Surface Characterization of Chalcopyrite Dissolution in Hypochlorite Medium
Abstract
1. Introduction
2. Materials and Methods
3. Results
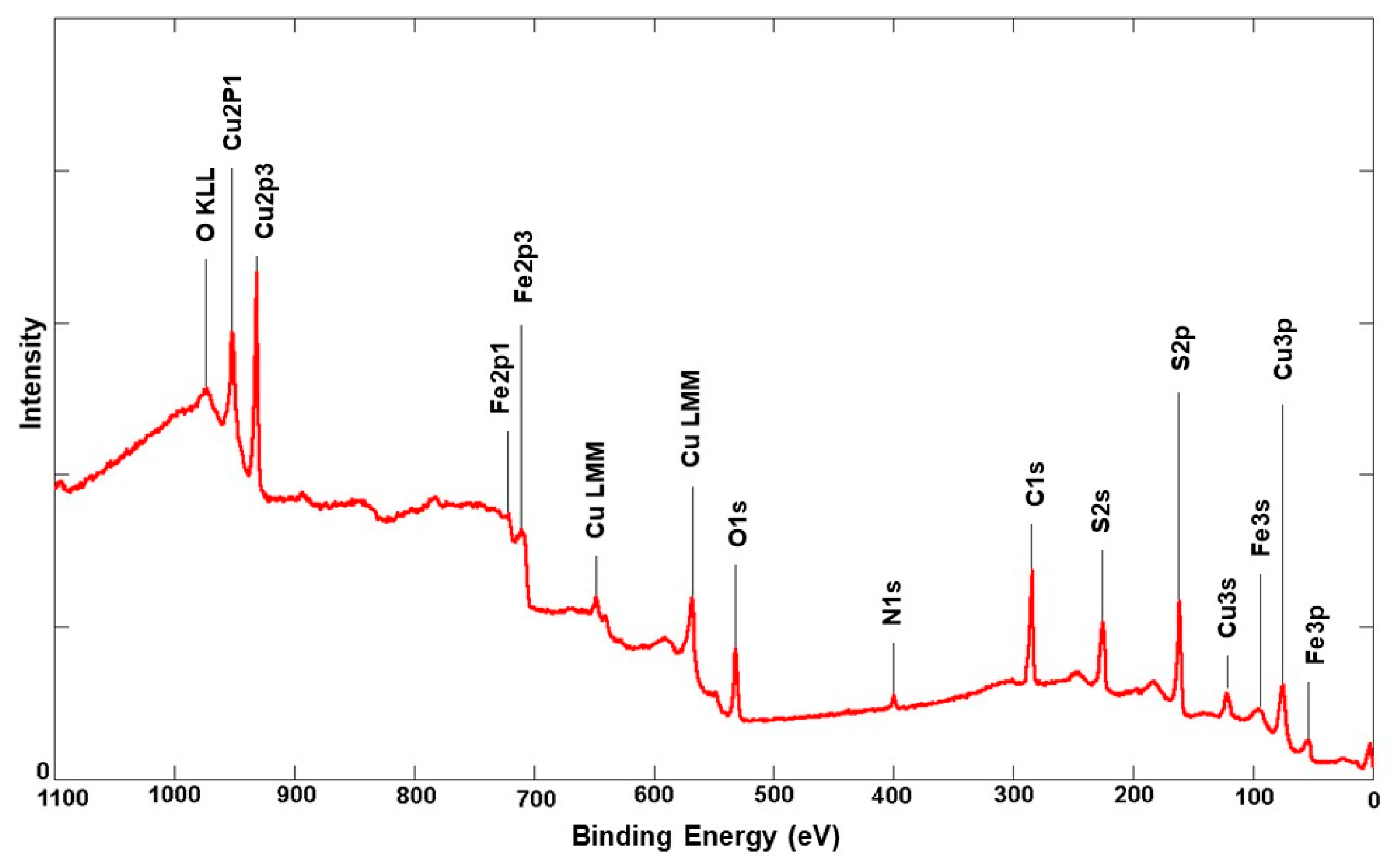
3.1. Surface Analysis of Unleached Chalcopyrite
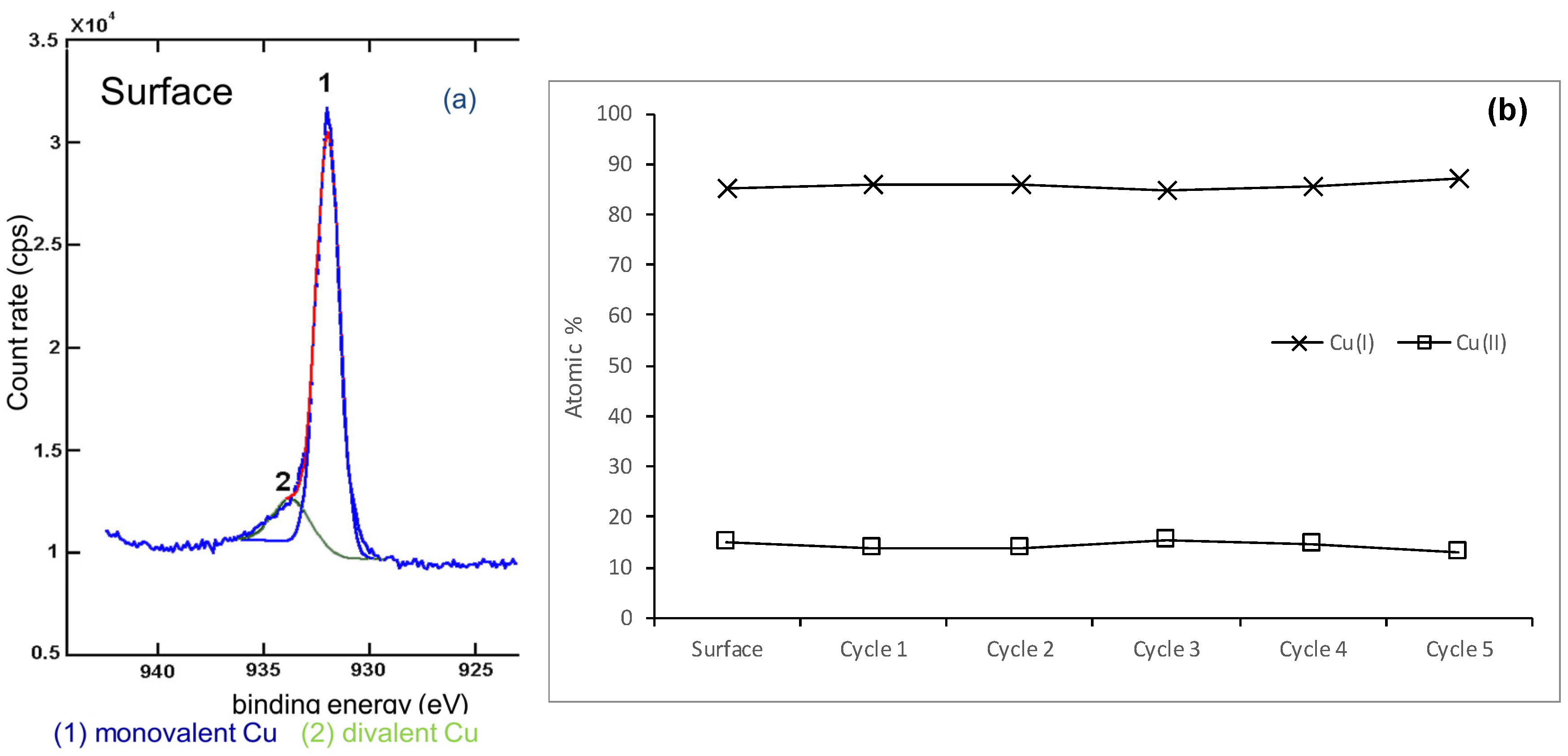
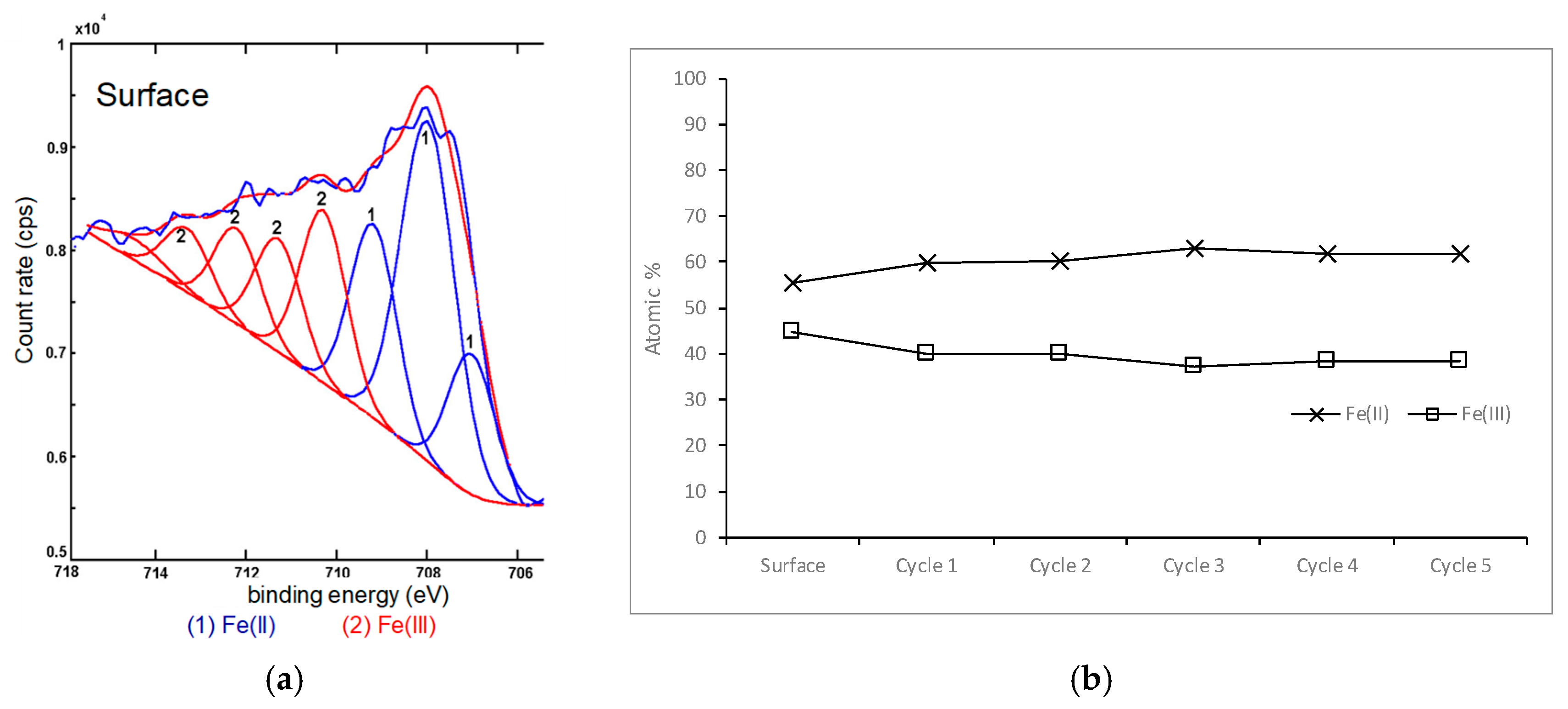
3.1.1. Analysis of the Atomic Oxidation States of Chalcopyrite
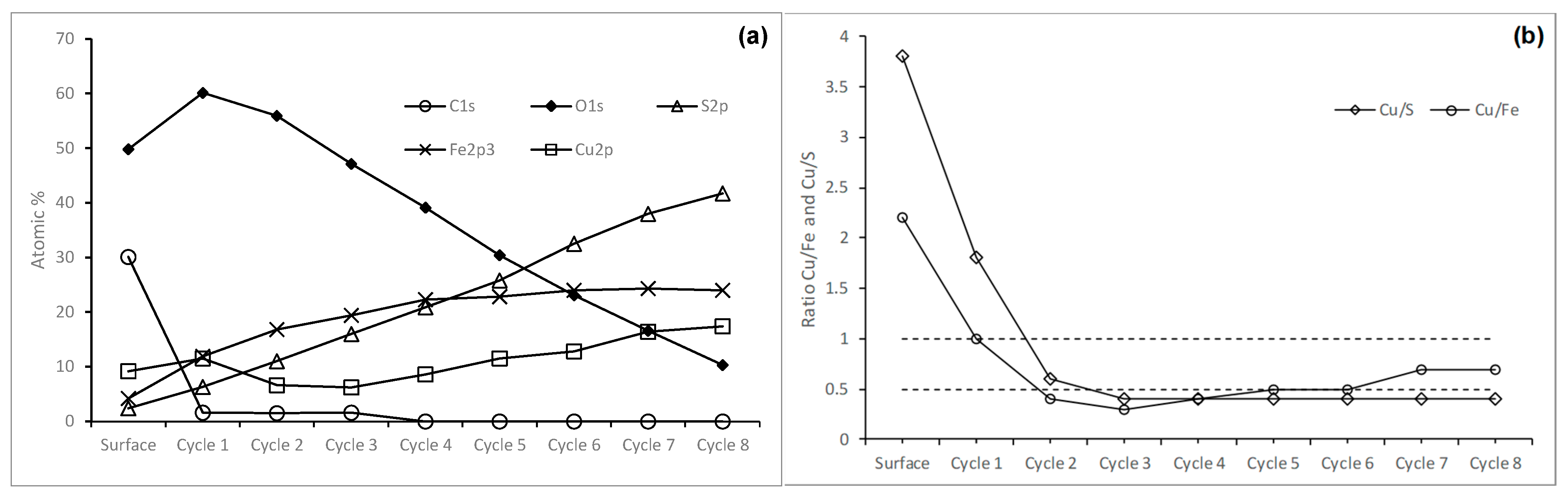
3.1.2. Chalcopyrite Composition
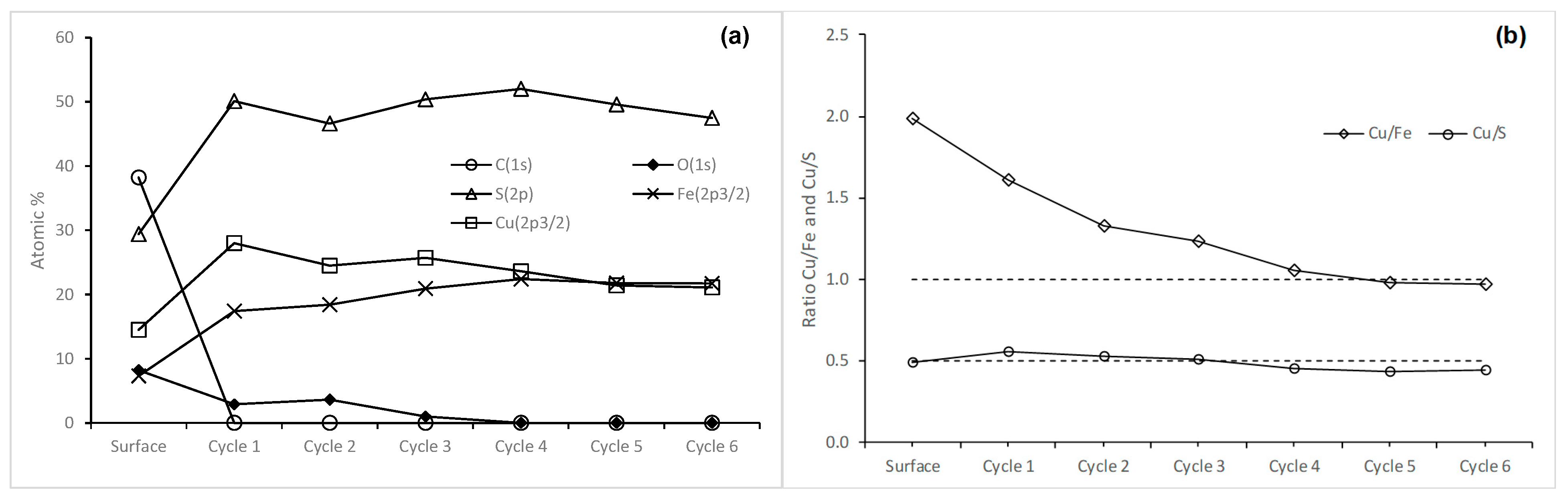
3.2. Surface Analysis of Chalcopyrite Leached with Sodium Hypochlorite
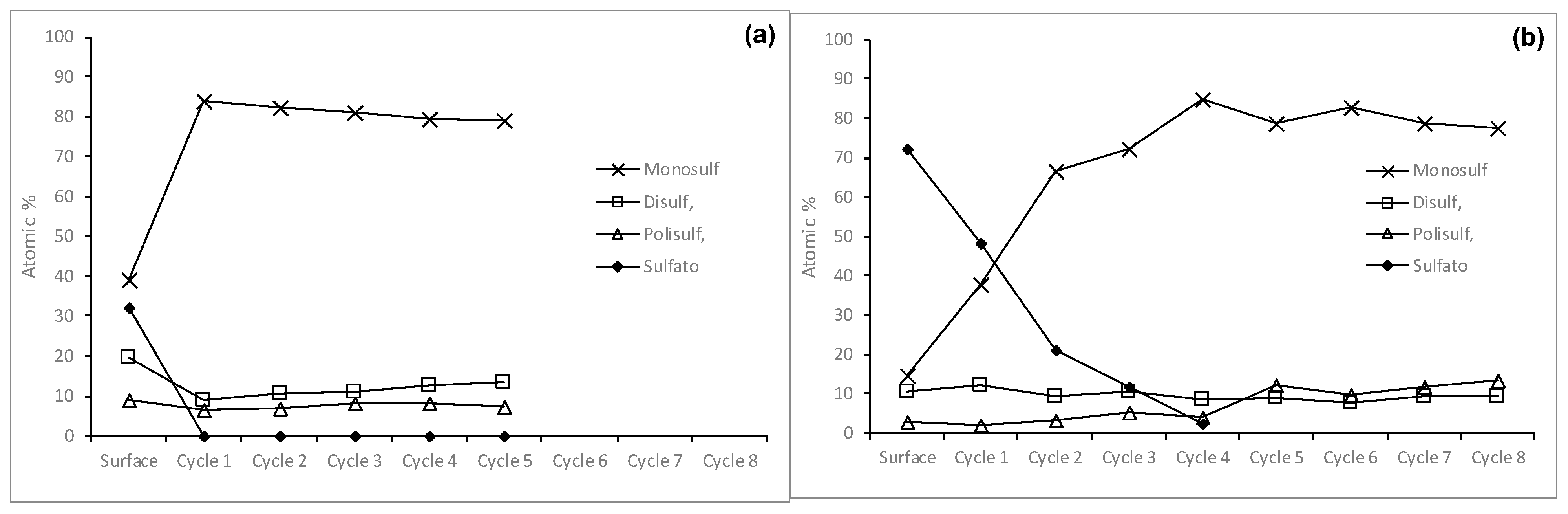
3.2.1. Behavior of Chalcopyrite Sulfur When Leached with Hypochlorite
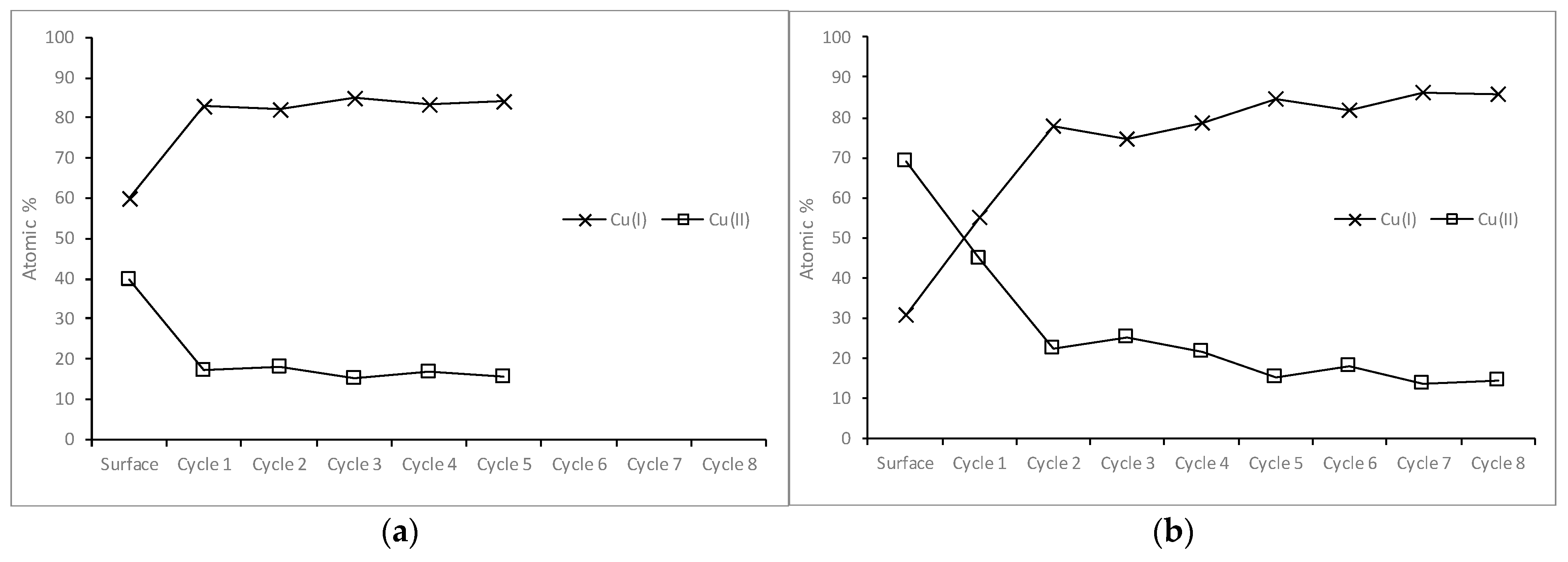
3.2.2. Copper in the Samples Leached for Five Seconds and Five Minutes
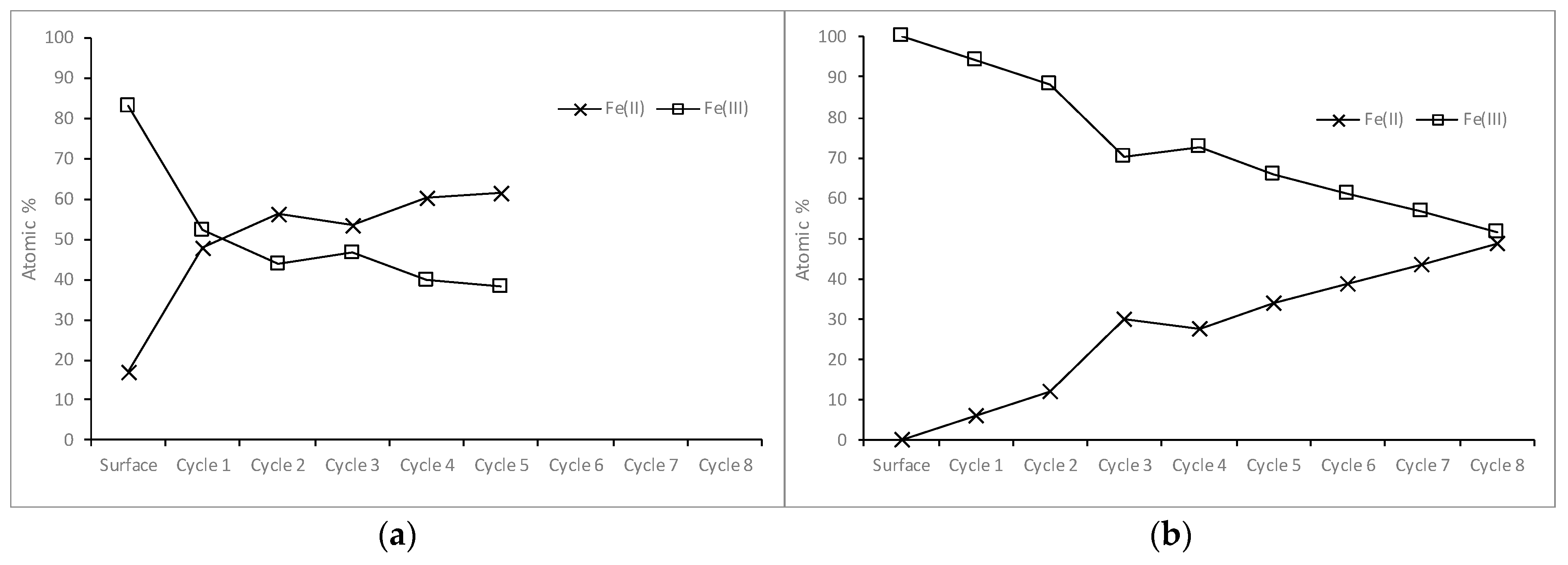
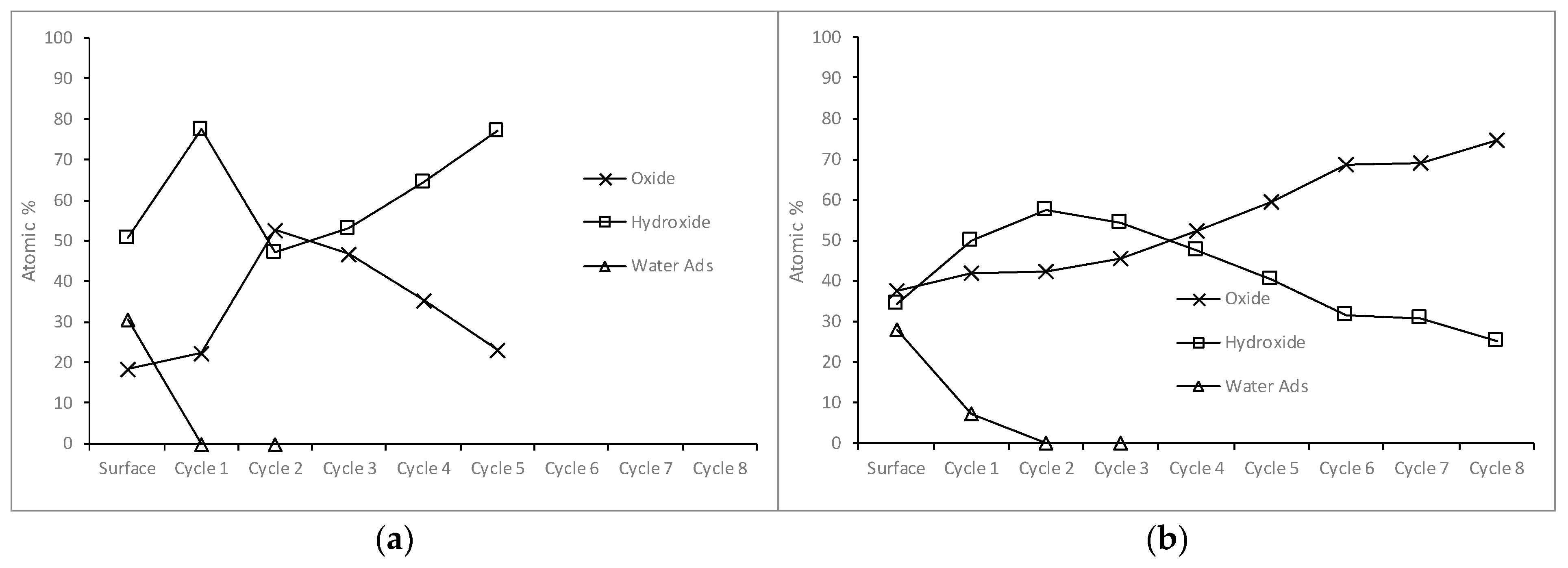
3.2.3. Iron in the Samples Leached for Five Seconds and Five Minutes
3.2.4. Sodium and Chlorine in the Samples Leached for Five Seconds and Five Minutes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkomirsky, I.; Parra, R.; Parada, F.; Balladares, E.; Etcheverry, J.; Díaz, R. Partial Roasting of High-Arsenic Copper Concentrates. Metall. Mater. Trans. B 2020, 51, 2030–2038. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Hydrometallurgy Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Zhang, B.; Zhang, J.; Qin, W. Selective leaching of arsenic from enargite concentrate using alkaline leaching in the presence of pyrite. Hydrometallurgy 2018, 181, 143–147. [Google Scholar] [CrossRef]
- Choubey, P.K.; Lee Jchun Kim Mseuk Kim, H.S. Conversion of chalcopyrite to copper oxide in hypochlorite solution for selective leaching of copper in dilute sulfuric acid solution. Hydrometallurgy 2018, 178, 224–230. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Keith, B.; Melo, E. Effect of pretreatment prior to leaching on a chalcopyrite mineral in acid media using NaCl and KNO3. J. Mater. Res. Technol. 2020, 9, 10316–10324. [Google Scholar] [CrossRef]
- Pratt, A.R.; Muir, I.J.; Nesbitt, H.W. X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar] [CrossRef]
- Havlík, T.; Škrobian, M.; Baláž, P.; Kammel, R. Leaching of chalcopyrite concentrate with ferric chloride. Int. J. Miner. Process 1995, 43, 61–72. [Google Scholar] [CrossRef]
- Crundwell, F.K. The influence of the electronic structure of solids on the anodic dissolution and leaching of semiconducting sulphide minerals. Hydrometallurgy 1988, 21, 155–190. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Mikhlin, Y. X-ray photoelectron spectroscopy in mineral processing studies. Appl. Sci. 2020, 10, 5138. [Google Scholar] [CrossRef]
- Tehrani, M.E.H.N.; Naderi, H.; Rashchi, F. Electrochemical study and XPS analysis of chalcopyrite dissolution in sulfuric acid in the presence of ethylene glycol. Electrochim. Acta 2021, 369, 137663. [Google Scholar] [CrossRef]
- Hernández, M.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; Van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Pettifer, Z.E.; Quinton, J.S.; Skinner, W.M.; Harmer, S.L. New interpretation and approach to curve fitting synchrotron X-ray photoelectron spectra of (Fe,Ni)9S8 fracture surfaces. Appl. Surf. Sci. 2020, 504, 144458. [Google Scholar] [CrossRef]
- Termes, S.C.; Buckley, A.N.; Gillard, R.D. 2P Electron Binding Energies for the Sulfur Atoms in Metal Polysulfides. Inorganica Chim. Acta 1987, 126, 79–82. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray photoelectron spectroscopic study of copper minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, N.S. Studies of the oxidation of iron by water vapour using X-ray photoelectron spectroscopy and QUASESTM. Surf. Sci. 2004, 572, 217–227. [Google Scholar] [CrossRef]
- Mycroft, J.R.; Nesbitt, H.W.; Pratt, A.R. X-ray photoelectron and Auger electron spectroscopy of air-oxidized pyrrhotite: Distribution of oxidized species with depth. Geochim. Cosmochim. Acta 1995, 59, 721–733. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Cases, J.M.; Alnot, M.; Ehrhardt, J.J. XPS characterization of chalcopyrite, tetrahedrite, and tennantite surface products after different conditioning. 2. amyl xanthate solution at pH 10. Langmuir 1996, 12, 2531–2543. [Google Scholar] [CrossRef]
- Puvvada, G.V.K.; Murthy, D.S.R. Selective precious metals leaching from a chalcopyrite concentrate using chloride/hypochlorite media. Hydrometallurgy 2000, 58, 185–191. [Google Scholar] [CrossRef]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of Chalcopyrite during oxidative leaching in sulfate media. Hidrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Ren, Z.; Chao Ch Krishnamoorthy, P.; Asselin, E.; Dixon, D.; Mora, N. The overlooked mechanism of chalcopyrite passivation. Acta Miner. 2022, 236, 118111. [Google Scholar] [CrossRef]
- Yin, Q.; Vaughan, D.J.; England, K.E.R.; Kelsall, G.H.; Brandona, N.P. Surface Oxidation of Chalcopyrite (CuFeS2) in Alkaline Solutions. J. Electrochem. Soc. 2000, 147, 2945–2951. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, O.; Hernández, M.-C.; Melo, E.; Quezada, V.; Vignals, J.; Roca, A. Surface Characterization of Chalcopyrite Dissolution in Hypochlorite Medium. Minerals 2025, 15, 1268. https://doi.org/10.3390/min15121268
Benavente O, Hernández M-C, Melo E, Quezada V, Vignals J, Roca A. Surface Characterization of Chalcopyrite Dissolution in Hypochlorite Medium. Minerals. 2025; 15(12):1268. https://doi.org/10.3390/min15121268
Chicago/Turabian StyleBenavente, Oscar, María-Cecilia Hernández, Evelyn Melo, Víctor Quezada, Joan Vignals, and Antoni Roca. 2025. "Surface Characterization of Chalcopyrite Dissolution in Hypochlorite Medium" Minerals 15, no. 12: 1268. https://doi.org/10.3390/min15121268
APA StyleBenavente, O., Hernández, M.-C., Melo, E., Quezada, V., Vignals, J., & Roca, A. (2025). Surface Characterization of Chalcopyrite Dissolution in Hypochlorite Medium. Minerals, 15(12), 1268. https://doi.org/10.3390/min15121268








