Copper Dissolution from Sulfide Ore with Deep Eutectic Solvents Based on Choline Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore
2.2. Reagents
2.3. Preparation of DES
2.4. Leaching Procedure
3. Results and Discussions
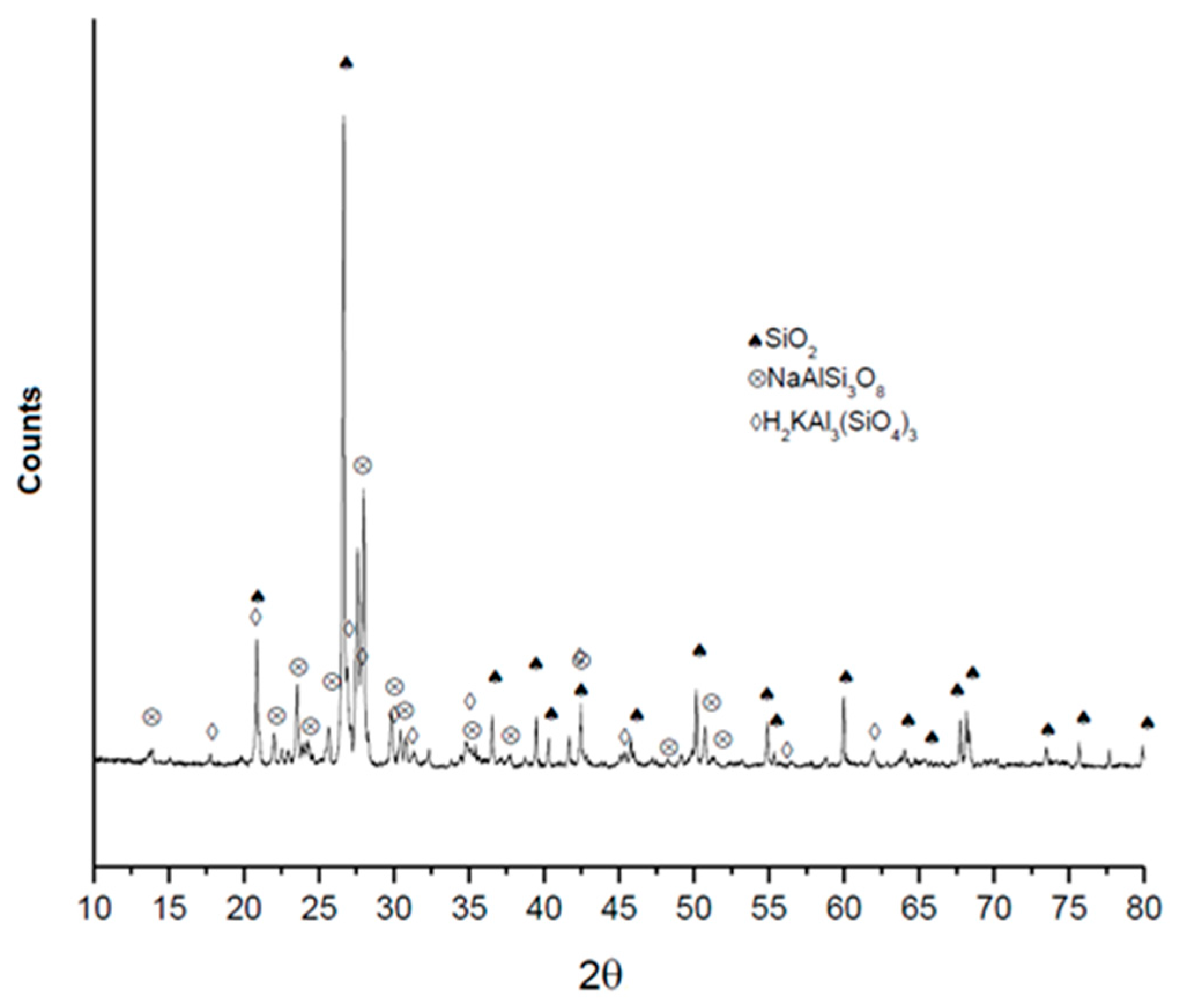
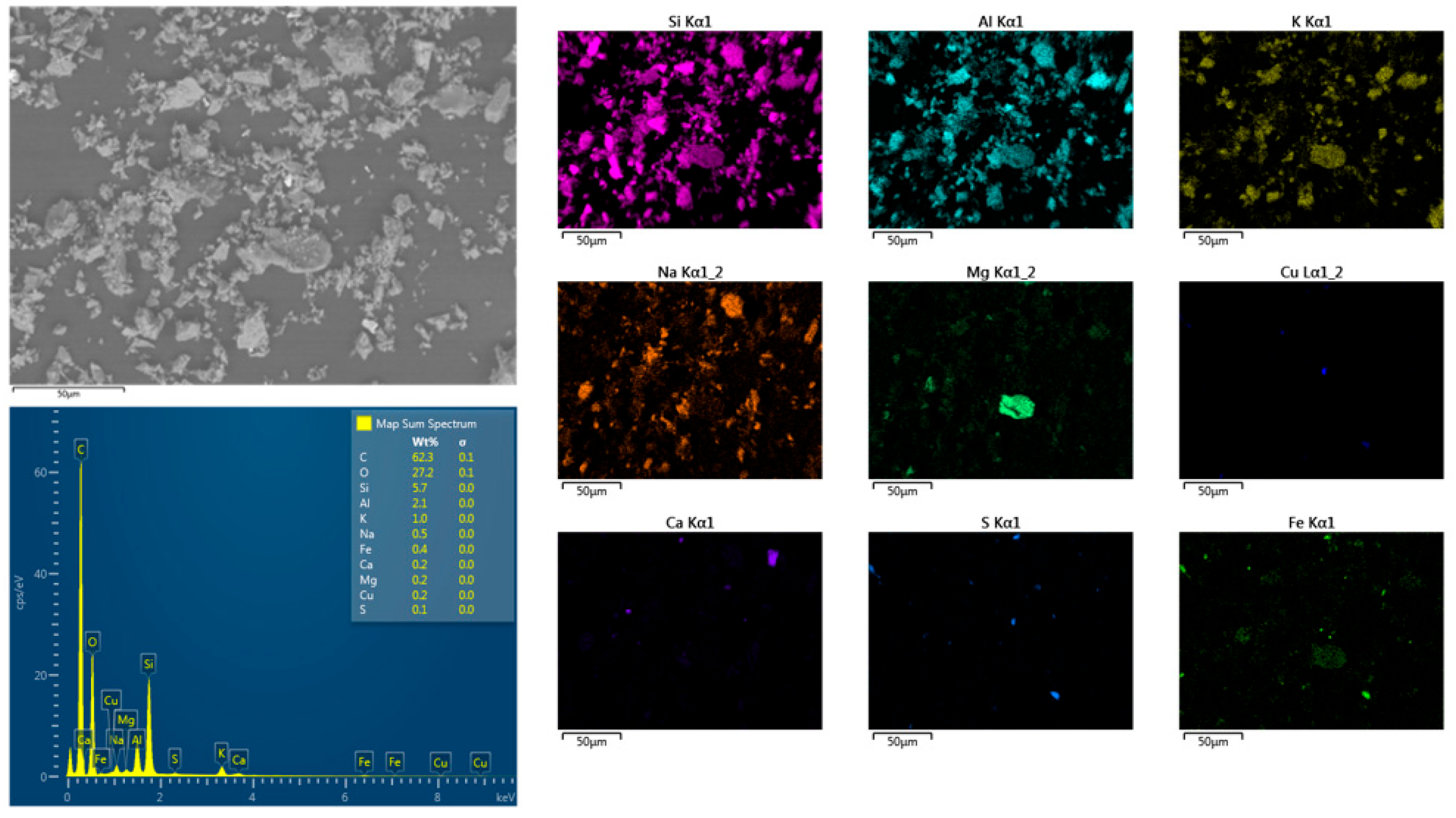
3.1. Characterization of the Ore
3.2. Leaching Results
3.3. Effect of the Type of DES and Temperature
3.4. Effect of Water Addition
3.5. Effect of Combining Two DES
3.6. Effect Hydrogen Peroxide
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DES | Deep eutectic solvent |
| SDGs | Sustainable Development Goals |
| HBD | Hydrogen bond donor |
| HBA | Hydrogen bond acceptor |
| PCB | Printed circuit board |
| ChCl | Choline chloride |
| MA | Malonic acid |
| EG | Ethylene glycol |
| XRD | X-ray diffractometer |
| SEM-EDS | Scanning electron microscope with an energy dispersive analyzer |
| AAS | Atomic absorption spectrometry |
| CA | Citric acid |
| U | Urea |
| ρDES | Density |
| ηDES | Viscosity |
References
- Hák, T.; Janoušková, S.; Moldan, B. Sustainable Development Goals: A need for relevant indicators. Ecol. Indic. 2016, 60, 565–573. [Google Scholar] [CrossRef]
- United Nation. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nation: New York, NY, USA, 2015.
- Ministerio del Medio Ambiente (Ed.) Hoja de Ruta para un Chile Circular al 2040; Gobierno-de-Chile: Santiago, Chile, 2021.
- Coorporación-Alta-Ley. Minería Verde. Oportunidades y Desafíos; Coorporación-Alta-Ley: Santiago, Chile, 2021. [Google Scholar]
- SERNAGEOMIN. Anuario de la Minería de Chile 2024; Servicio Nacional de Geología y Minería: Santiago, Chile, 2025. [Google Scholar]
- Coorporación-Alta-Ley. Hoja de Ruta 2.0 de la Minería Chilena. Actualización y Consensos para una Mirada Renovada; Coorporación-Alta-Ley: Santiago, Chile, 2019. [Google Scholar]
- Tian, G.; Liu, H. Review on the mineral processing in ionic liquids and deep eutectic solvents. Miner. Process. Extr. Metall. Rev. 2024, 45, 130–153. [Google Scholar]
- Anastas, P.T.; Warner, J.C. (Eds.) Principles of green chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; pp. 29–56. [Google Scholar]
- Cvjetko Bubalo, M.; Vidović, S.; Redovniković, I.R.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Carlesi, C.; Harris, R.C.; Abbott, A.P.; Jenkin, G.R. Chemical dissolution of chalcopyrite concentrate in choline chloride ethylene glycol deep eutectic solvent. Minerals 2022, 12, 65. [Google Scholar] [CrossRef]
- Dias, R.M.; da Costa, M.C.; Jimenez, Y.P. Perspectives of using DES-Based systems for solid–liquid and liquid–liquid extraction of metals from E-Waste. Minerals 2022, 12, 710. [Google Scholar]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Damilano, G.; Laitinen, A.; Willberg-Keyriläinen, P.; Lavonen, T.; Häkkinen, R.; Dehaen, W.; Binnemans, K.; Kuutti, L. Effects of thiol substitution in deep-eutectic solvents (DESs) as solvents for metal oxides. RSC Adv. 2020, 10, 23484–23490. [Google Scholar] [CrossRef]
- Sakamoto, T.; Hanada, T.; Sato, H.; Kamisono, M.; Goto, M. Hydrophobic deep eutectic solvents for the direct leaching of nickel laterite ores: Selectivity and reusability investigations. Sep. Purif. Technol. 2024, 331, 125619. [Google Scholar] [CrossRef]
- Bidari, E.; Winardhi, C.W.; Godinho, J.R.D.A.; Frisch, G. Role of Oxidants in Metal Extraction from Sulfide Minerals in a Deep Eutectic Solvent. ACS Omega 2024, 9, 14592–14603. [Google Scholar] [CrossRef]
- Topçu, M.A.; Kalem, V.; Rüşen, A. Processing of anode slime with deep eutectic solvents as a green leachant. Hydrometallurgy 2021, 205, 105732. [Google Scholar] [CrossRef]
- Li, X.; Shen, B.; Gao, Y.; Liang, D.; Yang, Y.; Xu, X.; Xu, C.; Xiang, M.; Tian, G. Efficiently leaching rare earth metal yttrium in deep eutectic solvents from waste phosphors based on a novel single-mode bottom-focused microwave reaction system. Waste Manag. 2025, 204, 114957. [Google Scholar] [PubMed]
- Rüşen, A.; Özel, F.; Topçu, M.A. Solvometallurgical Recovery of Zinc and Lead from Çin–Kur Leaching Residue Using Deep Eutectic Solvent. J. Sustain. Metall. 2025, 1–16. [Google Scholar] [CrossRef]
- Topçu, M.A.; Çeltek, S.A.; Rüşen, A. Green leaching and predictive model for copper recovery from waste smelting slag with choline chloride-based deep eutectic solvent. Chin. J. Chem. Eng. 2024, 75, 14–24. [Google Scholar] [CrossRef]
- Liu, K.; Wang, M.; Zhang, Q.; Dutta, S.; Zheng, T.; Valix, M.; Tsang, D.C. Negative-carbon recycling of copper from waste as secondary resources using deep eutectic solvents. J. Hazard. Mater. 2024, 465, 133258. [Google Scholar]
- Guo, M.; Deng, R.; Gao, M.; Xu, C.; Zhang, Q. Sustainable recovery of metals from e-waste using deep eutectic solvents: Advances, challenges, and perspectives. Curr. Opin. Green Sustain. Chem. 2024, 47, 100913. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, J.H. Sustainable leaching of metals from waste printed circuit boards using efficient carboxylic acid-based deep eutectic solvents. Sep. Purif. Technol. 2025, 374, 133712. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, S.; Wang, Z.; Duan, H.; Chen, D.; Long, M.; Li, Y. Efficient leaching of valuable metals from spent lithium-ion batteries using green deep eutectic solvents: Process optimization, mechanistic analysis, and environmental impact assessment. J. Clean. Prod. 2024, 480, 144128. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Panda, P.; Mishra, S. Deep eutectic solvents: Physico-chemical properties and their use for recovery of metal values from waste products. J. Mol. Liq. 2023, 390, 123070. [Google Scholar] [CrossRef]
- Martín, M.I.; García-Díaz, I.; López, F. Properties and perspective of using deep eutectic solvents for hydrometallurgy metal recovery. Miner. Eng. 2023, 203, 108306. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Anggara, S.; Bevan, F.; Harris, R.C.; Hartley, J.M.; Frisch, G.; Jenkin, G.R.; Abbott, A.P. Direct extraction of copper from copper sulfide minerals using deep eutectic solvents. Green Chem. 2019, 21, 6502–6512. [Google Scholar] [CrossRef]
- Topçu, M.A.; Rüşen, A.; Küçük, Ö. Treatment of copper converter slag with deep eutectic solvent as green chemical. Waste Manag. 2021, 132, 64–73. [Google Scholar] [CrossRef]
- Aragón-Tobar, C.F.; Endara, D.; de la Torre, E. Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride. Molecules 2024, 29, 290. [Google Scholar] [CrossRef]
- Ghadamgahi, S.M.; Babakhani, A.; Darband, G.B.; Shalchian, H.; Behmadi, R. Solvometallurgical properties of choline chloride-based deep eutectic solvents for copper extraction from chalcopyrite: Optimization and analysis. Mining 2025, 5, 8. [Google Scholar] [CrossRef]
- Shiri, H.R.; Mokmeli, M.; Ghadamgahi, S.M.; Babakhani, A. Deep Eutectic Solvents (DESs) for Chalcopyrite Concentrate Extraction: Leaching, Optimization and Kinetics Mechanism. J. Environ. Chem. Eng. 2025, 13, 117779. [Google Scholar] [CrossRef]
- Oke, E.A.; Fedai, Y.; Potgieter, J.H. Hydrometallurgical Leaching of Copper and Cobalt from a Copper–Cobalt Ore by Aqueous Choline Chloride-Based Deep Eutectic Solvent Solutions. Minerals 2025, 15, 815. [Google Scholar]
- Karimi, S.; Mohammadpour, P.; Esmailzadeh, M.; Izadi, M. Sustainable synthesis and application of green deep eutectic solvent in chalcopyrite leaching: A combined experimental and molecular dynamic simulation approach. Nano-Struct. Nano-Objects 2025, 42, 101481. [Google Scholar] [CrossRef]
- Behnajady, B.; Najafi, M.; Karimi, S. A new approach to direct chemical leaching of Sungun chalcopyrite concentrate via green deep eutectic solvent choline chloride-ρ-toluenesulfonic acid and MD simulation. J. Taiwan Inst. Chem. Eng. 2025, 172, 106118. [Google Scholar] [CrossRef]
- Moradi, M.; Karimi, S.; Behnajady, B.; Esmailzadeh, M. Green Solvent-Driven Chalcopyrite Dissolution: Ternary DES (ChCl/MOA/PTSA) for High-Efficiency Copper Extraction via RSM Optimization, Kinetics, and Molecular Dynamics Insights. Miner. Eng. 2025, 233, 109606. [Google Scholar] [CrossRef]
- Svärd, M.; Ma, C.; Forsberg, K.; Schiavi, P.G. Addressing the reuse of deep eutectic solvents in li-ion battery recycling: Insights into dissolution mechanism, metal recovery, regeneration and decomposition. ChemSusChem 2024, 17, e202400410. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Yu, L.; Ji, X.; Xu, X.; Xu, C.; Qi, X.; Wang, G.; Zhang, S.; Cai, J.; Lv, G.; Yang, Z. Sustainable and selective recovery of copper from electroplating sludge via choline chloride-citric acid deep eutectic solvent: Mechanistic elucidation and process intensification. Sep. Purif. Technol. 2025, 376, 134195. [Google Scholar] [CrossRef]
- Pateli, I.M.; Thompson, D.; Alabdullah, S.S.; Abbott, A.P.; Jenkin, G.R.; Hartley, J.M. The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents. Green Chem. 2020, 22, 5476–5486. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, H.; Zhang, S.; Lu, X.; Ji, X. Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea. J. Chem. Eng. Data 2014, 59, 3344–3352. [Google Scholar] [CrossRef]
- Ninayan, R.; Levshakova, A.S.; Khairullina, E.M.; Vezo, O.S.; Tumkin, I.I.; Ostendorf, A.; Logunov, L.S.; Manshina, A.A.; Shishov, A.Y. Water-induced changes in choline chloride-carboxylic acid deep eutectic solvents properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132543. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Hammond, O.; van den Bruinhorst, A.; Mannu, A.; Padua, A.; Mele, A.; Gomes, M.C. Connecting chloride solvation with hydration in deep eutectic systems. Phys. Chem. Chem. Phys. 2021, 23, 107–111. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Yang, J.H.; Ooi, A.W.S.; Goodwin, Z.A.; Xie, Y.; Ding, J.; Falletta, S.; Park, A.-H.A.; Kozinsky, B. Room-temperature decomposition of the ethaline deep eutectic solvent. J. Phys. Chem. Lett. 2025, 16, 3039–3046. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; García, J.; Rodríguez, F. Thermal stability of choline chloride deep eutectic solvents by TGA/FTIR-ATR analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M.; Vukčević, M.; Kovačević, R. The Extraction of Copper from Chalcopyrite Concentrate with Hydrogen Peroxide in Sulfuric Acid Solution. Metals 2023, 13, 1818. [Google Scholar] [CrossRef]
- Antonijević, M.; Janković, Z.; Dimitrijević, M. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 2004, 71, 329–334. [Google Scholar] [CrossRef]
- Sahlabad, M.K.; Javanshir, S.; Honarmand, M. Improvement in atmospheric leaching of chalcopyrite concentrate using a new environmentally-friendly ionic liquid. Hydrometallurgy 2022, 211, 105893. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, Á.; Lapidus, G.T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Septioga, K.; Fajar, A.T.; Wakabayashi, R.; Goto, M. Deep Eutectic Solvent-Aqueous Two-Phase Leaching System for Direct Separation of Lithium and Critical Metals. ACS Sustain. Resour. Manag. 2024, 1, 2482–2491. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Shahid, M.; Sankarasubramanian, S.; Ramani, V. Solvometallurgy: Design of ternary deep eutectic solvents for the electrochemical recovery of nickel from lithium-ion cathode materials. J. Mater. Chem. A 2025, 13, 12625–12638. [Google Scholar] [CrossRef]
- Bakkar, A. Recycling of electric arc furnace dust through dissolution in deep eutectic ionic liquids and electrowinning. J. Hazard. Mater. 2014, 280, 191–199. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Branchi, M.; Zanoni, R.; Simonetti, G.; Navarra, M.A.; Pagnanelli, F. Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent. Chem. Eng. J. 2021, 417, 129249. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Sharov, A.V.; Zolotov, Y.A. New generation extraction solvents: From ionic liquids and aqueous biphasic systems to deep eutectic solvents. Russ. Chem. Rev. 2021, 90, 1109. [Google Scholar] [CrossRef]
 25 °C,
25 °C,  50 °C,
50 °C,  60 °C (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
 25 °C,
25 °C,  50 °C,
50 °C,  60 °C (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).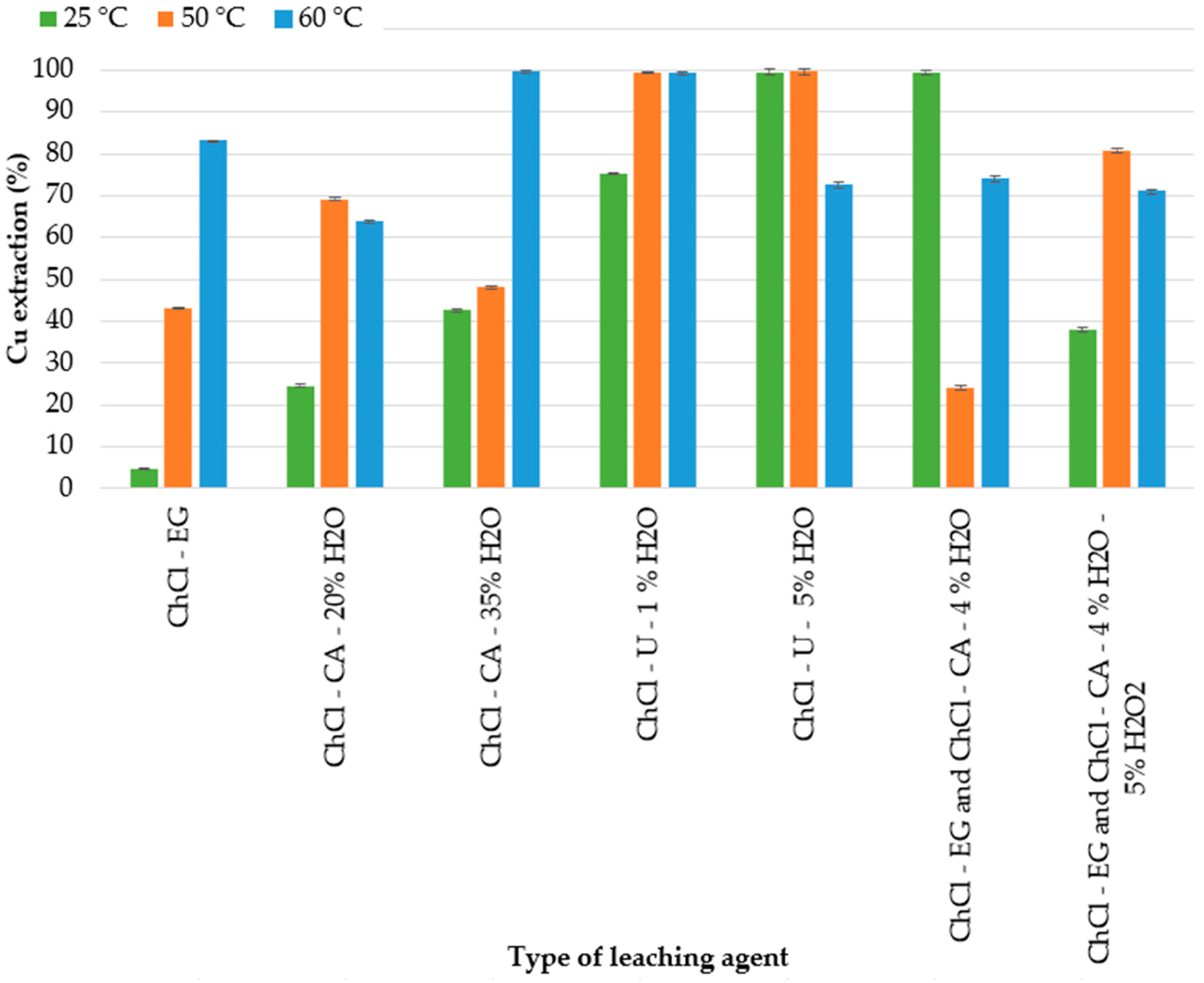
 25 °C and 20% H2O;
25 °C and 20% H2O;  25 °C and 35% H2O;
25 °C and 35% H2O;  50 °C and 20% H2O;
50 °C and 20% H2O;  50 °C and 35% H2O;
50 °C and 35% H2O;  60 °C and 20% H2O;
60 °C and 20% H2O;  60 °C and 35% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 35% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
 25 °C and 20% H2O;
25 °C and 20% H2O;  25 °C and 35% H2O;
25 °C and 35% H2O;  50 °C and 20% H2O;
50 °C and 20% H2O;  50 °C and 35% H2O;
50 °C and 35% H2O;  60 °C and 20% H2O;
60 °C and 20% H2O;  60 °C and 35% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 35% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).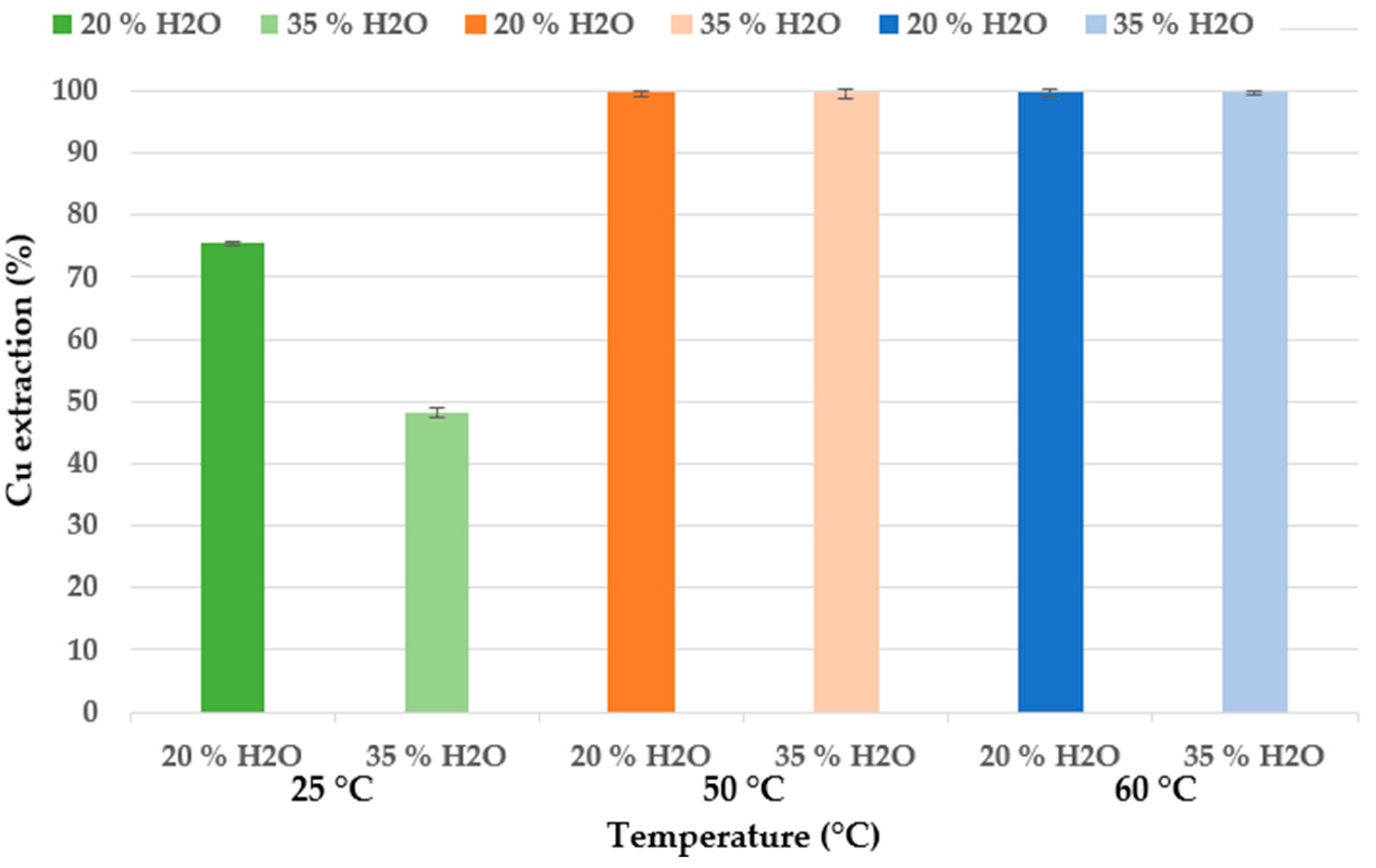
 25 °C and 1% H2O;
25 °C and 1% H2O;  25 °C and 5% H2O;
25 °C and 5% H2O;  50 °C and 1% H2O;
50 °C and 1% H2O;  50 °C and 5% H2O;
50 °C and 5% H2O;  60 °C and 1% H2O;
60 °C and 1% H2O;  60 °C and 5% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 5% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
 25 °C and 1% H2O;
25 °C and 1% H2O;  25 °C and 5% H2O;
25 °C and 5% H2O;  50 °C and 1% H2O;
50 °C and 1% H2O;  50 °C and 5% H2O;
50 °C and 5% H2O;  60 °C and 1% H2O;
60 °C and 1% H2O;  60 °C and 5% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 5% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).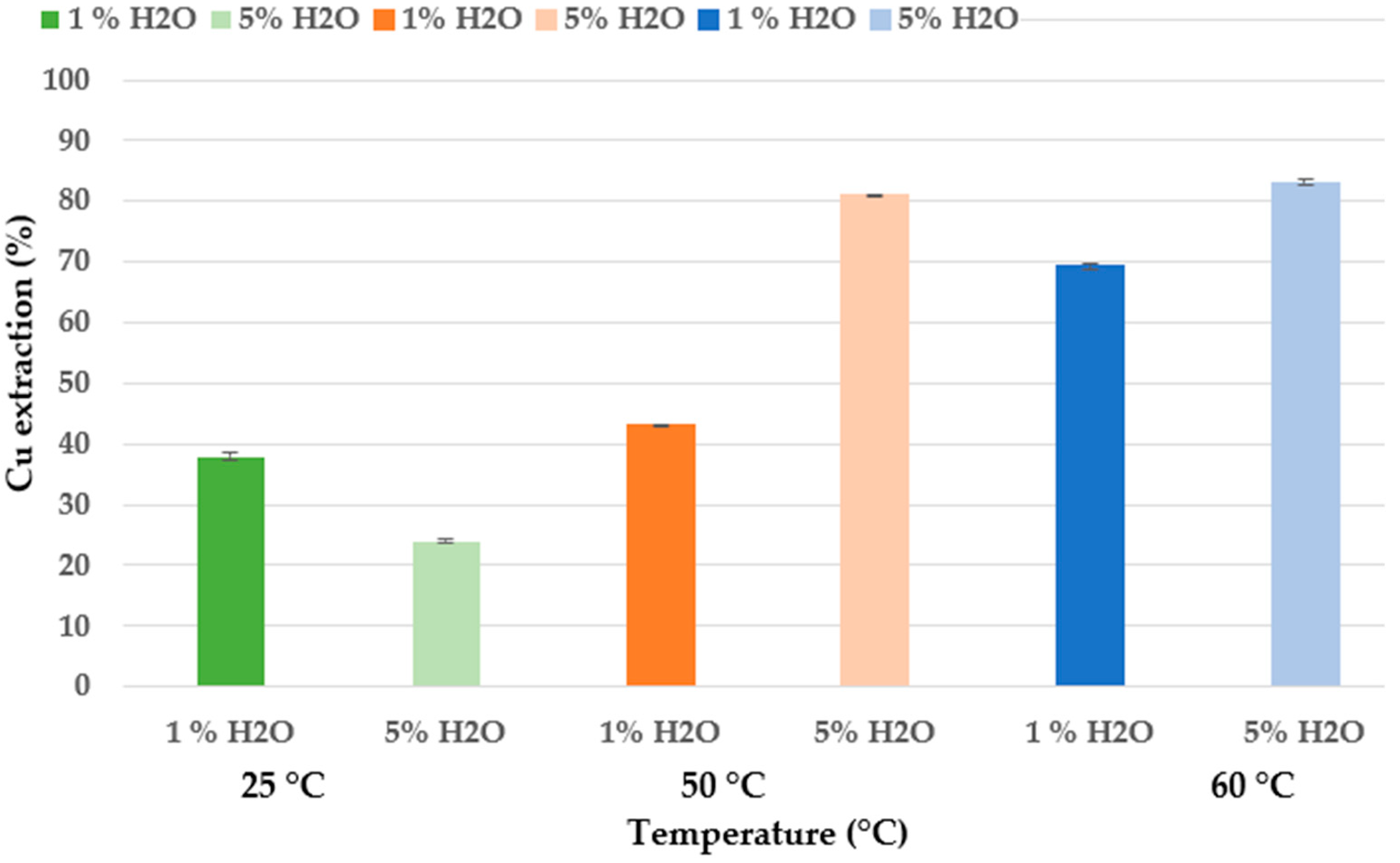
 25 °C,
25 °C,  50 °C,
50 °C,  60 °C. Types of DES: ChCl-EG; ChCl–CA-20% H2O; ChCl-EG and ChCl-CA-4% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C. Types of DES: ChCl-EG; ChCl–CA-20% H2O; ChCl-EG and ChCl-CA-4% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
 25 °C,
25 °C,  50 °C,
50 °C,  60 °C. Types of DES: ChCl-EG; ChCl–CA-20% H2O; ChCl-EG and ChCl-CA-4% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C. Types of DES: ChCl-EG; ChCl–CA-20% H2O; ChCl-EG and ChCl-CA-4% H2O (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).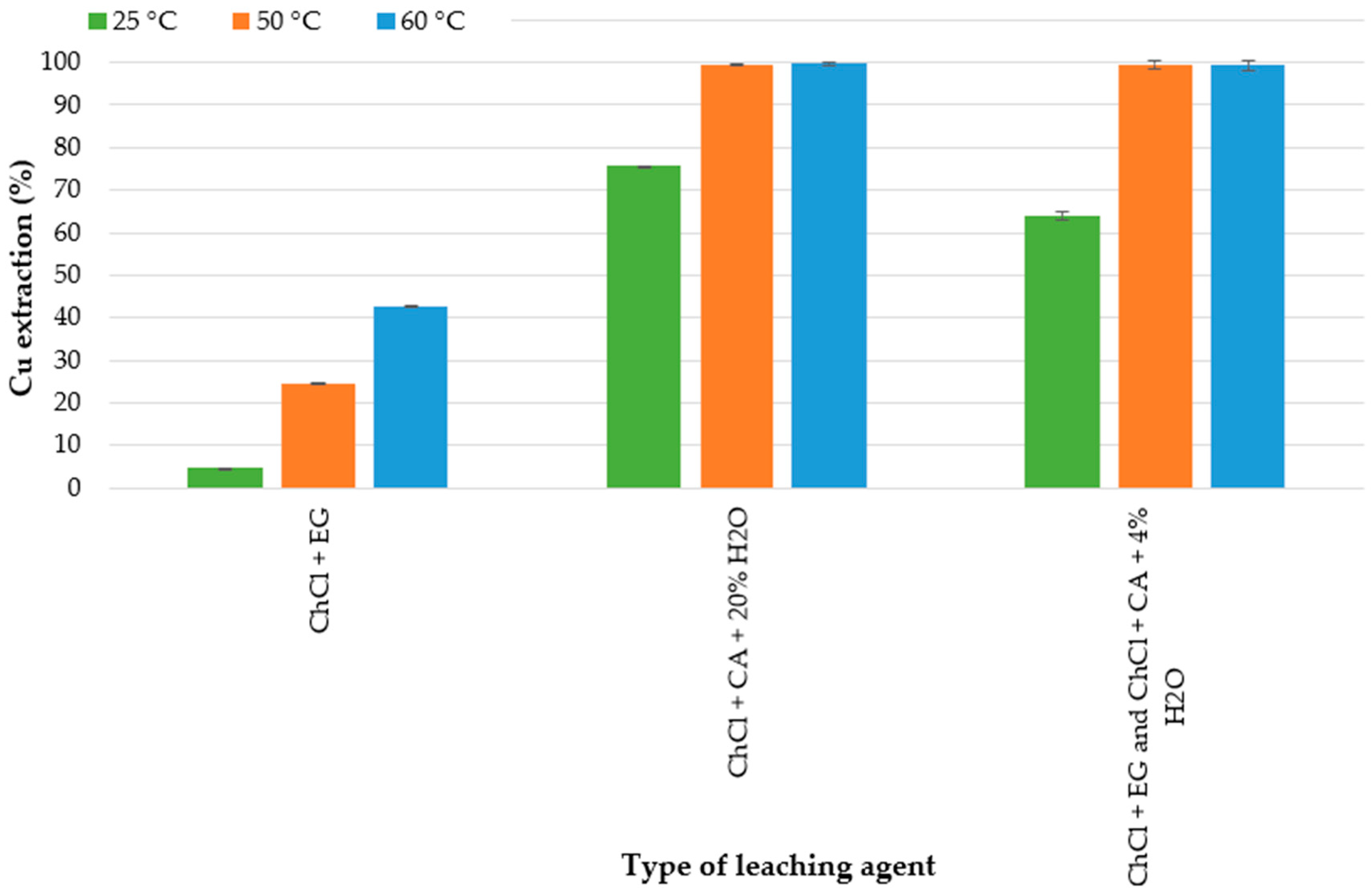
 25 °C and 0% H2O2;
25 °C and 0% H2O2;  25 °C and 5% H2O2;
25 °C and 5% H2O2;  50 °C and 0% H2O2;
50 °C and 0% H2O2;  50 °C and 5% H2O2;
50 °C and 5% H2O2;  60 °C and 0% H2O2;
60 °C and 0% H2O2;  60 °C and 5% H2O2 (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 5% H2O2 (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
 25 °C and 0% H2O2;
25 °C and 0% H2O2;  25 °C and 5% H2O2;
25 °C and 5% H2O2;  50 °C and 0% H2O2;
50 °C and 0% H2O2;  50 °C and 5% H2O2;
50 °C and 5% H2O2;  60 °C and 0% H2O2;
60 °C and 0% H2O2;  60 °C and 5% H2O2 (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).
60 °C and 5% H2O2 (experimental conditions: 72 h, 300 rpm, 3 g in 30 mL).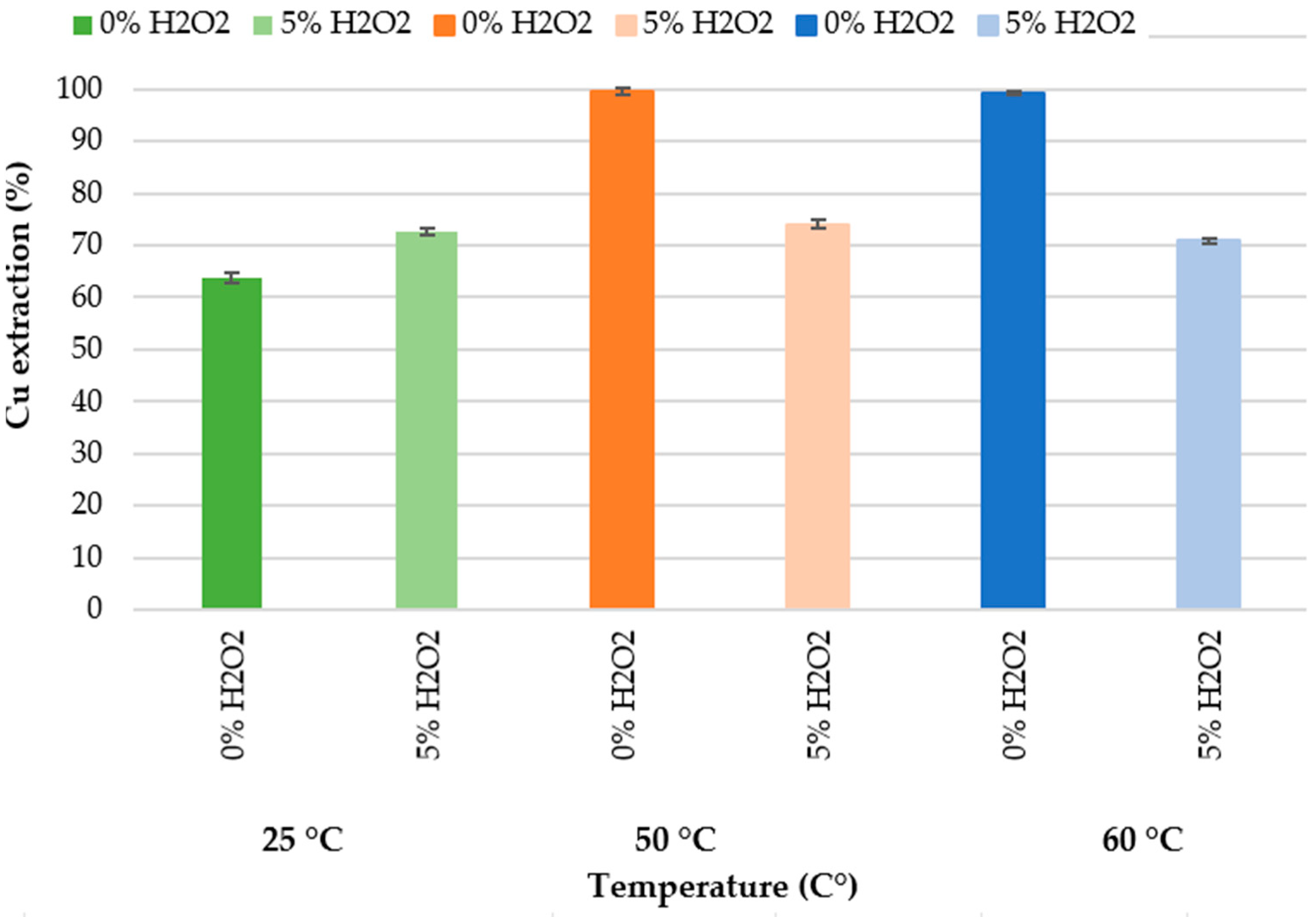
| DES Type | Solid Matrix | Experimental Conditions | Results | Authors |
|---|---|---|---|---|
| ChCl-ethylene glycol ChCl-urea ChCl-oxalic acid dihydrate. | Chalcocite, covellite, and chalcopyrite | Electrochemical measurements by cyclic voltammetry | Dissolution was achieved for all three solid samples | Anggara et al. [30] |
| ChCl-urea | Copper conversion slag | Leaching time (2–72 h), temperature (25–95 °C), and pulp density (1/10–1/40 g/mL). | 65.3% Zn and 89.9% Cu at 48 h, 95 °C, 600 rpm and 1/20 g/mL | Topçu et al. [31] |
| ChCl-based deep eutectic solvents (DES) with urea and ethylene glycol | Copper anode slime | Leaching time (4–48 h), temperature (25; 50; 75; 95 °C), solid/liquid ratio (1/10–1/25 g/mL), DES composition (ChCl-urea, ChCl-urea-water, ChCl-ethylene glycol, ChCl-ethylene glycol-urea) | 97% Cu in ChCl-urea, 95 °C, 4 h and 1 g in 25 mL 91% Ag in ChCl-urea, 95 °C, 48 h and 1 g in 10 mL. | Topçu et al. [18] |
| ChCl-ethylene glycol | Chalcopyrite concentrate (26.5% Cu) | Ambient pressure, temperature (19.5; 50; 80 and 90 °C) | 16% Cu at 90 °C, 9.88 mol DES in mol initial Cu in chalcopyrite | Carlesi et al. [11] |
| ChCl-ethylene glycol | Sulfide concentrate mainly composed by sphalerite, pyrite, chalcopyrite, and galena | Oxidants (CuCl2, FeCl3, H2O2, I2, and O2) in 300 rpm, 2/100 solid liquid ratio | Dissolution at 80 °C of the following:
| Bidari et al. [17] |
| ChCl-urea ChCl-ethylene glycol ChCl-glycerol | Polymetallic concentrates (sulfates, oxides, sulfides) composed of Cu, Fe, Pb, and Zn | 0.5 g/20 g DES, 30 °C, 100 rpm and 24 h | Sulfates dissolution:
| Aragón-Tobar et al. [32] |
| ChCl-ethylene glycol ChCl-oxalic acid ChCl-ethylene glycol-oxalic acid | Chalcopyrite concentrate | Temperature (50–80 °C), leaching time (24–72 h), 500 rpm, pulp density ratio 1:6 | 83% Cu at 80 °C, 72 h, ChCl-oxalic acid | Ghadamgahi et al. [33] |
| ChCl-ethylene glycol ChCl-oxalic acid ChCl-malonic acid ChCl-ethylene glycol-oxalic acid ChCl-ethylene glycol-malonic acid ChCl-oxalic acid-malonic acid | Chalcopyrite concentrate | 1st stage: 75 °C, 48 h, 1 g/20 mL, 400 rpm. 2nd stage: DES ChCl-ethylene glycol-oxalic acid, temperature (45, 55, 65 and 75 °C), leaching time (2, 6, 12, 24, 48 and 72 h), solid liquid ratio (0.025; 0.05; 0.075; 0.1 g/mL), water addition (5; 20; 35; 50%v/v) | 86% Cu at 75 °C, 48 h, 0.025 g/mL, ChCl-ethylene glycol-oxalic acid−20% vol. water | Shiri et al. [34] |
| ChCl-oxalic acid-30% water ChCl-ethylene glycol-30% water ChCl-urea-30% water ChCl-thiourea-30% water | CuO and CoO (analytical grade, ≥99%), Cu-Co ore | CuO and CoO: 60 °C, 400 rpm, 6 h, 1:10 solid/liquid ratio. One test was carried out using only 1 M H2SO4 as comparison. Cu-Co ore: temperature (30; 45; 60; and 75 °C), solid/liquid ratio (1:5; 1:10 and 1:20), leaching time (1–8 h), 400 rpm. | 89.2% Cu from CuO and 92.4% Co from CoO at 60 °C, 400 rpm, 6 h, 1:10 solid/liquid ratio, −75 + 53 µm, ChCl-oxalic acid-30% water | Oke et al. [35] |
| ChCl-maleic acid | Chalcopyrite concentrate | Leaching time (2–24 h), temperature (100–200 °C), mol ratio ChCl to maleic acid (1:2; 1:1 and 2:1) | 52.6% Cu obtained in ChCl-maleic acid ratio of 1:1, 150 °C, 24 h | Karimi et al. [36] |
| ChCl-ρ-toluenesulfonic acid | Chalcopyrite concentrate | Temperature (40; 60; 80; 100 and 120 °C), leaching time (1; 2; 3; 4 and 5 h), stirrer speed (100; 300; 500; 700 and 900 rpm), mass ratio DES/chalcopyrite (20; 40; 60; 80 and 100 g/g) | 73.6% Cu in 1 h, mass ratio DES/chalcopyrite of 100, 120 °C, 100 rpm | Behnajady et al. [37] |
| ChCl-maloni acid-ρ-toluenesulfonic acid | Chalcopyrite concentrate | Leaching time (5; 30; 55; 80 and 105 min), milling time (0; 2; 4; 6 and 8 h), temperature (40; 60; 80; 100 and 120 °C), mass ratio concentrate/DES (0.005; 0.03; 0.055; 0.08 and 0.105 g/g), 500 rpm | 83.9% Cu and 87.2% Fe in 80 min, 6 h, 100 °C, 0.03 g concentrate/g DES | Moradi et al. [38] |
| N° | DES | ρDES (g/mL) | ηDES (mPa s) | H2O (%) | H2O2 (%) | Temperature (°C) | Cu Ext. (%) |
|---|---|---|---|---|---|---|---|
| 1 | ChCl-EG | 1.11639 | 44.37 | 0 | 0 | 25 | 4.6 |
| 2 | ChCl-EG | 1.10221 | 18.12 | 0 | 0 | 50 | 24.6 |
| 3 | ChCl-EG | 1.09661 | 13.60 | 0 | 0 | 60 | 42.7 |
| 4 | ChCl-CA | 1.21891 | 254.24 | 20 | 0 | 25 | 75.4 |
| 5 | ChCl-CA | 1.20442 | 70.66 | 20 | 0 | 50 | 99.5 |
| 6 | ChCl-CA | 1.19864 | 46.76 | 20 | 0 | 60 | 99.6 |
| 7 | ChCl-U | 1.19375 | 597.28 | 1 | 0 | 25 | 37.9 |
| 8 | ChCl-U | 1.18019 | 96.09 | 1 | 0 | 50 | 43.1 |
| 9 | ChCl-U | 1.17486 | 18.80 | 1 | 0 | 60 | 69.3 |
| 10 | ChCl-CA | 1.17354 | 18.41 | 35 | 0 | 25 | 48.2 |
| 11 | ChCl-CA | 1.15939 | 8.15 | 35 | 0 | 50 | 99.5 |
| 12 | ChCl-CA | 1.15357 | 5.66 | 35 | 0 | 60 | 99.8 |
| 13 | ChCl-U | 1.18624 | 164.02 | 5 | 0 | 25 | 24.0 |
| 14 | ChCl-U | 1.17269 | 40.99 | 5 | 0 | 50 | 81.0 |
| 15 | ChCl-U | 1.16739 | 14.97 | 5 | 0 | 60 | 83.1 |
| 16 | ChCl-EG and ChCl-CA | 1.16013 | 198.44 | 4 | 0 | 25 | 63.9 |
| 17 | ChCl-EG and ChCl-CA | 1.14579 | 61.12 | 4 | 0 | 50 | 99.6 |
| 18 | ChCl-EG and ChCl-CA | 1.14016 | 41.97 | 4 | 0 | 60 | 99.3 |
| 19 | ChCl-EG and ChCl-CA | - | - | 4 | 5 | 25 | 72.6 |
| 20 | ChCl-EG and ChCl-CA | - | - | 4 | 5 | 50 | 74.1 |
| 21 | ChCl-EG and ChCl-CA | - | - | 4 | 5 | 60 | 71.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, P.C.; Muñoz V., M.; Jiménez, Y.P.; Coutinho, J.A.P.; Schaeffer, N.; Cortés, S.; Cerda, A.; Estay, H. Copper Dissolution from Sulfide Ore with Deep Eutectic Solvents Based on Choline Chloride. Minerals 2025, 15, 1176. https://doi.org/10.3390/min15111176
Hernández PC, Muñoz V. M, Jiménez YP, Coutinho JAP, Schaeffer N, Cortés S, Cerda A, Estay H. Copper Dissolution from Sulfide Ore with Deep Eutectic Solvents Based on Choline Chloride. Minerals. 2025; 15(11):1176. https://doi.org/10.3390/min15111176
Chicago/Turabian StyleHernández, Pía C., Matías Muñoz V., Yecid P. Jiménez, João A. P. Coutinho, Nicolas Schaeffer, Sonia Cortés, Alejandra Cerda, and Humberto Estay. 2025. "Copper Dissolution from Sulfide Ore with Deep Eutectic Solvents Based on Choline Chloride" Minerals 15, no. 11: 1176. https://doi.org/10.3390/min15111176
APA StyleHernández, P. C., Muñoz V., M., Jiménez, Y. P., Coutinho, J. A. P., Schaeffer, N., Cortés, S., Cerda, A., & Estay, H. (2025). Copper Dissolution from Sulfide Ore with Deep Eutectic Solvents Based on Choline Chloride. Minerals, 15(11), 1176. https://doi.org/10.3390/min15111176









