 26, 193–200. [Google Scholar]
- Abdaal, A.; Jordan, G.; Szilassi, P. Testing Contamination Risk Assessment Methods for Mine Waste Sites. Water Air Soil. Pollut. 2013, 224, 1–26. [Google Scholar] [CrossRef]
- Davis, J.C.; Sampson, R.J. Statistics and Data Analysis in Geology; Wiley: New York, NY, USA, 1986; Volume 646. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Statgraphics Technologies, Inc. STATGRAPHICS Centurion; Statgraphics Technologies, Inc.: The Plains, VI, USA, 2021. [Google Scholar]
- Fonseca, L.; Domingues, J.P. ISO 9001: 2015 Edition-Management, Quality and Value. Int. J. Qual. Res. 2017, 11, 149–158. [Google Scholar]
- Brough, C.P.; Warrender, R.; Bowell, R.J.; Barnes, A.; Parbhakar-Fox, A. The Process Mineralogy of Mine Wastes. Miner. Eng. 2013, 52, 125–135. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminum Hydroxysulfates from Acid Sulfate Waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar]
- THEODORE BOTINELLY, D. A Review of the Minerals of the Alunite-Jarosite, Beudantite, and Plumbogummite Groups. J. Res. US Geol. Surv. 1976, 4, 213–216. [Google Scholar]
- Stoffregen, R.E.; Alpers, C.N. Woodhouseite and Svanbergite in Hydrothermal Ore Deposits; Products of Apatite Destruction during Advanced Argillic Alteration. Can. Mineral. 1987, 25, 201–211. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory; VCH: Weinheim, Germany, 1991. [Google Scholar]
- Anawar, H.M.; Mihaljevič, M.; Garcia-Sanchez, A.; Akai, J.; Moyano, A. Investigation of Sequential Chemical Extraction of Arsenic from Sediments: Variations in Sample Treatment and Extractant. Soil Sediment Contam. 2010, 19, 133–141. [Google Scholar]
- Petronijević, N.; Stanković, S.; Radovanović, D.; Sokić, M.; Marković, B.; Stopić, S.R.; Kamberović, Ž. Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia). Metals 2019, 10, 16. [Google Scholar] [CrossRef]
- Armienta, M.A.; Zúñiga-Vázquez, D.; Cruz, O.; Aguayo, A.; Reséndiz, I. Mobility of Potentially Toxic Metals and Metalloids in Soils Impacted by Tailings with Distinct Oxidation Stages. In Proceedings of the EGU23, the 25th EGU General Assembly, Vienna, Austria, 23–28 April 2023. [Google Scholar]
- Hiller, E.; Tóth, R.; Kučerová, G.; Jurkovič, Ľ.; Šottník, P.; Lalinská-Voleková, B.; Vozár, J. Geochemistry of Mine Tailings from Processing of Siderite–Cu Ores and Mobility of Selected Metals and Metalloids Evaluated by a Pot Leaching Experiment at the Slovinky Impoundment, Eastern Slovakia. Mine Water Environ. 2016, 35, 447–461. [Google Scholar] [CrossRef]
- You, G.; Gu, S.; Li, Q.; Xie, X.; Guo, Z.; Zhao, F.; Zhang, T.; Deng, G.; Zhang, X. Heavy Metals Mobilization and Attenuation in Cd-Rich Niujiaotang Legacy Pb-Zn Tailings of Southwestern China. Geochem. J. 2024, 58, 80–93. [Google Scholar] [CrossRef]
- Lubkova, T.; Dogadina, L.; Yablonskaya, D.; Orlova, O.; Nikolaeva, I. Acid Generation and Metal Leaching Potential of Sulfide-Bearing Rocks in the Verhne-Krichalskaya Area (Western Chukotka, Russia). Int. Multidiscip. Sci. GeoConference SGEM 2017, 17, 31–38. [Google Scholar]
- Fosu, S.; Owusu, C.; Ntsiful, F.; Ackah, K. Determining Acid and Metalliferous Drainage Potential of Waste Rock on a Mine. Ghana Min. J. 2020, 20, 49–59. [Google Scholar] [CrossRef]
- Tong, L.; Peng, X.; Chen, D.; Chen, Y.; Wen, Y.; Wang, W.; Liu, X. Characterization and Risk Assessment of Soil around Waste Rock Heaps Affected by Acid Rock Drainage in an Abandoned Pyrite Mining Area. Front. Environ. Sci. 2022, 10, 1017809. [Google Scholar] [CrossRef]
- Szebényi, G. Ércbányászati Eredetű Környezetföldtani Tényezők És Veszélyforrások Recsk–Parádfürdő Térségében (Environmental Geological Factors and Hazards of Ore Mining Origin in the Recsk–Parádfürdő). Földtani Kut. 2002, 39, 28–35. [Google Scholar]
- Jordan, G.; Van Rompaey, A.; Somody, A.; Fügedi, U.; Farsang, A. Spatial Modelling of Contamination in a Catchment Area Impacted by Mining: A Case Study of the Recsk Copper Mine, Hungary. Land Contam. Reclam. 2009, 17, 413. [Google Scholar] [CrossRef]
- Smuda, J.; Dold, B.; Spangenberg, J.E.; Pfeifer, H.-R. Geochemistry and Stable Isotope Composition of Fresh Alkaline Porphyry Copper Tailings: Implications on Sources and Mobility of Elements during Transport and Early Stages of Deposition. Chem. Geol. 2008, 256, 62–76. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable Rehabilitation of Mining Waste and Acid Mine Drainage Using Geochemistry, Mine Type, Mineralogy, Texture, Ore Extraction and Climate Knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Palumbo-Roe, B.; Klinck, B.; Cave, M. Arsenic Speciation and Mobility in Mine Wastes from a Copper–Arsenic Mine in Devon, UK: A SEM, XAS, Sequential Chemical Extraction Study. Trace Met. Other Contam. Environ. 2007, 9, 441–471. [Google Scholar]
- Yousefi, S.; Doulati Ardejani, F.; Ziaii, M.; Esmaeil Zadeh, E.; Abedi, A.; Karamoozian, M. Identification of the Origin and Behaviour of Arsenic in Mine Waste Dumps Using Correlation Analysis: A Case Study Sarcheshmeh Copper Mine. Int. J. Min. Geo-Eng. 2013, 47, 139–149. [Google Scholar]
- Bech, J.; Poschenrieder, C.; Llugany, M.; Barceló, J.; Tume, P.; Tobias, F.J.; Barranzuela, J.L.; Vásquez, E.R. Arsenic and Heavy Metal Contamination of Soil and Vegetation around a Copper Mine in Northern Peru. Sci. Total Environ. 1997, 203, 83–91. [Google Scholar] [CrossRef]
- Bogolubov, A.; Kamshilin, A.; Volkova, E. Possibilities of Geoelectrical and Seismo-Electrical Monitoring in Investigations of the Karst Phenomena. Environ. Geol. 2002, 41, 760–764. [Google Scholar] [CrossRef]
- Dybowska, A.; Farago, M.; Valsami-Jones, E.; Thornton, I. Operationally Defined Associations of Arsenic and Copper from Soil and Mine Waste in South-West England. Chem. Speciat. Bioavailab. 2005, 17, 147–160. [Google Scholar] [CrossRef]
- Chukwura, U.O.; Hursthouse, A.S. Evaluating Controls on Potentially Toxic Element Release in Circum-Neutral Mine Water: A Case Study from the Abandoned Pb–Zn Mines of Leadhills and Wanlockhead, South of Scotland, United Kingdom. Environ. Earth Sci. 2020, 79, 363. [Google Scholar]
- Cappuyns, V.; Campen, V.A.; Bevandić, S.; Helser, J.; Muchez, P. Characterization of Mine Waste from a Former Pb–Zn Mining Site: Reactivity of Minerals during Sequential Extractions. J. Sustain. Metall. 2021, 7, 1456–1468. [Google Scholar] [CrossRef]
- Tlil, H.; Souissi, R.; Souissi, F.; Lattanzi, P.; Podda, F.; Concas, S.; Ardau, C.; Cidu, R. Environmental Mineralogy and Geochemistry of Pb–Zn Mine Wastes, Northern Tunisia. Rend. Lincei 2017, 28, 133–141. [Google Scholar]
- Schaider, L.A.; Senn, D.B.; Brabander, D.J.; McCarthy, K.D.; Shine, J.P. Characterization of Zinc, Lead, and Cadmium in Mine Waste: Implications for Transport, Exposure, and Bioavailability. Environ. Sci. Technol. 2007, 41, 4164–4171. [Google Scholar] [CrossRef]
- Bao, Z.; Al, T.; Bain, J.; Shrimpton, H.K.; Finfrock, Y.Z.; Ptacek, C.J.; Blowes, D.W. Sphalerite Weathering and Controls on Zn and Cd Migration in Mine Waste Rock: An Integrated Study from the Molecular Scale to the Field Scale. Geochim. Cosmochim. Acta 2022, 318, 1–18. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical Features of Surface Water and Groundwater Contaminated with Acid Mine Drainage (AMD) in Coal Mining Areas: A Case Study in Southern Brazil. Environ. Sci. Pollut. Res. 2016, 23, 18911–18927. [Google Scholar]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Genty, T. Spatial Mapping of Acidity and Geochemical Properties of Oxidized Tailings within the Former Eagle/Telbel Mine Site. Minerals 2019, 9, 180. [Google Scholar] [CrossRef]
- Jamieson, H.E. Geochemistry and Mineralogy of Solid Mine Waste: Essential Knowledge for Predicting Environmental Impact. Elements 2011, 7, 381–386. [Google Scholar]
- Drahota, P.; Chatziantoniou, A.; Mihaljevič, M.; Culka, A.; Melfou, M.; Melfos, V.; Voudouris, P. A Mineralogical and Geochemical Assessment of the As-, Cu-, In-, Pb-, Sb-, and Zn-Rich Mine Wastes at the Pefka Epithermal Deposit, Greece. J. Geochem. Explor. 2024, 256, 107336. [Google Scholar]
- Kumkrong, P.; Dy, E.; Tyo, D.D.; Jiang, C.; Gedara Pihilligawa, I.; Kingston, D.; Mercier, P.H.J. Investigation of Metal Mobility in Gold and Silver Mine Tailings by Single-Step and Sequential Extractions. Environ. Monit. Assess 2022, 194, 423. [Google Scholar]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Metal Extractability in Acidic and Neutral Mine Tailings from the Cartagena-La Unión Mining District (SE Spain). Appl. Geochem. 2008, 23, 1232–1240. [Google Scholar]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy Metal Distribution and Chemical Speciation in Tailings and Soils around a Pb–Zn Mine in Spain. J. Environ Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Larios, R.; Fernández-Martínez, R.; Álvarez, R.; Rucandio, I. Arsenic Pollution and Fractionation in Sediments and Mine Waste Samples from Different Mine Sites. Sci. Total Environ. 2012, 431, 426–435. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Comparison of Two Sequential Extraction Procedures for Heavy Metal Partitioning in Mine Tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar]
- Cappuyns, V.; Swennen, R.; Niclaes, M. Application of the BCR Sequential Extraction Scheme to Dredged Pond Sediments Contaminated by Pb–Zn Mining: A Combined Geochemical and Mineralogical Approach. J. Geochem. Explor. 2007, 93, 78–90. [Google Scholar] [CrossRef]
- Serra, A.V.; Botté, S.E.; Cuadrado, D.G.; La Colla, N.S.; Negrin, V.L. Metals in Tidal Flats Colonized by Microbial Mats within a South-American Estuary (Argentina). Environ. Earth Sci. 2017, 76, 1–10. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid Mine Drainage Formation and Arsenic Mobility under Strongly Acidic Conditions: Importance of Soluble Phases, Iron Oxyhydroxides/Oxides and Nature of Oxidation Layer on Pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [PubMed]
- Qureshi, A.; Maurice, C.; Öhlander, B. Potential of Coal Mine Waste Rock for Generating Acid Mine Drainage. J. Geochem. Explor. 2016, 160, 44–54. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Du, S.; Zhang, Z.; Lu, W.; Su, P.; Jiao, Y.; Zhao, Y. A Review: The Formation, Prevention, and Remediation of Acid Mine Drainage. Environ. Sci. Pollut. Res. 2023, 30, 111871–111890. [Google Scholar]
- Dold, B. Acid Rock Drainage Prediction: A Critical Review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar]
- Kiswanto, K.; Wintah, W. Analysis of Iron (Fe), Manganese (Mn), and PH of Coal Mine Acidic Water in Aceh Province. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2023; Volume 69, p. 02008. [Google Scholar]
- Bouzahzah, H.; Benzaazoua, M.; Bussiere, B.; Plante, B. Prediction of Acid Mine Drainage: Importance of Mineralogy and the Test Protocols for Static and Kinetic Tests. Mine Water Environ. 2014, 33, 54–65. [Google Scholar]
- Heikkinen, P.M.; Räisänen, M.L.; Johnson, R.H. Geochemical Characterisation of Seepage and Drainage Water Quality from Two Sulphide Mine Tailings Impoundments: Acid Mine Drainage versus Neutral Mine Drainage. Mine Water Environ. 2009, 28, 30–49. [Google Scholar]
- Deon, F.; van Ruitenbeek, F.; van der Werff, H.; van der Meijde, M.; Marcatelli, C. Detection of Interlayered Illite/Smectite Clay Minerals with XRD, SEM Analyses and Reflectance Spectroscopy. Sensors 2022, 22, 3602. [Google Scholar] [CrossRef]
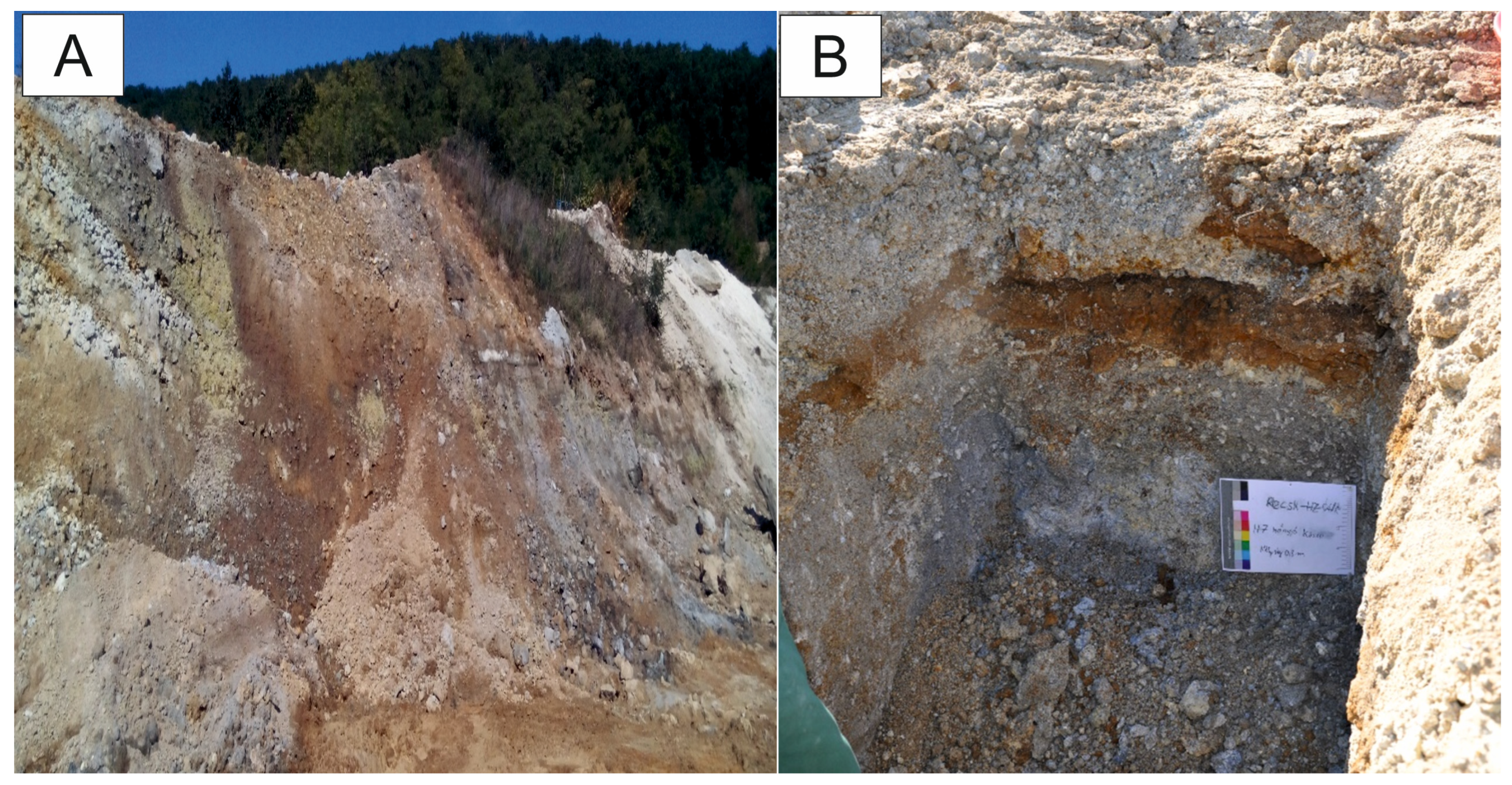
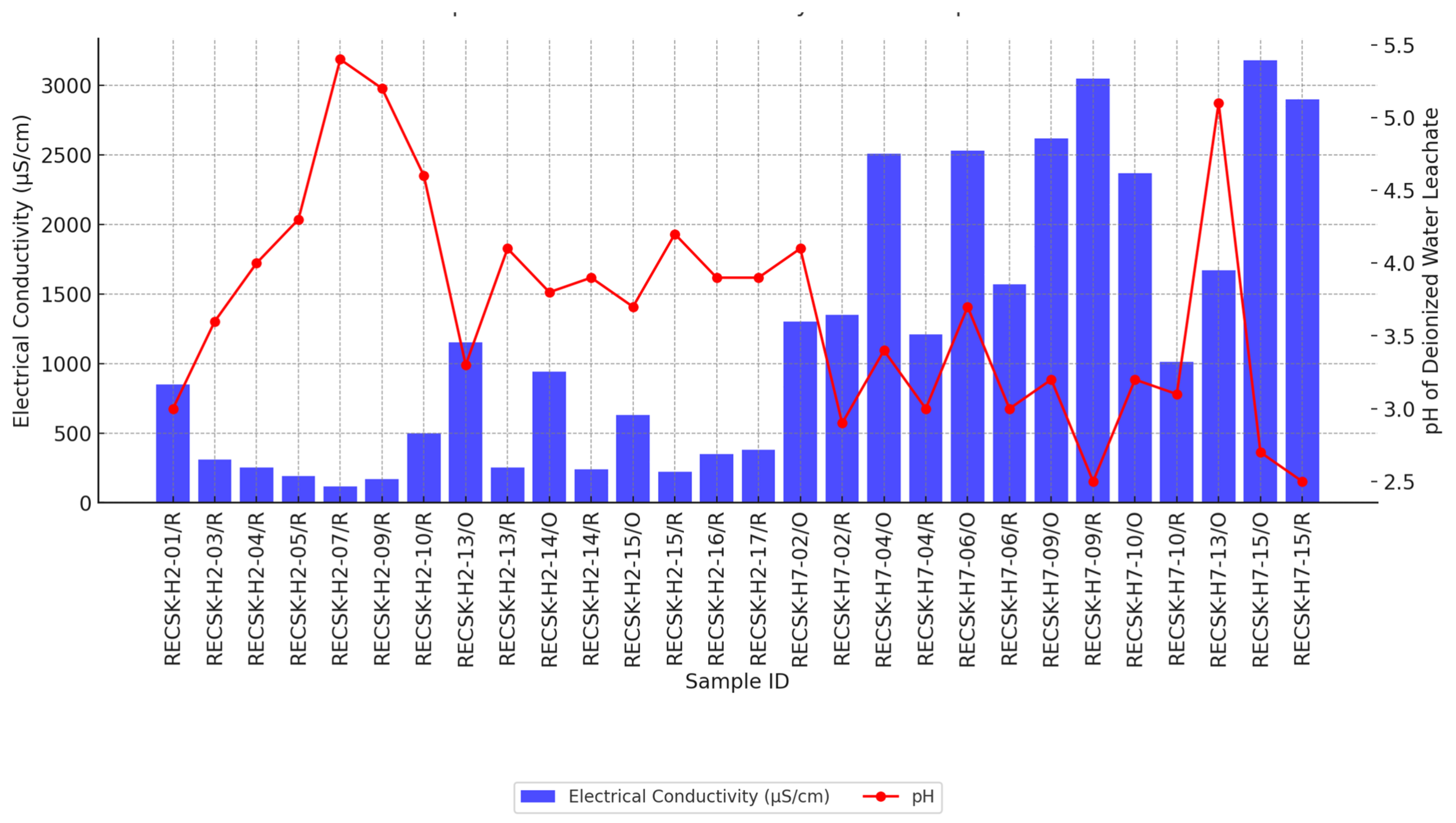
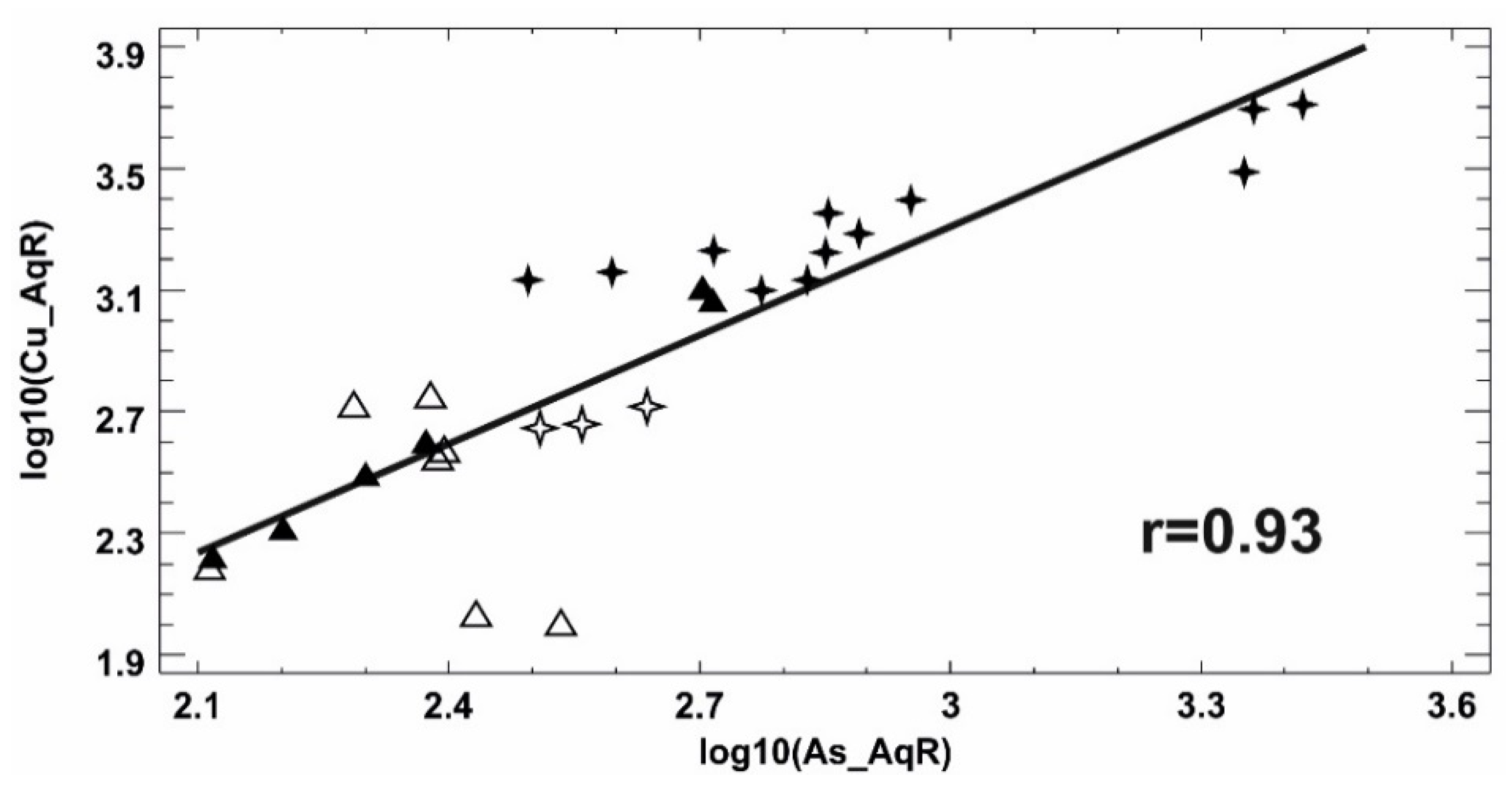
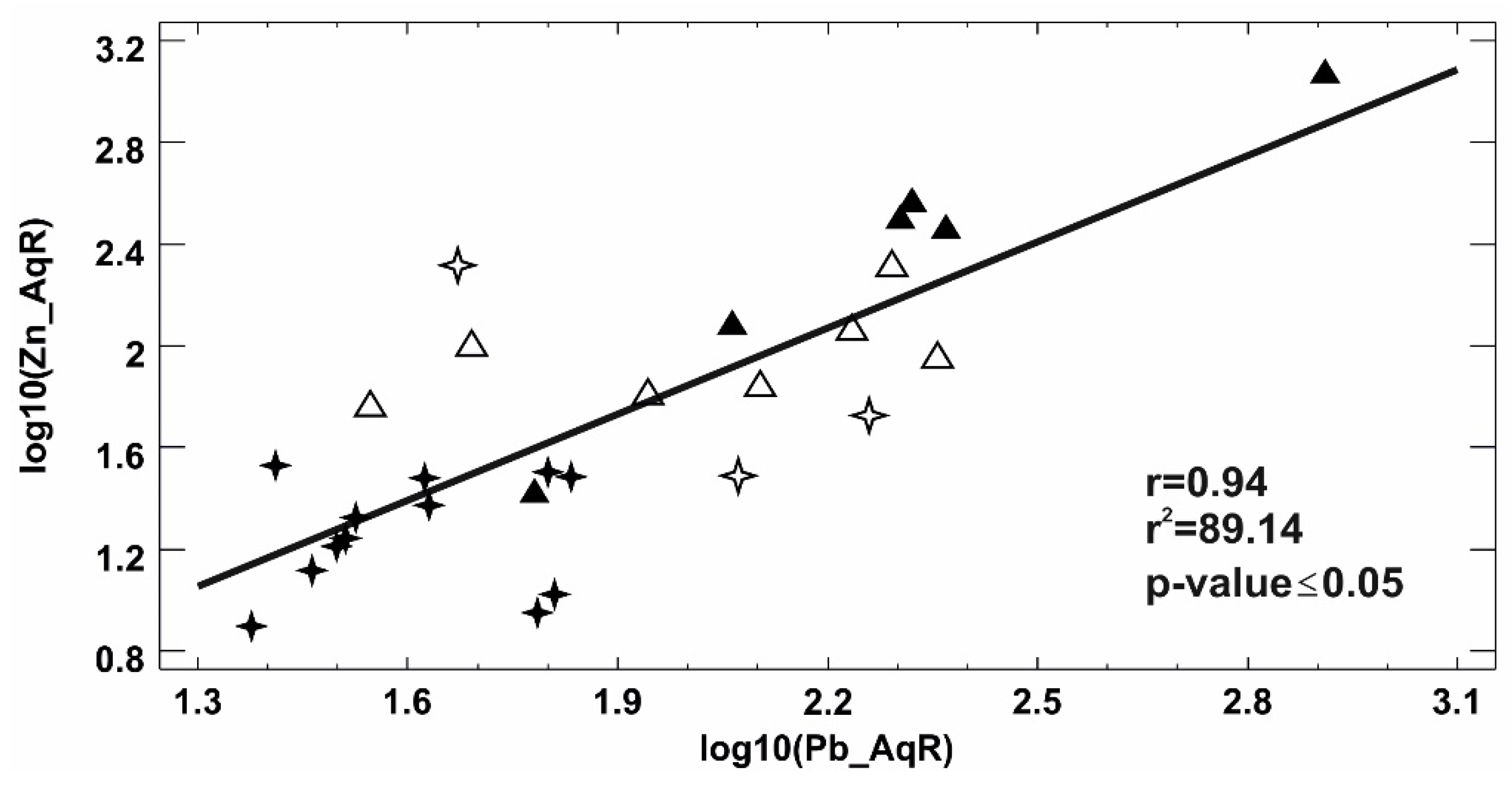
| Minerals | H2-03/R | H2-13/R | H2-14/R | H2-17/R | H2-14/O | H2-15/O | H7-10/R | H7-15/R | H7-10/O | H7-15/O |
|---|---|---|---|---|---|---|---|---|---|---|
| Barite BaSO4 | 7.49 | <1 | 3.5 | <1 | ||||||
| Gypsum CaSO4·2H2O | 1.1 | 1.4 | 2 | 25 | 1.4 | <1 | 1.2 | 1.3 | 9.2 | |
| Kaolinite Al2(Si2O5)(OH)4 | 6.6 | 11.4 | 13.1 | 23.5 | 19 | 20.7 | <1 | 2.3 | 1.1 | 3.9 |
| lllite/Smectite 11A | 3.5 | 9.8 | 3.34 | 6.1 | 6.6 | 10.2 | ||||
| Illite 1M (K,H3O)Al2Si3AlO10(OH)2 | 1.4 | 2 | 5.64 | 15.9 | 9.1 | 11.2 | ||||
| Smectite 12A | 2.5 | 3.1 | 4.7 | |||||||
| Andesine (Ca,Na)(AlSi3O8) | 13 | |||||||||
| Microcline K(AlSi3O8) | 12.5 | |||||||||
| Pyrite FeS2 | 4.8 | 1.3 | 1.2 | 3 | <1 | <1 | 1.5 | 3.5 | <1 | |
| Chalcopyrite CuFeS2 | <1 | |||||||||
| Tennantite (Cu,Fe)As4S13 | <1 | <1 | <1 | <1 | <1 | |||||
| Jarosite (K,H3O)Fe3(SO4)2(OH)6 | 0.6 | 6 | 0.80 | 1.7 | 4.8 | 4 | 8 | |||
| Anatase TiO2 | <1 | 1.3 | <1 | 2.1 | <1 | <1 | <1 | <1 | <1 | |
| Plumbogummite PbAl3(PO4)(PO3OH)(OH)6 | <1 | <1 | ||||||||
| Woodhouseite CaAl3(PO4)(SO4)(OH)6 | <1 | <1 | <1 | |||||||
| Quartz SiO4 | 82.8 | 75.9 | 79.6 | 51.6 | 49.48 | 40.6 | 75.7 | 46.5 | 66.1 | 40.7 |
| Amorphous | 4 | 3 | N/D * | 5 | 18 | 18 | 3 | 6 | 7 | 16 |
| Chemical Element | Statistical Parameters (Aqua Regia Extraction)—H2 and H7 Waste Heap | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| H2 Flotation Mud Waste Heap | H7 Waste Heap | Legal Limit (6/2009 Decree) | |||||||
| Minimum | Median | Maximum | MAD | Minimum | Median | Maximum | MAD | ||
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| Cu | 443 | 1660 | 5060 | 591 | 99 | 347 | 1237 | 182 | 75 |
| Zn | 7.8 | 24 | 205 | 8.3 | 27 | 114 | 1156 | 57 | 200 |
| Cd | 0.005 | 0.097 | 19.3 | 0.092 | 0.13 | 0.83 | 12.7 | 0.62 | 1 |
| Pb | 24 | 42.8 | 181 | 17 | 35 | 172 | 813 | 56 | 100 |
| As | 313 | 674 | 2625 | 239.5 | 130 | 238 | 518 | 44 | 15 |
| Fe | 9833 | 23,305 | 47,596 | 6249 | 10,914 | 28,007 | 43,549 | 7877 | n.a. 1 |
| Mn | 5.5 | 8.7 | 115.7 | 2.4 | 18.4 | 53 | 2.24 | 30 | n.a. 1 |
| Al | 2647 | 7098 | 26,281 | 3642 | 5347 | 10,589 | 16,703 | 2610 | n.a. 1 |
| Ca | 195 | 427 | 2457 | 140 | 1086 | 4137 | 19,666 | 2682 | n.a. 1 |
| Mg | 38 | 139 | 874 | 64 | 258 | 612 | 1090 | 220 | n.a. 1 |
| Chemical Element | Statistical Parameters (Deionized-water extraction)—H2 and H7 Waste Heap | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 Flotation Mud Waste Heap | H7 Waste Heap | Legal Limit (6/2009 Decree) | Legal Limit (20/2006 Decree) | |||||||
| Minimum | Median | Maximum | MAD | Minimum | Median | Maximum | MAD | |||
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| Cu | 19 | 52 | 385.5 | 32.7 | 11.9 | 30 | 79.7 | 10.8 | 75 | 2 |
| Zn | 0.93 | 2 | 5.2 | 0.78 | 3.3 | 10.5 | 36.7 | 6.5 | 200 | 4 |
| Cd | 0.00005 | 0.0027 | 0.049 | 0.0027 | 0.018 | 0.043 | 0.16 | 0.02 | 1 | 0.04 |
| Pb | 0.0014 | 0.0023 | 0.019 | 0.00078 | 0.0001 | 0.015 | 3.3 | 0.01 | 100 | 0.5 |
| As | 0.014 | 0.21 | 3.13 | 0.1 | 0.016 | 0.2 | 3.1 | 0.1 | 15 | 0.5 |
| Fe | 3.2 | 49 | 646 | 35 | 0.5 | 229 | 2392 | 190 | n.a. 2 | n.a. 2 |
| Mn | 0.01 | 0.7 | 4.7 | 0.65 | 3.5 | 14 | 33 | 8 | n.a. 2 | n.a. 2 |
| Al | 1.8 | 58 | 173 | 27 | 10.8 | 155 | 550.9 | 78 | n.a. 2 | n.a. 2 |
| Ca | 37.8 | 135 | 1558 | 81 | 837 | 2690 | 6460 | 1627 | n.a. 2 | n.a. 2 |
| Mg | 1.8 | 6.9 | 51 | 3.4 | 10.4 | 39.3 | 70.4 | 21 | n.a. 2 | n.a. 2 |
| Element | Total Content | Water-Soluble Content | Mobility (%) | |||
|---|---|---|---|---|---|---|
| p-Value | Sig | p-Value | Sig | p-Value | Sig | |
| Cu | 0.0001 | ** | 0.0156 | * | 0.0087 | ** |
| Zn | 0.0006 | ** | 0.0002 | ** | 0.9812 | ns |
| Cd | 0.0032 | ** | 0.0005 | ** | 0.0314 | * |
| Pb | 0.0014 | ** | 0.0011 | ** | 0.0236 | * |
| As | 0.0022 | ** | 0.7642 | ns | 0.0062 | ** |
| Fe | 0.4131 | ns | 0.0008 | ** | 0.0022 | ** |
| Mn | <0.0001 | ** | <0.0001 | ** | 0.0004 | ** |
| Al | 0.0563 | ns | 0.0004 | ** | 0.0412 | * |
| Ca | <0.0001 | ** | <0.0001 | ** | <0.0001 | ** |
| Mg | 0.0002 | ** | <0.0001 | ** | 0.2136 | ns |
| Chemical Element | H2 (%) | H7 (%) |
|---|---|---|
| Ca | 32 | 65 |
| Zn | 9 | 9 |
| Mn | 8 | 27 |
| Mg | 5 | 6 |
| Cu | 3 | 9 |
| Cd | 3 | 5 |
| Al | 0.8 | 1 |
| Fe | 0.2 | 0.8 |
| As | 0.03 | 0.09 |
| Pb | 0.005 | 0.009 |
| Element | H2 Tailings Heap | H7 Waste Rock Heap | ||
|---|---|---|---|---|
| Reactive Fraction (R1 + R2 + R3) | Residual Fraction (R4) | Reactive Fraction (R1 + R2 + R3) | Residual Fraction (R4) | |
| Cu | 74.4 | 1585.6 | 182.5 | 164.5 |
| Zn | 2.2 | 21.8 | 10.5 | 103.5 |
| Cd | 0.01 | 0.09 | 0.08 | 0.75 |
| Pb | 4.6 | 38.2 | 13.3 | 158.7 |
| As | 24.8 | 649.2 | 60.0 | 178.0 |
| Fe | 1094.7 | 22,210.3 | 2780.7 | 25,226.3 |
| Mn | 0.7 | 8.0 | 14.0 | 39.0 |
| Al | 291.3 | 6806.7 | 740.0 | 9849.0 |
| Ca | 135.0 | 292.0 | 2690.0 | 1447.0 |
| Sample ID | NAG pH | MPA (kg H2SO4/t) |
|---|---|---|
| RECSK-H2-03/R | 2.23 | 105.57 |
| RECSK-H2-13/R | 2.62 | 31.824 |
| RECSK-H2-14/O | 3.05 | 44.676 |
| RECSK-H2-14/R | 2.67 | 35.496 |
| RECSK-H2-15/O | 3.23 | 26.316 |
| RECSK-H2-17/R | 2.48 | 53.55 |
| RECSK-H7-10/O | 2.61 | 59.364 |
| RECSK-H7-10/R | 2.33 | 73.134 |
| RECSK-H7-15/O | 3.00 | 94.248 |
| RECSK-H7-15/R | 2.06 | 105.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwani, N.; Szabó, P.; Horváth-Mezőfi, Z.; Jókainé Szatura, Z.; Ban, M.; Nguyen, Q.D.; Hitka, G. Comparative Analysis of Potentially Toxic Elements (PTEs) in Waste Rock and Tailings: A Case Study from the Recsk Mining Area, Hungary. Minerals 2025, 15, 360. https://doi.org/10.3390/min15040360
Alwani N, Szabó P, Horváth-Mezőfi Z, Jókainé Szatura Z, Ban M, Nguyen QD, Hitka G. Comparative Analysis of Potentially Toxic Elements (PTEs) in Waste Rock and Tailings: A Case Study from the Recsk Mining Area, Hungary. Minerals. 2025; 15(4):360. https://doi.org/10.3390/min15040360
Chicago/Turabian StyleAlwani, Naji, Péter Szabó, Zsuzsanna Horváth-Mezőfi, Zsuzsanna Jókainé Szatura, My Ban, Quang Duc Nguyen, and Géza Hitka. 2025. "Comparative Analysis of Potentially Toxic Elements (PTEs) in Waste Rock and Tailings: A Case Study from the Recsk Mining Area, Hungary" Minerals 15, no. 4: 360. https://doi.org/10.3390/min15040360
APA StyleAlwani, N., Szabó, P., Horváth-Mezőfi, Z., Jókainé Szatura, Z., Ban, M., Nguyen, Q. D., & Hitka, G. (2025). Comparative Analysis of Potentially Toxic Elements (PTEs) in Waste Rock and Tailings: A Case Study from the Recsk Mining Area, Hungary. Minerals, 15(4), 360. https://doi.org/10.3390/min15040360









