Mapping Bronze Disease Onset by Multispectral Reflectography
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. VIS-NIR Multispectral Imaging
IR False-Color (IR-FC) Imaging, Principal Component Analysis (PCA), Color Difference (∆E*) Mapping, and Spectral Correlation Mapper (SCM)
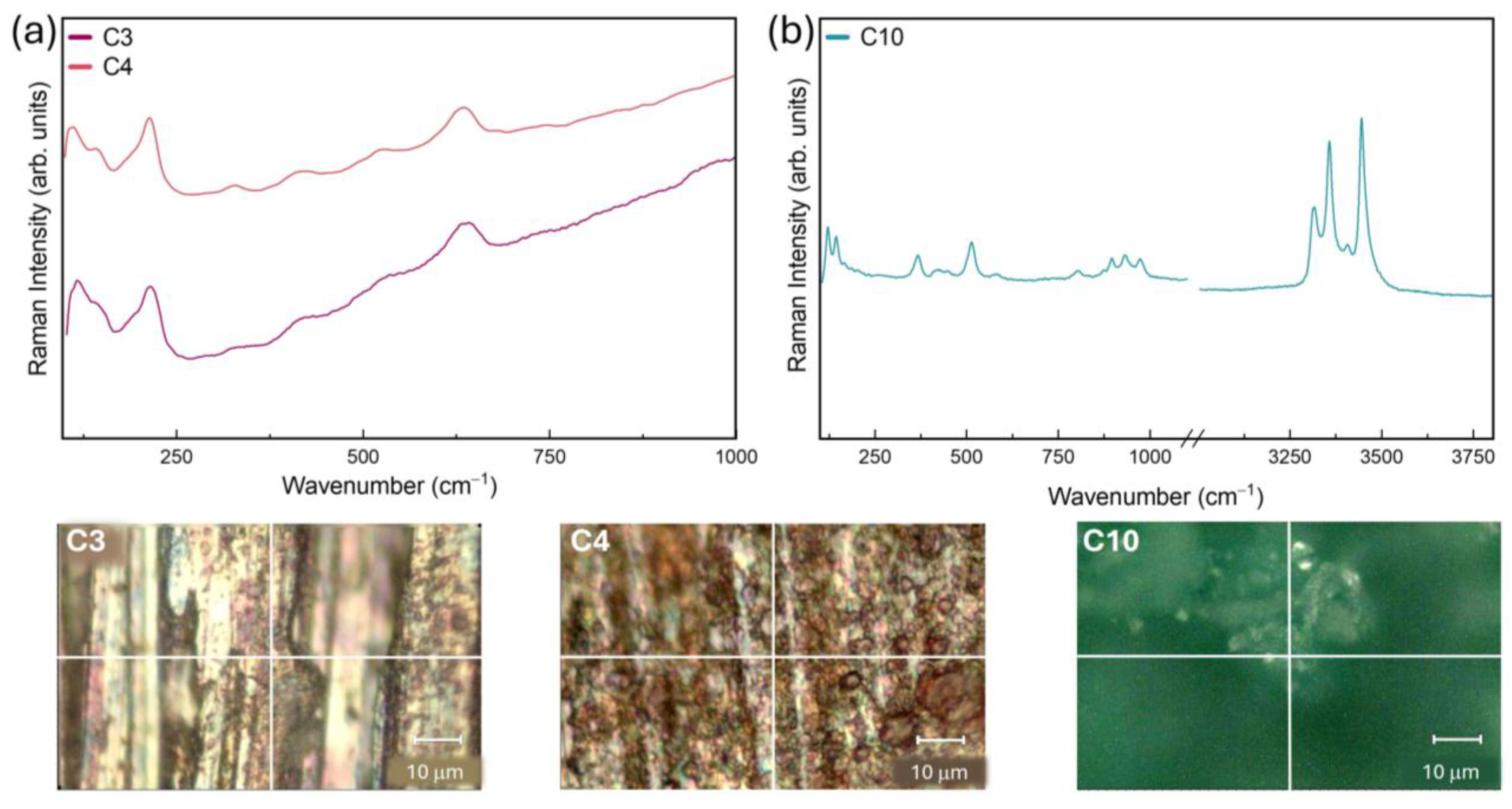
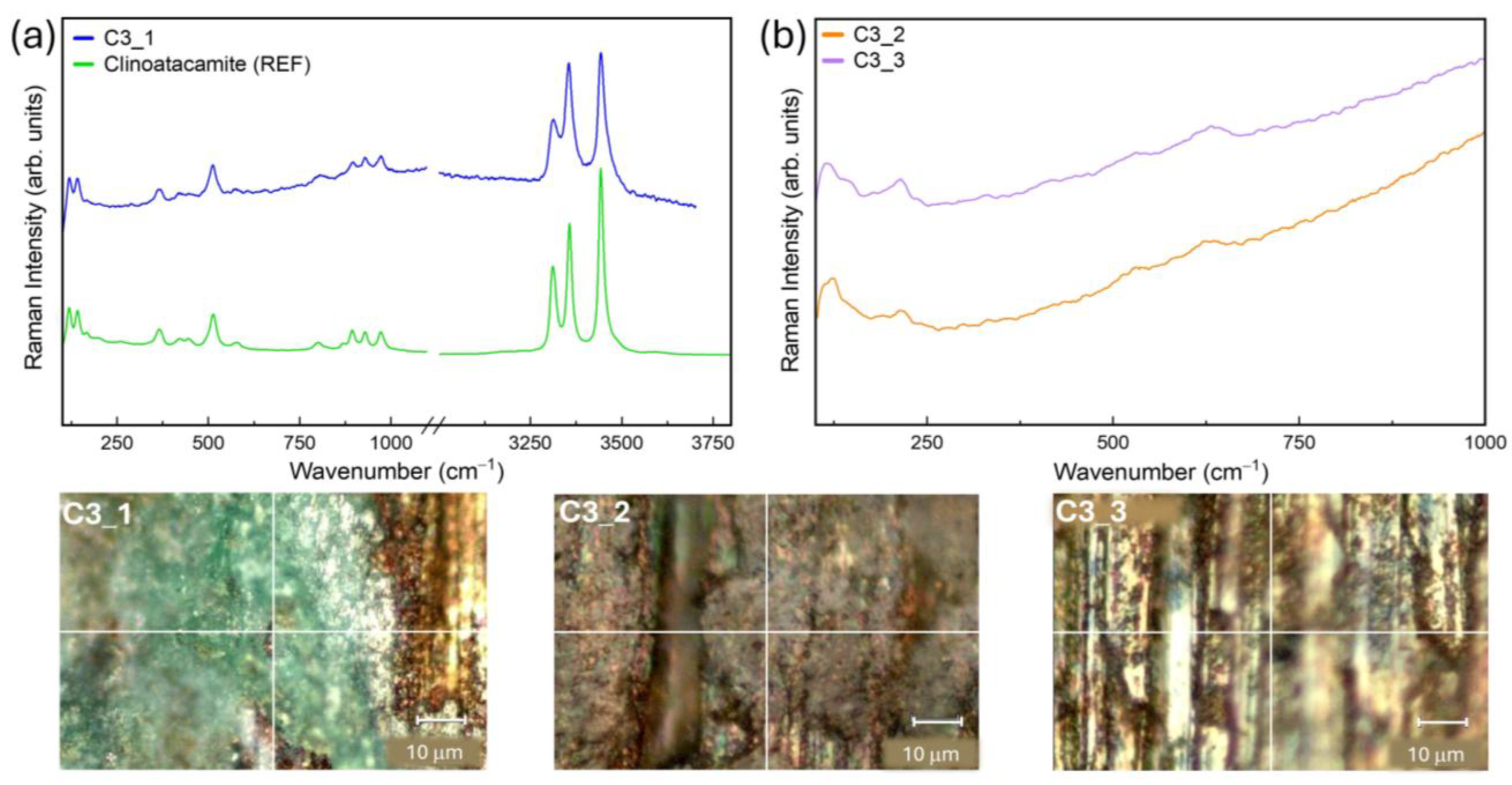
2.3. Confocal Raman Microspectroscopy (CRM)
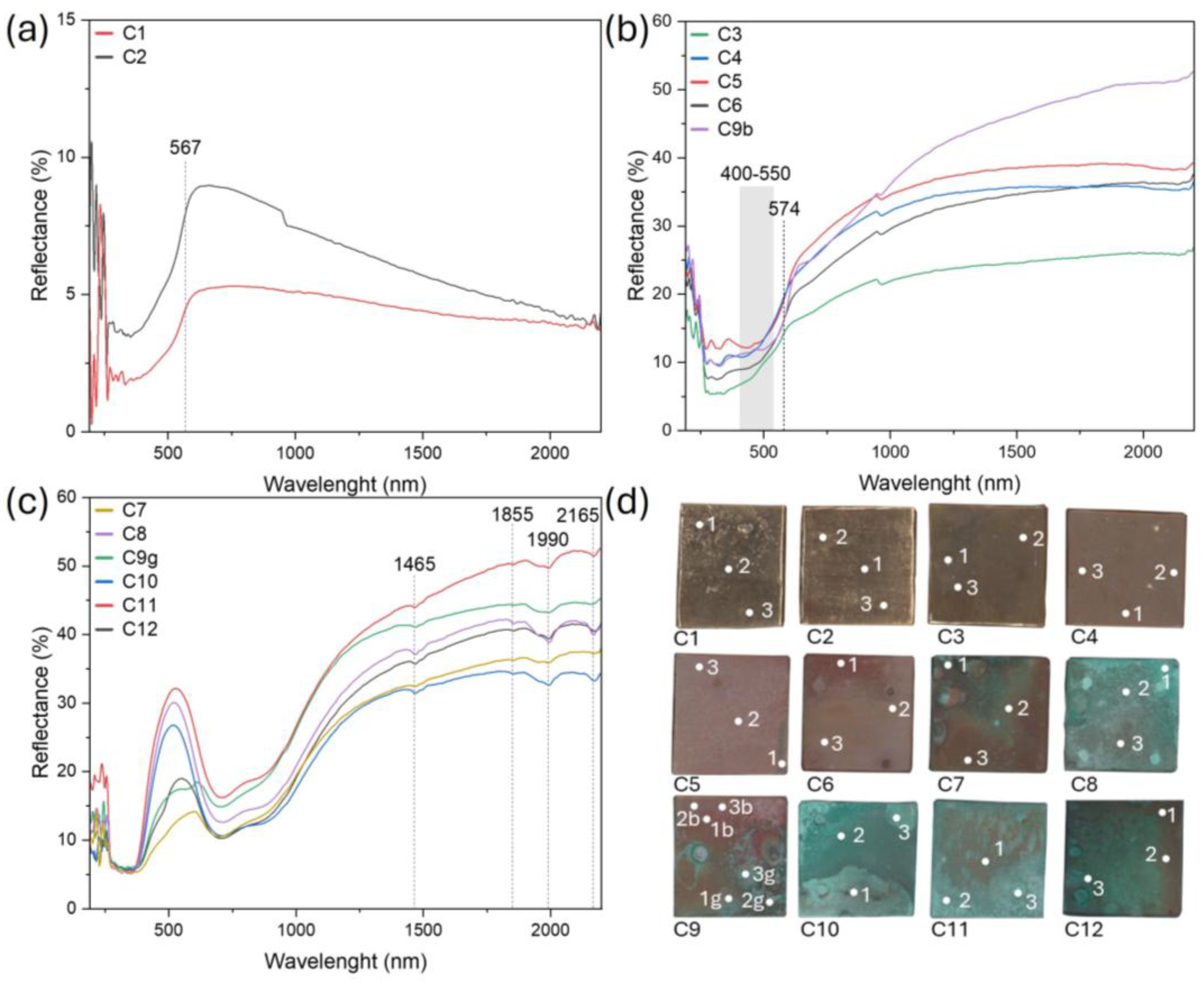
2.4. Fiber Optics Reflectance Spectroscopy (FORS)
3. Results and Discussion
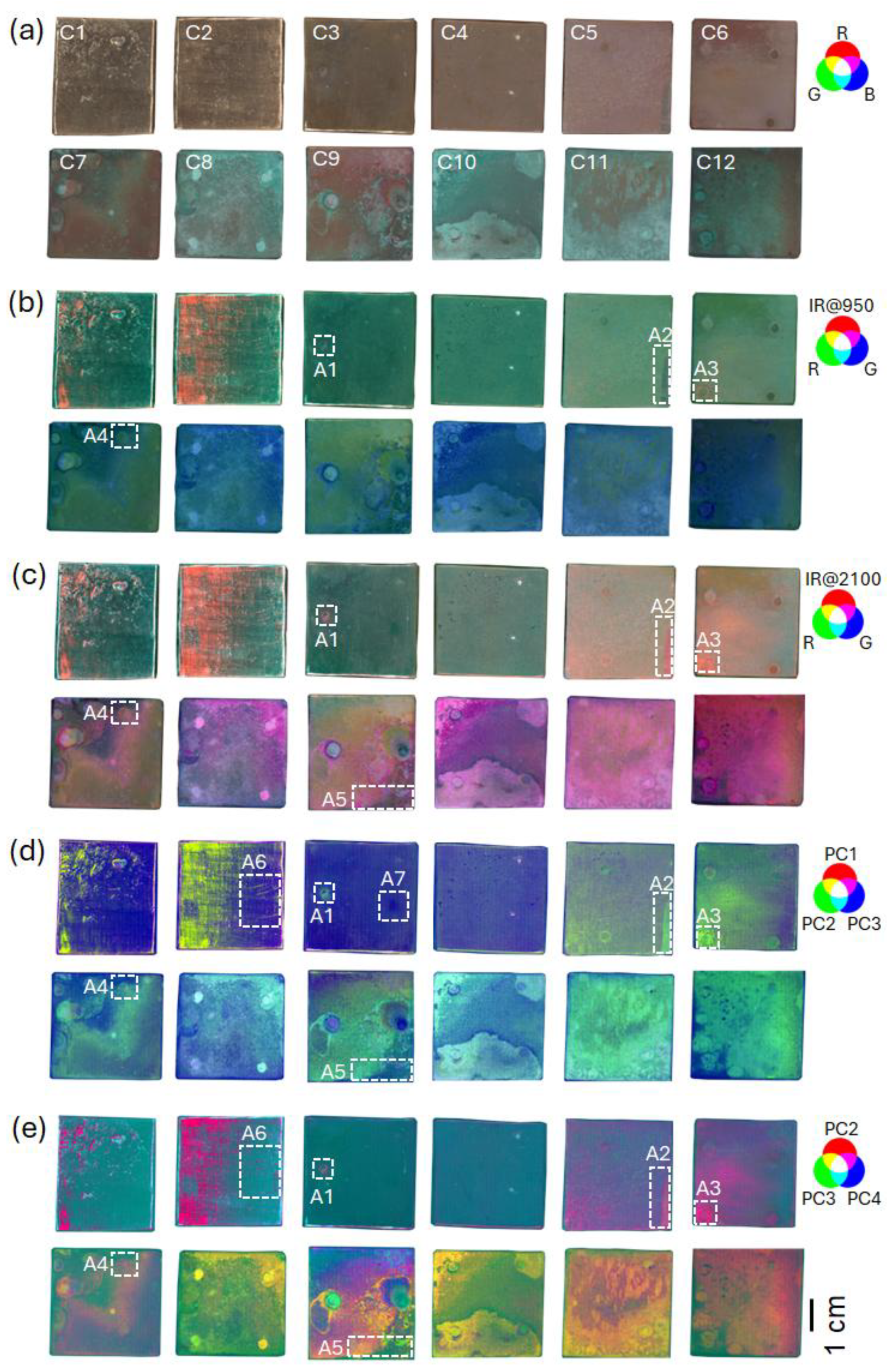
3.1. Patina Visualization
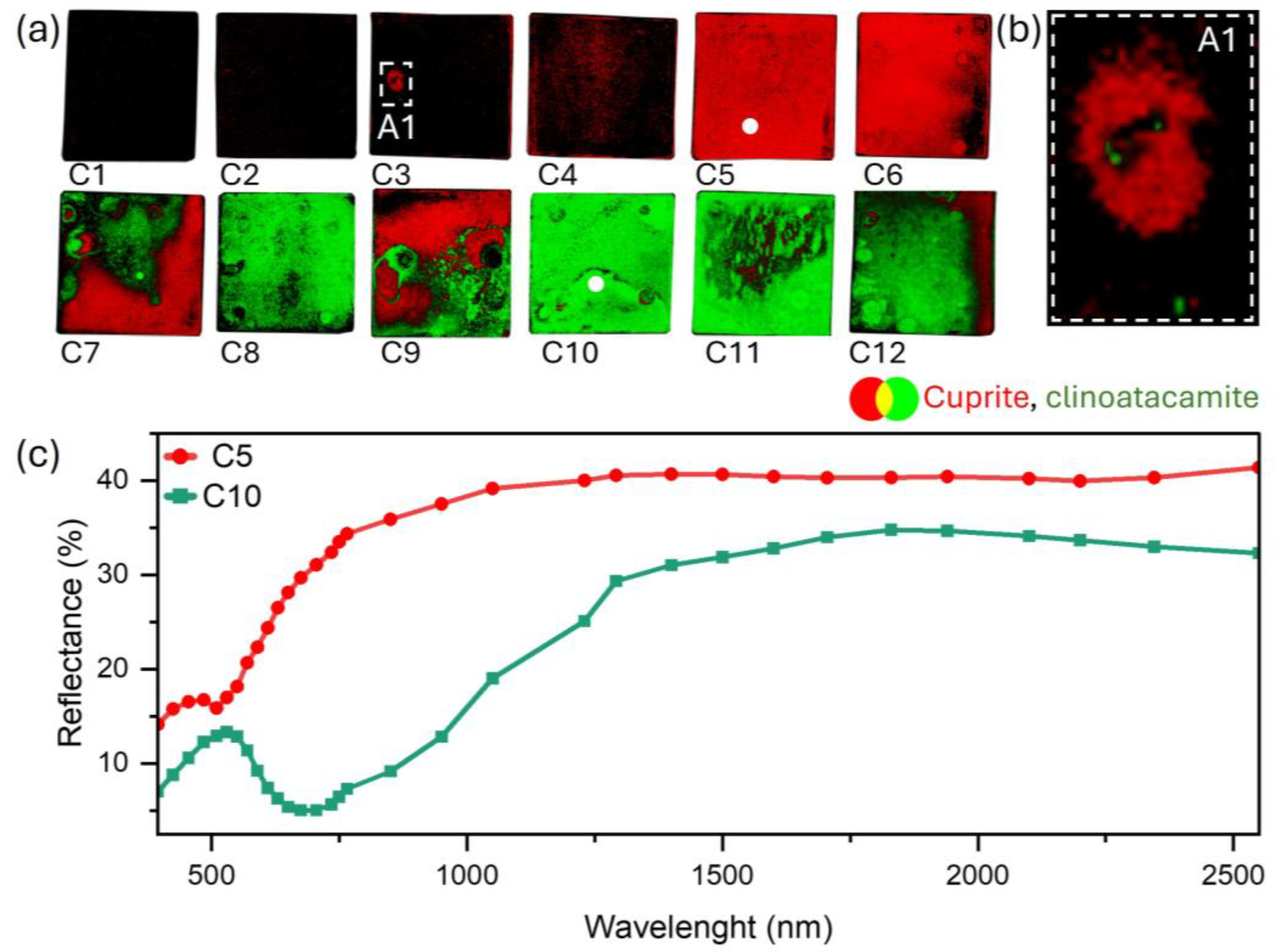
3.2. Patina Component Identification and Mapping
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehmann, E.H.; Vontobel, P.; Deschler-Erb, E.; Soares, M. Non-Invasive Studies of Objects from Cultural Heritage. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2005, 542, 68–75. [Google Scholar] [CrossRef]
- Di Turo, F. Limits and Perspectives of Archaeometric Analysis of Archaeological Metals: A Focus on the Electrochemistry for Studying Ancient Bronze Coins. J. Cult. Herit. 2020, 43, 271–281. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Sepiacci, L.; Innocenti, S.; Striova, J.; Fontana, R. Botticelli-Style Tempera Portrait on Tile: Imaging and Specific on-Site Spectroscopic Analysis and Cleaning Examination. J. Cult. Herit. 2024, 66, 229–235. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A New Application of Fiber Optics Reflection Spectroscopy (FORS): Identification of “Bronze Disease” Induced Corrosion Products on Ancient Bronzes. J. Cult. Herit. 2021, 49, 19–27. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Morello, M.; Mazzinghi, A.; Toso, C.; Pampaloni, E.; Fontana, R. Disclosure of a Concealed Michelangelo-Inspired Depiction in a 16th-Century Painting. J. Imaging 2024, 10, 175. [Google Scholar] [CrossRef]
- Cucci, C.; Delaney, J.K.; Picollo, M. Reflectance Hyperspectral Imaging for Investigation of Works of Art: Old Master Paintings and Illuminated Manuscripts. Acc. Chem. Res. 2016, 49, 2070–2079. [Google Scholar] [CrossRef]
- Delaney, J.K.; Thoury, M.; Zeibel, J.G.; Ricciardi, P.; Morales, K.M.; Dooley, K.A. Visible and Infrared Imaging Spectroscopy of Paintings and Improved Reflectography. Herit. Sci. 2016, 4, 6. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, W.; Liu, S.; Chen, J. Detecting Copper Trihydroxychlorides with Reflectance Spectroscopy and Machine Learning Methods. J. Cult. Herit. 2023, 59, 49–56. [Google Scholar] [CrossRef]
- Liggins, F.; Vichi, A.; Liu, W.; Hogg, A.; Kogou, S.; Chen, J.; Liang, H. Hyperspectral Imaging Solutions for the Non-Invasive Detection and Automated Mapping of Copper Trihydroxychlorides in Ancient Bronze. Herit. Sci. 2022, 10, 142. [Google Scholar] [CrossRef]
- Moffa, C.; Merola, C.; Magboo, F.P., Jr.; Chiadroni, E.; Giuliani, L.; Curcio, A.; Palumbo, L.; Felici, A.C.; Petrarca, M. Pigments, Minerals, and Copper-Corrosion Products: Terahertz Continuous Wave (THz-CW) Spectroscopic Characterization of Antlerite and Atacamite. J. Cult. Herit. 2024, 66, 483–490. [Google Scholar] [CrossRef]
- De Haro, A.; Córdova, M.; Rua Landa, C.; Huck-Iriart, C.; Siracusano, G.; Maier, M.S.; Tomasini, E. Methodologies for the Characterization and Identification of Natural Atacamite as a Pigment in Andean Colonial Painting. Heritage 2023, 6, 5116–5129. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Landa, C.R.; Siracusano, G.; Maier, M.S. Atacamite as a Natural Pigment in a South American Colonial Polychrome Sculpture from the Late XVI Century. J. Raman Spectrosc. 2013, 44, 637–642. [Google Scholar] [CrossRef]
- MacLeod, I.D. Bronze Disease: An Electrochemical Explanation. ICCM Bull. 1981, 7, 16–26. [Google Scholar] [CrossRef]
- Grayburn, R.; Dowsett, M.; Hand, M.; Sabbe, P.-J.; Thompson, P.; Adriaens, A. Tracking the Progression of Bronze Disease—A Synchrotron X-Ray Diffraction Study of Nantokite Hydrolysis. Corros. Sci. 2015, 91, 220–223. [Google Scholar] [CrossRef]
- Bozzini, B.; Alemán, B.; Amati, M.; Boniardi, M.; Caramia, V.; Giovannelli, G.; Gregoratti, L.; Kazemian Abyaneh, M. Novel Insight into Bronze Disease Gained by Synchrotron-Based Photoelectron Spectro-Microscopy, in Support of Electrochemical Treatment Strategies. Stud. Conserv. 2017, 62, 465–473. [Google Scholar] [CrossRef]
- Porcu, D.; Innocenti, S.; Galeotti, M.; Striova, J.; Dei, L.; Carretti, E.; Fontana, R. Spectroscopic and Morphologic Investigation of Bronze Disease: Performance Evaluation of Portable Devices. Heritage 2022, 5, 3548–3561. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wu, Y. The Inhibition Effect and Mechanism of L-Cysteine on the Corrosion of Bronze Covered with a CuCl Patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Kwon, H. Corrosion Behaviors of Artificial Chloride Patina for Studying Bronze Sculpture Corrosion in Marine Environments. Coatings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Mezzi, A.; Angelini, E.; Riccucci, C.; Grassini, S.; De Caro, T.; Faraldi, F.; Bernardini, P. Micro-Structural and Micro-Chemical Composition of Bronze Artefacts from Tharros (Western Sardinia, Italy). Surf. Interface Anal. 2012, 44, 958–962. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Ingo, G.M.; Albini, M.; Lapenna, A.; Pierigè, I.; Riccucci, C.; Faraldi, F. An Integrated Analytical Characterization of Corrosion Products on Ornamental Objects from the Necropolis of Colle Badetta-Tortoreto (Teramo, Italy). Appl. Phys. A 2010, 100, 801–808. [Google Scholar] [CrossRef]
- Faraldi, F.; Çilingirǒglu, A.; Angelini, E.; Riccucci, C.; De Caro, T.; Batmaz, A.; Mezzi, A.; Caschera, D.; Cortese, B. Micro-Chemical and Micro-Structural Investigation of Archaeological Bronze Weapons from the Ayanis Fortress (Lake Van, Eastern Anatolia, Turkey). Appl. Phys. A 2013, 113, 911–921. [Google Scholar] [CrossRef]
- Ingo, G.M.; Bustamante, A.D.; Alva, W.; Angelini, E.; Cesareo, R.; Gigante, G.E.; Zambrano, S.D.P.A.; Riccucci, C.; Di Carlo, G.; Parisi, E.I.; et al. Gold Coated Copper Artifacts from the Royal Tombs of Sipán (Huaca Rajada, Perù): Manufacturing Techniques and Corrosion Phenomena. Appl. Phys. A 2013, 113, 877–887. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Lavorgna, M.; Salzano de Luna, M.; Pascucci, M.; Di Carlo, G. Surface Investigation of Naturally Corroded Gilded Copper-Based Objects. Appl. Surf. Sci. 2016, 387, 244–251. [Google Scholar] [CrossRef]
- Ingo, G.M.; Albini, M.; Bustamante, A.D.; Zambrano Alva, S.d.P.; Fernandez, A.; Giuliani, C.; Messina, E.; Pascucci, M.; Riccucci, C.; Staccioli, P.; et al. Microchemical Investigation of Long-Term Buried Gilded and Silvered Artifacts From Ancient Peru. Front. Mater. 2020, 7, 230. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman Approach to the Forensic Study of Bronze Patinas. J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- de Caro, T.; Susanna, F.; La Russa, M.F.; Macchia, A. The Fontanamare Discovery (Sardinia Coast, Italy), a Case of Underwater Corrosion of Bronze Coins. Minerals 2023, 13, 1085. [Google Scholar] [CrossRef]
- de Caro, T.; Angelini, E.; Sebar, L.E. Application of U-Raman Spectroscopy to the Study of the Corrosion Products of Archaeological Coins. ACTA IMEKO 2021, 10, 234–240. [Google Scholar] [CrossRef]
- Robotti, S.; Rizzi, P.; Soffritti, C.; Garagnani, G.L.; Greco, C.; Facchetti, F.; Borla, M.; Operti, L.; Agostino, A. Reliability of Portable X-Ray Fluorescence for the Chemical Characterisation of Ancient Corroded Coppertin Alloys. Spectrochim. Acta Part B At. Spectrosc. 2018, 146, 41–49. [Google Scholar] [CrossRef]
- Soffritti, C.; Fabbri, E.; Merlin, M.; Garagnani, G.L.; Monticelli, C. On the Degradation Factors of an Archaeological Bronze Bowl Belonging to a Private Collection. Appl. Surf. Sci. 2014, 313, 762–770. [Google Scholar] [CrossRef]
- Oudbashi, O. Multianalytical Study of Corrosion Layers in Some Archaeological Copper Alloy Artefacts. Surf. Interface Anal. 2015, 47, 1133–1147. [Google Scholar] [CrossRef]
- Arafat, A.; Na’es, M.; Kantarelou, V.; Haddad, N.; Giakoumaki, A.; Argyropoulos, V.; Anglos, D.; Karydas, A.-G. Combined in Situ Micro-XRF, LIBS and SEM-EDS Analysis of Base Metal and Corrosion Products for Islamic Copper Alloyed Artefacts from Umm Qais Museum, Jordan. J. Cult. Herit. 2013, 14, 261–269. [Google Scholar] [CrossRef]
- Abate, F.; De Bernardin, M.; Stratigaki, M.; Franceschin, G.; Albertin, F.; Bettuzzi, M.; Brancaccio, R.; Bressan, A.; Morigi, M.P.; Daniele, S.; et al. X-Ray Computed Microtomography: A Non-Invasive and Time-Efficient Method for Identifying and Screening Roman Copper-Based Coins. J. Cult. Herit. 2024, 66, 436–443. [Google Scholar] [CrossRef]
- Wang, Z.; Xi, X.; Li, L.; Zhang, Z.; Han, Y.; Wang, X.; Sun, Z.; Zhao, H.; Yuan, N.; Li, H.; et al. Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-Ray Microtomography Study. Molecules 2023, 28, 4933. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.; Crespo, A.; Lafuente, D.; Ramirez Barat, B. A Novel Gel Polymer Electrolyte Cell for In-Situ Application of Corrosion Electrochemical Techniques. Electrochem. Commun. 2014, 41, 16–19. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Doménech-Carbó, M.; Martínez-Lázaro, I. Electrochemical Identification of Bronze Corrosion Products in Archaeological Artefacts. A Case Study. Microchim Acta 2008, 162, 351–359. [Google Scholar] [CrossRef]
- Yang, X.; Wu, W.; Chen, K. Investigation on the Electrochemical Evolution of the Cu-Sn-Pb Ternary Alloy Covered with CuCl in a Simulated Atmospheric Environment. J. Electroanal. Chem. 2022, 921, 116636. [Google Scholar] [CrossRef]
- Grazzi, F.; Cucci, C.; Casini, A.; Stefani, L.; Kardjilov, N.; Thiele, A.; Hošek, J.; Picollo, M. Merging of Imaging Techniques Based on Reflectance Hyperspectral and Neutron Tomography for Characterization of a Modern Replica of a 13th Century Knife from Croatia. In Optics for Arts, Architecture, and Archaeology VII; SPIE: Munich, Germany, 2019; Volume 11058, pp. 161–173. [Google Scholar]
- Orsilli, J.; Caglio, S. Combined Scanned Macro X-Ray Fluorescence and Reflectance Spectroscopy Mapping on Corroded Ancient Bronzes. Minerals 2024, 14, 192. [Google Scholar] [CrossRef]
- Hayem-Ghez, A.; Ravaud, E.; Boust, C.; Bastian, G.; Menu, M.; Brodie-Linder, N. Characterizing Pigments with Hyperspectral Imaging Variable False-Color Composites. Appl. Phys. A 2015, 121, 939–947. [Google Scholar] [CrossRef]
- Moon, T.; Schilling, M.R.; Thirkettle, S. A Note on the Use of False-Color Infrared Photography in Conservation. Stud. Conserv. 1992, 37, 42–52. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Bonacini, I.; Mazzeo, R. New Frontiers in Application of FTIR Microscopy for Characterization of Cultural Heritage Materials. In Analytical Chemistry for Cultural Heritage; Mazzeo, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 129–160. ISBN 978-3-319-52804-5. [Google Scholar]
- Marengo, E.; Manfredi, M.; Zerbinati, O.; Robotti, E.; Mazzucco, E.; Gosetti, F.; Bearman, G.; France, F.; Shor, P. Development of a Technique Based on Multi-Spectral Imaging for Monitoring the Conservation of Cultural Heritage Objects. Anal. Chim. Acta 2011, 706, 229–237. [Google Scholar] [CrossRef]
- Deborah, H.; George, S.; Hardeberg, J.Y. Pigment Mapping of the Scream (1893) Based on Hyperspectral Imaging. In Image and Signal Processing; Elmoataz, A., Lezoray, O., Nouboud, F., Mammass, D., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 247–256. [Google Scholar]
- de Carvalho, O.A.; Menese, P.R. Spectral Correlation Mapper (SCM): An Improvement on the Spectral Angle Mapper (SAM). In Proceedings of the Summaries of the 9th JPL Airborne Earth Science Workshop, Pasadena, CA, USA, 23–25 February 2000; pp. 65–74. [Google Scholar]
- Castelle, M.; Bormand, M.; Vandenberghe, Y.; Bourgarit, D. Two of a Kind: Shining New Light on Bronze Spiritelli Attributed to Donatello. Stud. Conserv. 2020, 65, 200–211. [Google Scholar] [CrossRef]
- Salvioli, N.; Sarri, S.; Agresti, J.; Osticioli, I.; Siano, S. Conservation Treatments and Archaeometallurgical Insights on the Medici Riccardi Horse Head. In Artistry in Bronze; Daehner, J.M., Lapatin, K., Spinelli, A., Eds.; The Greeks and Their Legacy XIXth International Congress on Ancient Bronzes; Getty Publications: Los Angeles, CA, USA, 2017; pp. 319–328. ISBN 978-1-60606-541-9. [Google Scholar]
- Daffara, C.; Pampaloni, E.; Pezzati, L.; Barucci, M.; Fontana, R. Scanning Multispectral IR Reflectography SMIRR: An Advanced Tool for Art Diagnostics. Acc. Chem. Res. 2010, 43, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Striova, J.; Ruberto, C.; Barucci, M.; Blažek, J.; Kunzelman, D.; Dal Fovo, A.; Pampaloni, E.; Fontana, R. Spectral Imaging and Archival Data in Analysing Madonna of the Rabbit Paintings by Manet and Titian. Angew. Chem. 2018, 130, 7530–7534. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The Spectral Image Processing System (SIPS)—Interactive Visualization and Analysis of Imaging Spectrometer Data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Ohno, Y. CIE Fundamentals for Color Measurements. In Proceedings of the Digital Printing Technologies, IS&T’s NIP16, International Conference, Vancouver, CA, USA, 16–20 October 2000. No. 16. [Google Scholar]
- Quintero Balbas, D.; Dal Fovo, A.; Montalbano, L.; Fontana, R.; Striova, J. Non-Invasive Contactless Analysis of an Early Drawing by Raffaello Sanzio by Means of Optical Methods. Sci. Rep. 2022, 12, 15602. [Google Scholar] [CrossRef]
- Dal Fovo, A.; Mattana, S.; Ramat, A.; Riitano, P.; Cicchi, R.; Fontana, R. Insights into the Stratigraphy and Palette of a Painting by Pietro Lorenzetti through Non-Invasive Methods. J. Cult. Herit. 2023, 61, 91–99. [Google Scholar] [CrossRef]
- Pellis, G.; Bertasa, M.; Ricci, C.; Scarcella, A.; Croveri, P.; Poli, T.; Scalarone, D. A Multi-Analytical Approach for Precise Identification of Alkyd Spray Paints and for a Better Understanding of Their Ageing Behaviour in Graffiti and Urban Artworks. J. Anal. Appl. Pyrolysis 2022, 165, 105576. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-04904-4. [Google Scholar]
- Ropret, P.; Kosec, T. Raman Investigation of Artificial Patinas on Recent Bronze—Part I: Climatic Chamber Exposure. J. Raman Spectrosc. 2012, 43, 1578–1586. [Google Scholar] [CrossRef]
- Cosano, D.; Esquivel, D.; Mateos, L.D.; Quesada, F.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Spectroscopic Analysis of Corrosion Products in a Bronze Cauldron from the Late Iberian Iron Age. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 489–496. [Google Scholar] [CrossRef]
- Choi, H.; Yu, Y.; Cho, N. Chemical Composition, Crystal Structure, and Microstructure of Slags on the Korean Peninsula from the First Copper Production Remains of the 9th Century. Crystals 2024, 14, 327. [Google Scholar] [CrossRef]
- Kwon, H.; Cho, N. In-Situ Non Destructive Investigation of Contemporary Outdoor Bronze Sculptures. Herit. Sci. 2024, 12, 167. [Google Scholar] [CrossRef]
- Ospitali, F.; Chiavari, C.; Martini, C.; Bernardi, E.; Passarini, F.; Robbiola, L. The Characterization of Sn-Based Corrosion Products in Ancient Bronzes: A Raman Approach. J. Raman Spectrosc. 2012, 43, 1596–1603. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.; Kloprogge, J.T.; Williams, P.A. Raman Spectroscopy of the Basic Copper Chloride Minerals Atacamite and Paratacamite: Implications for the Study of Copper, Brass and Bronze Objects of Archaeological Significance. J. Raman Spectrosc. 2002, 33, 801–806. [Google Scholar] [CrossRef]
- Frost, R.L. Raman Spectroscopy of Selected Copper Minerals of Significance in Corrosion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- de Ferri, L.; Mazzini, F.; Vallotto, D.; Pojana, G. In Situ Non-Invasive Characterization of Pigments and Alteration Products on the Masonry Altar of S. Maria Ad Undas (Idro, Italy). Archaeol. Anthropol. Sci. 2019, 11, 609–625. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M.; Marcello, P.; Ricciardi, P.; Delaney, J. Characterisation of Colourants on Illuminated Manuscripts by Portable Fibre Optic UV-Visible-NIR Reflectance Spectrophotometry. Anal. Methods 2014, 6, 1488–1500. [Google Scholar] [CrossRef]

| Ni (Kα) | Cu (Kα) | Zn (Kα) | Sn (Kα) | Pb (Lα) | Tot. | |
|---|---|---|---|---|---|---|
| Bronze composition | 0.38 | 87.44 | 2.92 | 6.57 | 2.69 | 100 |
| St. dev. | 0.02 | 0.35 | 0.01 | 0.01 | 0.35 |
| Sample | Aging Time (Hours) | Sample | Aging Time (Hours) |
|---|---|---|---|
| C1 | 0 | C7 | 62 |
| C2 | 0 | C8 | 65 |
| C3 | 14 | C9 | 68 |
| C4 | 24 | C10 | 72 |
| C5 | 38 | C11 | 134 |
| C6 | 48 | C12 | 143 |
| Sample | Measurement Point | ∆L* | ∆a* | ∆b* | ∆E* |
|---|---|---|---|---|---|
| C3 | 1 | 0.04 | −5.76 | −8.68 | 11.72 |
| 2 | −2.57 | 0.28 | 2.45 | 4.92 | |
| 3 | 11.54 | −8.57 | −11.79 | 20.22 | |
| C4 | 1 | 2.04 | −0.02 | −1.37 | 3.64 |
| 2 | 6.99 | 1.35 | −1.06 | 7.92 | |
| 3 | 3.46 | 1.47 | −0.07 | 5.21 | |
| C5 | 1 | 1.57 | −3.82 | −11.25 | 12.30 |
| 2 | 13.92 | 5.37 | −2.82 | 15.30 | |
| 3 | 9.58 | 4.22 | 2.53 | 11.39 | |
| C6 | 1 | −11.05 | 9.17 | −5.41 | 15.51 |
| 2 | 11.26 | 1.24 | −1.89 | 11.69 | |
| 3 | 9.57 | 3.63 | −1.82 | 8.42 | |
| C7 | 1 | −7.58 | 0.36 | −6.01 | 13.74 |
| 2 | −6.48 | −4.97 | 1.14 | 6.29 | |
| 3 | −8.48 | −2.34 | −1.82 | 6.72 | |
| C8 | 1 | 0.90 | −6.36 | −6.61 | 9.87 |
| 2 | 23.00 | −28.94 | −12.97 | 39.32 | |
| 3 | 2.55 | −17.89 | −16.27 | 26.28 | |
| C9 | 1b | 1.01 | 8.30 | −4.35 | 10.26 |
| 2b | 1.06 | 2.95 | −7.23 | 8.79 | |
| 3b | 3.82 | 8.25 | −7.93 | 12.59 | |
| 1g | 4.45 | −23.77 | −11.82 | 27.85 | |
| 2g | 10.31 | −12.45 | −15.19 | 23.13 | |
| 3g | 16.99 | −20.53 | −9.23 | 28.54 | |
| C10 | 1 | 10.30 | −18.22 | −9.93 | 23.25 |
| 2 | 15.07 | −14.82 | −11.13 | 24.10 | |
| 3 | 4.19 | −20.31 | −15.37 | 27.35 | |
| C11 | 1 | 6.67 | −8.46 | −7.32 | 13.46 |
| 2 | 8.92 | −17.98 | −17.55 | 27.14 | |
| 3 | 19.98 | −21.97 | −14.84 | 33.32 | |
| C12 | 1 | −4.07 | −8.47 | −4.08 | 10.43 |
| 2 | −5.76 | −6.00 | −7.23 | 10.46 | |
| 3 | −1.79 | −18.42 | −8.54 | 20.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, D.; Innocenti, S.; Striova, J.; Carretti, E.; Fontana, R. Mapping Bronze Disease Onset by Multispectral Reflectography. Minerals 2025, 15, 252. https://doi.org/10.3390/min15030252
Porcu D, Innocenti S, Striova J, Carretti E, Fontana R. Mapping Bronze Disease Onset by Multispectral Reflectography. Minerals. 2025; 15(3):252. https://doi.org/10.3390/min15030252
Chicago/Turabian StylePorcu, Daniela, Silvia Innocenti, Jana Striova, Emiliano Carretti, and Raffaella Fontana. 2025. "Mapping Bronze Disease Onset by Multispectral Reflectography" Minerals 15, no. 3: 252. https://doi.org/10.3390/min15030252
APA StylePorcu, D., Innocenti, S., Striova, J., Carretti, E., & Fontana, R. (2025). Mapping Bronze Disease Onset by Multispectral Reflectography. Minerals, 15(3), 252. https://doi.org/10.3390/min15030252










