Abstract
This study investigates the environmental stability of ferronickel slag (FNS) and primary lead slags (GCS and FCS) from historical metallurgical complexes in Greece, in rainwater and seawater media. Leaching experiments revealed that nickel is the most mobile element from FNS (43.5 μg·g−1 in seawater after 90 days). Chromium release, on the other hand, is very limited, not exceeding 0.04 μg·g−1. In lead slags, zinc and lead exhibit significant leaching (up to 650 and 230 μg·g−1, respectively), while arsenic release reaches 22.6 μg·g−1. GCS contains pores primarily in the range of 50–90 Å. The majority of pore volume in FCS is centered around 30 Å. The porosity appears to have a significant effect on the element’s leachability. Pb, Zn, As, Sb, and Cd are released in significantly higher amounts from the finely porous FCS compared to GCS. Thermodynamic modeling was used to identify the pollutant speciation in water media in relation to the oxygen concentration. The release of toxic elements such as Cr from FNS and As from lead slags is enhanced under oxic (open-air) conditions. Therefore, their land disposal poses a greater environmental threat compared to sea disposal, where anoxic conditions prevail.
1. Introduction
1.1. Production and Toxicity of Ferronickel and Lead Slags
Enormous quantities of metallurgical solid residues are generated globally. According to rough estimates, approximately 400 million tonnes (Mt) of ferrous slags and 50 Mt of non-ferrous slags are accumulated annually [1,2]. The ferronickel and lead industries significantly contribute to the production of these residues. Around 150 Mt of ferronickel slag is generated each year through the processing of oxidized nickeliferous ores (laterites) [3], while over 5.5 Mt of primary and secondary lead slags are produced annually from the processing of lead concentrates and lead acid battery scrap [4]. In general, slags may pose risks to both ecosystems and human health. Various toxic trace metals and metalloids contained in slags can be released under slightly acidic or alkaline conditions and subsequently transported into surface water, groundwater, or seawater [5,6]. These dissolved elements can bioaccumulate in plants consumed by livestock, thereby entering the human food chain, or be taken up by aquatic organisms and passed on to higher-order animals and humans. A secondary, less common contamination pathway involves the dispersion of fine slag particles—via water or air—into surrounding soils, especially in the case of finely granulated slags [5,6]. The chemical and solid phase composition of slags is directly related to the melt chemistry within the metallurgical furnace and the subsequent cooling process. These factors serve as key indicators of the slags’ chemical stability under various environmental conditions. Water-quenched electric arc furnace (EAF) ferronickel slags are primarily composed of a dominant amorphous glassy matrix, with a minor crystalline fraction consisting of olivine, pyroxene, feldspar, spinel-group phases, and unseparated ferronickel alloy particles [7,8,9]. In contrast, air-cooled primary lead slags are predominantly crystalline, with a smaller proportion of coexisting amorphous material. Commonly observed mineral phases include zinc and lead oxides and sulfides, olivines, willemite (Zn2SiO4), fayalite (Fe2SiO4), spinels such as franklinite (ZnFe2O4), and unseparated metallic lead [10]. Nearly all of these phases are potential carriers of trace element pollutants. Lead, apart from micro-sized droplets, Pb-sulfides, and other secondarily formed Pb species, is present at concentrations of up to 4 wt.% in the glass phase [11], forming PbO4 pyramid polymeric chain networks [12]. The presence of sub-micron lead particles is also possible [5]. Zinc is the most abundant detected trace element contained in pyroxenes, melilite, and glass. In glass, it forms ZnO4 tetrahedra within the silicate network [13]. High nickel concentrations have been reported in spinels and olivine (up to 2 wt.%) [14]. Franklinite and lead sulfides are the main arsenic-bearing phases [6,10]. Chromium occurs in spinels and pyroxenes [5]. The presence of the rarer but extremely toxic cadmium has been identified in the silicate minerals ardystonite (Ca2ZnSi2O7) and alamosite (Pb12Si12O36) [15].
1.2. Hazard Assessment of Ferronickel and Lead Slags
The corrosion/weathering of slags, in natural aqueous media (such as rainwater and seawater), and the subsequent release of hazardous metals and metalloids in ionic form are enhanced in natural environments by several factors, including pH changes, periodic climate alteration, acid rain, redox conditions, dissolved oxygen, the presence of Fe3+ as an oxidant, organic compounds, and microbial activity [5,6]. The SPLP (Synthetic Precipitation Leaching Procedure) and TCLP (Toxicity Characteristic Leaching Procedure) techniques are used to simulate and quantify the release of hazardous elements in aqueous media. SPLP is mainly used to simulate long-term acid rain conditions, while TCLP is primarily applied to simulate short-term aggressive leaching conditions. Both techniques provide a toxicity assessment of a material; however, they cannot accurately reproduce real environmental conditions because they use simplified leaching media and do not take into account the dissolution kinetics. The literature results of the TCLP and SPLP tests, as well as tests following alternative methodologies on primary lead and ferronickel slags are summarized in Table 1. A direct comparison with leachability data obtained from other studies was not possible due to the fact that materials with different physicochemical properties were used and different leaching methodologies were applied. Nevertheless, it can be generally concluded that (a) lead and zinc are the main released contaminants in the case of lead slags, while arsenic and cadmium—at concentrations below 1 mg/L—may also be released; (b) nickel, chromium, and lead are the primary pollutants in ferronickel slags; and (c) the particle size of the tested residues significantly impacts the degree of leachability. Lead concentrations differ by an order of magnitude in solutions derived from the leaching of lead slags with particle sizes of up to 9.5 mm and 0.8 mm, respectively, at pH = 5 [16,17].

Table 1.
Literature results of leachability tests on primary lead and ferronickel slags.
Despite the abundance of leachability assessment tests on ferronickel and primary lead slags using synthetic acid solutions or following the TCLP and SPLP procedures, the environmental stability of ferrous and lead slags in real rainwater and seawater environments has been studied to a limited extent. The environmental stability of ferrous and lead slags in real rainwater and seawater environments has been studied to a limited extent. However, a number of leaching studies conducted under prolonged or dynamic conditions using pure water or artificial seawater simulate the behavior of slags in terrestrial or marine ecosystems and provide valuable data. Regarding prolonged leaching (up to 6 months), a basic oxygen furnace (BOF) steel slag with deionized water under both aerobic and anaerobic (i.e., inert atmosphere) conditions showed that vanadium (V) release approximately doubled under aerobic conditions (859 µg/L at 6 months) [26]. Manganese was released at concentrations of up to 0.0029 mg/L after 25 h of leaching from basic steel slag in artificial seawater [27]. The leaching of BOF slag in artificial seawater under dynamic conditions (160 shaking cycles per minute) revealed limited Fe release not exceeding 1.5 µg/L after 192 h [28]. The exposure of BOF slag to marine mesocosm conditions resulted in the release of vanadium at concentrations up to 70 µg/L after 14 days [29]. The stability of blast furnace (BF) and BOF slags in seawater under dynamic conditions was also examined in a recent study, showing the mobilization of V and Cr at concentrations of 0.055 mg/L and 0.0025 mg/L, respectively, after 24 h [30]. Concerning the behavior of lead slags, column leaching tests using pure water at a flow rate of 60 mL/h revealed a cumulative release of Pb and Zn of 0.4 g/m2 and 0.35 g/m2, respectively, after 2500 h. Microscopic analysis of the leached samples showed the dissolution of lead droplets and the formation of secondary Pb species [31]. Column leaching tests on Pb-containing zinc slags using slightly acidic water, corresponding to the half the annual rainfall in the slag sampling region, revealed the mobilization sequence: Zn > Cr > Cd, Cu > Pb. The maximum Pb concentration released after five leaching cycles was approximately 370 µg/L [32]. The long-term stability of primary lead slags over prolonged leaching durations (up to 12 years) using deionized water as a leaching mean has been studied. After 12 years, a significant mobilization of Zn (8460 mg/kg) was observed, while the mobilization of Pb and As was limited (15.3 and 0.06 mg/kg, respectively). The release of Pb is hindered by the formation of secondary lead carbonates, sulfates, and phosphates [33].
The present study aims to contribute to the knowledge of the environmental stability of ferronickel and primary lead slags deposited in two major formed metallurgical complexes in Greece (Figure 1a) through (a) the performance of leaching tests using real rainwater, seawater and deionized water media and (b) the examination of pollutants speciation in water media in relation to the oxygen concentration and their dissolution behavior using thermodynamic modeling. Furthermore, the effect of porosity and pore size on the leachability was examined. It should be stressed that the behavior of electric arc furnace nickel slags and Zn-containing primary lead slags in seawater has not yet been studied.
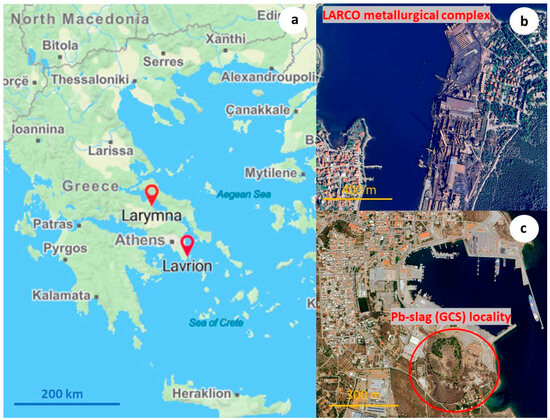
Figure 1.
Lavrion and Larymna former metallurgical complexes in the broader region of Central Greece administrative region: (a) ferronickel production plant in Larymna (Evoikos gulf-Aegean Sea) where the cooling of slag is performed (b), and Pb-slag (GCS) dumps in proximity to Lavrion town (c) (modified pictures sourced from Google Maps).
2. Materials and Methods
2.1. Slag Sites and History
2.1.1. Ferronickel Slag
The ferronickel slag was produced by the metallurgical company LARCO GMMSA in Larymna, Greece, located approximately 72 km northwest of Athens, from 1969 to 2022 through the reductive smelting of pre-roasted nickeliferous laterite in electric arc furnaces (EAFs). The average annual slag production was approximately 2 million tonnes [34]. The slag is discharged from the EAFs through tapholes at temperatures of about 1300–1400 °C and directed vertically into a pressurized seawater jet, where it undergoes granulation. The material then flows into seawater reservoirs, where it is further cooled (Figure 1b). Finally, it is loaded—using clamshell cranes—onto barges and either disposed of on the deeper seabed or partially valorized (about 10% of total annual production) as aggregate for road construction [35]. According to rough estimates, more than 80 million tonnes of ferronickel slag have been deposited in the Aegean Sea (north Evoikos gulf) near LARCO’s facilities.
2.1.2. Primary Lead Slags
Primary lead slags in Lavrion historic mining area, located approximately 40 km southeast of Athens, were generated through the mining and metallurgical exploitation of the region’s Ag-Pb-Zn ore. These activities took place during the 19th and 20th centuries, and the produced slags are accumulated near the town of Lavrion and its harbor in land sites and seawater environment (Figure 2a–c). Slags from the “Greek Company” (GCS) originated from the re-smelting of ancient slags, which were primarily produced during the 5th and 4th centuries BC. GCS were deposited approximately 1 km south of Lavrion, covering an area of 90,000 m2 (Figure 1c). Slags from the “French Company” (FCS) resulted from the pyrometallurgical processing of Pb-Zn-rich concentrates, carried out from the late 19th century until the 1970s. FCSs were deposited approximately 1.5 km north of Lavrion, currently covering an area of 110,000 m2. The reductive smelting of ancient slags and Pb-Zn concentrates was carried out in Castilian-type blast furnaces and water-jacket furnaces, respectively. The slags were cooled slowly in the atmosphere. As of the late 1990s, the total quantity of GCSs and FCSs was estimated at approximately 7 million tonnes [36]; however, a significant portion of this material has since been utilized as aggregate in road and port construction.

Figure 2.
GCS dumps in Lavrion area: (a) FCSs on a coastline; (b) weathered/rounded FCSs from the interaction with seawater; (c) GCS ranging in size from a few millimeters to several centimeters; (d) and water-quenched FNSs (e).
2.2. Materials and Sampling
A homogeneous amount (20 kg) of a water-quenched FNS sample (Figure 2e) was supplied by LARCO General Mining & Metallurgical Co. prior to the termination of the company’s operations in 2021. GCS and FCS (100 kg of each material) were collected from at least 30 different points across the surface of the dumps (Figure 2a,d). Representative samples (5 kg) were obtained using riffle splitter. GCS and FCS samples exhibiting inhomogeneous particle sizes, ranging from few millimeters to several centimeters (Figure 2d), were crushed using a jaw crusher (Retsch BB 100 Series, Retsch GmbH, Haan, Germany) and subsequently were ball milled with a Retsch PM100 (Retsch GmbH, Haan, Germany) machine to reduce their particle size to levels comparable to that of the FNS sample.
2.3. Analytical Techniques
The phase characterization of the slags was carried out by X-ray diffraction using a Bruker D8-Focus (Bruker Corporation, Billerica, MA, USA) diffractometer with nickel filtered CuKa radiation (l = 1.5406 Å), at 40 kV and 40 mA. The angular interval scans were carried out over the 5–70° 2θ range in 0.02° steps and counts of 4 s per step. Point analyses in microscale were carried out on a Scanning Electron Microscopy (SEM) JEOL JSM-5600 (JEOL Ltd., Akishima, Tokyo, Japan), combined with the energy dispersive system OXFORD LINK ISIS 300, along with its respective software for ZAF correction quantitative analysis. The system was operating at 20 kV, 0.5 nA and an analysis duration of 60 s. The bulk chemical analysis of the solid slag samples was conducted via X-ray fluorescence spectrometry (XRF) using a SPECTRO XEPOS ED-XRF (SPECTRO Analytical Instruments, Kleve, Germany) spectrometer. Prior to the analysis, the samples were vitrificated using a flux mixture of Li-tetraborate and Li-metaborate flux at a mass ratio 1:1 and 1% LiBr as a binding agent. The chemical analyses of the initial solid ferronickel (after fusion with Li-borate/-tetraborate and subsequent dissolution), the rainwater, seawater, and all fluid leachates were conducted using an Agilent 7900 ICP-MS (Agilent Technologies, Santa Clara, CA, USA) instrument. Specifically, undiluted seawater was analyzed using the Ultra High Matrix Introduction (UHMI) aerosol dilution system with a 25-fold online dilution. Helium was used as the collision gas to minimize polyatomic interferences, and all samples were acidified with nitric acid to a final concentration of 2% total acid. Calibration standards were obtained from CPAChem, and calibration curves were established over a concentration range of 0.5–20 μg/L. ICP measurements were repeated twice. The relative standard deviations (%RSD) for the Ni, Co, Cr, Pb, Zn, As, Sb, and Cd concentrations were approximately 4%, 3%, 4%, 4%, 5%, 2%, 5%, and 6%, respectively. Leaching tests were repeated twice for the shorter durations (15 and 30 days), as slightly elevated %RSD values were observed for these time periods. The %RSD of the pollutant concentrations in the leachate replicates was below 5% in all cases, indicating the repeatability of the entire procedure. The reference materials were as follows: SX66-06 tundish slag (NiO: 0.216 wt.%, Mg-containing), 77 Ferro-Chrome slag and SX62-03 cupola slag (1.05 wt.% PbO, 12.32 wt.% ZnO), all provided by the Fluxana GmbH & Co. KG (Bedburg-Hau, Germany) were used for the measurement standardization of Ni in FNS, Cr in FNS and Pb/Zn in the lead slags. The analyses of the reference materials and slag samples were performed in triplicate. The standard deviation values did not exceed 5% in any case.
The Brunauer–Emmett–Teller (BET) analysis was employed to investigate the specific surface area and the pore size distribution using an Autosorb-1 MP (Paar Group AG, Graz, Austria) gas analyzer. The pore size distributions of the three materials were extracted with the DFT method (Calc. Model: N2 at 77 K on silica).
2.4. Leaching Methodology
Slag samples (amount of 10 g) were leached with seawater and rainwater (volume of 1 L) at static conditions and open-air atmosphere for durations of 15, 30, 45, 60, 75, and 90 days. Seawater was collected from the Evoikos gulf in the center of Aegean Sea. Rainwater was collected in the nearby region (Evia Island), for the avoidance of possible acid rain effect near to industrial and urban regions, during the month of March. Seawater was collected in plastic containers from at a depth of 5 m, 100 m off the nearby coastline. Rainwater was collected using a 2 × 2 × 0.2 m plastic trough placed on the roof of an isolated building. A total volume of 60 L was collected in each case. Leachates were submitted to nanofiltration, using a 0.45 μm membrane filter. Nanofiltration was considered necessary in order microorganisms (such as microscopic plankton) be removed. Leaching with deionized water was performed according to the EN 12457-2 methodology appropriate for the testing of granular waste materials and sludges [37].
2.5. Thermodynamic Study
The thermodynamic investigation of the slags leaching with seawater and rainwater was conducted using HSC 10 software and more specifically via its module “Equilibrium compositions”. The leaching process was simulated in respect to the realistic (a) slag/water amount ratios and (b) concentration of the main contaminants (lead, arsenic, nickel and chromium) in the slags. The effect of oxygen concentration in the aqueous media was taken into consideration.
3. Results and Discussion
3.1. Physicochemical Characterization of Ferronickel and Lead Slags
Water-quenched FNS exhibits a standard particle size distribution due to the relatively homogeneous physicochemical characteristics of the laterite feed and the well-standardized pyrometallurgical process (Figure 2e, Table 2). On the contrary, the granulometry of GCS and FCS (Figure 2d) is extremely inhomogeneous. Aiming to obtain of comparable leaching results, as it was previously described, GCSs and FCSs were crushed and milled. Granulated lead slag samples were subsequently screened and particle size fractions corresponding to the respective fractions of FNS (Table 2) were obtained.

Table 2.
Particle size range of the FNS sample.
The surface and pore structural characteristics of the initial materials are summarized in Table 3. As it can be observed, the BET area of the slags was relatively small, ranging between 0.36 and 2.07 m2/g. The N2 adsorption–desorption curves show that maximum adsorption capacity of FNS reached 1.03 cm3 (STP)/g, while the respective values for GCS and FCS were 2.57 and 2.04 cm3 (STP)/g (Figure 3). The shape of the isotherm of the FNS sample was indicative of a non-porous or macroporous material, whereas the isotherms of the lead samples were characteristic of mesoporous materials with slit-like pores. In particular, the sample FCS exhibited a well-shaped adsorption isotherm with the characteristic inflection point at low relative pressures which may be relevant to the existence of phyllosilicate phases. The pore size distributions of the three materials are comparatively presented in Figure 4. Amongst the three samples, FCS exhibited a multimodal pore size distribution with a pore size in the range of 20–90 Å, while the major volume of the pores was centered around 30 Å. The sample GCS possessed larger pores, with the main portion of them having a size in the range of 50–90 Å. The pore size distribution of FNS confirms its moderately developed pore structure.

Table 3.
BET surface area characteristics of ferronickel and lead slags.
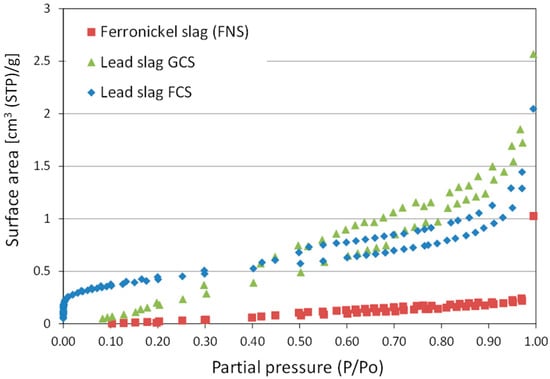
Figure 3.
BET adsorption isotherms of ferronickel and lead slags.
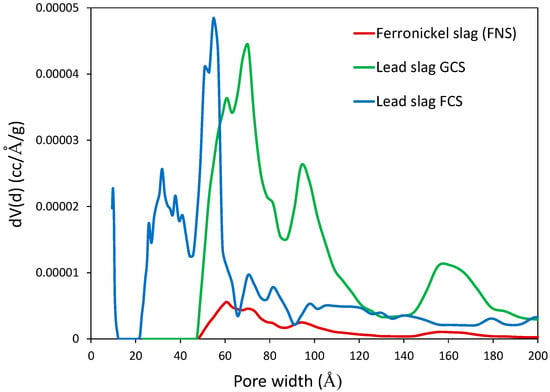
Figure 4.
Pore size distribution of ferronickel and lead slags.
The bulk chemical analysis of both lead slags showed (a) elevated zinc concentrations (ZnO around 4 wt.%) originating through the direct roasting and reductive smelting of the initial (Ag)-Pb-Zn flotation concentrate; (b) a relatively low content of lead (PbO < 1 wt%.), indicating an efficient separation between the (Ag)-Pb alloy and the slag phase even in case of old technology blast furnaces operating during the late 19th and early 20th centuries; and (c) a limited arsenic content (As2O3 ≤ 0.1 wt%.). Ferronickel slag contains a notable amount of chromium (2.27 wt.%) and relatively low nickel and cobalt concentrations (0.16 and 0.02 wt.%, respectively) (Table 4). GCS and FCS originate from the processing of Pb-Zn-containing concentrates with variable chemical and mineralogical compositions (i.e., different Pb concentrations and presence of various gangue minerals). This results in the production of slags with diverse chemical and mineralogical characteristics. Therefore, their presented bulk analyses, despite the multiple sampling and their crushing and homogenization, can be considered as indicative. On the other hand, ferronickel slag consists of a solid residue with a stable chemical composition in a specific metallurgical plant due to the simple mineralogy of the laterite raw material. FNS, taking into account its homogeneity, was further analyzed via ICP-MS (Table 5). The analysis exhibits: (a) the absence of toxic elements (i.e., As, Cd, etc.) and natural occurring radionuclides (i.e., U, Th) at notable concentrations and (b) an elevated total content of REEs + Y + Sc = 137.13 ppm (76 + 19.1 + 42 ppm).

Table 4.
Bulk–XRF chemical analyses of the ferronickel and lead slags. The major pollutants in the slags are highlighted in bold.

Table 5.
ICP-MS trace element analysis of the ferronickel slag.
The solid phase composition of the ferronickel slag was revealed through x-ray diffractometry and scanning electron microscopy. FNS is a mainly an amorphous (glassy) material, and only a multi-element (Mg-Cr-Al-Ti) spinel-type was identified as the crystalline phase (Figure 5). Scanning electron microscopy further revealed the presence of isolated non-separated, through the reductive smelting step, Fe-Ni alloy droplets. The size of both spinel and Fe-Ni phases ranged from few μm to 300 μm. Energy-dispersive spectroscopy analyses indicate that the chromium content in the spinel was elevated (Cr2O3 exceeds 37 wt.%). The nickel concentration in the Fe-Ni droplets was in the range of 17 wt.%, while other trace elements such as cobalt and chromium were not identified (Figure 6). The approximate chemical composition (wt.%) of the amorphous phase, based on the average of several EDS analyses, was as follows: SiO2 = 45.0, Fe2O3 = 26.2, CaO = 9.9, Al2O3 = 9.8, and MgO = 8.6.
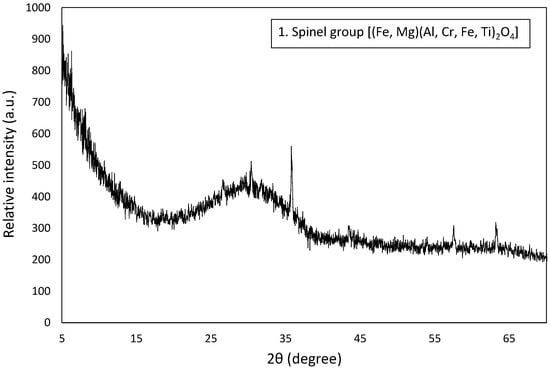
Figure 5.
X-ray diffraction pattern of the FNS sample.
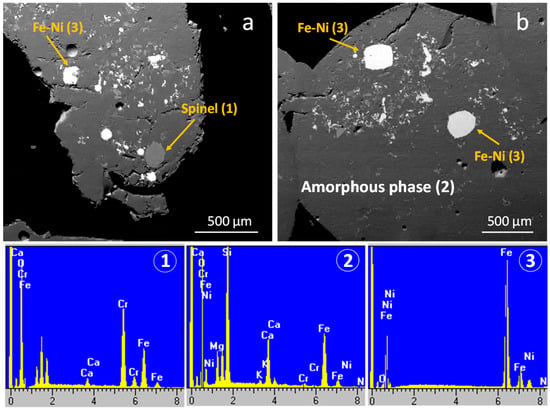
Figure 6.
Scanning electron microscopy and representative energy dispersive spectroscopy analyses of FNS sample. The representative EDS analyses (1), (2) and (3) correspond to spinel, the amorphous phase and Fe-Ni, as shown in the micrographs.
Primary lead slags, both GCS and FCS, exhibited a high degree of crystallization. Ca-Fe-/Fe-silicates, Zn-Fe-oxides and Fe-chlorides (esseneite, fayalite, franklinite, hydromolysite), and fluorite are the major crystalline phases. The main solid phase differences between GCS and FCS were as follows: (a) the presence of an additional illite phase in the FCS, and (b) the presence of fluorite and higher x-ray intensity in the GCS (Figure 7). The presence of the layer silicates (mainly illite) in the FCS is consistent with its microporosity, as revealed by the BET analysis. Metallic lead was scarcely observed, while Pb and Zn sulfides, as well as other secondarily formed phases, were not detected.
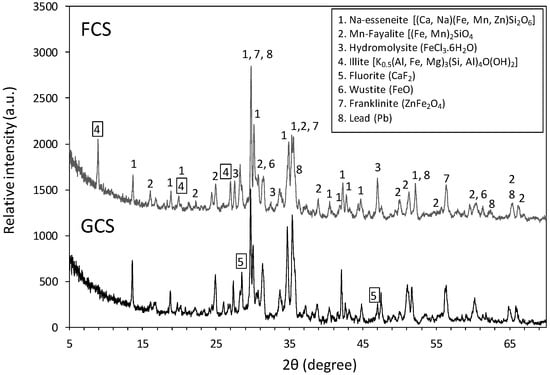
Figure 7.
X-ray diffraction of Lavrion primary lead slags (GCS and FCS).
The micro-scale topography of GCS and FCS provides additional insights into their solid-state phases. In addition to the dominant crystalline phase, an amorphous phase was dispersed throughout both slags, exhibiting an almost identical qualitative elemental composition. SEM-EDS semiquantitative analyses revealed the average chemical composition (wt.%) of the glass phase in GCS as follows: 39.7 SiO2, 31.0 Fe2O3, 13.2 Al2O3, 9.7 MgO, and 6.4 Na2O. The corresponding composition in FCS (wt.%) was as follows: 35.5 SiO2, 36.4 Fe2O3, 12.6 Al2O3, 8.6 MgO, and 6.9 Na2O. The majority of the species quoted in the x-ray diffractometry diagram of Figure 7 were fine-grained (a few micrometers or sub-micron in size); therefore, their identification via SEM-EDS was challenging. The dendritic intergrowths of wüstite within fine-grained or sub-micron phases were particularly well-developed in the GCS material (Figure 8b,d). Phases with exceptionally high lead content—such as metallic lead, Ag-Pb alloy droplets (with silver content up to 3 wt.%), lead sulfides, and Pb-Fe arsenates (PbO: 48.7 wt.%, As2O3: 30.4 wt.%, Fe2O3: 20.9 wt.%)—were present either as isolated particles or as aggregates within both amorphous and crystalline fractions. The particle size of Pb alloy droplets ranged from a few micrometers to 100 μm. Lead sulfides and arsenates were typically observed in aggregated form (Figure 8a,c,d).
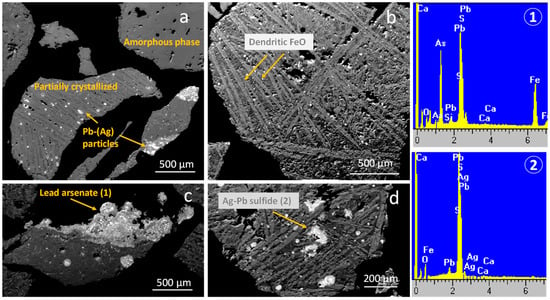
Figure 8.
Scanning electron microscopy and representative energy dispersive spectroscopy analyses of GCS (a,c) and FCS (b,d). The representative EDS analyses (1) and (2) correspond to lead arsenate and Ag-Pb sulfide, as shown in the micrographs.
3.2. Leaching Behavior of Slags
The environmental stability behavior of the slag materials was examined through their leaching in an open-air atmosphere using rainwater from central Greece and Aegean seawater media (Table 6). Prior to this investigation, a preliminary evaluation concerning the liberation degree of heavy and/or hazardous trace elements was performed following EN leaching tests (Table 7). Lead, zinc, antimony, manganese, and aluminum are the most leachable elements in lead slags, whereas nickel and zinc are the most leachable elements in ferronickel slag. In the same table, the concentrations of elements that exceed the limits for water intended for human consumption, as defined by European Union (EU) legislation and World Health Organization (WHO), are indicated. In the FCS leachate, the concentrations of Pb, Mn, and Al exceeded the limits according to the most recent directive (Pb = 5 μg·L−1, Al = 200 μg·L−1, Mn = 50 μg·L−1) [38]. In the case of the GCS leachate, concentration limits are exceeded for Pb and Sb. EU Directive 2003/40/EC establishes 5 μg·L−1 as the maximum acceptable concentration for antimony [39]. Nickel is the only element found to exceed the permissible limit (20 μg·L−1) in the FNS leachate. The EU has not established specific limits for zinc. Despite the elevated Zn amounts in lead slag leachates, none exceeded the maximum proposed concentration by the WHO (3 mg·L−1) [40]. Elements leachability determined using the EN methodology, which provides information on the leaching behavior of granular wastes and sludge, cannot be directly compared to the threshold values of the pollutants in water intended for human consumption. However, this comparison targets the identification of potentially the most leachable pollutants that could impact the ecosystems in the vicinity of slag deposition sites.

Table 6.
Major metal ion and anion concentrations, pH, and electrical conductivity of rainwater in Athens and Sea Water from Aegean Sea.

Table 7.
Summary of the metal concentrations of EN leachates originated by the leaching of FNS, GCS, and FCS materials. The metal concentrations that exceed the limits set by the European Union for water intended for human consumption are marked with bold.
The dissolution kinetics of the Ni, Co, Zn, and Cr contained in ferronickel slag are depicted in Figure 9. Nickel is the most released element with concentrations ranging from 2 to 10 μg·g−1, followed by zinc (up to approximately 8 μg·g−1) and cobalt (up to about 1 μg·g−1). Despite its notable presence in the residue, primarily in the spinel phase, chromium release is negligible. The dissolution behavior of nickel and cobalt is similar. A substantial release was observed between 30 and 45 days, after which the release rate stabilizes. These data suggest a common origin for the two metals, likely within the (Co)-Ni-Fe phase. The zinc release rate presented a continuous quick decrease after 15 days stabilizing at low levels (<3 μg·g−1) at 75 days. Chromium was released at extremely low levels (<0.043 μg·g−1) with a constant rate. Seawater favored the liberation of nickel and cobalt, whereas rainwater is a more efficient mean for the Zn liberation. The origin of zinc in the FNS, with a concentration not exceeding 300 ppm, was unclear and would require a transmission electron microscopy–EDS study.
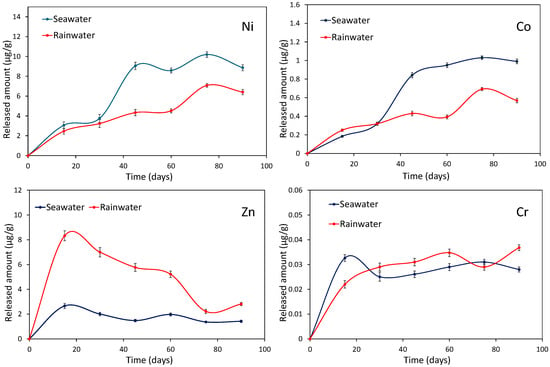
Figure 9.
Evolution of the Ni, Co, Zn, and Cr concentrations during the leaching of the FNS sample in seawater and rainwater media. The released amounts are expressed as micrograms per gram of slag.
The leaching behavior of all examined elements can be correlated to the formation of secondary mineral phases on the surface of the primary slag components, which can either impede or facilitate further leaching, depending on their solubility product constant (Ksp). Thin-layered secondary phases could potentially be identified using spectroscopic techniques such as infrared (IR) or Raman spectroscopy; however, the application of these methods is beyond the scope of the current work.
The dissolution kinetics of the most abundantly occurring heavy metals (e.g., Pb, Zn, Cr, Cd, Co and Ni) and metalloids (e.g., As, Sb) contained in the primary lead and ferronickel slags using seawater and rainwater as leaching media are depicted in Figure 10. Lead and zinc were the metals released at the highest amounts in the ranges of 25–43 μg·g−1 and 16–164 μg·g−1, respectively. Arsenic and antimony are released at concentration levels of few μg·g−1, while the maximum cadmium liberation did not exceed 0.2 μg·g−1. The analyzed metals and metalloids exhibited distinct leaching behaviors. The release rate of Pb in both seawater and rainwater media slowed down and stabilized after 30 days. In contrast, the release rate of Zn tended to plateau after 90 days. Arsenic, antimony, and cadmium showed intermediate behavior, with their release rates decreasing after about 75 days. Seawater appears to enhance the extraction of Pb, Zn, As, and Cd, while rainwater seems to favor the leaching of Sb (Figure 10). The origin of the leached lead can be traced to multiple sources, including residual, unseparated Ag-Pb droplets, lead sulfides, lead arsenates, and lead incorporated within the amorphous phase structure. The latter phases also contained arsenic at elevated concentrations. Esseneite and franklinite were identified as the main Zn-bearing phases; however, the liberation of the metal from these species should be considered less probable due to their high chemical stability.
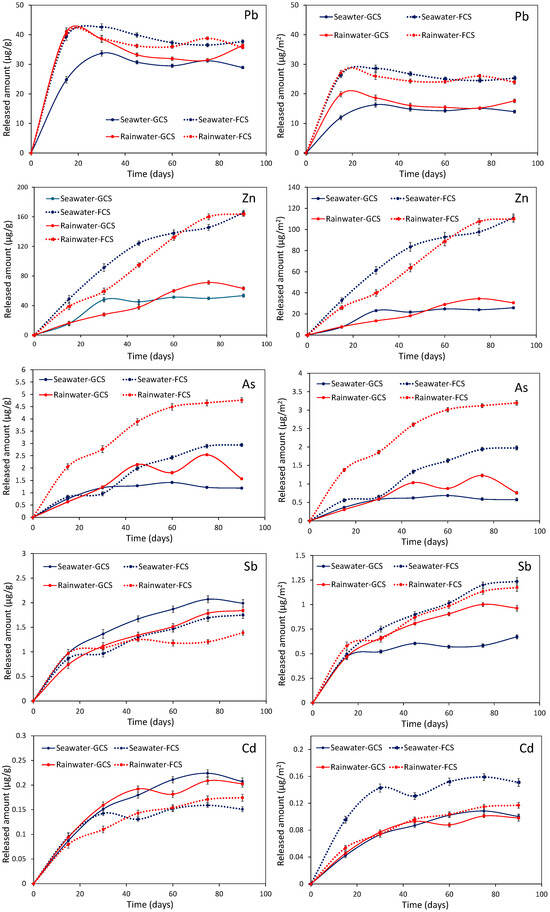
Figure 10.
Evolution of the Pb, Zn, As, Sb, and Cd concentrations during the leaching of lead slags in seawater and rainwater media. The released amounts are expressed as micrograms per gram of slag in the curves on the left, and as micrograms per square meter (based on the specific surface areas, SSAs) in the curves on the right.
More conclusive data regarding the comparative leaching efficiency of seawater and rainwater, as well as the influence of the material’s porosity on the overall process, were obtained by normalizing the released amounts of metals and metalloids to the specific surface area (μg/m2) of the slag samples (Figure 10). Within this framework, it is evident that FCS is more leachable in comparison to GCS. As BET analysis showed (Figure 4), the pore size distribution of the FCS material occurs in the micropore region, subsequently its increased active surface enhances the dissolution kinetics. As shown by the data, the amounts of Pb, Zn, As, Sb, and Cd released from the FCS material—expressed in micrograms per square meter—were approximately 50%, five times, up to four times, up to two times, and 70% higher, respectively, compared to the corresponding amounts released from GCS material (Figure 10). Furthermore, in all cases, seawater has proven to be a more effective leaching medium. Seawater has a higher ionic strength in comparison to rainwater, while, additionally, the elevated concentration of SO4− anions is a key-factor.
Unfortunately, there is a lack of data on the leaching behavior of lead from lead slags in seawater and rainwater environments. Certain data on the leachability of primary lead slags in ultra-pure water indicate that Pb and Zn are released in cumulative amounts of approximately 15 and 240 μg·g−1, respectively, after 120 days of leaching [20,41]. Furthermore, these studies show that the release rate of lead stabilizes after 40 days, whereas the decrease in the zinc release rate takes place more gradually. Conclusively, these findings are consistent with the results obtained in the present study.
3.3. Investigation of Pollutant Speciation and Thermodynamic Stability in Water Media
The speciation of pollutants from ferronickel and lead slags in rainwater and seawater media as a function of oxygen concentration was investigated from a thermodynamic perspective. These data can be correlated with the solubility potential of the pollutants and, consequently, with their release amounts, as shown in Figure 9 and Figure 10. Particular emphasis was placed on the thermodynamic stability of Cr and Ni phases present in FNS, as well as the Pb and As phases found in the primary lead slags (GCS and FCS). Two leaching scenarios were simulated using HSC 10 software:
- Case 1 examines the thermodynamic behavior of pollutants from FNS;
- Case 2 examines the thermodynamic behavior of pollutants from GCS and FCS.
Equilibrium composition diagrams were constructed as a function of oxygen concentration. Dissolved oxygen (DO) plays a crucial role in the formation of secondary species during the leaching process. The enhancement of the dissolution kinetics of chromite and sulfides under oxic conditions has been presented in several studies [42,43,44]. Additionally, the thermodynamic systems of slag in rainwater or seawater at elevated DO amounts simulates their stability under more realistic weathering conditions such as slags disposed of in open-air environments exposed to rainfall or located near coastal areas. Due to limitations in the species included in the HSC database and the lack of published data on their fundamental thermodynamic properties, simplified mineral phases were considered in Cases 1 and 2. For example, there is a lack of fundamental thermodynamic data for iron–nickel alloys for specific Fe-Ni mass ratios. Experimental measurements of the specific heat capacity (Cp) of Fe-Ni alloys have shown significant variation as a function of Ni content [45]. Additionally, there is a lack of thermodynamic data for the Mg-Al-Cr-spinel identified in FNS. Therefore, Cr-spinel (FeCr2O4) and metallic nickel were selected as the Cr- and Ni-bearing phases in ferronickel slag, while metallic lead, lead sulfide (PbS), and lead arsenate [Pb3(AsO4)2] were chosen as the Pb- and As-bearing phases in lead slags. The initial HSC input concentrations are presented in Table 8.

Table 8.
Initial amounts of the pollutant-bearing phases in ferronickel and lead slags used as an input in the HSC equilibrium system.
The first thermodynamic case (Case 1) simulates the dissolution of a chromite/metallic Ni mixture in rainwater and seawater using the same mineral amounts and water ionic concentrations as those exist in the ferronickel–rain/seawater systems. The equilibrium compositions of the nickel and chromium phases as a function of the oxygen content are presented in Figure 11. It can be observed that chromite in rainwater was oxidized to the thermodynamically stable chromium hydroxide species CrO(OH) and Cr(OH)3·3H2O, which are insoluble in aqueous media. A negligible amount of the soluble species CrO4−2 was formed when the dissolved oxygen concentration exceeded 18 ppm (i.e., 5.5 × 10−4 mol in equilibrium composition diagram), a value higher than the typical level in rainwater, which is around 10 ppm [46] (Figure 11a). Chromite exhibited similar thermodynamic behavior in seawater, where chromium hydroxide hydrate was the only major stable phase. The formation of the soluble species CrO42− is even more limited in this environment and requires an oxygen concentration exceeding typical seawater levels (approximately 8 ppm) [47] (Figure 11c). The aforementioned thermodynamic results indicate the formation thermodynamically stable and insoluble Cr species. From this perspective, the low amounts of Cr released during the leaching tests (Figure 9) can be explained. Supporting evidence is provided by a recent study investigating Cr release from urban contaminated soils using spectroscopic techniques. The study demonstrated that Cr release is indeed inhibited under anoxic seawater conditions, whereas it can occur under oxic freshwater conditions [48].
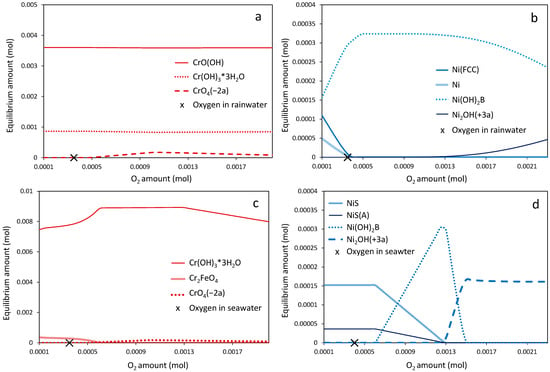
Figure 11.
Equilibrium compositions of chromium and nickel phases in rain water (a,b) and in seawater (c,d) simulants at 20 °C (Case 1). Typical concentrations of dissolved oxygen in rainwater and seawater (10 and 8 ppm, respectively) are indicated (X) in the graphs.
Metallic nickel in rainwater is almost entirely oxidized to nickel hydroxide [Ni(OH)2] even under mildly oxic conditions. A substantial oxygen excess (>38 ppm) is required for the formation of the aqueous Ni2OH3+ species, and even then, only in limited amounts (Figure 11b). It should be noted, however, that nickel hydroxide is slightly soluble in water. Metallic nickel is even less thermodynamically stable in seawater, where it is converted to both nickel sulfide and nickel hydroxide under mildly oxic conditions. These secondary phases are fully dissolved as Ni2OH3+ when the dissolved oxygen concentration exceeds 35 ppm (Figure 11d).
Conclusively, the equilibrium compositions of Cr and Ni carrier phases contained in the FNS sample indicate the following: (a) Cr stability due to the formation of stable insoluble chromium (oxy)hydroxide phases and (b) Ni mobilization in water media due to the formation of the slightly soluble nickel hydroxide phase. Both Cr and Ni mobilization increased with the increase in DO amount and the formation of CrO42− and Ni2OH3+ species, respectively. Therefore, under realistic conditions (i.e., FNS deposited in sites periodically exposed to seawater, such as coastal zones), the formation of Ni2OH3+ and CrO42− ions may be enhanced by the high atmospheric oxygen partial pressure.
According to scanning electron microscopy examination, metallic Pb droplets, Pb sulfides, and Pb arsenates are the primary carriers of lead and arsenic in GCS and FCS samples. Thermodynamic Case 2 simulates the dissolution of a Pbm/PbS/Pb3(AsO4)2 mixture at a mass ratio 0.5/0.5/0.5 into rainwater and seawater (Figure 12 and Figure 13). The results indicate that in rainwater with a typical DO amount, (a) 2PbO·PbSO4 was the dominant thermodynamically stable lead species, and (b) lead arsenate remained largely unconverted. Lead sulfates with varying Pb–S mass ratios, including PbSO4, PbO·PbSO4, and 2PbO·PbSO4, appeared to be metastable within an oxygen concentration range of 5–40 ppm. Under stronger oxic conditions, lead was immobilized as insoluble lead dioxide. Rainwater environments with DO concentrations between 30 and 40 ppm enhanced the mobilization of lead since. Under these conditions, the formation of the soluble species H3AsO4 and H2AsO4− seemed to be maximized (Figure 12). Lead-bearing phases exhibited a different thermodynamic behavior in seawater compared to rainwater. Lead sulfide remained stable under anoxic conditions and was gradually converted to lead sulfate and additionally to lead carbonate due to the presence of HCO3− ions in the seawater. At oxygen concentrations above 11.5 ppm (slightly higher than typical levels), PbS was completely eliminated, and PbSO4 and PbCO3 became the only stable lead phases. As in case of rainwater, a high oxygen excess required the mobilization of arsenic as H3AsO4 and H2AsO4− (Figure 13).
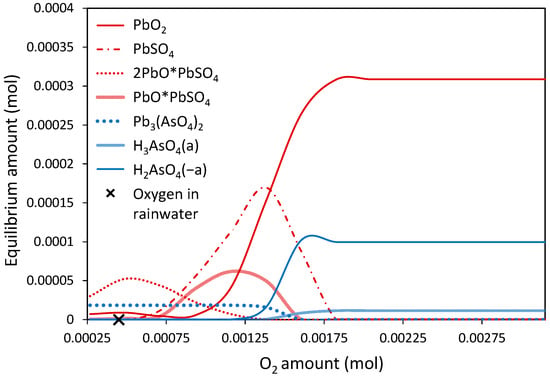
Figure 12.
Equilibrium compositions of lead and arsenic phases in rainwater simulant at 20 °C (Case 2). The typical concentration of dissolved oxygen in rainwater (10 ppm) is indicated in the graph (X).
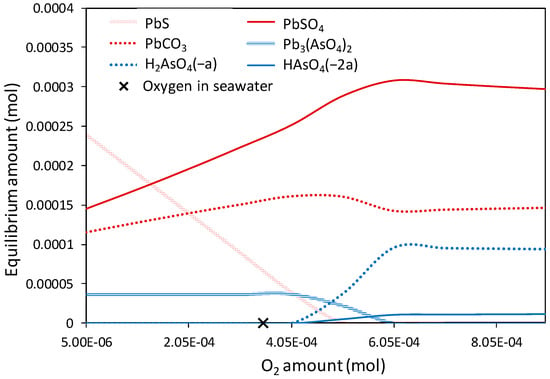
Figure 13.
Equilibrium compositions of lead and arsenic phases in seawater simulant at 20 °C (Case 2). The typical concentration of dissolved oxygen in seawater (8 ppm) is indicated in the graph (X).
4. Conclusions
The environmental stability of ferronickel and primary lead slags in rainwater and seawater environments was investigated. These residues occurred in proximity to major historical metallurgical complexes in Central Greece, with a total estimated deposited amount of 7 and 80 Mt, respectively. Ferronickel slag (FNS) was primarily composed of an XRD–amorphous glassy phase (average semiquantitative chemical composition wt.%: SiO2 = 45.0, Fe2O3 = 26.2, CaO = 9.9, Al2O3 = 9.8, and MgO = 8.6), within which the Fe-Ni(Co) alloy and Cr-containing spinel phases are dispersed. It exhibited a small specific surface area (0.36 m2/g) and a pore size distribution ranging between 50 and 120 Å. Primary lead slags (GCS and FCS) are predominantly crystalline and contain typical Ca-Fe-Zn silicates, wustite and illite. Scanning electron microscopy identified micrometer-sized lead droplets, lead sulfides, and lead arsenates as the main Pb- and As-bearing phases. Lead slags show an irregular pore size distribution. FCS, produced from the processing of Ag-Pb-Zn concentrated in modern reductive smelting furnaces, was more microporous (around 30 Å) compared to GCS (50–90 Å), which resulted from the re-smelting of ancient slags in older-type blast furnaces.
The mobilization of hazardous metals and metalloids from FNS, GCS, and FCS materials was tested under static conditions and open-air exposure using rainwater and seawater media. Nickel was the most leachable metal cation from FNS, with cumulative releases reaching up to 43.5 μg·g−1 after 90 days in seawater. Chromium release was negligible (0.17 μg·g−1 after 90 days in seawater), indicating the chemical stability of Cr-bearing phases in the aqueous media. In lead slags, zinc and lead were the most leachable cations, with cumulative releases of up to 650 and 230 μg·g−1, respectively, after 90 days in seawater. The maximum arsenic release was 22.6 μg·g−1 after 90 days in seawater. Overall, the mobilization of hazardous elements was less intense in rainwater. Porosity was a key factor affecting leaching efficiency: Pb, Zn, and As were released in approximately 50%, five times, and four times greater amounts, respectively, from the microporous FCS material compared to GCS, when concentrations were normalized to surface area (μg·m−2).
The speciation of leached pollutants in rainwater and seawater was investigated through a thermodynamic study using the HSC software. Equilibrium composition diagrams for the FeCr2O4–Nim–H2O and Pbm–PbS–Pb3(AsO4)2–H2O systems, simulating the leaching behavior of ferronickel and lead slags respectively, revealed the following:
- (a)
- Ni(OH)2 and 2PbO·PbSO4 were the major secondary species formed from the leaching of FNS and GCS/FCS, respectively. Therefore, Ni and Pb can be mobilized via the partial dissolution of these phases.
- (b)
- The potential mobilization of Cr from FNS and As from GCS/FCS under anoxic conditions appeared negligible due to the formation of the insoluble in water secondary species Cr(OH)3·3H2O and Pb3(AsO4)2. These findings are consistent with the limited leachability of Cr and As observed in the kinetic leaching curves.
In conclusion, based on both experimental and thermodynamic results, it is supported that the presence of ferronickel and primary lead slags in static rainwater and seawater results in the moderate mobilization of Ni and Pb/Zn, respectively, and limited mobilization of Cr and As, respectively. However, the open-air disposal of FNS, GCS, and FCS may pose a more intense environmental risk. In these environments, where the oxygen’s partial pressure is higher compared to static water conditions, in addition to Ni and Pb, Cr and As may also become mobilized.
Author Contributions
Methodology and writing, M.S. (Michail Samouhos); Methodology, A.G. (Anastasia Gkika); Methodology (ICP analyses), M.G.K. (Marios. G. Kostakis); Methodology (ICP analyses), E.S. (Eirini Siandri); Methodology (BET analyses) and writing, G.R. (George Romanos); A.G. (Athanasios Godelitsas), supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FNS | Ferronickel slag |
| GCS | Greek company slag |
| FCS | French company slag |
References
- Wang, G.C. Nonferrous metal extraction and nonferrous slags. In The Utilization of Slag in Civil Infrastructure Construction, 1st ed.; Woodhead Publishing: Duxford, UK, 2016; pp. 35–61. [Google Scholar]
- Worldsteel. Available online: https://worldsteel.org/wp-content/uploads/Fact-sheet-Steel-industry-co-products.pdf (accessed on 3 August 2025).
- Nguyen, Q.D.; Castel, A.; Kim, T.; Khan, M.S.H. Performance of fly ash concrete with ferronickel slag fine aggregate against alkali-silica reaction and chloride diffusion. Cem. Concr. Res. 2021, 139, 106265. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Piatak, N.M. Environmental Characteristics and Utilization Potential of Metallurgical Slag. In Environmental Geochemistry, 2nd ed.; DeVivo, B., Belkin, H., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–519. [Google Scholar]
- Piatak, N.M.; Ettler, V. Metallurgical Slags: Environmental Geochemistry and Resource Potential; Series: Chemistry in the Environment; Royal Society of Chemistry Publisher: Cambridge, UK, 2021; p. 306. [Google Scholar]
- Fu, P.; Yang, H.; Zhang, G.; Fu, P.; Li, Z. In-Situ Immobilization of Cd-Contaminated Soils Using Ferronickel Slag as Potential Soil Amendment. Bull. Environ. Contam. Toxicol. 2019, 103, 756–762. [Google Scholar] [CrossRef]
- Prasetyo, A.B.; Khaerul, A.; Mayangsari, W.; Febriana, E.; Maksum, A.; Andinie, J.; Firdiyono, F.; Soedarsono, J.W. Magnesium extraction of ferronickel slag processed by alkali fusion and hydrochloric acid leaching. J. Min. Metall. Sect. B Metall. 2021, 57, 225–233. [Google Scholar] [CrossRef]
- Đorđević, T.; Tasev, G.; Aicher, C.; Potysz, A.; Nagl, P.; Lengauer, C.L.; Pędziwiatr, A.; Serafimovski, T.; Boev, I.; Boev, B. Mineralogy and environmental stability of metallurgical slags from the Euronickel smelter, Vozarci, North Macedonia. Appl. Geochem. 2024, 170, 106068. [Google Scholar] [CrossRef]
- Nowińska, K.; Kokowska-Pawłowska, M. Mineralogy of zinc and lead metallurgical slags in terms of their impact on the environment: A review. Minerals 2024, 14, 852. [Google Scholar] [CrossRef]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Buatier, M.; Recourt, P.; Damidot, D.; Potdevin, J.L. Effect of Pb-rich and Fe-rich entities during alteration of a partially vitrified metallurgical waste. J. Hazard. Mater. 2007, 149, 418–431. [Google Scholar] [CrossRef]
- Wang, P.W.; Zhang, L. Structural role of lead in lead silicate glasses derived from XPS spectra. J. Non-Cryst. Sol. 1996, 194, 129–134. [Google Scholar] [CrossRef]
- Calas, G.; Cormier, L.; Galoisy, L.; Jollivet, P. Structure–property relationships in multicomponent oxide glasses. Comptes Rendus Chim. 2002, 5, 831–843. [Google Scholar] [CrossRef]
- Kierczak, J.; Néel, C.; Puziewicz, J.; Bril, H. The mineralogy and weathering of slag produced by the smelting of the lateritic Ni ores, Szklary, Southwestern Poland. Can. Mineral. 2009, 47, 557–572. [Google Scholar] [CrossRef]
- Tyszka, R.; Pietranik, A.; Kierczak, J.; Zieliński, G.; Darling, J. Cadmium distribution in Pb-Zn slags from Upper Silesia, Poland: Implications for cadmium mobility from slag phases to the environment. J. Geochem. Explor. 2018, 186, 215–224. [Google Scholar] [CrossRef]
- De Andrade Lima, L.R.P.; Bernardez, L.A. Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef]
- Barcos-Arias, M.; Vazquez, M.J.; Maldonado, V.M.; Alarcon, A.; Peña-Cabriales, J.J. Chemical characterization and local dispersion of slag generated by a lead recovery plant in Central Mexico. Afr. J. Biotechnol. 2014, 13, 1973–1978. [Google Scholar] [CrossRef]
- Saikia, N.; Borah, R.R.; Konwar, K.; Vandecastelee, C. pH dependent leachings of some trace metals and metalloid species from lead smelter slag and their fate in natural geochemical environment. Groundw. Sustain. Dev. 2018, 7, 348–358. [Google Scholar] [CrossRef]
- Warchulski, R.; Mendecki, M.; Gawęda, A.; Sołtysiak, M.; Gadowski, M. Rainwater-induced migration of potentially toxic elements from a Zn–Pb slag dump in Ruda Śląska in light of mineralogical, geochemical and geophysical investigations. Appl. Geochem. 2019, 109, 104396. [Google Scholar] [CrossRef]
- Yin, N.H. Weathering of Metallurgical Slags: A Comprehensive Study on the Importance of Chemical and Biological Contributions. Ph.D. Thesis, Université Paris-Est, Paris, France, 2014. [Google Scholar]
- De Andrade Lima, L.R.P.; Bernardez, L.A. Evaluation of the chemical stability of a landfilled primary lead smelting slag. Environ. Earth Sci. 2013, 68, 1033–1040. [Google Scholar] [CrossRef]
- Baharuddin, I.I.; Imran, A.M.; Maulana, A.; Hamzah, A. Leaching time correlation with heavy metals (Fe, Cr, Ni, Mn) distribution in ferronickel slag leaching test. Int. J. Adv. Res. Eng. Technol. 2020, 11, 505–511. [Google Scholar]
- Susanto, I.; Irawan, R.R.; Hamdani, D. Nickel slag waste utilization for road pavement material as strategy to reduce environmental pollution. E3S Web Conf. 2020, 202, 05003. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Warmadewanthi, I.D.A.A.; Saptarini, D.; Pudjiastuti, L.; Tardan, M.A.M.; Luqman, A. Ferronickel slag performance from reclamation area in Pomalaa, Southeast Sulawesi, Indonesia. Adv. Chem. Eng. Sci. 2015, 5, 408–412. [Google Scholar] [CrossRef]
- Demotica, J.S.; Amparado, R.; Malaluan, R.M.; Demayo, C. Characterization and leaching assessment of ferronickel slag from a smelting plant in Iligan city, Philippines. Int. J. Environ. Sci. Dev. 2012, 3, 470–474. [Google Scholar] [CrossRef]
- Hobson, A.J.; Stewart, D.I.; Bray, A.W.; Mortimer, R.J.; Mayes, W.M.; Rogerson, M.; Burke, I.T. Mechanism of vanadium leaching during surface weathering of basic oxygen furnace steel slag blocks: A microfocus X-ray absorption spectroscopy and electron microscopy study. Environ. Sci. Technol. 2017, 51, 7823–7830. [Google Scholar] [CrossRef]
- Han, L.; Chen, B.; Liu, T.; Choi, Y. Leaching characteristics of iron and manganese from steel slag with repetitive replenishment of leachate. KSCE J. Civ. Eng. 2019, 23, 3297–3304. [Google Scholar] [CrossRef]
- Sakurai, Y.; Hisaka, Y.; Tsukihashi, F. Nutrient supply to seawater from steelmaking slag: The coupled effect of gluconic acid usage and slag carbonation. Metall. Mater. Trans. B 2020, 51, 1039–1047. [Google Scholar] [CrossRef]
- Foekema, E.M.; Tamis, J.E.; Blanco, A.; van der Weide, B.; Sonneveld, C.; Kleissen, F.; van den Heuvel-Greve, M.J. Leaching of metals from steel slag and their ecological effects on a marine ecosystem: Validating field data with mesocosm observations. Environ. Toxicol. Chem. 2021, 40, 2499–2509. [Google Scholar] [CrossRef]
- Riley, A.L.; Cameron, J.; Burke, I.T.; Onnis, P.; MacDonald, J.M.; Gandy, C.J.; Crane, R.A.; Byrne, P.; Comber, S.; Jarvis, A.P.; et al. Environmental behaviour of iron and steel slags in coastal settings. Environ. Sci. Pollut. Res. 2024, 31, 42428–42444. [Google Scholar] [CrossRef] [PubMed]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Damidot, D.; Potdevina, J.-L. Leaching of lead metallurgical slags and pollutant mobility far from equilibrium conditions. Appl. Geochem. 2008, 23, 3699–3711. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, T.; Yang, Y.; Jackson, D. Leaching of cadmium, chromium, copper, lead, and zinc from two slag dumps with different environmental exposure periods under dynamic acidic condition. Ecotoxicol. Environ. Saf. 2014, 104, 43–50. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z. 12 years of leaching of contaminants from Pb smelter slags: Geochemical/mineralogical controls and slag recycling potential. Appl. Geochem. 2014, 40, 97–103. [Google Scholar] [CrossRef]
- Sakaroglou, M.; Anastassakis, G.N. Nickel recovery from electric arc furnace slag by magnetic separation. J. Min. Metall. 2017, 53, 3–15. [Google Scholar] [CrossRef]
- Gaitanos, G.; Zevgolis, E.; Kontos, G. Utilization of Slags from (the Greek) Ferronickel Industry. 1999. Available online: https://www.researchgate.net/publication/320979826_E_Diacheirise_ton_Metallourgikon_Skorion_ste_GMM_AE_LARKO_Utilization_of_Slags_from_the_Greek_Ferronickel_Industry (accessed on 6 August 2025). (In Greek).
- Kontopoulos, A.; Komnitsas, K.; Xenidis, A.; Papassiopi, N. Environmental characterisation of the sulphidic tailings in Lavrion. Miner. Eng. 1995, 8, 1209–1219. [Google Scholar] [CrossRef]
- EN 12457-2; Characterization of Waste–Leaching–Compliance Test for Leaching of Granular Waste Material and Sludge—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg with Article Size Below 4 mm (Without or With Size Reduction). European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- Eur-lex.europa.eu. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32020L2184 (accessed on 6 August 2025).
- Eur-lex.europa.eu. Directive (EU) 2003/40/EC. Available online: https://eur-lex.europa.eu/eli/dir/2003/40/oj/eng (accessed on 6 August 2025).
- WHO. Zinc in Drinking-Water. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/zinc.pdf?sfvrsn=9529d066_4#:~:text=The%20daily%20requirement%20for%20adult,has%20an%20undesirable%20astringent%20taste.&text=1.,Weinheim%2C%20Verlag%20Chemie%2C%201989 (accessed on 6 August 2025).
- Van Hullebusch, E.D.; Yin, N.H.; Seignez, N.; Labanowski, J.; Gauthier, A.; Lens, P.N.L.; Avril, C.; Sivry, Y. Bio-alteration of metallurgical wastes by Pseudomonas aeruginosa in a semi flow-through reactor. J. Environ. Manag. 2015, 147, 297–305. [Google Scholar] [CrossRef]
- Hsieh, Y.; Huang, C. The dissolution of PbS(s) in dilute aqueous solutions. J. Colloid Interface Sci. 1989, 131, 537–549. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Bang, S.; Chang, J.-S.; Gyu Kim, M.; Kim, K.-W. Effects of pH and dissolved oxygen on Cr(VI) removal in Fe(0)/H2O systems. J. Hazard. Mater. 2011, 186, 855–862. [Google Scholar] [CrossRef]
- Chou, P.-I.; Ng, D.-Q.; Li, I.-C.; Lin, Y.-P. Effects of dissolved oxygen, pH, salinity and humic acid on the release of metal ions from PbS, CuS and ZnS during a simulated storm event. Sci. Total Environ. 2018, 624, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Biele, J.; Grott, M.; Zolensky, M.E.; Benisek, A.; Dachs, E. The specific heat of astro-materials: Review of theoretical concepts, materials, and techniques. Int. J. Thermophys. 2022, 43, 144. [Google Scholar] [CrossRef] [PubMed]
- DeBusk Gee, K.; Schimoler, D.; Charron, B.T.; Woodward, M.D.; Hunt, W.F. A comparison of methods to address anaerobic conditions in rainwater harvesting systems. Water 2021, 13, 3419. [Google Scholar] [CrossRef]
- Vargel, C. Chapter D.3—Seawater. In Corrosion of Aluminium; Elsevier Publisher: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Sricharoenvech, P.; Siebecker, M.G.; Tappero, R.; Landrot, G.; Fischel, M.H.H.; Sparks, D.L. Chromium speciation and mobility in contaminated coastal urban soils affected by water salinity and redox conditions. J. Hazard. Mater. 2024, 462, 132661. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).