Effect of Frother Type on Surface Properties and Flotation Performance of Galena: A Comparative Study of EH, PPG250, and MIBC
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Surface Tension Measurements
2.2.2. Bubble Coalescence Measurements
2.2.3. Dynamic Foam Stability Measurements
2.2.4. Micro-Flotation Experiments
3. Results and Discussion
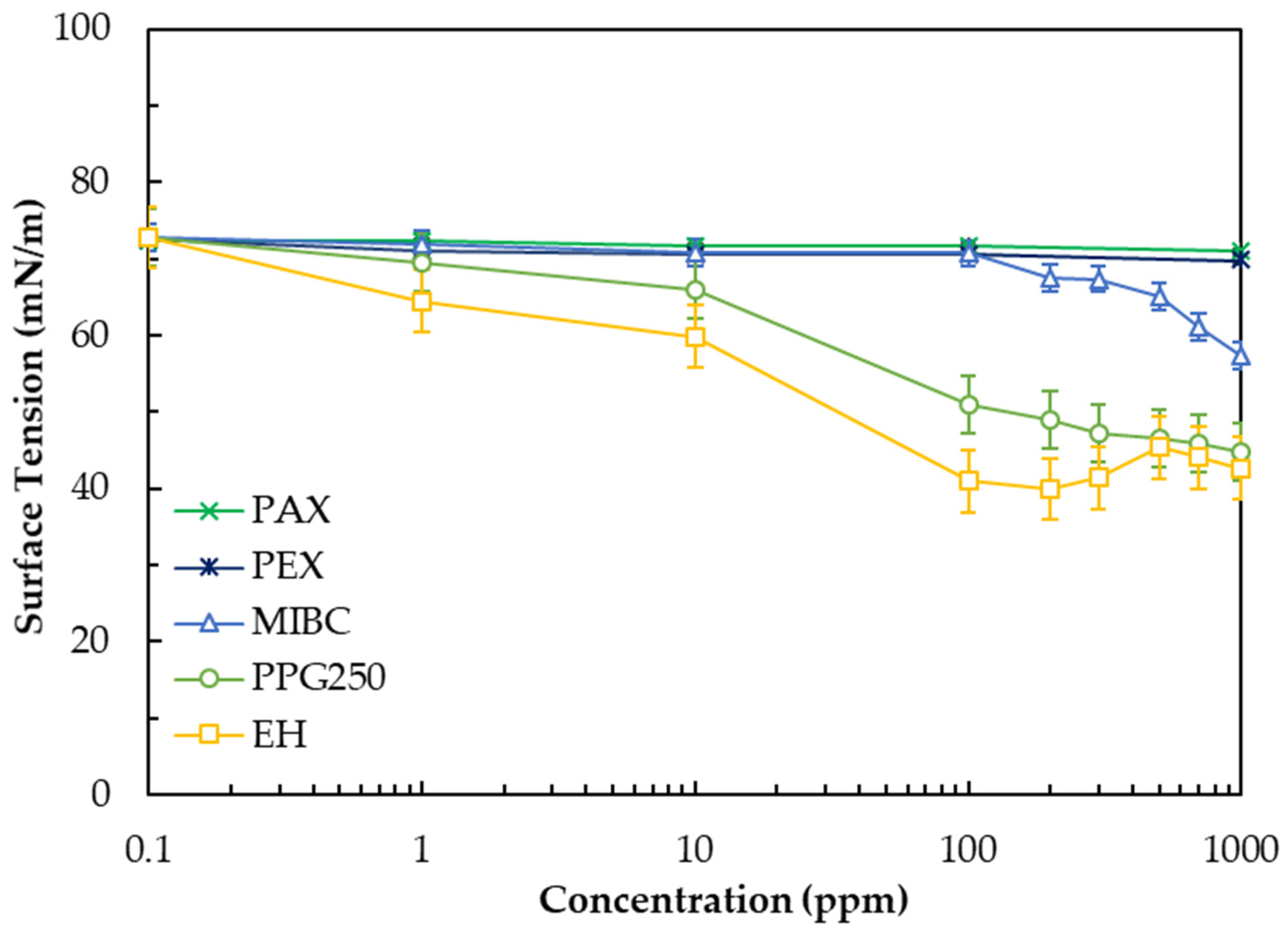
3.1. Effect of Reagent Type on Surface Tension
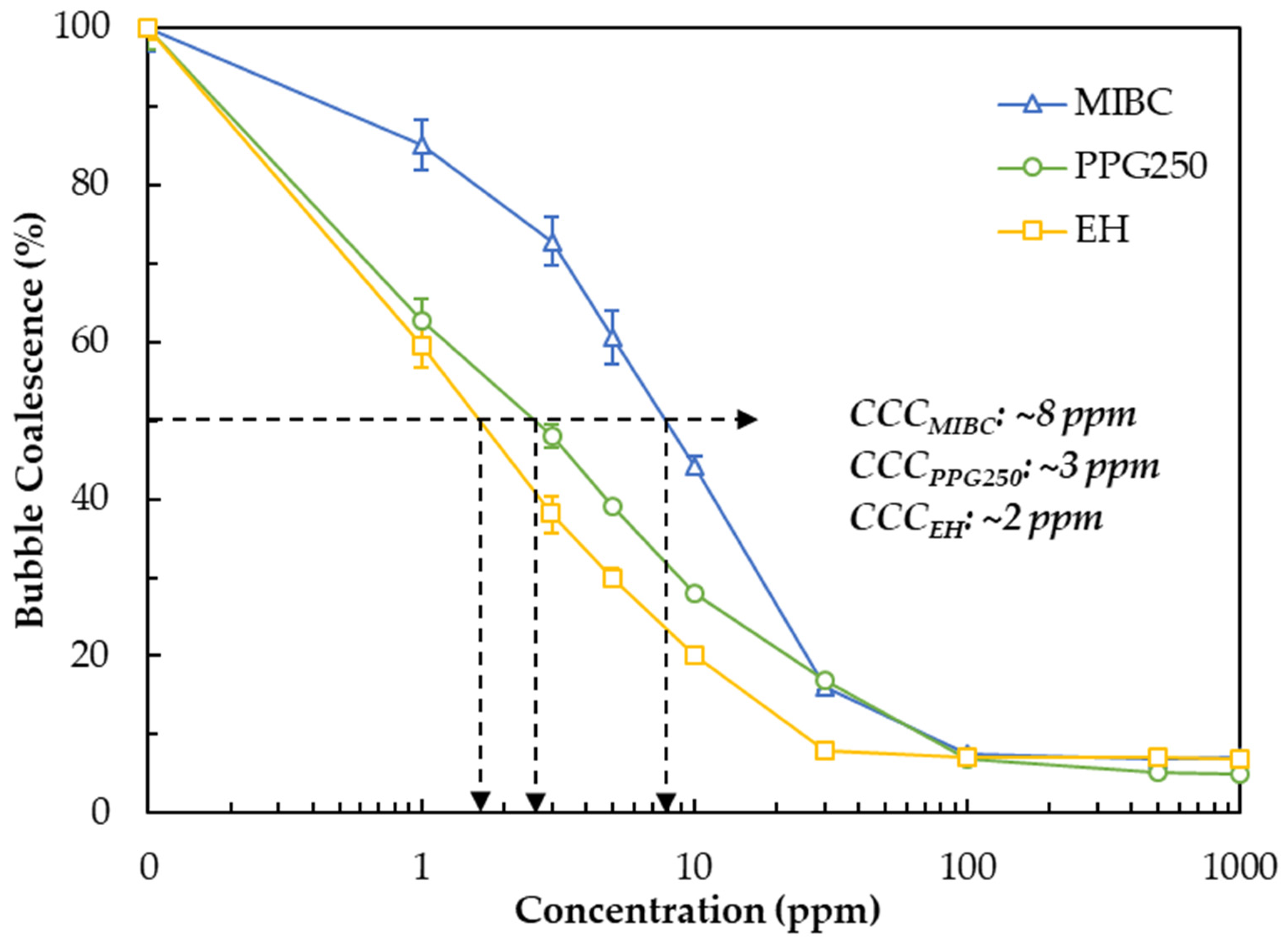
3.2. Determination of CCC Values of Different Types of Frothers
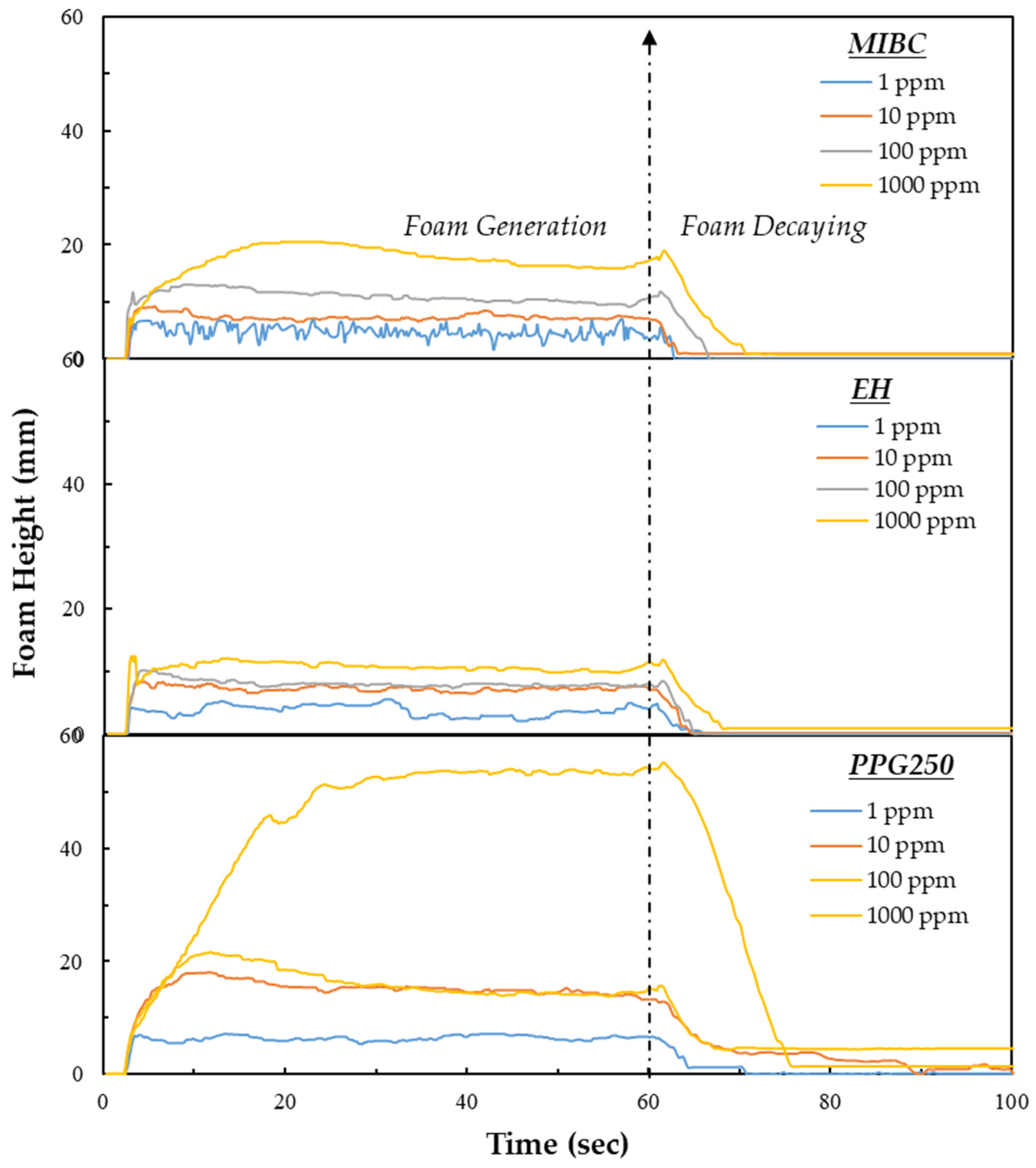
3.3. Dynamic Foam Stability Measurements
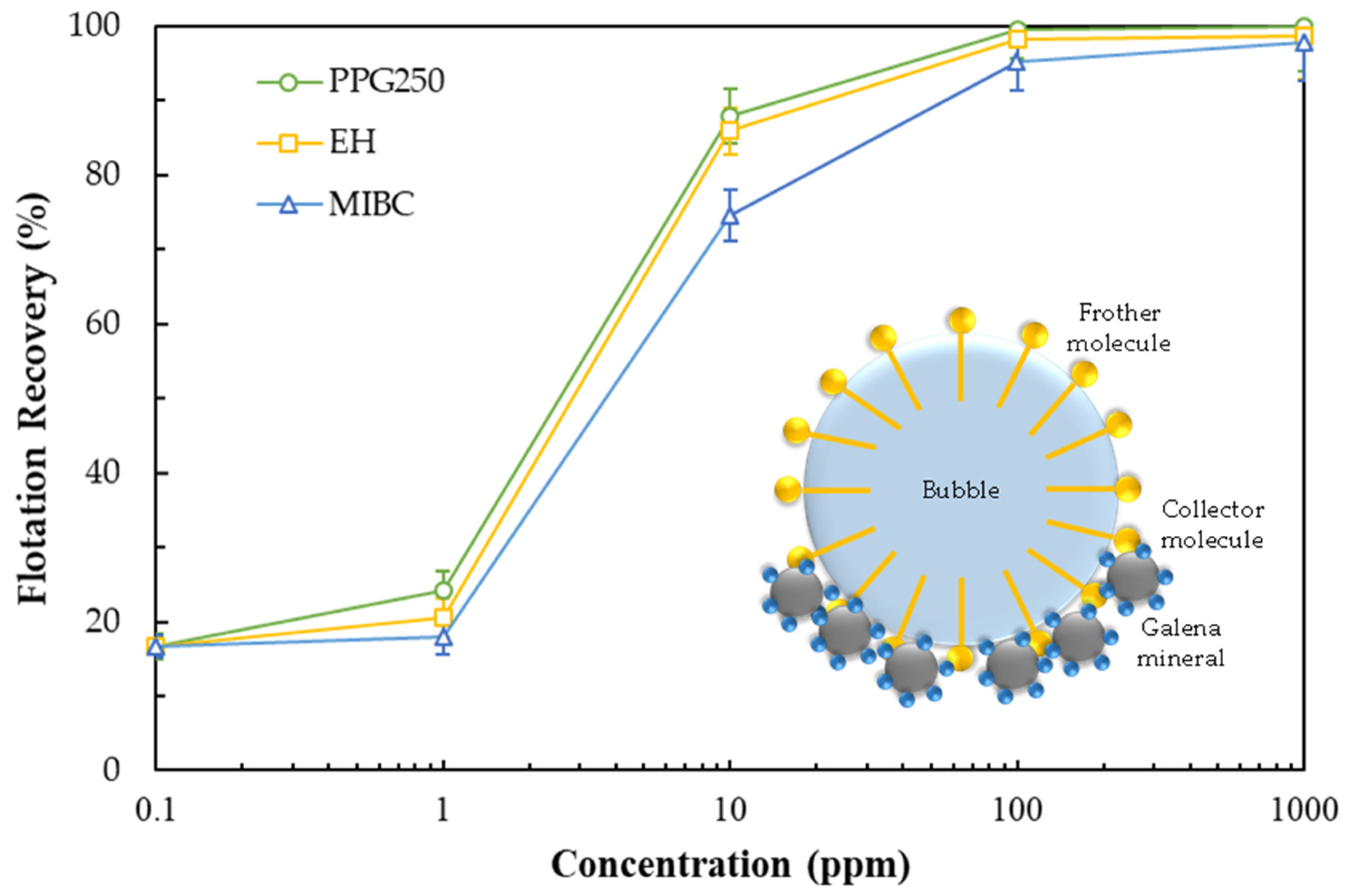
3.4. Effect of Frother Type on Flotation Recovery
4. Conclusions
- In this study, the interfacial behavior and flotation performance of three structurally distinct frothers (EH, PPG250, and MIBC) were systematically investigated using a high-purity galena sample. The results revealed the critical role of frother molecular structure and surface activity in determining surface tension, bubble coalescence, foam stability, and flotation efficiency.
- EH exhibited the highest surface activity, reached the lowest surface tension and CCC value (~2 ppm), indicating rapid interface adsorption and effective coalescence suppression.
- PPG250 provided the best dynamic foam stability and flotation recovery by forming persistent interfacial films even at low dosages.
- MIBC, despite its widespread industrial use, showed moderate performance; higher concentrations (~8 ppm CCC) were required to achieve comparable stability and recovery due to its weaker surface activity and lower film elasticity.
- The results of the micro-flotation experiments confirmed that all frothers followed a typical flotation recovery curve, with recovery increasing gradually as frother dosage increased; the highest recoveries were obtained with PPG250 (99.45%), EH (98.31%), and MIBC (95.17%).
- In this study, the experiments were carried out using a single high-purity galena mineral. However, industrial flotation processes often involve complex ore bodies and the presence of various gangue minerals, which can significantly affect reagent behavior and overall process performance. Therefore, future research should aim to validate these findings in multi-mineral flotation systems to better represent industrial conditions.
- The results also carry important practical implications for mineral processing operations. Selecting frothers with lower CCC values and higher interfacial activity (such as EH and PPG250) allows reduced reagent consumption. In addition to reducing operational costs and increasing process efficiency, lower dosages contribute to more sustainable flotation practices by minimizing chemical usage, reducing waste generation, and limiting environmental impacts, a critical factor especially in large-scale operations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wills, B.A.; Finch, J.A. Froth Flotation. In Wills’ Mineral Processing Technology; Wills, B.A., Finch, J.A., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 265–380. [Google Scholar]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An Overview of Oil-Water Separation Using Gas Flotation Systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, S.; Bennington, C.P.J.; Grace, J.R.; Kerekes, R.J. Column Flotation Deinking: State-of-the-Art and Opportunities. Resour. Conserv. Recycl. 2011, 55, 1154–1177. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Aggregating Fine Hydrophilic Materials in Froth Flotation to Improve Separation Efficiency through a Homo-Aggregation Flotation Process. Adv. Colloid Interface Sci. 2024, 325, 103110. [Google Scholar] [CrossRef]
- Tao, D. Role of Bubble Size in Flotation of Coarse and Fine Particles—A Review. Sep. Sci. Technol. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of Flotation Frothers on Bubble Size and Foam Stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Peleka, E.N.; Gallios, G.P.; Matis, K.A. A Perspective on Flotation: A Review. J. Chem. Technol. Biotechnol. 2018, 93, 615–623. [Google Scholar] [CrossRef]
- Melo, F.; Laskowski, J.S. Fundamental Properties of Flotation Frothers and Their Effect on Flotation. Proc. Miner. Eng. 2006, 19, 766–773. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of Flotation Frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of Frother on Bubble Production and Behaviour in Flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Effect of Frother on Initial Bubble Shape and Velocity. Int. J. Miner. Process. 2010, 94, 115–120. [Google Scholar] [CrossRef]
- Kosior, D.; Zawala, J.; Malysa, K. Influence of N-Octanol on the Bubble Impact Velocity, Bouncing and the Three Phase Contact Formation at Hydrophobic Solid Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 788–795. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S. Role of Frothers in Bubble Generation and Coalescence in a Mechanical Flotation Cell. Can. J. Chem. Eng. 2006, 84, 170–182. [Google Scholar] [CrossRef]
- Hadler, K.; Cilliers, J.J. The Relationship between the Peak in Air Recovery and Flotation Bank Performance. Miner. Eng. 2009, 22, 451–455. [Google Scholar] [CrossRef]
- Hadler, K.; Greyling, M.; Plint, N.; Cilliers, J.J. The Effect of Froth Depth on Air Recovery and Flotation Performance. Miner. Eng. 2012, 36–38, 248–253. [Google Scholar] [CrossRef]
- Pawliszak, P.; Bradshaw-Hajek, B.H.; Skinner, W.; Beattie, D.A.; Krasowska, M. Frothers in Flotation: A Review of Performance and Function in the Context of Chemical Classification. Miner. Eng. 2024, 207, 108567. [Google Scholar] [CrossRef]
- Farrokhpay, S. Editorial for the Special Issue: “Physical Separation and Enrichment”. Minerals 2020, 10, 173. [Google Scholar] [CrossRef]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A.; Satish, P.; Pradip. Effect of Alcohol and Polyglycol Ether Frothers on Foam Stability, Bubble Size and Coal Flotation. Int. J. Miner. Process. 2007, 82, 126–137. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Selectivity and Power of Frothers in Copper Ore Flotation. Physicochem. Probl. Miner. Process. 2017, 53, 515–523. [Google Scholar] [CrossRef]
- Maldonado, M.; Ramos, I.; Pinto, A.; Gomez, C.O. Effect of Air Profiling and Frother Concentration on a Flotation Bank Performance. Physicochem. Probl. Miner. Process. 2023, 59, 186274. [Google Scholar] [CrossRef]
- Wiese, J.; Harris, P. The Effect of Frother Type and Dosage on Flotation Performance in the Presence of High Depressant Concentrations. Miner. Eng. 2012, 36–38, 204–210. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Using Sound to Study Bubble Coalescence. J. Colloid Interface Sci. 2009, 332, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Hampton, M.A.; Nguyen, A.V.; Birkett, G.R. The Influence of Gas Velocity, Salt Type and Concentration on Transition Concentration for Bubble Coalescence Inhibition and Gas Holdup. Chem. Eng. Res. Des. 2012, 90, 33–39. [Google Scholar] [CrossRef]
- Grandon, F.; Alvarez, J.; Gomez, C. Frother Dosage in Laboratory Flotation Testing. In Proceedings of the 11th International Mineral Processing Conference, Santiago, Chile, 21–23 October 2015. [Google Scholar]
- Gungoren, C.; Islek, E.; Baktarhan, Y.; Unver, I.K.; Ozdemir, O. A Novel Technique to Investigate the Bubble Coalescence in the Presence of Surfactant (MIBC) and Electrolytes (NaCl and CaCl2). Physicochem. Probl. Miner. Process. 2018, 54, 1215–1222. [Google Scholar] [CrossRef]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental Procedure for the Determination of the Critical Coalescence Concentration (Ccc) of Simple Frothers. Minerals 2020, 10, 617. [Google Scholar] [CrossRef]
- Wang, J.; Forbes, G.; Forbes, E. Frother Characterization Using a Novel Bubble Size Measurement Technique. Appl. Sci. 2022, 12, 750. [Google Scholar] [CrossRef]
- Gahona, G.; Cisternas, L.A.; Araya-Gómez, N.; Lucay, F.A.; Gálvez, E.D.; Lopéz-Valdivieso, A.; Valdes, F. Bubble Size Characterization in the HydroFloat® Fluidized-Bed Flotation Cell Using Tap Water and Seawater. Minerals 2024, 14, 813. [Google Scholar] [CrossRef]
- Cui, H.; Cao, G.; Zhu, S.; Mu, J.; Liu, X.; Chou, X. Foaming Performance Evaluation of Frother Emulsions in the Slime Flotation: Foamability, Foam Stability, and Foam Flow. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128310. [Google Scholar] [CrossRef]
- Saavedra Moreno, Y.; Bournival, G.; Ata, S. Foam Stability of Flotation Frothers under Dynamic and Static Conditions. Sep. Purif. Technol. 2021, 274, 117822. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Min, F.; Wang, L.; Shu, Q. Research on the Flotation Efficiency of Alcohol/Ether Alcohol Frothers for Common Collectors: Insight of Molecular Dynamics Simulations. Appl. Surf. Sci. 2023, 614, 156233. [Google Scholar] [CrossRef]
- Nuorivaara, T.; Klemettinen, A.; Serna-Guerrero, R. Improving the Flotation Recovery of Cu from Flash Smelting Slags by Utilizing Cellulose-Based Frother Formulations. Miner. Eng. 2022, 181, 107522. [Google Scholar] [CrossRef]
- Page, P.W.; Hazell, L.B. X-Ray Photoelectron Spectroscopy (XPS) Studies of Potassium Amyl Xanthate (KAX) Adsorption on Precipitated PbS Related to Galena Flotation. Int. J. Miner. Process. 1989, 25, 87–100. [Google Scholar] [CrossRef]
- GUNGOREN, C.; BAKTARHAN, Y.; DEMIR, I.; OZKAN, S.G. Enhancement of Galena-Potassium Ethyl Xanthate Flotation System by Low Power Ultrasound. Trans. Nonferrous Met. Soc. China 2020, 30, 1102–1110. [Google Scholar] [CrossRef]
- Gungoren, C.; Muse, S.M.; Terzi, M.; Eskibalci, M.F.; Kursun, I.; Ozdemir, O. Understanding the Collective Effect of Dissolved Ions on the Surface Chemistry of Galena Flotation. JOM 2025, 77, 7713–7723. [Google Scholar] [CrossRef]
- Çavdar, Y.E.; Karakashev, S.I.; Donchev, S.; Yotova, I.; Ozdemir, O. Ion-Specific Effects on the Bubble Coalescence in Aqueous Medium. Physicochem. Probl. Miner. Process. 2025, 61, 204586. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Batjargal, K.; Guven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Correlations for Easy Calculation of the Critical Coalescence Concentration (CCC) of Simple Frothers. Coatings 2020, 10, 612. [Google Scholar] [CrossRef]
- Özün, S.; Ergen, G. Determination of Optimum Parameters for Flotation of Galena: Effect of Chain Length and Chain Structure of Xanthates on Flotation Recovery. ACS Omega 2019, 4, 1516–1524. [Google Scholar] [CrossRef]
- Lu, Y.; Tong, X.; Xie, X.; Yang, B.; Hua, Z. Effect of Particle Size on the Oxidation and Flotation Behavior of Galena Particles. Physicochem. Probl. Miner. Process. 2019, 55, 208–216. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals 2021, 11, 304. [Google Scholar] [CrossRef]
- Batjargal, K.; Güven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers. Minerals 2025, 15, 849. [Google Scholar] [CrossRef]
- Lin, R.; Wu, S.; Liu, Q.; Deng, R.; Li, X. Effects of Surface Oxidation on Floatability and Separation of Galena and Sphalerite and Its Elimination. JOM 2025, 77, 4838–4849. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Mkhonto, P.P.; Meng, Y.; McFadzean, B.; Ngoepe, P.E.; O’Connor, C. The Flotation Separation of Chalcopyrite and Galena: Crystal, Surface, Floatability and Reagents. Sep. Purif. Rev. 2024, 54, 289–309. [Google Scholar] [CrossRef]
- Jameson, G.J.; Emer, C. Effect of Bubble Loading on the Recovery of Coarse Mineral Particles by Flotation. Miner. Eng. 2024, 215, 108788. [Google Scholar] [CrossRef]
- Pashkevich, D.; Li, R.; Kökkılıç, O.; Waters, K.E. Investigations of Monomineralic Flotation of Galena, Sphalerite, and Pyrite at Different Temperatures. Minerals 2023, 13, 615. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Phan, C.M.; Ang, H.M.; Nakahara, H.; Shibata, O.; Moroi, Y. Surface Potential of 1-Hexanol Solution: Comparison with Methyl Isobutyl Carbinol. J. Phys. Chem. B 2013, 117, 7615–7620. [Google Scholar] [CrossRef]
- Qu, J.; Tao, X.; He, H.; Zhang, X.; Xu, N.; Zhang, B. Synergistic Effect of Surfactants and a Collector on the Flotation of a Low-Rank Coal. Int. J. Coal Prep. Util. 2015, 35, 14–24. [Google Scholar] [CrossRef]
- Cappuccitti, F.; Finch, J.A. Development of New Frothers through Hydrodynamic Characterization. Miner. Eng. 2008, 21, 944–948. [Google Scholar] [CrossRef]
- Finch, J.A.; Zhang, W. Frother Function-Structure Relationship: Dependence of CCC95 on HLB and the H-Ratio. Miner. Eng. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Alvarado, O.; Quezada, G.R.; Saavedra, J.H.; Rozas, R.E.; Toledo, P.G. Species Surface Distribution and Surface Tension of Aqueous Solutions of MIBC and NaCl Using Molecular Dynamics Simulations. Polymers 2022, 14, 1967. [Google Scholar] [CrossRef]
- Malati, M.A.; Yousef, A.A. Surface Tension of Collector—Frother Mixtures. J. Appl. Chem. 1967, 17, 1–4. [Google Scholar] [CrossRef]
- Han, G.; Su, S.; Huang, Y.; Peng, W.; Cao, Y.; Liu, J. An Insight into Flotation Chemistry of Pyrite with Isomeric Xanthates: A Combined Experimental and Computational Study. Minerals 2018, 8, 166. [Google Scholar] [CrossRef]
- An, D.; Zhang, J. A Study of Temperature Effect on the Xanthate’s Performance during Chalcopyrite Flotation. Minerals 2020, 10, 426. [Google Scholar] [CrossRef]
- Gungoren, C.; Muse, S.M.; Terzi, M.; Eskibalci, M.F.; Kursun Unver, I.; Ozdemir, O. Synergistic Effect of Frequently Found Ions in the Flotation of Pb-Zn Sulfide Ores on Air/Water Interface. Minerals 2023, 13, 1236. [Google Scholar] [CrossRef]
- Szyszka, D. Critical Coalescence Concentration (Ccc) for Surfactants in Aqueous Solutions. Minerals 2018, 8, 431. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Tlhone, T.; Williams, P.; Ding, K. Fundamental Properties of the Polyoxypropylene Alkyl Ether Flotation Frothers. Int. J. Miner. Process. 2003, 72, 289–299. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Cho, Y.S.; Ding, K. Effect of Frothers on Bubble Size and Foam Stability in Potash Ore Flotation Systems. Can. J. Chem. Eng. 2003, 81, 63–69. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S.; Heiskanen, K. Effect of Frothers on Bubble Size. Int. J. Miner. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Alsafasfeh, A.; Alagha, L.; Al-Hanaktah, A. The Effect of Methyl Isobutyl Carbinol “MIBC” on the Froth Stability and Flotation Performance of Low-Grade Phosphate Ore. Min. Metall. Explor. 2024, 41, 353–361. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, S.; Wang, Y.; Xing, Y.; Ma, Z.; Cao, Y. Investigation on Properties of Aqueous Foams Stabilized by Aliphatic Alcohols and Polypropylene Glycol. J. Dispers. Sci. Technol. 2019, 40, 728–736. [Google Scholar] [CrossRef]
- Yuan, Z.; Herold, K.E. Surface Tension of Pure Water and Aqueous Lithium Bromide with 2-Ethyl-Hexanol. Appl. Therm. Eng. 2001, 21, 881–897. [Google Scholar] [CrossRef]
- Barbian, N.; Ventura-Medina, E.; Cilliers, J.J. Dynamic Froth Stability in Froth Flotation. Miner. Eng. 2003, 16, 1111–1116. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Finch, J.A. Correspondence of Bubble Size and Frother Partitioning in Flotation. J. Cent. South. Univ. 2014, 21, 2383–2390. [Google Scholar] [CrossRef]
- Ostadrahimi, M.; Farrokhpay, S.; Gharibi, K.; Dehghani, A.; Aghajanloo, M. Effects of Flotation Operational Parameters on Froth Stability and Froth Recovery. J. S. Afr. Inst. Min. Metall. 2021, 121, 11–20. [Google Scholar] [CrossRef]
- Contreras, F.; Yianatos, J.; Vinnett, L. On the Froth Transport Modelling in Industrial Flotation Cells. Miner. Eng. 2013, 41, 17–24. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Ozdemir, O.; Batjargal, K.; Guven, O.; Ata, S.; Bournival, G.; Boylu, F.; Sabri Çelik, M. On the Frother’s Strength and Its Performance. Miner. Eng. 2021, 171, 17–24. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavdar, Y.E.; Asil, I.; Muse, S.M.; Boylu, F.; Ozdemir, O. Effect of Frother Type on Surface Properties and Flotation Performance of Galena: A Comparative Study of EH, PPG250, and MIBC. Minerals 2025, 15, 1044. https://doi.org/10.3390/min15101044
Cavdar YE, Asil I, Muse SM, Boylu F, Ozdemir O. Effect of Frother Type on Surface Properties and Flotation Performance of Galena: A Comparative Study of EH, PPG250, and MIBC. Minerals. 2025; 15(10):1044. https://doi.org/10.3390/min15101044
Chicago/Turabian StyleCavdar, Yunus Emre, Ilayda Asil, Saleban Mohamed Muse, Feridun Boylu, and Orhan Ozdemir. 2025. "Effect of Frother Type on Surface Properties and Flotation Performance of Galena: A Comparative Study of EH, PPG250, and MIBC" Minerals 15, no. 10: 1044. https://doi.org/10.3390/min15101044
APA StyleCavdar, Y. E., Asil, I., Muse, S. M., Boylu, F., & Ozdemir, O. (2025). Effect of Frother Type on Surface Properties and Flotation Performance of Galena: A Comparative Study of EH, PPG250, and MIBC. Minerals, 15(10), 1044. https://doi.org/10.3390/min15101044









