1. Introduction
Quartz, as one of the primary mineral forms of silica, is widely distributed in igneous, metamorphic, and sedimentary rocks of the Earth’s crust [
1]. Its formation involves primary or secondary magmatic processes, hydrothermal activities, sedimentation, as well as diagenesis and metamorphism [
2]. Owing to its high hardness, chemical inertness, and high-temperature resistance, high-purity quartz sand is extensively used in high-end applications such as glass, ceramics, and electronics [
3].
Natural quartz sand often contains associated impurities such as clay, feldspar, and metal oxides [
4,
5,
6], making it difficult to directly meet the performance requirements of high-purity quartz sand. Thus, efficient purification is essential. Current mainstream purification techniques include traditional physical methods (e.g., washing and classification, magnetic separation, and flotation) and chemical methods (e.g., acid leaching and chlorination roasting) [
7,
8,
9,
10]. Conventional physical beneficiation methods (magnetic separation and flotation) primarily separate quartz sand from associated minerals based on differences in their physical and chemical properties, but they exhibit limited effectiveness in removing impurities embedded within the crystal lattice or present as inclusions [
9]. Chemical purification methods (acid leaching and chlorination roasting) remove internal impurities and fluid/gas inclusions through chemical reaction [
11].
Current research on whitening and purifying quartz sand primarily focuses on acid leaching. Zhong et al. noted that commonly used acids in the acid leaching process include H
2SO
4, HCl, oxalic acid (H
2C
2O
4), and HF. While the use of a single inorganic acid improves quartz sand purity to some extent, impurity levels-particularly of Al- remain relatively high, with very low removal efficiency observed for aluminum impurities [
12]. Zhong et al. discovered synergistic effects among mixed acids; using an HCl-HF-H
2C
2O
4 mixture, the removal efficiencies for Fe and Al impurities reached 93.31% and 47.06%, respectively [
13]. The removal of Al impurities during acid leaching mainly occurs through the dissolution of quartz by HF, which exposes inclusions and impurities within the quartz. However, HF poses severe environmental hazards, directly disrupting the ecological balance of water, soil, and atmosphere, and is difficult to remove by conventional methods (e.g., activated carbon adsorption, biodegradation). Under current national strategic goals, purification technologies are evolving toward green and high-efficiency approaches that balance effectiveness, cost, and environmental friendliness [
11].
In this context, chlorination roasting has garnered attention due to its potential for deep impurity removal. This technique involves introducing chlorinating agents in a specific atmosphere to convert impurity elements (e.g., Fe, Al) into volatile or separable gaseous/condensed-phase substances [
14,
15]. Notably, the chlorination mechanism of key impurity components like aluminosilicates and Fe-oxides in chlorination roasting has been well elaborated by high-temperature metallurgy studies. For instance, Holm and Kleppa systematically measured the thermodynamic parameters of aluminosilicate chlorination reactions, clarifying the temperature-dependent Gibbs free energy change that governs reaction spontaneity [
16]. Meanwhile, Majzlan et al. [
17] investigated the chlorination thermodynamics of Fe-oxides (e.g., goethite and maghemite), revealing the critical temperature thresholds for converting solid Fe-oxides into volatile chlorides. These thermodynamic studies provide fundamental theoretical support for optimizing chlorination roasting conditions of quartz sand. During the chlorination roasting of quartz sand, the sand is typically mixed with a chlorinating agent and heated. Chlorinating agents are classified into gaseous (e.g., HCl, Cl
2) and solid forms (e.g., NaCl, CaCl
2, NH
4Cl) [
18,
19]. Gaseous chlorinating agents are associated with issues such as high consumption, elevated costs, and safety concerns during application, and are mainly used in the chlorination purification of high-purity quartz sand (SiO
2 ≥ 99.998%). Solid chlorinating agents, on the other hand, are used in smaller quantities and are simpler to apply, typically requiring only mixing with quartz sand before heating [
14]. Solid chlorinating agents are widely used in impurity removal from minerals. Several researchers have employed CaCl
2 as a chlorinating agent in studies such as roasting copper tailings to remove gallium impurities, extracting gold and silver from cyanide tailings, and recovering lithium from β-spodumene [
20,
21,
22], all achieving promising results. Liang Xiaoliang et al. used sodium chloride chlorination combined with microwave processing to treat high-purity quartz sand with certain success [
14,
23]. However, current research on chlorination roasting of quartz sand primarily targets high-purity quartz sand, with insufficient studies on its application to panel-grade sand.
The quality of panel-grade sand depends on the whiteness and impurity contents of Al, Fe, and Ca in panel-grade quartz sand, and the control of these indicators is crucial to ensuring panel performance. The industry requires panel-grade sand to have a whiteness of ≥80%; otherwise, it tends to cause discoloration or yellowing of panel surfaces, affecting their appearance and grade. Al needs to be controlled at a low content (e.g., A2O3 ≤ 0.7%), as excessive Al impairs the thermal stability and mechanical properties of panels. Fe must be strictly limited (e.g., Fe2O3 < 0.24%) because it easily causes panel discoloration and interferes with light transmittance. Ca requires an extremely low level (usually ≤ 100 ppm); high Ca content leads to “alkali efflorescence” of panels, reduced strength, and poor corrosion resistance. Precise control of these indicators is key to ensuring artificial quartz stone panels meet standards and remain competitive.
To achieve a low-cost and safe chlorination purification process for high-quality panel-grade sand, this study focuses on a solid-state chlorination roasting process using CaCl2 as the primary agent. We systematically investigate its mechanism for removing key impurities such as iron and aluminum from quartz sand, and elucidate the influence of roasting conditions (temperature, duration, and mixing ratio) on whiteness and impurity content. The aim is to provide theoretical support for the development of green and efficient purification technologies.
2. Experimental
2.1. Experimental Materials
To assess the effectiveness of CaCl2 as a solid chlorinating agent for impurity removal and whiteness enhancement of quartz sand, high-aluminum quartz ore from Suichuan County of the Jiangxi Province in China was used as the feedstock. The sample was ultrasonically cleaned in anhydrous ethanol for 1 h to remove surface-adhered impurities and organics, then dried, sieved, and the 80–120 mesh fraction was collected for use. The chlorinating reagent was CaCl2 (analytical grade, Sinopharm, Beijing, China), pre-dried at 400 °C for 24 h to eliminate residual moisture. Hydrochloric acid for leaching was HCl (analytical grade, Xilong Scientific, Shantou, China), and all solutions were prepared with in-house ultrapure water (18.2 MΩ·cm).
2.2. Experimental Apparatus
A three-zone tube furnace was employed for the high-temperature chlorination roasting experiments in this study. A schematic diagram of the experimental setup is presented in
Figure 1. The tube furnace features three independently controlled heating zones, allowing for precise temperature regulation in each section. The system (Model OTF-1200X, Hefei Kejing Co., Ltd., Hefei, China) consists of a quartz tube with an inner diameter of 100 mm and a length of 1200 mm, integrated with corresponding heating elements.
2.3. Experimental Procedure
To ensure thorough mixing of the quartz sand with anhydrous CaCl2, the materials were first ground and homogenized in an agate mortar. Due to the high hygroscopicity of anhydrous CaCl2 in air, the mixed samples were stored in a drying oven immediately after preparation to prevent moisture absorption. For the experiments, the mixture was loaded into a quartz boat and positioned at the center of the three heating zones in the tube furnace. Chlorination roasting was conducted under varying process parameters, including CaCl2 dosage, roasting temperature, and holding time, a heating rate of 5 °C/min, a cooling rate of 10 °C/min, and a gas atmosphere of air. Subsequently, acid leaching was performed to remove the residual calcium compounds introduced during the roasting process.
2.4. Analytical Methods
The main testing and analysis methods for quartz sand impurities included whiteness measurement (Benchtop Whiteness Meter: WSB-2A), Microscopy (OLYMPUS CX43 Optical Microscope), TIMA (Field Emission Scanning Electron Microscope, TESCAN Mira3 LMH), ICP testing (Experimental equipment: AGILENT ICP-OES-730; Standard test solution: National standard reference materials with calibration points at 0, 0.5, 1.0, 2.0, and 5.0 mg/L; Digestion method: a certain amount of sample was weighed into a PTFE container, followed by the addition of 5 mL concentrated nitric acid, 3 mL HCl, 1 mL HF, and 2 mL H2O2. The container was sealed and placed in a microwave digestion oven. The temperature was raised to 130 °C over 20 min at 1200 W, held at this temperature for 5 min, then further raised to 180 °C over another 20 min and held for 40 min, before finally cooling to room temperature), and TG-DSC (Simultaneous Thermal Analyzer: PerkinElmer STA 8000, Shelton, CT, USA; Heating rate: 20 °C/min).
3. Results and Discussion
3.1. Mineralogical Analysis of the Raw Material
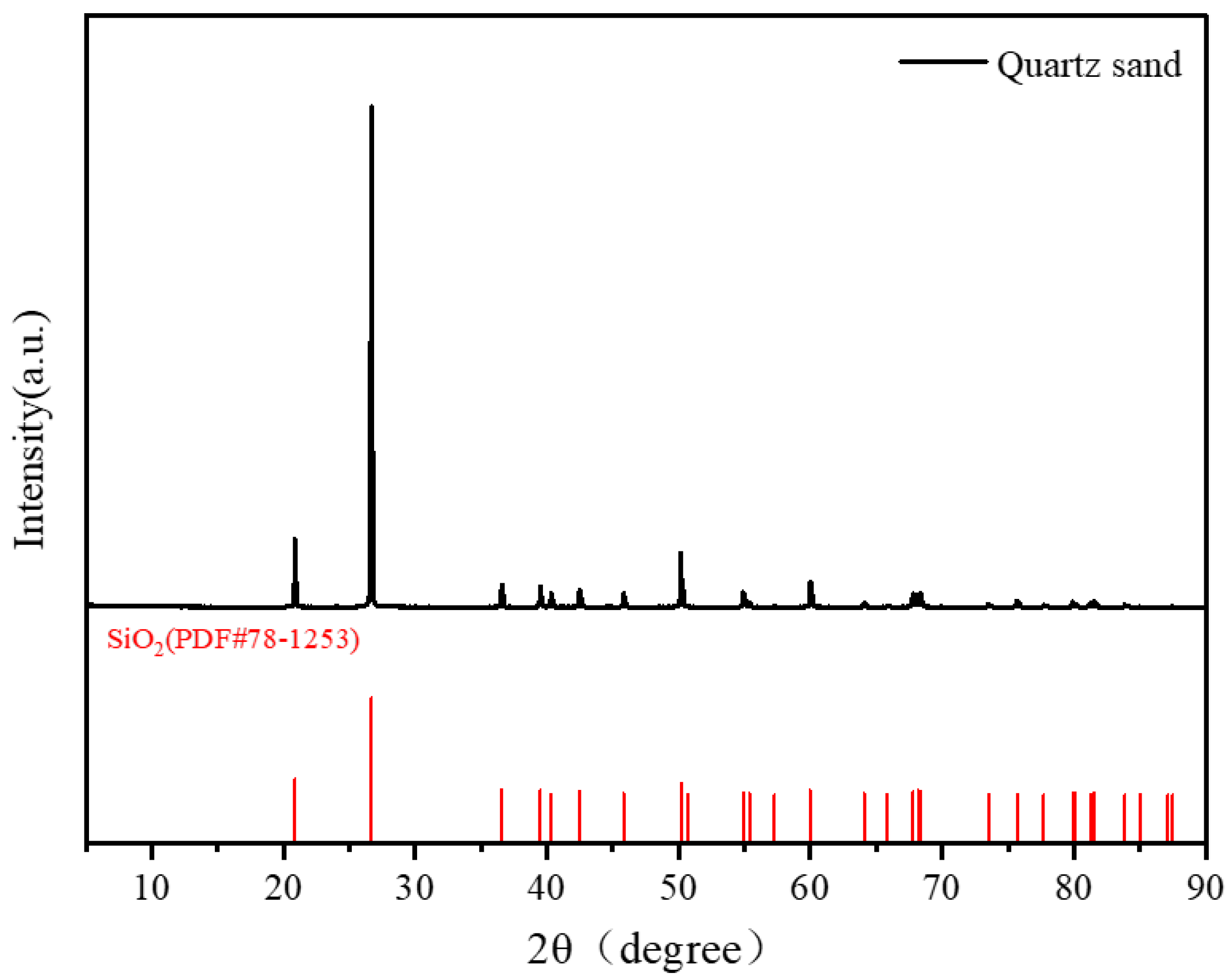
The sample is overwhelmingly quartz as the dominant phase. As shown by XRD (
Figure 2), the pattern matches the reference card PDF #78-1253 for α-quartz, with no reflections from phases other than SiO
2. TIMA quantification (
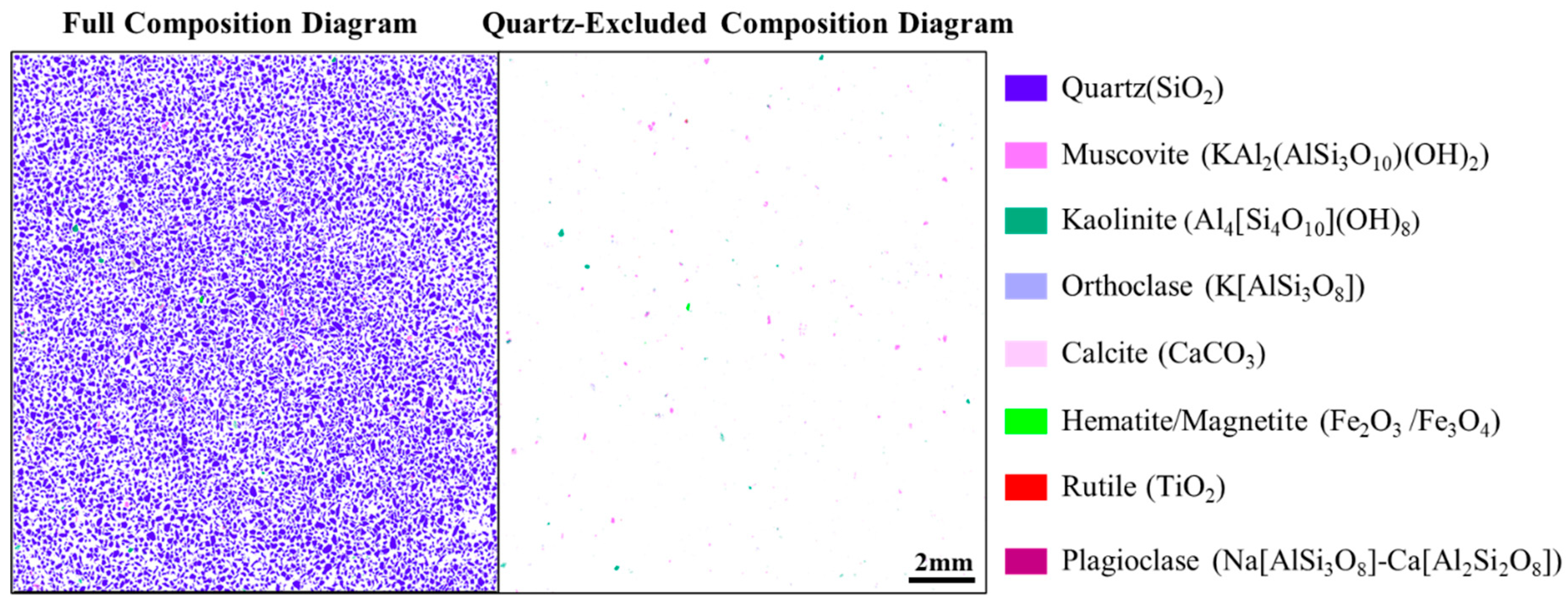
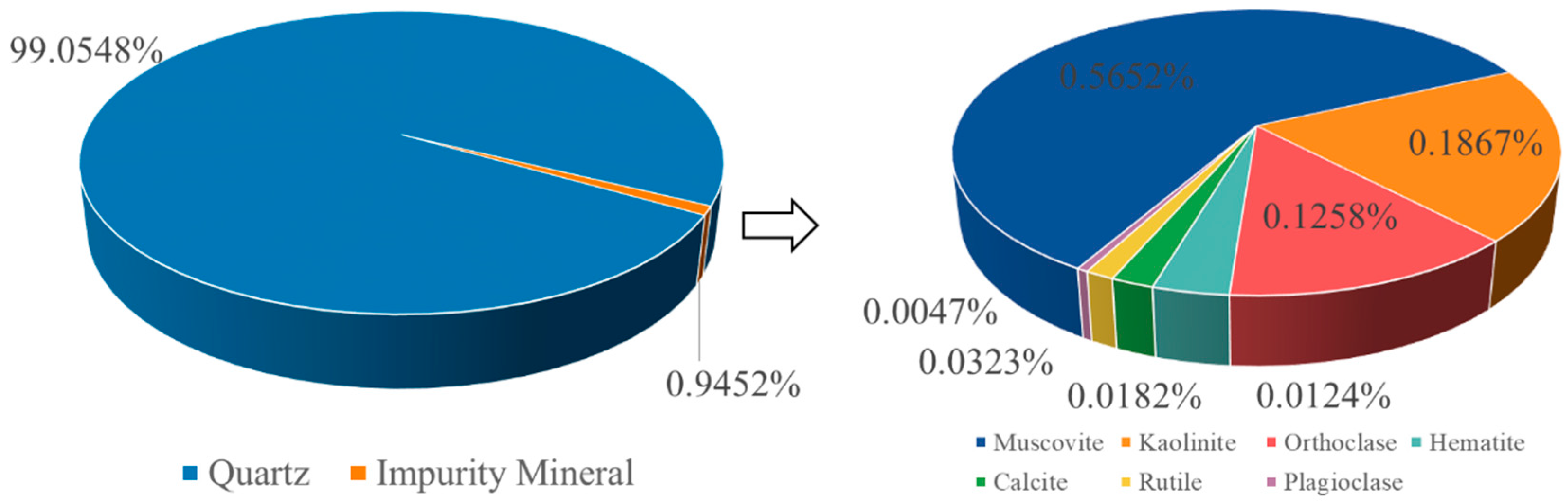
Figure 3 and
Figure 4) indicates 99.05 wt% quartz and 1 wt% total accessory minerals, chiefly muscovite (0.57 wt%), kaolinite (0.13 wt%), and orthoclase (0.19 wt%), with trace rutile, calcite, hematite, and minor plagioclase, these impurities are uniformly disseminated within quartz grains. ICP analysis further shows that bulk impurities are dominated by Al, K, and Fe (4519, 1177, and 496.3 ppm), with Mg, Ca, and Ti present at the 100 ppm level (
Table 1), consistent with an impurity budget controlled by aluminosilicate phases and minor Fe/Ti-bearing species that govern purity and whiteness.
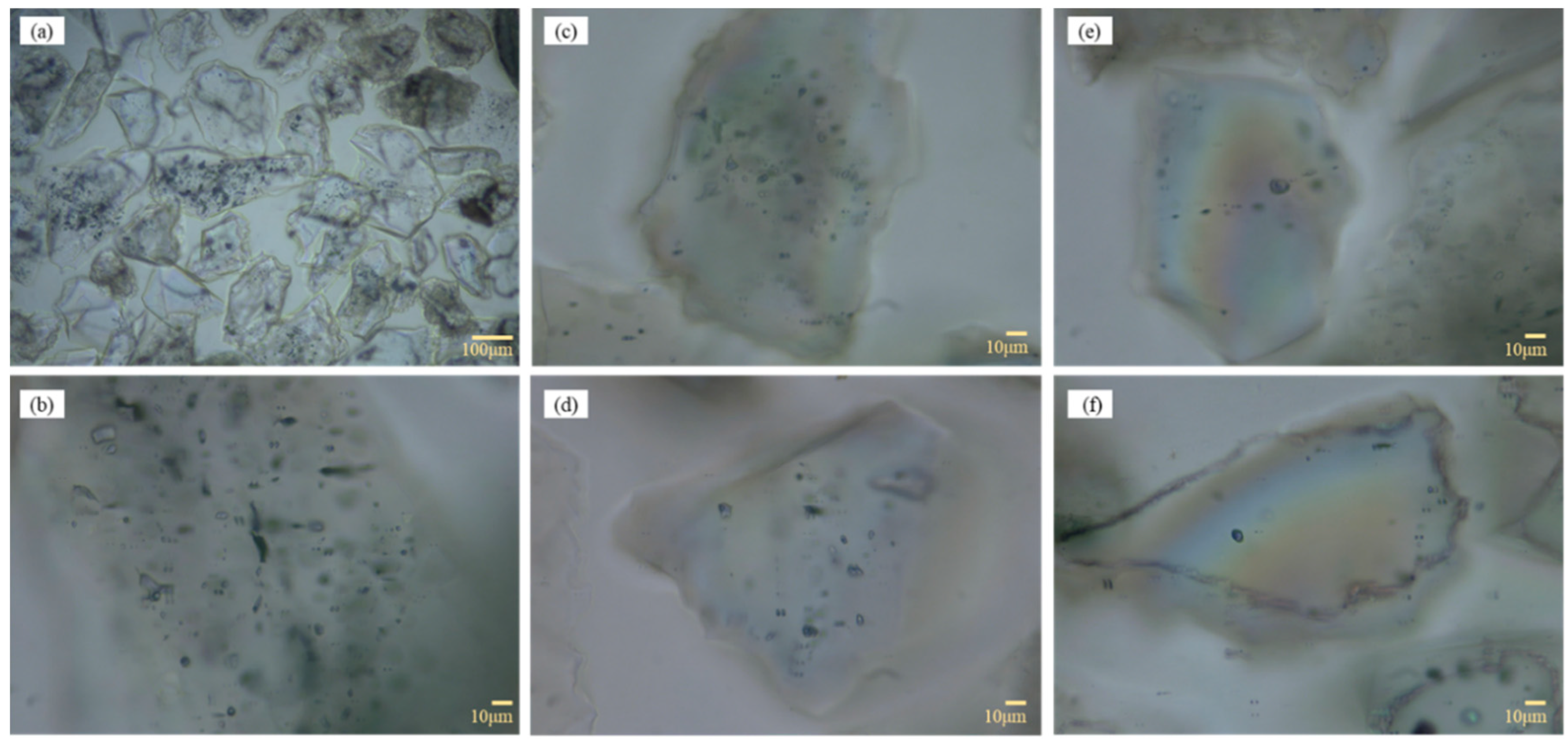
Optical microscopy (
Figure 5) reveals that 80% of particles host abundant micro-fluid inclusions, occurring both as discrete clusters and banded enrichments, indicating that internal inclusions and fine intergrowths are important hosts for impurities. Overall, despite the high SiO
2 content and simple diffraction signature, the presence of aluminosilicate impurities, Fe/Ti-bearing microphases, and pervasive inclusions increases the difficulty of deep purification; physical beneficiation alone is of limited effectiveness, and chemical routes are required to mobilize and remove the key impurities.
3.2. Analysis of Chlorination Mechanism by Calcium Chloride
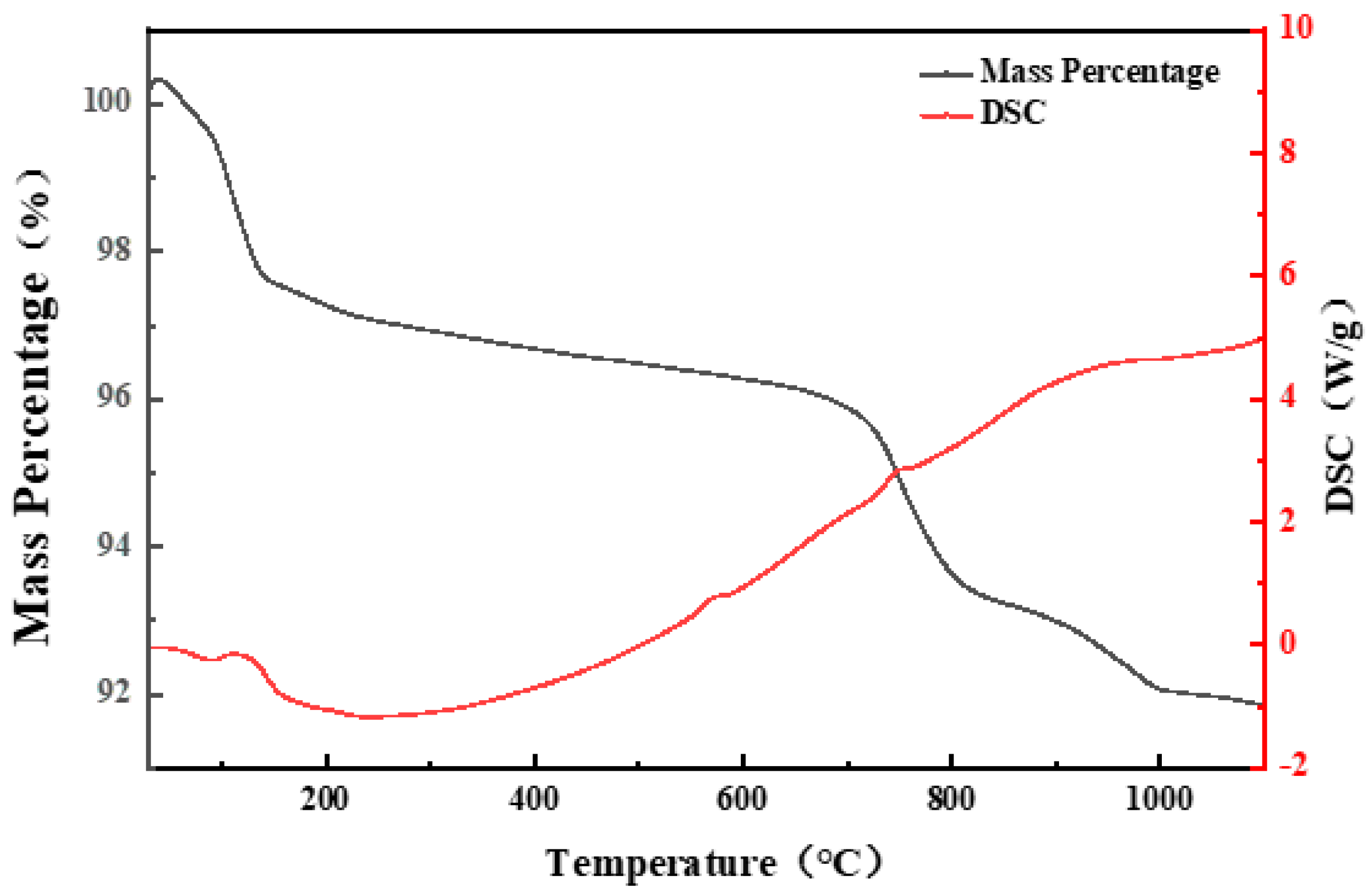
As shown in
Figure 6, a mixture of SiO
2 and CaCl
2 with a mass ratio of 1:0.1 was heated under air conditions. When the temperature exceeded room temperature, a significant mass loss was observed. It was caused by the dehydration reaction of CaCl
2, which readily absorbs moisture at ambient temperature to form hydrates (e.g., CaCl
2·2H
2O, CaCl
2·4H
2O, CaCl
2·6H
2O). As the temperature increased, these hydrates underwent decomposition, leading to continuous mass reduction [
24,
25].
The observed endothermic peak at 778 °C corresponds to the melting point of CaCl
2. During the melting process, a significant amount of heat is absorbed, leading to a noticeable endothermic event on the DSC curve [
26]. Simultaneously, a pronounced mass loss is observed, which can be attributed to the reaction of molten CaCl
2 with both H
2O and O
2 (when present), resulting in the release of HCl and Cl
2 [
21,
24,
26]. The relevant reactions (Equations (1)–(3)) are shown as follows:
Moreover, since both CaO and Ca(OH)
2 can react with SiO
2, the calcium is ultimately retained in the form of CaSiO
3 [
27]. Calcium silicate (CaSiO
3) possesses high thermodynamic stability, with a standard Gibbs free energy of formation of approximately −1548 kJ/mol at 25 °C. Consequently, it needs to be removed through a subsequent acid washing step. The relevant reactions (Equations (1)–(3)) are shown as follows:
The released chlorine gas (Cl2) and hydrogen chloride (HCl) react with the impurities in the quartz sand, converting the impurities into volatile chlorides that then separate from the system, ultimately achieving the goal of impurity removal.
3.3. Effect of Calcium Chloride Dosage on Impurity Removal Efficiency
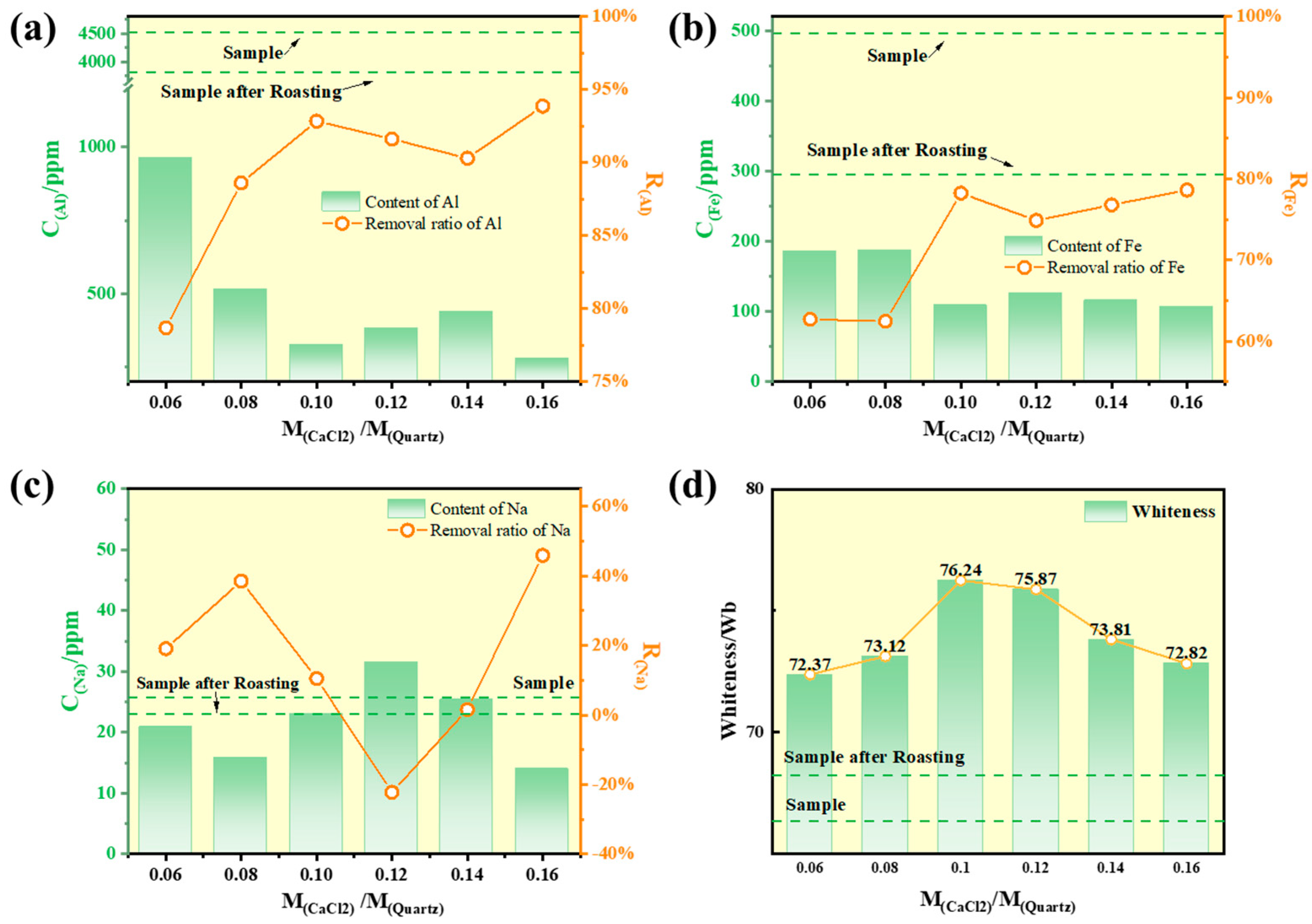
Calcium chloride was mixed with quartz sand samples (10 g each) with mass ratios range from 0.06 to 0.16, respectively. The mixtures were heated at 900 °C for 2 h. The whiteness and impurity content of the roasted samples were analyzed, and the results are shown in
Figure 7. As observed in the figure, direct roasting of quartz sand without additives resulted in only marginal improvements in both whiteness and impurity removal efficiency. This limited enhancement is primarily attributed to the fact that roasting quartz without additives can only volatilize a minor fraction of impurities, while the majority remain embedded within the quartz matrix.
In contrast, with the addition of calcium chloride, the aluminum and iron content in the quartz sand were significantly reduced. When the mass ratio of calcium chloride to quartz sand reached 0.10, the removal rates of aluminum and iron reached 92.85% and 79.2%, respectively. This significant improvement is primarily attributed to the chlorination capability of calcium chloride. As shown in
Figure 6, at 800 °C, calcium chloride decomposes and releases active chlorinating agents such as HCl and Cl
2. Under high-temperature conditions, impurity elements within the quartz sand react with these gases to form volatile chlorides (e.g., AlCl
3 and FeCl
3), which volatilize and escape from the quartz sand, thereby achieving separation of impurity elements [
14]. Compared with aluminum and iron, the removal efficiency of sodium was lower and exhibited considerable variability. This inconsistency can be attributed to the inherent differences in impurity content across sample batches and the measurement uncertainties associated with ICP analysis. Furthermore, when the dosage of CaCl
2 exceeded the optimal ratio, no further reduction in aluminum and iron content was observed, indicating that the purification limit of this particular quartz sand had been reached. The residual aluminum and iron impurities are likely embedded within the quartz lattice, making them recalcitrant to removal [
28].
Except removing impurity elements, the introduction of calcium chloride also significantly enhances the whiteness of quartz sand. As shown in
Figure 7d, when the addition ratio was below 0.10, only a marginal improvement in the whiteness of the roasted product was observed. This limited enhancement can be attributed to superficial melting of the quartz surface at elevated temperatures, which reduces surface porosity and micro-cracks, thereby increasing light reflectivity and resulting in moderate whiteness improvement. When the calcium chloride addition ratio exceeded 0.10, the whiteness of the quartz sand increased from 66.32 Wb to 76.24 Wb. During high-temperature roasting, mineral impurities such as muscovite and hematite fracture and become exposed on the quartz sand surface. These impurities subsequently react with HCl and Cl
2 released by the decomposition of CaCl
2. Since iron impurities are a critical factor affecting quartz sand whiteness, the reduction in iron content directly contributes to the observed whiteness improvement [
14]. However, when the CaCl
2 dosage exceeded 0.10, no further whiteness enhancement was observed; instead, a decline occurred. This is attributed to excessive CaCl
2, which can absorb moisture and form agglomerates on the quartz sand surface. These agglomerates compromise the uniformity of the sand and reduce surface reflectivity, ultimately leading to decreased whiteness [
24].
From the perspectives of both quartz sand purity and whiteness, the addition of calcium chloride during roasting proves beneficial for enhancing product quality. However, it simultaneously leads to the introduction of calcium as an impurity element.
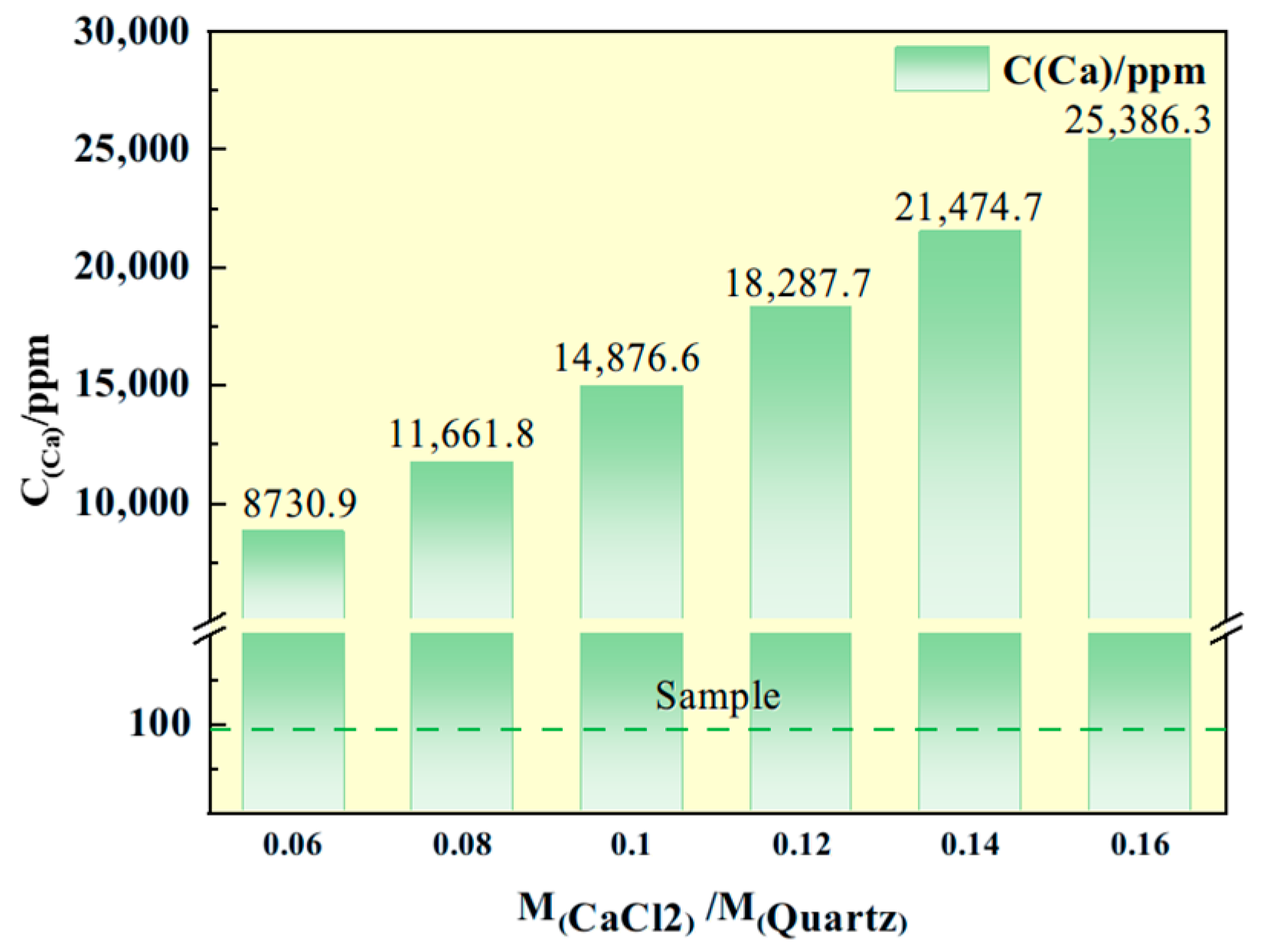
Figure 8 illustrates the calcium content within the quartz sand following the addition of calcium chloride. As evident from
Figure 8, the calcium content exhibits a linear increase with higher dosages of calcium chloride, which complicates subsequent calcium removal efforts. Therefore, the dosage of calcium chloride should be optimized to the minimal level necessary to achieve target impurity removal. Considering both purification efficiency and economic feasibility, a mass ratio of quartz sand to calcium chloride of 0.10 is determined to be optimal. Subsequent experiments were therefore conducted using this specific dosage.
3.4. Effect of Roasting Time on Impurity Removal Efficiency
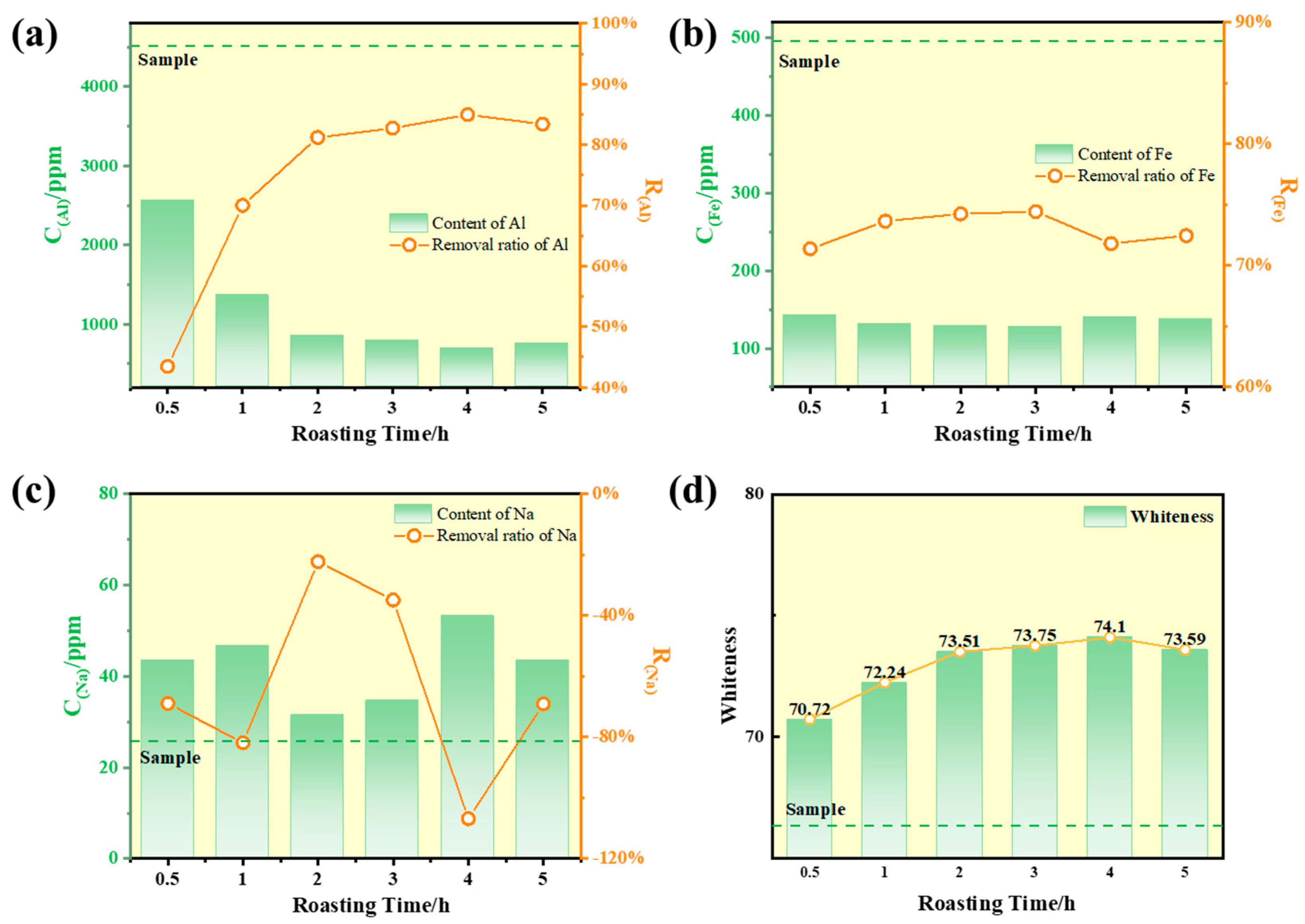
Calcium chloride was mixed with calcium chloride at a mass ratio of 0.10 and subjected to chlorination roasting at 900 °C with different holding times (0.5 h, 1 h, 2 h, 3 h, 4 h, and 5 h). The whiteness and impurity content of the roasted samples were analyzed, and the results are presented in
Figure 9. As shown in the figure, the aluminum impurity content gradually decreased with prolonged roasting time. When the roasting time reached 4 h, the removal rates of aluminum and iron reached 81.2% and 74.2%, respectively. This phenomenon is primarily attributed to the insufficient decomposition of CaCl
2 and the consequent low release of HCl at shorter holding times, leading to incomplete reactions with impurities. As the roasting time extended, the increased release of HCl from CaCl
2 enhanced the reaction efficiency with metallic impurities in the quartz sand. Simultaneously, iron impurities within the quartz particles diffused to the surface and underwent chlorination reactions with active chlorine species [
29], further improving impurity removal efficiency. However, beyond a certain point, further extension of the roasting time did not yield additional improvements in purification efficiency. This is because the remaining impurities are embedded within the quartz lattice and are recalcitrant to removal [
30].
Extending the holding time also influenced the whiteness of the quartz sand. As shown in
Figure 9d, the whiteness initially increased and then decreased with prolonged roasting time. The maximum whiteness of 74.10 Wb was achieved at a roasting time of 4 h. This trend is correlated with the iron impurity content in the quartz sand. After 2 h of roasting, the iron content stabilized, and the whiteness also reached a stable range, with minor fluctuations likely attributable to measurement errors during testing.
Although the highest aluminum removal efficiency and whiteness were achieved at 4 h, the improvements compared to the results at 2 h were marginal. Prolonging the roasting time beyond this point did not significantly enhance either impurity removal or whiteness. Moreover, extended roasting at high temperatures consumes additional energy, leading to increased operational costs. Therefore, considering both efficiency and economic feasibility, the optimal roasting time was determined to be 2 h. Subsequent experiments were conducted using this duration.
3.5. Effect of Roasting Temperature on Impurity Removal Efficiency
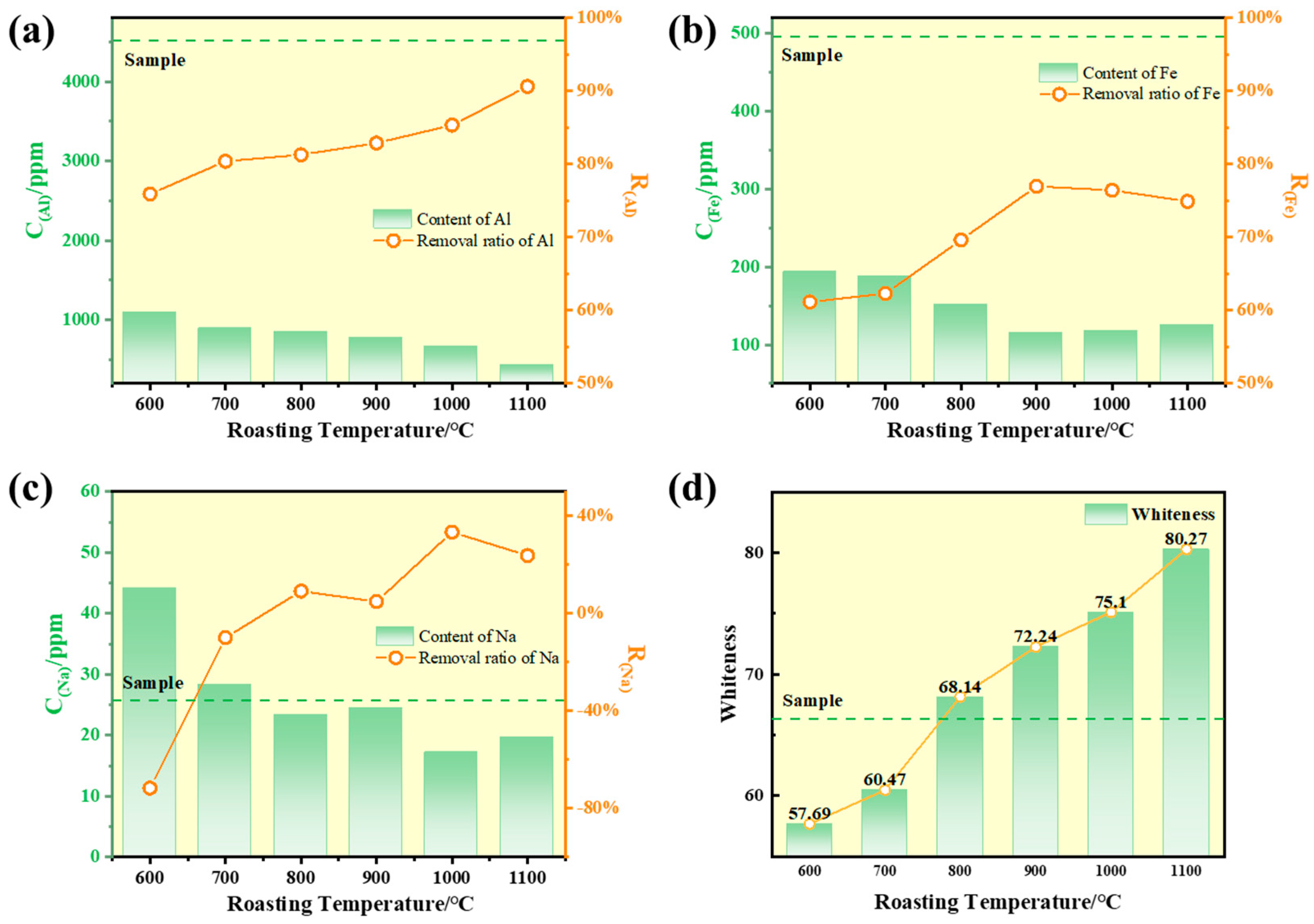
Calcium chlorides are mixed with quartz sand samples (10 g each) at a mass ratio of 0.10 and subjected to chlorination roasting at different temperatures (600 °C, 700 °C, 800 °C, 900 °C, 1000 °C, and 1100 °C) for 2 h. The whiteness and impurity content of the roasted samples were analyzed, and the results are shown in
Figure 10. As observed in the figure, the aluminum and iron impurity contents significantly decreased with the roasting temperature increasing. When the temperature reached 1100 °C, the removal rates of aluminum and iron reached 90.6% and 74.9%, respectively. This phenomenon is primarily attributed to the enhanced decomposition of CaCl
2 at higher temperatures, which releases more chlorinating gases. The aluminum and iron impurities are fully converted into volatile chlorides (e.g., AlCl
3 and FeCl
3) and subsequently removed via volatilization, leading to a continuous reduction in impurity content.
The increase in roasting temperature also positively influenced the whiteness of the quartz sand, as shown in
Figure 10d. At temperatures of 600 °C and 700 °C, the whiteness of the quartz sand was lower than that of the raw material. However, when the temperature was raised to 800 °C, the whiteness significantly improved and exceeded the raw material value. It continued to increase with further temperature elevation, reaching a maximum of 80.27 Wb at 1100 °C. This phenomenon can be explained as follows: at lower temperatures (600 °C and 700 °C), CaCl
2 did not reach its melting point and instead coated the quartz sand particles. The limited release of chlorinating gases at these temperatures prevented the complete conversion of hematite (Fe
2O
3) into chlorides. Conversely, sintering caused the aggregation of iron oxides, intensifying the reddish-yellow coloration, thereby reducing whiteness compared to the raw material [
31]. When the temperature exceeded 800 °C, CaCl
2 decomposed rapidly, releasing abundant chlorinating gases. This promoted the full chlorination of aluminum and iron impurities into volatile AlCl
3 and FeCl
3, which were subsequently removed via volatilization. The continuous reduction in impurity content led to whiteness improvement. Additionally, the high temperature induced slight surface melting of the quartz particles, forming a smooth layer that reduced diffuse reflection and further enhanced whiteness [
6].
As the roasting temperature increased, whiteness improved concurrently with the reduction in aluminum and iron impurity contents. However, when the temperature exceeds 1200 °C, quartz may undergo transformation to cristobalite [
14], resulting in volume expansion and a consequent decrease in quartz sand strength. Therefore, the experimental temperature was controlled within 1200 °C and not further increased. Considering all factors, the optimal roasting temperature was determined to be 1100 °C, and subsequent experiments were conducted at this temperature.
3.6. Acid Leaching for Removal of Impurities Introduced by Chlorinating Agent
As indicated in
Figure 8, the addition of CaCl
2, while effective in removing certain impurities, simultaneously introduces significant amounts of calcium. The presence of Ca in panel-grade quartz sand can significantly impair the performance of quartz panels, such as causing “alkali efflorescence” on the panels, damaging their appearance and stain resistance, and reducing their strength and chemical resistance. Therefore, an acid leaching step is necessary to eliminate the introduced Ca. The leaching process was conducted using 4 mol/L hydrochloric acid at 70 °C with a liquid-to-solid ratio of 5:1 for 6 h. The impurity content and whiteness of the roasted samples processed by different methods were shown in
Figure 11.
Compared with chlorination roasting process, although the acid leaching process alone had limited effectiveness in removing aluminum Al impurities, with the aluminum content remaining as high as 2184.99 ppm, it could effectively remove. In contrast, iron and calcium Ca and Fe impurities contents were reduced to relatively lower levels of 130.4 ppm and 35.96 ppm, respectively, which means the acid leaching process could remove the Ca impurity introduced by chlorination roasting process. In the chlorination roasting process alone, significant removal of aluminum and iron impurities was achieved, with residual contents of 422.62 ppm and 124.43 ppm, respectively. However, this process introduced and enriched calcium impurities, reaching a high level of 13,157.9 ppm.
The combined process of chlorination roasting followed by acid leaching (C + A process) could further decrease impurities, especially for Fe and Ca. During chlorination roasting, aluminum and iron impurities migrate to the surface of the quartz sand particles, where they react with calcium chloride to form volatile low-boiling-point chlorides that are subsequently removed via volatilization [
32]. A minor fraction of unvolatilized residues remains on the surface and is effectively eliminated during the subsequent acid leaching step [
33]. Simultaneously, calcium introduced during roasting—primarily in the form of CaSiO
3 on the quartz surface [
27]—reacts with HCl during acid leaching to form H
2SiO
3, thereby enabling efficient removal of calcium impurities.
When the combined process of chlorination roasting followed by acid leaching was employed, all impurity elements were significantly reduced. The aluminum, iron, and calcium contents decreased to 401.44 ppm, 46.3 ppm, and 48.39 ppm, respectively. During chlorination roasting, aluminum and iron impurities migrate to the surface of quartz sand particles and react with calcium chloride to form low-boiling-point chlorides that volatilize and are removed [
32]. A small amount of unvolatilized residues remain on the quartz sand surface and can be further removed by acid leaching [
31]. Simultaneously, the calcium impurities introduced during chlorination roasting attach to the quartz sand surface in the form of CaSiO
3 [
27]. During acid leaching, CaSiO
3 reacts with HCl to form H
2SiO
3, thereby facilitating the removal of calcium elements.
Regarding the improvement in whiteness, as shown in
Figure 11, the sample treated with acid leaching alone exhibited a whiteness of 77.56 Wb. After treatment by chlorination roasting alone, the whiteness increased to 80.27 Wb. When the combined process of chlorination roasting followed by acid leaching was applied, the whiteness was further enhanced to 85.2 Wb. The chlorination roasting process converts the most recalcitrant encapsulated impurity elements (e.g., Fe, Ti) within the quartz sand into gaseous species that volatilize [
32], thereby addressing the coloration issues originating from the interior of the quartz particles. However, certain residues generated during roasting, such as CaCl
2, CaO, and CaSiO
3, remain on the surface. The subsequent acid leaching step effectively dissolves and removes these soluble residues, achieving an optimally clean surface and consequently further enhancing the whiteness.
The comparison of different process was shown in
Figure 11c. From the figure, the combined process of chlorination roasting and acid leaching proved exhibits highly effective in enhancing sample whiteness and reducing impurity content, which has potential for application of purification of quartz sand. It also successfully eliminates the calcium impurities introduced during the chlorination roasting stage.
A comparison of the aluminum removal efficiency between traditional purification processes and the proposed process reveals the following: the aluminum removal rates of conventional gaseous chlorination roasting and HF leaching are approximately 44.4% and 87.6%, respectively. In contrast, the CaCl2-based chlorination roasting-acid leaching combined process proposed in this study achieves an aluminum removal rate of 91.1% under optimal experimental conditions, which is significantly superior to the two traditional processes.
Furthermore, traditional processes suffer from notable application limitations: gaseous Cl2 and HCl not only incur high storage costs but also exhibit strong corrosiveness and toxicity, posing substantial safety risks during operation. Meanwhile, HF is highly corrosive to equipment and generates waste liquids that are difficult to treat, easily leading to severe environmental hazards. In comparison, the combined process in this study uses solid CaCl2 as the chlorinating agent and eliminates the need for the aforementioned hazardous reagents. This fundamentally avoids the safety and environmental issues associated with traditional processes, endowing it with greater potential for industrial application.
4. Conclusions
To address the industrial pain points of high impurity content and low whiteness in quartz sand—key bottlenecks for its application in high-value fields like panel manufacturing—this study develops a combined process of solid chlorination roasting and acid leaching. The process uses calcium chloride (CaCl2), a safe, low-cost solid chlorinating agent, mixed with quartz sand for roasting, effectively removing aluminum, iron, and other impurities to boost whiteness. Under optimized conditions (quartz sand-to-CaCl2 mass ratio 1:0.1, 1100 °C roasting for 2 h), aluminum and iron contents dropped to 422.62 ppm and 124.43 ppm, respectively, though calcium content increased significantly. Subsequent acid leaching further reduced residual impurities and introduced calcium, ultimately achieving 91.1% Al removal, 90.7% Fe removal, 50.2% Ca removal, and a whiteness of 85.2 Wb—outperforming standalone acid leaching or chlorination roasting. Notably, the use of low-cost, easily accessible CaCl2 and straightforward process steps (roasting + leaching) lays a foundation for scalability in industrial production, supporting its potential for large-scale application in high-panel-grade quartz sand manufacturing.