Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
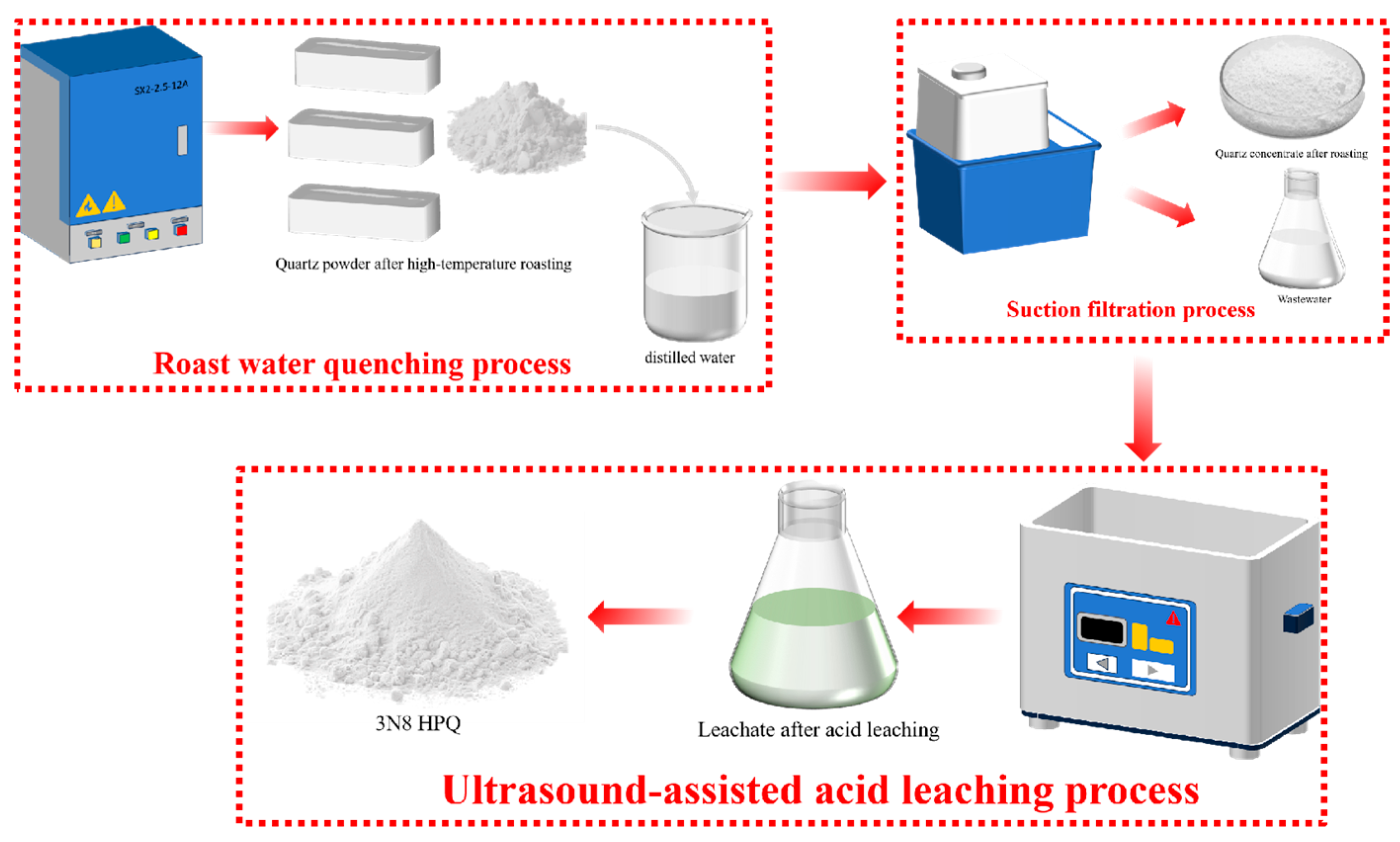
2.2.1. Raw Material Pretreatment
2.2.2. Roasting and Acid Leaching Experiments
2.3. Equipment and Characterization
3. Results and Discussion
3.1. Characterization of Quartz Conglomerate Ore
3.2. Research on Impurity Removal Effect of Quartz by Roasting
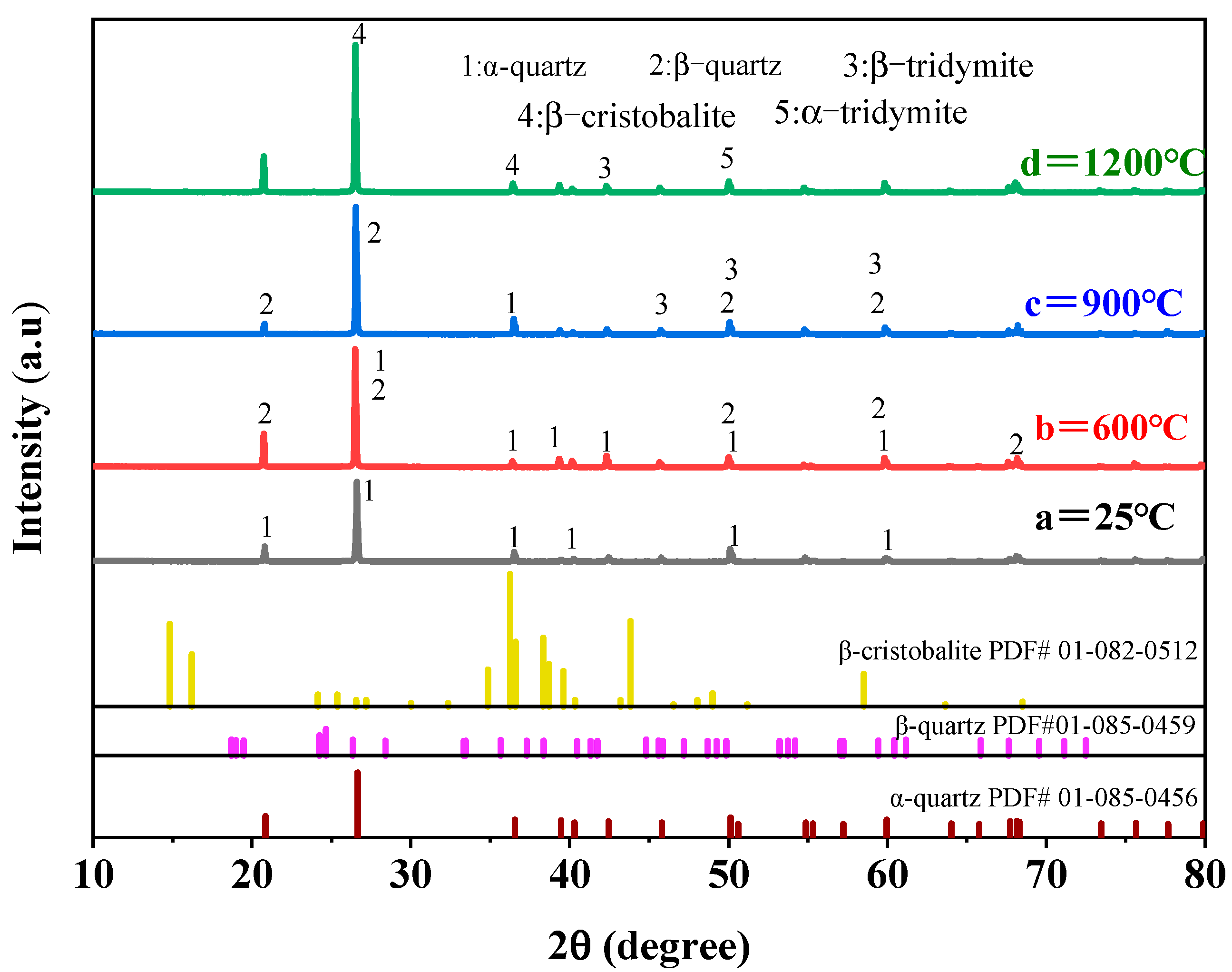
3.2.1. Effect of Roasting on Crystal Structure of Quartz
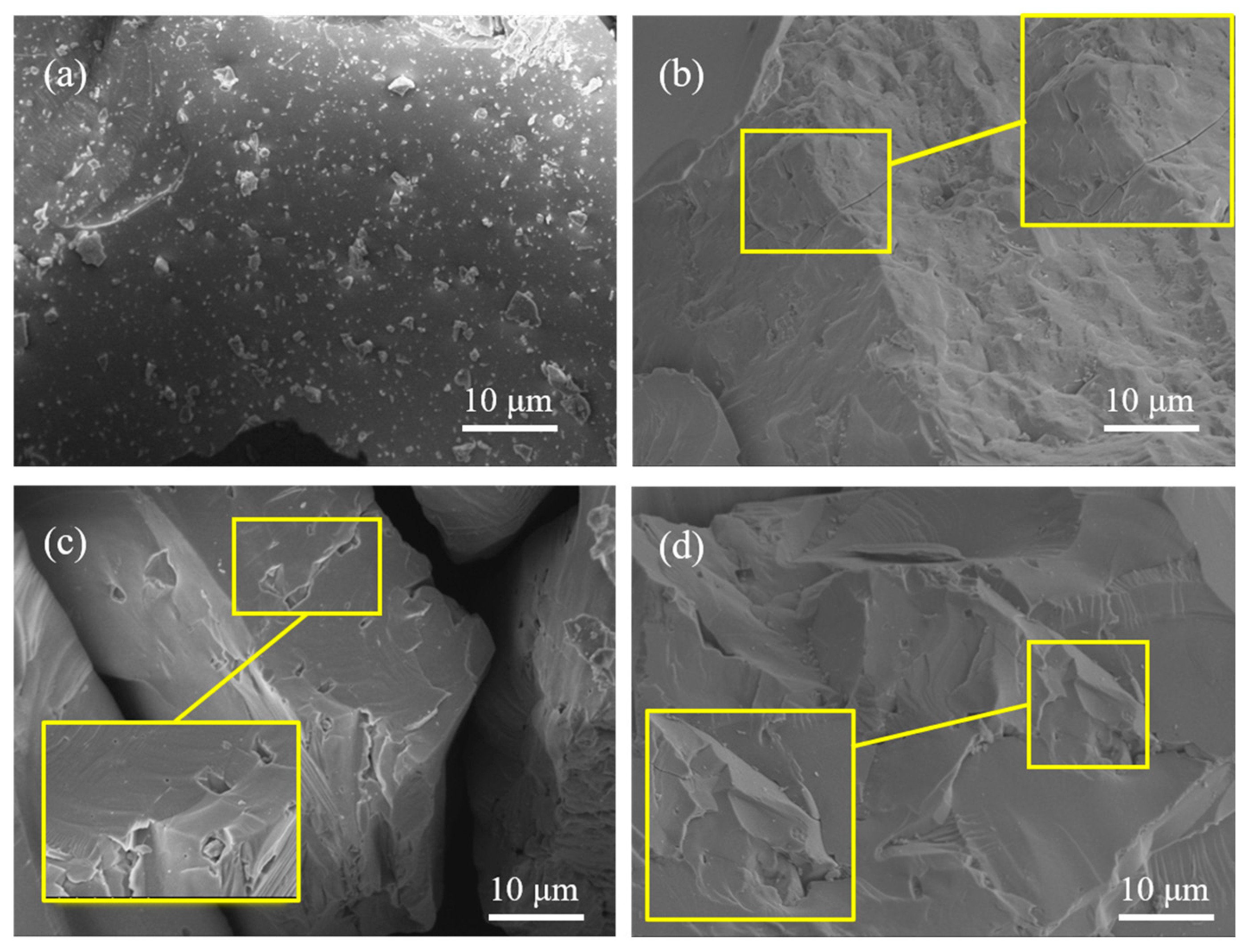
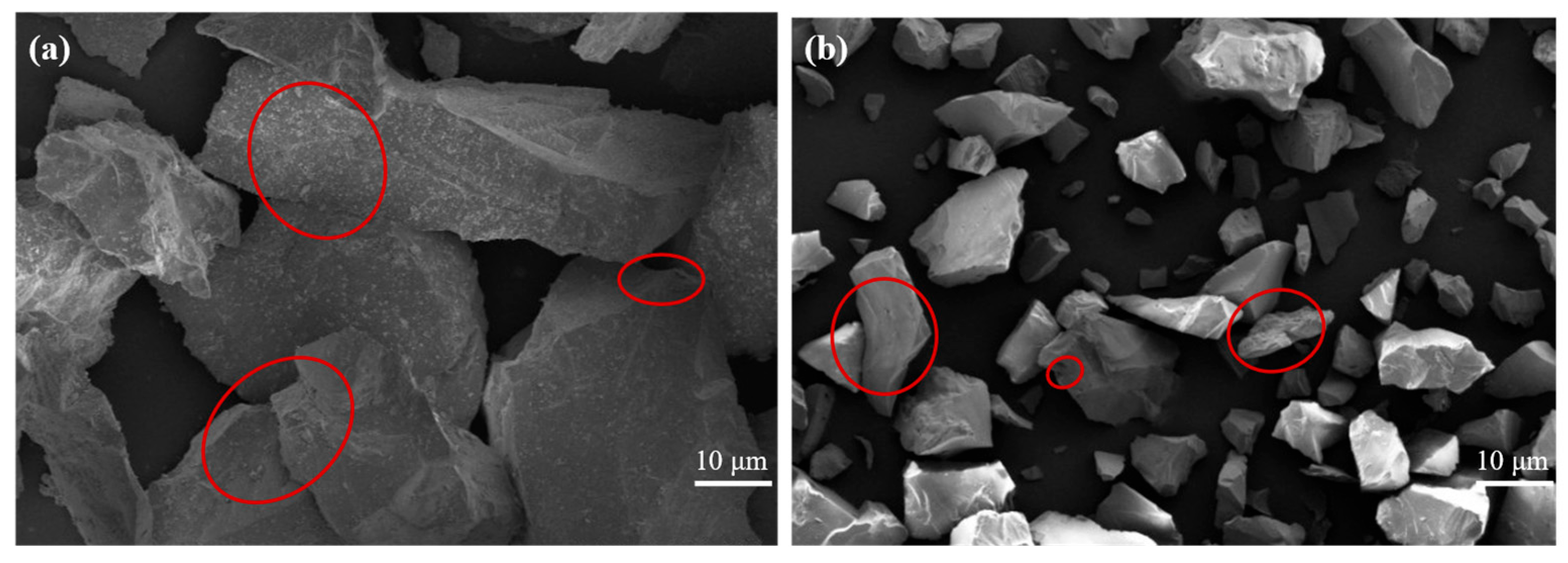
3.2.2. Effect of Roasting on Morphology of Quartz
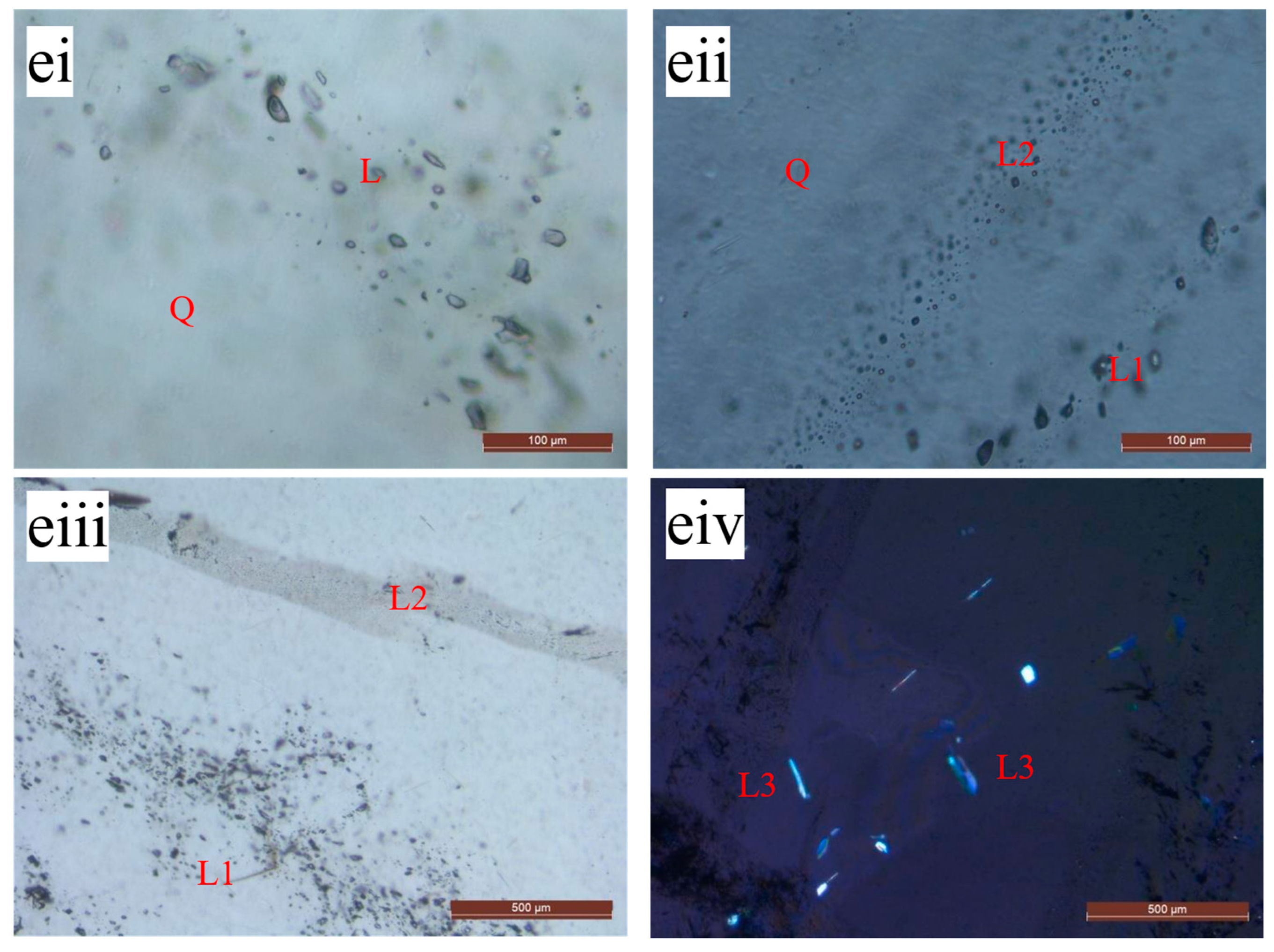
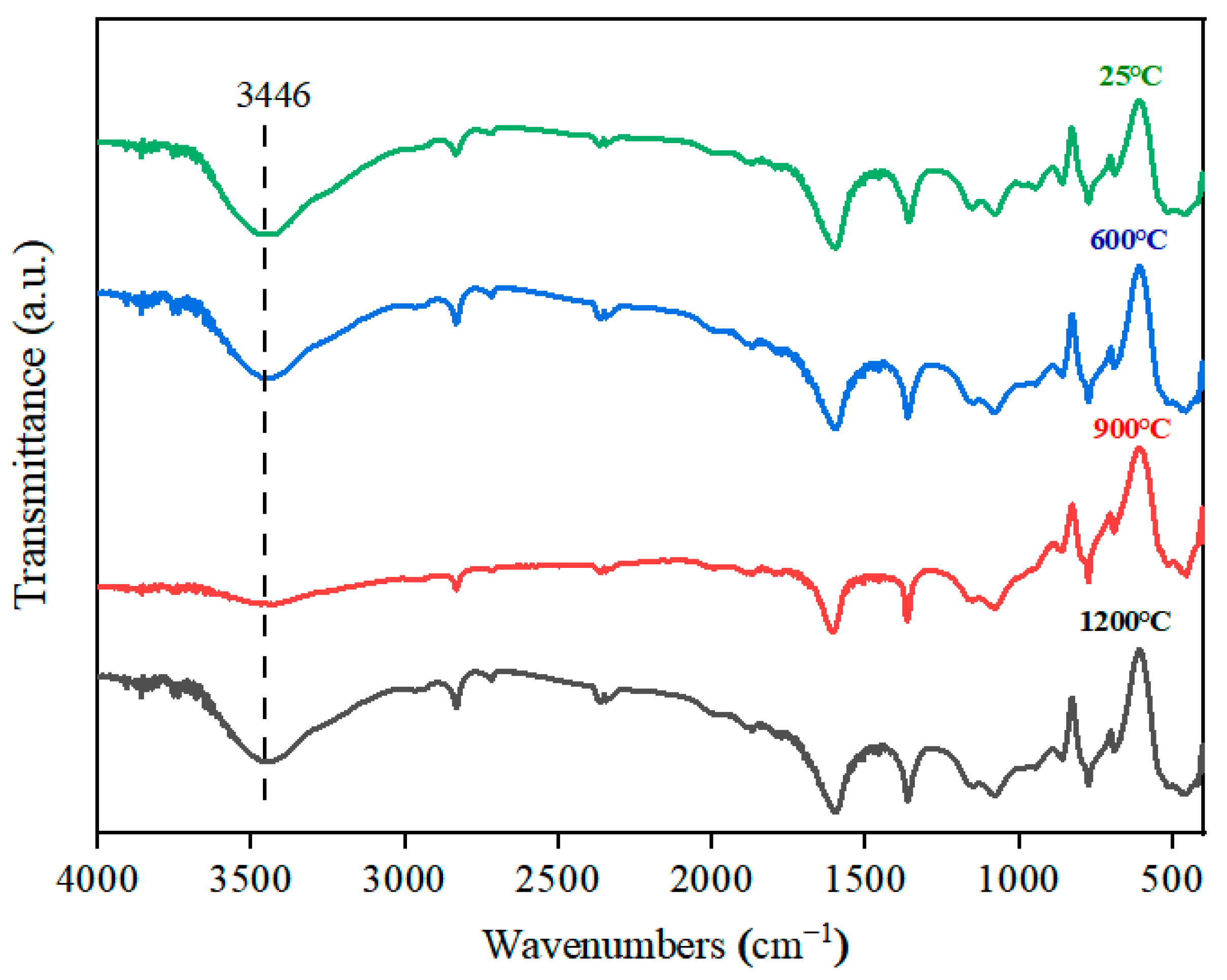
3.2.3. Effect of Roasting on Fluid Inclusion Removal in Quartz
3.2.4. Effect of Roasting on Impurity Removal of Quartz by Acid Leaching
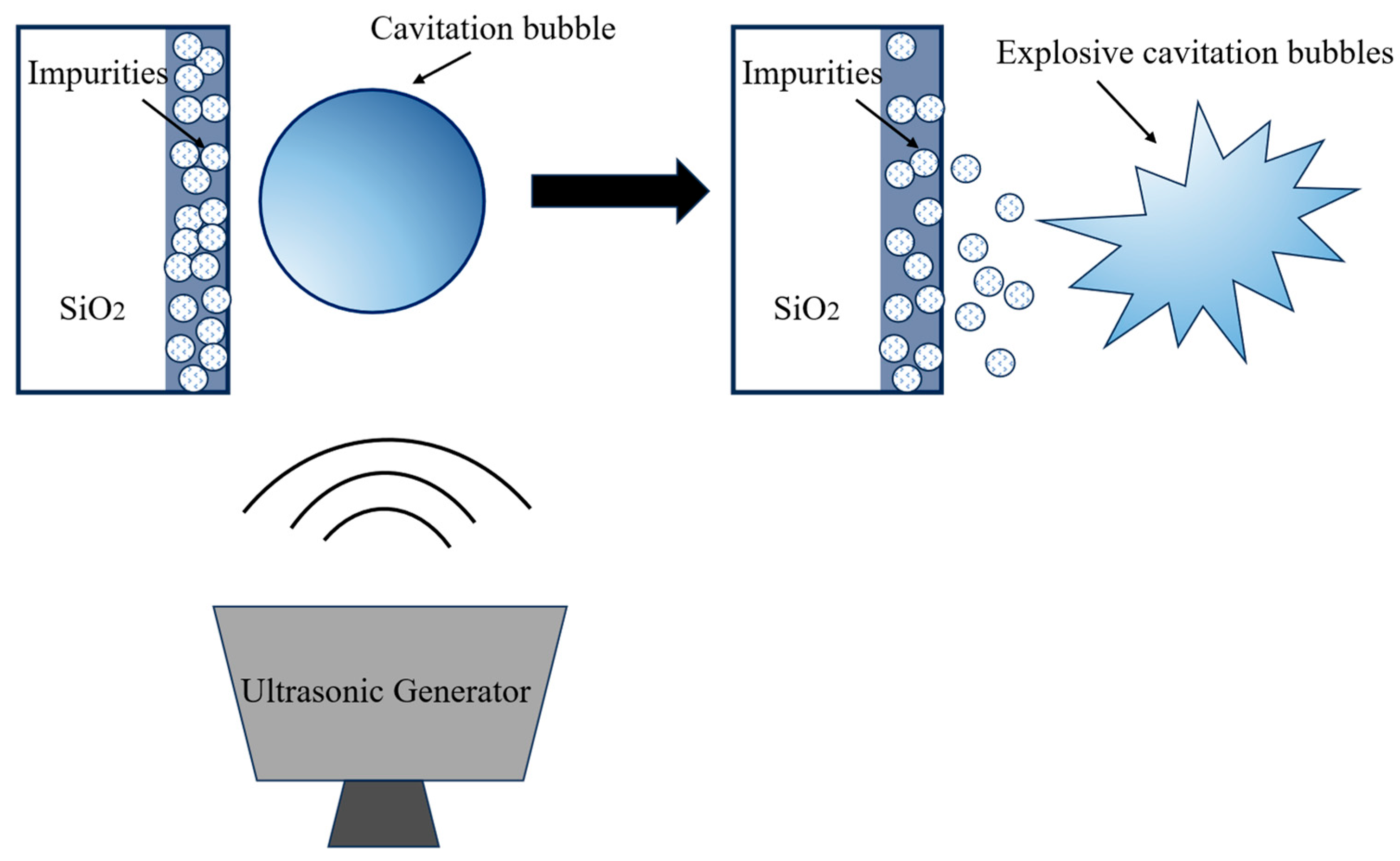
3.3. Research on Fe Removal from Quartz by Ultrasonic-Assisted Acid Leaching
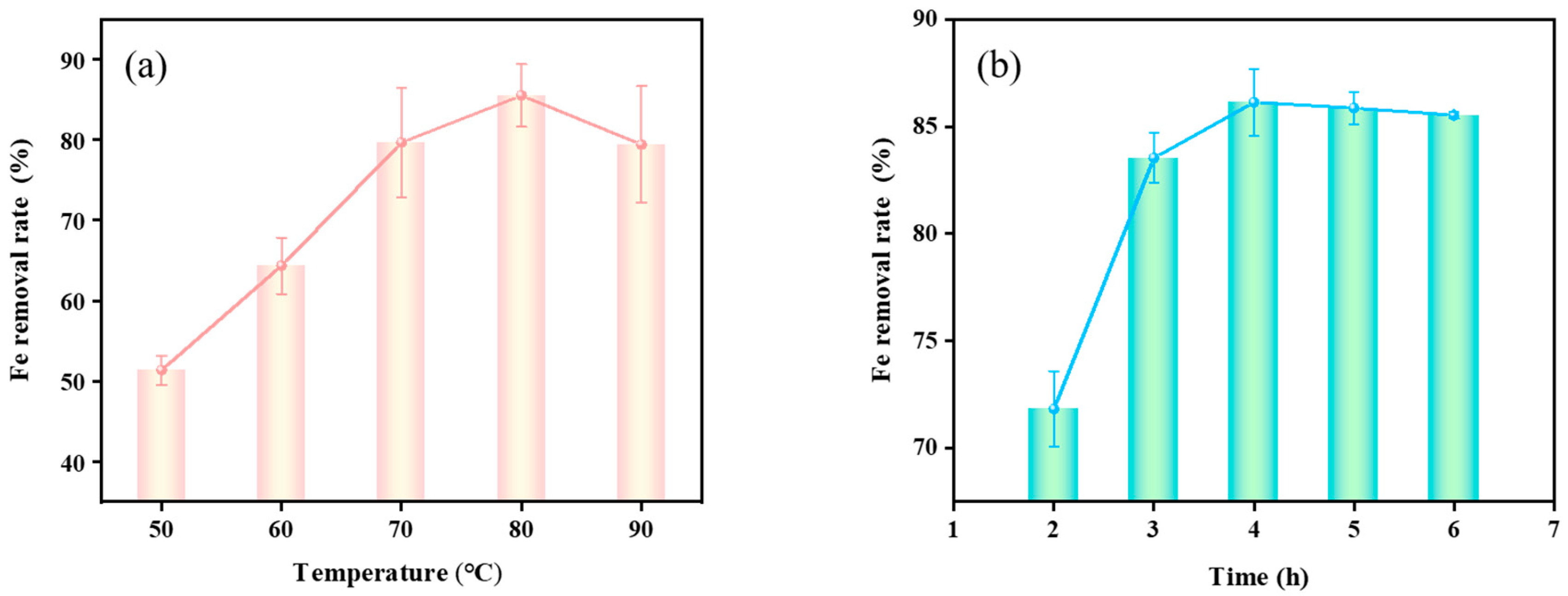
3.3.1. Effect of Acid Leaching Temperature on Fe Removal Rate
3.3.2. Effect of Acid Leaching Time on Fe Removal Rate
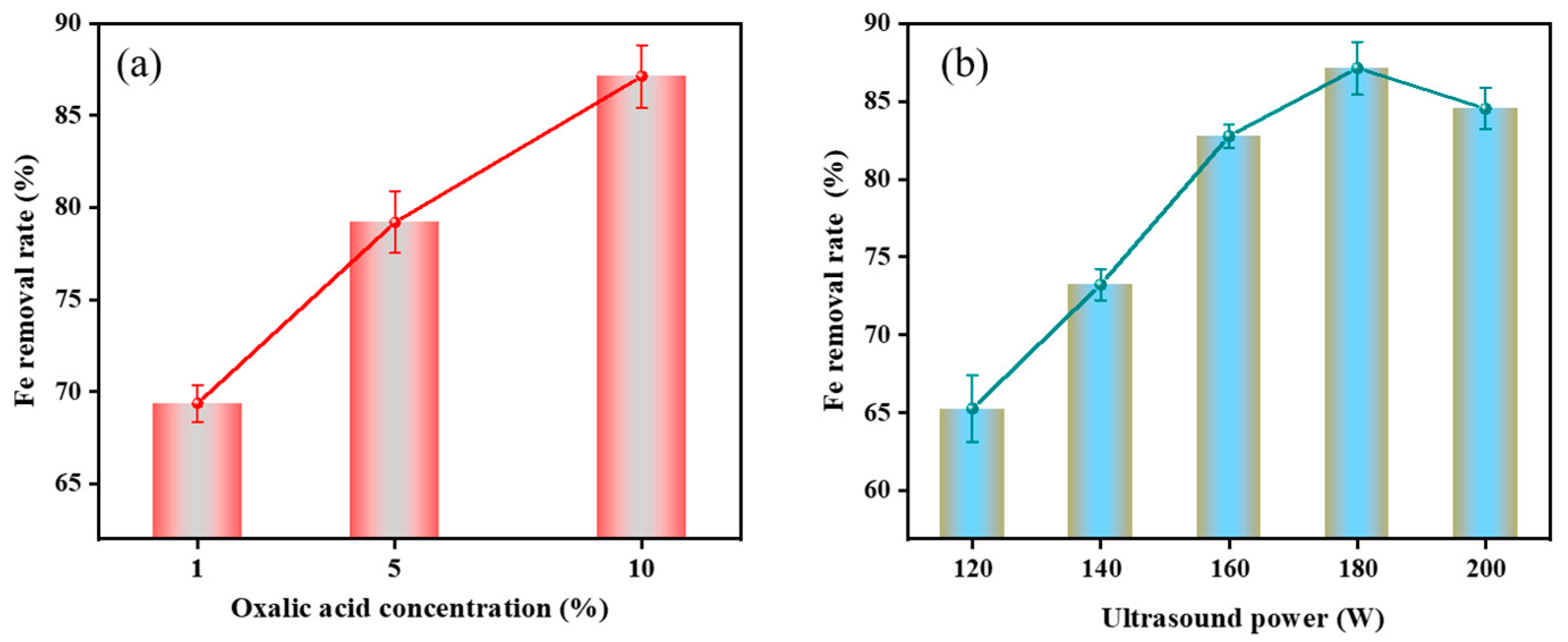
3.3.3. Effect of Oxalic Acid Concentration on Fe Removal Rate
3.3.4. Effect of Ultrasonic Power on Fe Removal Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mecchia, M.; Sauro, F.; Piccini, L.; De Waele, J.; Sanna, L.; Tisato, N.; Lira, J.; Vergara, F. Geochemistry of Surface and Subsurface Waters in Quartz-Sandstones: Significance for the Geomorphic Evolution of Tepui Table Mountains (Gran Sabana, Venezuela). J. Hydrol. 2014, 511, 117–138. [Google Scholar] [CrossRef]
- Xia, M.; Sun, C.; Yang, X.Y.; Chen, J. Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification. Minerals 2023, 13, 261. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Liang, G.B.; Li, Y.H.; Yang, C.; Zi, C.Y.; Zhang, Y.Q.; Hu, X.; Zhao, W.B. Production of biosilica nanoparticles from biomass power plant fly ash. Waste Manag. 2020, 105, 8–17. [Google Scholar] [CrossRef]
- Prakash, V.; Agarwal, A.; Mussada, E.K. Processing Methods of Silicon to its Ingot: A Review. Silicon 2019, 11, 1617–1634. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wang, H.; Zhu, C.; Ren, B. A Review on Removal of Iron Impurities from Quartz Mineral. Minerals 2023, 13, 1128. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, M.; Yang, X.; Khan, I.; Hou, Z. Research on 4N8 High-Purity Quartz Purification Technology Prepared Using Vein Quartz from Pakistan. Minerals 2024, 14, 1049. [Google Scholar] [CrossRef]
- Qi, D.; Ren, Z.; Song, Y.; He, Y.; Li, P.; Yin, H. Purification of Different-Sized Quartz Crystals in Granitic Pegmatite. Miner. Eng. 2024, 216, 108856. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Li, P.; Pan, L.; Hu, X.; Gu, Y.; Tian, Y. Migration Mechanisms of Al3+/Li+ Lattice Impurities during Phase Transition from α-Quartz to β-Quartz: An Implication for Purification of High-Purity Quartz. Minerals 2023, 13, 1280. [Google Scholar] [CrossRef]
- Li, J.S.; Li, X.X.; Shen, Q.; Zhang, Z.Z.; Du, F.H. Further Purification of Industrial Quartz by Much Milder Conditions and a Harmless Method. Environ. Sci. Technol. 2010, 44, 7673–7677. [Google Scholar] [CrossRef]
- Li, Y.K.; Li, S.Q.; Zhao, X.; Pan, X.D.; Guo, P.H. Separation and purification of high-purity quartz from high-silicon iron ore tailing: An innovative strategy for comprehensive utilization of tailings resources. Process Saf. Environ. Prot. 2023, 169, 142–148. [Google Scholar] [CrossRef]
- Hagemann, S.G.; Angerer, T.; Duuring, P.; Rosière, C.A.; Figueiredo e Silva, R.C.; Lobato, L.; Hensler, A.; Walde, D. BIF-Hosted Iron Mineral System: A Review. Ore Geol. Rev. 2016, 76, 317–359. [Google Scholar] [CrossRef]
- Ortiz-Mosquera, J.F.; Nieto-Muñoz, A.M.; Rodrigues, A.C.M. Influence of Al3+ on Glass-Forming Ability, Structural and Electrical Properties of the Na3.4Sc22Si0.4P2.6O12 Superionic Conductor. J. Alloys Compd. 2021, 850, 156670. [Google Scholar] [CrossRef]
- Song, W.; Jiang, X.; Chen, C.; Ban, B.; Wan, S.; Chen, J. Purification of Quartz via Low-Temperature Microwave Chlorinated Calcination Combined with Acid Leaching and Its Mechanism. Silicon 2023, 15, 971–981. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of Quartz from Quartz-Feldspar Mixtures by the HF Method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Gui, Y.Z.Y.; Zhu, X.X.; Fu, H.; Jiang, X.S.; Sun, J.F.; Wang, L.; Ban, B.; Chen, J. Preparation of Silica Powder from Low-Grade Quartz by Mechanical Activation Acid Leaching and Its Mechanism. Silicon 2025, 17, 835–849. [Google Scholar] [CrossRef]
- Yang, S.C.; Han, S.F.; Wan, H.L.; Wei, K.X.; Ma, W.H. Silicon and Quartz Recovery and Purification from Waste Quartz Crucible Ash with a Combined Process of Electric Separation and Hydrometallurgy. Silicon 2024, 16, 4025–4035. [Google Scholar] [CrossRef]
- Qu, J.Y.; Chen, Z.J.; Wu, D.D.; Ma, W.H. Study on the Purification Mechanism of Low-Grade Silicon Ore through a Combination of Direct Roasting and Pressure Leaching. Silicon 2024, 16, 5257–5271. [Google Scholar] [CrossRef]
- Deng, L.J.; Ma, F.Y. Study on Dynamic Behavior and Mechanism of Reverse Flotation of Micro-fine Hematite (MFH) Enhanced by Nanobubbles (NBs). JOM 2019, 76, 7387–7397. [Google Scholar]
- Xiong, K.; Pei, Z.Y.; Zang, F.F.; Lin, M. Study on Preparation Process and Mechanism of High-Purity Quartz by Mixed Acid Leaching. Non-Met. Mines 2016, 39, 60–62. (In Chinese) [Google Scholar]
- Tuncuk, A.; Akcil, A. Removal of Iron from Quartz Ore Using Different Acids: A Laboratory-Scale Reactor Study. Miner. Process. Extr. Metall. Rev. 2014, 35, 217–228. [Google Scholar] [CrossRef]
- Shao, H.; Zang, F.; Ji, M.; Yu, M. Preparation and Mechanism of High Purity Quartz by Alkali Corrosion and Acid Leaching Process Using Vein Quartz. Silicon 2022, 14, 12475–12483. [Google Scholar] [CrossRef]
- Wu, X. Study on Evaluation and Purification Process of High Purity Quartz Raw Materials. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2016. (In Chinese). [Google Scholar]
- Liu, A.; Fan, P.P.; Qiao, X.X.; Li, Z.H.; Wang, H.F.; Fan, M.Q. Synergistic effect of mixed DDA/surfactants collectors on flotation of quartz. Miner. Eng. 2020, 159, 106605. [Google Scholar] [CrossRef]
- Guo, W.; Lu, H.; Zhang, Z.; Jiang, L.; Wu, H.; Liu, D.; Chi, R. Crystal Structure Transformation and Lattice Impurities Migration of Quartz During Chlorine Roasting. Int. J. Min. Sci. Technol. 2024, 34, 1465–1474. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P.; Zhao, Z. Recovery and preparation of high-grade silica from iron ore tailings by S-HGMS coupling with acid leaching technology: Description of separation mechanism and leaching kinetics. Powder Technol. 2023, 424, 118523. [Google Scholar] [CrossRef]
- Wang, M.; Yao, H. A Novel Organic–Inorganic Hybrid Admixture for Increasing Flowability and Reducing Viscosity of Ultra-High Performance Paste. Materials 2020, 13, 3385. [Google Scholar] [CrossRef]
- Khanlary, M.R.; Rahmani, E.; Pasalari, Z.; Reyhani, A. Post-Annealing Effect on the Structural and Optical Properties of Tungsten Trioxide Thin Films Prepared by Thermal Evaporation. Mater. Chem. Phys. 2025, 338, 130670. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Xie, A.; Chen, K.; Yang, Y.; Liu, L.; Zhu, S. Microstructure Study of Phase Transformation of Quartz in Potassium Silicate Glass at 900 °C and 1000 °C. Crystals 2021, 11, 1481. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Xia, Z.; Li, W.; Lei, S. Influences of Na2CO3 Roasting and H3PO4 Hot-Pressure Leaching on the Purification of Vein Quartz to Achieve High-Purity Quartz. Hydrometallurgy 2023, 218, 106065. [Google Scholar]
- Chayab Draa, A.; Mokhtari, F.; Lasloudji, I.; Zermout, S.; Lebbou, K. Internal Radiation Effect on Semiconductor β-Ga2O3 Crystals Grown by the VB Method and Anisotropic Thermal Stress. J. Cryst. Growth. 2024, 648, 127910. [Google Scholar] [CrossRef]
- Austrheim, H.; Dunkel, K.G.; Plümper, O.; Ildefonse, B.; Liu, Y.; Jamtveit, B. Fragmentation of Wall Rock Garnets during Deep Crustal Earthquakes. Sci. Adv. 2017, 3, e1602067. [Google Scholar] [CrossRef] [PubMed]
- Adeagbo, W.A.; Doltsinis, N.L.; Klevakina, K.; Renner, J. Transport Processes at α-Quartz-Water Interfaces: Insights from First-Principles Molecular Dynamics Simulations. ChemPhysChem 2008, 9, 994–1002. [Google Scholar] [PubMed]
- Sanchez-Navas, A. Sequential Kinetics of a Muscovite-Out Reaction: A Natural Example. Am. Miner. 1999, 84, 1270–1286. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Arstad, B.; Muggerud, A.M.F.; Erbe, A. Structural Evolution of Water and Hydroxyl Groups during Thermal, Mechanical and Chemical Treatment of High Purity Natural Quartz. RSC Adv. 2020, 10, 29061–29069. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Muggerud, A.M.F.; Erbe, A. In Situ High Temperature Spectroscopic Study of Liquid Inclusions and Hydroxyl Groups in High Purity Natural Quartz. Miner. Eng. 2021, 174, 107238. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, X.; Zhu, X.; Fu, H.; Jiang, X.; Sun, J.; Wang, L.; Ban, B.; Chen, J. Quartz Lattice Al Impurity Removal: A Simple Annealing-Leaching Process and Analysis. Mater. Lett. 2025, 357, 135712. [Google Scholar] [CrossRef]
- Ai, G.; Guo, S.; Zhao, J.; Deng, X.; Wei, K.; Ma, W. Hot-Pressure Acid Leaching Changes Grain Boundaries to Deeply Remove Impurities in Quartz Sand. J. Mater. Res. Technol. 2024, 28, 4321–4332. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Li, S.; Zhao, X.; Guo, P.; He, T. Innovative Technology for Preparation of High-Purity Silica from Vein Quartz Ore through S-HGMS Coupling Acid Leaching Process. Process Saf. Environ. Prot. 2023, 179, 632–643. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zuo, Q.; Li, J.; Ban, B.; Chen, J. Purification Mechanism of Quartz Sand by Combination of Microwave Heating and Ultrasound Assisted Acid Leaching Treatment. Silicon 2021, 13, 531–541. [Google Scholar]
- Lin, M.; Pei, Z.Y.; Li, Y.B.; Liu, Y.Y.; Wei, Z.L.; Lei, S.M. Separation Mechanism of Lattice-Bound Trace Elements from Quartz by KCl-Doping Calcination and Pressure Leaching. Miner. Eng. 2018, 125, 42–49. [Google Scholar]
- Yuan, J.; Xiao, J.; Li, F.; Wang, B.; Yao, Z.; Yu, B.; Zhang, L. Co-Treatment of Spent Cathode Carbon in Caustic and Acid Leaching Process under Ultrasonic Assisted for Preparation of SiC. Ultrason. Sonochem 2017, 39, 654–662. [Google Scholar] [CrossRef] [PubMed]



| Element | Na | Mg | K | Ca | Al | Ti | Fe | Mn | Cr | Others | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 171 | 38.72 | 190.67 | 168.07 | 421.45 | 16.04 | 1204.73 | 54.29 | 19.71 | 118.92 | 2403.6 |
| Samples | Content (mg/kg) | % Removal | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Fe | K | Na | Al | Fe | K | Na | |
| Wraw | 254.56 | 25.09 | 128.44 | 113.06 | 49.86 | 53.6 | 30.43 | 49.00 |
| W600 | 219.15 | 15.87 | 71.33 | 77.36 | 57.30 | 68.92 | 28.15 | 56.91 |
| W900 | 183.82 | 10.59 | 56.74 | 42.74 | 65.60 | 86.95 | 36.41 | 64.03 |
| W1200 | 221.13 | 20.72 | 72.52 | 63.58 | 50.69 | 76.60 | 30.93 | 53.75 |
| Element | Na | Mg | K | Ca | Al | Ti | Fe | Mn | Cr | Others | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content after acid leaching | 32.72 | 1.65 | 46.74 | 0.29 | 108.23 | 3.97 | 6.95 | 0.13 | 0.76 | 9.07 | 210.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Huang, Y.; Zhang, Y.; Li, S.; Liu, Y.; Wei, G.; Wei, L. Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process. Minerals 2025, 15, 1028. https://doi.org/10.3390/min15101028
Jiao L, Huang Y, Zhang Y, Li S, Liu Y, Wei G, Wei L. Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process. Minerals. 2025; 15(10):1028. https://doi.org/10.3390/min15101028
Chicago/Turabian StyleJiao, Liran, Yong Huang, Yingshuang Zhang, Sining Li, Yubin Liu, Guirong Wei, and Linlong Wei. 2025. "Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process" Minerals 15, no. 10: 1028. https://doi.org/10.3390/min15101028
APA StyleJiao, L., Huang, Y., Zhang, Y., Li, S., Liu, Y., Wei, G., & Wei, L. (2025). Preparation of High-Purity Quartz by Roasting–Water Quenching and Ultrasound-Assisted Acid Leaching Process. Minerals, 15(10), 1028. https://doi.org/10.3390/min15101028







