Hydrometallurgical Recovery of Critical Metal Indium from Scrap LCD Panels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Leaching
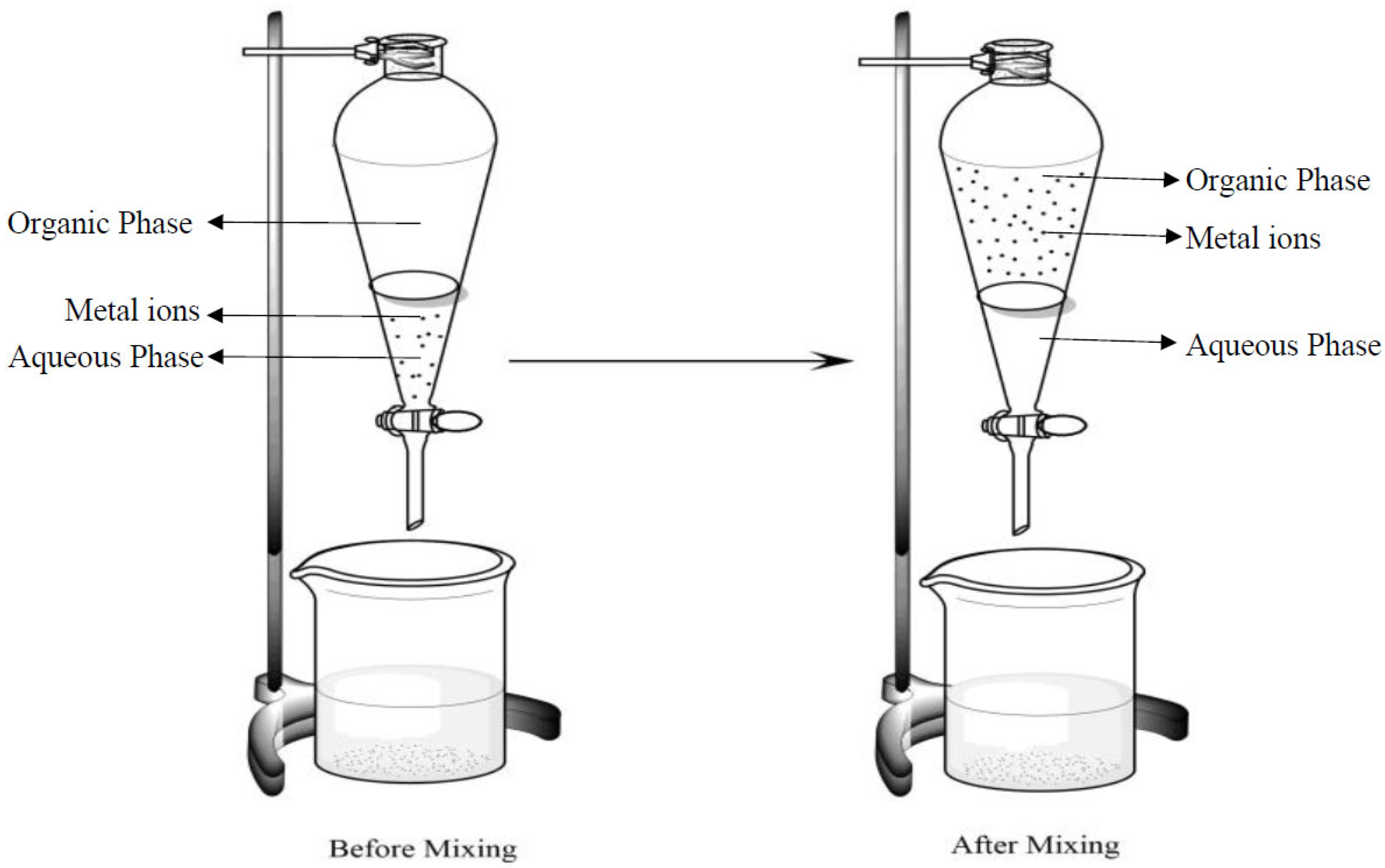
2.2.2. Solvent Extraction
3. Result and Discussion
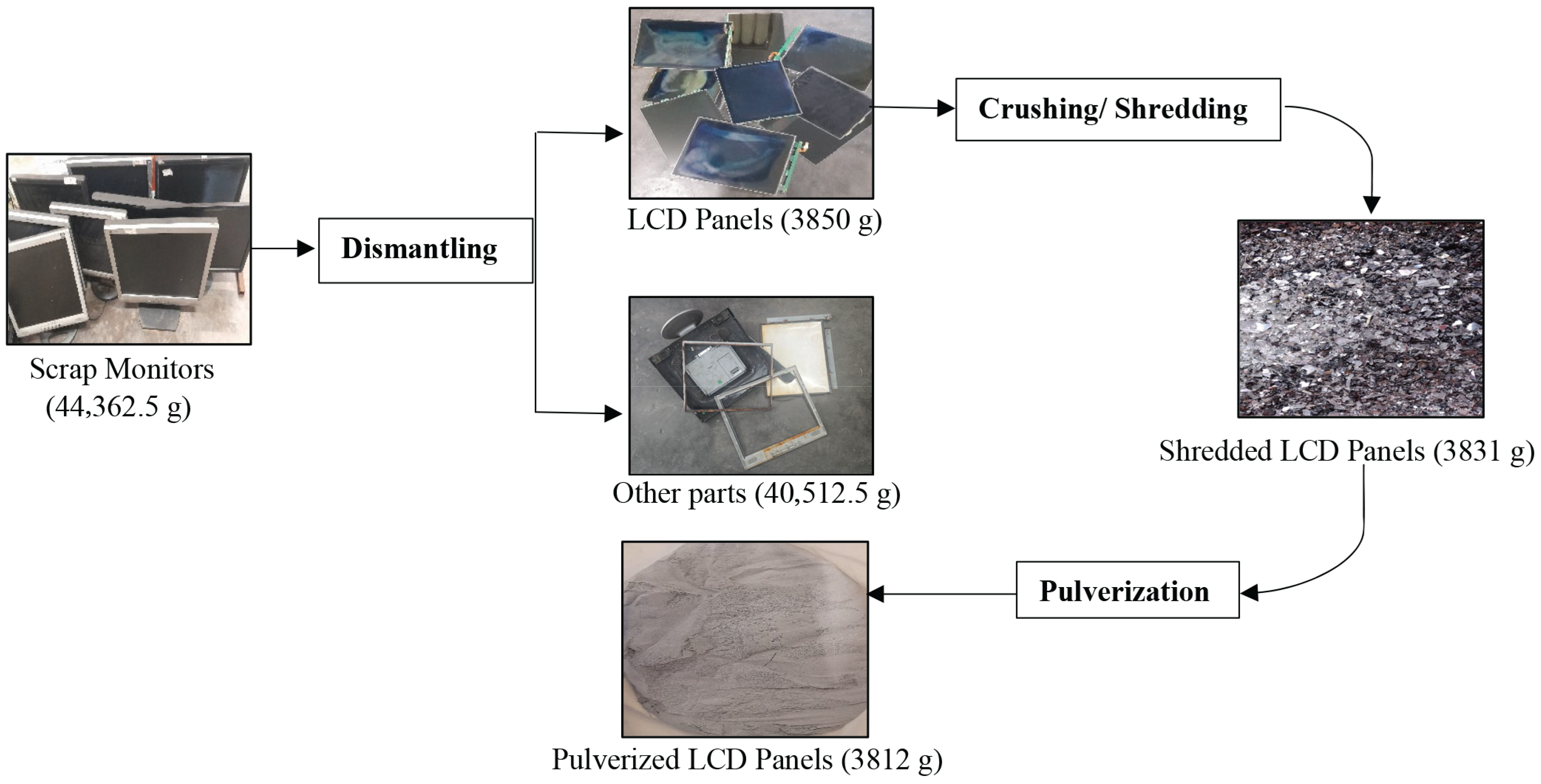
3.1. Pre-Treatment
3.2. Leaching Studies
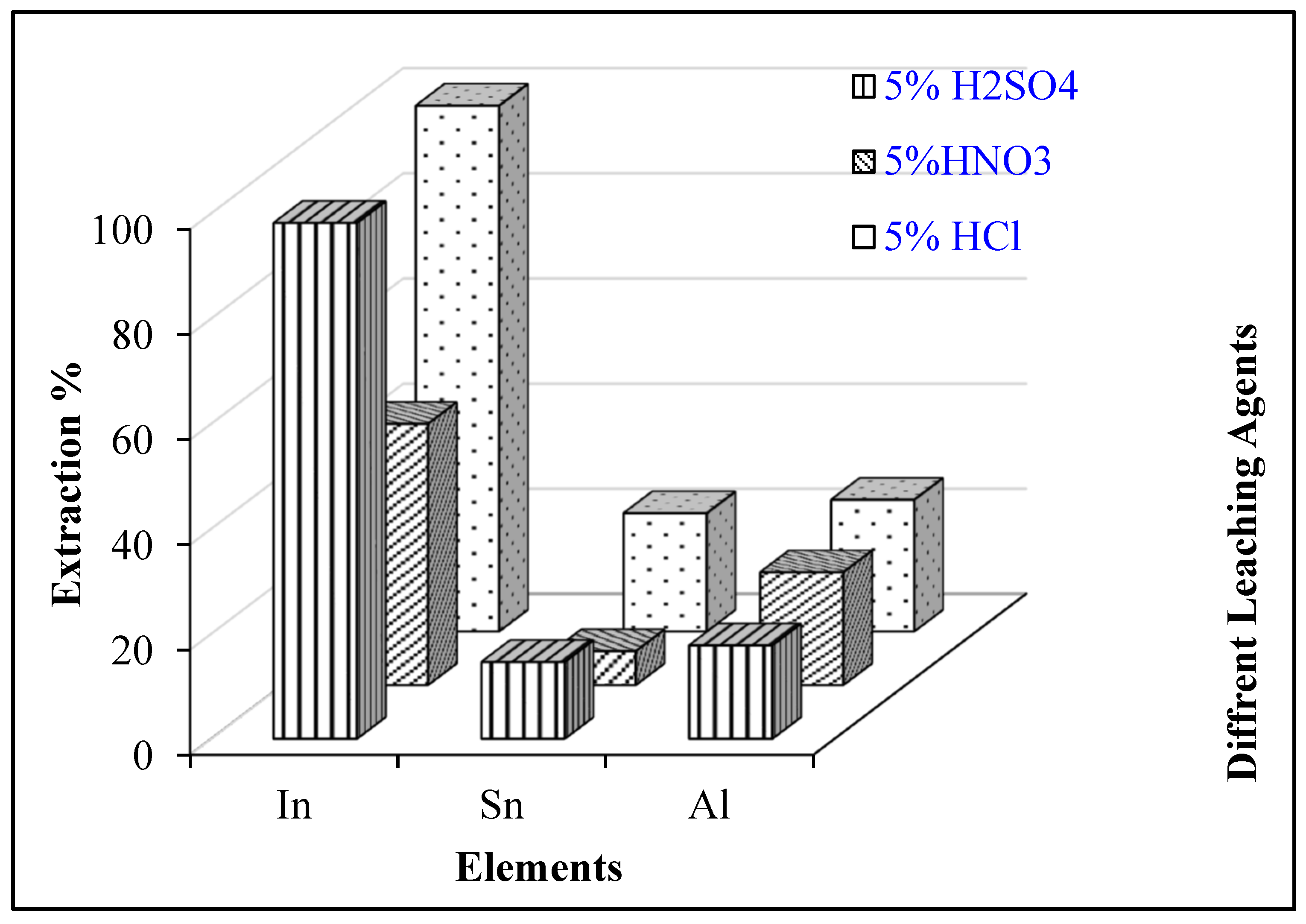
3.2.1. Selection of Leaching Agent
3.2.2. Effect of Acid Concentration
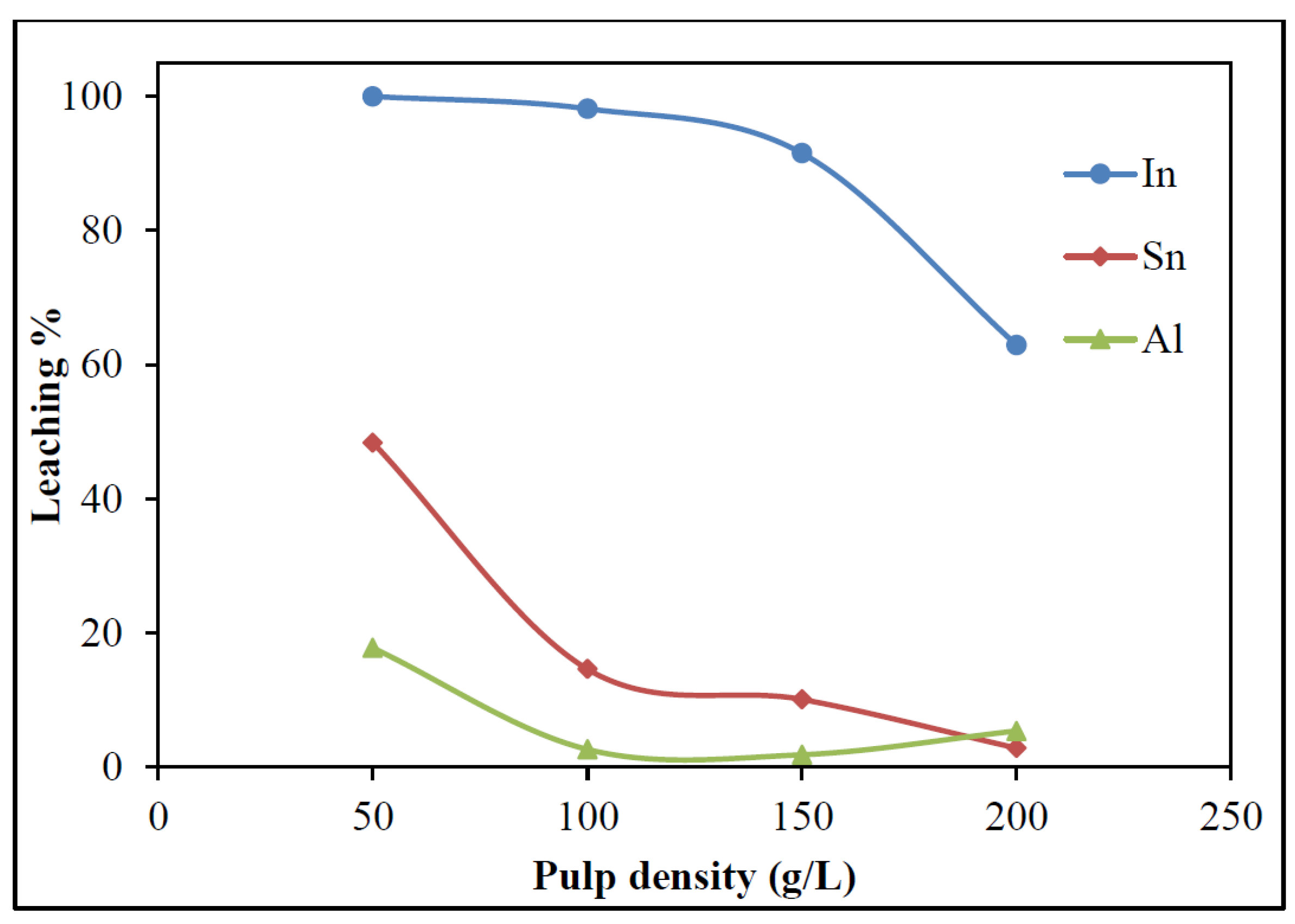
3.2.3. Effect of Pulp Density
3.2.4. Effect of Temperature
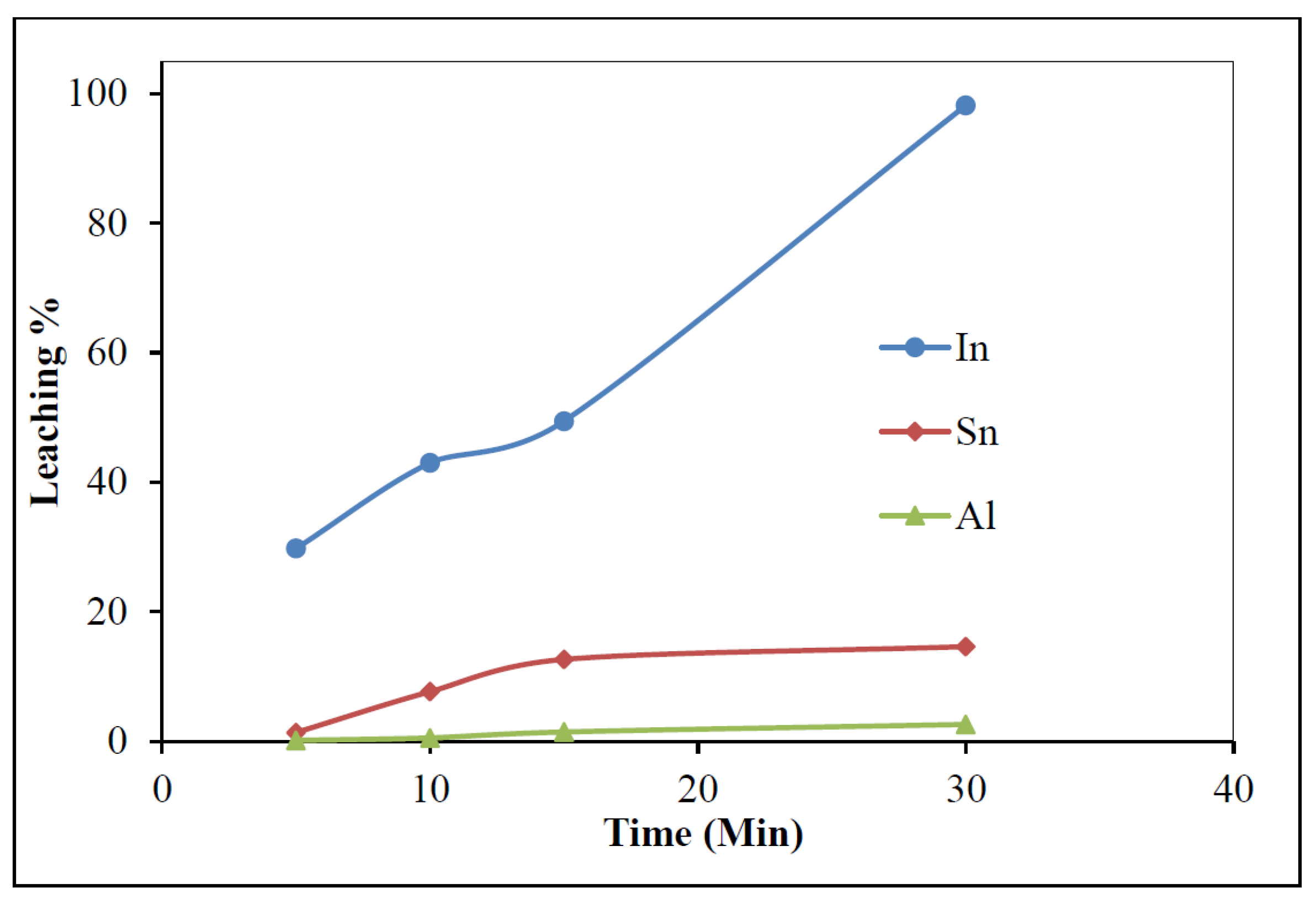
3.2.5. Effect of Time
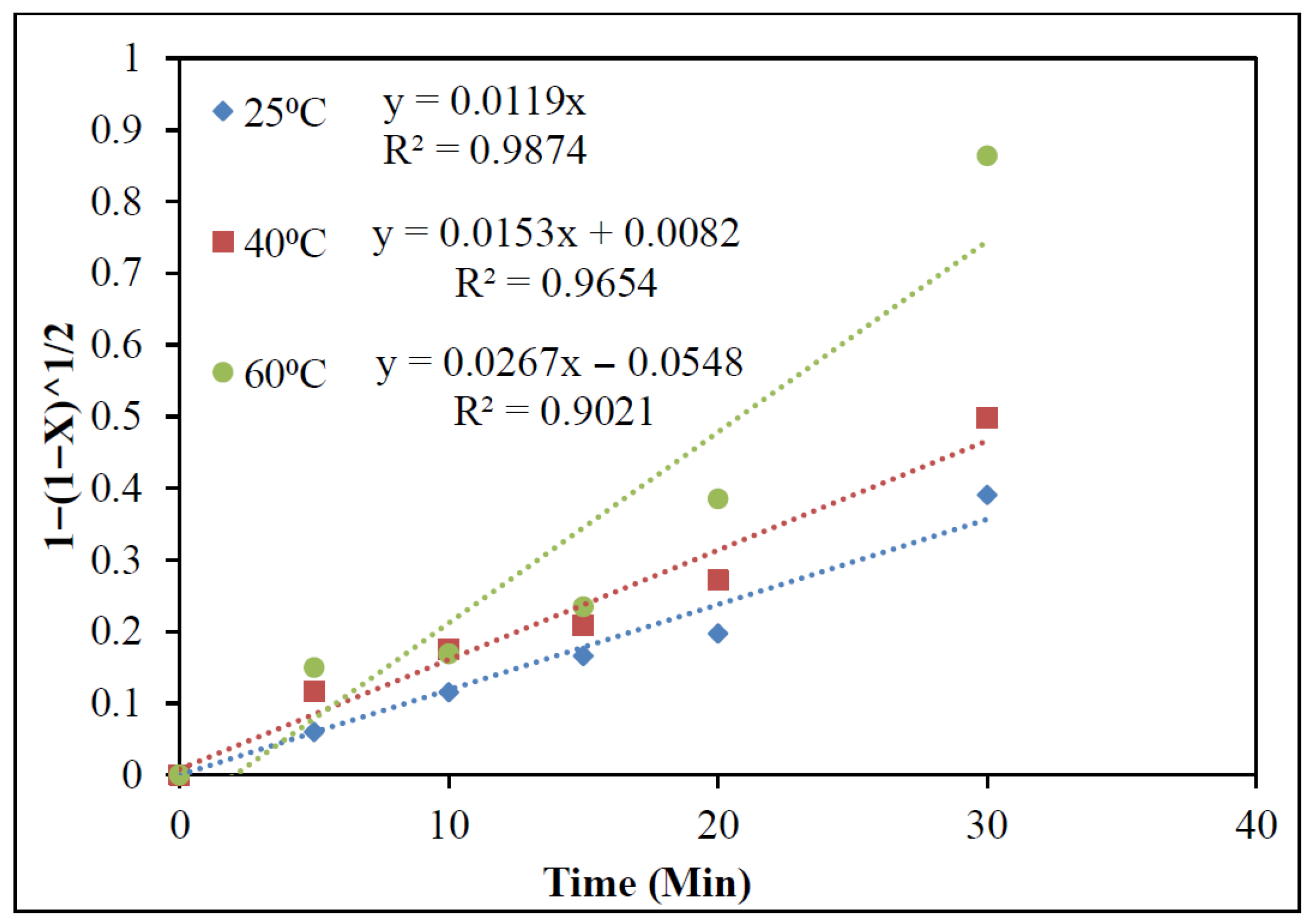
3.2.6. Kinetics Studies
- k = rate constant
- A = frequency factor (pre-exponential factor)
- Ea = activation energy (kJ/mol)
- R = gas constant (8.314 J/mol·K)
- T = absolute temperature (K)
3.3. Solvent Extraction Studies
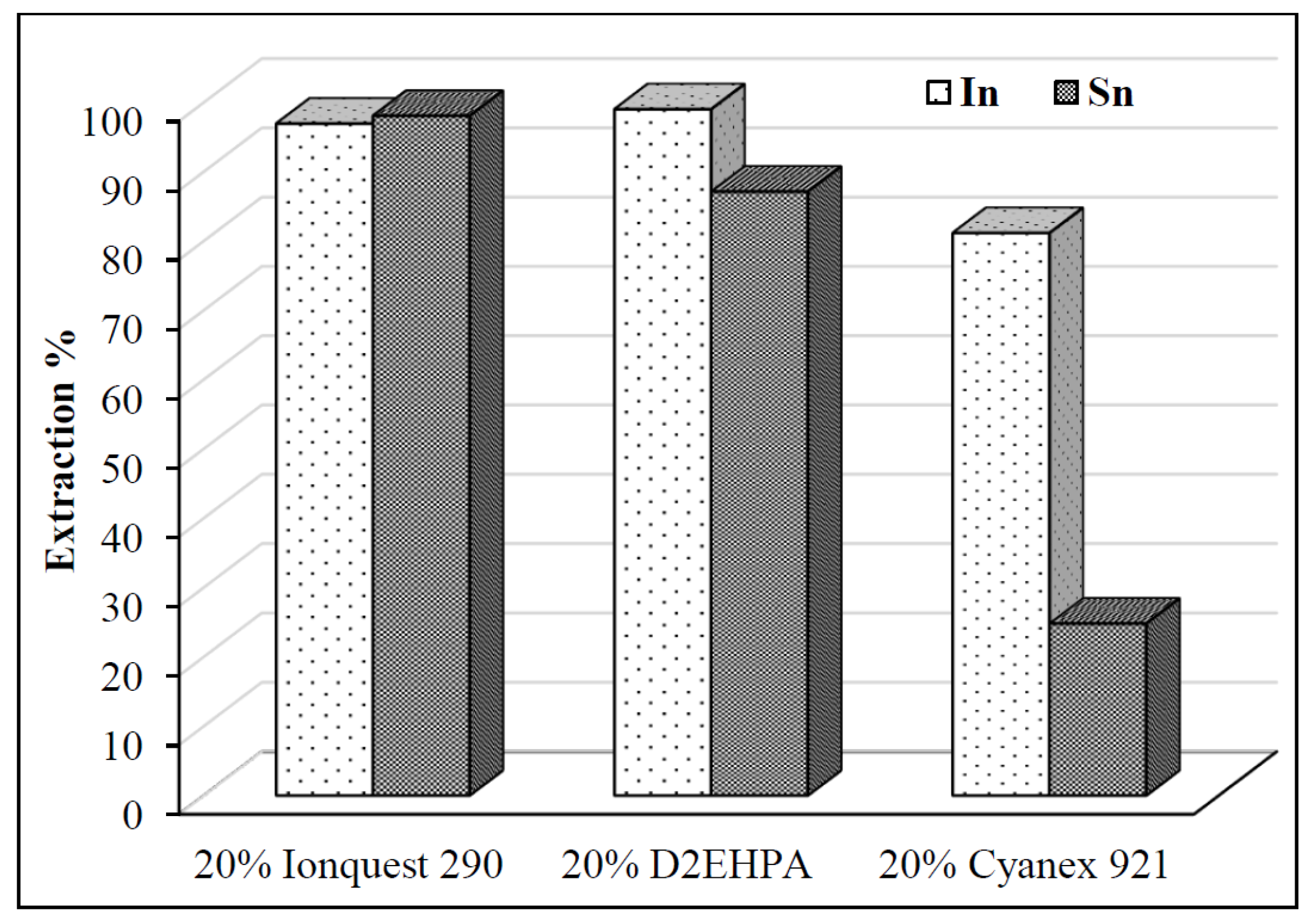
3.3.1. Selection of Extractant
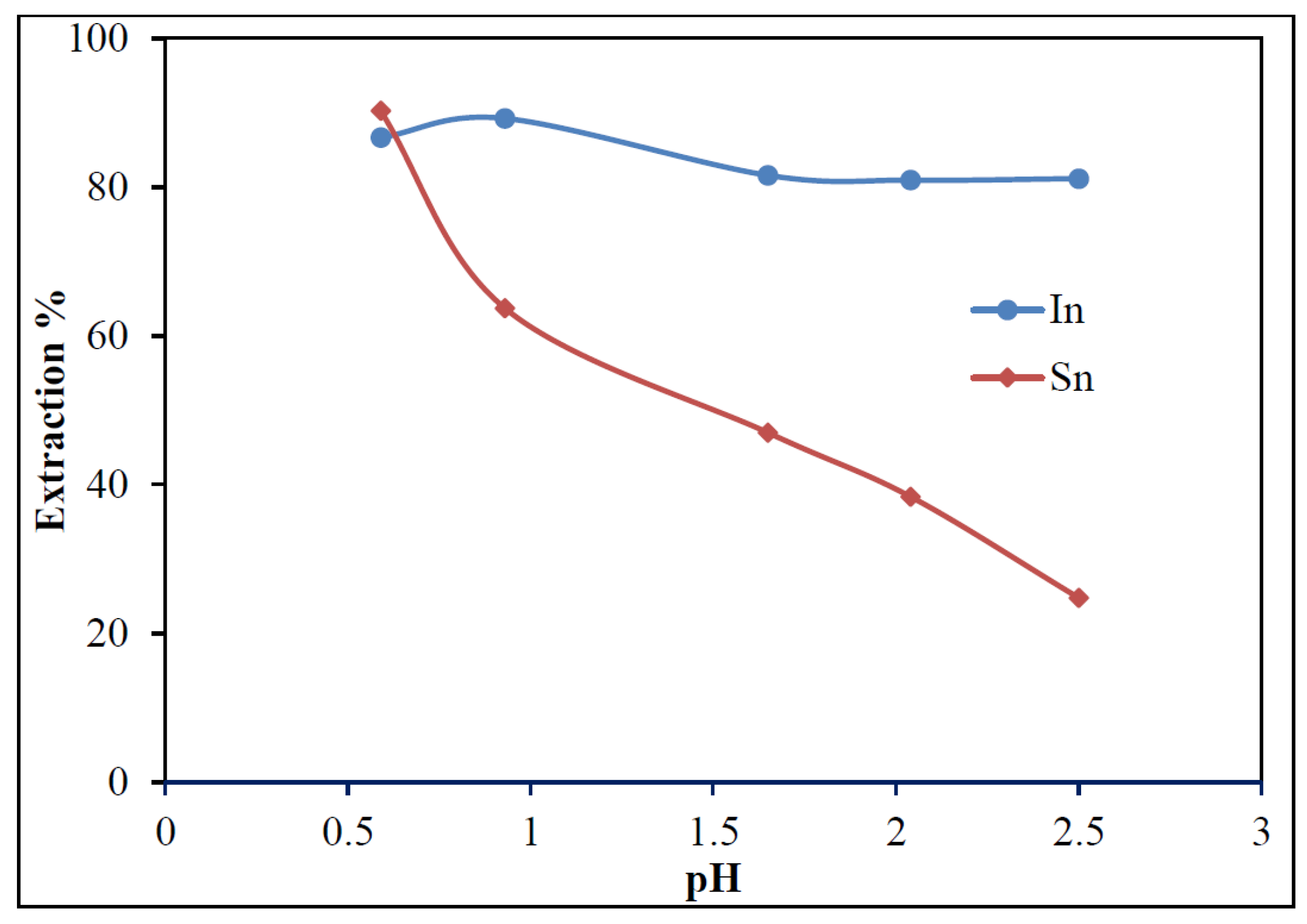
3.3.2. Effect of pH on Extraction
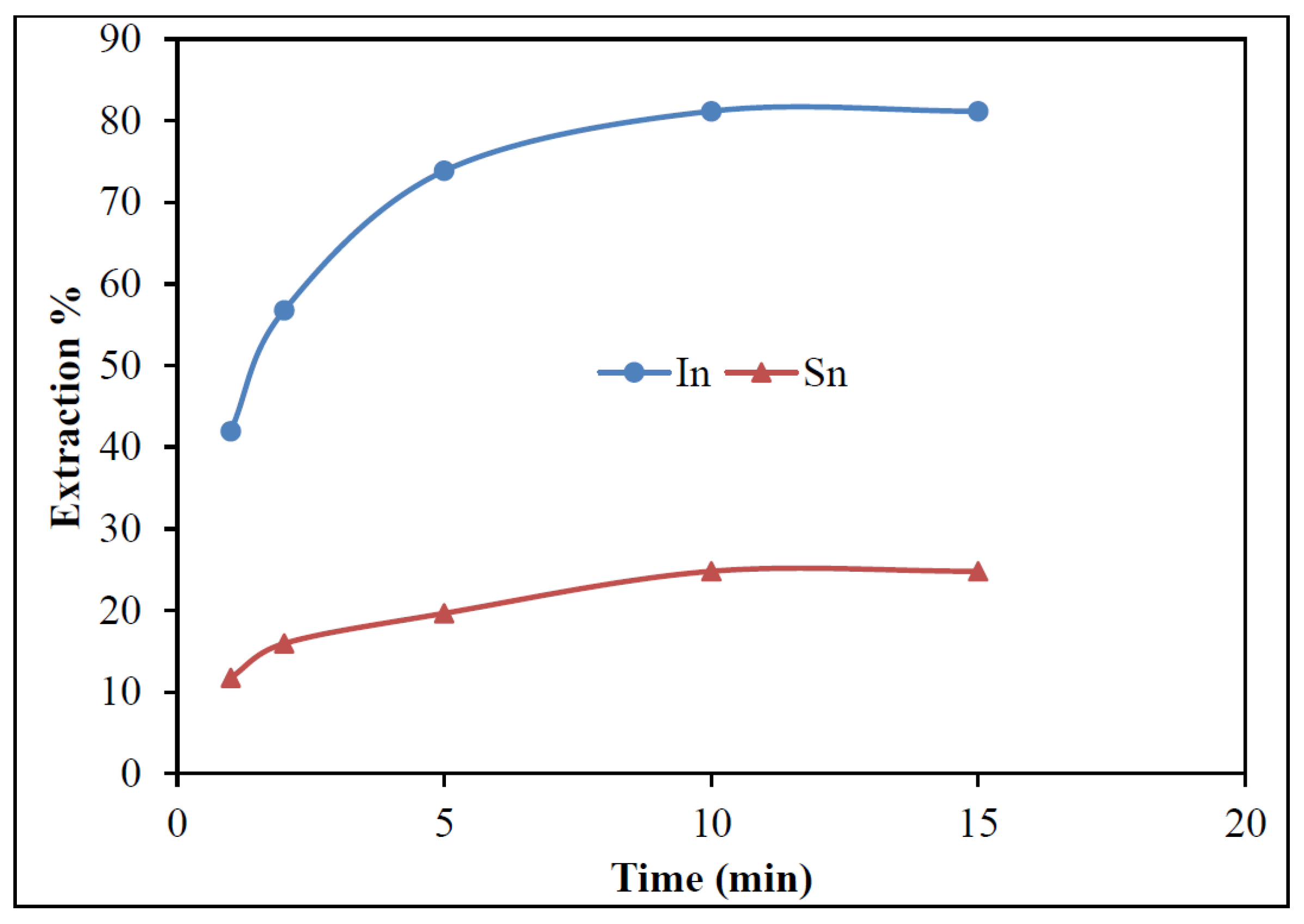
3.3.3. Effect of Time
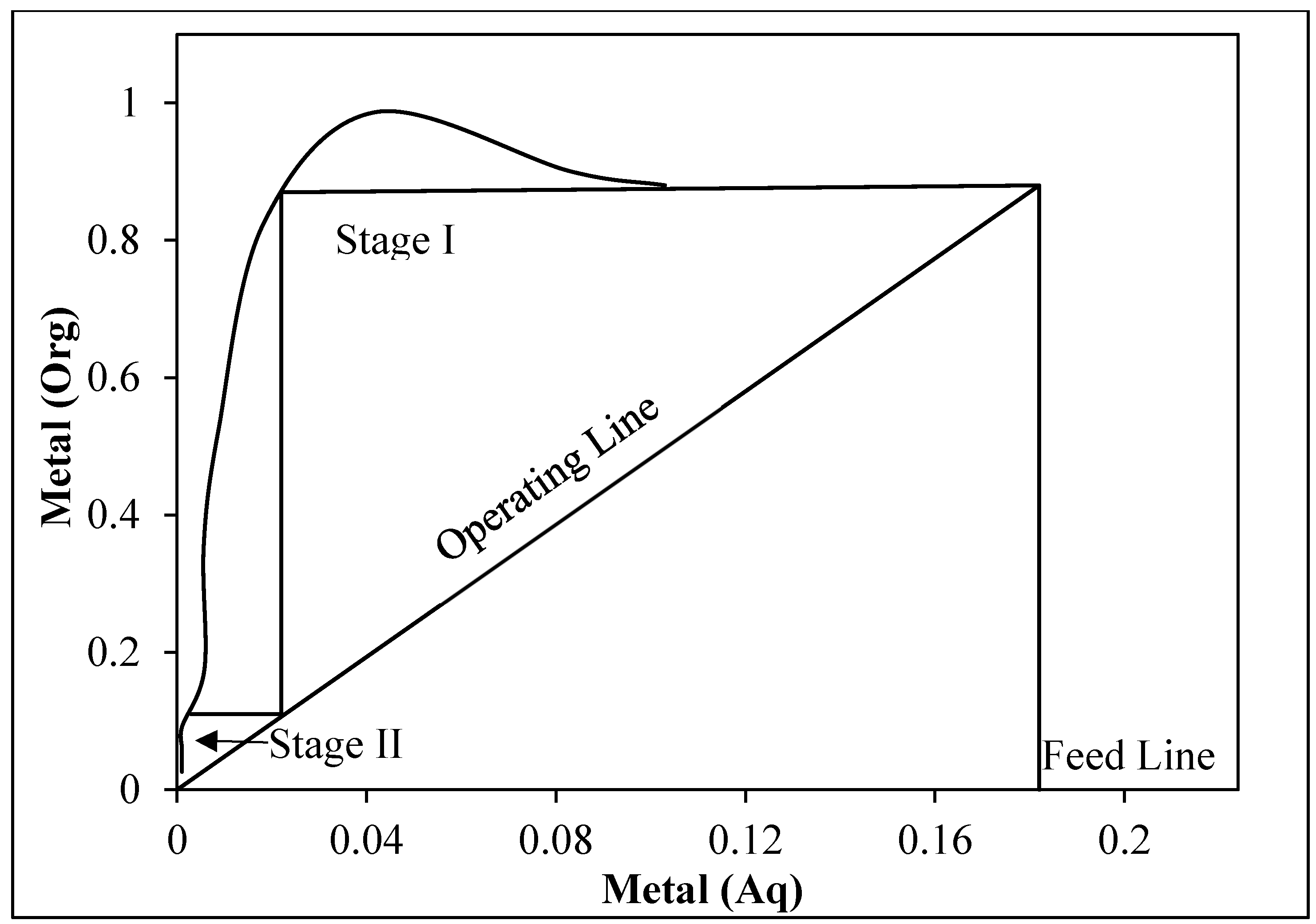
3.3.4. Effect of Phase Ratio and McCabe–Thiele Plot
3.3.5. Separation Factor
- β = Separation factor
- D(In) = Distribution co-efficient for indium
- D(Sn) = Distribution co-efficient for tin
- In(O) = In in organic phase
- In(A) = In in aqueous phase
- Sn(O) = Sn in organic phase
- Sn(A) = Sn in aqueous phase
| pH | In(O)/In(A) | Sn(O)/Sn(A) | Separation Factor |
|---|---|---|---|
| 0.59 | 6.5 | 9.27 | 0.7 |
| 0.93 | 8.28 | 1.75 | 4.71 |
| 1.65 | 4.43 | 0.88 | 4.98 |
| 2.04 | 4.24 | 0.56 | 7.55 |
| 2.5 | 4.30 | 0.33 | 13.01 |
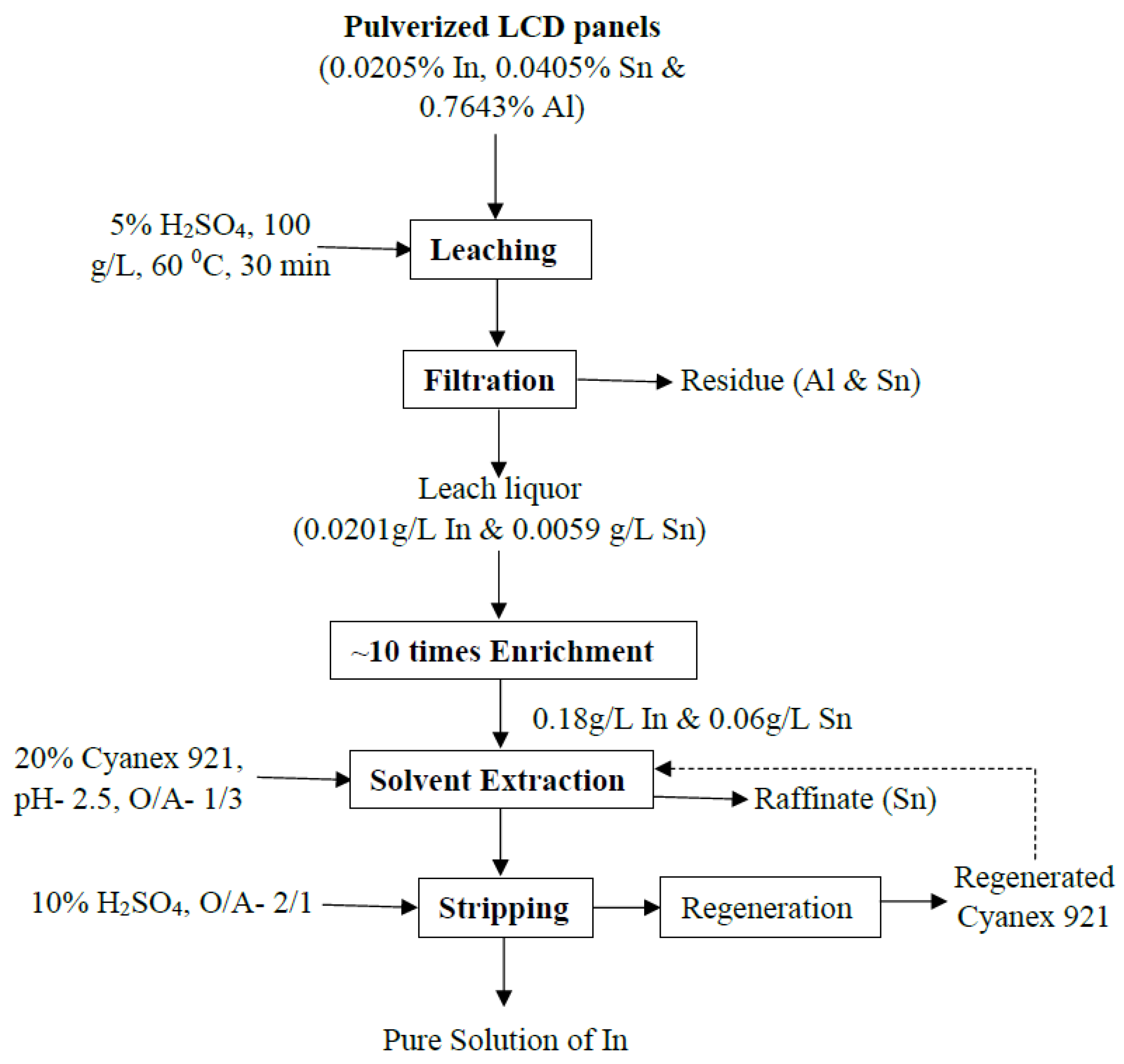
3.3.6. Developed Process Flowsheet for Indium Recovery
3.3.7. FT-IR of Loaded Organic
4. Conclusions
- Waste LCD panels were confirmed to be a promising secondary source of indium. Leaching studies established the dissolution of indium with 5% H2SO4, a 60 °C temperature, at a 100 g/L pulp density, and 30 min of mixing time, 98.1% In dissolution could be achieved.
- The mechanism of indium dissolution was clarified through kinetic studies. Analysis revealed that the process followed a diffusion-controlled kinetic model (1 − (1 − X)1/2 = kct) with an activation energy of 21.2 kJ mol−1.
- In downstream purification, solvent extraction proved to be most effective. Comparative tests with 20% D2EHPA, 20% Cyanex 921, and 20% Ionquest 290 showed that 20% Cyanex 921 provided the best results for In extraction. Optimum extraction occurred at pH 2.5 with an O/A ratio of 1/3, and equilibrium was reached within 15 min.
- McCabe–Thiele diagram demonstrated that two stages are required for complete In loading using 20% Cyanex 921. The separation factor was calculated to be β = 13.01.
- FT-IR studies further confirm the In extractant bonding at optimized pH.
- This approach demonstrates strong potential for industrial application. The process combines efficiency, selectivity, and scalability while relying on inexpensive reagents and elementary equipment. These advantages position the developed flow sheet as a practical solution for indium recovery from LCD waste, with clear economic and environmental benefits that align with sustainable resource management.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Critical Raw Materials Act: Proposal for a Regulation on Critical Raw Materials; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- U.S. Geological Survey. Draft List of Critical Minerals 2025. U.S. Department of the Interior. 2025. Available online: https://www.usgs.gov (accessed on 26 September 2025).
- World Bank. India imports of Gallium, Hafnium, Indium, Niobium, Rhenium, or Thall (HS 811299) by Partner Country [Data Set]. WITS/UN Comtrade. 2023. Available online: https://wits.worldbank.org/trade/comtrade/en/country/IND/year/2023/tradeflow/Imports/partner/ALL/product/811299 (accessed on 26 September 2025).
- Vučinić, A.A.; Šimunić, S.; Radetić, L.; Presečki, I. Indium Recycling from Waste Liquid Crystal Displays: Is It Possible? Processes 2023, 11, 1662. [Google Scholar] [CrossRef]
- Silveira, A.V.M.; Fuchs, M.S.; Pinheiro, D.K.; Tanabe, E.H.; Bertuol, D.A. Recovery of indium from LCD screens of discarded cell phones. Waste Manag. 2015, 45, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Willner, J.; Fornalczyk, A.; Gajda, B.; Saternus, M. Bioleaching of indium and tin from used LCD panels. Physicochem. Probl. Miner. Process. 2018, 54, 639–645. [Google Scholar] [CrossRef]
- Guan, J.; Wang, S.; Ren, H.; Guo, Y.; Yuan, H.; Yan, X.; Guo, J.; Gu, W.; Su, R.; Liang, B.; et al. Indium recovery from waste liquid crystal displays by polyvinyl chloride waste. RSC Adv. 2015, 5, 102836–102843. [Google Scholar] [CrossRef]
- Ma, E.; Xu, Z. Technological process and optimum design of organic materials vacuum pyrolysis and indium chlorinated separation from waste liquid crystal display panels. J. Hazard. Mater. 2013, 263 Pt 2, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Fortin-Lecomte, C.; Tran, L.-H.; Rioux, G.; Coudert, L.; Blais, J.-F. Recovery of indium from acidic leach solutions of spent LCD panels using ion exchange. Hydrometallurgy 2022, 210, 105845. [Google Scholar] [CrossRef]
- Lahti, J.; Vázquez, S.; Virolainen, S.; Mänttäri, M.; Kallioinen, M. Membrane filtration enhanced hydrometallurgical recovery process of indium from waste LCD panels. J. Sustain. Metall. 2020, 6, 576–588. [Google Scholar] [CrossRef]
- Souada, M.; Louage, C.; Doisy, J.-Y.; Meunier, L.; Benderrag, A.; Ouddane, B.; Bellayer, S.; Nuns, N.; Traisnel, M.; Maschke, U. Extraction of indium-tin oxide from end-of-life LCD panels using ultrasound assisted acid leaching. Ultrason. Sonochemistry 2018, 40 Pt A, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhu, N.; Li, Y.; Cui, J.; Wu, P.; Wang, J. Simultaneous leaching and extraction of indium from waste LCDs with acidic ionic liquids. Hydrometallurgy 2019, 189, 105146. [Google Scholar] [CrossRef]
- Moutiy, E.H.; Tran, L.-H.; Mueller, K.K.; Coudert, L.; Blais, J.-F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Waste Manag. 2020, 114, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tuck, D.G. Critical survey of stability constants of complexes of indium. Pure Appl. Chem. 1983, 55, 1477–1528. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction: Principles and Applications to Process Metallurgy (Vol. 2); Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Schuster, J.; Ebin, B. Investigation of indium and other valuable metals leaching from unground waste LCD screens by organic and inorganic acid leaching. Sep. Purif. Technol. 2021, 279, 119659. [Google Scholar] [CrossRef]
- Abd El Fatah, A.I.L.; Elashry, S.M. La (III) separation by tri octyl phosphine oxide (Cyanex 921) based on Amberlite Xad-4 chelating resin. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2793–2805. [Google Scholar] [CrossRef]
- Ferella, F.; Belardi, G.; Marsilii, A.; De Michelis, I.; Vegliò, F. Separation and recovery of glass, plastic and indium from spent LCD panels. Waste Manag. 2017, 60, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Zhang, B.; Zhou, S.; Wei, Y.; Li, B.; Wang, H. Efficient recovery of indium from waste indium tin oxide (ITO) targets by pressure leaching with sulfuric acid. React. Chem. Eng. 2023, 8, 2332–2341. [Google Scholar] [CrossRef]
- Virolainen, S.; Ibana, D.; Paatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometallurgy 2011, 107, 56–61. [Google Scholar] [CrossRef]
- Oliveira GFRde Botelho Junior, A.B.; Tenório, J.A.S. Separation of cobalt from the nickel-rich solution from HPAL process by synergism using organic extracts Cyanex 272 and Ionquest 290. Tecnol. Metal. Mater. Mineração 2019, 16, 478–486. [Google Scholar] [CrossRef]
- Sreevani, K.; Thangaraj, K.; Ramamurthi, K.; Selvanayagam, S.; Sridhar, B.; Anierudhe, V.V. Growth and characterization of dichloro tris(triphenyl phosphine oxide)cadmium(II)—Second harmonic generation from a centrosymmetric crystal. J. Miner. Mater. Charact. Eng. 2013, 1, 20–23. [Google Scholar] [CrossRef]





| Elements | Sn | In | Al | Balance |
|---|---|---|---|---|
| wt.% | 0.0405 | 0.0205 | 0.7643 | Silica |
| g/ton | 405 | 205 | 7643 | Silica |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, K.; Panda, R.; Sharma, A.; Meher, A.K.; Ambade, B.; Yoo, K.; Jha, M.K. Hydrometallurgical Recovery of Critical Metal Indium from Scrap LCD Panels. Minerals 2025, 15, 1084. https://doi.org/10.3390/min15101084
Rani K, Panda R, Sharma A, Meher AK, Ambade B, Yoo K, Jha MK. Hydrometallurgical Recovery of Critical Metal Indium from Scrap LCD Panels. Minerals. 2025; 15(10):1084. https://doi.org/10.3390/min15101084
Chicago/Turabian StyleRani, Karina, Rekha Panda, Ankur Sharma, Alok Kumar Meher, Balram Ambade, Kyoungkeun Yoo, and Manis Kumar Jha. 2025. "Hydrometallurgical Recovery of Critical Metal Indium from Scrap LCD Panels" Minerals 15, no. 10: 1084. https://doi.org/10.3390/min15101084
APA StyleRani, K., Panda, R., Sharma, A., Meher, A. K., Ambade, B., Yoo, K., & Jha, M. K. (2025). Hydrometallurgical Recovery of Critical Metal Indium from Scrap LCD Panels. Minerals, 15(10), 1084. https://doi.org/10.3390/min15101084









