Waste Quartz Crucible Crystallization-Induced Purification to Prepare High-Purity Cristobalite Sand
Abstract
1. Introduction
2. Material and Experiment
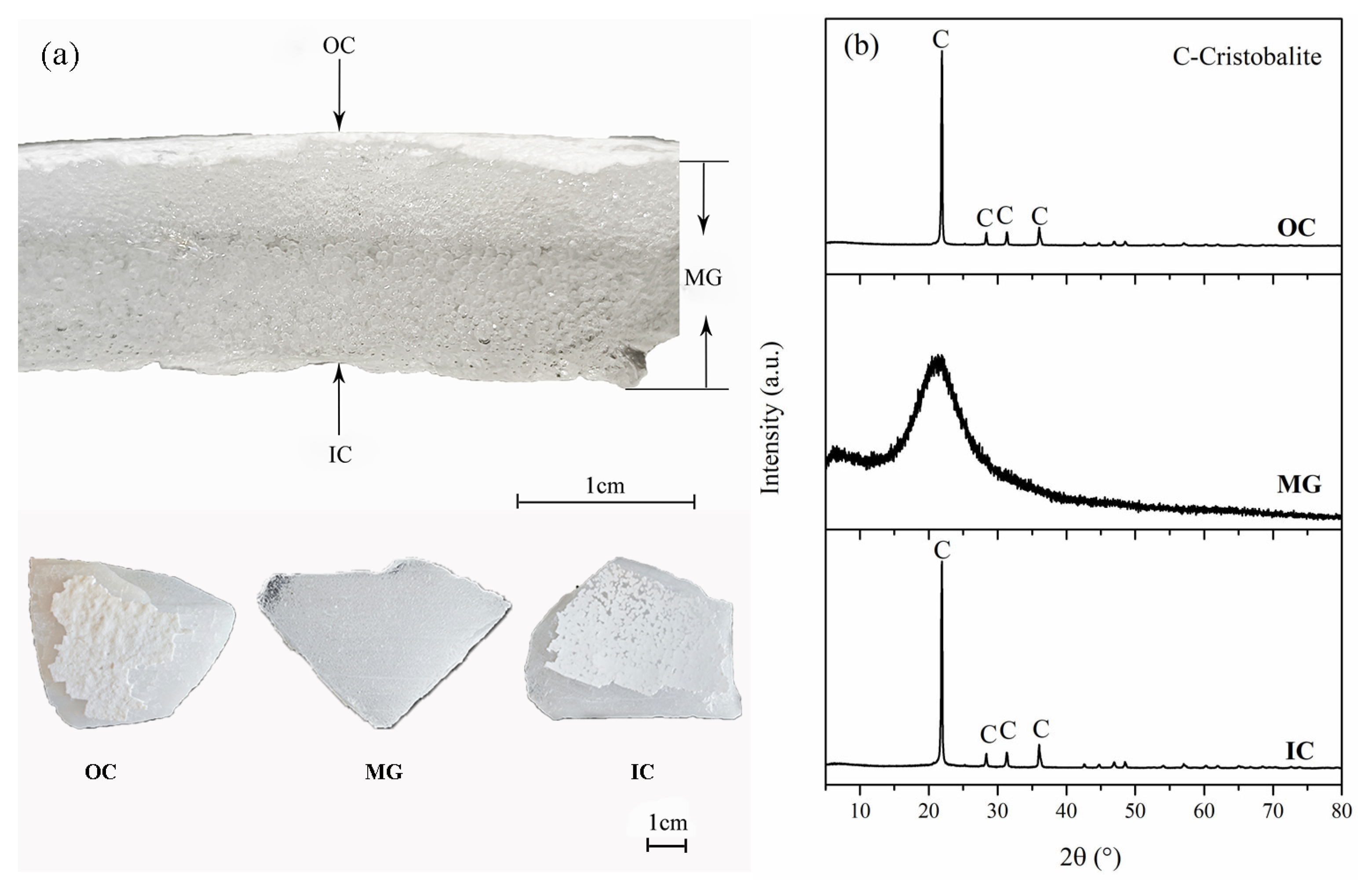
2.1. Material and Pretreatment
2.2. Crystallization and Impurity Distribution Analysis
2.3. Acid Leaching for Impurity Removal Assessment
2.4. Analytical Characterization Phase
3. Results and Discussion
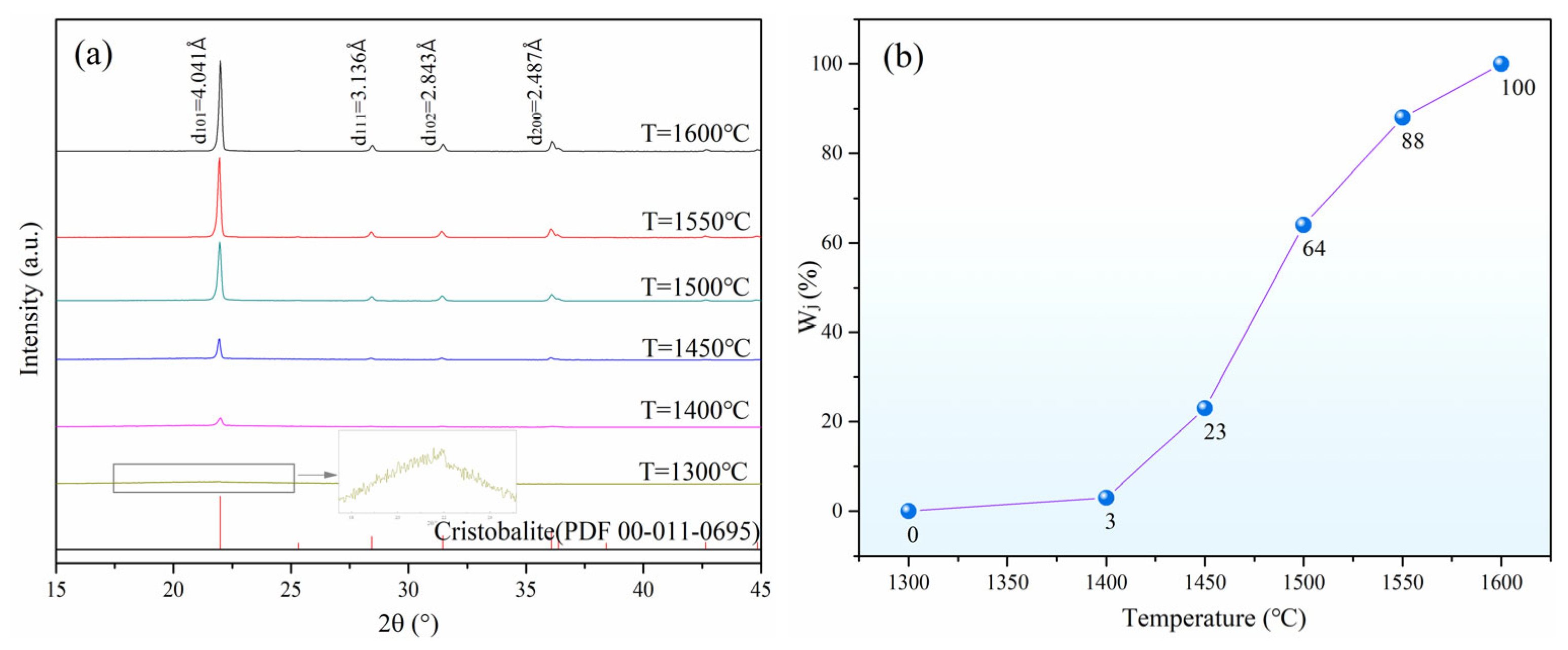
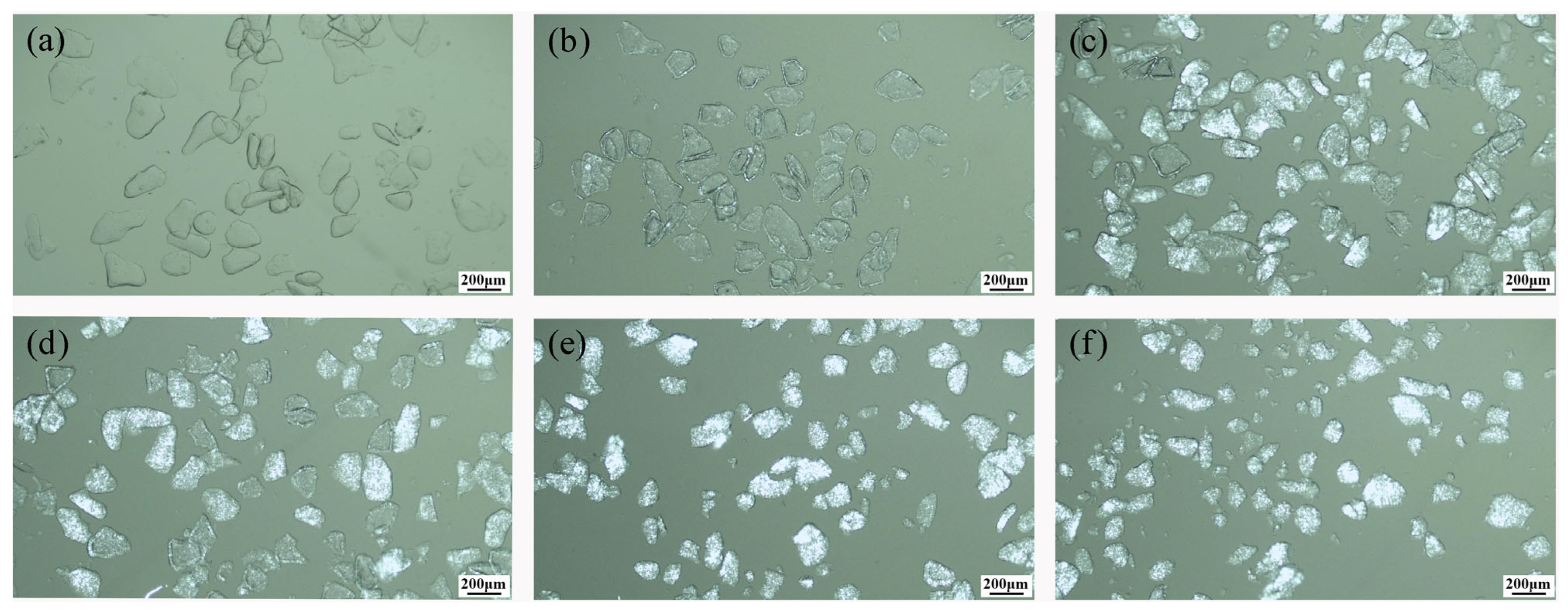
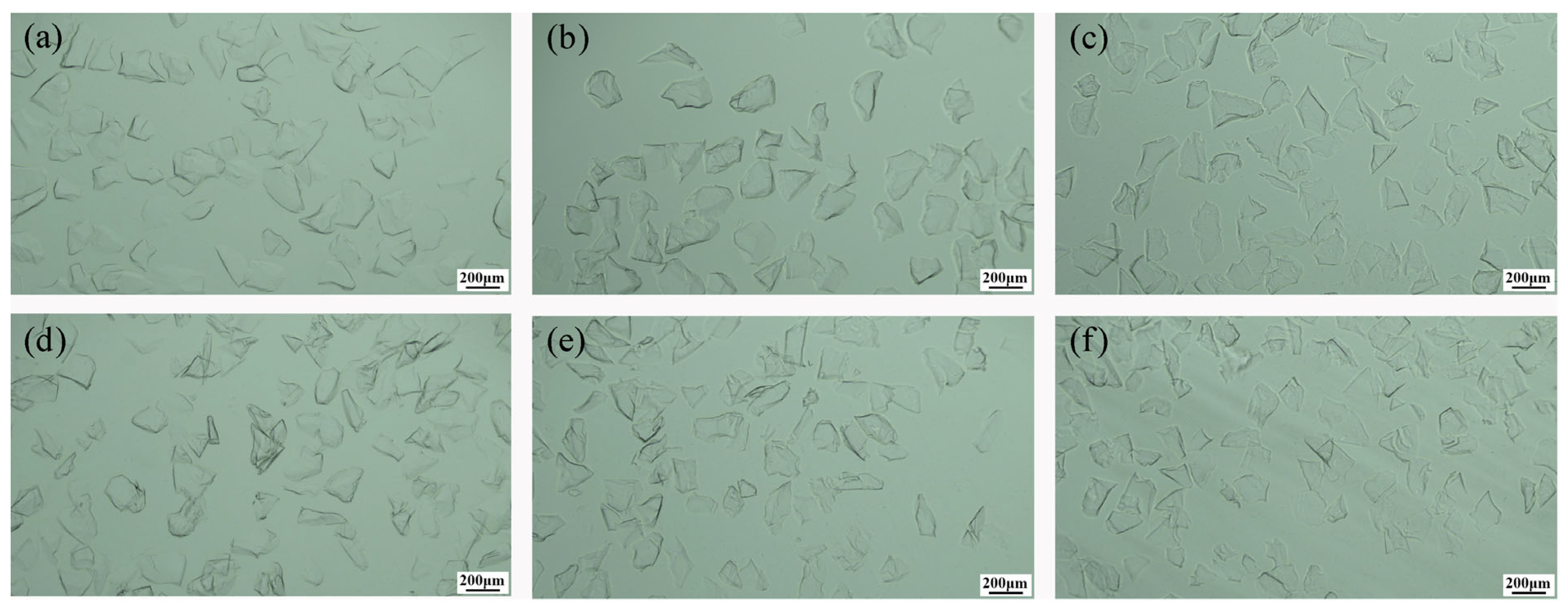
3.1. Effect of Calcination Temperature on Crystallization
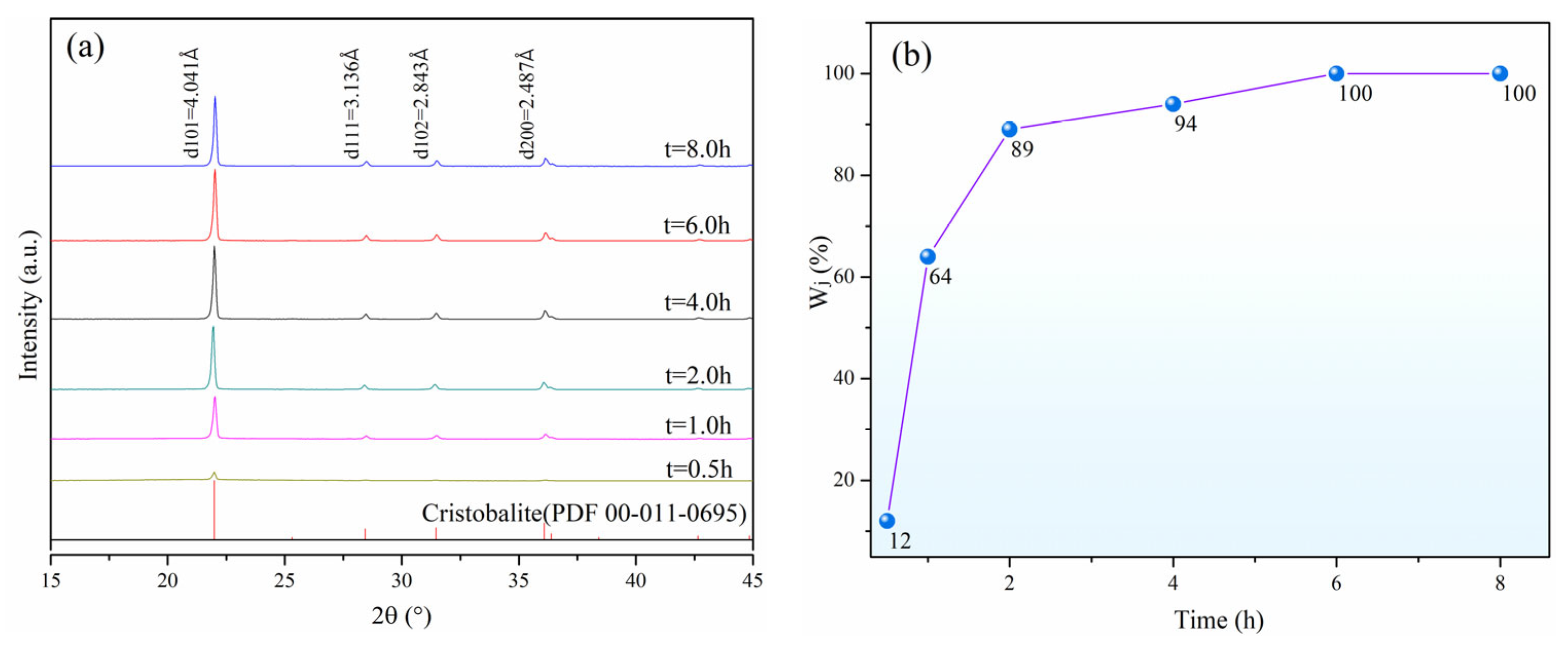
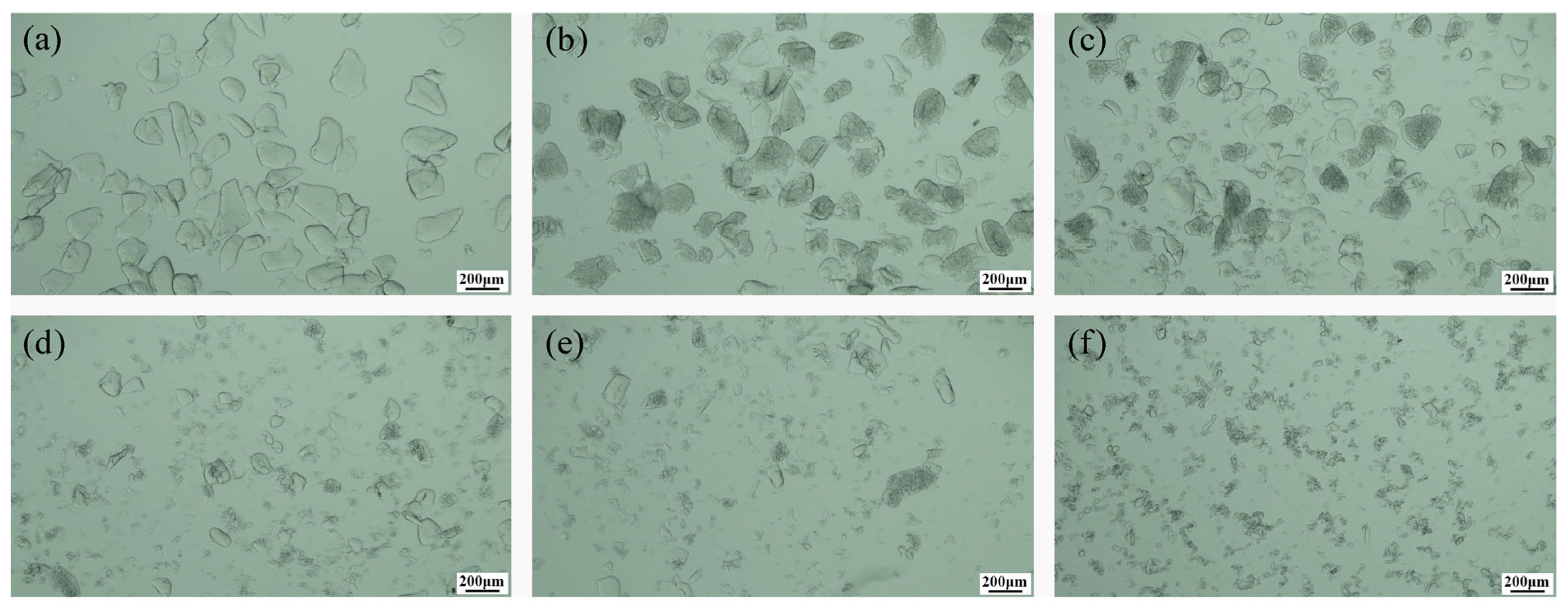
3.2. Effect of Calcination Time on Crystallization
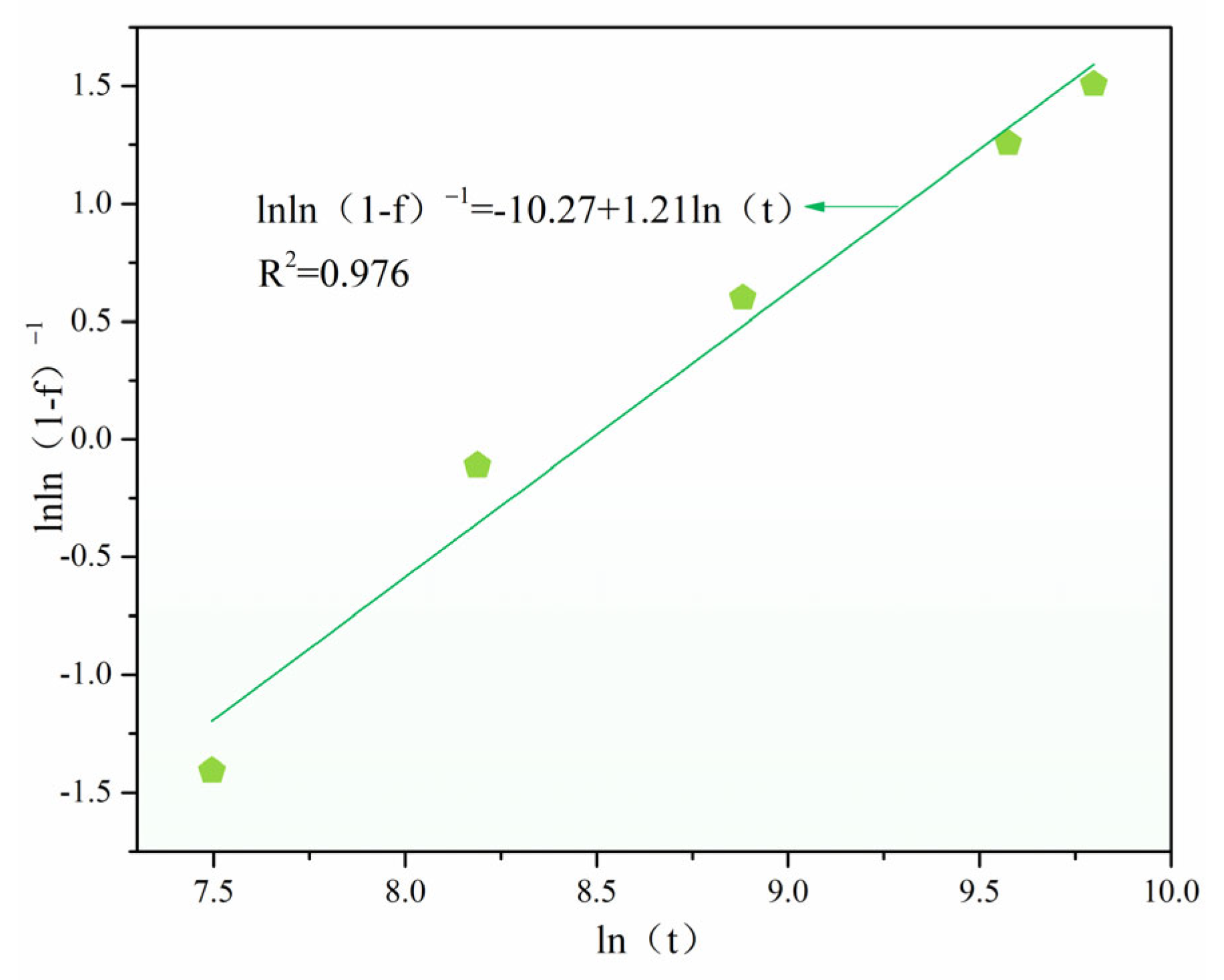
3.3. Crystalline Kinetics and Impurity Redistribution Mechanism
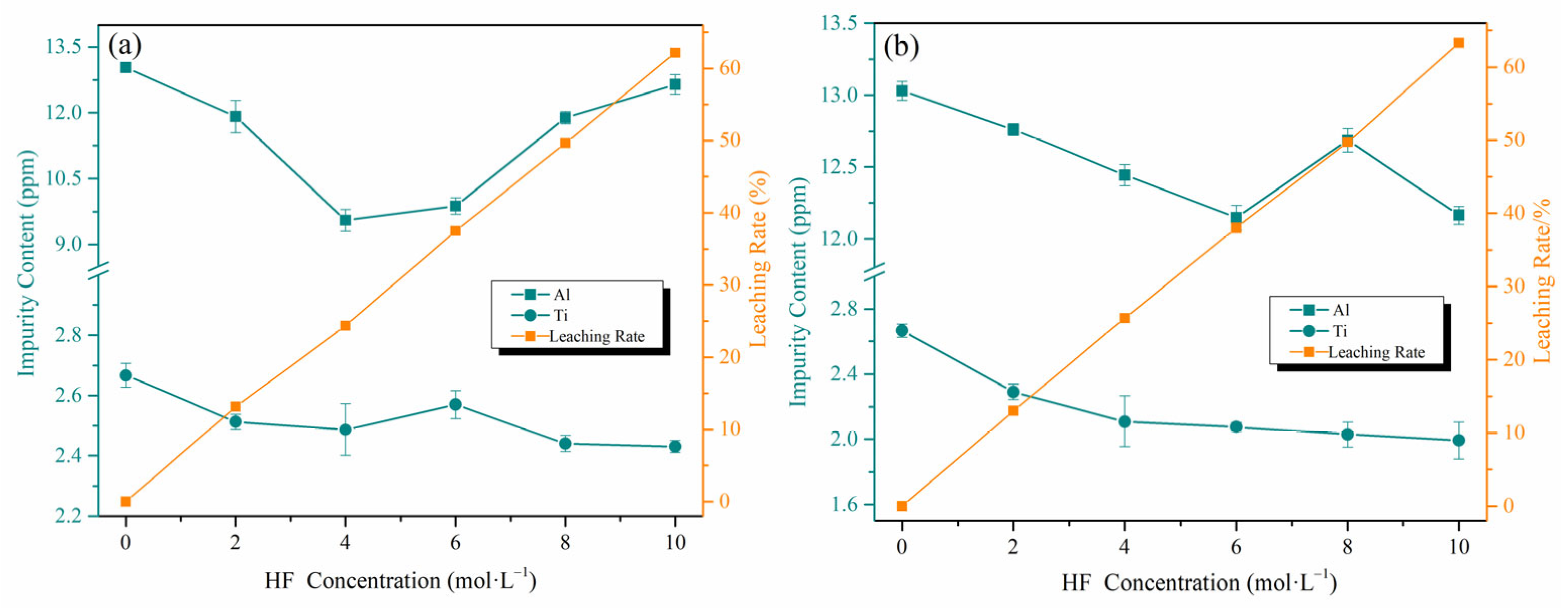
3.4. Purity Enhancement via Crystallization: Post-Treatment Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, R.D.; Zha, X.D.; Hu, H.W.; Niu, C. A review on the influencing factors of solar pavement power generation efficiency. Appl. Energy 2025, 379, 124897. [Google Scholar] [CrossRef]
- Koichi, K.; Gao, B.; Liu, X.; Nakano, S. Growth of semiconductor silicon crystals. Prog. Cryst. Growth Charact. Mater. 2016, 62, 273–285. [Google Scholar] [CrossRef]
- Yang, S.C.; Han, S.F.; Chen, J.; Wei, K.X.; Ma, W.H. A sustainable mineral process for silicon and quartz recovery from quartz crucible waste ash via electrical separation. Miner. Eng. 2024, 216, 108887. [Google Scholar] [CrossRef]
- Warden, G.K.; Juel, M.; Gawet, B.A.; Sabatino, M.D. Recent developments on manufacturing and characterization of fused quartz crucibles for monocrystalline silicon for photovoltaic applications. Open Ceram. 2023, 13, 100321. [Google Scholar] [CrossRef]
- Breneman, R.C.; JOHN, W.; Sglao, V. Effect of cristobalite on the strength of sintered fused silica above and below the cristobalite transformation. J. Am. Ceram. Soc. 2015, 98, 1611–1617. [Google Scholar] [CrossRef]
- Huang, X.M.; Kishi, H.; Oishi, S.; Watanabe, H.; Sanpei, K.; Sakai, S.; Hoshikawa, K. Expansion behavior of bubbles in silica glass concerning Czochralski (CZ) Si Growth. Jpn. J. Appl. Phys. 1999, 38, 353–355. [Google Scholar] [CrossRef]
- Hatch, D.M.; Subrata, G. The α-β phase transition in cristobalite, SiO2. Phys. Chem. Miner. 1991, 17, 554–562. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, L.C. Quartz Glass; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- Aswathappa, S.; Dai, L.D.; Martin, B.D.; Raju, S.K.; Vasanthi, T. Assessment of acoustic shock wave resistance of SiO2 (α-cristobalite): A potential material for aerospace and defense industry applications. Ceram. Int. 2024, 50, 35647–35656. [Google Scholar] [CrossRef]
- Lucia, P.; Monica, D.; Alessandro, P.; Fernando, F. A kinetic study of the quartz–cristobalite phase transition. J. Eur. Ceram. Soc. 2013, 33, 3403–3410. [Google Scholar] [CrossRef]
- Huang, X.M.; Kon, S.; Wu, K.H.; Chen, M.G.; Hoshikawa, T.; Hoshikawa, K.; Uda, S. Reaction at the interface between Si melt and a Ba-doped silica crucible. J. Cryst. Growth 2005, 277, 154–161. [Google Scholar] [CrossRef]
- Antje, H.; Manuel, S.; Felix, S.; Matthias, T.; Christian, R.; Jochen, F. Factors influencing the gas bubble evolution and the cristobalite formation in quartz glass Cz crucibles for Czochralski growth of silicon crystals. J. Cryst. Growth 2021, 570, 126231. [Google Scholar] [CrossRef]
- Pan, X.D.; Li, S.Q.; Li, Y.K.; Guo, P.H.; Zhao, X.; Cai, Y.S. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xi, W.; Wang, L. Mineral material science characteristics of high quality raw material of granite pegmatite high purity quartz from Spruce Pine, United States. Mineral. Petrol. 2025, 16, 10. [Google Scholar]
- Wang, D.H.; Wang, Y.; Chen, Z.H. Discussion on the Prospecting Direction of High-purity Quartz Deposit. Acta Geosci. Sin. 2025, 46, 8–14. [Google Scholar]
- Zhang, W.F.; Zhang, G.L.; Xie, G.G. Genesis and prospecting potential of the Haoping hydrothermal vein-type high-purity quartz deposit in the Wudang Uplift, South Qinling. Geol. China 2024, 8, 1–25. [Google Scholar]
- Liu, M.; Wang, G.; Zhao, F.; Li, W.; Zhu, G.; Liang, G.; Jian, W.; Liao, L.; Lv, G. Advances in purification technologies and applications of high-purity quartz resource. Prog. Nat. Sci. Mater. Int. 2025, 35, 51–64. [Google Scholar] [CrossRef]
- Sang, L.K.; Ma, C.Q. Petrology; Geological Press: Beijing, China, 2001. [Google Scholar]
- Amir, R.; Zary, A. Adjustment of oxygen transport phenomena for Czochralski silicon crystal growth. Heliyon 2024, 10, e29346. [Google Scholar] [CrossRef]
- Wang, L.Y.; Hon, M.H. The effect of cristobalite seed on the crystallization of fused silica based ceramic core—A kinetic study. Ceram. Int. 1995, 21, 187–193. [Google Scholar] [CrossRef]
- Hurst, V.J.; Schroeder, P.A.; Styron, R.W. Accurate quantification of quartz and other phases by powder X-ray diffractometry. Anal. Chim. Acta 1997, 337, 233–252. [Google Scholar] [CrossRef]
- Müller, R.; Zanotto, E.D.; Fokin, V.M. Surface crystallization of silicate glasses: Nucleation sites and kinetics. J. Non-Cryst. Solids 2013, 274, 208–231. [Google Scholar] [CrossRef]
- Oldenbourg, R. Polarized Light Microscopy: Principles and Practice; Cold Spring Harbor Protocols: New York, NY, USA, 2013. [Google Scholar]
- Gomes, O.D.; Iglesias, J.C.A.; Paciornik, S.; Vieira, M.B. Classification of hematite types in iron ores through circularly polarized light microscopy and image analysis. Miner. Eng. 2013, 52, 191–197. [Google Scholar] [CrossRef]
- Wagstaff, F.E.; Richards, K.J. Kinetics of Crystallization of Stoichiometric SiO2 Glass in H2O Atmospheres. J. Am. Ceram. Soc. 1966, 49, 118–121. [Google Scholar] [CrossRef]
- Cantor, B. The Avrami equation: Phase transformations. Equ. Mater. 2020, 07, 180–206. [Google Scholar]
- Shirzand, K.; Christopher, V.A. A critical review on applications of the Avrami equation beyond materials science. J. R. Soc. Interface 2023, 20, 230242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.T. Fundamentals of Inprganic Materials Science; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Wang, L. Principles of Mineral Materal Science; Geological Press: Beijing, China, 2021. [Google Scholar]
- Xv, L.H.; Tian, J.; Wu, H.Q. New insights into the oleate flotation response of feldspar particles of different sizes: Anisotropic adsorption model. J. Colloid Interface Sci. 2017, 505, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; Chen, J.; Wei, M.N.; Li, F.F.; Ban, B.; Li, J.W. Effect of impurity content difference between quartz particles on flotation behavior and its mechanism. Powder Technol. 2020, 375, 504–512. [Google Scholar] [CrossRef]


| Sample Name | Al | Ti | Fe | K | Li | Na | Mg | Mn | Ni | Ba | Zr | Cr | Ge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OC | 19.45 | 5.09 | 2.53 | 3.47 | 0.07 | 2.57 | 1.35 | 0.01 | 0.01 | 0.11 | 1.80 | 0.06 | 0.02 |
| MG | 12.96 | 2.86 | 1.23 | 0.43 | 0.52 | - | 0.41 | 0.02 | 0.05 | - | 0.40 | 0.05 | 1.01 |
| IC | 13.21 | 1.44 | 1.96 | 1.57 | - | - | 0.15 | 0.08 | 0.03 | 2.83 | 0.86 | 0.03 | 0.23 |
| Calcination Time (h) | a (Å) | b (Å) | c (Å) | V (Å3) | ΔV/V0 (%) |
|---|---|---|---|---|---|
| PDF 00-011-0695 | 4.9710 | 4.9710 | 6.9180 | 170.95 | 0 |
| 6 | 4.9722 | 4.9722 | 6.9257 | 171.22 | 0.158 |
| 10 | 4.9723 | 4.9723 | 6.9260 | 171.23 | 0.164 |
| 16 | 4.9728 | 4.9728 | 6.9256 | 171.26 | 0.181 |
| 24 | 4.9724 | 4.9724 | 6.9280 | 171.30 | 0.205 |
| Sample | Al | Ti | Fe | K | Li | Na | Mg | Mn | Ni | Ba | Zr | Cr | Ge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG70–150 | 13.01 | 2.85 | 0.51 | - | 0.60 | - | - | - | - | - | 0.80 | - | 0.89 |
| MG-C | 10.40 | 2.20 | 0.54 | - | 0.51 | - | - | 0.01 | - | - | 0.44 | - | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Huang, Y.; Sun, H.; Tang, Y.; Peng, T. Waste Quartz Crucible Crystallization-Induced Purification to Prepare High-Purity Cristobalite Sand. Minerals 2025, 15, 1184. https://doi.org/10.3390/min15111184
Zhang T, Huang Y, Sun H, Tang Y, Peng T. Waste Quartz Crucible Crystallization-Induced Purification to Prepare High-Purity Cristobalite Sand. Minerals. 2025; 15(11):1184. https://doi.org/10.3390/min15111184
Chicago/Turabian StyleZhang, Tanlu, Yehao Huang, Hongjuan Sun, Yu Tang, and Tongjiang Peng. 2025. "Waste Quartz Crucible Crystallization-Induced Purification to Prepare High-Purity Cristobalite Sand" Minerals 15, no. 11: 1184. https://doi.org/10.3390/min15111184
APA StyleZhang, T., Huang, Y., Sun, H., Tang, Y., & Peng, T. (2025). Waste Quartz Crucible Crystallization-Induced Purification to Prepare High-Purity Cristobalite Sand. Minerals, 15(11), 1184. https://doi.org/10.3390/min15111184





