Mechanisms of Thermal Color Change in Brown Elbaite–Fluorelbaite Tourmaline: Insights from Trace Elements and Spectral Signatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Standard Gemological Methods
2.2. Sample Preparation and Heat Treatment
2.3. EMPA and LA-ICP-MS
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.5. Laser Raman (LR) Spectroscopy
2.6. Ultraviolet–Visible (UV-Vis) Spectroscopy
3. Results
3.1. Gemological Characteristics
3.2. Chemical Composition and Species
3.3. Heat-Treated Samples’ Features
3.4. Infrared Spectroscopy
3.4.1. Reflective MIR Spectra (1600–400 cm−1)
3.4.2. Transmission NIR Spectra (9000–4000 cm−1)
| This Study | Green Elbaite [20] | Green Elbaite [4] | Pink Tourmaline [24] | Green Tourmaline [24] | Brownish-Green [24] | Assignment | ||
|---|---|---|---|---|---|---|---|---|
| Unheated | Heat-Treated | |||||||
| 8012 | 8012 ↑ | 8000 | First overtones of hydroxyl stretching modes | |||||
| 7185 | ||||||||
| 7138 | 7138 ↑ | 7132 | 7140 | |||||
| 6997 | 6997 ↑ | 6995 | 7000 | |||||
| 6783 | 6795 | |||||||
| 5183 | 5183 | 5180 | The bending vibration of the water and the stretching vibration | |||||
| 4871 | 4871 | 4873 | νas Si–O–Si | |||||
| 4721 | 4721 ↓ | |||||||
| 4597 | 4597 | 4598 | 4597 | 4604 | 4604 | 4596 | Combination of stretching and bending modes of M-OH units | YM-OH1 |
| 4536 | 4536 | 4544 | 4538 | 4541 | 4545 | 4541 | YM-OH1 | |
| 4440 | 4440 | 4444 | 4433 | 4444 | 4448 | 4444 | YM-OH1 | |
| 4344 | 4344 | 4344 | 4347 | 4347 | 4347 | 4344 | ZM-OH3 | |
| 4201 | 4201 | 4206 | 4211 | 4214 | YM-OH3 | |||
| 4170 | 4170↓ | 4164 | 4171 | 4146 | 4177 | 4175 | YM-OH3 | |
3.5. Raman Spectroscopy
3.6. UV-Vis Spectroscopy
4. Discussion
4.1. Tourmaline Species of Samples
4.2. Spectral Response to 500 °C Heating and Structural Stability
4.3. Color Origin and Color Change Mechanism Induced by Heat Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hawthorne, F.C.; Henry, D.J. Classification of the Minerals of the Tourmaline Group. Eur. J. Mineral. 1999, 11, 201–216. [Google Scholar] [CrossRef]
- Prasad, P.S.R. Study of Structural Disorder in Natural Tourmalines by Infrared Spectroscopy. Gondwana Res. 2005, 8, 265–270. [Google Scholar] [CrossRef]
- Castaneda, C.; Eeckhout, S.G.; Da Costa, G.M.; Botelho, N.F.; De Grave, E. Effect of Heat Treatment on Tourmaline from Brazil. Phys. Chem. Min. 2006, 33, 207–216. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Martens, W.N.; Wain, D.L.; Kloprogge, J.T. Spectroscopic Characterization of Mn-Rich Tourmalines. Vib. Spectrosc. 2007, 44, 42–49. [Google Scholar] [CrossRef]
- Henry, D.J.; Dutrow, B.L. Tourmaline Studies through Time: Contributions to Scientific Advancements. J. Geosci. 2018, 63, 77–98. [Google Scholar] [CrossRef]
- Pasetti, L.; Fornasini, L.; Mantovani, L.; Andò, S.; Raneri, S.; Palleschi, V.; Bersani, D. Study of Mg–Fe Content in Tourmalines from the Dravite–Schorl Series by Raman Spectroscopy. J. Raman Spectrosc. 2024, 55, 276–286. [Google Scholar] [CrossRef]
- Hoang, L.H.; Hien, N.T.M.; Chen, X.B.; Van Minh, N.; Yang, I. Raman Spectroscopic Study of Various Types of Tourmalines. J. Raman Spectrosc. 2011, 42, 1442–1446. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, P.L.; Yue, S.W. Spectroscopic Characteristics and Coloring Mechanism of Greenish-Yellow Beryl Under Heating Treatment. Spectrosc. Spectr. Anal. 2020, 40, 3795–3800, (In Chinese with English Abstract). [Google Scholar]
- Wu, W.Y.; Yue, S.W. Influence of Heat Treatment on Color of Amethyst and Its Spectral Characteristic. J. Gems Gemol. 2016, 18, 47–55, (In Chinese with English Abstract). [Google Scholar]
- Wang, P.L.; Yue, S.W.; Li, J.Y. Spectral Characteristics and Color Mechanism of Heat-Treated Gem-Quality Yellow Sphene. Spectrosc. Spectr. Anal. 2024, 44, 2545–2550, (In Chinese with English Abstract). [Google Scholar]
- Yue, S.W.; Yan, X.X.; Chen, S.M.; Luo, J. The Heat Treatment Process and Color Mechanism of Green Fluor-Hydroxyapatite. Spectrosc. Spectr. Anal. 2025, 45, 1961–1967, (In Chinese with English Abstract). [Google Scholar]
- Pezzotta, F.; Laurs, B.M. Tourmaline: The Kaleidoscopic Gemstone. Elements 2011, 7, 333–338. [Google Scholar] [CrossRef]
- Ahn, Y.K.; Seo, J.Y.; Park, J.W. Electronic and Vibrational Spectra of Tourmaline-The Impact of Electron Beam Irradiation and Heat Treatment. Vib. Spectrosc. 2013, 65, 165–175. [Google Scholar] [CrossRef]
- Maneewong, A.; Seong, B.S.; Shin, E.J.; Kim, J.S.; Kajornrith, V. Color Change of Tourmaline by Heat Treatment and Electron Beam Irradiation: UV-Visible, EPR, and Mid-IR Spectroscopic Analyses. J. Korean Phys. Soc. 2016, 68, 83–92. [Google Scholar]
- Thongnopkun, P.; Bamrungpol, J. Color Change of Green Tourmaline from Madagascar by Heat Treatment. Burapha Sci. J. 2017, 22, 299–308. [Google Scholar]
- Thongnopkun, P.; Naowabut, P. Effect of Heat Treatment on Madagascar Dravite Tourmaline: UV-Visible and Diffuse Reflectance Infrared Spectroscopic Characterization. J. Appl. Spectrosc. 2018, 85, 616–623. [Google Scholar] [CrossRef]
- Abduriyim, A.; Kitawaki, H.; Furuya, M.; Schwarz, D. “Paraíba”-Type Copper-Bearing Tourmaline from Brazil, Nigeria, and Mozambique: Chemical Fingerprinting By LA-ICP-MS. Gems Gemol. 2006, 42, 4–21. [Google Scholar]
- Laurs, B.M.; Zwaan, J.C.; Breeding, C.M.; Simmons, W.B.; Beaton, D.; Rijsdijk, K.F.; Befi, R.; Falster, A.U. Copper-Bearing (Paraíba-Type) Tourmaline from Mozambique. Gems Gemol. 2008, 44, 294–320. [Google Scholar] [CrossRef]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the Tourmaline-Supergroup Minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Chen, Y.F.; Xu, D.; Zhou, Z.Y.; Schwarz, D.; Zheng, J.H.; Zhang, L.M. Chemical Composition and Spectral Variation in Gem-Quality Blue Iron-Bearing Tourmaline from Brazil. Crystals 2024, 14, 877. [Google Scholar]
- Cui, L.Y.; Guo, Y.; Tang, J.; Yang, Y.S. Spectroscopy Characteristics and Color-Influencing Factors of Green Iron-Bearing Elbaite. Crystals 2023, 13, 1461. [Google Scholar]
- Makreski, P.; Jovanovski, G. Minerals from Macedonia: XXIII. Spectroscopic and Structural Characterization of Schorl and Beryl Cyclosilicates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.W.; Yan, X.X.; Lin, J.Q.; Wang, P.L.; Liu, J.F. Spectroscopic Characteristics and Coloring Mechanism of Brown Tourmaline under Heating Treatment. Spectrosc. Spectr. Anal. 2021, 41, 2524–2529, (In Chinese with English Abstract). [Google Scholar]
- Liu, X.L.; Feng, X.Q.; Fan, J.L.; Guo, S.G. Optical Absorption Spectra of Tourmaline Crystals from Altay, China. Chin. Opt. Lett. 2011, 9, 083001–083014. [Google Scholar] [CrossRef][Green Version]
- Phichaikamjornwut, B.; Pongkrapan, S.; Intarasiri, S.; Bootkul, D. Conclusive Comparison of Gamma Irradiation and Heat Treatment for Color Enhancement of Rubellite from Mozambique. Vib. Spectrosc. 2019, 103, 102926. [Google Scholar] [CrossRef]
- Bosi, F.; Celata, B.; Skogby, H.; Hålenius, U.; Tempesta, G.; Ciriotti, M.E.; Bittarello, E.; Marengo, A. Mn-Bearing Purplish-Red Tourmaline from the Anjanabonoina Pegmatite, Madagascar. Miner. Mag 2021, 85, 242–253. [Google Scholar] [CrossRef]
- Li, X.J.; Zu, E.D. Near-Infrared Spectrum Analysis of Cyclosilicates Gem Minerals. Bull. Chin. Ceram. Soc. 2016, 35, 1318–1321. [Google Scholar]
- Ertl, A.; Kolitsch, U.; Dyar, M.D.; Hughes, J.M.; Rossman, G.R.; Pieczka, A.; Henry, D.J.; Pezzotta, F.; Prowatke, S.; Lengauer, C.L. Limitations of Fe2+ and Mn2+ Site Occupancy in Tourmaline: Evidence from Fe2+- and Mn2+-Rich Tourmaline. Am. Mineral. 2012, 97, 1402–1416. [Google Scholar] [CrossRef]
- Robinson, G.W.; Chamberlain, S.C.; Walter, M. The History and Mineralogy of the Classic Brown Tourmaline Locality, Gouverneur, New York. Rocks Miner. 2016, 91, 520–529. [Google Scholar] [CrossRef]
- Fonseca-Zang, W.A.d.; Zang, J.W.; Hofmeister, W. The Ti-Influence on the Tourmaline Color. J. Braz. Chem. Soc. 2008, 19, 1186–1192. [Google Scholar] [CrossRef]
- Manning, P.G. An Optical Absorption Study of the Origin of Colour and Pleochroism in Pink and Brown Tourmalines. Can. Miner. 1969, 9, 678–690. [Google Scholar]
- Faye, G.H.; Manning, P.G.; Gosselin, J.R.; Tremblay, R.J. The Optical Absorption Spectra of Tourmaline; Importance of Charge-Transfer Processes. Can. Miner. 1974, 12, 370–380. [Google Scholar]
- Burns, R.G.; Simon, H.F. Cation Disorder in Tourmalines. Geol. Soc. Am. Ann. Meet. Dallas Abstr. 1973, 5, 563–564. [Google Scholar]
- de Camargo, M.B.; Isotani, S. Optical Absorption Spectroscopy of Natural and Irradiated Pink Tourmaline. Am. Mineral. 1988, 73, 172–180. [Google Scholar]
- da Silva, S.F.; Moura, M.A.; Queiroz, H.d.A.; Ardisson, J.D. Chemical and Spectroscopic Characterization of Tourmalines from the Mata Azul Pegmatitic Field, Central Brazil. J. Geosci. 2018, 63, 155–165. [Google Scholar] [CrossRef]
- Smith, G. Evidence for Absorption by Exchange-Coupled Fe2+-Fe3+ Pairs in the near Infra-Red Spectra of Minerals. Phys. Chem. Min. 1978, 3, 375–383. [Google Scholar] [CrossRef]
- Smith, G. A Reassessment of the Role of Iron in the 5,000-30,000 Cm-1 Region of the Electronic Absorption Spectra of Tourmaline. Phys. Chem. Min. 1978, 3, 343–373. [Google Scholar] [CrossRef]
- Rossman, G.R.; Mattson, S.M. Yellow, Mn-Rich Elbaite with Mn-Ti Intervalence Charge Transfer. Am. Mineral. 1986, 71, 599–602. [Google Scholar]
- Vigier, M.; Evans, H.; Rossman, G.R.; Jobic, S.; Fritsch, E. Fe-Ti vs. Fe-Fe Charge Transfers: A Comprehensive Review and Its Applications in Minerals and Glasses. Am. Mineral. 2025, 110. [Google Scholar] [CrossRef]
- Wilkins, R.W.T.; Farrell, E.F.; Naiman, C.S. The Crystal Field Spectra and Dichroism of Tourmaline. J. Phys. Chem. Solids 1969, 30, 43–56. [Google Scholar] [CrossRef]
- Kaewtip, M.; Limtrakun, P. Gemological and Chemical Characteristics of Green Tourmaline from Madagascar, Mozambique, and Tanzania. Walailak J Sci Technol 2016, 13, 985–992. [Google Scholar]
- Laurs, B.M.; Simmons, W.B.; Rossman, G.R.; Fritz, E.A.; Koivula, J.I.; Anckar, B.; Falster, A.U. Yellow Mn-Rich Tourmaline from the Canary Mining Area, Zambia. Gems Gemol. 2007, 43, 314–331. [Google Scholar] [CrossRef][Green Version]
- Fritz, E.A.; Breeding, C.M.; Rossman, G.R.; Laurs, B.M.; Simmons, W.B.; Falster, A.U. Yellow-Green Clinohumite and Yellow Chondrodite from Tanzania. Gems Gemol. 2007, 43, 377–379. [Google Scholar] [CrossRef]
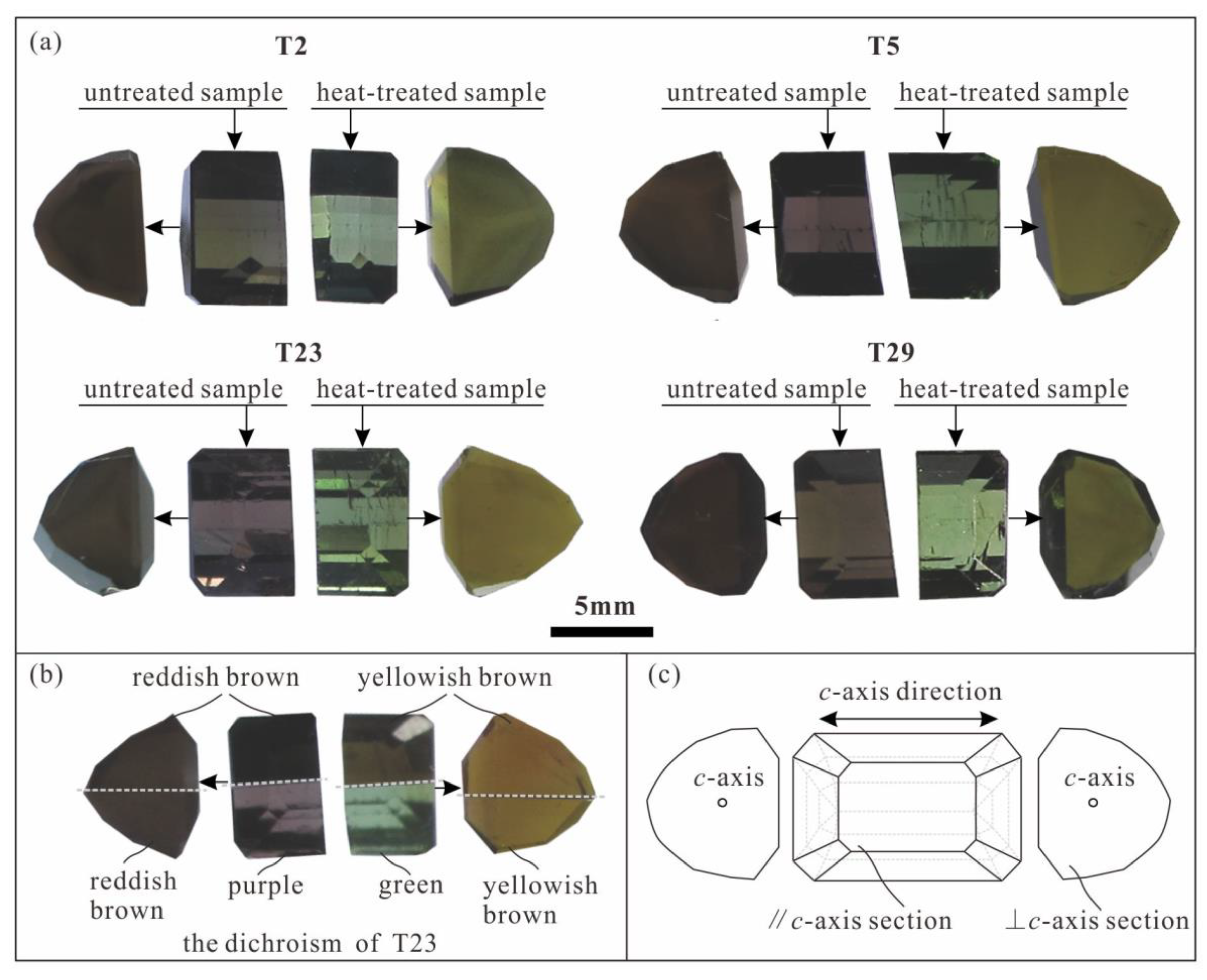
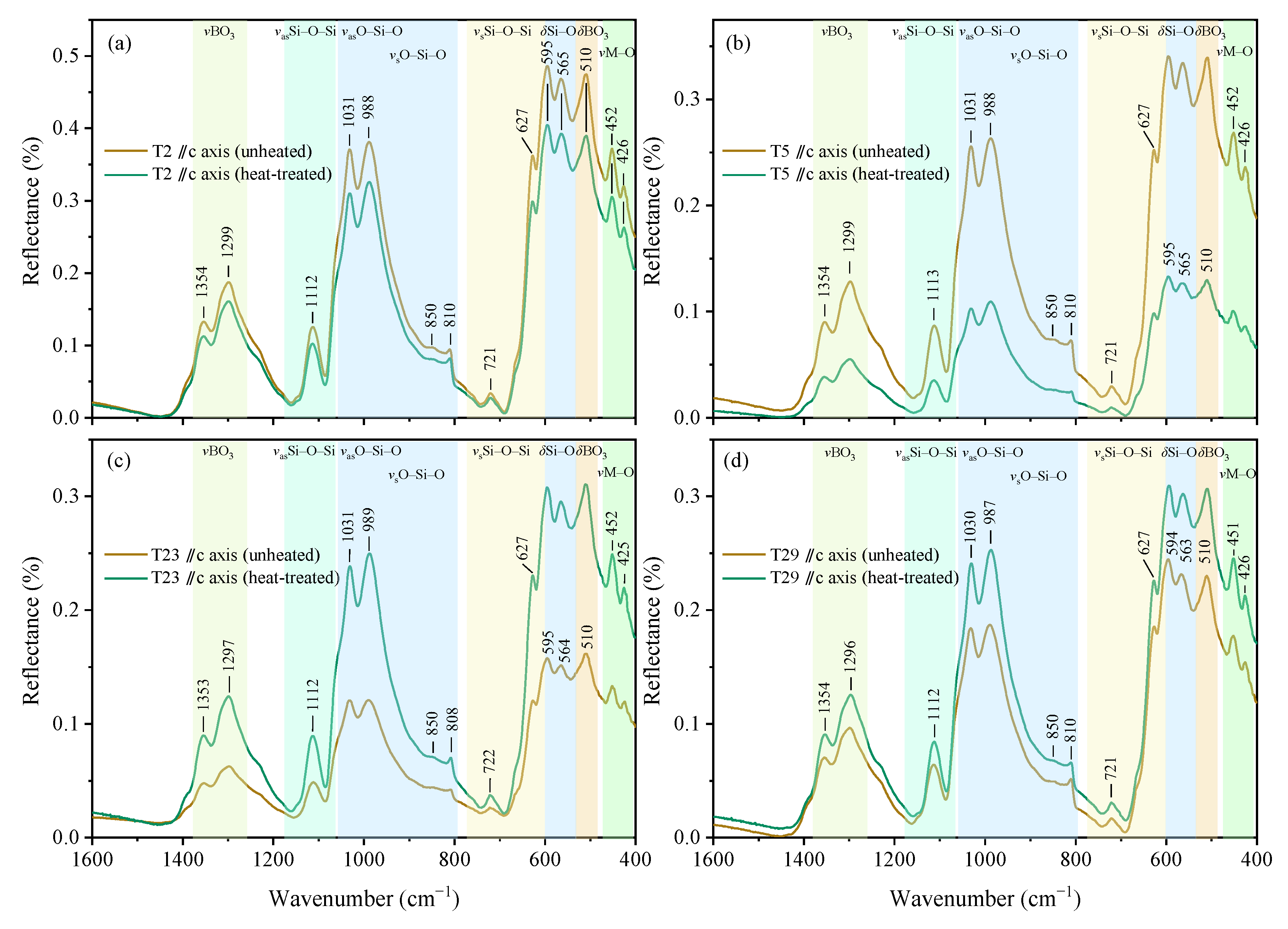

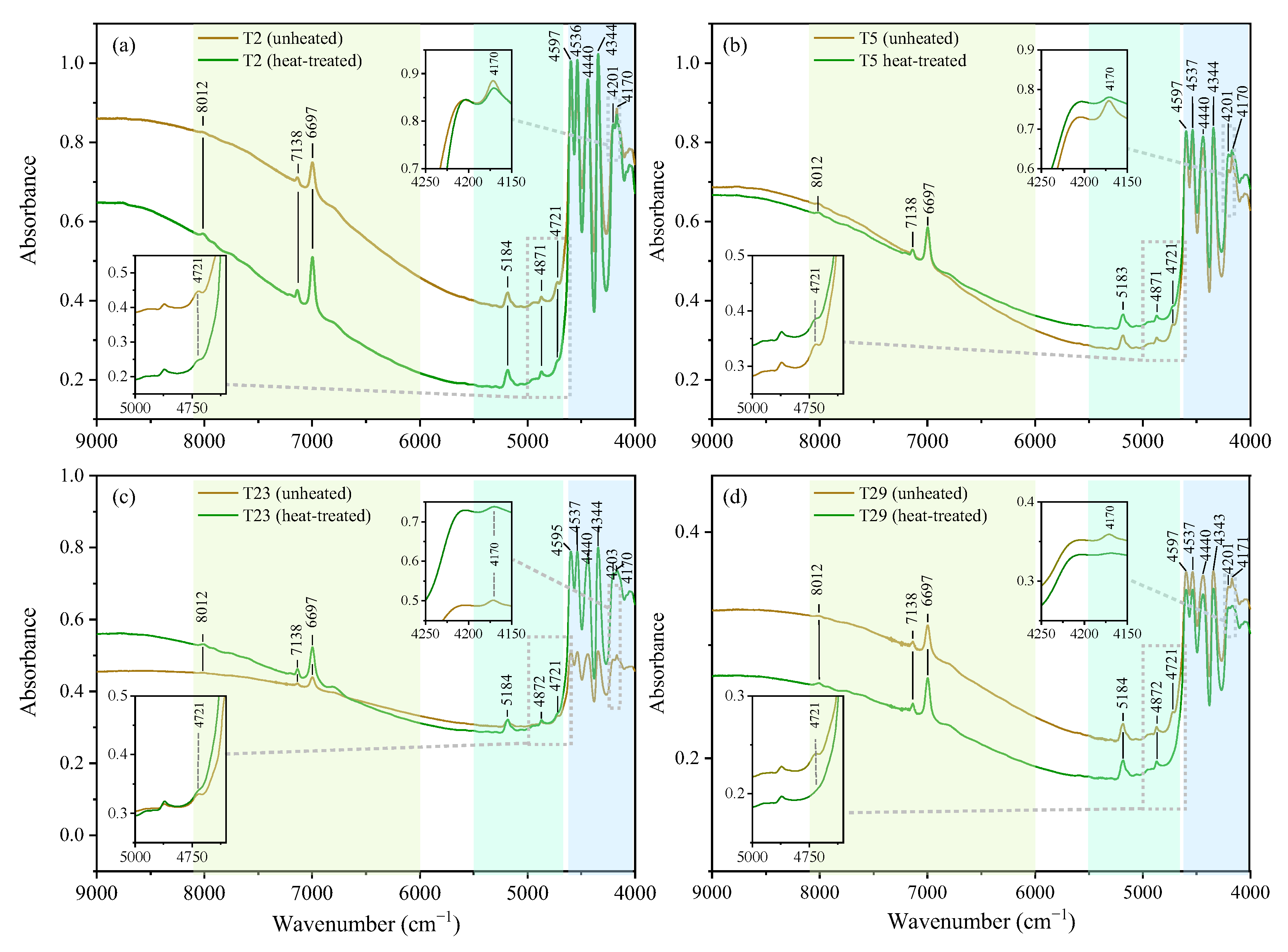


| Sample No. | Temperature (°C) | Heating Rate | Holding Time (h) | Atmosphere |
|---|---|---|---|---|
| °C/min | ||||
| T2 | 500 | 1 | 0.5 | reduction |
| T5 | 500 | 3 | 0.5 | reduction |
| T23 | 500 | 4 | 1 | reduction |
| T29 | 500 | 2 | 0.5 | reduction |
| Sample No. | Weight (g) | Transparency | Refractive Index | Birefringence | UV Fluorescence | Color | Dichroism | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Ne | Long | Short | Unheated | Heat-Treated | Unheated | Heat-Treated | ||||
| T2 | 0.511 | transparent | 1.640 | 1.620 | 0.020 | inert | inert | yellowish brown | yellowish green | reddish brown + yellowish brown | yellowish brown + green |
| T5 | 0.470 | transparent | 1.640 | 1.620 | 0.020 | inert | inert | reddish brown | yellowish green | Brown + light green | yellowish brown + green |
| T23 | 0.442 | transparent | 1.640 | 1.620 | 0.020 | inert | inert | reddish brown | yellowish green | purple + reddish brown | yellowish brown + green |
| T29 | 0.407 | transparent | 1.640 | 1.620 | 0.020 | inert | inert | yellowish brown | yellowish green | pink green + brown | yellowish brown + green |
| Sample No. | T2 | T5 | T23 | T29 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| SiO2 | 38.94 | 38.79 | 38.76 | 38.82 | 38.59 | 38.67 | 38.79 | 38.63 |
| TiO2 | 0.09 | 0.07 | 0.06 | 0.06 | 0.02 | 0.04 | 0.05 | 0.06 |
| Al2O3 | 38.57 | 38.72 | 38.79 | 38.85 | 38.60 | 38.58 | 38.47 | 38.69 |
| FeO | 1.41 | 1.46 | 1.15 | 1.13 | 1.03 | 1.04 | 1.10 | 1.08 |
| CaO | 0.40 | 0.42 | 0.46 | 0.43 | 0.53 | 0.50 | 0.50 | 0.50 |
| MnO | 2.19 | 2.14 | 2.18 | 2.29 | 2.62 | 2.52 | 2.43 | 2.43 |
| ZnO | 0.11 | 0.07 | 0.07 | 0.07 | 0.00 | 0.04 | 0.04 | 0.03 |
| Na2O | 2.32 | 2.26 | 2.11 | 2.18 | 2.10 | 2.18 | 2.12 | 2.05 |
| F | 1.04 | 0.96 | 1.02 | 0.94 | 0.10 | 1.03 | 0.10 | 0.94 |
| H2O * | 3.36 | 3.40 | 3.37 | 3.41 | 3.37 | 3.36 | 3.37 | 3.40 |
| B2O3 * | 11.69 | 11.71 | 11.74 | 11.74 | 11.77 | 11.77 | 11.68 | 11.68 |
| Li2O * | 2.04 | 2.04 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 | 2.07 |
| Total | 102.18 | 102.05 | 101.79 | 102.01 | 101.72 | 101.80 | 101.63 | 101.57 |
| O=F | 0.44 | 0.41 | 0.43 | 0.40 | 0.42 | 0.43 | 0.42 | 0.39 |
| Total * | 101.74 | 101.64 | 100.37 | 101.61 | 101.30 | 101.37 | 101.21 | 101.17 |
| T: Si | 6.06 | 6.04 | 6.04 | 6.04 | 6.02 | 6.03 | 6.06 | 6.03 |
| B | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Z: Al | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Y: Al | 1.07 | 1.10 | 1.12 | 1.12 | 1.10 | 1.09 | 1.08 | 1.12 |
| Ti | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 |
| Mn | 0.29 | 0.28 | 0.29 | 0.30 | 0.35 | 0.33 | 0.32 | 0.32 |
| Fe2+ | 0.18 | 0.19 | 0.15 | 0.15 | 0.13 | 0.14 | 0.14 | 0.14 |
| Zn | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Li | 1.28 | 1.28 | 1.30 | 1.29 | 1.30 | 1.30 | 1.30 | 1.30 |
| X: Ca | 0.07 | 0.07 | 0.08 | 0.07 | 0.09 | 0.08 | 0.08 | 0.08 |
| Na | 0.70 | 0.68 | 0.64 | 0.66 | 0.64 | 0.66 | 0.64 | 0.62 |
| V: OH | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| W: OH | 0.49 | 0.53 | 0.50 | 0.54 | 0.51 | 0.49 | 0.51 | 0.54 |
| F | 0.51 | 0.47 | 0.50 | 0.46 | 0.49 | 0.51 | 0.49 | 0.46 |
| Samples No. | Li | B | Na | Mg | K | Ca | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 | 9530.01 | 36,297.61 | 18,037.89 | 5.21 | 165.33 | 3187.23 | 1111.77 | — | — | 17,346.71 | 10,750.68 | — | — | 10.87 | 736.06 |
| T5 | 9571.51 | 36,381.28 | 17,977.81 | 4.47 | 162.35 | 3480.91 | 942.31 | 0.40 | — | 17,871.53 | 9001.68 | — | — | 10.02 | 569.89 |
| T23 | 9572.69 | 36,533.50 | 17,886.37 | 3.70 | 167.76 | 3948.25 | 582.43 | 0.36 | — | 20,669.52 | 8396.46 | — | — | 8.27 | 276.87 |
| T29 | 9636.87 | 36,172.70 | 17,710.79 | 3.81 | 162.99 | 4100.36 | 748.28 | — | — | 19,608.02 | 8612.76 | — | — | 10.25 | 331.12 |
| This Study | Elbaite S5 [20] | Elbaite Tg-1 [21] | Schorl [22] | Dravite [22] | Assignment | |
|---|---|---|---|---|---|---|
| ‖c-Axis Section | ⊥c-Axis Section | |||||
| 1354(m) | 1353(w) | 1355 | 1357 | 1353 | 1345 | vs BO3 or vas BO3 |
| 1299(s) | 1298(s) | 1309 | 1297 | 1255 | 1312 | |
| 1165 | v Si–O–Al | |||||
| 1112(m) | 1110(s) | 1106 | 1112 | 1085 | 1112 | vas Si–O–Si |
| 1053(w) | 1056 | vas Si–O–Si | ||||
| 1031(s) | 1031(s) | 1031 | 1031 | 1039 | 1065 | vas O–Si–O |
| 988(s) | 992(s) | 996 | 988 | 985 | 1040 | vas O–Si–O |
| 850(w) | vs O–Si–O | |||||
| 810(w) | 800 | 802 | vs O–Si–O | |||
| 791(s) | 794 | 790 | 780 | 787 | vs Si–O–Si | |
| 753(w) | 755 | 752 | 758 | 727 | vs Si–O–Si | |
| 721(w) | 716(s) | 717 | 716 | 713 | 720 | vs Si–O–Si |
| 651 | 650 | v M–O (M = Fe, Mg, Al) | ||||
| 627(s) | 631(s) | 629 | 636 | 631 | vs Si–O–Si | |
| 595(s) | 596(m) | 593 | 595 | 608 | δ Si–O | |
| 565(s) | 535 | 565 | 590 | δ Si–O | ||
| 510(s) | 508(s) | 515 | 503 | 510 | 520 | δ BO3 |
| 483 | 475 | δ Si–O | ||||
| 452(m) | 453(w) | 458 | 449 | 449 | v M–O | |
| 426(m) | 429(w) | 431 | 428 | 422 | 430 | v M–O |
| This Study | Elbaite [4,7] | Liddicoatite [7] | Uvite [7] | Assignment |
|---|---|---|---|---|
| ⊥c-Axis Section | ||||
| 223(s) | 222 | YO6 vibrations | ||
| 242(w) | 244 | O–YAl–O bond bending | ||
| 376(s) | 373 | 383 | ZAl–O bond stretching | |
| 408(w) | 407 | Onon–Si–Onon bond bending | ||
| 512(w) | 508 | Oxygen vibrations in Si–O rings | ||
| 637 | Si–Obr bond rocking | |||
| 727 | “Breathing” of Obr in SiO rings | |||
| 730(vs) | 731 | 734 | 728 | B–O bond stretching and O–B–O bond bending |
| 756(sh) | 760 | Si–O bond stretching and Si–O–Si bond bending | ||
| 837(w) | 840 | 839 | Si–Onon bond stretching | |
| 1063(s) | 1059 | 1068 | 1070 | Si–Onon bond stretching |
| 1131 | δMgOH | |||
| 1395(w) | 1400 | 1400 | 1400 | B–O stretching mode |
| 3460 | ZAlZAlYAl-OH3 | |||
| 3498(m) | 3490 | 3500 | Two Z-site cations and one Y-site cation link OH3: ZAlZAlYAl-OH3 or ZAlZAlYLi-OH3 in liddicoatite; ZAlZAlYMg-OH3 in uvite | |
| 3560(sh) | 3560 | 3570 | ||
| 3593(m) | 3585 | 3597 | ||
| 3653(w) | 3655 | 3640 | 3728 | Three Y-site cations link OH1: YAlYAlYLi-OH1 orYAlYLiYLi-OH1 in liddicoatite; YMgYMgYMg-OH1 in uvite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Yue, S. Mechanisms of Thermal Color Change in Brown Elbaite–Fluorelbaite Tourmaline: Insights from Trace Elements and Spectral Signatures. Minerals 2025, 15, 1032. https://doi.org/10.3390/min15101032
Li K, Yue S. Mechanisms of Thermal Color Change in Brown Elbaite–Fluorelbaite Tourmaline: Insights from Trace Elements and Spectral Signatures. Minerals. 2025; 15(10):1032. https://doi.org/10.3390/min15101032
Chicago/Turabian StyleLi, Kun, and Suwei Yue. 2025. "Mechanisms of Thermal Color Change in Brown Elbaite–Fluorelbaite Tourmaline: Insights from Trace Elements and Spectral Signatures" Minerals 15, no. 10: 1032. https://doi.org/10.3390/min15101032
APA StyleLi, K., & Yue, S. (2025). Mechanisms of Thermal Color Change in Brown Elbaite–Fluorelbaite Tourmaline: Insights from Trace Elements and Spectral Signatures. Minerals, 15(10), 1032. https://doi.org/10.3390/min15101032





