Recovery of Rare Earth Elements from Calciothermic Reduction Slag by Sulfation Roasting–Water Leaching Method
Abstract
1. Introduction
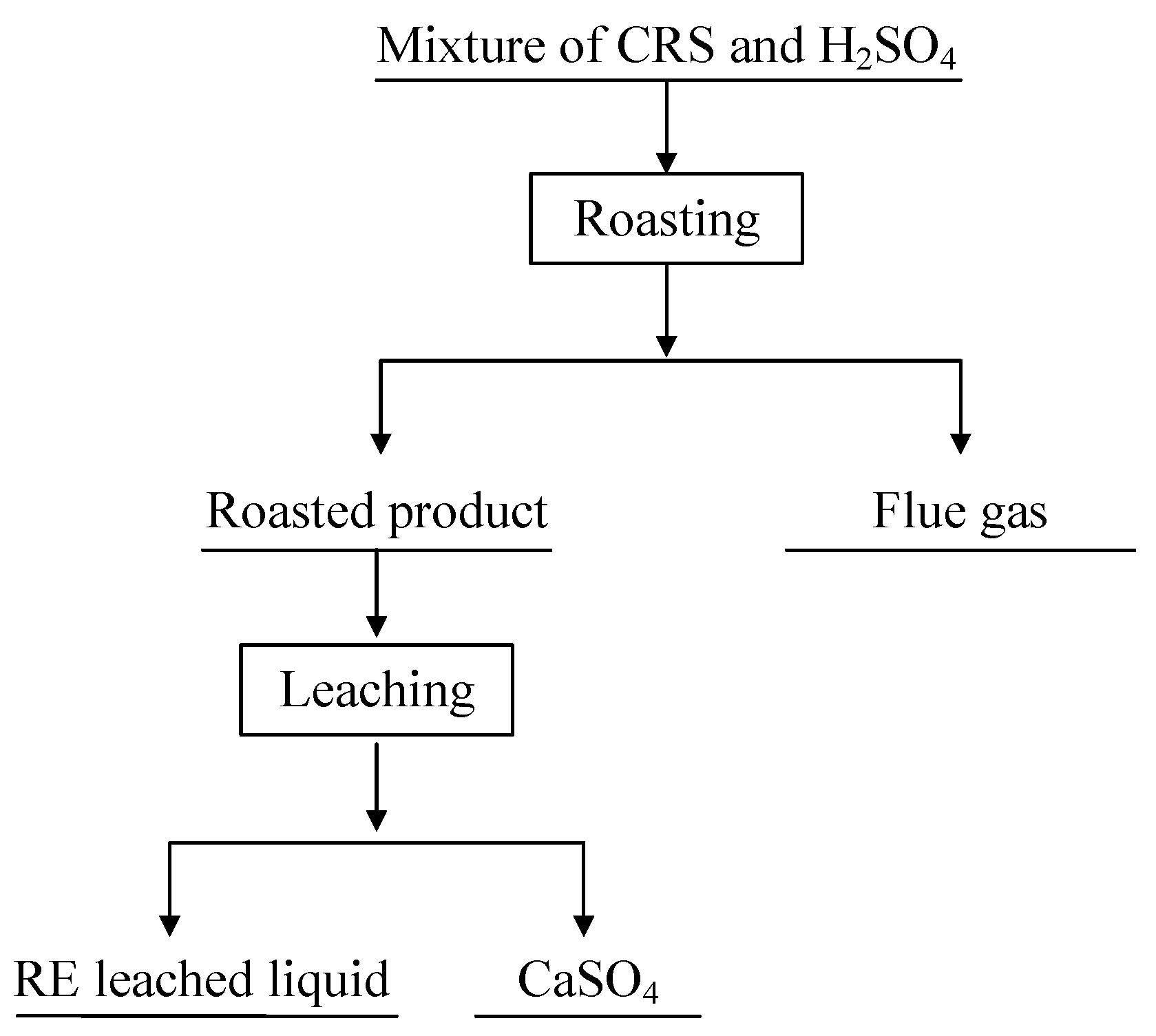
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
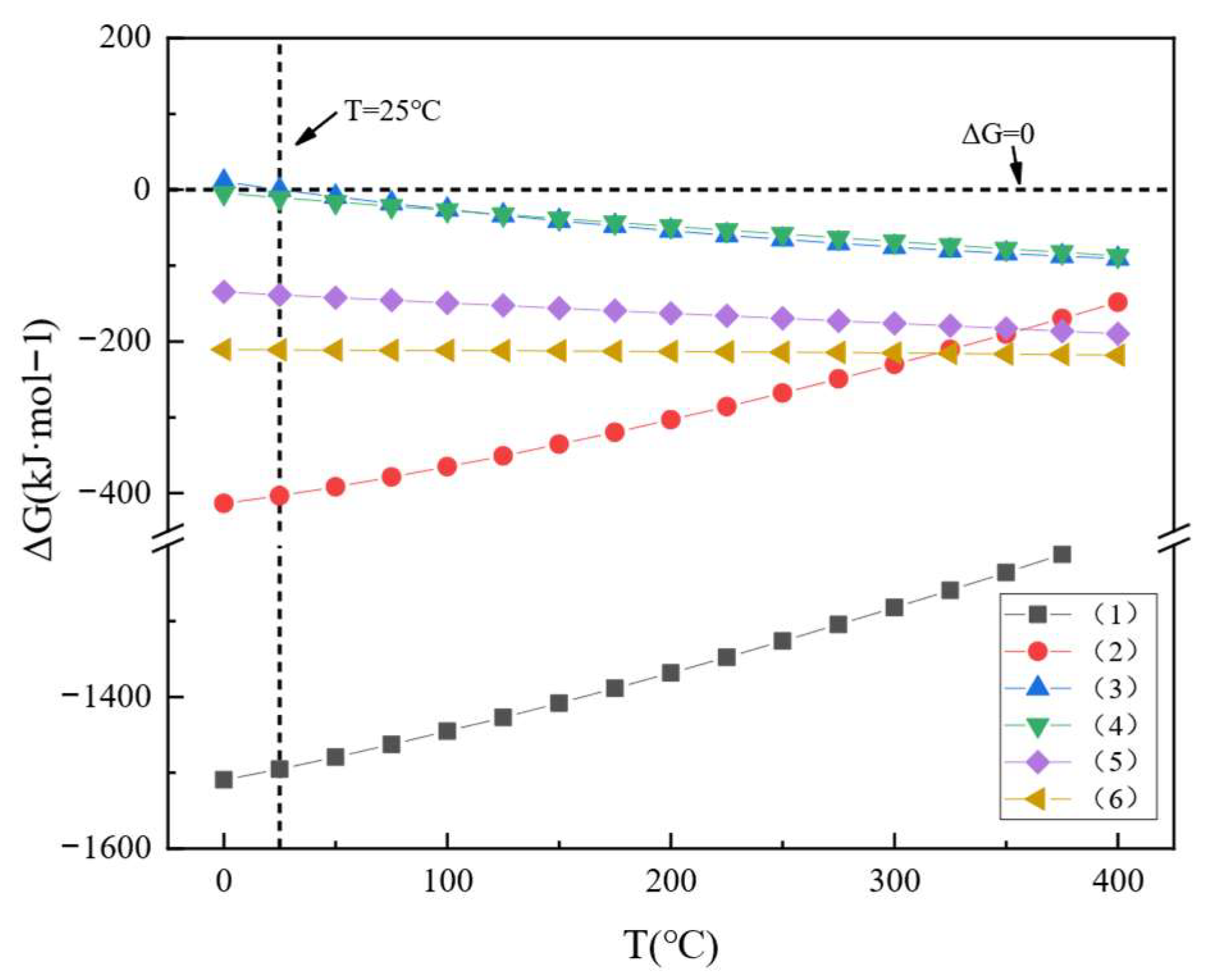
3.1. Thermodynamic Analysis
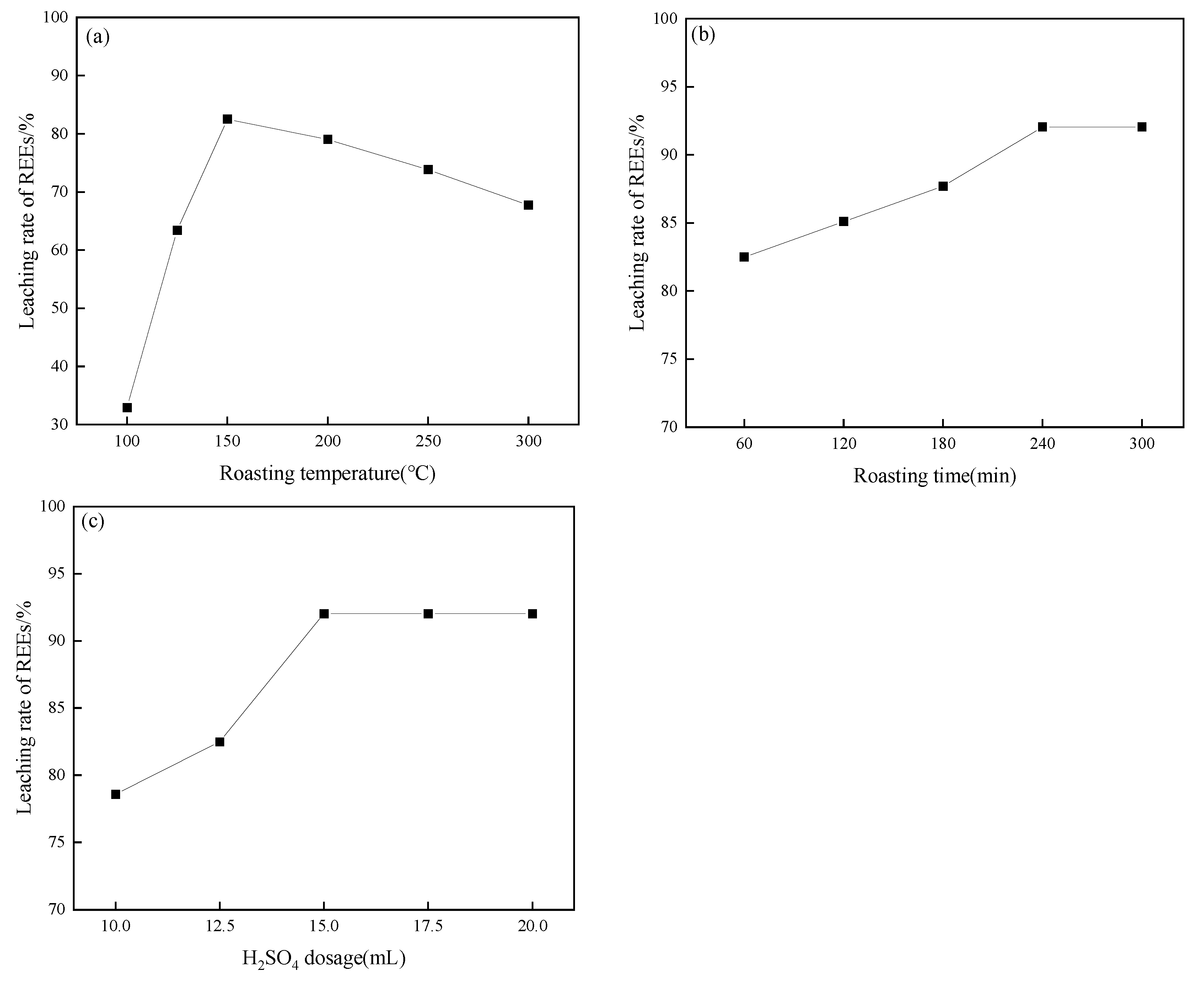
3.2. Influence of Roasting Parameters on REE Leaching
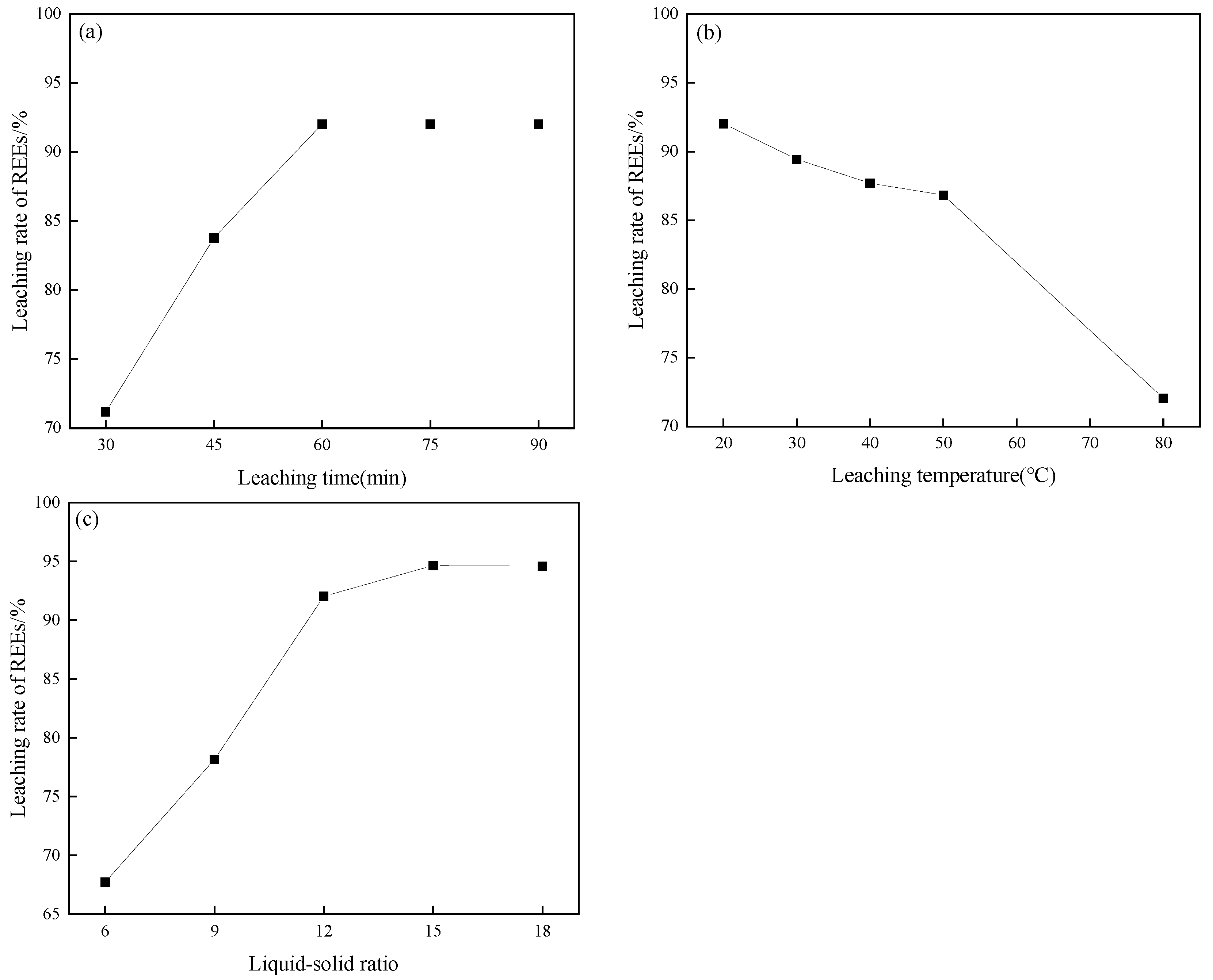
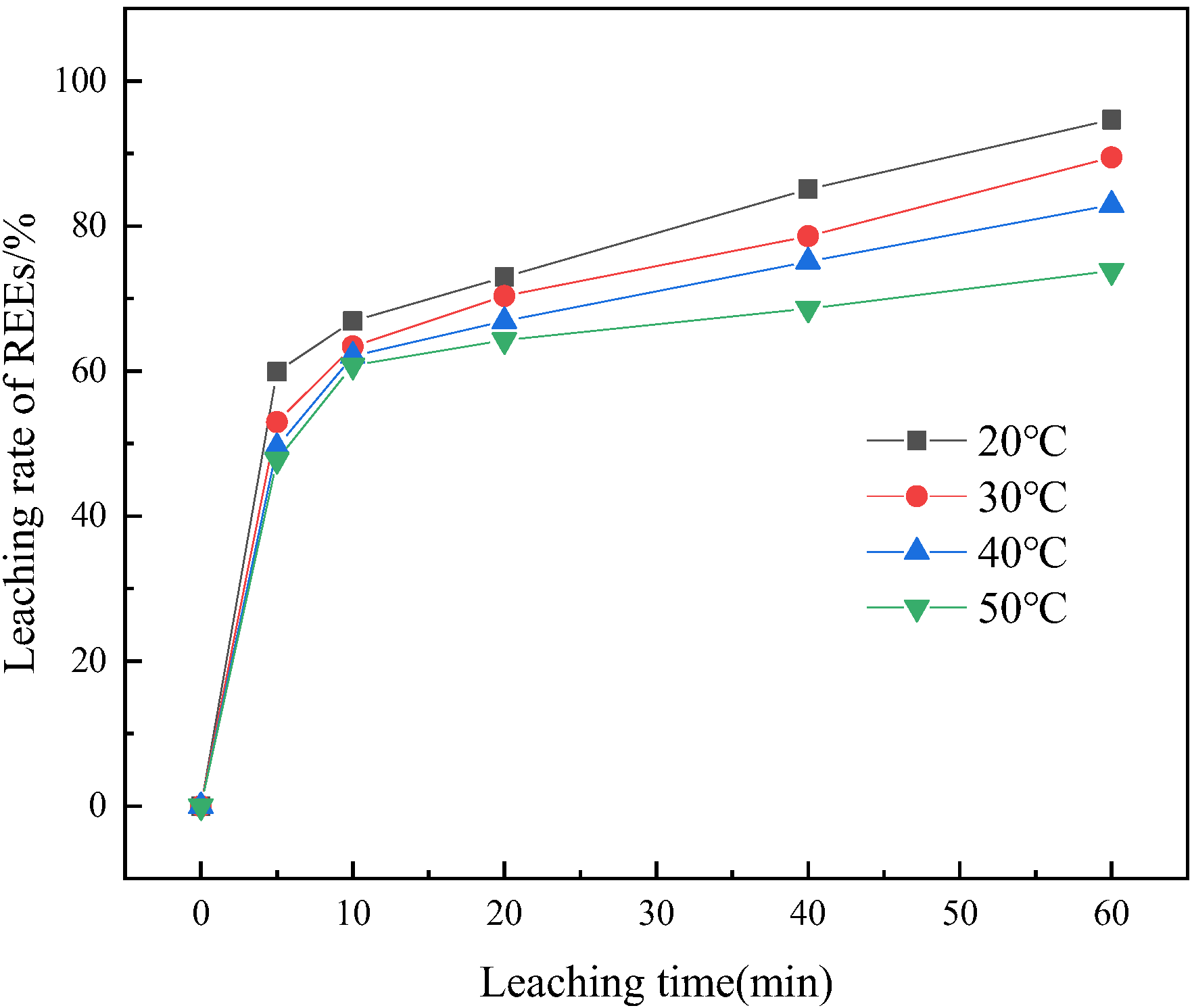
3.3. Influence of Leaching Parameters on REE Leaching
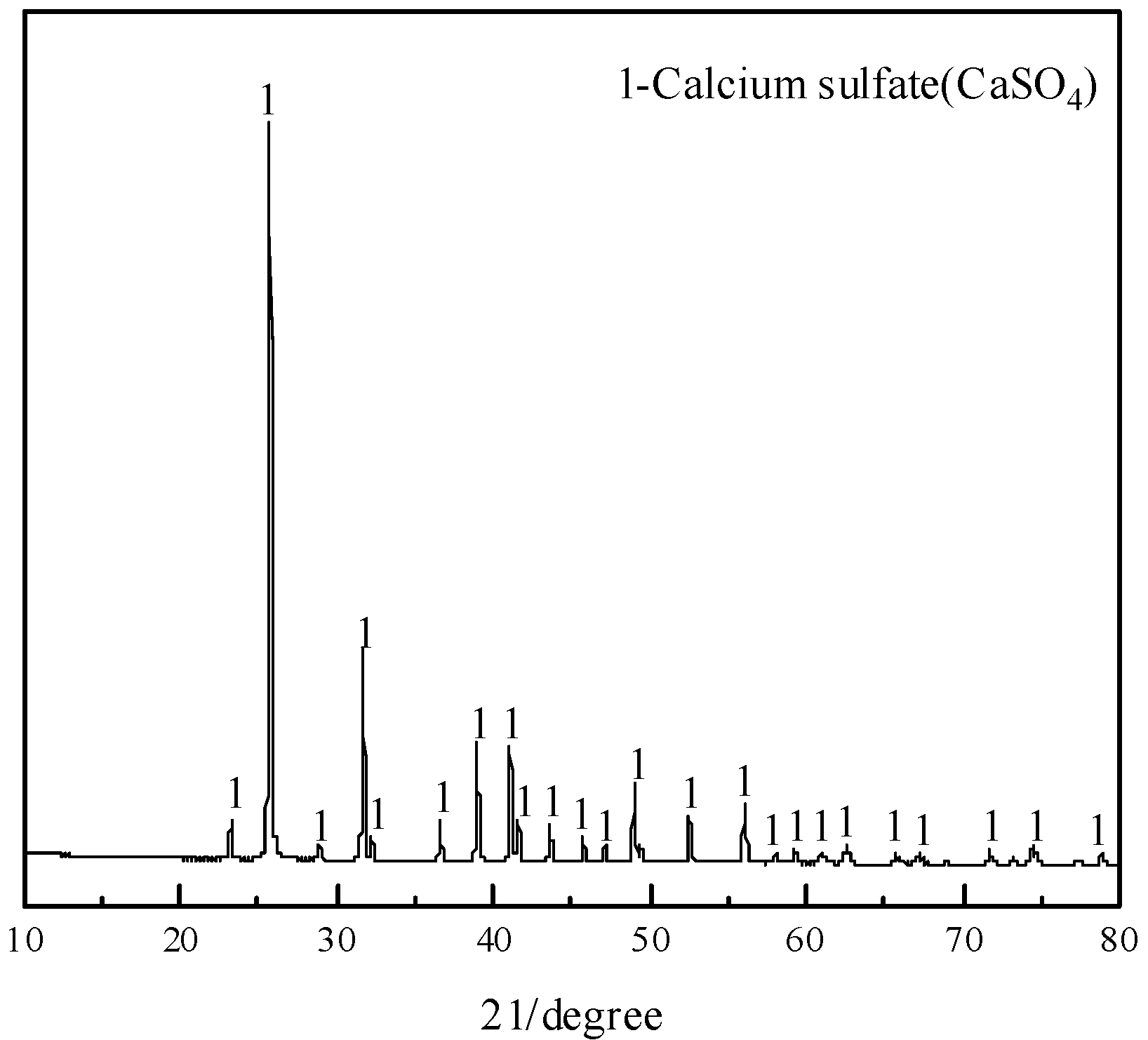
3.4. Characterization of Leached Slag
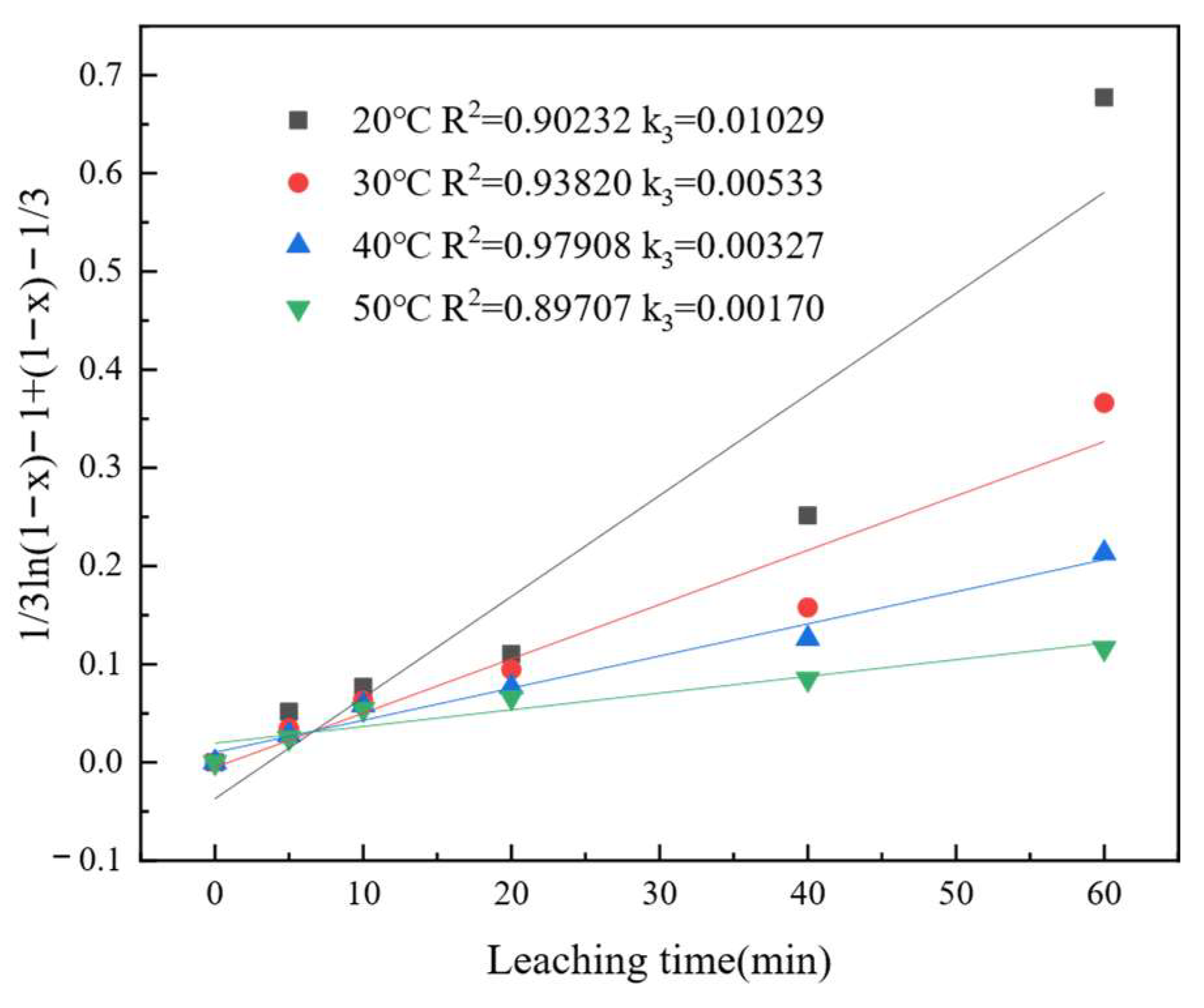
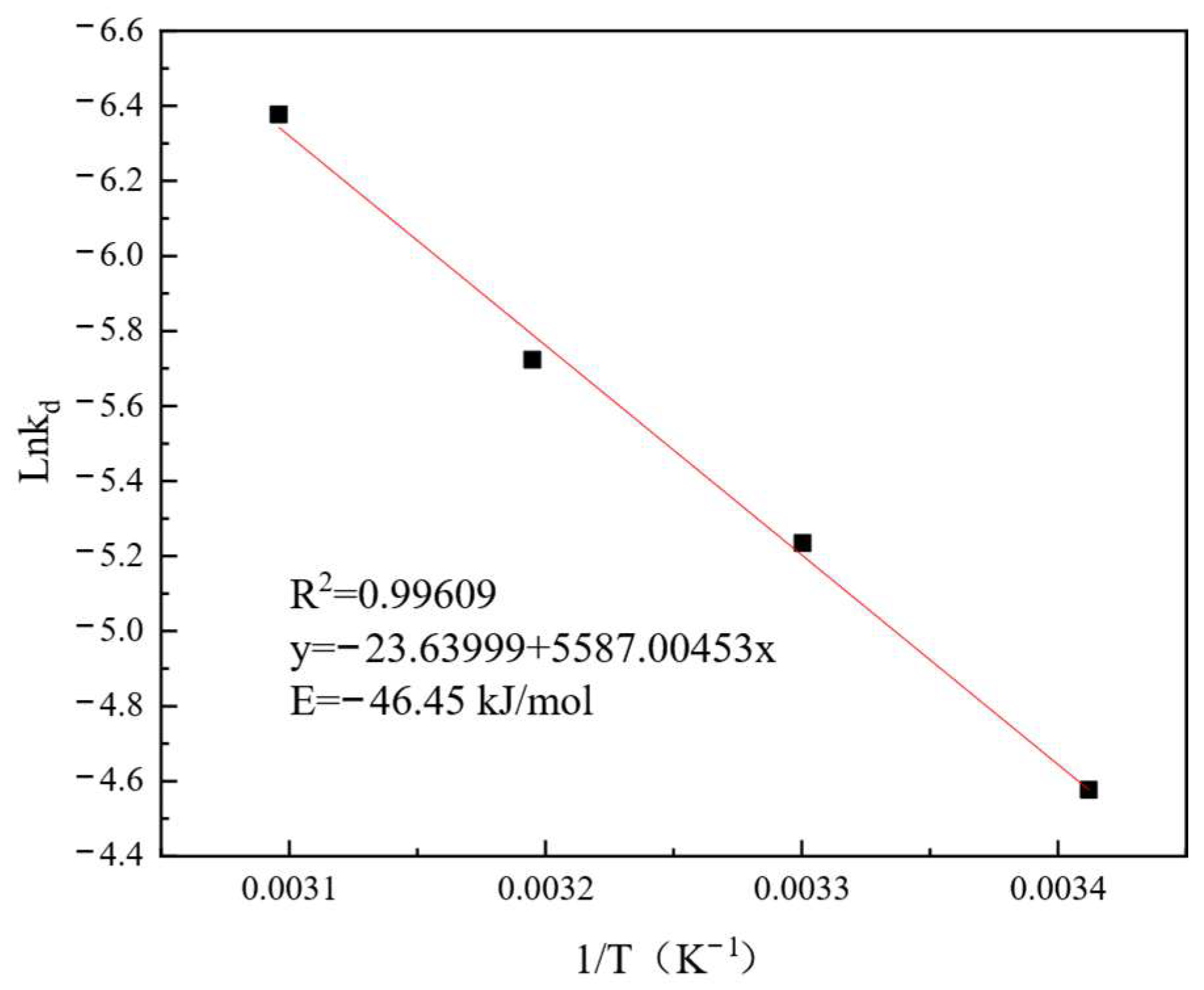
3.5. Leaching Kinetic Analysis
4. Conclusions
- (1)
- The leaching efficiency of REEs is highly dependent on both roasting and leaching parameters. The optimal conditions were determined to be 15 mL of H2SO4, roasting at 150 °C for 240 min, followed by water leaching at 20 °C for 60 min with a liquid–solid ratio of 15:1, achieving a maximum REE extraction efficiency of 94.65%.
- (2)
- REE in the CRS were completely transformed into water-soluble sulfates during roasting, which were subsequently dissolved during water leaching.
- (3)
- A CaSO4 product with 98.10% purity was obtained with a calcium recovery of 90.79%. The removal rate of fluorine in the CRS was 99.99%, which makes the comprehensive utilization of the CRS possible.
- (4)
- The leaching kinetics of REEs were best described by a combined diffusion and interfacial mass transfer model, with an apparent activation energy of –46.45 kJ·mol−1.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Keefe, M.J.; Geng, S.; Joshi, S. Cerium-Based Conversion Coatings as Alternatives to Hex Chrome: Rare-Earth Compounds Provide Resistance Against Corrosion for Aluminum Alloys in Military Applications. Met. Finish. 2007, 105, 25–28. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Laghzaoui, S.; Lamrani, A.F.; Laamara, R.A. Robust half-metallic ferromagnet in doped double perovskite Sr2TiCoO6 by rare-earth elements for photovoltaic and thermoelectric conversion: A DFT method. J. Phys. Chem. Solids 2023, 183, 111639. [Google Scholar] [CrossRef]
- Gad, S.M.; Jin, Z.; Emad, S.; Vergara, J.E.; Yawas, D.S.; Dagwa, I.M.; Omiogbemi, I.M.-B. Potential of rare-earth compounds as anticorrosion pigment for protection of aerospace AA2198-T851 alloy. Heliyon 2023, 9, e14693. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Oxide reduction processes in the preparation of rare-earth metals. Min. Metall. Explor. 2013, 30, 38–44. [Google Scholar] [CrossRef]
- Han, W.; Li, M.; Zhang, M.-L.; Yan, Y.-D. Progress in preparation of rare earth metals and alloys by electrodeposition in molten salts. Rare Met. 2016, 35, 811–825. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Luan, Y.; Yu, H.; Li, D. Research Progress in Preparation and Purification of Rare Earth Metals. Metals 2020, 10, 1376. [Google Scholar] [CrossRef]
- Huang, M.; Liu, K.; Zhang, H.; Zhang, X.; Li, J.; Xie, Y.; Lai, Y.; Huang, Z.; Qi, T. A Novel Process for the Recovery of Rare Earth and Fluoride Compounds from Calciothermic Reduction Slag. JOM 2023, 75, 3577–3586. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Xue, L.; Zou, Y. Extraction of rare earth elements from fluoride molten salt electrolytic slag by mineral phase reconstruction. J. Clean. Prod. 2018, 177, 567–572. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Chen, T.; Wang, C.; Hu, K.; Zhang, C.; Huang, Z.; Li, X.; Wan, Y. Effective recovery of rare earth and fluorine from REF3 smelting slag by a mechanochemical process. Sep. Purif. Technol. 2023, 316, 123832. [Google Scholar] [CrossRef]
- Chen, D.; Ou, Y.; Lu, D. Study on Comprehensive Utilization of Slag from Vacuum Calcium Thermal Reduction. Jiangxi Nonferrous Met. 2004, 3, 27–30. [Google Scholar]
- Xia, H.; Liu, Y.; Liu, D.; Wu, X. Thermodynamics Analysis for NaOH Roasting of Reduction Residue from Calcium Thermal Reduction of Fluorinated Rare Earth. Min. Metall. Eng. 2021, 4, 109–112. [Google Scholar]
- Liang, Y.; Li, Y.; Lin, R.; Shao, L.; Liu, Y. Extracting REEs from Smelter Slag of Calcium Thermal Reduction by Roasting with Sodium Metasilicate Nonahydrate. J. Chin. Soc. Rare Earths 2018, 6, 739–744. [Google Scholar]
- Liu, Y.; Liang, Y.; Liu, D.; Tang, H. Thermodynamic Analysis on Extracting REEs from Smelter Slag of Calcium Thermal Reduction of Rare Earth Fluoride. J. Chin. Soc. Rare Earths 2021, 5, 759–765. [Google Scholar]
- Huang, J.; Zhang, L.; Yu, W.; Chen, J.; Le, C.; Ren, S. Extraction of Rare Earth and CaF2 from Rare Earth Calcium Thermal Reduction Slag by Using CaO Roasting–Acid Leaching Method. Minerals 2024, 14, 1001. [Google Scholar] [CrossRef]
- Zeng, G.; Ling, B.; Li, Z.; Luo, S.; Sui, X.; Guan, Q. Fluorine removal and calcium fluoride recovery from rare-earth smelting wastewater using fluidized bed crystallization process. J. Hazard. Mater. 2019, 375, 313–320. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- GB/T 14635-2020; Standardization Administration of China. Rare Earth Metals and Their Compounds—Determination of Total Rare Earth Content. China Standards Press: Beijing, China, 2020.
- Xing, P.; Li, H.; Ye, C.; Zhong, L. Recovery of Rare-Earth Elements from Molten Salt Electrolytic Slag by Fluorine Fixation Roasting and Leaching. J. Sustain. Metall. 2022, 8, 522–531. [Google Scholar] [CrossRef]
- Lin, J.; He, Q.; Li, C. A Method for Separating and Recovering Rare Earth Elements from Waste Rare Earth Molten Salt Electrolysis. Chinese Patent CN101956078A, 26 January 2011. [Google Scholar]
- Bale, C.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Ben Mahfoud, R.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010-2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Duan, X.; Lu, S.; Yang, J.; Yang, H.; Liu, T. Study on the synergistic mechanism of sulfuric acid and ammonium sulfate on the extraction of metals from ferronickel slag by roasting. Miner. Eng. 2023, 202, 108300. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Z.; Wang, M.; Zhao, L.; Yu, Z.; Xia, C.; Huang, X. Leaching kinetic study of sulfuric acid roasted mixed-type rare earth concentrate for reducing the solid-waste production and chemical consumption. J. Clean. Prod. 2020, 260, 120989. [Google Scholar] [CrossRef]
- Das, G.; Lencka, M.M.; Eslamimanesh, A.; Wang, P.; Anderko, A.; Riman, R.E.; Navrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. J. Chem. Thermodyn. 2019, 131, 49–79. [Google Scholar] [CrossRef]
- Speight, J. Lange’s Handbook of Chemistry, Sixteenth Edition (McGraw-Hill Education: New York, Chicago, San Francisco, Lisbon, London, Madrid, Mexico City, Milan, New Delhi, San Juan, Seoul, Singapore, Sydney, Toronto, 2005); McGraw-Hill Education: Columbus, OH, USA, 2005; Available online: https://www.accessengineeringlibrary.com/content/book/9780071432207 (accessed on 23 September 2025).
- Faraji, F.; Alizadeh, A.; Rashchi, F.; Mostoufi, N. Kinetics of leaching: A review. Rev. Chem. Eng. 2022, 38, 113–148. [Google Scholar] [CrossRef]
- Lushnikova, N.; Dvorkin, L. 25—Sustainability of gypsum products as a construction material. In Sustainability of Construction Materials; Woodhead Publishing Series in Civil and Structural Engineering: Cambridge, UK, 2016; pp. 643–681. [Google Scholar] [CrossRef]
- Ritchey, K.; Norton, L.; Hass, A.; Gonzalez, J.; Snuffer, D. Effect of selected soil conditioners on soil properties, erosion, runoff, and rye growth in nonfertile acid soil. J. Soil. Water Conserv. 2012, 67, 264–274. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Liu, C. 12—Beers. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–351. [Google Scholar] [CrossRef]
- Kammerlander, C.; Neuerburg, C.; Verlaan, J.-J.; Schmoelz, W.; Miclau, T.; Larsson, S. The use of augmentation techniques in osteoporotic fracture fixation. Injury 2016, 47, s36–s43. [Google Scholar] [CrossRef]
- Schmidt, L.D. The Engineering of Chemical Reactions; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Dickinson, C.F.; Heal, G.R. Solid–liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340, 89–103. [Google Scholar] [CrossRef]
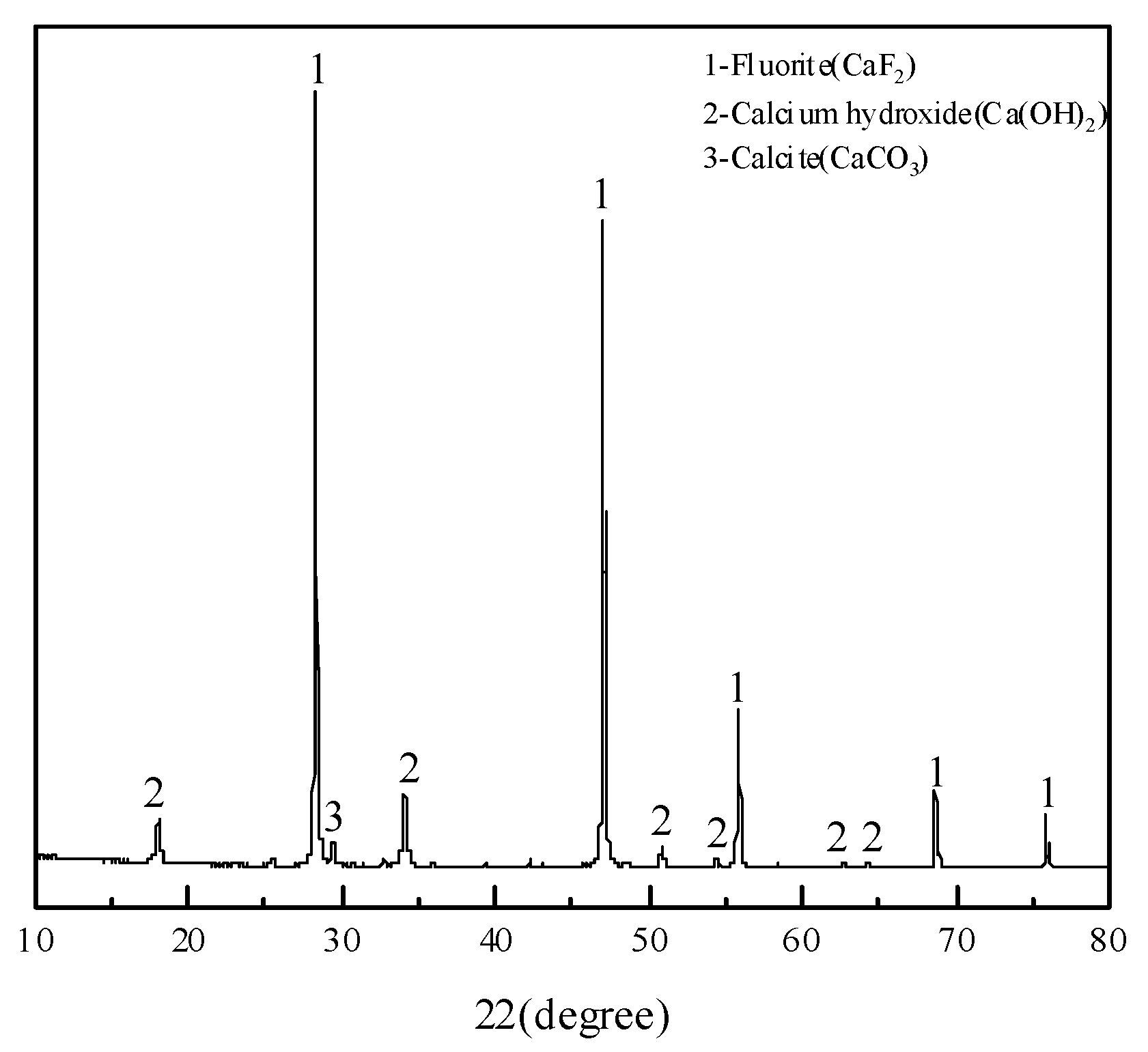
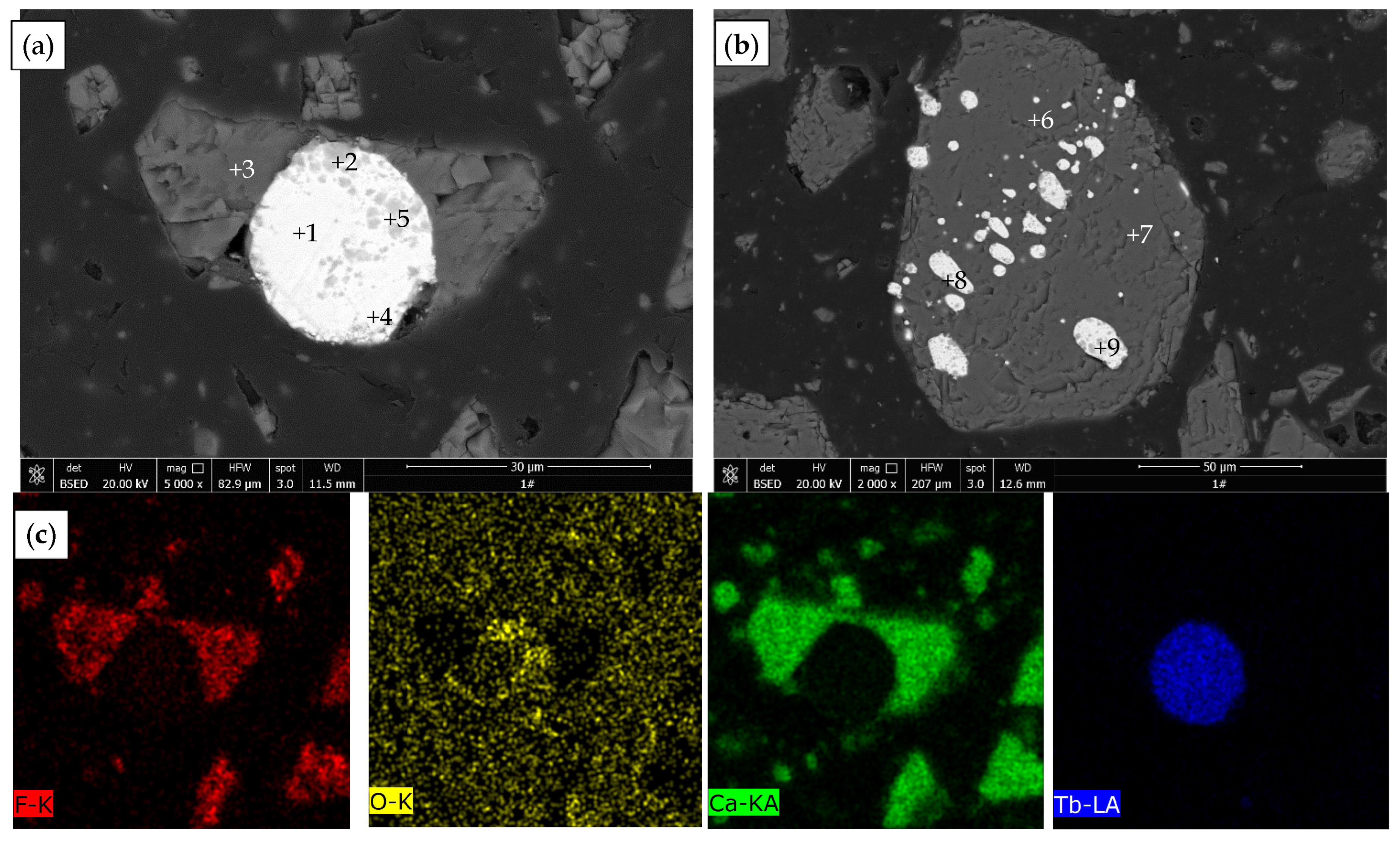
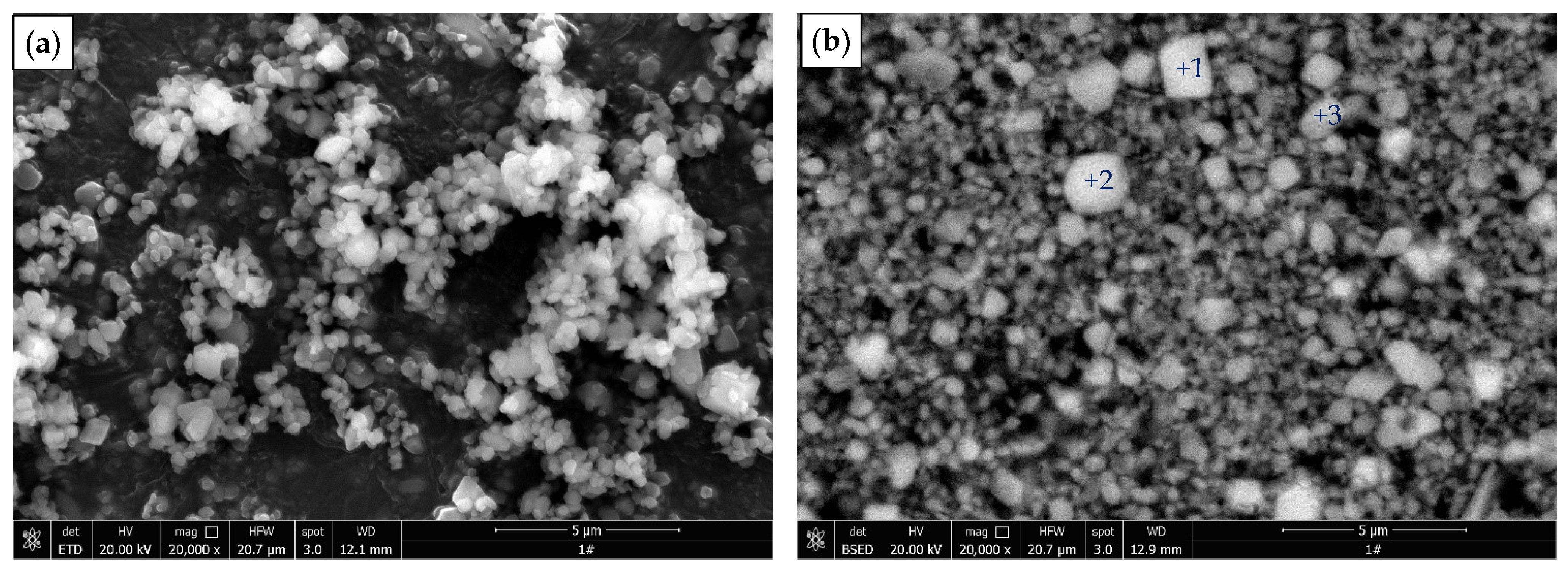
| Point | O | F | Ca | Tb | Phase |
|---|---|---|---|---|---|
| 1 | 0.17 | - | 0.99 | 98.83 | Terbium metal |
| 2 | 12.80 | 3.18 | 16.29 | 67.73 | Terbium oxide |
| 3 | 1.79 | 38.67 | 59.24 | 0.29 | Fluorite |
| 4 | 0.60 | - | 1.19 | 98.20 | Terbium metal |
| 5 | 6.72 | - | 1.30 | 91.98 | Terbium oxide |
| 6 | 2.14 | 41.93 | 55.61 | 0.32 | Fluorite |
| 7 | 1.92 | 41.85 | 55.96 | 0.27 | Fluorite |
| 8 | 1.20 | - | 1.46 | 97.33 | Terbium metal |
| 9 | 0.22 | - | 1.79 | 97.99 | Terbium metal |
| Equation | Reactions | ΔG(kJ·mol−1) |
|---|---|---|
| (1) | 2Tb + 3H2SO4 = Tb2(SO4)3 + 3H2(g) | 0.0005T2 + 0.6122T − 1510.80 |
| (2) | Tb2O3 + 3H2SO4 = Tb2(SO4)3 + 3H2O(g) | 0.0005T2 + 0.4496T − 415.04 |
| (3) | 2TbF3 + 3H2SO4 = Tb2(SO4)3 + 6HF(g) | 0.0003T2 − 0.3870T + 9.57 |
| (4) | CaF2 + H2SO4 = CaSO4 + 2HF(g) | 0.00006T2 − 0.2307T − 4.65 |
| (5) | CaCO3 + H2SO4 = CaSO4 + H2O(g) + CO2(g) | 0.00001T2 − 0.1421T − 135.01 |
| (6) | Ca(OH)2 + H2SO4 = CaSO4 + 2H2O(g) | −0.00003T2 − 0.0048T − 211.04 |
| Elements | REO | Ca | F | |
|---|---|---|---|---|
| Assay | 0.068 | 28.94 | 0.084 | 69.79 |
| Point | O | F | S | Ca | Tb | Phase |
|---|---|---|---|---|---|---|
| 1 | 45.14 | 0.48 | 22.45 | 31.85 | 0.07 | Calcium sulfate |
| 2 | 44.78 | 0.23 | 22.74 | 32.14 | 0.10 | Calcium sulfate |
| 3 | 43.82 | 0.40 | 22.50 | 33.19 | 0.09 | Calcium sulfate |
| Parameters | Values | Chemical Reaction Control | Three-Dimensional Diffusion Control | New Kinetic Model | |||
|---|---|---|---|---|---|---|---|
| 1 − (1 − x)1/3 = k1t | 1 − 2x/3 − (1 − x)2/3 = k2t | 1/3ln(1 − x) − 1+(1 − x)−1/3 = k3t | |||||
| K1 | R2 | K2 | R2 | K3 | R2 | ||
| T/℃ | 20 | 0.00828 | 0.83915 | 0.00334 | 0.96147 | 0.01029 | 0.90232 |
| 30 | 0.00689 | 0.80181 | 0.00263 | 0.94651 | 0.00533 | 0.93820 | |
| 40 | 0.00573 | 0.73771 | 0.00203 | 0.91232 | 0.00327 | 0.97908 | |
| 50 | 0.00433 | 0.60014 | 0.00135 | 0.78336 | 0.00170 | 0.89707 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, L.; Yu, W.; Chen, J.; Li, X.; Li, Q.; Liao, T.; Mo, X. Recovery of Rare Earth Elements from Calciothermic Reduction Slag by Sulfation Roasting–Water Leaching Method. Minerals 2025, 15, 1025. https://doi.org/10.3390/min15101025
Huang J, Zhang L, Yu W, Chen J, Li X, Li Q, Liao T, Mo X. Recovery of Rare Earth Elements from Calciothermic Reduction Slag by Sulfation Roasting–Water Leaching Method. Minerals. 2025; 15(10):1025. https://doi.org/10.3390/min15101025
Chicago/Turabian StyleHuang, Jinqiu, Lizhi Zhang, Wen Yu, Jiangan Chen, Xinwei Li, Qizhi Li, Ting Liao, and Xiaoning Mo. 2025. "Recovery of Rare Earth Elements from Calciothermic Reduction Slag by Sulfation Roasting–Water Leaching Method" Minerals 15, no. 10: 1025. https://doi.org/10.3390/min15101025
APA StyleHuang, J., Zhang, L., Yu, W., Chen, J., Li, X., Li, Q., Liao, T., & Mo, X. (2025). Recovery of Rare Earth Elements from Calciothermic Reduction Slag by Sulfation Roasting–Water Leaching Method. Minerals, 15(10), 1025. https://doi.org/10.3390/min15101025








