Artificial Aggregates from Metallurgical Waste as a Potential Source of Groundwater and Soil Contamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Chemical Composition
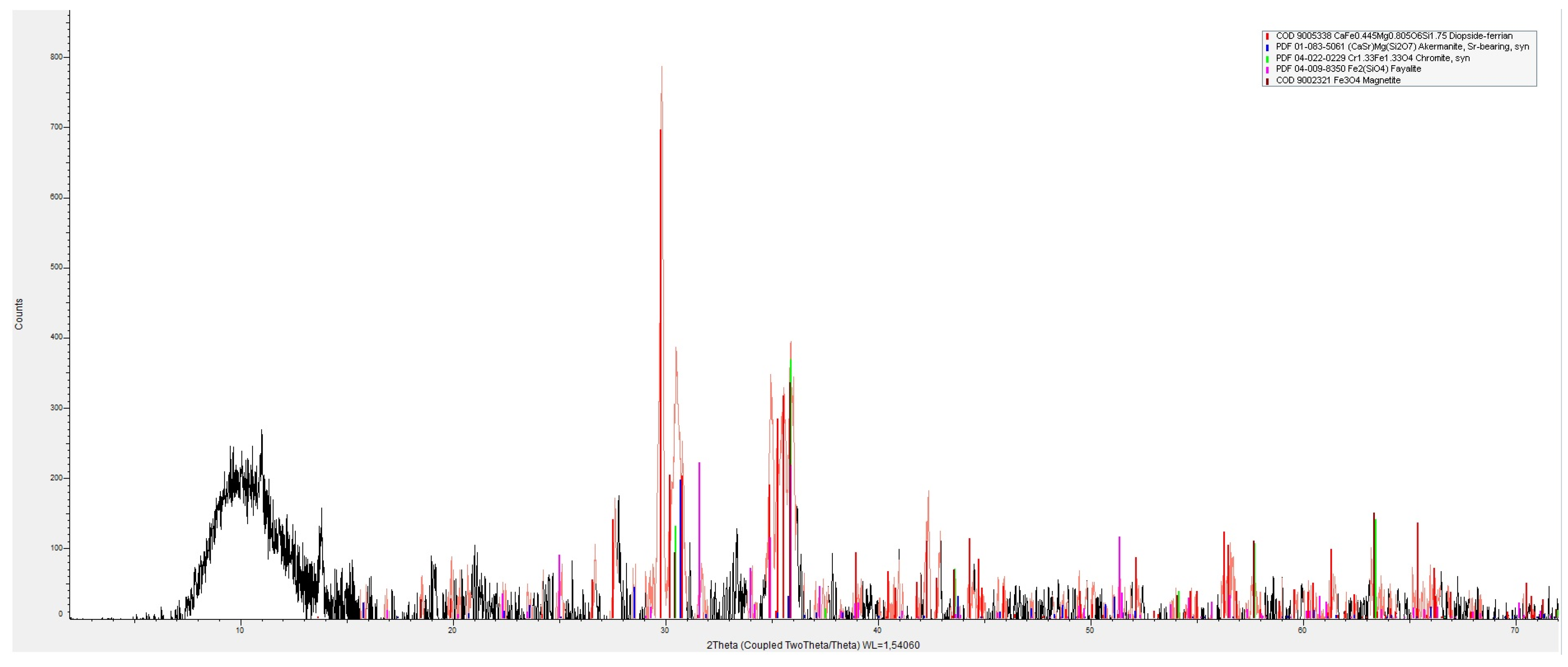
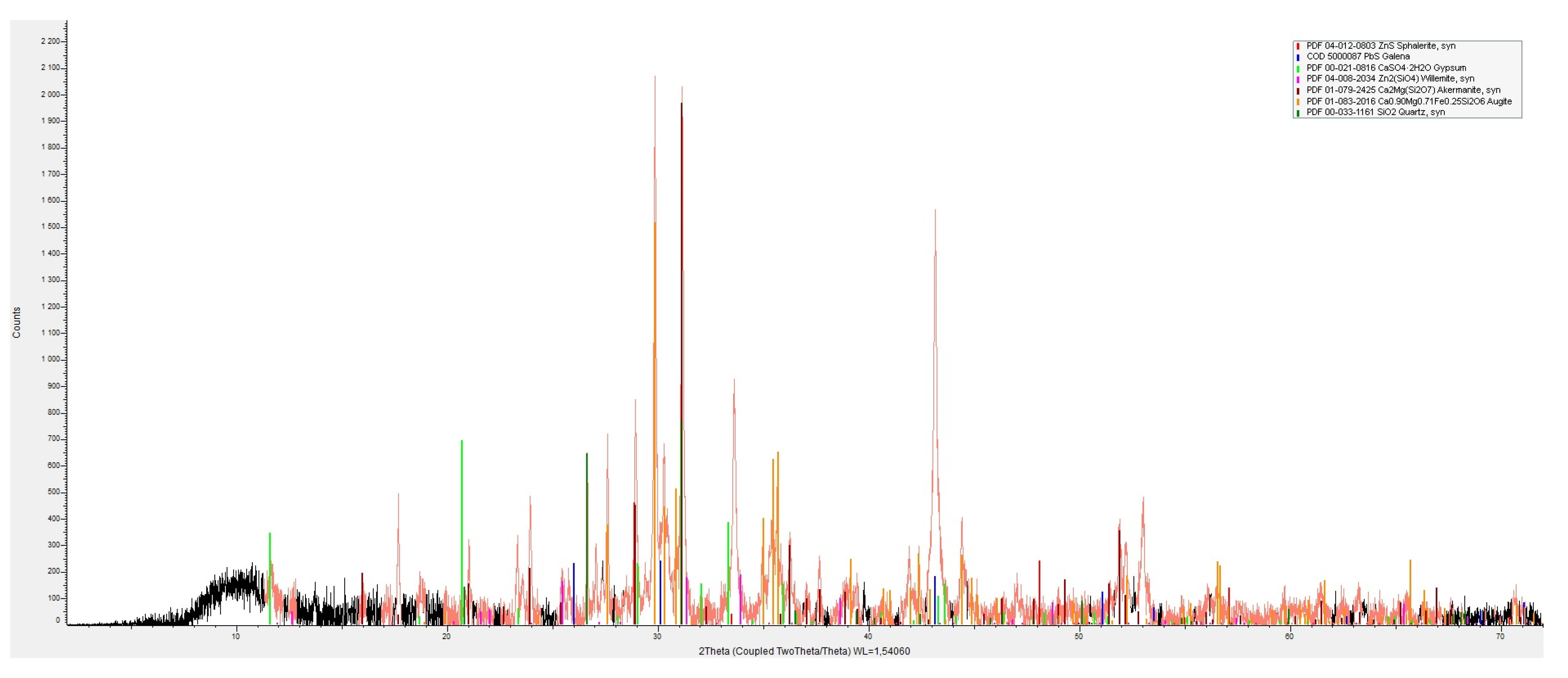
2.3. Determination of Phase Composition
2.4. Leaching Tests
- −
- pH using a CPC 502 pH/conductivity metre kit;
- −
- total dissolved solids using the conventional gravimetric method;
- −
- Ca2+ and Mg2+ ion content using conventional complexometry;
- −
- SO42−, Fe2+/Fe3+ and Mn2+ ion content using a SLANDI LF205 (LED) photometer (Slandi Sp. z o.o., Michałowice, Poland);
- −
- Al, Na, V, Cr, Mn, Ni, Pb, Zn, Sr, As, and Cu content using an ICP-AES JY 2000 spectrometer (Horiba Jobin Yvon, Paris, France).
2.5. Determination of the Content of Selected Elements
3. Results
3.1. Chemical and Phase Composition of Artificial Aggregates
3.1.1. Aggregate from Nickel Smelting Slag
3.1.2. Aggregate from Zinc Smelting Slag
3.1.3. Aggregate from Blast Furnace Slag
3.1.4. Aggregate from Steelmaking Slag
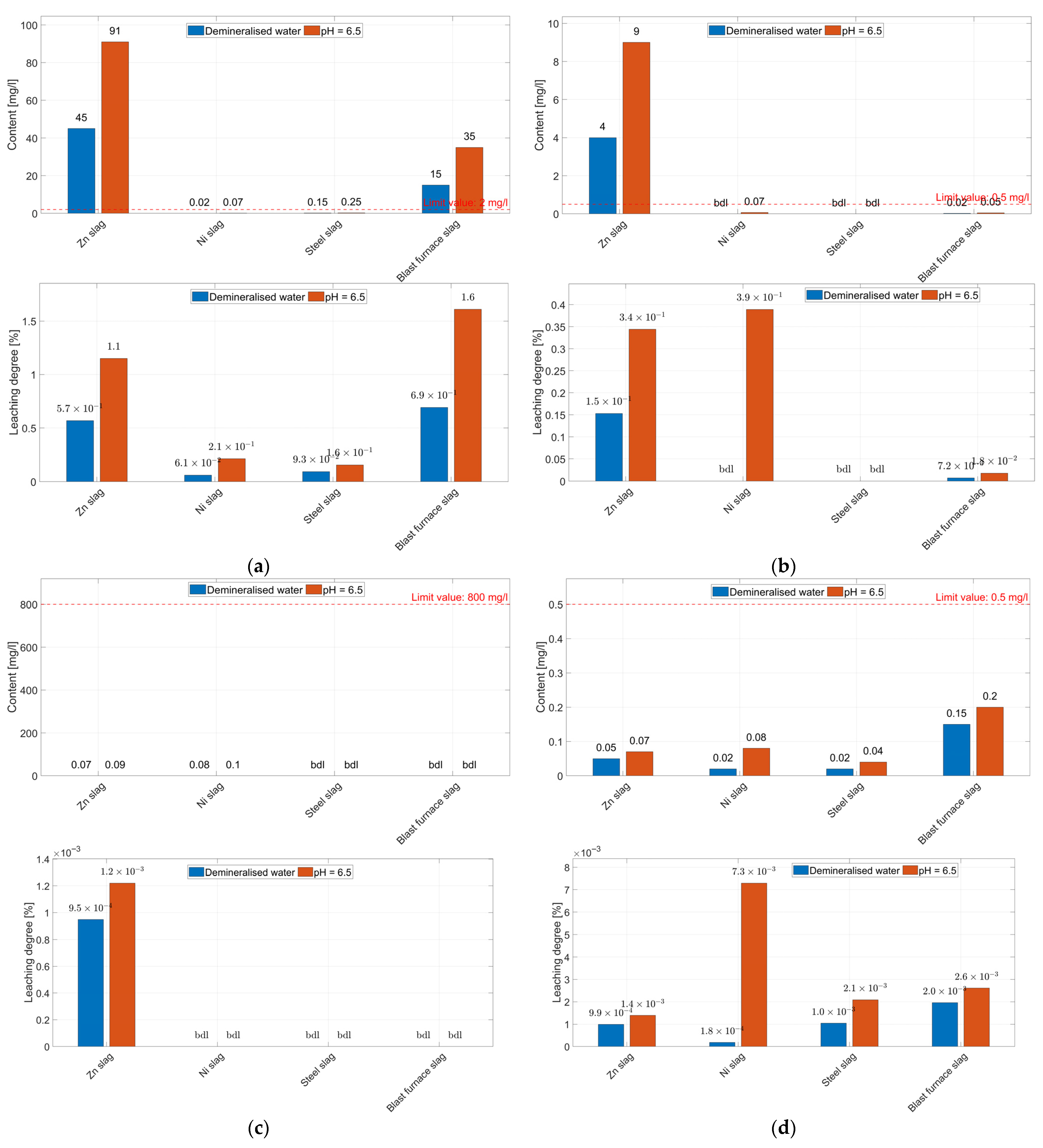
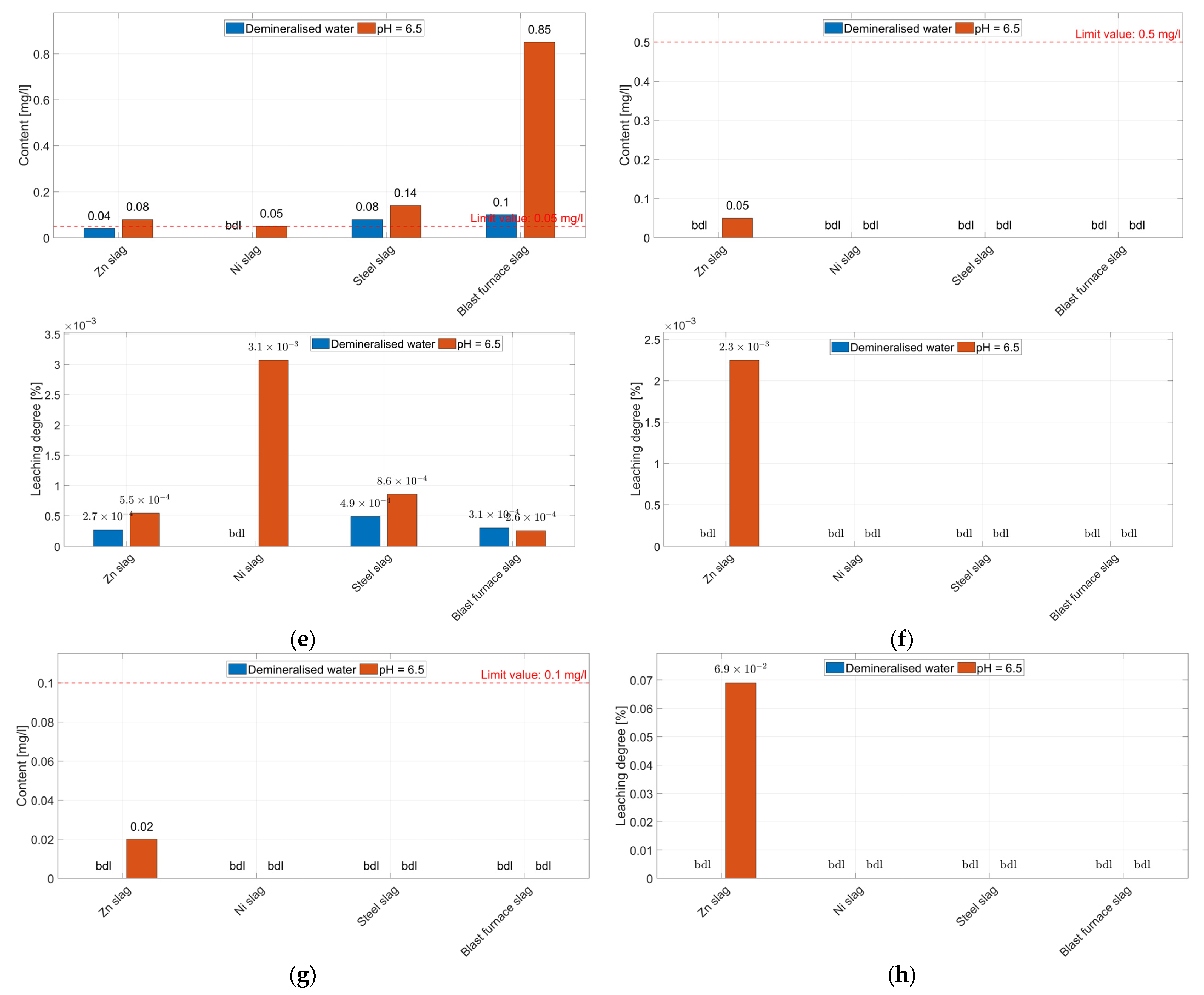
3.2. Leaching Tests
3.2.1. pH of Water Extracts
- −
- nickel smelting slag, pH = 6.55,
- −
- zinc smelting slag, pH = 5.7,
- −
- blast furnace slag, pH = 11.65,
- −
- steelmaking slag, pH = 13.06.
3.2.2. Total Dissolved Solids Content and Macroconstituent Content in Eluates
3.2.3. Degree of Leaching of Minor Elements from the Aggregates
4. Discussion
5. Conclusions
- The studied aggregates are characterised by differences in chemical and phase compositions, resulting from the type of slag from which they originate; however, the dominant chemical components of all the aggregates are SiO2 and Fe2O3.
- In the phase composition of aggregates, in terms of their origin, the following components were identified:
- −
- components derived from input material (ore or fluxes): sphalerite, galena, quartz (Zn and Pb slags); quartz (blast furnace slags, steel slags);
- −
- components formed during the metallurgical process: diopside, akermanite, fayalite, magnetite, chromite (Ni slags); willemite, åkermanite, augite, brownmillerite (Zn and Pb slags); larnite, monticellite, srebrodolskite, hematite, magnesioferrite (blast furnace slags); larnite, brownmillerite, hematite, wüstite (steel slags);
- −
- components formed through the action of hypergenic processes on waste after its disposal: gypsum (Zn and Pb slags); brucite, calcite (blast furnace slags); portlandite (steel slags).
- The leachability tests showed low leaching degrees of the studied elements (<1.65%), resulting from the phase composition of aggregates, which are dominated by low-solubility components. The leaching degrees are higher in acidic environments. Despite the low leaching degree, the concentrations of some of the elements in acid eluates, i.e., Mn (all aggregates), Zn and Pb (zinc slags and blast furnace slags), exceed the permissible values specified in the Ordinance on wastewater discharge, making these aggregates a potential threat to soil and water environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Research and Markets. Construction Aggregates—Global Strategic Business Report; Research and Markets: Dublin, Ireland, 2025. [Google Scholar]
- Global Construction Aggregates Industry Report. 2025. Available online: https://www.gminsights.com/industry-analysis/construction-aggregates-market (accessed on 15 August 2025).
- Worldwide Aggregates Market Research Report. 2025. Available online: https://www.marketreportsworld.com/market-reports/aggregates-market-14716374 (accessed on 15 August 2025).
- Pizoń, J.; Gołaszewski, J.; Alwaeli, M.; Szwan, P. Properties of Concrete with Recycled Concrete Aggregate Containing Metallurgical Sludge Waste. Materials 2020, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Paldyna, J.; Krasnodebska-Ostrega, B.; Kregielewska, K.; Kowalska, J.; Jedynak, L.; Golimowski, J.; Grobelski, T.; Farbiszewska-Kiczma, J.; Farbiszewska, T. The Assessment of Environmental Pollution Caused by Mining and Metallurgy Wastes from Highly Polluted Post-Industrial Regions in Southern Poland. Environ. Earth Sci. 2013, 68, 439–450. [Google Scholar] [CrossRef][Green Version]
- Vaněk, A.; Grösslová, Z.; Mihaljevič, M.; Ettler, V.; Trubač, J.; Chrastný, V.; Penížek, V.; Teper, L.; Cabala, J.; Voegelin, A. Thallium Isotopes in Metallurgical Wastes/Contaminated Soils: A Novel Tool to Trace Metal Source and Behavior. J. Hazard. Mater. 2018, 343, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Vijerathne, D.; Wahala, S.; Illankoon, C. Impact of Crushed Natural Aggregate on Environmental Footprint of the Construction Industry: Enhancing Sustainability in Aggregate Production. Buildings 2024, 14, 2770. [Google Scholar] [CrossRef]
- Pal, S.; Mandal, I. Impacts of Stone Mining and Crushing on Environmental Health in Dwarka River Basin. Geocarto Int. 2019, 36, 392–420. [Google Scholar] [CrossRef]
- Anees, M.; Khan, E.; Shah, W. Environmental Impact Assessment of a Typical Stone Crushing Plant. Int. Rev. Soc. Sci. 2023, 11, 11–18. [Google Scholar]
- Nowińska, K.; Grzesik, B.; Kokowska-Pawłowska, M.; Nowak, J. Use of Mineral Waste for the Production of Artificial Aggregates. Appl. Sci. 2024, 14, 11734. [Google Scholar] [CrossRef]
- Nowińska, K.; Kokowska-Pawłowska, M. Mineralogy of Zinc and Lead Metallurgical Slags in Terms of Their Impact on the Environment: A Review. Minerals 2024, 14, 852. [Google Scholar] [CrossRef]
- Wowkonowicz, P.; Bojanowicz-Bablok, A.; Gworek, B. Wykorzystanie Odpadów z Przemysłu Wydobywczego i Hutnictwa w Drogownictwie. Rocz. Ochr. Sr. 2018, 20, 1335–1349. [Google Scholar]
- Yao, Y.; Hong, B. Evolution of Recycled Concrete Research: A Data-Driven Scientometric Review. Low-Carbon Mater. Green Constr. 2024, 2, 16. [Google Scholar] [CrossRef]
- Skotniczny, G.; Kozioł, M.; Korol, J.; Poneta, P. Production and Evaluation of Synthetic Lightweight Aggregates Based on Mixture of Fluidized Bed Fly Ash and Post-Mining Residues. Materials 2022, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Bekkeri, G.B.; Shetty, K.K.; Nayak, G. Synthesis of Artificial Aggregates and Their Impact on Performance of Concrete: A Review. J. Mater. Cycles Waste Manag. 2023, 25, 1988–2011. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, W.; Zou, S.H.; Liu, H.W.; Zhao, W.; Chen, W. A Critical Review on Cold-Bonded Artificial Aggregate Developed from Solid Wastes: From Granulation Analysis to Performance Evaluation. J. Build. Eng. 2025, 99, 111588. [Google Scholar] [CrossRef]
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and Metallurgical Wastes: A Review of Recycling and Re-Use Practices. J. S. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Dhanalakshmi, K.; Alex, A.G.; Jose, P.A.; Kumar, R.D. Effect of Using Recycled E-Waste Plastic as Coarse Aggregate with Supplementary Nano-Fills in Concrete. Int. J. Concr. Struct. Mater. 2025, 19, 44. [Google Scholar] [CrossRef]
- Loureiro, C.D.A.; Moura, C.F.N.; Rodrigues, M.; Martinho, F.C.G.; Silva, H.M.R.D.; Oliveira, J.R.M. Steel Slag and Recycled Concrete Aggregates: Replacing Quarries to Supply Sustainable Materials for the Asphalt Paving Industry. Sustainability 2022, 14, 5022. [Google Scholar] [CrossRef]
- Olofinnade, O.; Morawo, A.; Okedairo, O.; Kim, B. Solid Waste Management in Developing Countries: Reusing of Steel Slag Aggregate in Eco-Friendly Interlocking Concrete Paving Blocks Production. Case Stud. Constr. Mater. 2021, 14, e00532. [Google Scholar] [CrossRef]
- Rani, K.; Senthil, K. WITHDRAWN: Performance of Plastic Aggregate Along with Steel Slag in the Concrete Through the Mechanical Tests and Microstructural Analysis. Preprint. Research Square. 2024; Version 1. Available online: https://www.researchsquare.com/article/rs-4354714/v1 (accessed on 30 September 2025).
- Zhang, X.; Gao, M.; Zhang, D.; Zhang, B.; Wang, M. Experimental Study on the Mechanical Properties of Metallurgical Slag Aggregate Concrete and Artificial Aggregate Concrete. Buildings 2024, 14, 2548. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O.; Mihaljevic, M. Mineralogy and Environmental Stability of Slags from the Tsumeb Smelter, Namibia. Appl. Geochem. 2009, 24, 1–15. [Google Scholar] [CrossRef]
- De Andrade Lima, L.R.P.; Bernardez, L.A. Characterization of the Lead Smelter Slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef]
- Nowińska, K.; Adamczyk, Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials 2023, 16, 7295. [Google Scholar] [CrossRef] [PubMed]
- Piatak, N.M. Environmental Characteristics and Utilization Potential of Metallurgical Slag. In Environmental Geochemistry; De Vivo, B., Belkin, H.E., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–519. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.A.; Aciu, C. Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability 2022, 14, 5488. [Google Scholar] [CrossRef]
- Đorđević, T.; Tasev, G.; Aicher, C.; Potysz, A.; Nagl, P.; Lengauer, C.L.; Pędziwiatr, A.; Serafimovski, T.; Boev, I.; Boev, B. Mineralogy and Environmental Stability of Metallurgical Slags from the Euronickel Smelter, Vozarci, North Macedonia. Appl. Geochem. 2024, 170, 106068. [Google Scholar] [CrossRef]
- Mahieux, P.Y.; Aubert, J.E.; Cyr, M.; Coutand, M.; Husson, B. Quantitative Mineralogical Composition of Complex Mineral Wastes—Contribution of the Rietveld Method. Waste Manag. 2010, 30, 378–388. [Google Scholar] [CrossRef]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R. Characteristics and Environmental Aspects of Slag: A Review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
- Kwiecień, S.; Grzesik, B.; Adamczyk, Z.; Nowak, J.; Grzyb, K.; Zając, J.; Drobiec, Ł. The Impact of Slag Swelling on Building Structures Demonstrated through Long-Term Measurement Analysis: A Case Study Lesson. Sci. Rep. 2025, 15, 33474. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Liu, J.; Guo, R. Applications of Steel Slag Powder and Steel Slag Aggregate in Ultra-High Performance Concrete. Adv. Civ. Eng. 2018, 2018, 1426037. [Google Scholar] [CrossRef]
- Nicolae, M.; Vîlciu, I.; Zăman, F. X-ray Diffraction Analysis of Steel Slag and Blast Furnace Slag Viewing Their Use for Road Construction. UPB Sci. Bull. 2007, 69, 99–108. [Google Scholar]
- Piatak, N.M.; Ettler, V.; Hoppe, D. Geochemistry and Mineralogy of Slags. In Metallurgical Slags: Environmental Geochemistry and Resource Potential, 1st ed.; Piatak, N.M., Ettler, V., Eds.; Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- PN-EN 12457-2:2002; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2. Polish Standardization Committee: Warsaw, Poland, 2002.
- Doschek-Held, K.; Krammer, A.C.; Steindl, F.R.; Gatschlhofer, C.; Raonic, Z.; Wohlmuth, D. Treatment of Metallurgical Residues by Chemical Modification, Reduction, and Phase Modification for Metal Recovery and Slag Utilization. Minerals 2025, 15, 408. [Google Scholar] [CrossRef]
- Kasina, M.; Michalik, M. Iron Metallurgy Slags as a Potential Source of Critical Elements—Nb, Ta and REE. Mineralogia 2016, 47, 15–28. [Google Scholar] [CrossRef]
- Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. [Google Scholar] [CrossRef]
- Nocoń, M.; Korus, I.; Łoska, K. Quantitative and Qualitative Analysis of Slags from Zinc and Lead Metallurgy. Arch. Environ. Prot. 2023, 49, 26–37. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Mineralogical Characteristics and Preliminary Beneficiation of Nickel Slag from Reduction Roasting–Ammonia Leaching. Minerals 2017, 7, 98. [Google Scholar] [CrossRef]
- Kierczak, J.; Néel, C.; Puziewicz, J.; Bril, H. The Mineralogy and Weathering of Slag Produced by the Smelting of Lateritic Ni Ores, Szklary. Can. Mineral. 2009, 47, 557–572. [Google Scholar] [CrossRef]
- Nang-Htay, Y.; Sivry, Y.; Guyot, F.; Lens, P.N.; van Hullebusch, E.D. Evaluation on Chemical Stability of Lead Blast Furnace (LBF) and Imperial Smelting Furnace (ISF) Slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef]
- Gomes, J.F.P.; Pinto, C.G. Leaching of Heavy Metals from Steelmaking Slags. Rev. Metal. 2006, 42, 409–416. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Nguyen, T.D.; Tran, T.V.; Nguyen, D.L.; Tran, H.S.; Nguyen, T.L.; Nguyen, T.H.; Nguyen, H.G.; Nguyen, T.P.; Nguyen, N.T.; et al. Steel Slag Quality Control for Road Construction Aggregates and Its Environmental Impact: Case Study of Vietnamese Steel Industry—Leaching of Heavy Metals from Steel-Making Slag. Environ. Sci. Pollut. Res. 2022, 29, 41983–41991. [Google Scholar] [CrossRef]
- Lim, B.; Alorro, R.D.; Aylmore, M.; Grimsey, D. Complexation Leaching of Critical and Strategic Metals from Nickel Converter Slag Using Organic Acids. Miner. Eng. 2023, 201, 108167. [Google Scholar] [CrossRef]
- He, X.; Shi, J.; Li, J.; Wang, J. Leaching Characteristics of Heavy Metals from Nickel Slag. Chin. J. Environ. Eng. 2014, 8, 3385–3389. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The Efficiency of Quartz Addition on Electric Arc Furnace (EAF) Carbon Steel Slag Stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Cabała, J.; Warchulski, R.; Rozmus, D.; Środek, D.; Szełęg, E. Pb-Rich Slags, Minerals, and Pollution Resulted from a Medieval Ag-Pb Smelting and Mining Operation in the Silesian-Cracovian Region (Southern Poland). Minerals 2020, 10, 28. [Google Scholar] [CrossRef]
- Riley, A.L.; Cameron, J.; Burke, I.T.; Onnis, P.; MacDonald, J.M.; Gandy, C.J.; Crane, R.A.; Byrne, P.; Comber, S.; Jarvis, A.P.; et al. Environmental Behaviour of Iron and Steel Slags in Coastal Settings. Environ. Sci. Pollut. Res. 2024, 31, 42428–42444. [Google Scholar] [CrossRef] [PubMed]
- Strandkvist, I.; Björkman, B.; Engström, F. Synthesis and Dissolution of Slag Minerals—A Study of β-Dicalcium Silicate, Pseudowollastonite and Monticellite. Can. Metall. Q. 2015, 54, 446–454. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z. 12 Years of Leaching of Contaminants from Pb Smelter Slags: Geochemical/Mineralogical Controls and Slag Recycling Potential. Appl. Geochem. 2014, 40, 97–103. [Google Scholar] [CrossRef]
- Ettler, V.; Komarkova, M.; Jechlicka, J.; Coufal, P.; Hradil, D.; Machovic, V.; Delorme, F. Leaching of Lead Metallurgical Slag in Citric Solutions—Implications for Disposal and Weathering in Soil Environments. Chemosphere 2004, 57, 567–577. [Google Scholar] [CrossRef]
- Perederiy, I.; Papangelakis, V.G. Why Amorphous FeO–SiO2 Slags Do Not Acid-Leach at High Temperatures. J. Hazard. Mater. 2017, 321, 737–744. [Google Scholar] [CrossRef]
- Eker, H.; Şahin, D.D.; Çullu, M. Effect of Reduced Fineness of Fly Ash Used on the Alkali–Silica Reaction (ASR) of Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2023, 47, 2203–2217. [Google Scholar] [CrossRef]
- Juckes, L.M. Dicalcium Silicate in Blast-Furnace Slag: A Critical Review of the Implications for Aggregate Stability. Miner. Process. Extr. Metall. 2002, 111, 120–128. [Google Scholar] [CrossRef]
- The Ordinance of the Minister of the Environment of 16 December 2014 on the Conditions to Be Met When Discharging Wastewater into Water or Ground, and on Substances Particularly Harmful to the Aquatic Environment. Journal of Laws 2014, Item 1800. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20140001800 (accessed on 19 August 2025).



| Constituent | Aggregate from Zinc Smelting Slag | Aggregate from Nickel Smelting Slag | Aggregate from Steelmaking Slag | Aggregate from Blast Furnace Slag |
|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | |
| SiO2 | 24.89 | 36.1 | 14.6 | 20.53 |
| TiO2 | 0.13 | 0.51 | 0.23 | 0.30 |
| Al2O3 | 3.68 | 11.03 | 1.84 | 4.57 |
| Fe2O3 | 24.89 | 31.09 | 32.13 | 18.66 |
| MnO | 1.89 | 0.21 | 2.11 | 4.20 |
| CaO | 15.93 | 12.03 | 36.72 | 27.06 |
| MgO | 3.88 | 5.11 | 3.40 | 14.00 |
| Na2O | 1.00 | bdl | bdl | bdl |
| K2O | 0.21 | 0.73 | 0.05 | 0.16 |
| P2O5 | 0.3 | bdl | 1.38 | 0.38 |
| SO3 | 20.91 | 0.9 | 0.14 | 0.69 |
| Cl | 0.03 | bdl | 0.01 | 0.05 |
| Cr2O3 | 0.74 | 1.6 | 0.28 | 1.12 |
| NiO | bdl | 0.67 | bdl | bdl |
| ZnO | 0.99 | bdl | 0.02 | 0.27 |
| CuO | 0.28 | bdl | bdl | bdl |
| PbO | 0.27 | bdl | bdl | 0.03 |
| V2O5 | bdl | bdl | 0.08 | 0.08 |
| SrO | bdl | bdl | 0.01 | 0.02 |
| ZrO2 | bdl | bdl | 0.01 | 0.02 |
| Nb2O5 | bdl | bdl | 0.02 | bdl |
| LOI | −2.97 | −2.47 | 6.90 | 7.86 |
| Aggregate Base Type | Constituent Content in Eluate [mg/dm3]/Leaching Degree [%] | Total Dissolved Solids [mg/dm3] | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | SO42− | Fe2+/3+ | |||||||
| Zinc smelting slag | 75.2 | 0.07 | 9.1 | 0.04 | 90.0 | 0.11 | 29.0 | 0.02 | 227 | 5.70 |
| Nickel smelting slag | 10.2 | 0.01 | 52.7 | 0.17 | <10 | bdl | <0.05 | - | 76 | 6.55 |
| Steelmaking slag | 545.1 | 0.2 | 2.4 | 0.02 | 11.4 | 0.8 | <0.05 | - | 1182 | 13.06 |
| Blast furnace slag | 76.2 | 0.04 | 2.4 | 0.004 | 115.0 | 1.4 | <0.05 | - | 307 | 11.65 |
| (a) | |||||||
| Aggregate Constituents | Eluate | Leaching Degree | |||||
| Component | Content [wt.%] | Element | Content [mg/dm3] | Demineralised Water [mg/dm3] | pH = 6.5 [mg/dm3] | Neutral Medium [%] | Acidic Medium [%] |
| Aggregate from zinc smelting slag | |||||||
| Al2O3 | 3.68 | Al | 19,494 | 0.07 | 0.1 | 3.59∙10−4 | 5.13∙10−4 |
| Na2O | 1.00 | Na | 7385 | 0.07 | 0.09 | 9.48∙10−4 | 1.22∙10−3 |
| Cr2O3 | 0.74 | Cr | 5041 | 0.05 | 0.07 | 9.92∙10−4 | 1.39∙10−3 |
| MnO | 1.89 | Mn | 14,648 | 0.04 | 0.08 | 2.73∙10−4 | 5.46∙10−4 |
| PbO | 0.27 | Pb | 2616 | 4 | 9.00 | 1.53∙10−1 | 3.44∙10−1 |
| ZnO | 0.99 | Zn | 7917 | 45 | 91.0 | 5.68∙10−1 | 1.15 |
| CuO | 0.28 | Cu | 2226 | bdl | 0.05 | bdl | 2.25∙10−3 |
| - | As | 29 | bdl | 0.02 | - | - | |
| Aggregate from nickel smelting slag | |||||||
| Al2O3 | 11.03 | Al | 58,381 | 0.08 | 0.1 | 1.37∙10−4 | 1.71∙10−4 |
| Na2O | bdl | Na | bdl | 0.08 | 0.1 | bdl | bdl |
| Cr2O3 | 1.60 | Cr | 10,978 | 0.02 | 0.08 | 1.82∙10−4 | 7.29∙10−3 |
| MnO | 0.21 | Mn | 1631 | bdl | 0.05 | bdl | 3.07∙10−3 |
| NiO | 0.67 | Ni | 5280 | 0.6 | 1.4 | 1.14∙10−2 | 2.65∙10−2 |
| PbO | - | Pb | 18 | bdl | 0.07 | bdl | 3.89∙10−1 |
| ZnO | - | Zn | 33 | 0.02 | 0.07 | 6.06∙10−2 | 2.12∙10−1 |
| (b) | |||||||
| Aggregate Constituents | Eluate | Leaching Degree | |||||
| Content [wt.%] | Element | Content [mg/dm3] | Demineralised Water [mg/dm3] | pH = 6.5 [mg/dm3] | Neutral Medium [%] | Acidic Medium [%] | |
| Aggregate from steelmaking slag | |||||||
| Al2O3 | 1.84 | Al | 9738 | 0.15 | 0.2 | 1.54∙10−3 | 2.05∙10−3 |
| Na2O | bdl | Na | bdl | bdl | bdl | bdl | bdl |
| Cr2O3 | 0.28 | Cr | 1916 | 0.02 | 0.04 | 1.04∙10−3 | 2.09∙10−3 |
| MnO | 2.11 | Mn | 16,341 | 0.08 | 0.14 | 4.90∙10−4 | 8.57∙10−4 |
| NiO | bdl | Ni | bdl | bdl | bdl | bdl | bdl |
| PbO | bdl | Pb | bdl | bdl | bdl | bdl | bdl |
| ZnO | 0.02 | Zn | 160.7 | 0.15 | 0.25 | 9.33∙10−2 | 1.56∙10−1 |
| CuO | bdl | Cu | bdl | bdl | bdl | bdl | bdl |
| SrO | 0.01 | Sr | 85 | bdl | 0.03 | bdl | 3.53∙10−2 |
| - | As | bdl | bdl | bdl | - | - | |
| Aggregate from blast furnace slag | |||||||
| Al2O3 | 4.57 | Al | 24,186 | 0.25 | 0.35 | 1.03∙10−3 | 1.45∙10−3 |
| Na2O | bdl | Na | bdl | bdl | bdl | bdl | bdl |
| Cr2O3 | 1.12 | Cr | 7663 | 0.15 | 0.2 | 1.96∙10−3 | 2.61∙10−3 |
| MnO | 4.20 | Mn | 32,527 | 0.1 | 0.85 | 3.07∙10−4 | 2.61∙10−4 |
| NiO | bdl | Ni | bdl | bdl | bdl | bdl | bdl |
| PbO | 0.03 | Pb | 278 | 0.02 | 0.05 | 7.19∙10−3 | 1.80∙10−2 |
| ZnO | 0.27 | Zn | 2169 | 15 | 35 | 6.92∙10−1 | 1.61 |
| CuO | bdl | Cu | bdl | bdl | bdl | bdl | bdl |
| SrO | 0.02 | Sr | 169 | bdl | 0.04 | bdl | 2.37∙10−2 |
| - | As | bdl | bdl | bdl | - | - | |
| Aggregate Type | Phase Group | Phase Constituent | Genetic Classification |
|---|---|---|---|
| Aggregate from nickel smelting slag | Silicates | Diopside CaMg(Si2O6) | II |
| Akermanite Ca2Mg(Si2O7) | II | ||
| Fayalite Fe2(SiO4) | II | ||
| Oxides | Magnetite Fe2+Fe3+ 2O4 | II | |
| Chromite FeCr2O4 | II | ||
| Aggregate from zinc smelting slag | Sulphides | Sphalerite ZnS | I |
| Galena PbS | I | ||
| Silicates | Willemite Zn2(SiO)4 | II | |
| Akermanite Ca2Mg(Si2O7) | II | ||
| Augite (Ca,Mg,Fe)2Si2O6 | II | ||
| Quartz SiO2 | I | ||
| Oxides | Brownmillerite Ca2(Al,Fe)2O5 | II | |
| Hydrated sulphates | Gypsum CaSO4·2H2O | III | |
| Aggregate from blast furnace slag | Silicates | Quartz SiO2 | I |
| Larnite Ca2SiO4 | II | ||
| Monticellite Ca(Mg,Fe)SiO4 | II | ||
| Oxides | Srebrodolskite Ca2Fe3+2O5 | II | |
| Hematite Fe2O3 | II | ||
| Magnesioferrite Mg(Fe3+)2O4 | II | ||
| Hydroxides | Brucite Mg(OH)2 | III | |
| Carbonates | Calcite CaCO3 | III | |
| Aggregate from steelmaking slag | Silicates | Quartz SiO2 | I |
| Larnite Ca2SiO4 | II | ||
| Oxides | Brownmillerite Ca2(Al,Fe)2O5 | II | |
| Hematite Fe2O3 | II | ||
| Wüstite FeO | II | ||
| Hydroxides | Portlandite Ca(OH)2 | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowińska, K.; Nowak, J.; Bartyzel, A.; Kokowska-Pawłowska, M.; Kuliński, K. Artificial Aggregates from Metallurgical Waste as a Potential Source of Groundwater and Soil Contamination. Minerals 2025, 15, 1082. https://doi.org/10.3390/min15101082
Nowińska K, Nowak J, Bartyzel A, Kokowska-Pawłowska M, Kuliński K. Artificial Aggregates from Metallurgical Waste as a Potential Source of Groundwater and Soil Contamination. Minerals. 2025; 15(10):1082. https://doi.org/10.3390/min15101082
Chicago/Turabian StyleNowińska, Katarzyna, Jacek Nowak, Aleksandra Bartyzel, Magdalena Kokowska-Pawłowska, and Krzysztof Kuliński. 2025. "Artificial Aggregates from Metallurgical Waste as a Potential Source of Groundwater and Soil Contamination" Minerals 15, no. 10: 1082. https://doi.org/10.3390/min15101082
APA StyleNowińska, K., Nowak, J., Bartyzel, A., Kokowska-Pawłowska, M., & Kuliński, K. (2025). Artificial Aggregates from Metallurgical Waste as a Potential Source of Groundwater and Soil Contamination. Minerals, 15(10), 1082. https://doi.org/10.3390/min15101082








