4.1. Major Mineral Characteristics
The lateritic nickel ore sample contains major minerals such as chromite, limonite, and other silicate minerals. Chromite has a hardness of 5.5 to 6.5, and a density of 4.3 to 4.8 g/cm
3, with an average magnetic susceptibility of 286 [
24]. Chromite content is relatively low and predominantly occurs in euhedral to subhedral granular texture, often featuring irregular fractures (
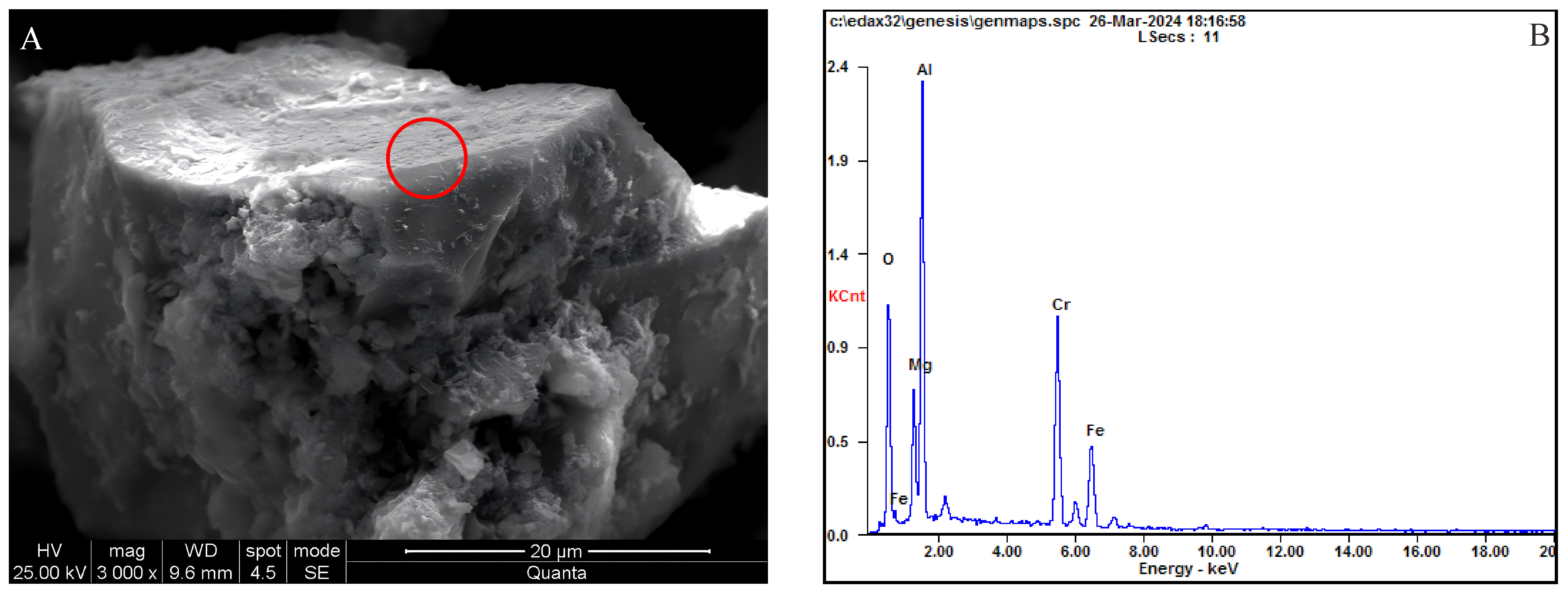
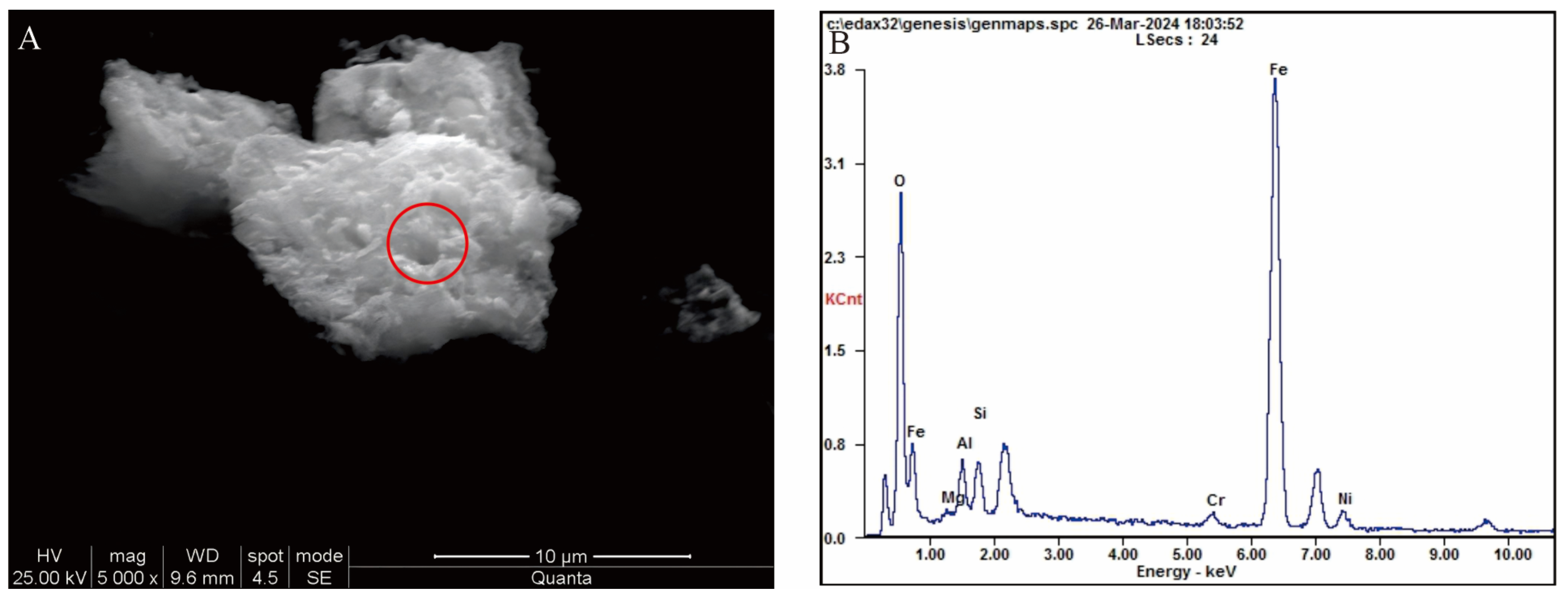
Figure 4A,B). A small portion of the chromite is cemented with magnetite and limonite, and some are cemented with clayey limonite (
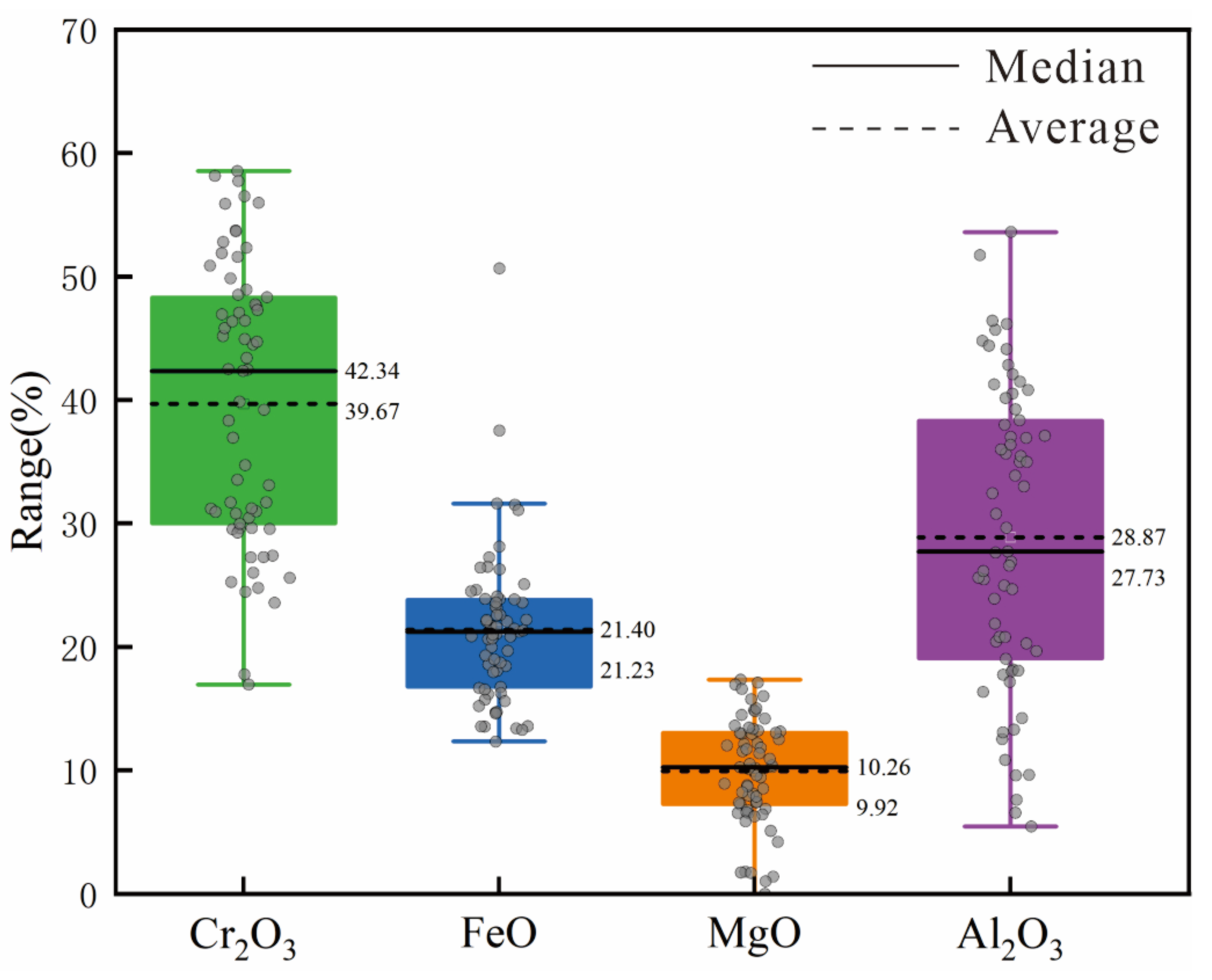
Figure 5A,B). The Cr content in chromite varies widely, with Cr
2O
3 levels primarily ranging from 16.95% to 58.53% (
Figure 6 and
Table 3). Partial SEM pictures of chromite are attached as
Supplementary Figures S1–S5.
As the predominant mineral component in the sample, Limonite manifests in two categories: cryptocrystalline limonite and limonite (
Figure 7A,B). The former is the easily observable clayey limonite mineral, while the latter is the most common mud-like material and the dominant component in this sample (
Figure 8). Limonite is characterized by its extremely fine-grained texture, comprising primarily of various iron oxide minerals such as acicular ferrite, hydrous acicular ferrite, hydrous hematite, and fibrous ferrite. Additionally, limonite hosts a diverse array of adsorbed substances including silicate minerals, manganese oxide minerals, and very fine-grained chromium minerals (
Figure 9 and
Table 4). Partial SEM pictures of limonite are attached as
Supplementary Figures S6–S10. In nickel laterite ores, limonite often serves as the main source of nickel. Due to its composition and structure, it is particularly amenable to extraction via wet metallurgical methods. Its adaptability makes limonite the key to the processing of nickel laterites, emphasizing its industrial and economic importance.
In addition to chromite and limonite, the samples also contain metallic minerals such as manganese oxides and magnetite. The manganese oxides are epithermal. Manganese-bearing minerals dominated by MnO2 and include diverse elements like Fe, Co, and Ni (but notably exclude Cr), exhibit varying crystallinities. While some particles display poor crystallinity and form fine-grained, aggregate-like mixtures, others exhibit good crystallinity. These manganese oxides are often intimately associated with limonite, appearing in argillation and irregular grains with blurred mineral boundaries, typically cemented with limonite.
Magnetite is another significant mineral in the samples, which consist a relative density of 4.9 to 5.2 and a Mohs hardness of 5.5 to 6.5 [
25]. Its high specific magnetization coefficient, averaging 92,000, makes it readily separable by magnetic methods. However, the presence of magnetite in the ore is minor, typically occurring as euhedral grains embedded within limonite or cemented alongside limonite and manganese oxides. X-ray spectroscopy analysis reveals that magnetite contains traces amount of Cr and Ni, highlighting its compositional complexity.
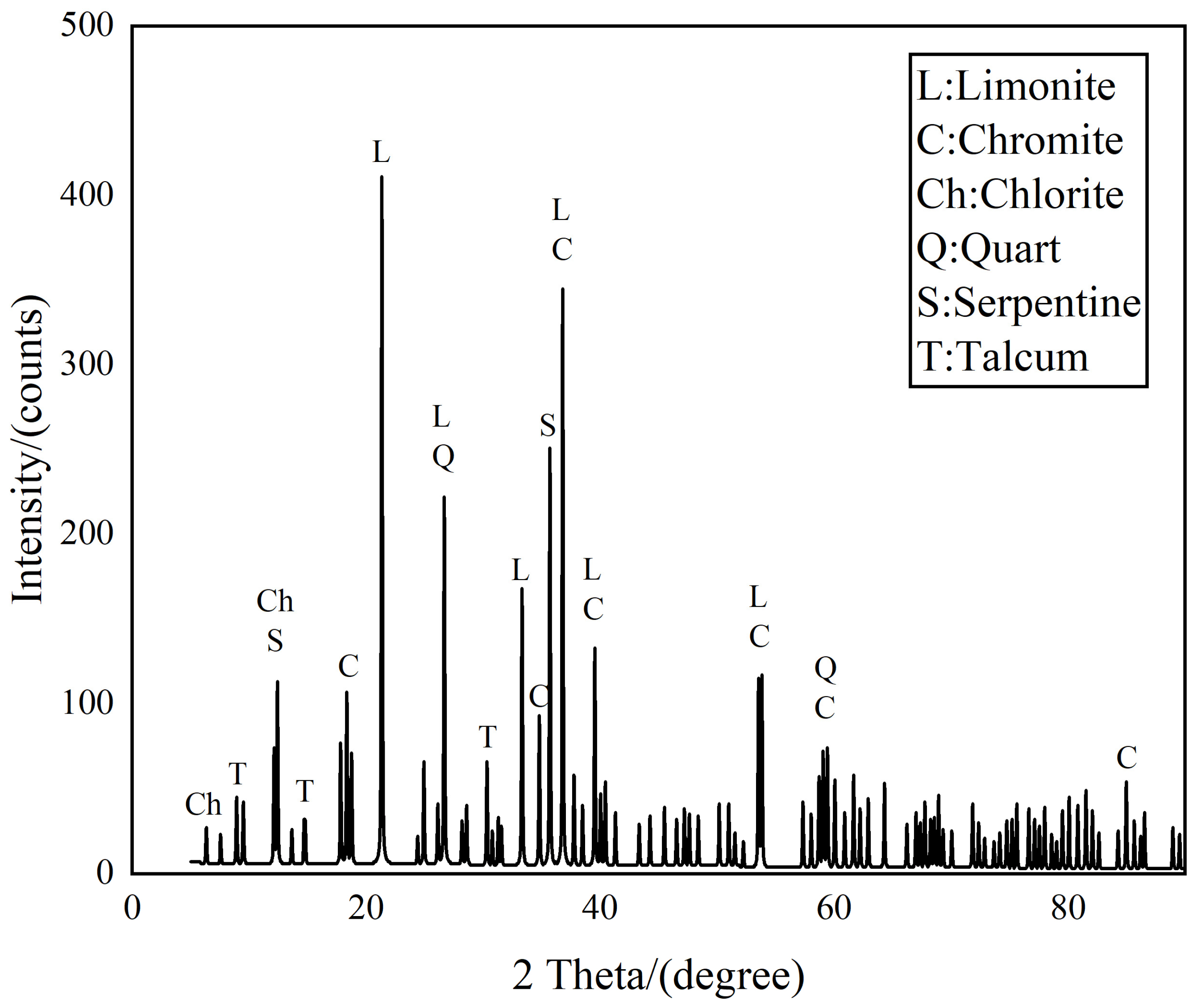
Beyond the primary minerals discussed, the sample also comprises amounts of silicate minerals, including chlorite, quartz, serpentine, and talcum, along with lesser amounts of tremolite, diopside, and fayalite. These silicates contain traces amount of nickel, yet notably lack chromium. Although the silicate minerals are present in low concentrations, their influence on the recovery processes, particularly on flotation, should not be overlooked. While they minimally affect the chromium and nickel recovery directly, their impact on the flotation process requires careful consideration to ensure optimal recovery efficiency and process selectivity.
4.2. Influencing Factors of Chromium Recovery
The weathering characteristics and material composition of lateritic deposits provide insights into their genesis. Additionally, the Ultra Magnesian Iron Alteration Index (UMIA) is utilized to assess the geochemistry of lateritic nickel deposits [
26,
27]:
The UMIA quantifies the loss of Si and Mg elements and the enrichment of Fe elements in lateritic nickel deposits. The UMIA value for fresh peridotite is typically below 10, indicating minimal alteration. Values for the saprolite zone and lateritic zone progressively increase, usually ranging between 40 and 50. The current sample from a lateritic nickel deposit has a UMIA of 81.71, which reflects a substantial loss of Mg and Si and a significant enrichment of Fe. The high UMIA value suggests that the deposit underwent intensive physical and chemical weathering and mineral alteration. The degree of alteration is directly correlated with particle size. More intense alteration typically results in finer particles, which complicates mineral processing. Strong alteration significantly alters the particle size composition and structure of minerals.
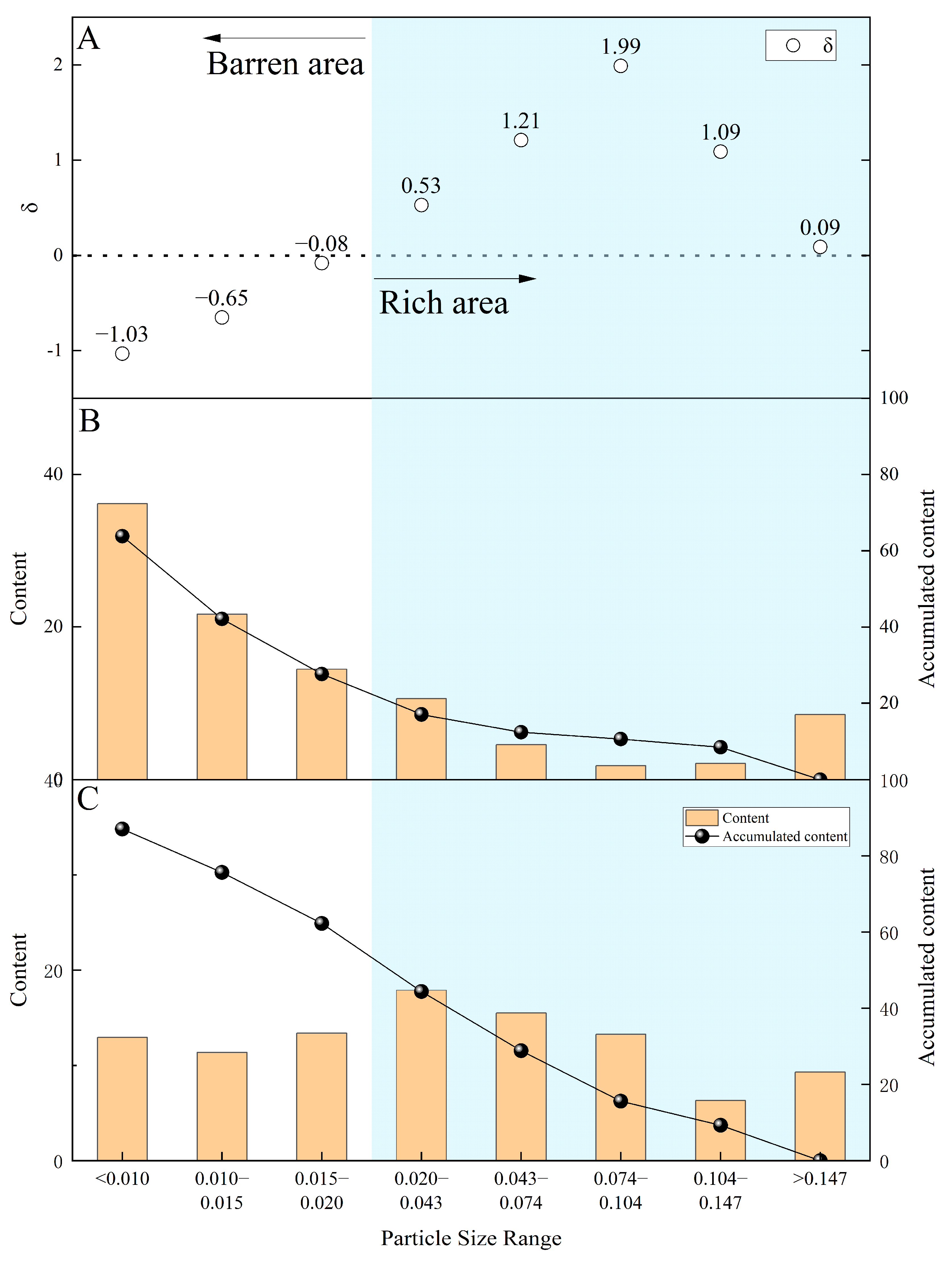
Particle size analysis reveals that 72.34% of the total mass distributed under 0.02 mm, which indicates a high proportion of fine particles within the lateritic nickel deposits (
Table 5). For the target minerals, coarse and concentrated particle distributions generally reduce the difficulty of beneficiation. However, the particle size distribution of chromite shows low concentrated distribution. While coarser particles can be recovered through beneficiation, but about 37.74% of the chromite is finer than 0.02 mm, making physical separation challenging. The fine-grained minerals smaller than 0.02 mm are primarily nickel-containing cryptocrystalline limonite. This fraction of limonite will encapsulate microfine-grained chromite, making it difficult to separate from each other. We used δ as a measure of chromite enrichment based on the particle size alone. If the ratio of chromite content to total chromite content of the same grain size is significantly greater than the mineral content of the same grade, the recovery value of that grade is considered likely to be greater. The range of grades is called the enriched area. In contrast, the other grain size ranges are barren area. The ratio can be better represented by applying a natural logarithmic process. The calculations show that the interval with a particle size larger than 0.02 mm is the relatively enriched area for chromite, which means that it is more feasible to recover chromite with a particle size larger than 0.02 mm only from the aspect of particle size (
Figure 10).
This lateritic nickel deposit was formed through intense weathering, resulting in ore samples with a mud-like consistency. Chromite shows more resistant to weathering than other minerals such as limonite or silicate minerals and exhibits distinct characteristics. By analyzing the primary samples, it was determined that the chromite is predominantly liberated. The percentage of liberated chromite particles was found to be 97% or higher.
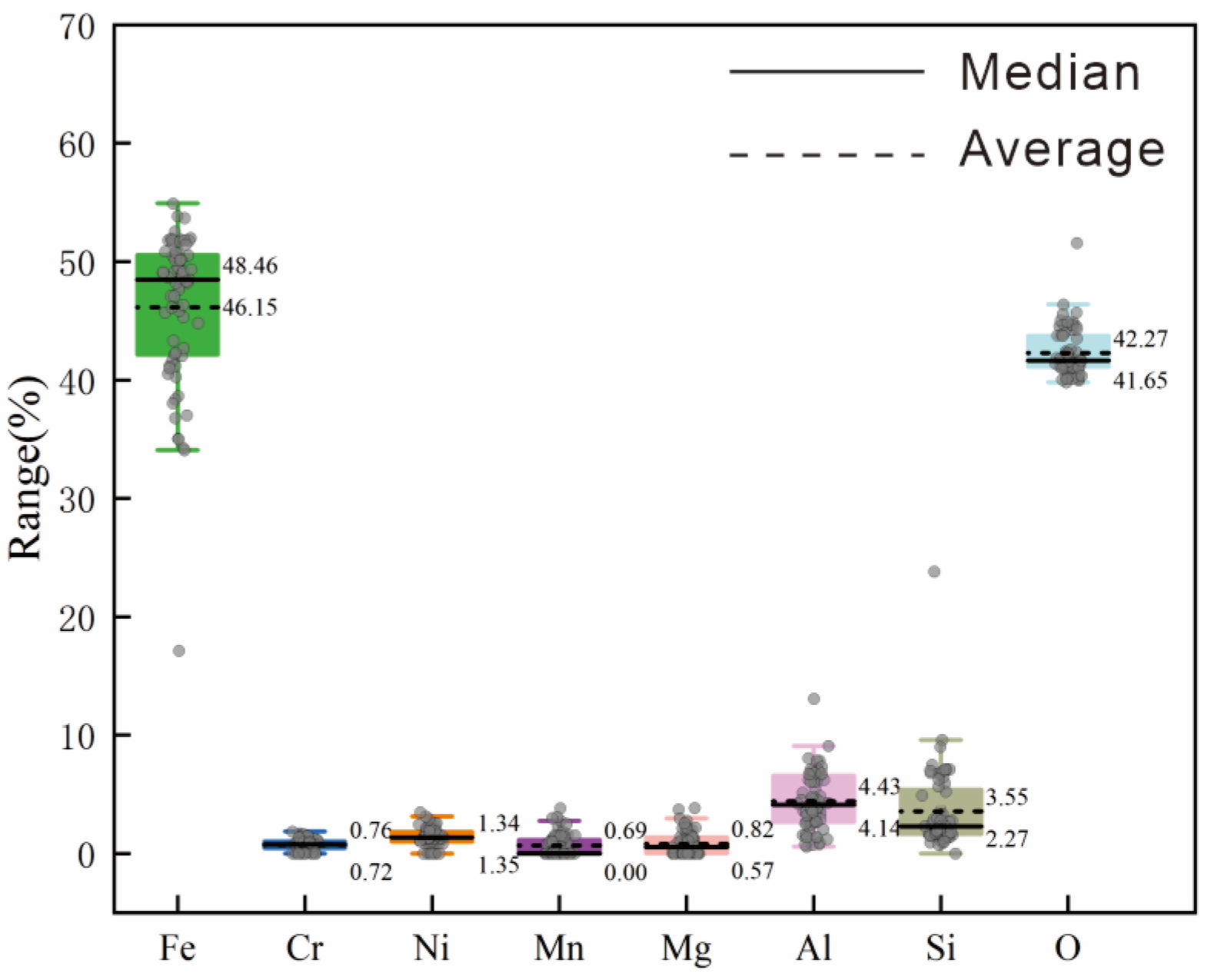
Phase analysis of chromium, nickel, and iron reveals distinct mineral associations (
Table 6). Nickel is predominantly found in limonite, while chromium mainly occurs as chromite, existing as an independent mineral. The primary iron-bearing mineral is limonite, which is accompanied by minor quantities of silicate. Additionally, trace amounts of magnetite and chromite are present. Phases analysis suggested that the deposit is primarily composed of weakly magnetic minerals.
We synthesized SEM analysis of the major minerals in the sample and the results of phase analysis to understand the occurrence state of nickel and chromium. Nickel exhibits a complex occurrence state, predominantly associated with limonite, followed by manganese oxides and trace amount of Ni found in serpentine, talcum, and magnetite. Chromium primarily exists in distinct mineral forms like chromite. Notably, 32.56% of the chromium content is found in limonite, with additional traces in chlorite and magnetite. The equilibrium composition of chromium and nickel across these minerals are detailed in
Table 7 and
Table 8, respectively.
The occurrence state of nickel and chromium suggests targeted recovery approaches: chromium primarily from chromite and nickel from limonite. The subsequently designed recovery experiment aims to physically separate chromite and to isolate chromite from chromite and other silicate minerals. In the sample, 63.40% of the chromium is located in chromite, which is our primary recycling target. However, 37.74% of the chromite is finer than 0.02 mm, complicating the recovery of this chromium fraction. Except for chromite, 34.18% of the chromium is hosted in mud-like limonite. Its fine-grained characteristic made it unapproachable for physical beneficiation. In addition, manganese oxide is a class of minerals, which means its density and specific magnetization coefficient are difficult to determine [
28]. During the processes of gravity separation and magnetic methods, manganese oxides exhibit a relatively uniform behavior alongside chromite, which complicates their separation. This similarity in behavior adversely impacts the quality of the chrome concentrate, as the separation was inefficient. Furthermore, nickel is not associated with chromite, thus ensuring no nickel loss during the recovery of chromite.
In summary, there are mainly two reasons that affect the improvement of the recovery rate of chromite. The fine-grained nature of chromite is the primary reason that makes it difficult to increase the recovery efficiency. A large amount of fine-grained chromite is encapsulated within limonite, which makes the recovery of this portion of chromite challenging. Secondly, the distribution rate of chromium elements in limonite reaches 34.18%. This particular portion of chromium is hard to recover, which is also one of the major issues that elevate the difficulty to improve the chromium recovery rate. In addition, due to the similarity in magnetism and density to chromite, the manganese oxides in lateritic nickel ores, made physical beneficiation methods indistinguishable between the them, which affects the quality of the chromite concentrate.
4.3. Comprehensive Recycling Process
By carrying the process mineralogy of this deposit, we aim to emphasize two primary issues. First, it is essential to gain a comprehensive understanding of all minerals present in the sample. This facilitates the identification of impurity minerals that need to be eliminated during the chromite enrichment process, thereby defining the optimal processing sequence. Second, by investigating the granularity and liberation of minerals, along with the occurrence states of elements, the theoretical maximum recovery rate can be estimated. This estimate serves as a critical reference for subsequent beneficiation trials, providing a basis for evaluating the efficacy of the beneficiation outcomes. The exploration of these issues is crucial for determining the target of recovery, the sequence of processing steps, and the potential recovery outcomes. It is imperative to conduct such feasibility research on the development of a mineral deposit as it can substantially reduce the temporal and resource expenditures associated with beneficiation and smelting.
Through the aforementioned mineralogical research, we can confirm that the target mineral for chromium recovery is chromite. The impurity minerals that need to be disseminated include limonite, manganese oxides, magnetite, and silicate minerals. The mineral composition of this nickel laterite ore is predominantly limonite, characterized by an extremely fine-grained size and significant argillation. For effective recovery of chromite, initial and deeply desliming of the sample is imperative. Although the desliming process results in the loss of some chromite, it directly removes the majority of limonite and incidentally removes silicate minerals. The minerals that still need to be separated from chromite include manganese oxides and magnetite. Given its high specific magnetization coefficient, magnetite separation necessitates the introduction of magnetic separation technique. After the removal of magnetite, 1.73% of the remaining impurities are manganese oxides. Unfortunately, it is physically challenging to separate manganese oxides from chromite during mineral processing, which may affect the grade of the final product. The granularity and liberation of major minerals, as well as the occurrence state of elements, can guide the prediction of the theoretical recovery rate of chromium. According to the distribution of chromium elements, 63.40% of chromium exists in the form of chromite, and we are only able to recover chromium from chromite. Therefore, considering only the occurrence state of elements, the maximum theoretical recovery rate is 63.40%. In addition,
Figure 10 indicates that chromite is relatively more enriched in minerals with a particle size greater than 0.02 mm. The substantial amount of clayey limonite surrounding the fine-grained chromite in the raw ore lead to an inevitable outflow during desliming. The fine chromite fraction (with a particle size less than 0.02 mm) accounts for 37.74% of the total chromite. Since chromite is almost completely liberated, the recovery of chromite will hardly decrease due to liberation. The effective liberation of chromite facilitates beneficiation without the need for further grinding. In summary, it is theoretically possible to recover 39.47% of the chromium in the sample through physical beneficiation methods.
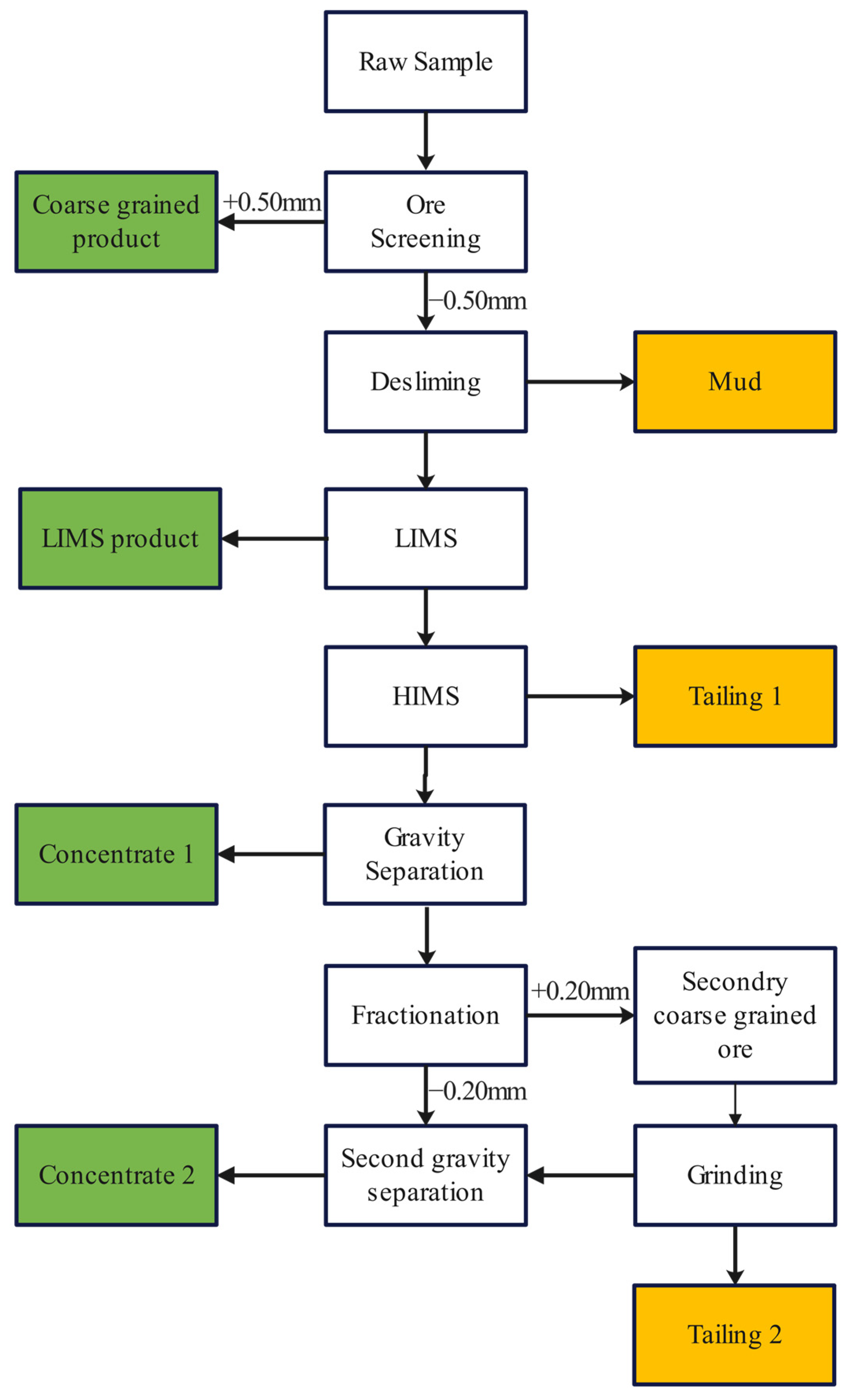
Based on these findings, we propose a process for the recovery of chromium, as illustrated in
Figure 11. The beneficiation recovery process for chromium minerals includes several key steps: ore screening, intensive desliming, low intensity magnetic separation (LIMS), high intensity magnetic separation (HIMS), gravity separation, and lastly, the regrinding of middling with a second gravity separation [
29,
30,
31]. Initially, the ore is screened to select coarse chromite particles larger than 0.50 mm. The finer fractions undergo extensive desliming, followed by both LIMS and HIMS, leading to the initial gravity separation [
32]. Given chromite’s high density, its separation in this stage is highly effective. The first round of gravity separation produces Concentrate 1. Subsequently, the remaining material is subjected to further fractionation, grinding, and a second gravity separation to produce Concentrate 2. This two-stage approach aims to enhance the overall recovery rate. Following the aforementioned beneficiation process, we executed a series (five rounds) of beneficiation experiments, the details are delineated in
Supplementary Table S1. The aggregate outcomes of these experiments revealed that the combined yield of Concentrates 1 and 2 averaged 1.39%, with a mean grade of 33.82% chromium oxide. Additionally, the anticipated recovery rate for chromium oxide (Cr
2O
3) was determined to be 20.36%. The recovery rate of the experiment only reached 51.58% of the theoretical recovery rate, indicating that it is difficult to recover chromium from laterite nickel ore and there is still great potential for improvement in the future.