An Occurrence of Pyroxmangite in the NYF Granitic Pegmatite of the Gabal El-Bakriya Intrusion, Arabian–Nubian Shield
Abstract
1. Introduction
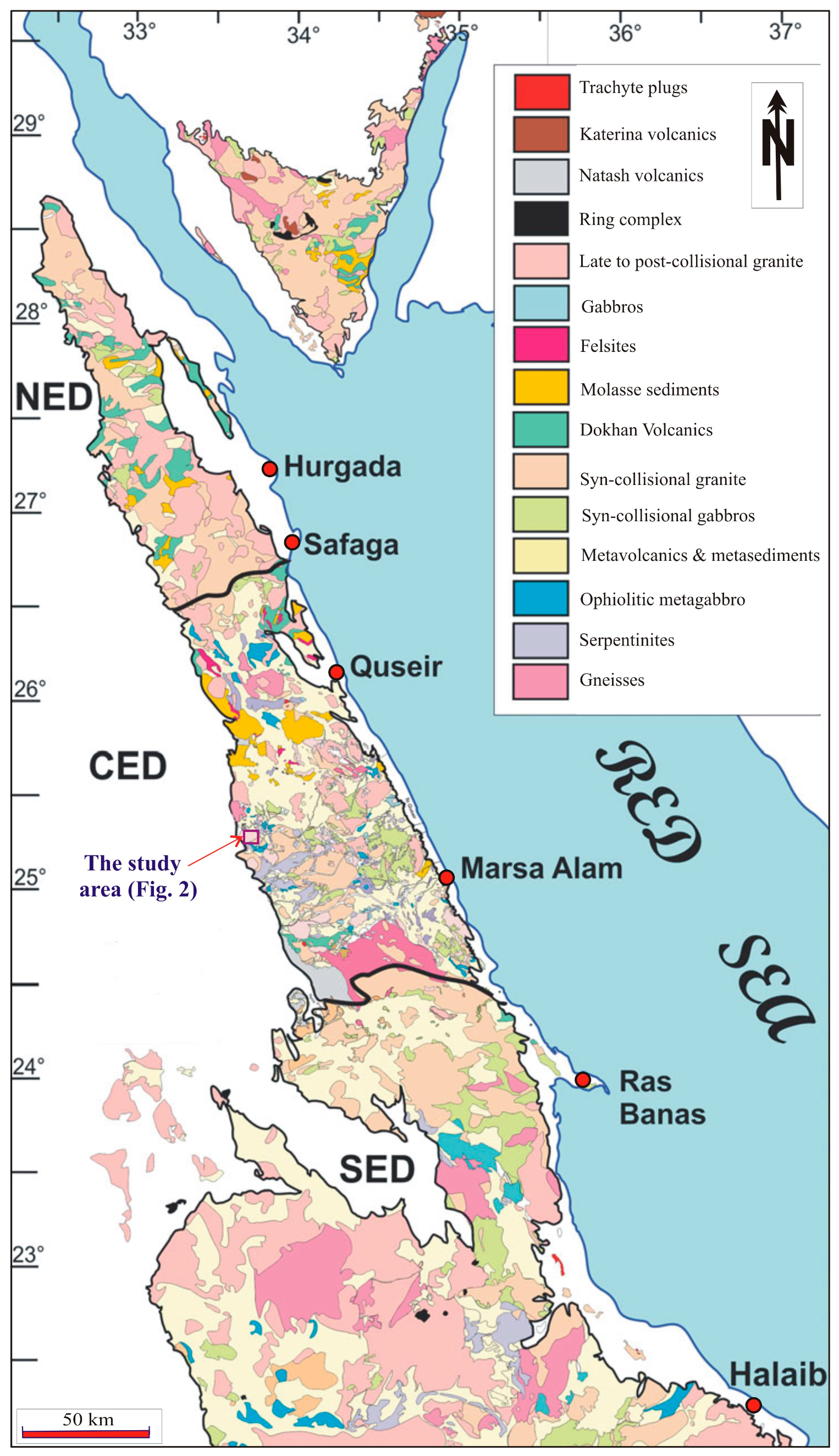
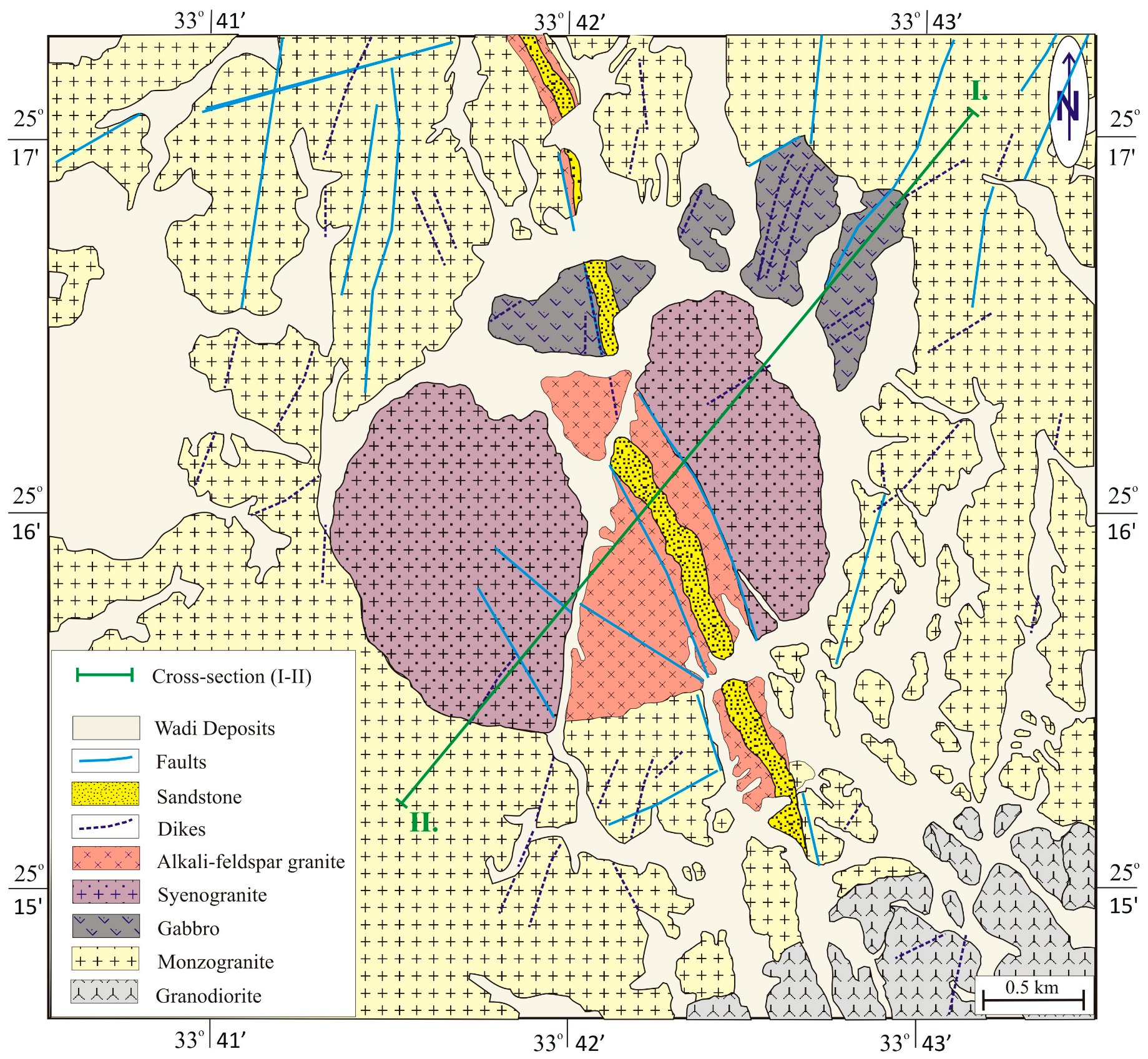
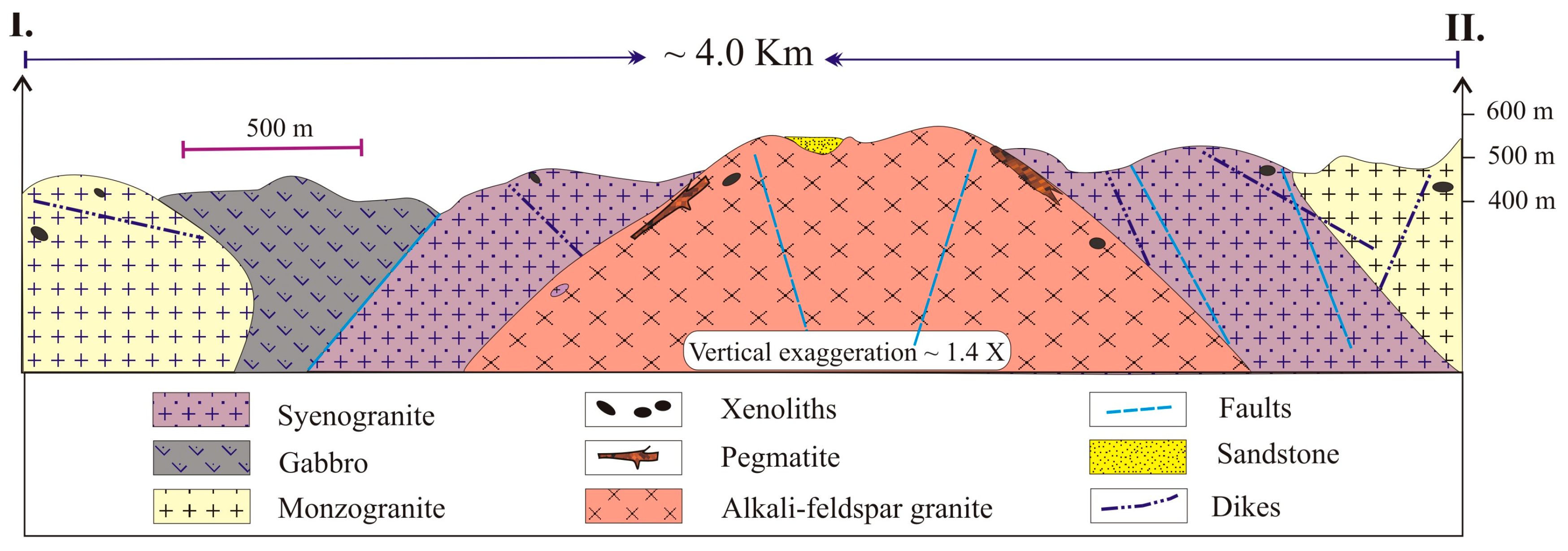
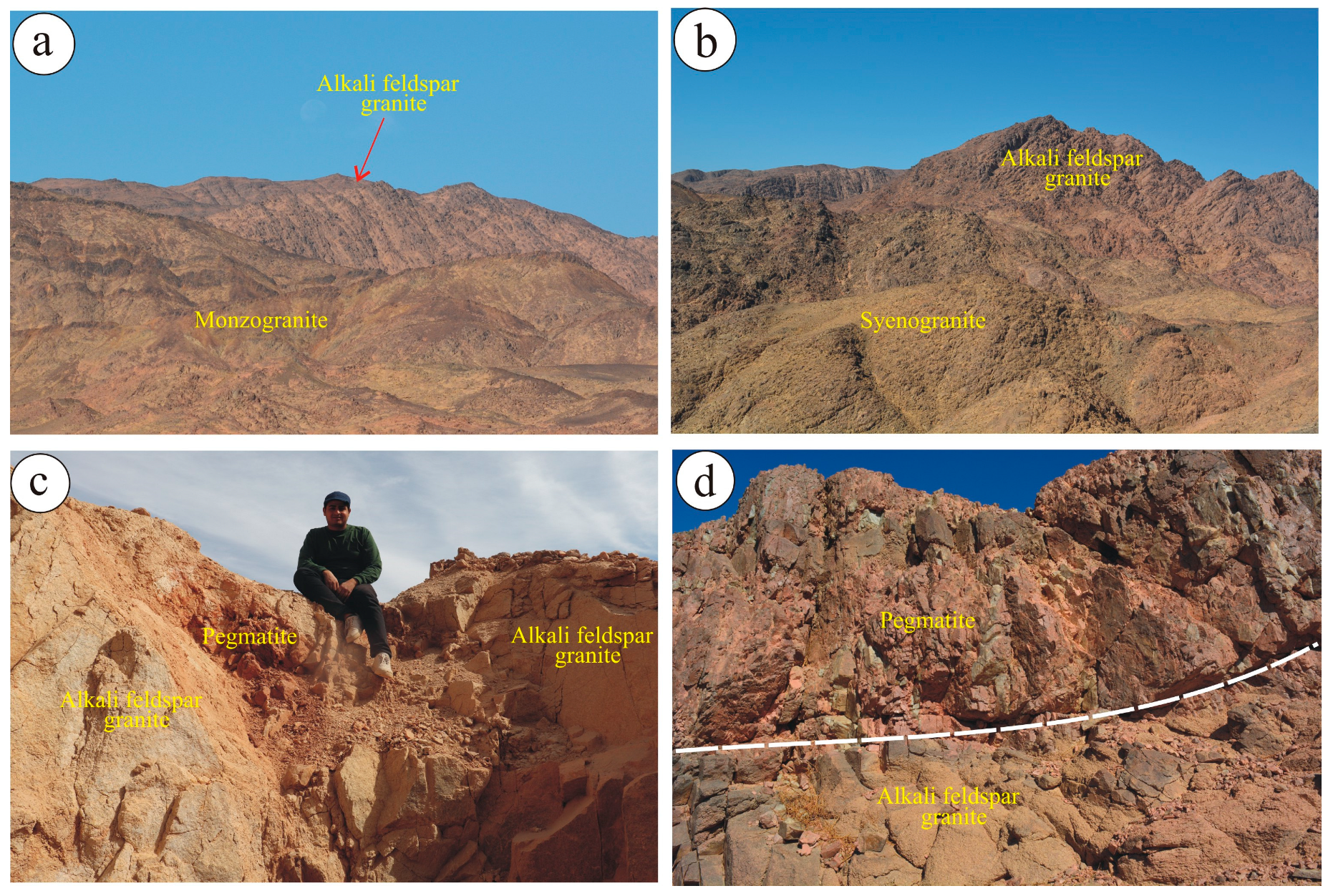
2. Geologic Setting
3. Petrography
3.1. Alkali-Feldspar Granite
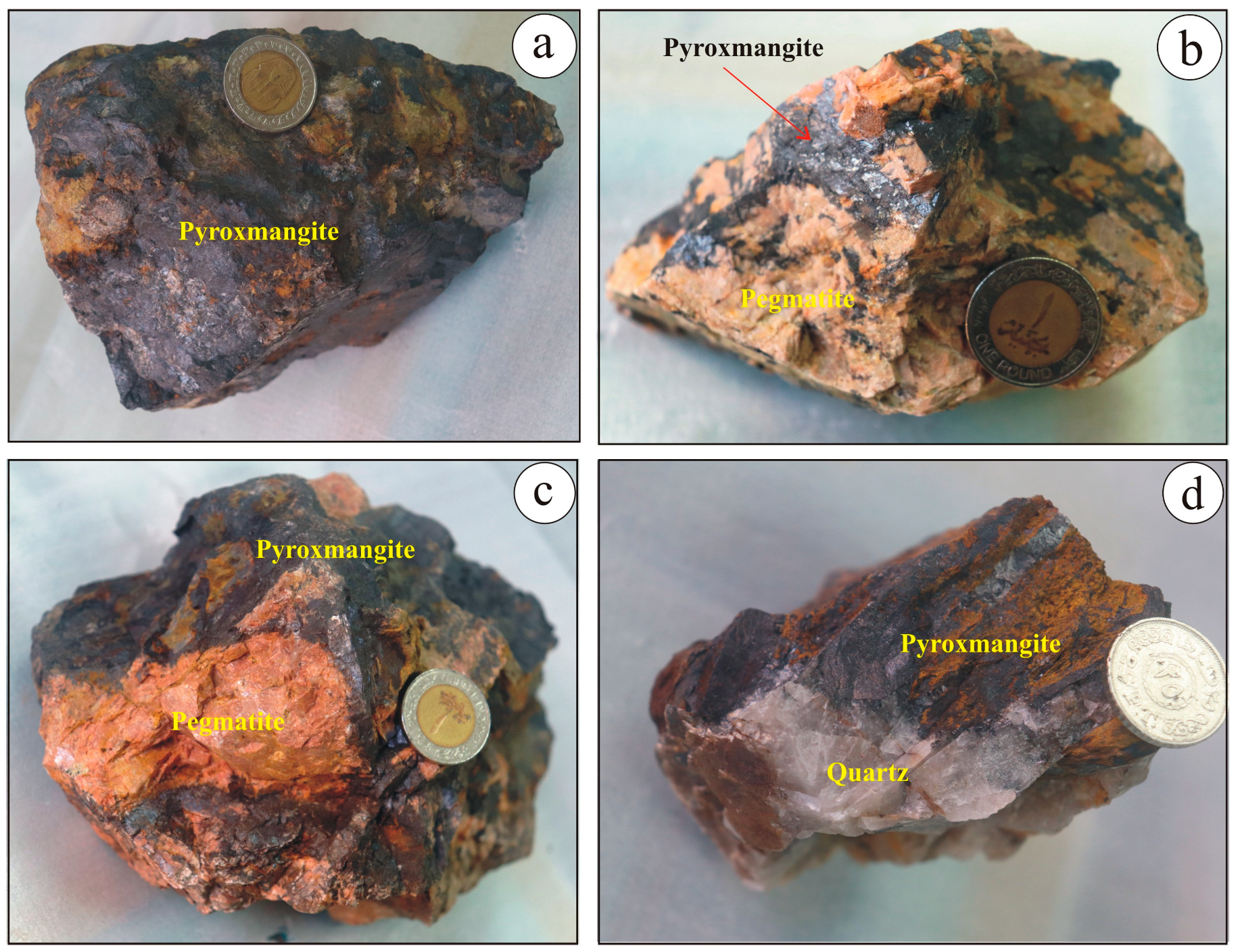
3.2. Pegmatite
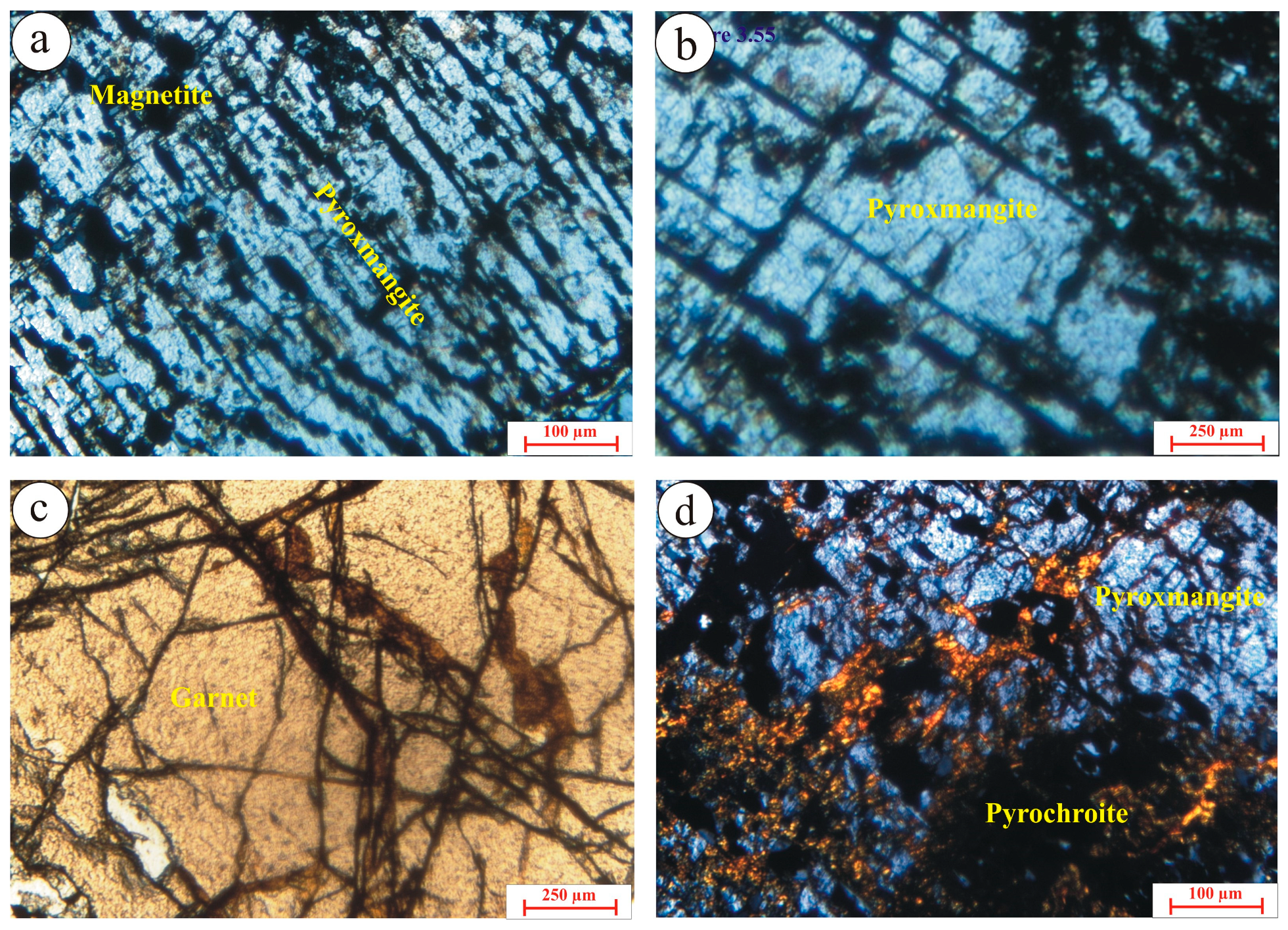
3.3. Pyroxmangite
4. Analytical Method
5. Mineral Composition
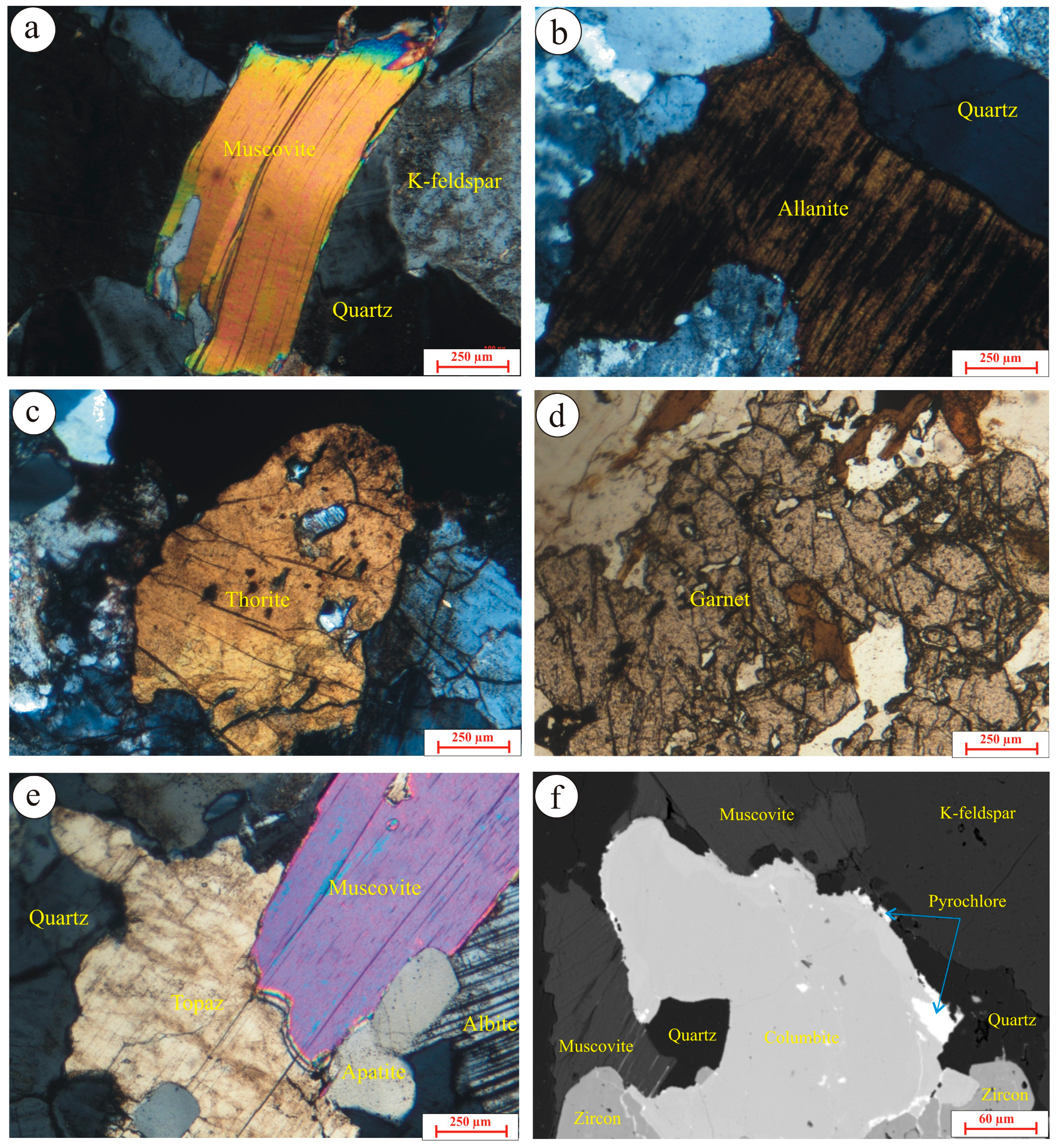
5.1. Silicate Minerals
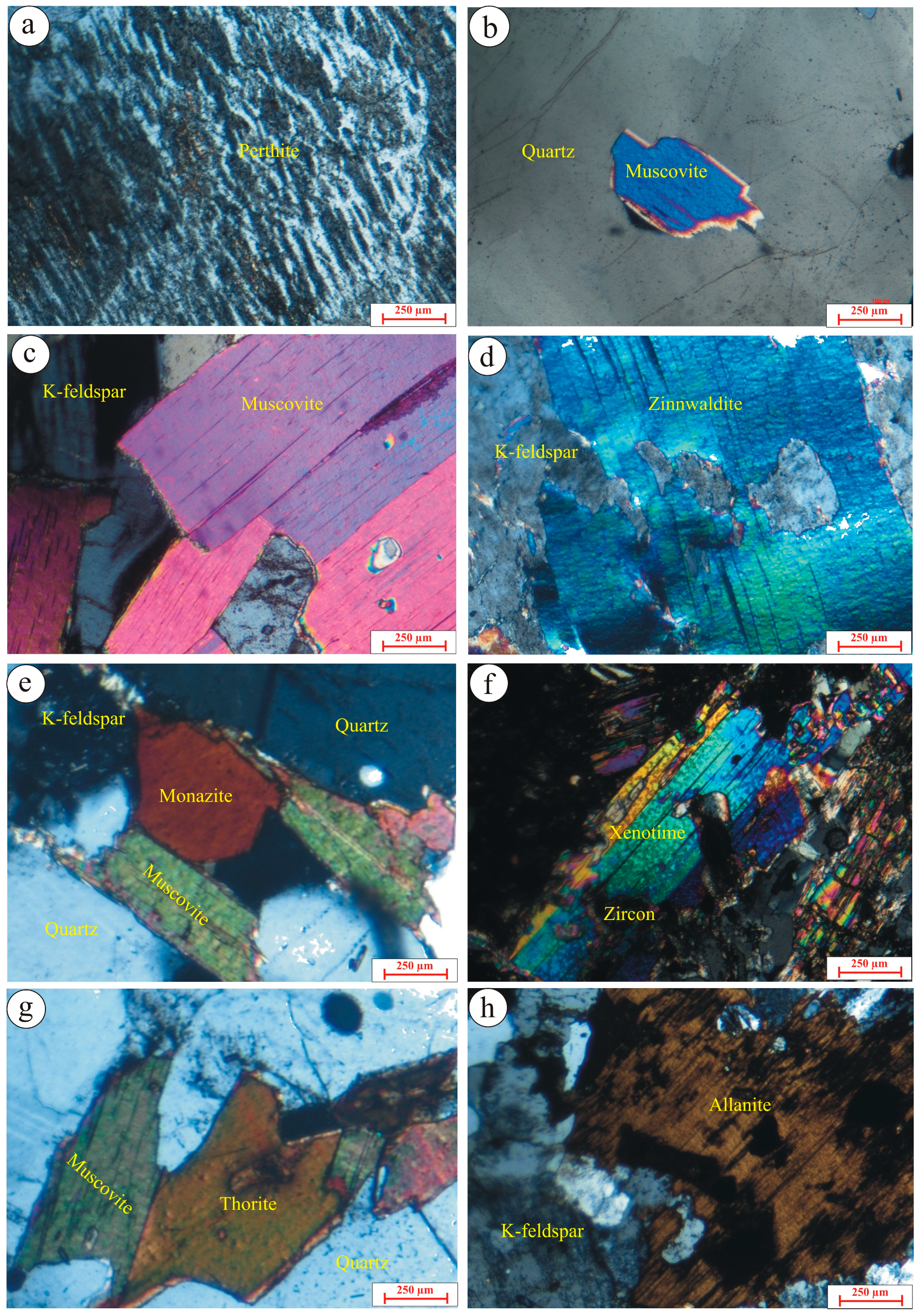
5.1.1. Feldspars
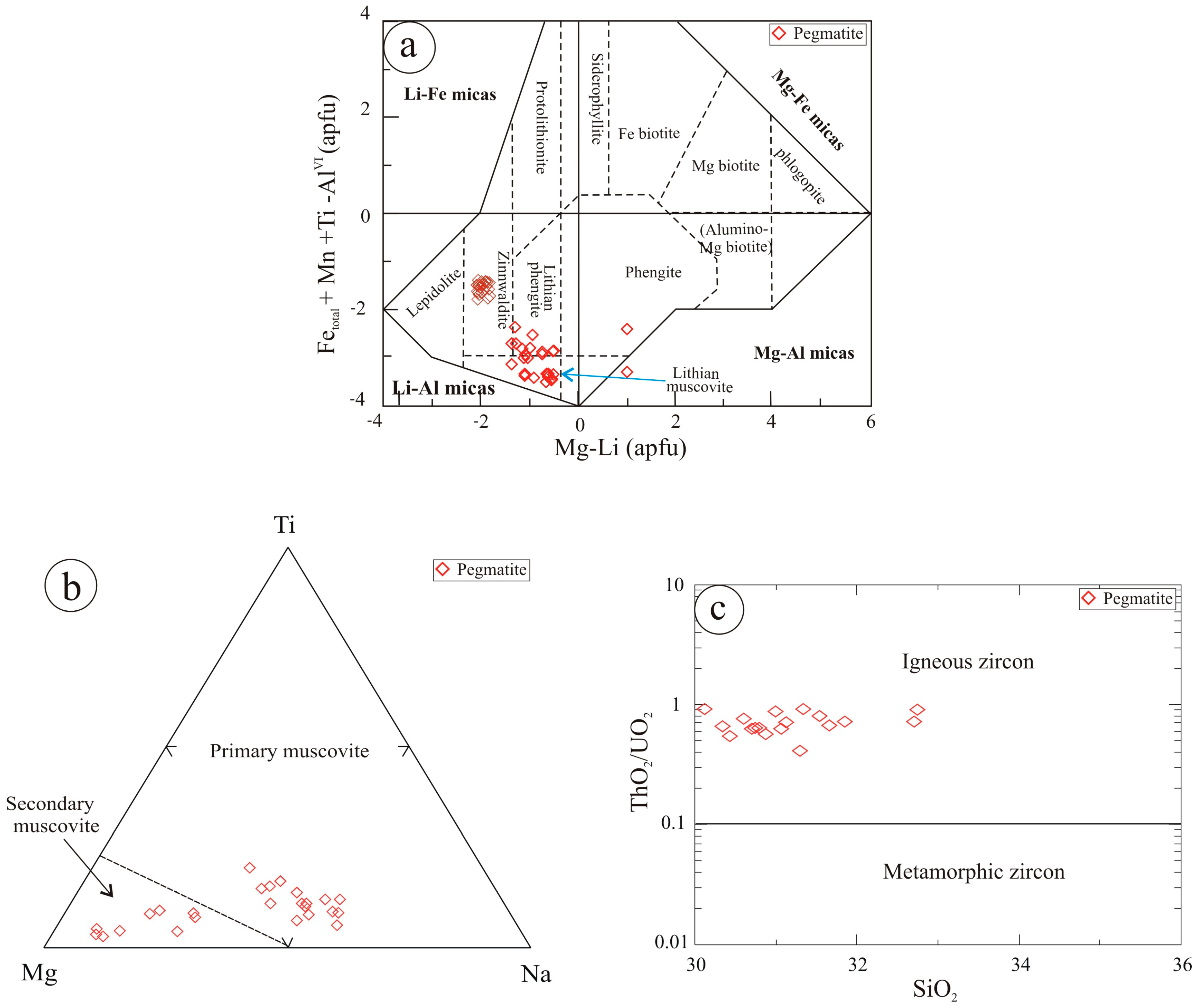
5.1.2. Micas
5.1.3. Pyroxmangite
5.1.4. Zircon
5.1.5. Thorite
5.1.6. Topaz
5.1.7. Chlorite
5.2. Non-Silicate Minerals
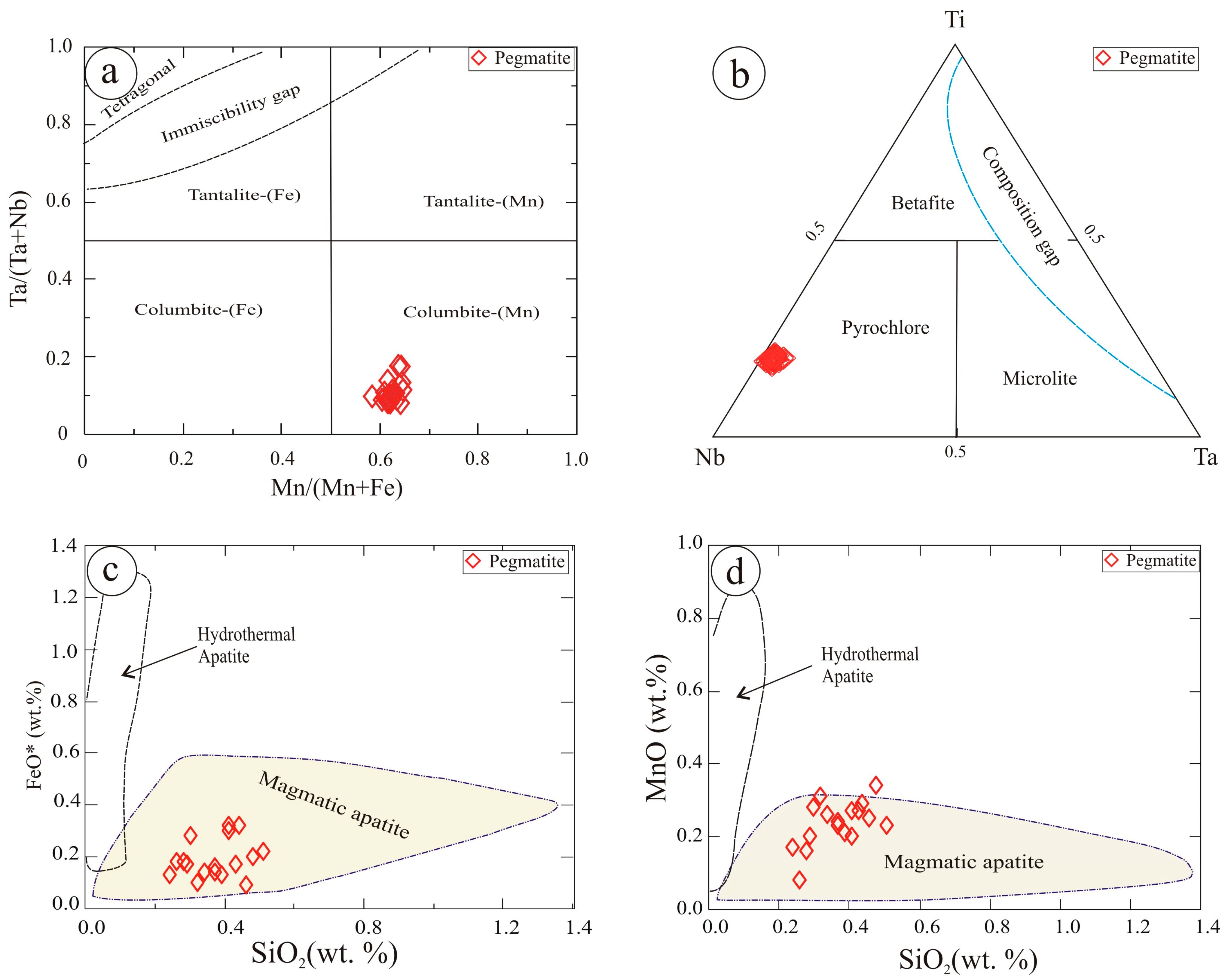
5.2.1. Nb-Ta Oxides
5.2.2. Niobian Rutile
5.2.3. Pyrochroite
5.2.4. Cassiterite
5.2.5. Beryl
5.2.6. Bastnäsite-(Y)
5.2.7. Monazite-(Ce)
5.2.8. Apatite
5.2.9. Fluorite
Fe-Ti Oxides
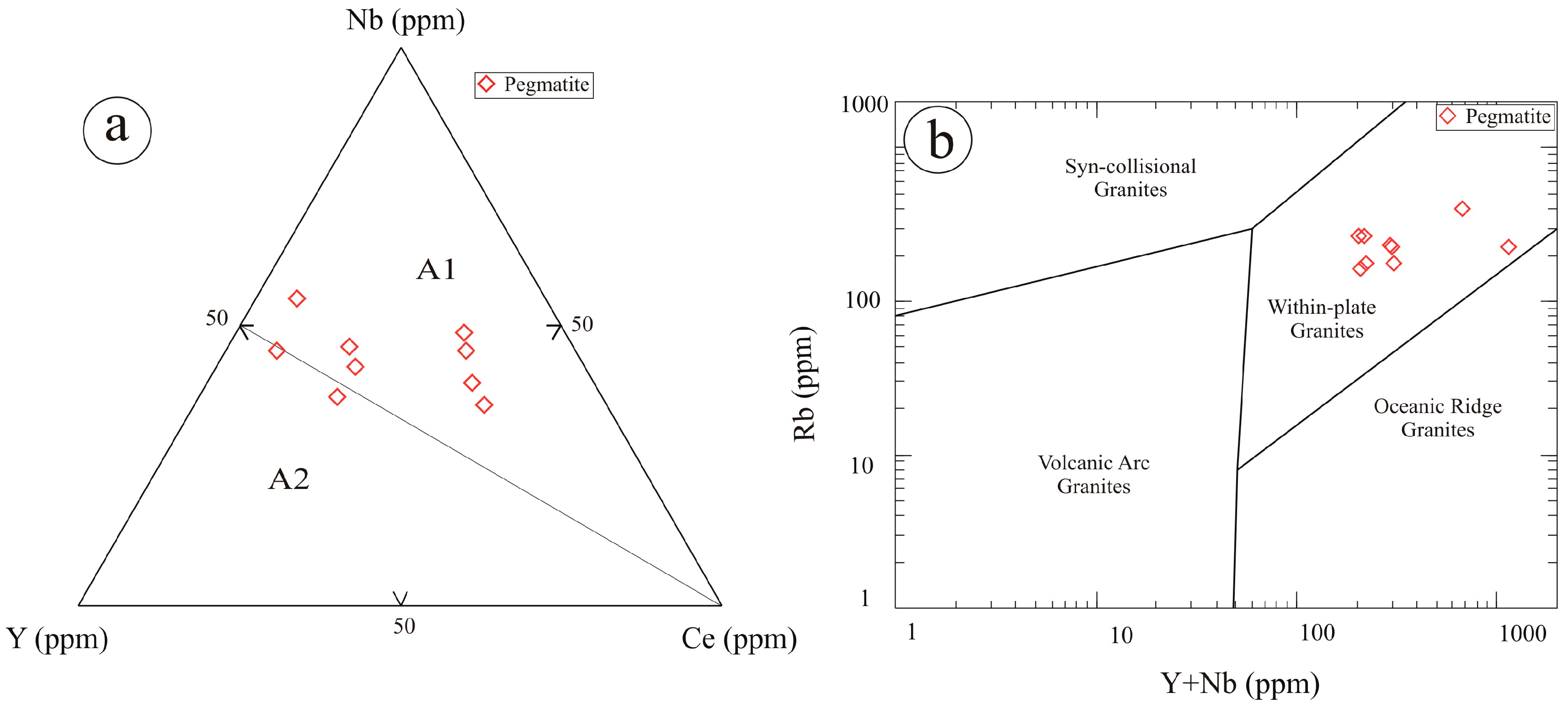
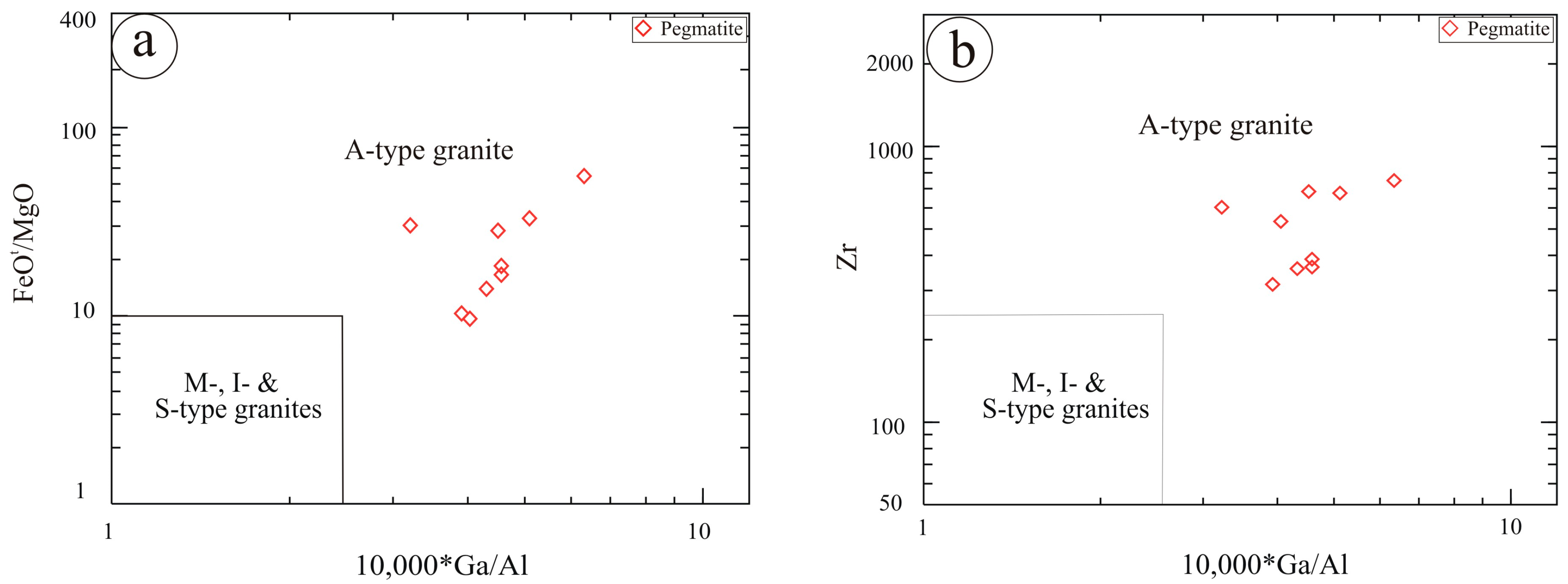
6. Geochemical Characteristics
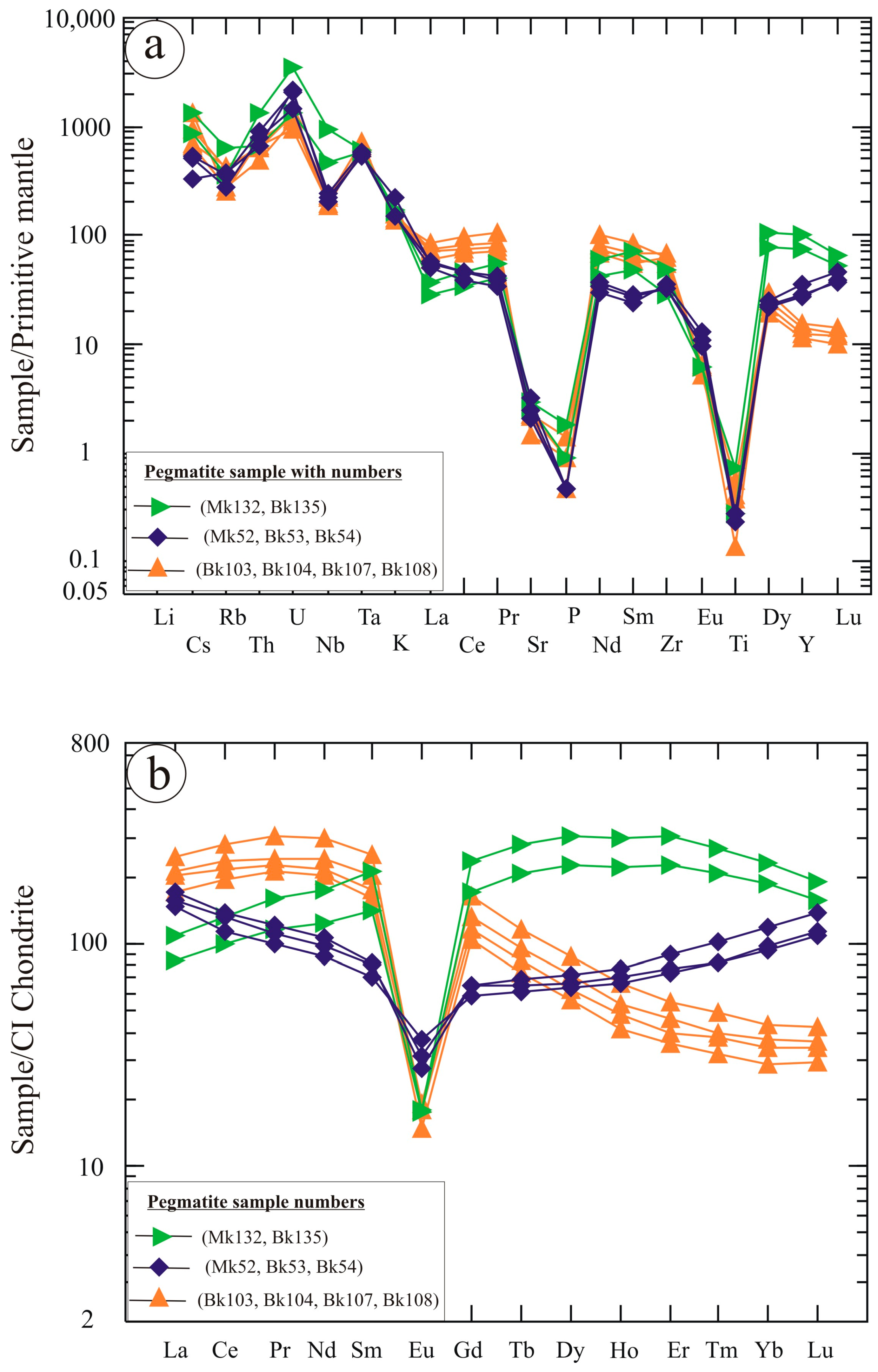
7. Discussion
7.1. Petrogenesis
7.1.1. Pegmatite
7.1.2. Pyroxmangite
7.2. Mineralization of the GEBI
Dynamic Model of Mineralization Enrichment
7.3. Ore Minerals
7.3.1. Nb-Ta Oxide Minerals
7.3.2. Bastnäsite
7.3.3. Cassiterite
7.3.4. Beryl
8. Conclusions
- Pyroxmangite is reported for the first time in the Gabal El-Bakriya pegmatites and on the whole Nubian Shield. The pegmatites are hosted by post-collisional, A-type granites of the Gabal El-Bakriya intrusion (GEBI). They were emplaced as dikes and plugs along the margin of the alkali-feldspar granite.
- The pyroxmangite pockets consist essentially of pyroxmangite mineral alongside garnet, pyrochroite, and fluorite. The composition of pyroxmangite reflects Mn-rich, low-Ca, and high-silica fluids that infiltrated at the hydrothermal stage.
- The pegmatites are NYF-type, characterized by high SiO2 content and significant enrichment in Nb, Y, REE, Zr, Th, U, and F. These geochemical characteristics indicate advanced magmatic differentiation under oxidizing, fluorine-rich conditions.
- The NYF-pegmatites host a diverse suite of REE- and HFSE-bearing accessory minerals, including monazite-(Ce), xenotime, bastnäsite, fergusonite, and pyrochlore, indicating favorable conditions for the concentration of critical metals. The evolved nature of the GEBI system, combined with its complex mineralogy and REE–Nb–Ta–U–F enrichment, highlights its high potential for mineral exploration for critical minerals, particularly in the greisens, pegmatites, and quartz-fluorite veins. In addition, the studied pyroxmangite has potential economic value for its use in jewelry and decorative applications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, P.R.; Woldehaimanot, B. Development of the Arabian–Nubian Shield: Perspectives on accretion and deformation in the northern East African Orogen and the assembly of Gondwana. Geol. Soc. Lond. Spec. Publ. 2003, 206, 289–325. [Google Scholar] [CrossRef]
- Stern, R.J. Arc assembly and collision in the Neoproterozoic African Orogen: Implications for the consolidation of Gondwanaland. Annu. Rev. Earth Planet. Sci. 1994, 22, 319–351. [Google Scholar] [CrossRef]
- Stern, R.J. Crustal evolution in the East African Orogen: A neodymium isotopic perspective. J. Afr. Earth Sci. 2002, 34, 109–117. [Google Scholar] [CrossRef]
- Meert, J.G.; Nédélec, A.; Hall, C. The stratoid granites of central Madagascar: Paleomagnetism and further age constraints on Neoproterozoic deformation. Precambrian Res. 2003, 120, 101–129. [Google Scholar] [CrossRef]
- Hargrove, U.; Stern, R.; Kimura, J.-I.; Manton, W.; Johnson, P. How juvenile is the Arabian–Nubian Shield? Evidence from Nd isotopes and pre-Neoproterozoic inherited zircon in the Bi’r Umq suture zone, Saudi Arabia. Earth Planet. Sci. Lett. 2006, 252, 308–326. [Google Scholar] [CrossRef]
- Liégeois, J.-P.; Stern, R.J. Sr–Nd isotopes and geochemistry of granite-gneiss complexes from the Meatiq and Hafafit domes, Eastern Desert, Egypt: No evidence for pre-Neoproterozoic crust. J. Afr. Earth Sci. 2010, 57, 31–40. [Google Scholar] [CrossRef]
- Ali, K.A.; Azer, M.K.; Gahlan, H.A.; Wilde, S.A.; Samuel, M.D.; Stern, R.J. Age constraints on the formation and emplacement of Neoproterozoic ophiolites along the Allaqi–Heiani Suture, South Eastern Desert of Egypt. Gondwana Res. 2010, 18, 583–595. [Google Scholar] [CrossRef]
- Johnson, P.; Andresen, A.; Collins, A.; Fowler, A.; Fritz, H.; Ghebreab, W.; Kusky, T.; Stern, R. Late Cryogenian–Ediacaran history of the Arabian–Nubian Shield: A review of depositional, plutonic, structural, and tectonic events in the closing stages of the northern East African Orogen. J. Afr. Earth Sci. 2011, 61, 167–232. [Google Scholar] [CrossRef]
- Khalil, A.E.S.; Obeid, M.A.; Azer, M.K.; Asimow, P.D. Geochemistry and petrogenesis of post-collisional alkaline and peralkaline granites of the Arabian-Nubian Shield: A case study from the southern tip of Sinai Peninsula, Egypt. Int. Geol. Rev. 2017, 60, 998–1018. [Google Scholar] [CrossRef]
- Azer, M.K.; Abdelfadil, K.M.; Asimow, P.D.; Khalil, A.E. Tracking the transition from subduction-related to post-collisional magmatism in the north Arabian–Nubian Shield: A case study from the Homrit Waggat area of the Eastern Desert of Egypt. Geol. J. 2019, 55, 4426–4452. [Google Scholar] [CrossRef]
- Abuamarah, B.A.; Azer, M.K.; Seddik, A.M.; Asimow, P.D.; Guzman, P.; Fultz, B.T.; Wilner, O.; Dalleska, N.; Darwish, M.H. Magmatic and post-magmatic evolution of post-collisional rare-metal bearing granite: The Neoproterozoic Homrit Akarem Granitic Intrusion, south Eastern Desert of Egypt. Geochemistry 2022, 82, 125840. [Google Scholar] [CrossRef]
- Elsagheer, M.A.; Azer, M.K.; Moussa, H.E.; Maurice, A.E.; Sami, M.; El Maaty, M.A.A.; Akarish, A.I.M.; Heikal, M.T.S.; Khedr, M.Z.; Elnazer, A.A.; et al. Late Neoproterozoic Rare-Metal Pegmatites with Mixed NYF-LCT Features: A Case Study from the Egyptian Nubian Shield. Minerals 2025, 15, 495. [Google Scholar] [CrossRef]
- Bentor, Y. The crustal evolution of the Arabo-Nubian Massif with special reference to the Sinai Peninsula. Precambrian Res. 1985, 28, 1–74. [Google Scholar] [CrossRef]
- Jarrar, G.; Stern, R.; Saffarini, G.; Al-Zubi, H. Late-and post-orogenic Neoproterozoic intrusions of Jordan: Implications for crustal growth in the northernmost segment of the East African Orogen. Precambrian Res. 2003, 123, 295–319. [Google Scholar] [CrossRef]
- Moussa, E.M.; Stern, R.J.; Manton, W.I.; Ali, K.A. SHRIMP zircon dating and Sm/Nd isotopic investigations of Neoproterozoic granitoids, Eastern Desert, Egypt. Precambrian Res. 2008, 160, 341–356. [Google Scholar] [CrossRef]
- Azer, M.; Obeid, M.; Ren, M. Geochemistry and petrogenesis of late Ediacaran (605–580 Ma) post-collisional alkaline rocks from the Katherina ring complex, south Sinai, Egypt. J. Asian Earth Sci. 2014, 93, 229–252. [Google Scholar] [CrossRef]
- Be’ERi-Shlevin, Y.; Katzir, Y.; Valley, J.W. Crustal evolution and recycling in a juvenile continent: Oxygen isotope ratio of zircon in the northern Arabian Nubian Shield. Lithos 2009, 107, 169–184. [Google Scholar] [CrossRef]
- Be’eRi-Shlevin, Y.; Samuel, M.D.; Azer, M.; Rämö, O.; Whitehouse, M.; Moussa, H. The Ediacaran Ferani and Rutig volcano-sedimentary successions of the northernmost Arabian-Nubian Shield (ANS): New insights from zircon U–Pb geochronology, geochemistry and O–Nd isotope ratios. Precambrian Res. 2011, 188, 21–44. [Google Scholar] [CrossRef]
- Azer, M.K.; Stern, R.J.; Kimura, J.I. Origin of a late Neoproterozoic (605 ± 13 Ma) intrusive carbonate–albitite complex in Southern Sinai, Egypt. Int. J. Earth Sci. 2010, 99, 245–267. [Google Scholar] [CrossRef]
- Eyal, M.; Litvinovsky, B.; Jahn, B.; Zanvilevich, A.; Katzir, Y. Origin and evolution of post-collisional magmatism: Coeval Neoproterozoic calc-alkaline and alkaline suites of the Sinai Peninsula. Chem. Geol. 2010, 269, 153–179. [Google Scholar] [CrossRef]
- Abu El-Rus, M.A.; Mohamed, M.A.; Lindh, A. Mueilha rare metals granite, Eastern Desert of Egypt: An example of a magmatic-hydrothermal system in the Arabian-Nubian Shield. Lithos 2017, 294–295, 362–382. [Google Scholar] [CrossRef]
- Azer, M.K.; Abdelfadil, K.M.; Ramadan, A.A. Geochemistry and petrogenesis of Late Ediacaran rare-metal albite granite of the Nubian Shield: Case study of Nuweibi intrusion, Eastern Desert, Egypt. J. Geol. 2019, 127, 665–689. [Google Scholar] [CrossRef]
- Seddik, A.M.; Darwish, M.H.; Azer, M.K.; Asimow, P.D. Assessment of magmatic versus post-magmatic processes in the Mueilha rare-metal granite, Eastern Desert of Egypt, Arabian-Nubian Shield. Lithos 2020, 366–367, 105542. [Google Scholar] [CrossRef]
- Moussa, H.E.; Asimow, P.D.; Azer, M.K.; El Maaty, M.A.A.; Akarish, A.I.; Yanni, N.N.; Mubarak, H.S.; Wilner, O.; Elsagheer, M.A. Magmatic and hydrothermal evolution of highly-fractionated rare-metal granites at Gabal Nuweibi, Eastern Desert, Egypt. Lithos 2021, 400–401, 106405. [Google Scholar] [CrossRef]
- Heikal, M.T.S.; Shereif, A.S.; Azer, M.K. Gamma activity concentrations (226Ra, 232Th, 40K) of mineralized Homret Akarem composite granitic pluton, Egyptian Nubian Shield: Environmental hazards assessment. Euro-Mediterranean J. Environ. Integr. 2024, 9, 1629–1658. [Google Scholar] [CrossRef]
- Heikal, M.T.S.; Azer, M.K.; Kamar, M.S.; Ibrahim, M.O.; El Monsef, M.A. Petrogenesis and geodynamic model for (Ta, Nb)-fertilized Nuweibi albite granite, Egyptian Nubian Shield: Juvenile crust-mantle mixing and metasomatic enhancement. J. Afr. Earth Sci. 2024, 223, 105530. [Google Scholar] [CrossRef]
- Zoheir, B.; Carr, P.; Xu, X.; Zeh, A.; Kraemer, D.; McAleer, R.; Steele-MacInnis, M.; Ragab, A.; Deshesh, F. The Igla Sn-(W-Be) deposit, Egypt: Prolonged magmatic-metasomatic processes during the middle stage evolution of the Arabian-Nubian Shield. Gondwana Res. 2025, 142, 20–43. [Google Scholar] [CrossRef]
- Saleeb-Roufaiel, G.S. Note on the possible occurrence of contact metamorphism Nubian sandstone at Bakriya, Eastern Desert of Egypt. BNRC Egypt 1977, 2, 283–286. [Google Scholar]
- Moussa, H.E. Contribution to the Geology and Mineralization in the Area of Bakriya, Eastern Desert, Egypt. Master’s Thesis, Ain Shams University, Cairo, Egypt, 1979; 129p. [Google Scholar]
- Saleeb-Roufaiel, G.S.; Samuel, M.D.; Hilmy, M.E.; Moussa, H.E. Fluorite mineralization at El-Bakriya, Eastern Desert of Egypt. Egypt. J. Geol. 1982, 26, 9–18. [Google Scholar]
- Samuel, M.D.; Roufaiel, G.S.; Hilmy, M.E.; Moussa, H. Study of Cretaceous fore-arc granite and associated rocks at Bakriya, Eastern Desert. Egypt. J. Geol. 1983, 27, 1–11. [Google Scholar]
- El-Fatah, A.A.A.; Surour, A.A.; Azer, M.K.; Madani, A.A. Integration of Whole-Rock Geochemistry and Mineral Chemistry Data for the Petrogenesis of A-Type Ring Complex from Gebel El Bakriyah Area, Egypt. Minerals 2023, 13, 1273. [Google Scholar] [CrossRef]
- Abd El-Fatah, A.A.; Abd El-Dayiem, A.; Madani, A.A.; Surour, A.A.A.; Azer, M.K. Integration of Landsat-8 and reflectance spectroscopy data for mapping of late Neoproterozoic igneous ring complexes in an arid environment: A case study of Gebel El-Bakriyah Area, Eastern Desert, Egypt. JME 2023, 14, 13–31. [Google Scholar]
- El-Sayed, M.; Mohamed, F.; Furnes, H. Petrological and geochemical constraints on the evolution of late Pan-African Bakriya post-orogenic ring complex, Central Eastern Desert, Egypt. In Proceedings of the 7th EUREGEO, Zolder, Belgium, 11–12 September 2004; p. 688. [Google Scholar]
- Abd EL-Wahed, M.; Hamimi, Z. Neoproterozoic tectonic events of Egypt. Acta Geol. Sin.-Engl. 2021, 95, 1366–1405. [Google Scholar] [CrossRef]
- El-Amin, H. Radiometric and Geological Investigations of El-Bakriya Area, Eastern Desert, Egypt. Ph.D. Thesis, Cairo University, Cairo, Egypt, 1975. [Google Scholar]
- Miller, C.F.; Stoddard, E.F. The role of manganese in the paragenesis of magmatic garnet: An example from the Old Woman-Piute Range, California. J. Geol. 1981, 89, 233–246. [Google Scholar] [CrossRef]
- Tischendorf, G.; Förster, H.-J.; Gottesmann, B. Minor-and trace-element composition of trioctahedral micas: A review. Miner. Mag. 2001, 65, 249–276. [Google Scholar] [CrossRef]
- Zen, E.A. Phase relations of peraluminous granitic rocks and their petrogenetic implications. Annu. Rev. Earth Planet. Sci. 1988, 16, 21–51. [Google Scholar] [CrossRef]
- Mokhtar, H.; Surour, A.A.; Azer, M.K.; Ren, M.; Said, A. Petrogenesis and possible fingerprints of the Najd shear system on the evolution of deformed granitic rocks in the west Wadi Nugrus area, Egypt. J. Afr. Earth Sci. 2023, 207, 105045. [Google Scholar] [CrossRef]
- Mokhtar, H.; Surour, A.A.; Azer, M.K.; Ren, M.; Said, A. Geochemistry and mineral chemistry of granitic rocks from west Wadi El Gemal area, southern Eastern Desert of Egypt: Indicators for highly fractionated syn-to post-collisional Neoproterozoic felsic magmatism. Acta Geochim. 2024, 44, 163–188. [Google Scholar] [CrossRef]
- Vavra, G.; Gebauer, D.; Schmid, R.; Compston, W. Multiple zircon growth and recrystallization during polyphase Late Carboniferous to Triassic metamorphism in granulites of the Ivrea Zone (Southern Alps): An ion microprobe (SHRIMP) study. Contrib. Miner. Pet. 1996, 122, 337–358. [Google Scholar] [CrossRef]
- Hoskin, P.; Black, L. Metamorphic zircon formation by solid-state recrystallization of protolith igneous zircon. J. Metamorph. Geol. 2000, 18, 423–439. [Google Scholar] [CrossRef]
- Mücke, A. Chamosite, siderite and the environmental conditions of their formation in chamosite-type Phanerozoic ooidal ironstones. Ore Geol. Rev. 2006, 28, 235–249. [Google Scholar] [CrossRef]
- Inoue, A. Formation of clay minerals in hydrothermal environments. In Origin and Mineralogy of Clays: Clays and the Environment; Springer: Berlin/Heidelberg, Germany, 1995; pp. 268–329. [Google Scholar]
- Černý, P.; Ercit, T.S.; Wise, M.A. The tantalite-tapiolite gap; natural assemblages versus experimental data. Can. Mineral. 1992, 30, 587–596. [Google Scholar]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Giere, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Z.; Wang, Z.Q.; Wang, K.M. Characteristics of apatite from 160–140 Ma Cu (Mo) and Mo (W) deposits in East Qinling. Acta Geol. Sin. 2017, 91, 1925–1941. [Google Scholar]
- Du, J.; Wang, G.; Jia, L. In situ major and trace element compositions of apatites from Luanchuan orecluster: Implications for porphyry Mo mineralization. Ore Geol. Rev. 2019, 115, 103174. [Google Scholar] [CrossRef]
- Yang, F.; Santosh, M.; Glorie, S.; Xue, F.; Zhang, S.; Zhang, X. Apatite geochronology and chemistry of Luanchuan granitoids in the East Qinling Orogen, China: Implications for petrogenesis, metallogenesis and exploration. Lithos 2020, 378–379, 105797. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, W.; Xiao, B.; Wu, C.; Zheng, H. Apatite geochronology and geochemistry of Gucheng granites: Implications for petrogenesis and REE metallogenesis in South China. Ore Geol. Rev. 2023, 163, 105791. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.-Y.; Zhao, Y.-H. Trace elements in apatite from Gejiu Sn polymetallic district: Implications for petrogenesis, metallogenesis and exploration. Ore Geol. Rev. 2022, 145, 104880. [Google Scholar] [CrossRef]
- Li, J.; Yan, Q.; Li, P.; Jacobson, M.I. Formation of granitic pegmatites during orogenies: Indications from a case study of the pegmatites in China. Ore Geol. Rev. 2023, 156, 105391. [Google Scholar] [CrossRef]
- Stormer, J.C. The effects of recalculation on estimates of temperature and oxygen fugacity from analyses of multicomponent iron-titanium oxides. Am. Mineral. 1983, 68, 586–594. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman Scientific & Technical: London, UK, 1992. [Google Scholar]
- Černý, P. Distribution, affiliation and derivation of rare-element granitic pegmatites in the Canadian Shield. Geol. Rundsch. 1990, 79, 183–226. [Google Scholar] [CrossRef]
- Cerny, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Hanson, S.L. A tectonic evaluation of pegmatite parent granites. Can. Mineral. 2016, 54, 917–933. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Wu, F.-Y.; Sun, D.-Y.; Li, H.; Jahn, B.-M.; Wilde, S. A-type granites in northeastern China: Age and geochemical constraints on their petrogenesis. Chem. Geol. 2002, 187, 143–173. [Google Scholar] [CrossRef]
- Liégeois, J.P.; Black, R. Alkaline magmatism subsequent to collision in the Pan-African belt of the Adrar des Iforas (Mali). Geol. Soc. Lond. Spec. Publ. 1987, 30, 381–401. [Google Scholar] [CrossRef]
- Bonin, B. A-type granites and related rocks: Evolution of a concept, problems and prospects. Lithos 2007, 97, 1–29. [Google Scholar] [CrossRef]
- Eby, G. The A-type granitoids: A review of their occurrence and chemical characteristics and speculations on their petrogenesis. Lithos 1990, 26, 115–134. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivision of the A-type granitoids:Petrogenetic and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.F.M.; El-Kibbi, M.M. Anorogenic magmatism: Chemical evolution of the Mount El-Sibai A-type complex (Egypt), and implications for the origin of within-plate felsic magmas. Geol. Mag. 2001, 138, 67–85. [Google Scholar] [CrossRef]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Pet. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- London, D.; Evensen, J.M. Beryllium in silicic magmas and the origin of beryl-bearing pegmatites. Rev. Mineral. Geochem. 2002, 50, 445–486. [Google Scholar] [CrossRef]
- Maphumulo, M.S. Mineralogical and Geochemical Characterisation of Mineralised and Regular NYF-Type Pegmatites from the Namaqualand Pegmatite Belt, Northern Cape, South Africa. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2020. [Google Scholar]
- Bonzi, W.M.-E.; Van Lichtervelde, M.; Vanderhaeghe, O.; André-Mayer, A.-S.; Salvi, S.; Wenmenga, U. Insights from mineral trace chemistry on the origin of NYF and mixed LCT+ NYF pegmatites and their mineralization at Mangodara, SW Burkina Faso. Miner. Depos. 2022, 58, 75–104. [Google Scholar] [CrossRef]
- Simmons, W.B.; Webber, K.L.; Falster, A.U. Pegmatites. Rocks Miner. 2024, 99, 18–32. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.; Rao, L.; Wu, F. Discovery of a petalite-type pegmatite in the eastern Himalaya and implications for rare-metal mineralization. Lithos 2025, 510–511, 108112. [Google Scholar] [CrossRef]
- Dill, H.G. Pegmatitic Rocks and Economic Geology. In The Hagendorf-Pleystein Province: The Center of Pegmatites in an Ensialic Orogen; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–54. [Google Scholar]
- Xiong, Y.-Q.; Fan, Z.-W.; Yu, H.-Y.; Di, H.; Cao, Y.-H.; Wen, C.-H.; Jiang, S.-Y. Genetic linkage between parent granite and zoned rare metal pegmatite in the Renli-Chuanziyuan granite-pegmatite system, South China. GSA Bull. 2024, 137, 1607–1627. [Google Scholar] [CrossRef]
- Zheng, S.; Su, J.-H.; Wang, J.; Liu, S.-J.; Zhao, X.-F. Origin and evolution of granitic pegmatite rare metal deposits in the northern Mufushan batholith, South China: Insights from muscovite chemistry. Am. Mineral. 2025. [Google Scholar] [CrossRef]
- Černý, P.; London, D.; Novák, M. Granitic pegmatites as reflections of their sources. Elements 2012, 8, 289–294. [Google Scholar] [CrossRef]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Kaeter, D.; Barros, R.; Menuge, J.F.; Chew, D.M. The magmatic–hydrothermal transition in rare-element pegmatites from southeast Ireland: LA-ICP-MS chemical mapping of muscovite and columbite–tantalite. Geochim. Cosmochim. Acta 2018, 240, 98–130. [Google Scholar] [CrossRef]
- Michaud, J.A.-S.; Pichavant, M. Magmatic fractionation and the magmatic-hydrothermal transition in rare metal granites: Evidence from Argemela (Central Portugal). Geochim. Cosmochim. Acta 2020, 289, 130–157. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Wang, R.-C.; Wang, C.Y.; Linnen, R.; Wu, F.-Y. Highly fractionated granites and rare-metal mineralization. Lithos 2021, 398–399, 106262. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, B.; Zhu, W.; Chen, Y.; Li, G.; Gao, J.; Che, X.; Zhang, R.; Wei, H.; Li, W.; et al. Geologic scenario from granitic sheet to Li-rich pegmatite uncovered by Scientific Drilling at the Jiajika lithium deposit in eastern Tibetan Plateau. Ore Geol. Rev. 2023, 161, 105636. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.-C.; Linnen, R.L.; Wu, F.-Y.; Xie, L.; Liu, X.-C. Continuous Be mineralization from two-mica granite to pegmatite: Critical element enrichment processes in a Himalayan leucogranite pluton. Am. Mineral. 2023, 108, 31–41. [Google Scholar] [CrossRef]
- Yan, Q.-G.; Li, J.-K.; Wang, D.-H.; Zhu, Z.-Y.; Li, C.; Chen, Z.-Y. Petrogenesis of Ke’eryin granitic pegmatites and associated Li mineralization in the Songpan–Ganze orogenic belt, China: Evidence from apatite and bulk-rock chemistry. Miner. Depos. 2024, 60, 723–742. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Shaw, R.A.; Borst, A.M.; Nex, P.; Kinnaird, J.A.; van Lichtervelde, M.; Essaifi, A.; Koopmans, L.; Deady, E.A. Lithium pegmatites in Africa: A review. Econ. Geol. 2025, 120, 513–539. [Google Scholar] [CrossRef]
- Müller, A.; Romer, R.L.; Szuszkiewicz, A.; Ilnicki, S.; Szełęg, E. Can pluton-related and pluton-unrelated granitic pegmatites be distinguished by their chemistry? In Proceedings of the 2nd Eugene E. Foord Pegmatite Symposium, Golden, CO, USA, 15–19 July 2016; p. 67. [Google Scholar]
- Webber, K.; Simmons, W.B.; Falster, A.U.; Hanson, S.L. Anatectic Pegmatites of the Oxford County Pegmatite Field, Maine, USA. Can. Mineral. 2019, 57, 811–815. [Google Scholar] [CrossRef]
- Martin, R.F.; De Vito, C. The patterns of enrichment in felsic pegmatites ultimately depend on tectonic setting. Can. Mineral. 2005, 43, 2027–2048. [Google Scholar] [CrossRef]
- Konzett, J.; Schneider, T.; Nedyalkova, L.; Hauzenberger, C.; Melcher, F.; Gerdes, A.; Whitehouse, M. Anatectic granitic pegmatites from the Eastern Alps: A case of variable rare-metal enrichment during high-grade regional metamorphism–I: Mineral assemblages, geochemical characteristics, and emplacement ages. Can. Mineral. 2018, 56, 555–602. [Google Scholar] [CrossRef]
- Fei, G.; Menuge, J.F.; Li, Y.; Yang, J.; Deng, Y.; Chen, C.; Yang, Y.; Yang, Z.; Qin, L.; Zheng, L.; et al. Petrogenesis of the Lijiagou spodumene pegmatites in Songpan-Garze Fold Belt, West Sichuan, China: Evidence from geochemistry, zircon, cassiterite and coltan U-Pb geochronology and Hf isotopic compositions. Lithos 2020, 364–365, 105555. [Google Scholar] [CrossRef]
- Adoze, U.; Abubakar, F.; Ochu, G.; Danga, O.; Adamu, M.; Baba, Y. Geological and geochemical analyses of pegmatites in Egbe, Isanlu (sheet 225), Southwestern Nigeria. Sci. Afr. 2024, 23, 183–202. [Google Scholar] [CrossRef]
- Sirbescu, M.-L.C.; Nabelek, P.I. Crystallization conditions and evolution of magmatic fluids in the Harney Peak Granite and associated pegmatites, Black Hills, South Dakota—Evidence from fluid inclusions. Geochim. Cosmochim. Acta 2003, 67, 2443–2465. [Google Scholar] [CrossRef]
- London, D. Reading pegmatites: Part 1—What beryl says. Rocks Miner. 2015, 90, 138–153. [Google Scholar] [CrossRef]
- Wu, F.-Y.; Liu, X.-C.; Liu, Z.-C.; Wang, R.-C.; Xie, L.; Wang, J.-M.; Ji, W.-Q.; Yang, L.; Liu, C.; Khanal, G.P.; et al. Highly fractionated Himalayan leucogranites and associated rare-metal mineralization. Lithos 2020, 352–353, 105319. [Google Scholar] [CrossRef]
- Teixeira, L.M.F.; Troch, J.; Allaz, J.; Bachmann, O. Magmatic to hydrothermal conditions in the transition from the A-type Pikes Peak granite (Colorado) to its related pegmatite. Front. Earth Sci. 2022, 10, 976588. [Google Scholar] [CrossRef]
- London, D. Granitic pegmatites: An assessment of current concepts and directions for the future. Lithos 2005, 80, 281–303. [Google Scholar] [CrossRef]
- Stilling, A.; Ćerný, P.; Vanstone, P.J. The Tanco pegmatite at Bernic Lake, Manitoba. XVI. Zonal and bulk compositions and their petrogenetic significance. Can. Mineral. 2006, 44, 599–623. [Google Scholar] [CrossRef]
- Buick, I.S.; Storkey, A.; Williams, I.S. Timing relationships between pegmatite emplacement, metamorphism and deformation during the intra-plate Alice Springs Orogeny, central Australia. J. Metamorph. Geol. 2008, 26, 915–936. [Google Scholar] [CrossRef]
- London, D. A petrologic assessment of internal zonation in granitic pegmatites. Lithos 2014, 184, 74–104. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil-Crespo, P.; Torres-Ruiz, J. From granite to highly evolved pegmatite: A case study of the Pinilla de Fermoselle granite–pegmatite system (Zamora, Spain). Lithos 2012, 153, 192–207. [Google Scholar] [CrossRef]
- Simmons, W.B.S.; Webber, K.L. Pegmatite genesis: State of the art. Eur. J. Miner. 2008, 20, 421–438. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Asimow, P.D.; Azer, M.K.; Ma, C.; Al-Kahtany, K.M.; Hakeem, A.Y. Geochemistry and mineralogy of the Jebel Aja Igneous Intrusion and the associated exotic pegmatites, Arabian Shield, Saudi Arabia. Lithos 2021, 400–401, 106395. [Google Scholar] [CrossRef]
- Partington, G.A.; McNaughton, N.J.; Williams, I.S. A review of the geology, mineralization, and geochronology of the Greenbushes pegmatite, Western Australia. Econ. Geol. 1995, 90, 616–635. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Shaw, R.A.; Smith, M.; Estrade, G.; Marqu, E.; Bernard, C.; Nex, P. Economic mineralization in pegmatites: Comparing and contrasting NYF and LCT examples. Can. Mineral. 2019, 57, 753–755. [Google Scholar] [CrossRef]
- Elbialy, M.Y. Origin and Economic Profits of Pegmatites: A Case Study from Egypt. Ph.D. Thesis, Tanta University, Tanta, Egypt, 2022. [Google Scholar]
- Maresch, W.V.; Mottana, A. The pyroxmangite-rhodonite transformation for the MnSiO3 composition. Contrib. Miner. Pet. 1976, 55, 69–79. [Google Scholar] [CrossRef]
- Azer, M.K.; Surour, A.A.; Moussa, H.E.; Maurice, A.E.; Sami, M.; El Maaty, M.A.A.; Akarish, A.I.M.; Heikal, M.T.S.; Elnazer, A.A.; Elsagheer, M.A.; et al. Homrit Akarem Post-Collisional Intrusion, Southeastern Desert, Egypt: Petrogenesis of Greisen Formed in a Cupola Structure and Enrichment in Strategic Minerals. Geosciences 2025, 15, 200. [Google Scholar] [CrossRef]
- El Hadek, H.H.; Mohamed, M.A.; El Habaak, G.H.; Bishara, W.W.; Ali, K.A. Geochemical constraints on petrogenesis of Homrit Waggat rare metal granite, Egypt. Int. J. Geo. Geochem. 2016, 3, 33–48. [Google Scholar]
- Kotlánová, M.K.; Dolníček, Z.; René, M.; Prochaska, W.; Ulmanová, J.; Kapusta, J.; Mašek, V.; Kropáč, K. Fluid evolution of greisens from Krupka Sn-W ore district, Bohemian Massif (Czech Republic). Minerals 2024, 14, 86. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Bartels, A.; Holtz, F.; Linnen, R.L. Solubility of manganotantalite and manganocolumbite in pegmatitic melts. Am. Miner. 2010, 95, 537–544. [Google Scholar] [CrossRef]
- Fiege, A.; Simon, A.; Linsler, S.A.; Bartels, A.; Linnen, R.L. Experimental constraints on the effect of phosphorous and boron on Nb and Ta ore formation. Ore Geol. Rev. 2018, 94, 383–395. [Google Scholar] [CrossRef]
- Lahti, S.I. Zoning in columbite-tantalite crystals from the granitic pegmatites of the Eräjärvi area, southern Finland. Geoch. Cosmochim. Acta 1987, 51, 509–517. [Google Scholar] [CrossRef]
- Roda, E.; Keller, P.; Pesquera, A.; Fontan, F. Micas of the muscovite–lepidolite series from Karibib pegmatites, Namibia. Miner. Mag. 2007, 71, 41–62. [Google Scholar] [CrossRef]
- Van Lichtervelde, M.; Holtz, F.; Melcher, F. The effect of disequilibrium crystallization on Nb-Ta fractionation in pegmatites: Constraints from crystallization experiments of tantalite-tapiolite. Am. Mineral. 2018, 103, 1401–1416. [Google Scholar] [CrossRef]
- Chevychelov, V.Y.; Borodulin, G.P.; Zaraisky, G.P. Solubility of columbite,(Mn, Fe)(Nb, Ta)2O6, in granitoid and alkaline melts at 650–850 °C and 30–400 MPa: An experimental investigation. Geochem. Int. 2010, 48, 456–464. [Google Scholar] [CrossRef]
- McNeil, A.G.; Linnen, R.L.; Flemming, R.L. Solubility of wodginite, titanowodginite, microlite, pyrochlore, columbite-(Mn) and tantalite-(Mn) in flux-rich haplogranitic melts between 700° and 850 °C and 200 MPa. Lithos 2020, 352–353, 105239. [Google Scholar] [CrossRef]
- Chevychelov, V.Y.; Zaraisky, G.P.; Borisovsky, S.E.; Nekrasov, A.N. Solubility of Columbite and Diffusion of Ta, Nb, Fe, and Mn in Li–F Granite Melts at 740–980 C and 1 kbar. In Proceedings of the 15th Russian Conference on Experimental Mineralogy, Syktyvkar, Russia, 22–24 June 2005; pp. 123–125. [Google Scholar]
- London, D. The application of experimental petrology to the genesis and crystallization of granitic pegmatites. Can. Min. 1992, 30, 499–540. [Google Scholar]
- Van Lichtervelde, M.; Salvi, S.; Beziat, D.; Linnen, R.L. Textural features and chemical evolution in tantalum oxides: Magmatic versus hydrothermal origins for Ta mineralization in the Tanco Lower pegmatite, Manitoba, Canada. Econ. Geol. 2007, 102, 257–276. [Google Scholar] [CrossRef]
- Helba, H.; Trumbull, R.B.; Morteani, G.; Khalil, S.O.; Arslan, A. Geochemical and petrographic studies of Ta mineralization in the Nuweibi albite granite complex, Eastern Desert, Egypt. Miner. Depos. 1997, 32, 164–179. [Google Scholar] [CrossRef]
- Abuamarah, B.A.; Azer, M.K.; Asimow, P.D.; Ghrefat, H.; Mubarak, H.S. Geochemistry and petrogenesis of late Ediacaran rare-metal albite granites of the Arabian-Nubian Shield. Acta Geol. Sin. Engl. 2019, 95, 459–480. [Google Scholar] [CrossRef]
- Heikal, M.T.S.; Khedr, M.Z.; El Monsef, M.A.; Gomaa, S.R. Petrogenesis and geodynamic evolution of neoproterozoic Abu Dabbab albite granite, central Eastern Desert of Egypt: Petrological and geochemical constraints. J. Afr. Earth Sci. 2019, 158, 103518. [Google Scholar] [CrossRef]
- Zozulya, D.; Macdonald, R.; Bagiński, B. REE fractionation during crystallization and alteration of fergusonite-(Y) from Zr-REE-Nb-rich late-to post-magmatic products of the Keivy alkali granite complex, NW Russia. Ore Geol. Rev. 2020, 125, 103693. [Google Scholar] [CrossRef]
- Gawad, A.E.A.; Panova, E.G.; Ghoneim, M.M.; Yanson, S.Y.; Alsufyani, S.J.; Saftah, A.; Alresheedi, N.M.; Hanfi, M.Y. Radioactive Assessment and Th-, Nb-Ta-, Zr-, REE-Bearing Minerals in Alkaline Syenite: Environmental Implications for Radiological Safety. Geosciences 2025, 15, 138. [Google Scholar] [CrossRef]
- Hisinger, W. Lethoea Svecica: Seu, Petrificata Svecioe, Iconibus et Characteribus Illustrata; PA Norstedt et filii: Stockholm, Sweden, 1838. [Google Scholar]
- Andersen, T. Compositional variation of some rare earth minerals from the Fen complex (Telemark, SE Norway): Implications for the mobility of rare earths in a carbonatite system. Miner. Mag. 1986, 50, 503–509. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can. Mineral. 1996, 34, 1111–1126. [Google Scholar]
- Christiansen, E.H.; Stuckless, J.S.; Funkhouser, M.J.; Howell, K.A.; Taylor, R.P.; Strong, D.F. Petrogenesis of rare-metal granites from depleted crustal sources: An example from the Cenozoic of western Utah, USA. Recent Advances in the Geology of Granite-Related Mineral Deposits. CIM 1988, 39, 307–321. [Google Scholar]
- Ballouard, C.; Elburg, M.A.; Tappe, S.; Reinke, C.; Ueckermann, H.; Doggart, S. Magmatic-hydrothermal evolution of rare metal pegmatites from the Mesoproterozoic Orange River pegmatite belt (Namaqualand, South Africa. Ore Geol. Rev. 2020, 116, 103252. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.-Y.; Ge, W.-C.; Yang, H.; Wu, H.-R.; Chen, J.-C. Fluid evolution and physicochemical constraints of the Nasigatu greisen-type Be deposit in Inner Mongolia. Ore Geol. Rev. 2024, 176, 106431. [Google Scholar] [CrossRef]
| Rock Type | Pegmatite | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample No | BK52 | BK53 | BK54 | BK103 | BK104 | BK107 | BK108 | BK132 | BK135 |
| SiO2 | 80.69 | 79.35 | 78.07 | 78.83 | 80.05 | 79.22 | 78.47 | 77.54 | 76.51 |
| TiO2 | 0.06 | 0.06 | 0.05 | 0.09 | 0.03 | 0.12 | 0.08 | 0.06 | 0.16 |
| Al2O3 | 7.69 | 8.75 | 7.62 | 9.69 | 9.72 | 8.18 | 9.2 | 9.46 | 10.76 |
| Fe2O3 | 1.28 | 1.32 | 1.24 | 1.18 | 1.39 | 1.62 | 1.93 | 1.43 | 1.64 |
| MnO | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.02 | 0.3 | 0 |
| MgO | 0.07 | 0.08 | 0.09 | 0.04 | 0.05 | 0.03 | 0.06 | 0.14 | 0.17 |
| CaO | 0.24 | 0.16 | 0.2 | 0.15 | 0.3 | 0.24 | 0.23 | 0.28 | 0.63 |
| Na2O | 3.08 | 3.04 | 1.96 | 2.94 | 2.98 | 3.94 | 3.18 | 3.19 | 3.12 |
| K2O | 4.42 | 4.47 | 6.76 | 4.4 | 4.09 | 4.77 | 4.75 | 5.01 | 5.04 |
| P2O5 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.04 |
| LOI | 0.95 | 1.09 | 1.03 | 0.78 | 0.82 | 0.54 | 0.61 | 0.71 | 0.83 |
| Total | 98.5 | 98.34 | 97.04 | 98.13 | 99.48 | 98.69 | 98.56 | 98.14 | 98.9 |
| Normative composition | |||||||||
| Quartz | 45.28 | 46.34 | 47.68 | 44.42 | 42.67 | 38.07 | 52.22 | 47.83 | 50.15 |
| Corundum | - | - | - | - | - | - | - | - | - |
| Orthoclase | 26.74 | 24.53 | 28.76 | 28.7 | 30.42 | 30.41 | 26.8 | 27.19 | 41.65 |
| Albite | 25.58 | 25.59 | 15.82 | 21.33 | 21.32 | 26.95 | 15.32 | 20.69 | 1.61 |
| Anorthite | 0.24 | 1.07 | - | - | - | 0.47 | - | - | - |
| Acmite | - | - | 0.78 | 0.9 | 0.81 | - | 0.6 | 0.62 | 0.59 |
| Na-Metasilicate | - | - | 4.03 | 1.2 | 1.28 | - | 2.5 | 1.18 | 3.49 |
| Diopside | 0.34 | 0.27 | 1.02 | 0.85 | 1.13 | 2.12 | 1.01 | 0.66 | 0.84 |
| Hypersthene | 1.3 | 1.77 | 1.66 | 2.35 | 2.2 | 1.16 | 1.39 | 1.67 | 1.52 |
| Magnetite | 0.29 | 0.32 | - | - | - | 0.4 | - | - | - |
| Ilmenite | 0.18 | 0.06 | 0.23 | 0.16 | 0.12 | 0.31 | 0.12 | 0.12 | 0.1 |
| Apatite | 0.04 | 0.04 | 0.02 | 0.07 | 0.04 | 0.09 | 0.02 | 0.02 | 0.02 |
| Some chemical parameters | |||||||||
| R1 | 3212 | 3125 | 2890 | 3145 | 3280 | 2717 | 2935 | 2822 | 2763 |
| R2 | 180 | 193 | 175 | 208 | 225 | 188 | 208 | 222 | 287 |
| AI | 1.28 | 1.12 | 1.38 | 0.99 | 0.96 | 1.42 | 1.13 | 1.13 | 0.98 |
| ASI | 0.75 | 0.86 | 0.70 | 0.98 | 0.98 | 0.68 | 0.85 | 0.89 | 0.92 |
| Ti | 360 | 360 | 300 | 540 | 180 | 719 | 480 | 360 | 959 |
| K | 36,692 | 37,107 | 56,117 | 36,526 | 33,952 | 39,597 | 39,431 | 41,590 | 41,839 |
| P | 44 | 44 | 44 | 87 | 87 | 44 | 131 | 87 | 175 |
| Mg# | 9.78 | 10.72 | 12.57 | 6.29 | 6.65 | 3.54 | 5.80 | 16.24 | 17.04 |
| Colour Index | 2.11 | 2.42 | 2.91 | 3.36 | 3.44 | 4 | 2.52 | 2.44 | 2.47 |
| Diff. Index | 97.59 | 96.45 | 92.25 | 94.46 | 94.41 | 95.43 | 94.34 | 95.72 | 93.42 |
| ANOR | 0.89 | 4.18 | 0.00 | 0.00 | 0.00 | 1.52 | 0.00 | 0.00 | 0.00 |
| Q/ | 46.28 | 47.51 | 51.68 | 47.03 | 45.20 | 39.70 | 55.35 | 49.97 | 53.69 |
| Rock Type | Pegmatite | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample No | BK52 | BK53 | BK54 | BK103 | BK104 | BK107 | BK108 | BK132 | BK135 |
| Cr | 2.33 | 4.11 | 2.45 | 3.09 | 2.37 | 3.77 | 1.89 | 2.25 | 1.54 |
| Ni | 4.59 | 2.74 | 3.32 | 5.11 | 3.68 | 14.02 | 2.75 | 3.21 | 12.63 |
| Co | 4.74 | 5.55 | 4.44 | 6.71 | 8.55 | 5.43 | 3.23 | 8.21 | 6.83 |
| Sc | 0.89 | 0.75 | 0.54 | 0.49 | 1.03 | 1.24 | 0.84 | 0.47 | 1.05 |
| V | 12.33 | 15.15 | 16.63 | 10.05 | 3.22 | 10.05 | 10.05 | 16.47 | 5.93 |
| Cu | 7.89 | 9.14 | 6.51 | 11.1 | 5.63 | 11.35 | 9.36 | 4.68 | 5.87 |
| Pb | 9.07 | 6.51 | 11.15 | 6.58 | 2.68 | 6.23 | 5.16 | 1.06 | 7.74 |
| Zn | 11.8 | 7.8 | 9.16 | 75.72 | 158.28 | 154.12 | 129.37 | 10.2 | 162.94 |
| Rb | 224.27 | 178.05 | 235.19 | 176.05 | 163.77 | 263.12 | 267.65 | 402.3 | 228.91 |
| Ba | 83.56 | 89.63 | 40.53 | 69.35 | 42.73 | 74.68 | 42.59 | 55.98 | 64.51 |
| Sr | 43.62 | 52.26 | 67.49 | 47.41 | 30.99 | 46.07 | 48.16 | 54.09 | 61.59 |
| Ga | 18.61 | 21.17 | 17.35 | 16.38 | 23.11 | 27.24 | 24.71 | 19.54 | 22.93 |
| Ta | 23.54 | 22.62 | 21.81 | 30.2 | 27.41 | 25.48 | 24.36 | 23.73 | 25.03 |
| Nb | 170.76 | 143.94 | 158.95 | 160.25 | 135.99 | 137.96 | 165.56 | 333.57 | 669.93 |
| Hf | 10.22 | 11.16 | 11.09 | 13.93 | 15.5 | 17.62 | 15.68 | 9.51 | 10.84 |
| Zr | 391.41 | 368.87 | 361.64 | 606.06 | 696.93 | 763.68 | 688.89 | 318.96 | 537.25 |
| Y | 126.36 | 157.96 | 130.98 | 57.62 | 70.64 | 63.21 | 51.32 | 334.53 | 464.47 |
| Th | 75.66 | 67.56 | 56.28 | 41.36 | 63.74 | 57.24 | 54.78 | 55.6 | 112.08 |
| U | 42.8 | 31.05 | 45.77 | 23.61 | 24.95 | 19.91 | 26.79 | 28.22 | 73.82 |
| Li | 39.66 | 34.46 | 34.9 | 35.13 | 30.58 | 30.61 | 34.56 | 33.41 | 35.91 |
| Be | 35.91 | 42.66 | 29.03 | 47.89 | 38.2 | 43.24 | 40.18 | 28.51 | 26.57 |
| Mo | 0.96 | 1.61 | 1.43 | 0.42 | 0.06 | 0.79 | 0.42 | 0.23 | 0.23 |
| Cs | 4.25 | 3.95 | 2.55 | 5.35 | 10.45 | 7.35 | 5.55 | 10.7 | 6.95 |
| Sn | 16.98 | 23.27 | 22.3 | 10.22 | 9.56 | 8.88 | 7.96 | 17.09 | 9.33 |
| La | 34.38 | 40.08 | 37.35 | 48.33 | 58.49 | 50.36 | 40.68 | 19.72 | 25.98 |
| Ce | 69.86 | 83.48 | 80.4 | 131.58 | 170.39 | 142.59 | 119.24 | 60.5 | 80.45 |
| Pr | 9.5 | 11.59 | 10.53 | 21.44 | 29.03 | 23.04 | 20.07 | 11 | 15.05 |
| Nd | 40.76 | 49.81 | 46.03 | 100.14 | 138.47 | 111.17 | 94.4 | 57.08 | 81.29 |
| Sm | 10.74 | 12.6 | 12.23 | 26.46 | 38.45 | 30.99 | 24.5 | 21.54 | 32.23 |
| Eu | 2.14 | 1.58 | 1.8 | 1.04 | 1.05 | 1.1 | 0.86 | 1.02 | 1.04 |
| Gd | 12.06 | 13.38 | 13.38 | 23.73 | 33.36 | 26.88 | 21.2 | 34.98 | 48.24 |
| Tb | 2.28 | 2.58 | 2.43 | 3.12 | 4.34 | 3.56 | 2.75 | 7.73 | 10.53 |
| Dy | 16.02 | 18.16 | 16.78 | 15.87 | 22.26 | 18.42 | 14.09 | 57.18 | 77.99 |
| Ho | 3.78 | 4.34 | 3.98 | 2.72 | 3.72 | 3.05 | 2.36 | 12.46 | 16.79 |
| Er | 12.26 | 14.76 | 12.65 | 6.92 | 9.05 | 7.55 | 5.87 | 37.59 | 49.83 |
| Tm | 2.1 | 2.6 | 2.1 | 0.96 | 1.25 | 1.02 | 0.81 | 5.32 | 6.84 |
| Yb | 16.42 | 20.28 | 15.95 | 5.85 | 7.28 | 6.29 | 4.91 | 31.5 | 39.45 |
| Lu | 2.26 | 3.46 | 2.78 | 0.87 | 1.07 | 0.92 | 0.74 | 3.96 | 4.85 |
| Geochemical parameters | |||||||||
| K/Rb | 163.61 | 208.41 | 238.60 | 207.47 | 207.32 | 150.49 | 147.32 | 103.38 | 182.77 |
| K/Ba | 439.11 | 414.00 | 1384.57 | 526.69 | 794.58 | 530.22 | 925.83 | 742.94 | 648.56 |
| Zr/Rb | 1.75 | 2.07 | 1.54 | 3.44 | 4.26 | 2.90 | 2.57 | 0.79 | 2.35 |
| Ba/Nb | 0.49 | 0.62 | 0.25 | 0.43 | 0.31 | 0.54 | 0.26 | 0.17 | 0.10 |
| Rb/Sr | 5.14 | 3.41 | 3.48 | 3.71 | 5.28 | 5.71 | 5.56 | 7.44 | 3.72 |
| Eu/Eu* | 0.57 | 0.37 | 0.43 | 0.13 | 0.09 | 0.12 | 0.12 | 0.11 | 0.08 |
| (La/Yb)n | 1.42 | 1.34 | 1.58 | 5.59 | 5.43 | 5.41 | 5.60 | 0.42 | 0.45 |
| (La/Sm)n | 2.02 | 2.01 | 1.93 | 1.15 | 0.96 | 1.03 | 1.05 | 0.58 | 0.51 |
| (Gd/Lu)n | 0.65 | 0.47 | 0.59 | 3.34 | 3.82 | 3.58 | 3.51 | 1.08 | 1.22 |
| (La/Lu)n | 1.56 | 1.19 | 1.38 | 5.69 | 5.60 | 5.61 | 5.63 | 0.51 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, D.M.; Abanumay, F.A.; Ali, S.; Farahat, E.S.; Bekker, A.; Azer, M.K. An Occurrence of Pyroxmangite in the NYF Granitic Pegmatite of the Gabal El-Bakriya Intrusion, Arabian–Nubian Shield. Minerals 2025, 15, 1027. https://doi.org/10.3390/min15101027
Fathy DM, Abanumay FA, Ali S, Farahat ES, Bekker A, Azer MK. An Occurrence of Pyroxmangite in the NYF Granitic Pegmatite of the Gabal El-Bakriya Intrusion, Arabian–Nubian Shield. Minerals. 2025; 15(10):1027. https://doi.org/10.3390/min15101027
Chicago/Turabian StyleFathy, Danial M., Faris A. Abanumay, Shehata Ali, Esam S. Farahat, Andrey Bekker, and Mokhles K. Azer. 2025. "An Occurrence of Pyroxmangite in the NYF Granitic Pegmatite of the Gabal El-Bakriya Intrusion, Arabian–Nubian Shield" Minerals 15, no. 10: 1027. https://doi.org/10.3390/min15101027
APA StyleFathy, D. M., Abanumay, F. A., Ali, S., Farahat, E. S., Bekker, A., & Azer, M. K. (2025). An Occurrence of Pyroxmangite in the NYF Granitic Pegmatite of the Gabal El-Bakriya Intrusion, Arabian–Nubian Shield. Minerals, 15(10), 1027. https://doi.org/10.3390/min15101027