Design of New Eco-Cementitious Material Based on Foundry Slag and Lime Sludge
Abstract
1. Introduction
| Component | Foundry Slag | Lime Sludge | ||||
|---|---|---|---|---|---|---|
| Cardoso et al. [4] | Ceccato et al. [5] | Devi et al. [14] | Singh et al. [8] | Maheswaran et al. [9] | Suthar, Aggarwa [15] | |
| CaO | 2.58 | 21.78 | 7.0 | 85.50 | 40.74 | 57.11 |
| SiO2 | 71.6 | 49.2 | 41.92 | 11.13 | 5.78 | 2.3 |
| MgO | 2.16 | 11.0 | 1.24 | 1.18 | - | 1.22 |
| MnO | 7.04 | 2.81 | 6.61 | - | - | - |
| Al2O3 | 11.0 | 10.68 | 20.55 | 0.24 | 0.18 | 0.21 |
| Na2O | 0.62 | - | - | 0.99 | - | 0.84 |
| K2O | 0.5 | - | 0.53 | 0.20 | 2.03 | 0.16 |
| P2O5 | - | 0.02 | - | 0.31 | 0.19 | 0.41 |
| ZrO2 | 0.27 | - | - | - | - | - |
| Fe2O3 | 3.39 | - | 18.92 | 0.18 | 0.15 | 0.28 |
| FeO | - | 2.97 | - | - | - | - |
| Cl | 0.11 | - | - | - | - | - |
| S | - | 0.64 | - | - | - | - |
| V2O5 | - | 0.67 | - | - | - | - |
| TiO2 | - | 0.67 | 1.49 | - | - | - |
| Cr2O3 | - | 0.05 | 0.53 | - | - | - |
| CuO | - | - | - | 0.02 | - | - |
| SO3 | - | - | - | 0.17 | 0.28 | 0.64 |
| Loss on ignition | - | - | - | - | 50.04 | 36.67 |
| CaO/SiO2 | 0.03 | 0.44 | 0.17 | 7.68 | 7.04 | 24.83 |
2. Materials and Methods
2.1. Raw Materials and Characterization
2.2. Manufacture of the Eco-Cementitious Material
2.3. Characterization of the Eco-Cementitious Material
2.4. Cement Preparation
2.5. Statistical Analysis
2.6. Test of Mechanical Strength
2.7. Superficial Area
2.8. Initial Setting Time
2.9. Hydration Heat Test
3. Results and Discussion
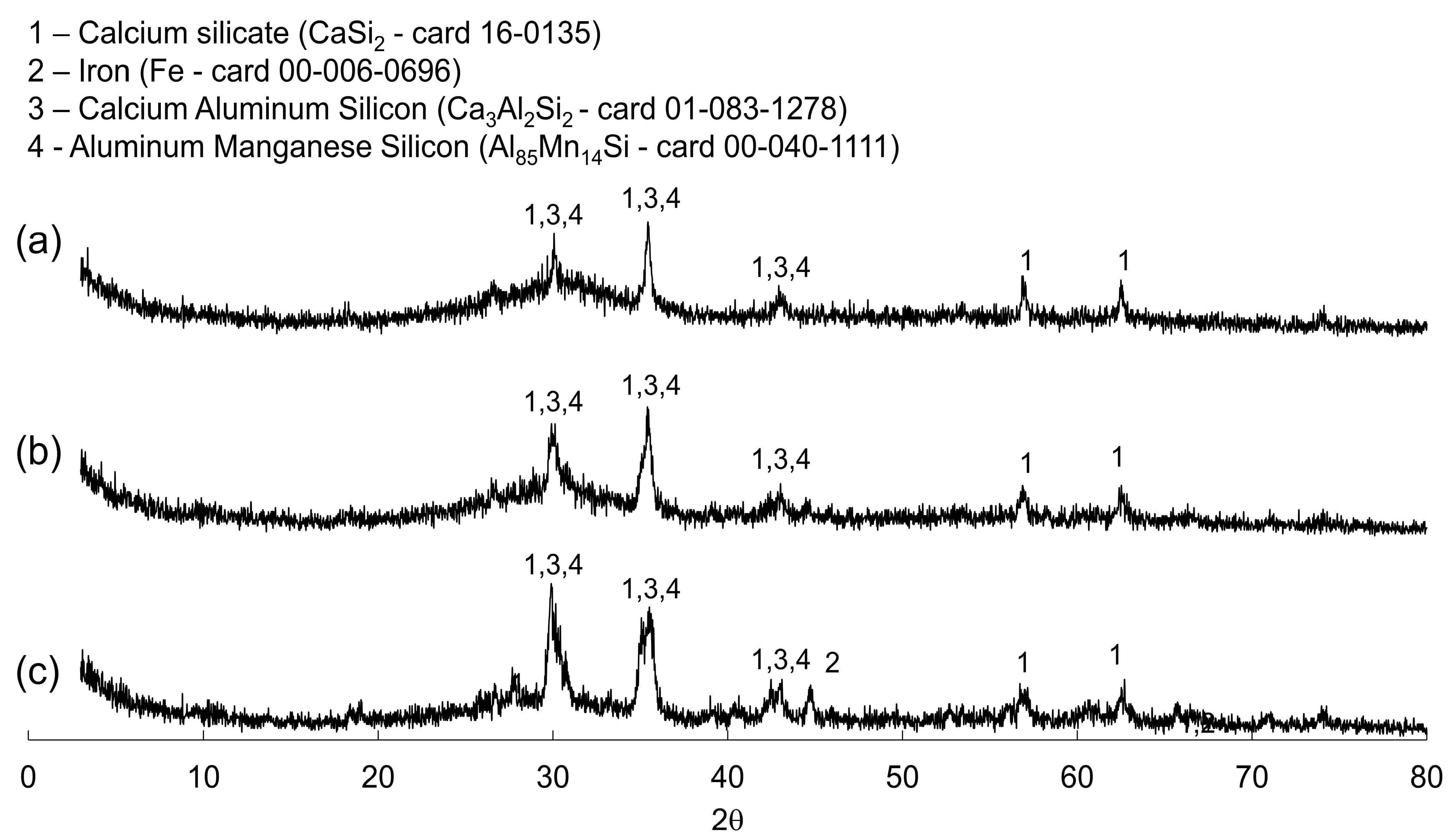
3.1. Wastes Characterization
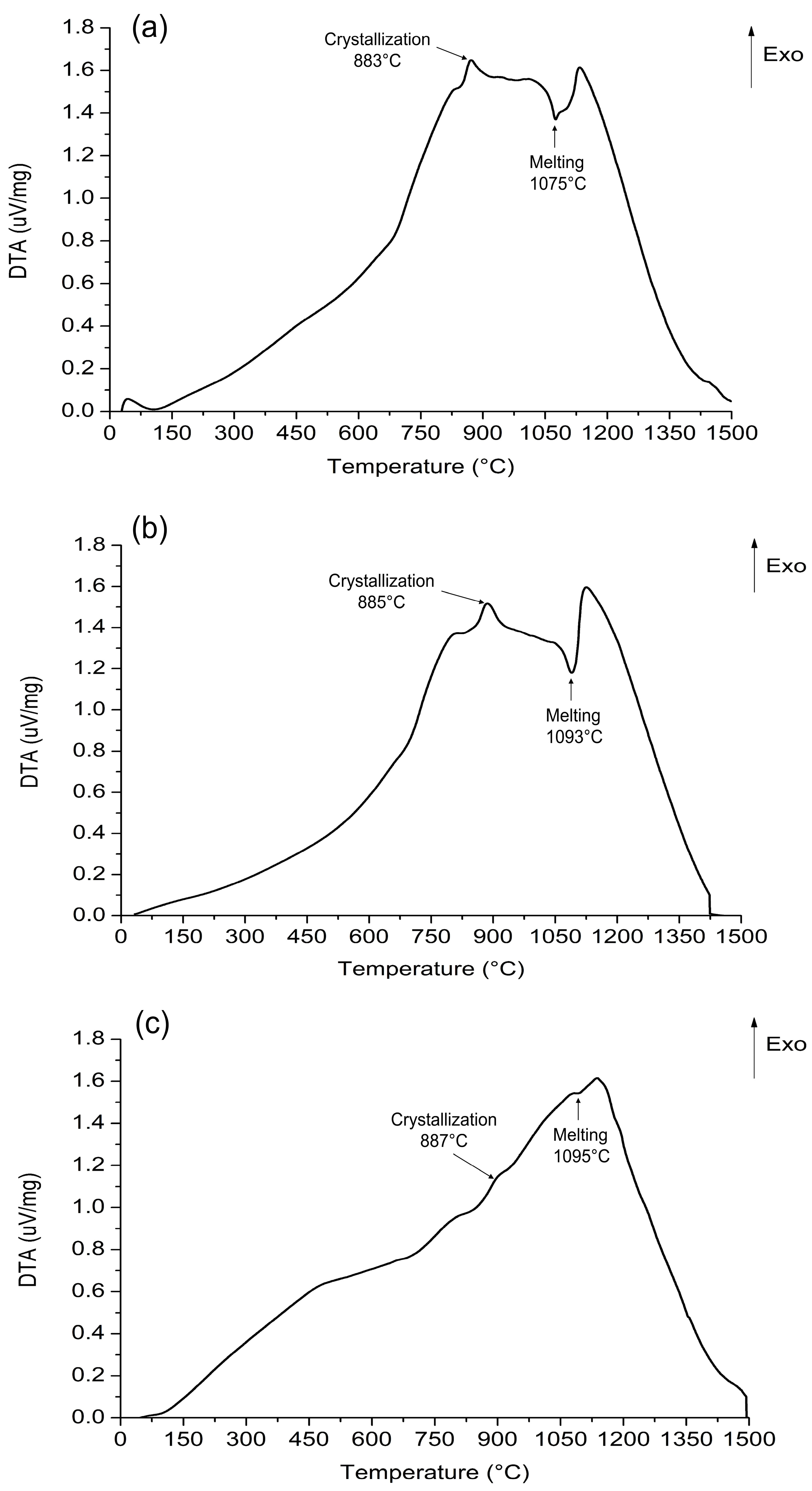
3.2. Characterization of the Eco-Cementitious Material
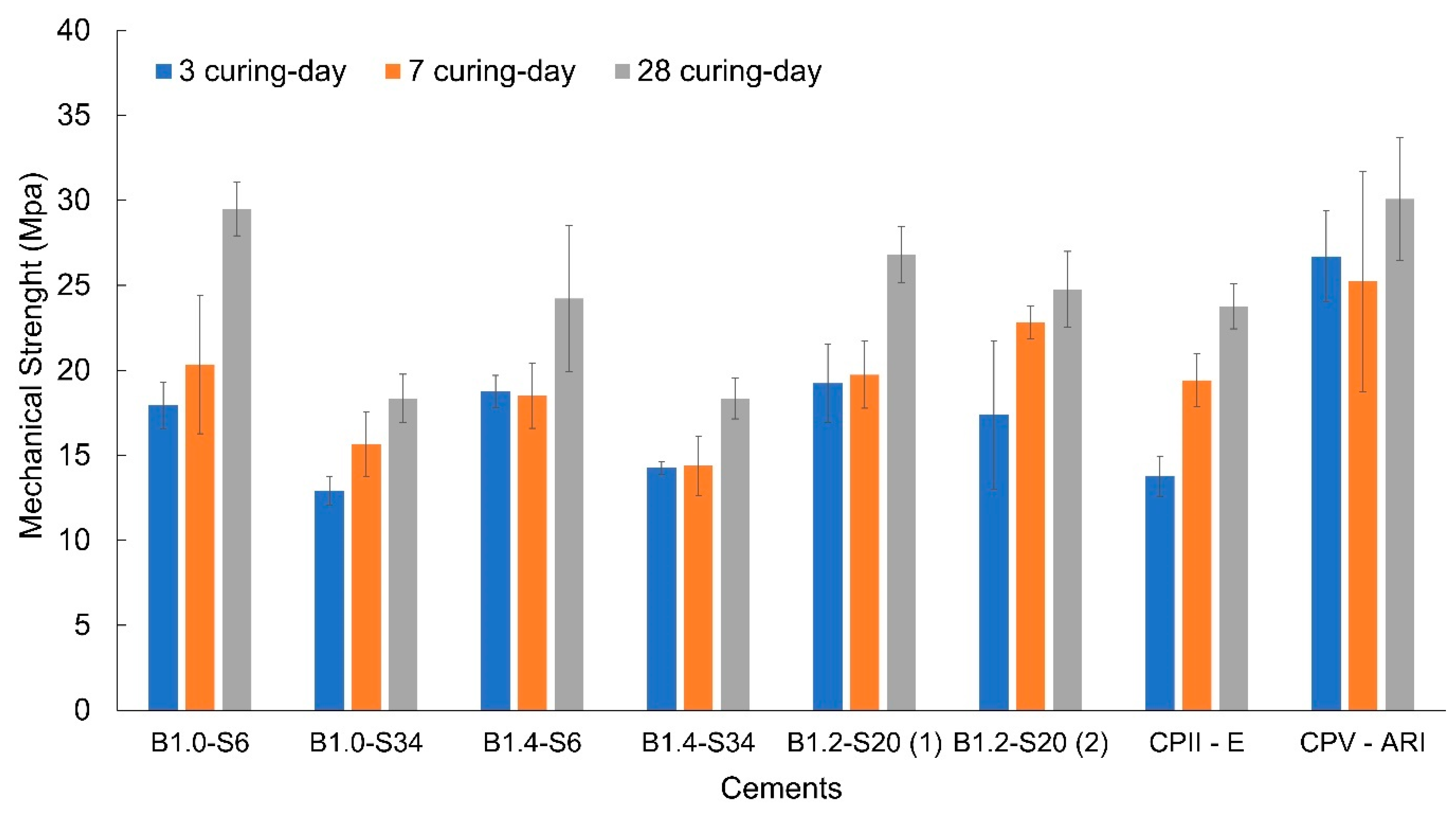
3.3. Cement Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markandeya, A.; Shanahan, N.; Gunatilake, D.M.; Riding, K.A.; Zayed, A. Influence of Slag Composition on Cracking Potential of Slag-Portland Cement Concrete. Constr. Build. Mater. 2018, 164, 820–829. [Google Scholar] [CrossRef]
- Carvalho, S.Z.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S.N. The Recycling Effect of BOF Slag in the Portland Cement Properties. Resour. Conserv. Recycl. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Mi, G. Effect of Blended Steel Slag-GBFS Mineral Admixture on Hydration and Strength of Cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Cardoso, C.; Camões, A.; Eires, R.; Mota, A.; Araújo, J.; Castro, F.; Carvalho, J. Using Foundry Slag of Ferrous Metals as Fine Aggregate for Concrete. Resour. Conserv. Recycl. 2018, 138, 130–141. [Google Scholar] [CrossRef]
- Ceccato, D.M.; Masuero, A.B.; Moraes, C.A.M.; Vilela, A.C.F. The Recycling of Foundry Granulated Slag (FGS) as a Partial Substitute of Cement in Concrete. Rev. Mater. 2009, 14, 737–748. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, S.; Goyal, A. Utilization of Waste Foundry Slag and Alccofine for Developing High Strength Concrete. Int. J. Electrochem. Sci. 2016, 11, 3190–3205. [Google Scholar] [CrossRef]
- Sachithanantham, P.; Dayakar, P.; Raju, K.V.B. Experimental Study on Eco Recycling of Ferrous Foundry Slag in Concrete—A Sustainable Development. Int. J. Eng. Trends Technol. 2012, 2, 6. [Google Scholar]
- Singh, S.K.; Singh, A.; Singh, B.; Vashistha, P. Application of Thermo-Chemically Activated Lime Sludge in Production of Sustainable Low Clinker Cementitious Binders. J. Clean. Prod. 2020, 264, 121570. [Google Scholar] [CrossRef]
- Maheswaran, S.; Kalaiselvam, S.; Saravana Karthikeyan, S.K.S.; Kokila, C.; Palani, G.S. β-Belite Cements (β-Dicalcium Silicate) Obtained from Calcined Lime Sludge and Silica Fume. Cem. Concr. Compos. 2016, 66, 57–65. [Google Scholar] [CrossRef]
- Ladomerský, J.; Janotka, I.; Hroncová, E.; Najdená, I. One-Year Properties of Concrete with Partial Substitution of Natural Aggregate by Cupola Foundry Slag. J. Clean. Prod. 2016, 131, 739–746. [Google Scholar] [CrossRef]
- NBR 16697; Cimento Portland—Requisitos. Associação Brasileira de Normas Técnicas ABNT: São Paulo, Brazil, 2011.
- Murali, G.; Wong, L.S.; Ramkumar, V.R.; Abid, S.R.; Karthik, S. From Waste to Resource: Recycled Lime Sludge Sustainable Low-Clinker Cementitious Binder—A Comprehensive Study on Hydration and Strength of Concrete. J. Build. Eng. 2024, 86, 108935. [Google Scholar] [CrossRef]
- Murali, G.; Prakash, S.S.; Vignesh, T.; Siva, A.; Manoj Kumar, R. Effect of Recycled Lime Sludge, Calcined Clay and Silica Fume on the Hydration and Performance of Sustainable Concrete. Case Stud. Constr. Mater. 2023, 19, e02202. [Google Scholar] [CrossRef]
- Devi, S.; Meena, S.; Joshi, S.; Sharma, S.K. Utilization of Foundry Slag as a Partial Replacement of Cement and Sand. SSRG Int. J. Civ. Eng. 2016, 3, 80–85. [Google Scholar]
- Suthar, M.; Aggarwal, P. Bearing Ratio and Leachate Analysis of Pond Ash Stabilized with Lime and Lime Sludge. J. Rock Mech. Geotech. Eng. 2018, 10, 769–777. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Zuo, Z.; Yang, F.; Han, Z.; Qin, Q. Reactivity and Performance of Dry Granulation Blast Furnace Slag Cement. Cem. Concr. Compos. 2019, 95, 19–24. [Google Scholar] [CrossRef]
- ASTM C150/C150M-22; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- EN 197-1:2011; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- NBR 7215; Cimento Portland—Determinação da Resistência à Compressão. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 1997.
- NBR 16372; Cimento Portland e Outros Materiais Em Pó—Determinação Da Finura Pelo Método de Permeabilidade Ao Ar (Método de Blaine). Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2015.
- NBR 16607; Cimento Portland—Determinação Dos Tempos de Pega. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2018.
- He, D.; Ou, Z.; Qin, C.; Deng, T.; Yin, J.; Pu, G. Understanding the Catalytic Acceleration Effect of Steam on CaCO3 Decomposition by Density Function Theory. Chem. Eng. J. 2020, 379, 122348. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors Affecting the Mechanical Properties of Alkali Activated Ground Granulated Blast Furnace Slag Concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Pinto Junior, L.A.B.; Berger, A.P.L.; Junca, E.; Grillo, F.F.; Sampaio, N.P.; de Oliveira, J.R. Characterization of Basic Oxygen Furnace Slag and Granite Waste Mixtures to Portland Cement Production. Rev. Esc. Minas 2016, 69, 459–464. [Google Scholar] [CrossRef]
- Aziz, A.; Stocker, O.; El Amrani El Hassani, I.E.; Laborier, A.P.; Jacotot, E.; El Khadiri, A.; El Bouari, A. Effect of Blast-Furnace Slag on Physicochemical Properties of Pozzolan-Based Geopolymers. Mater. Chem. Phys. 2021, 258, 123880. [Google Scholar] [CrossRef]
- Osmanovic, Z.; Haračić, N.; Zelić, J. Properties of Blastfurnace Cements (CEM III/A, B, C) Based on Portland Cement Clinker, Blastfurnace Slag and Cement Kiln Dusts. Cem. Concr. Compos. 2018, 91, 189–197. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Z.; Peng, C.; Li, X.; Jiang, H. Influence of Particle Size on Sinterability, Crystallisation Kinetics and Flexural Strength of Wollastonite Glass-Ceramics from Waste Glass and Fly Ash. Mater. Chem. Phys. 2014, 148, 449–456. [Google Scholar] [CrossRef]
- Saedi, M.; Behfarnia, K.; Soltanian, H. The Effect of the Blaine Fineness on the Mechanical Properties of the Alkali-Activated Slag Cement. J. Build. Eng. 2019, 26, 100897. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Su, Y. Hydration Kinetics of Cement-Quicklime System at Different Temperatures. Thermochim. Acta 2019, 673, 1–11. [Google Scholar] [CrossRef]
- Meng, T.; Hong, Y.; Wei, H.; Xu, Q. Effect of Nano-SiO2 with Different Particle Size on the Hydration Kinetics of Cement. Thermochim. Acta 2019, 675, 127–133. [Google Scholar] [CrossRef]
- Neville, A.M. Propriedades do Concreto, 5th ed.; Bookman: São Paulo, Brazil, 2016; ISBN 978-8582603659. [Google Scholar]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Kunhi Mohamed, A. Advances in Understanding Cement Hydration Mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Batog, M.; Giergiczny, Z. Influence of Mass Concrete Constituents on Its Properties. Constr. Build. Mater. 2017, 146, 221–230. [Google Scholar] [CrossRef]

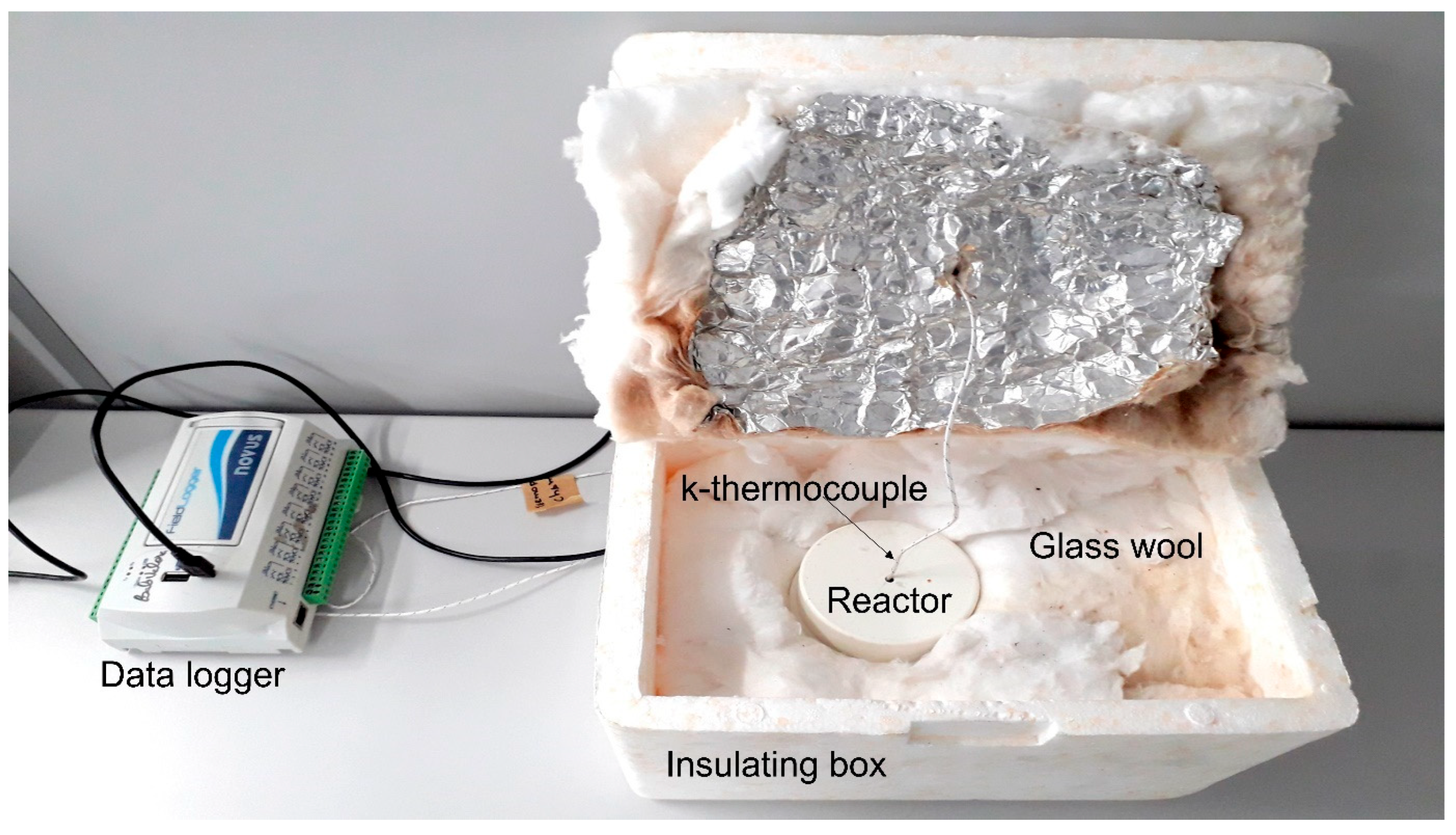
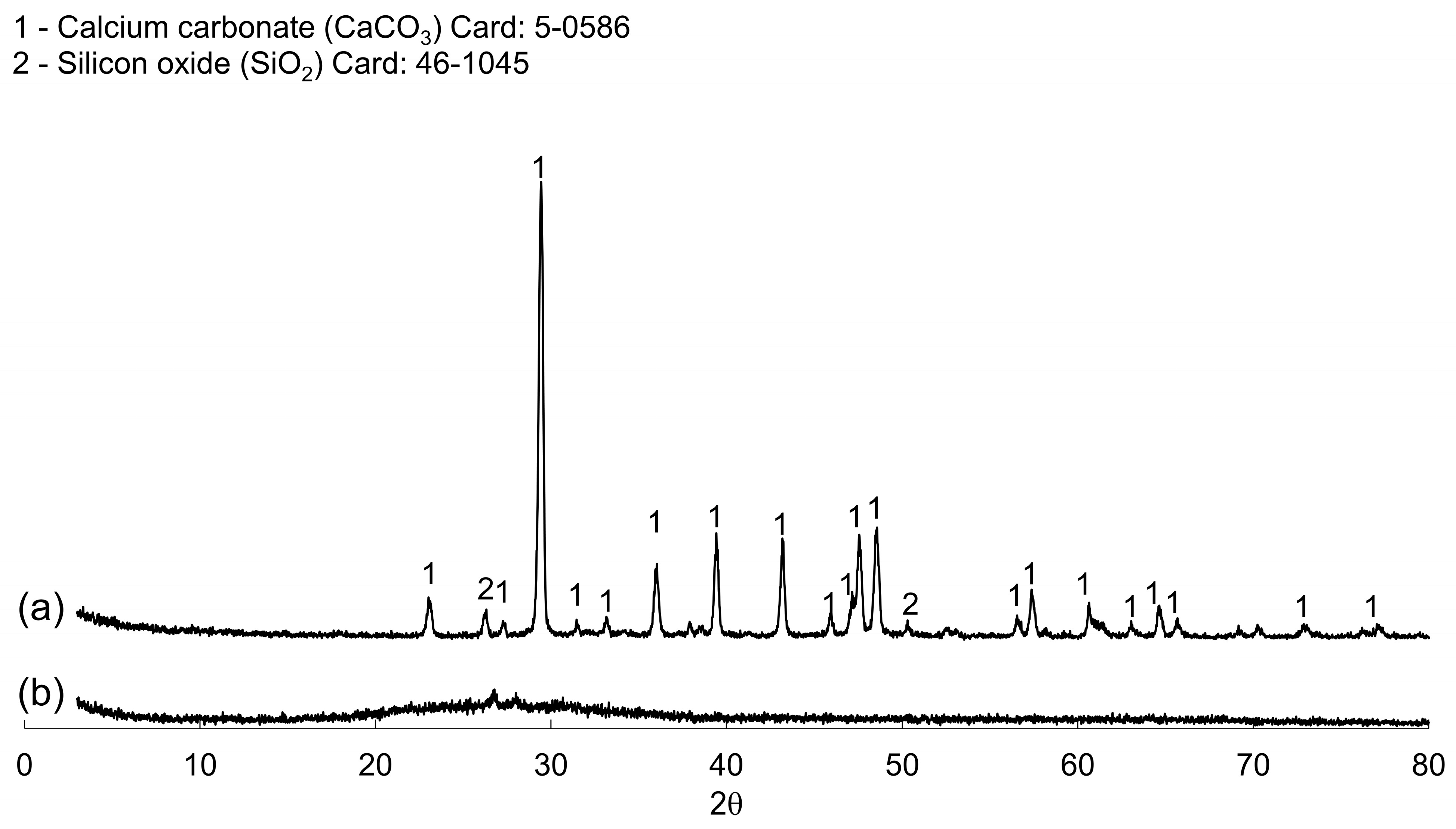
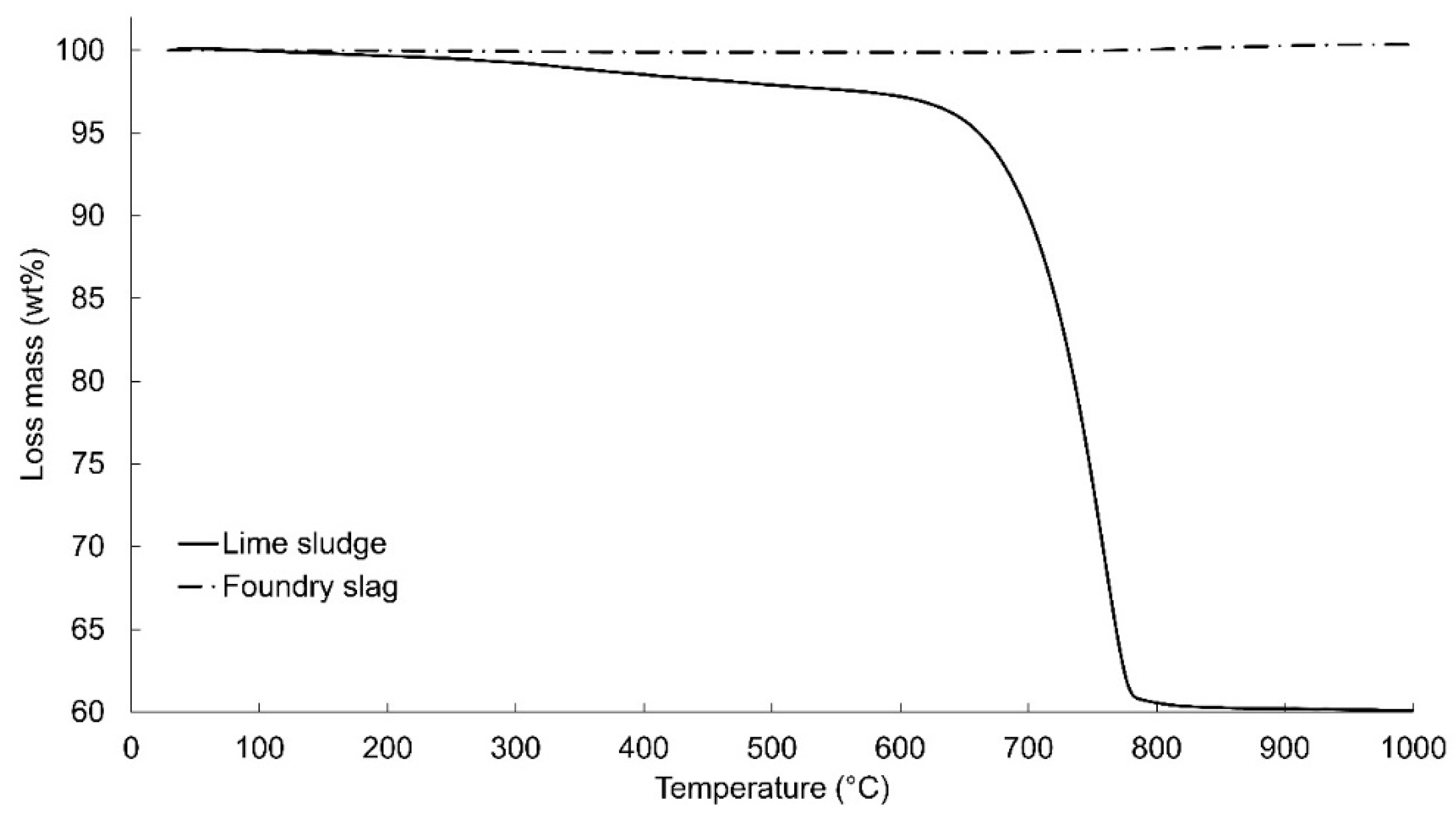
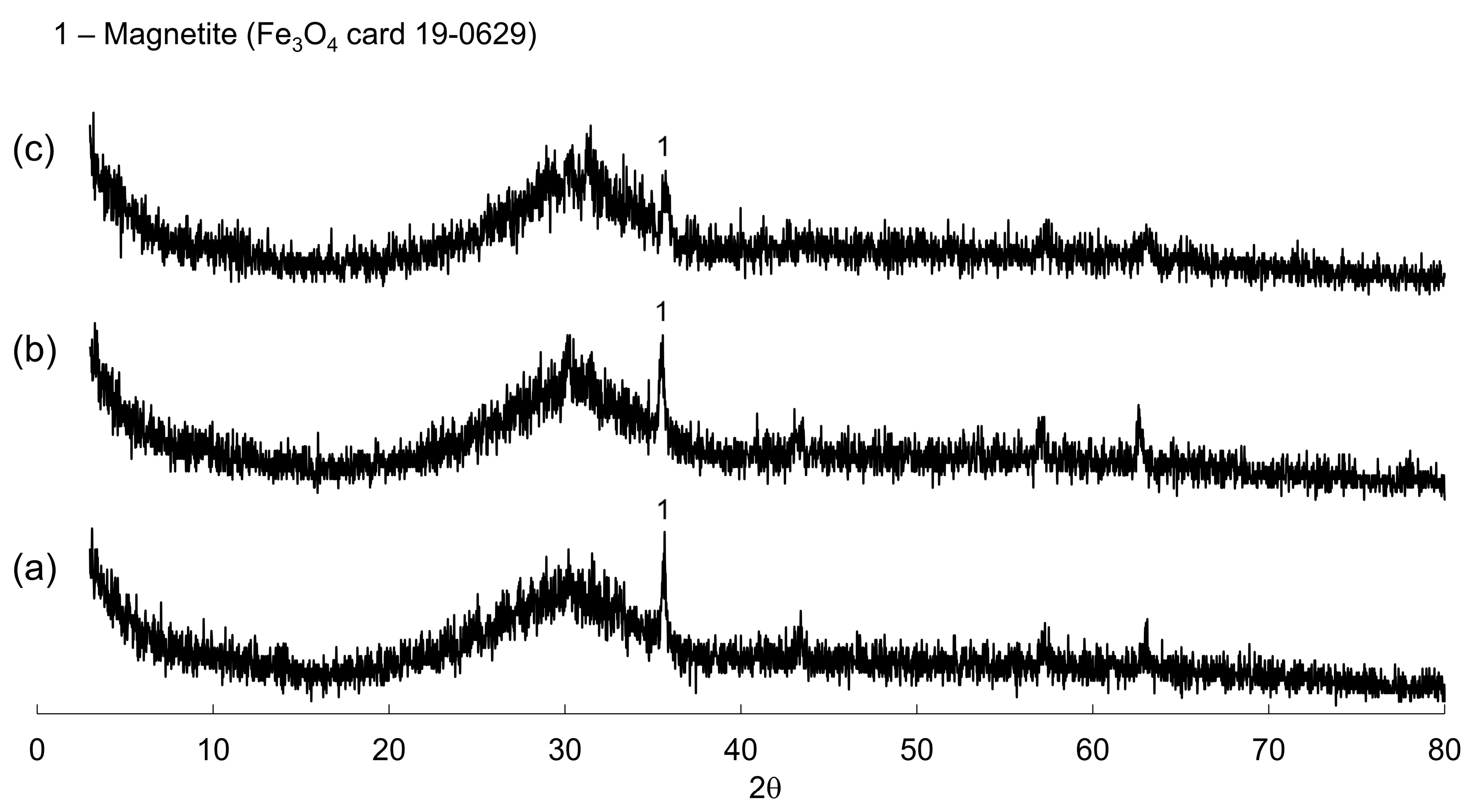

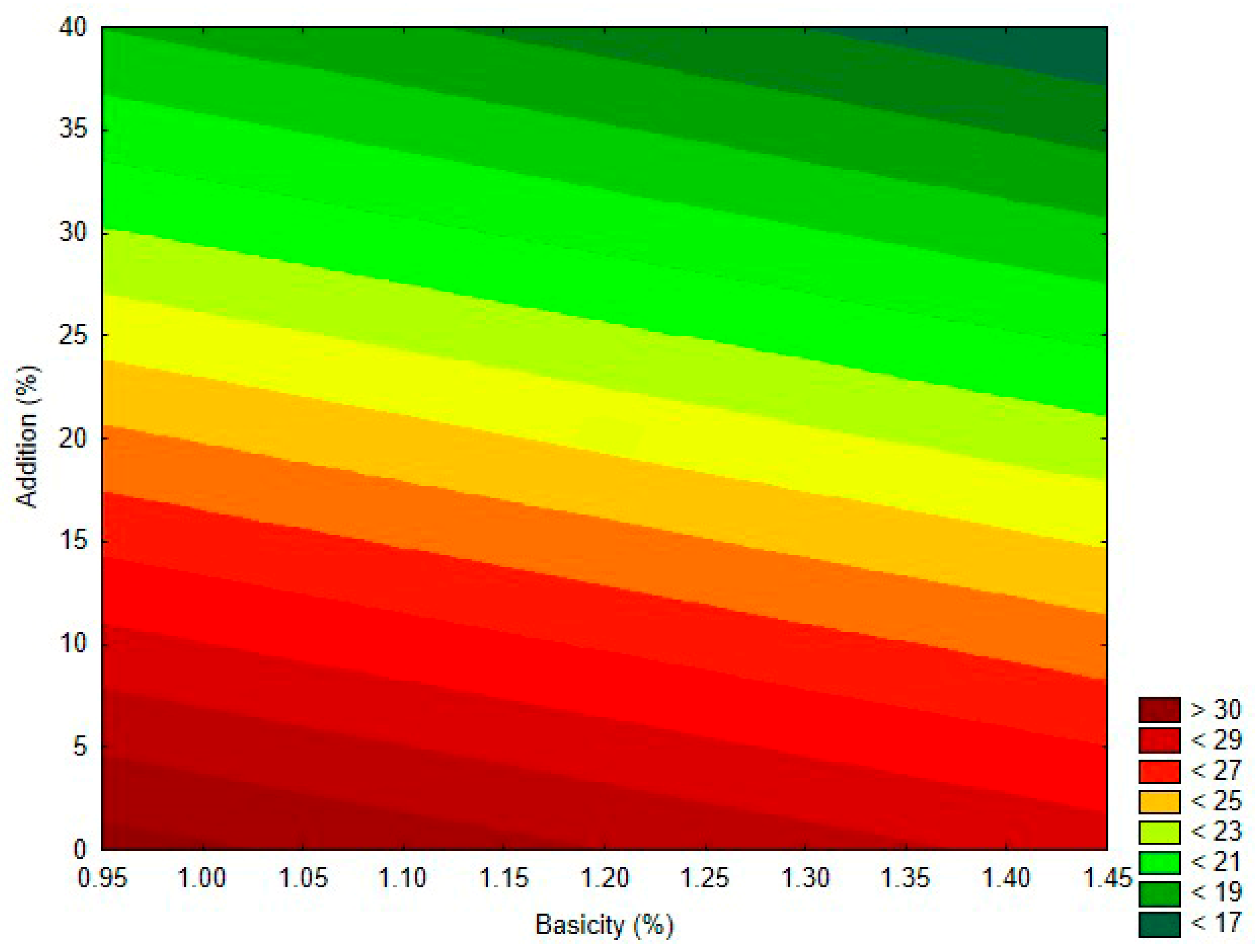

| Components | Basicity 1.0 | Basicity 1.2 | Basicity 1.4 |
|---|---|---|---|
| Al2O3 | 14.0 | 13.1 | 12.4 |
| CaO | 29.4 | 33.4 | 36.6 |
| Fe2O3 | 5.0 | 4.7 | 4.5 |
| MnO | 19.2 | 18.0 | 17.0 |
| MgO | 1.1 | 1.1 | 1.1 |
| K2O | 0.5 | 0.4 | 0.4 |
| Na2O | 1.5 | 1.7 | 1.9 |
| SiO2 | 29.2 | 27.5 | 26.1 |
| Types of Cement | Binary Basicity | Eco-Cementitious Material (wt.%) |
|---|---|---|
| B1.0-S6 | 1.0 | 6 |
| B1.0-S34 | 1.0 | 34 |
| B1.4-S6 | 1.4 | 6 |
| B1.4-S34 | 1.4 | 34 |
| B1.2-S20 (1) | 1.2 | 20 |
| B1.2-S20 (2) | 1.2 | 20 |
| Chemical Composition | Lime Sludge (wt%) | Foundry Slag (wt%) | Cement CPV—ARI (wt%) | Cement CPII—E (wt%) |
|---|---|---|---|---|
| Al2O3 | 0.18 | 19.95 | 6.46 | 5.49 |
| CaO | 55.53 | 1.96 | 52.21 | 55.03 |
| Fe2O3 | 0.18 | 7.04 | 2.97 | 2.2 |
| MnO | 0.03 | 27.51 | 0.08 | 0.2 |
| MgO | 0.34 | 1.36 | 5.78 | 2.41 |
| K2O | 0.01 | 0.61 | 1.12 | 0.93 |
| Na2O | 2.58 | 0.34 | 0.27 | 0.32 |
| SiO2 | 1.0 | 41.23 | 22.84 | 21.83 |
| TiO2 | - | - | 0.35 | 0.27 |
| P2O5 | - | - | - | 0.15 |
| Loss on Ignition | 40.07 | - | 5.15 | 8.72 |
| Cementitious Materials | Surface Área (m2/kg) |
|---|---|
| B1.0-S6 | 1438.56 |
| B1.0-S34 | 1337.56 |
| B1.4-S6 | 1519.79 |
| B1.4-S34 | 1342.54 |
| B1.2-S20 (1) | 1400.94 |
| B1.2-S20 (2) | 1396.17 |
| CP II | 1013.93 |
| CP V | 1222.84 |
| Components | B1.2-S20 (1) (wt%) |
|---|---|
| Al2O3 | 7.79 |
| CaO | 48.45 |
| Fe2O3 | 3.32 |
| MnO | 3.66 |
| MgO | 4.84 |
| K2O | 0.98 |
| Na2O | 0.56 |
| SiO2 | 23.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, C.L.; Rosso Neto, L.; Borgert, C.H.; Machado, J.P.; Grillo, F.F.; de Oliveira, J.R.; Zimmermann, M.V.G.; Milanez, M.; Keller, T.A.; Frizon, T.E.A.; et al. Design of New Eco-Cementitious Material Based on Foundry Slag and Lime Sludge. Minerals 2025, 15, 1059. https://doi.org/10.3390/min15101059
Eckert CL, Rosso Neto L, Borgert CH, Machado JP, Grillo FF, de Oliveira JR, Zimmermann MVG, Milanez M, Keller TA, Frizon TEA, et al. Design of New Eco-Cementitious Material Based on Foundry Slag and Lime Sludge. Minerals. 2025; 15(10):1059. https://doi.org/10.3390/min15101059
Chicago/Turabian StyleEckert, Camila Lopes, Lucio Rosso Neto, Carlos Henrique Borgert, Júlio Preve Machado, Felipe Fardin Grillo, José Roberto de Oliveira, Matheus Vinicius Gregory Zimmermann, Mateus Milanez, Tchesare Andreas Keller, Tiago Elias Allievi Frizon, and et al. 2025. "Design of New Eco-Cementitious Material Based on Foundry Slag and Lime Sludge" Minerals 15, no. 10: 1059. https://doi.org/10.3390/min15101059
APA StyleEckert, C. L., Rosso Neto, L., Borgert, C. H., Machado, J. P., Grillo, F. F., de Oliveira, J. R., Zimmermann, M. V. G., Milanez, M., Keller, T. A., Frizon, T. E. A., da Silva, B. A., De Noni Junior, A., & Junca, E. (2025). Design of New Eco-Cementitious Material Based on Foundry Slag and Lime Sludge. Minerals, 15(10), 1059. https://doi.org/10.3390/min15101059







