Characterization of Brazilian Tin Slag and Evaluation of Its Potential as a Secondary Source of Nb and Ta
Abstract
1. Introduction
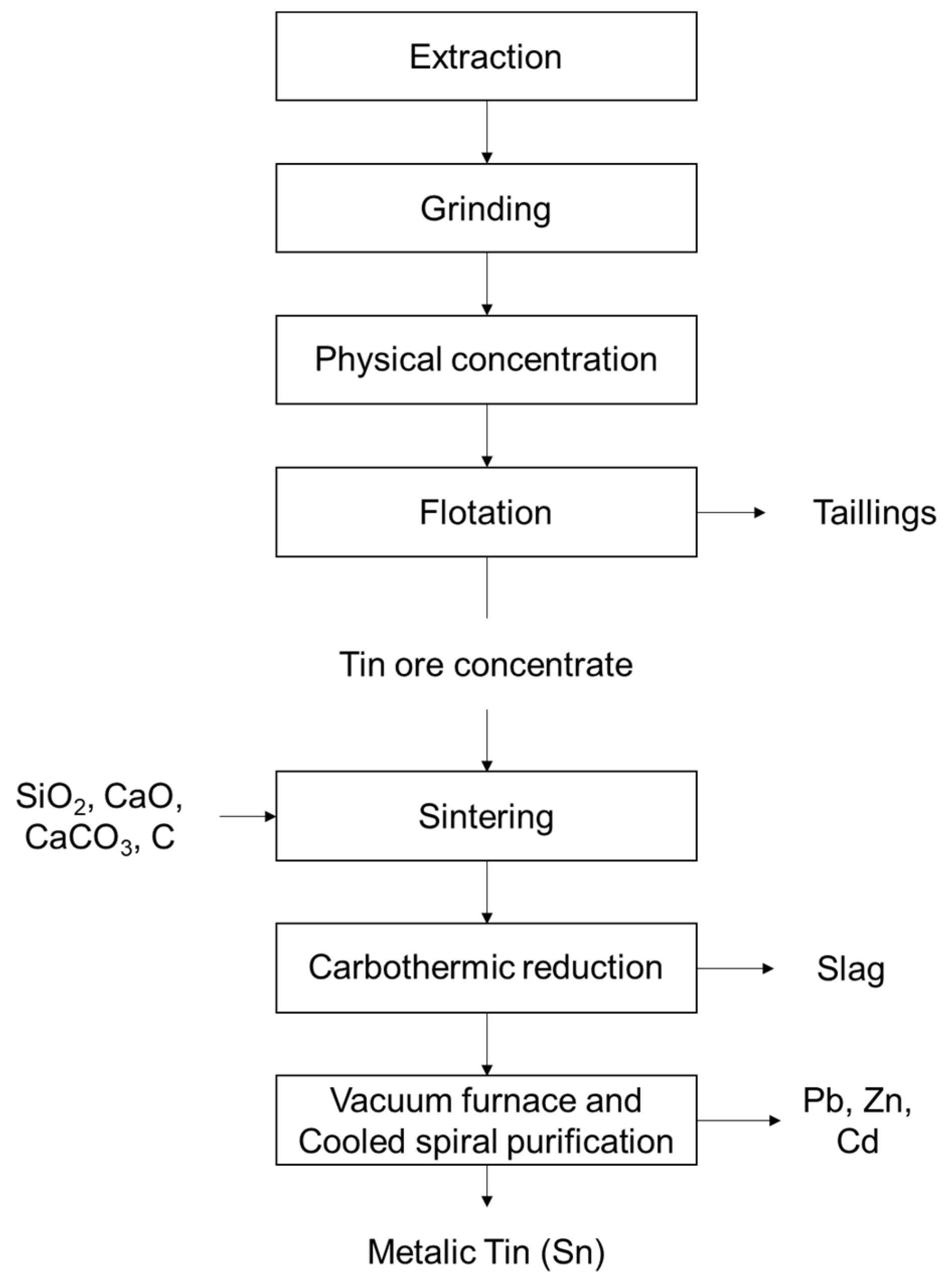
1.1. Production Process
1.2. Waste Disposal
1.3. Reuse of Slags
1.4. Recovery of Elements of Economic Interest
1.5. Brazilian Tin Slag
2. Materials and Methods
2.1. Homogenization and Quartering
2.2. Particle Size Distribution
2.3. Elemental Composition
2.4. Mineralogical Analysis
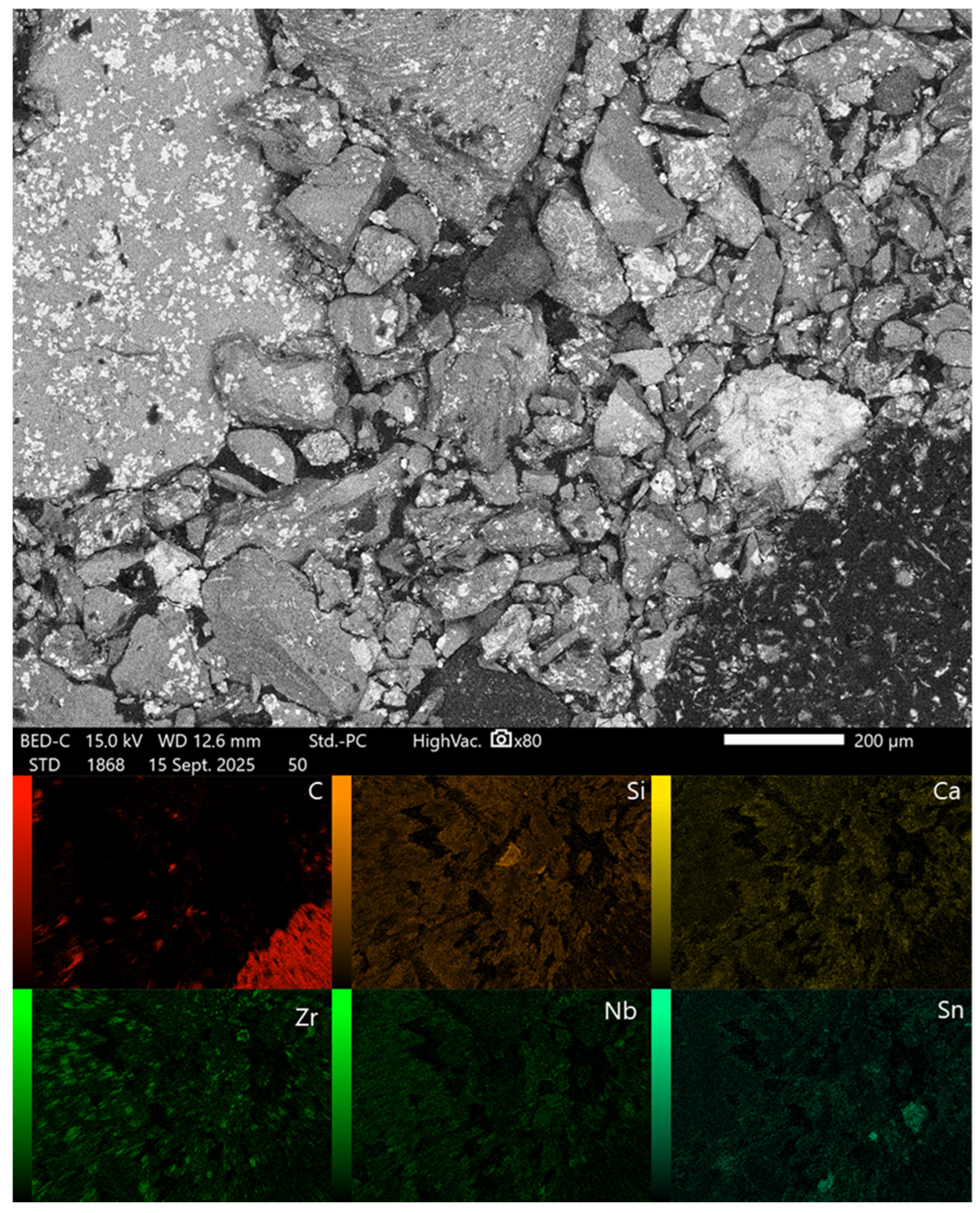
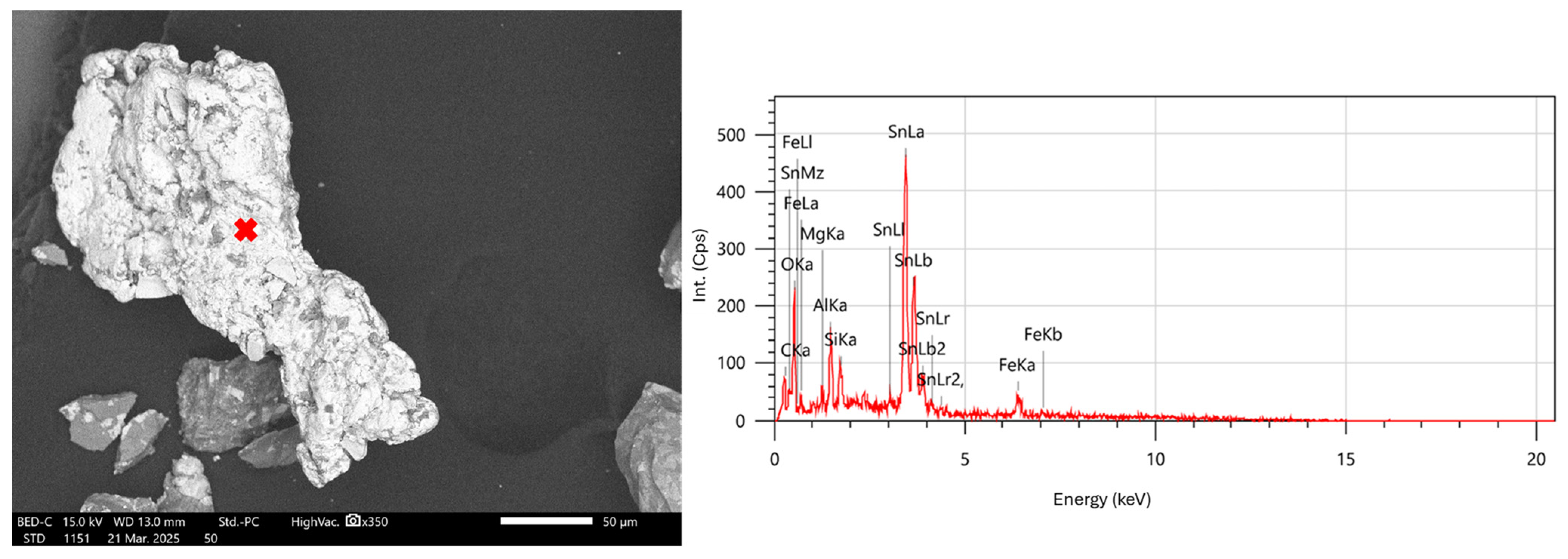
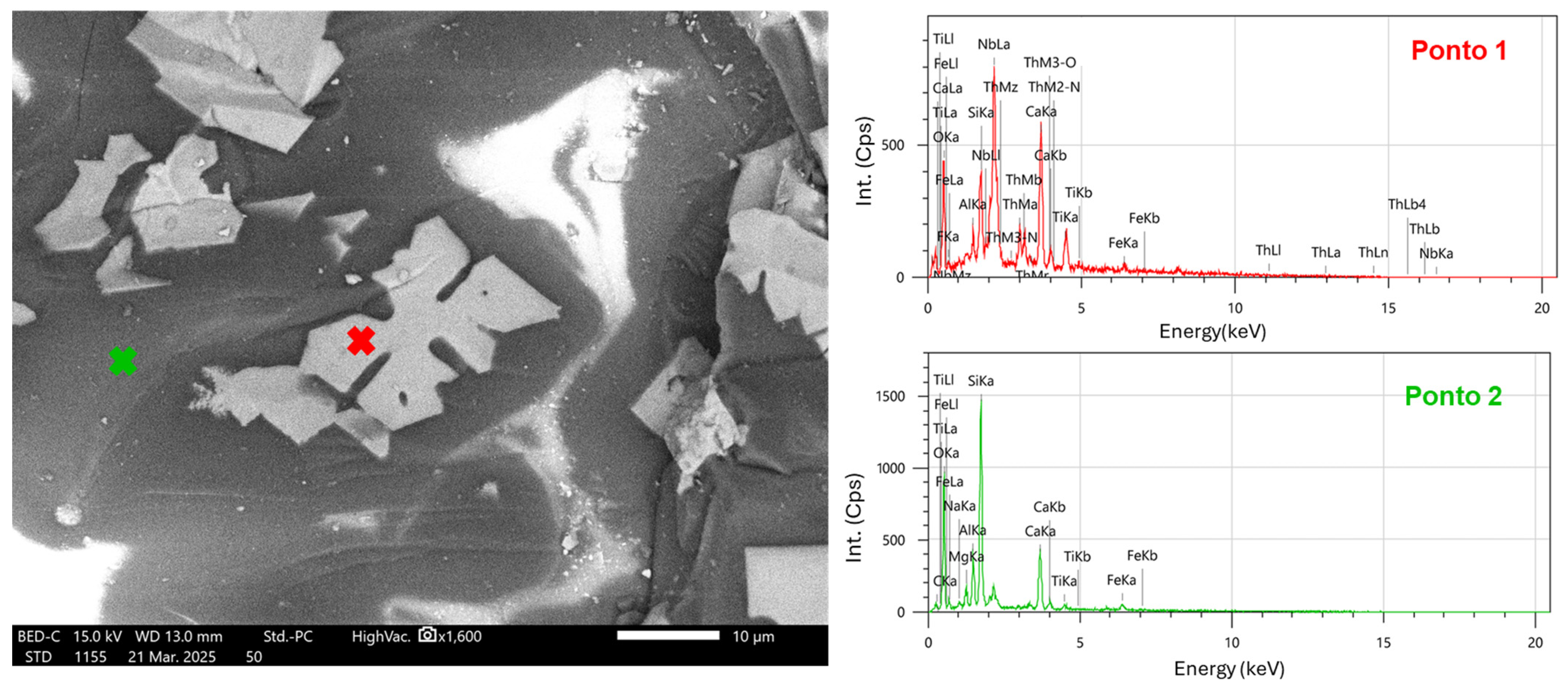
2.5. Morphological Characterization
3. Results and Discussion
3.1. Particle Size Distribution
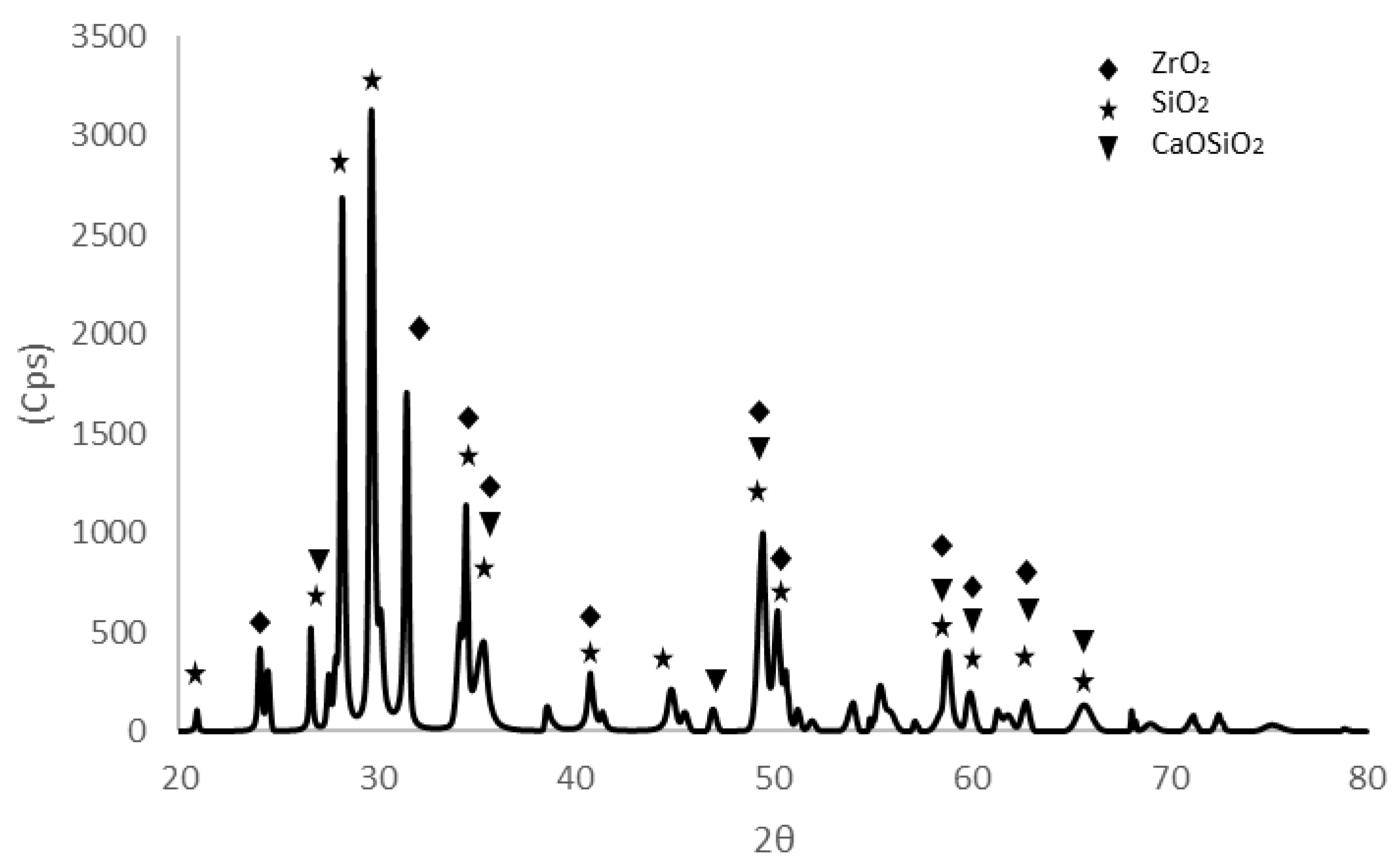
3.2. Mineralogical Analysis
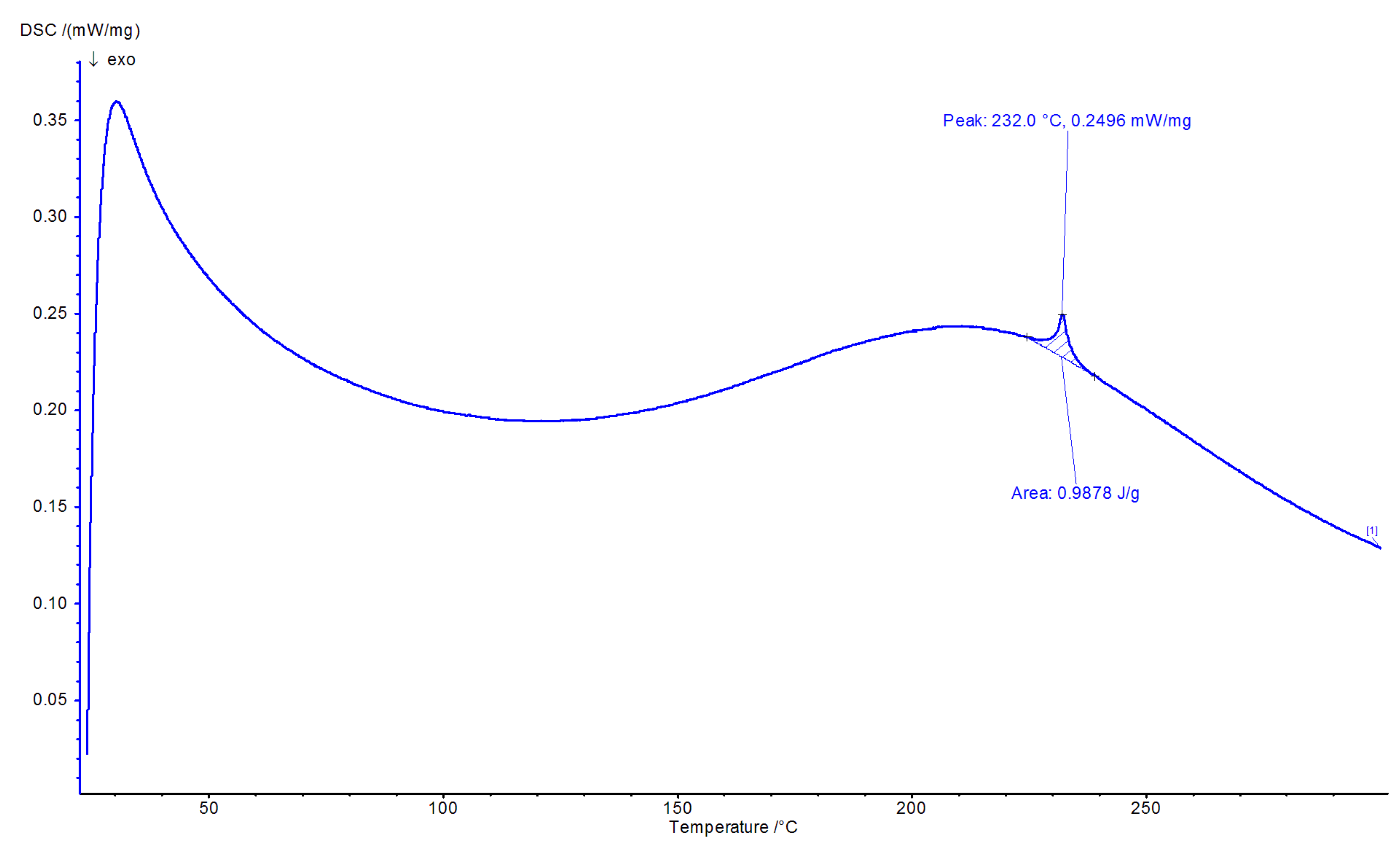
3.3. Morphological Characterization
3.4. Elemental Composition
3.5. What Does the Characterization of the Slag Indicate for a Future Recycling Process of Nb and Ta?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Shikika, A.; Sethurajan, M.; Muvundja, F.; Mugumaoderha, M.C.; Gaydardzhiev, S. A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 2020, 198, 105496. [Google Scholar] [CrossRef]
- Muñoz, N.F.S. Evaluación Comparativa del Crecimiento de una Planta de Rábano (Raphanus Sativus), Cultivada en Maceta Aplicando Compost Aditivado com Metales de Escoria Ferrosa. Master’s Thesis, FACULTAD DE INGENIERÍA Y CIENCIAS APLICADAS, Quito, Ecuador, 2021. Available online: https://repositorio.uisek.edu.ec/bitstream/123456789/4477/1/Sánchez%20Muñoz%20Nelson%20Francisco.pdf (accessed on 1 August 2025).
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Habib, A.; Bhatti, H.N.; Iqbal, M. Metallurgical Processing Strategies for Metals Recovery from Industrial Slags. Z. Für Phys. Chem. 2020, 234, 201–231. [Google Scholar] [CrossRef]
- Sobral, M.F.; Nascimento, C.W.; Cunha, K.P.; Ferreira, H.A.; Silva, A.J.; Silva, F.B. Escória de siderurgia e seus efeitos nos teores de nutrientes e metais pesados em cana-de-açúcar. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 867–872. [Google Scholar] [CrossRef]
- NBR 10004:2004; Resíduos sólidos—Classificação. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2004.
- European Parliament and Council. Directive 2008/98/EC of 19 November 2008 on Waste and Repealing Certain Directives. Official Journal of the European Union. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0098 (accessed on 1 August 2025).
- U.S. Environmental Protection Agency. Special Wastes; EPA: Washington, DC, USA, 2025. Available online: https://www.epa.gov/hw/special-wastes (accessed on 1 August 2025).
- Garcia, M.A.A. Caracterização Radioquímica e Impacto Radiológico Ambiental no Processo de Cassiterita para Produção de Estanho e Chumbo Metálico. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2009. [Google Scholar]
- Prasetyo, E.; Supriyatna, Y.I.; Bahfie, F.; Trinopiawan, K. Extraction of thorium from tin slag using acidic roasting and leaching method. AIP Conf. Proc. 2020, 2232, 040008. [Google Scholar] [CrossRef]
- Ryu, G.U.; Kim, H.J.; Yu, H.J.; Pyo, S. Utilization of steelmaking slag in cement clinker production: A review. J. CO2 Util 2024, 84, 102842. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. The Use of Steel Slags in Asphalt Pavements: A State-of-the-Art Review. Sustainability 2023, 15, 8817. [Google Scholar] [CrossRef]
- Wang, G.C. Nonferrous metal extraction and nonferrous slags. In The Utilization of Slag in Civil Infrastructure Construction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–61. [Google Scholar] [CrossRef]
- Fagundes, F.G.; Rocha, G.O.C.; Paulino, G.M. Tratamento de escória proveniente da redução da cassiterita visando à remoção de enxofre e o aproveitamento do tântalo. Revista Engenharia de Interesse Social. Rev. Eng. Interesse Soc. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Zulhan, Z.; Ryanta, I.G.P.A. Utilization of Gypsum Byproduct as Fuming Agent for Tin Smelting Slag. J. Sustain. Metall. 2018, 4, 388–394. [Google Scholar] [CrossRef]
- Clemente, D.M.; Menezes, R.d.O.T.; Damaso, H.V.; Peixoto, J.J.M.; Silva, C.A.d. Study of the compositional and melting characteristics of some Brazilian and Congolese tin slags. Tecnol. Metal. Mater. Mineração 2024, 21, e3061. [Google Scholar] [CrossRef]
- Akli, H.F.; Permana, S.; Maksum, A.; Soedarsono, J.W.; Widana, K.S.; Anggraini, M.; Munir, B. Enrichment of Tantalum and Niobium Contents in Bangka Tin Slag by NaOH and H3PO4 Leaching. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012050. [Google Scholar] [CrossRef]
- Anes, I.A.; Garjulli, F.; Siqueira de Carvalho, M.; Tenório, J.A.S.; Espinosa, D.C.R.; Coleti, J.L. Extraction of niobium in one step from tin slag by NH4F-HCl leaching process. Can. J. Chem. Eng. 2024, 102, 168–176. [Google Scholar] [CrossRef]
- Permana, S.; Soedarsono, J.W.; Rustandi, A.; Maksum, A. Other Oxides Pre-removed from Bangka Tin Slag to Produce a High Grade Tantalum and Niobium Oxides Concentrate. IOP Conf. Ser. Mater. Sci. Eng. 2016, 131, 012006. [Google Scholar] [CrossRef]
- de Souza, J.J.L.L.; Fontes, M.P.F.; Gilkes, R.; da Costa, L.M.; de Oliveira, T.S. Geochemical Signature of Amazon Tropical Rainforest Soils. Rev. Bras. Ciência Solo 2018, 42. [Google Scholar] [CrossRef]
- Cheje Machaca, D.M.; Juyo Salazar, R.B.; de Carvalho, T.C.; Romano Espinosa, D.C.; Soares Tenório, J.A. Recovery of niobium and tantalum from tin slags: An alternative approach using acid roasting and oxalic leaching. Miner. Eng. 2025, 232, 109564. [Google Scholar] [CrossRef]
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef]
- Ministério de Minas e Energia BN de M. Balanço Mineral Brasileiro 2001: Nióbio. Ministério de Minas e Energia 2001. Available online: https://www.gov.br/anm/pt-br/centrais-de-conteudo/anm/paginas/balanco-mineral/arquivos/balanco-mineral-brasileiro-2001-niobio (accessed on 1 August 2025).
- Lv, Z.; Cheng, H.; Wei, M.; Zhao, D.; Wu, D.; Liu, C. Mineralogical Characteristic and Beneficiation Evaluation of a Ta-Nb-Li-Rb Deposit. Minerals 2022, 12, 457. [Google Scholar] [CrossRef]
- Matinfar, M.; Nychka, J.A. A review of sodium silicate solutions: Structure, gelation, and syneresis. Adv. Colloid Interface Sci. 2023, 322, 103036. [Google Scholar] [CrossRef] [PubMed]



| Element | NBR 10004 Limit (µg/L) |
|---|---|
| Barium | 70,000.00 |
| Zinc | - |
| Selenium | 1000.00 |
| Manganese | - |
| Chromium | 5000.00 |
| Chromium | 1000.00 |
| Copper | - |
| Cadmium | 500.00 |
| Arsenic | 1000.00 |
| Industrial Segment | Application |
|---|---|
| Heavy construction | Asphalt |
| Heavy construction | Road bases |
| Heavy construction | Road drains |
| Civil construction | Portland cement |
| Civil construction | Embankments |
| Civil engineering | Mineral wool |
| Civil engineering | Railway ballast |
| Civil engineering | Yard covering |
| Steel industry | Furnace recycling |
| Steel | Iron ore |
| Agriculture | Agricultural corrective |
| Agriculture | Limestone substitute |
| Sanitation | River banks |
| Sanitation | Water and sewage treatment |
| Element | Composition (%) |
|---|---|
| Si | 18.2 ± 0.6 |
| Zr | 11 ± 2 |
| Ca | 9 ± 1 |
| Nb | 4.8 ± 0.8 |
| Fe | 3.3 ± 0.3 |
| Th | 2.7 ± 0.6 |
| Al | 2.6 ± 0.2 |
| Mg | 2.0 ± 0.2 |
| Sn | 1.5 ± 0.5 |
| Hf | 1.3 ± 0.6 |
| K | 1.2 ± 0.1 |
| Ta | 0.8 + 0.1 |
| Ti | 0.8 ± 0.2 |
| Na | 0.8 ± 0.2 |
| Zn | 0.4 ± 0.5 |
| Mn | 0.4 ± 0.2 |
| U | 0.3 ± 0 |
| Y | 0.10 ± 0.04 |
| Ba | 0.080 ± 0.004 |
| O (Balance) | 38.62 |
| Refrence | Zulhan and Ryanta, (2018) [16] | Permana et al. (2016) [20] | Clemente et al., (2024) [17] | Allain et al. (2019) [23] | Anes et al. (2024) [19] | Machaca et al. (2025) [22] | Present Work |
|---|---|---|---|---|---|---|---|
| Steps | 1 | 1 | Multiple | 2 | 1 | 1 | 1 |
| Origin | Indonesia | Indonesia | Brazil | Congo | Brazil | Brazil | Brazil |
| SnO2 (wt%) | 13.3 | NA | 0.5 | 0.7 | 2.5 | 2.6 | 1.9 |
| FeO (wt%) | 26.8 | 8.8 | 2 | 3.3 | 5.1 | 4.3 | 4.2 |
| SiO2 (wt%) | 15.7 | 34.3 | 46 | 41.9 | 42.8 | 35.5 | 38.9 |
| CaO (wt%) | 4.9 | 15.4 | 15.8 | 11.6 | 15.4 | 18.0 | 12.6 |
| ZrO2 (wt%) | 4.9 | 4.8 | 11.3 | 0.9 | 18.9 | 15.5 | 14.9 |
| Nb2O5 + Ta2O5 (wt%) | NA | 1 | 6.9 | 12.7 | 6.3 | 6.2 | 7.8 |
| Al2O3 (wt%) | 7.1 | 11.7 | 6.5 | 11.2 | 3.8 | 4.5 | 4.9 |
| TiO2 (wt%) | 7.3 | 11.9 | 1.4 | 1.3 | 1.7 | 1.5 | 1.3 |
| MgO (wt%) | 0.4 | NA | 1.4 | NA | 6.6 | 3.2 | 3.3 |
| MnO (wt%) | 0.4 | NA | NA | 3.7 | 0.8 | 0.6 | 0.5 |
| ThO2 (wt%) | 0.3 | NA | NA | NA | NA | 2.1 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garjulli, F.; Gonçalves, G.A.d.S.; Tenório, J.A.S.; Espinosa, D.C.R. Characterization of Brazilian Tin Slag and Evaluation of Its Potential as a Secondary Source of Nb and Ta. Minerals 2025, 15, 1126. https://doi.org/10.3390/min15111126
Garjulli F, Gonçalves GAdS, Tenório JAS, Espinosa DCR. Characterization of Brazilian Tin Slag and Evaluation of Its Potential as a Secondary Source of Nb and Ta. Minerals. 2025; 15(11):1126. https://doi.org/10.3390/min15111126
Chicago/Turabian StyleGarjulli, Franco, Gabriel Alves de Souza Gonçalves, Jorge Alberto Soares Tenório, and Denise Crocce Romano Espinosa. 2025. "Characterization of Brazilian Tin Slag and Evaluation of Its Potential as a Secondary Source of Nb and Ta" Minerals 15, no. 11: 1126. https://doi.org/10.3390/min15111126
APA StyleGarjulli, F., Gonçalves, G. A. d. S., Tenório, J. A. S., & Espinosa, D. C. R. (2025). Characterization of Brazilian Tin Slag and Evaluation of Its Potential as a Secondary Source of Nb and Ta. Minerals, 15(11), 1126. https://doi.org/10.3390/min15111126







