Electrodeposition of Metallic Magnesium in Ionic Liquids: A Systematic Review
Abstract
1. Introduction
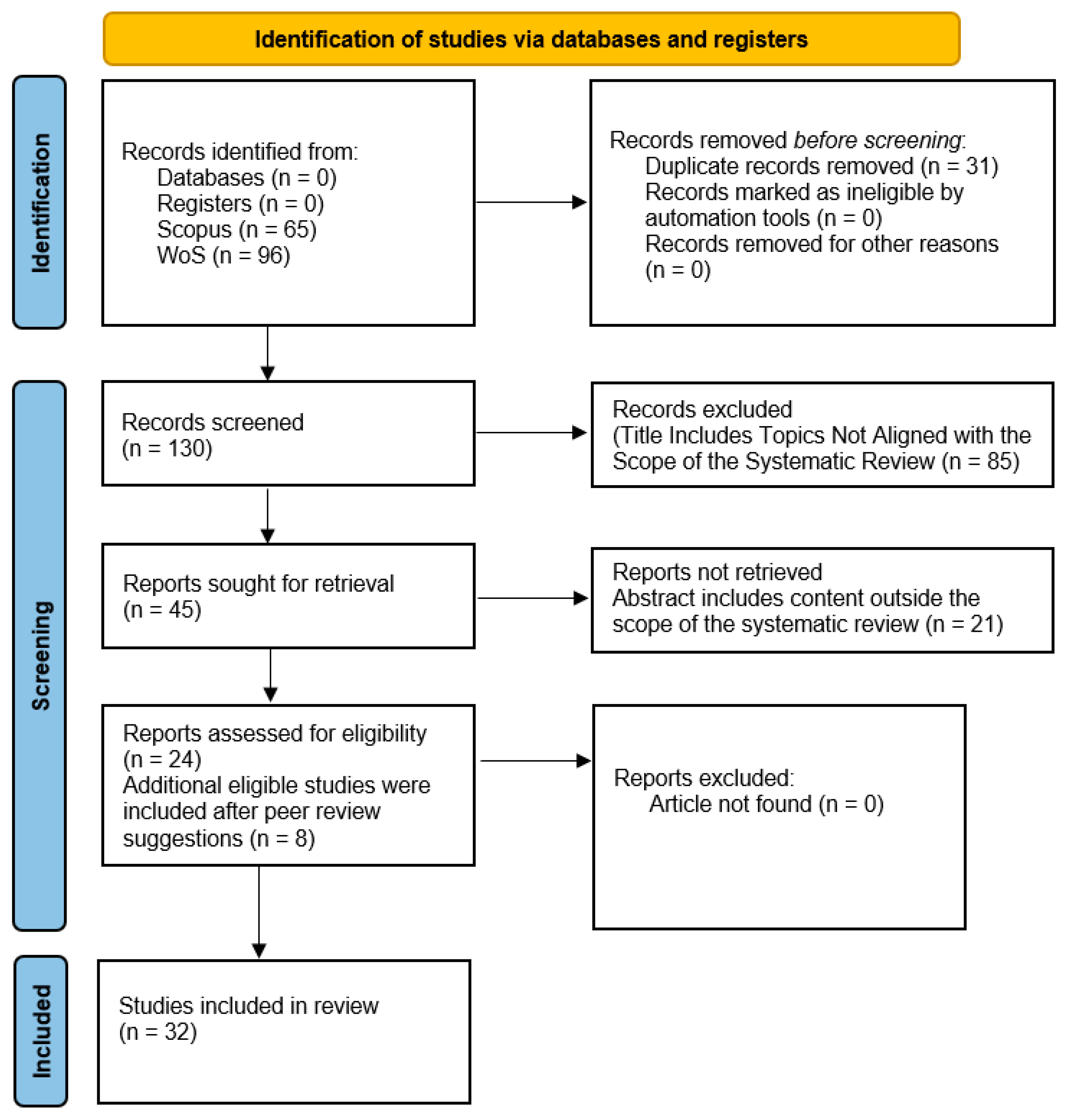
2. Materials and Methods
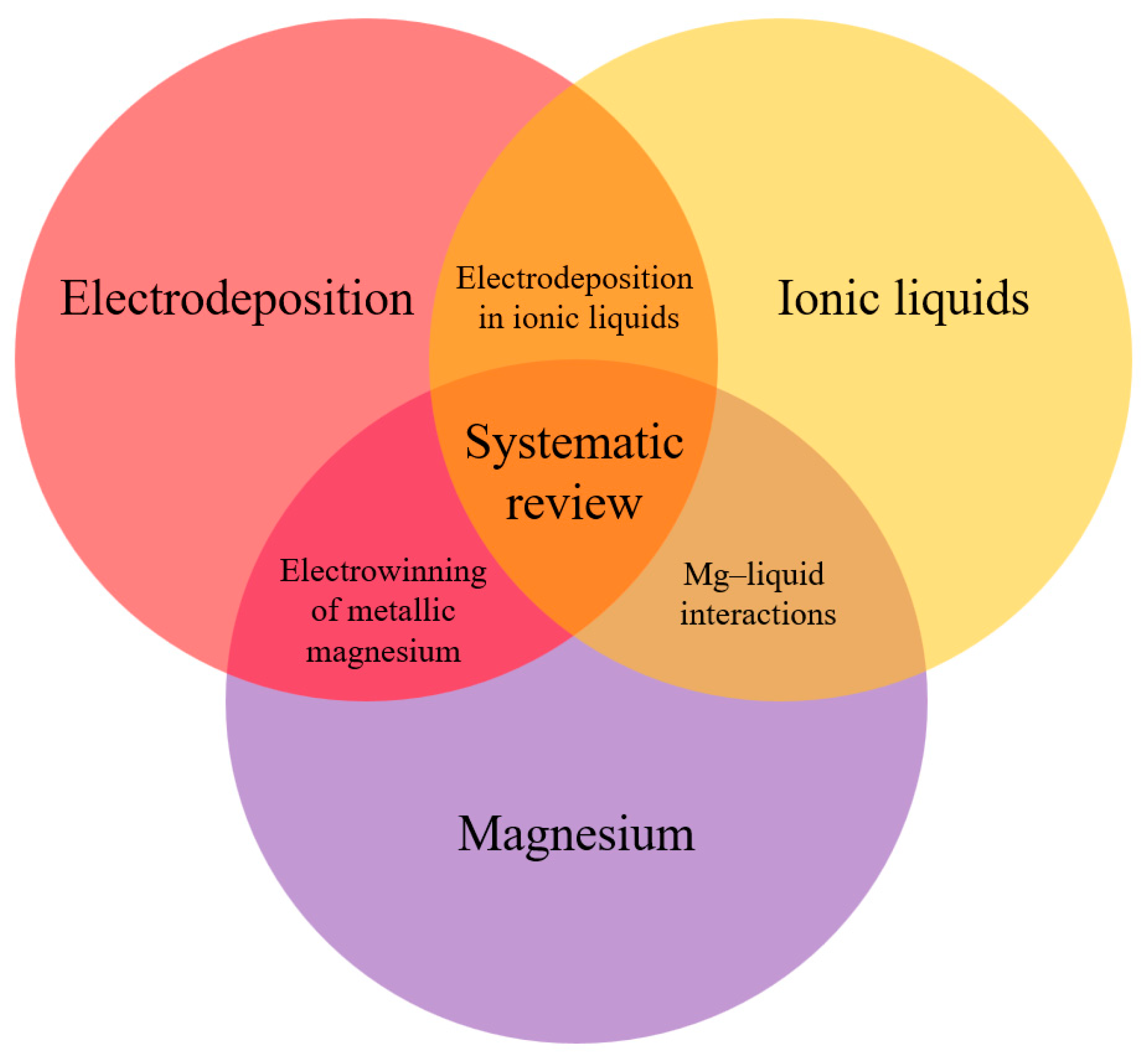
- General Research Question (GRQ):
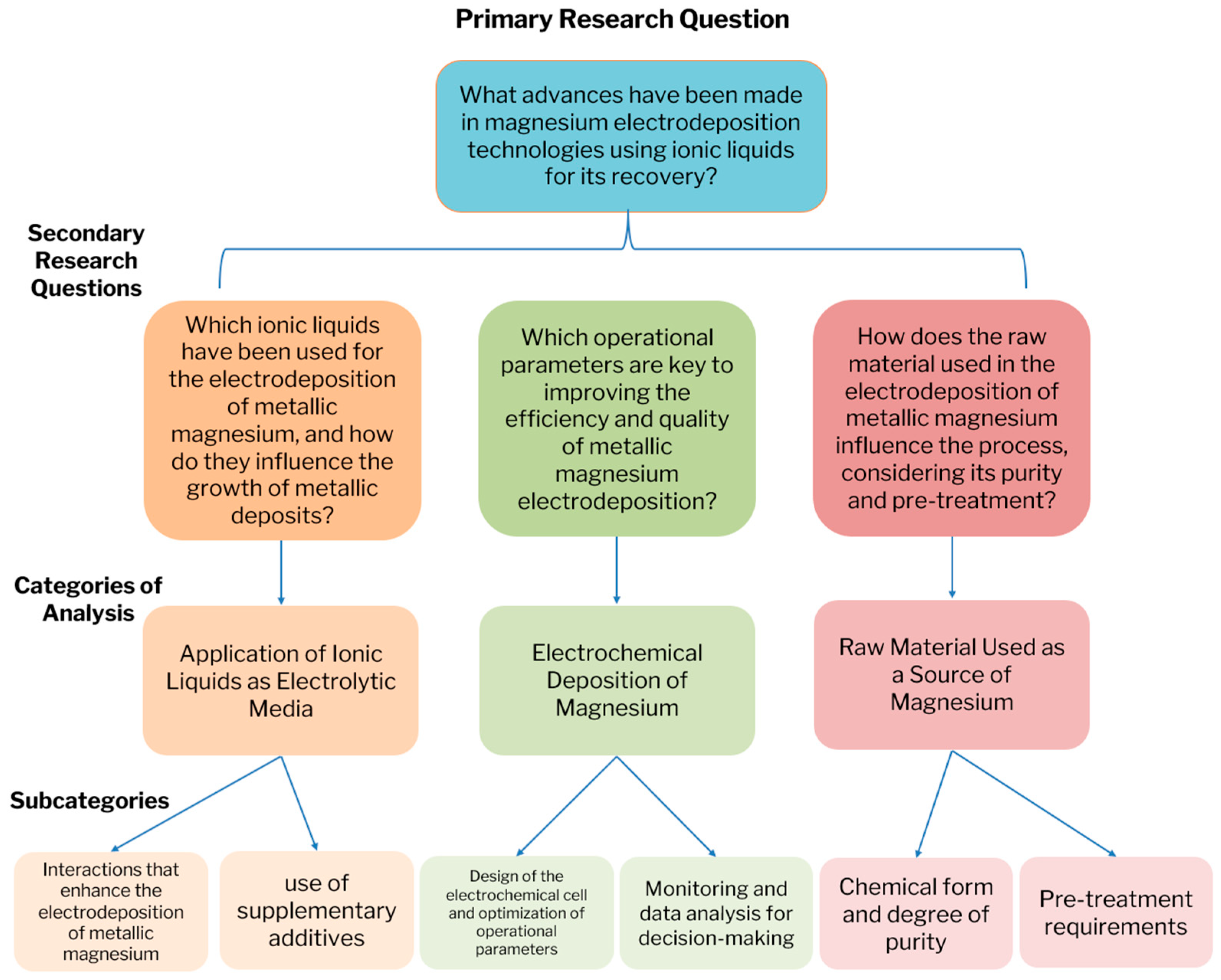
- GRQ: What are the recent advances in metallic magnesium electrodeposition technologies using ionic liquids?
- Specific Research Questions (SRQs):
- SRQ 1: What types of ionic liquids have been used in the electrodeposition of metallic magnesium, and how do they influence deposit growth?
- SRQ 2: What operational parameters are key to improving the efficiency and quality of metallic magnesium electrodeposition?
- SRQ 3: How does the raw material used in magnesium electrodeposition influence the process, considering its purity and pretreatment?
- GO: To describe electrodeposition technologies using ionic liquids for the recovery of metallic magnesium through a systematic review of the scientific literature.
- Ensure access to peer-reviewed publications;
- Enable replicability and traceability of the search process, in alignment with the transparency standards set by PRISMA 2020;
- Complement each other’s coverage, reduce publication bias and enhance thematic representativeness in the field under study.
- Subcategory: Interactions with the ionic liquid
- SC1.1—Interactions with magnesium cations that promote the electrodeposition of metallic magnesium.
- SC1.2—Interactions with supplementary additives present in the medium, with potential to mitigate side reactions that may affect process efficiency.
- Subcategory: Operational parameters in magnesium electrodeposition
- SC2.1—Design of the electrochemical cell and optimization of operational parameters to improve the efficiency of metallic magnesium electrodeposition.
- SC2.2—Monitoring and data analysis for decision-making, including sensors, automation and processing of relevant process information.
- Subcategory: Considerations regarding the raw material
- SC3.1—Chemical form and purity level, including the presence of impurities that could interfere with the electrodeposition process.
- SC3.2—Required pretreatment steps to optimize their use in the electrodeposition of metallic magnesium.
3. Results
3.1. Main Features of the Selected Articles
- (1)
- A word cloud generated from article titles to reveal dominant thematic terms (Figure 5).
- (2)
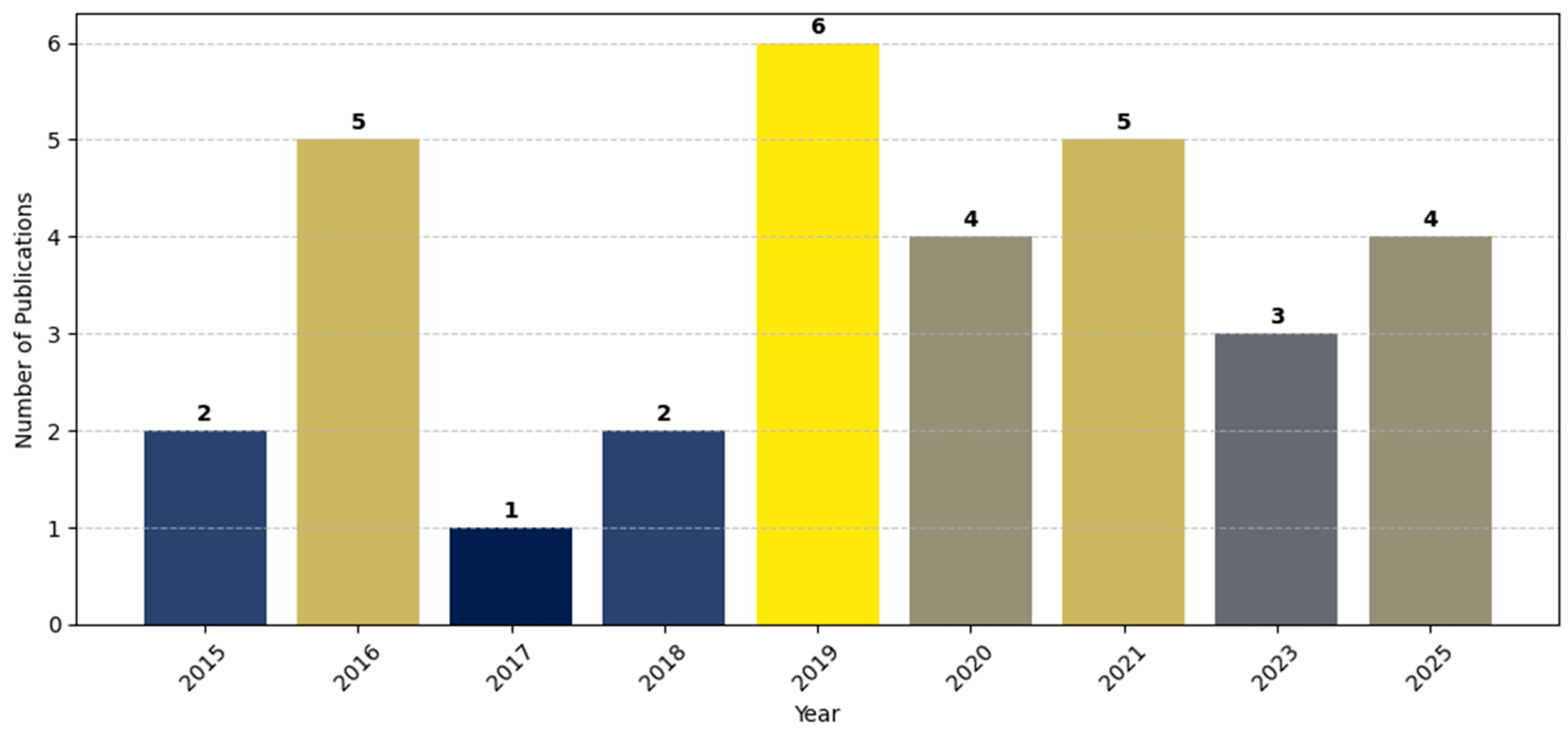
- A bar plot illustrating annual publication trends to show the temporal evolution of research activity (Figure 6).
- (3)
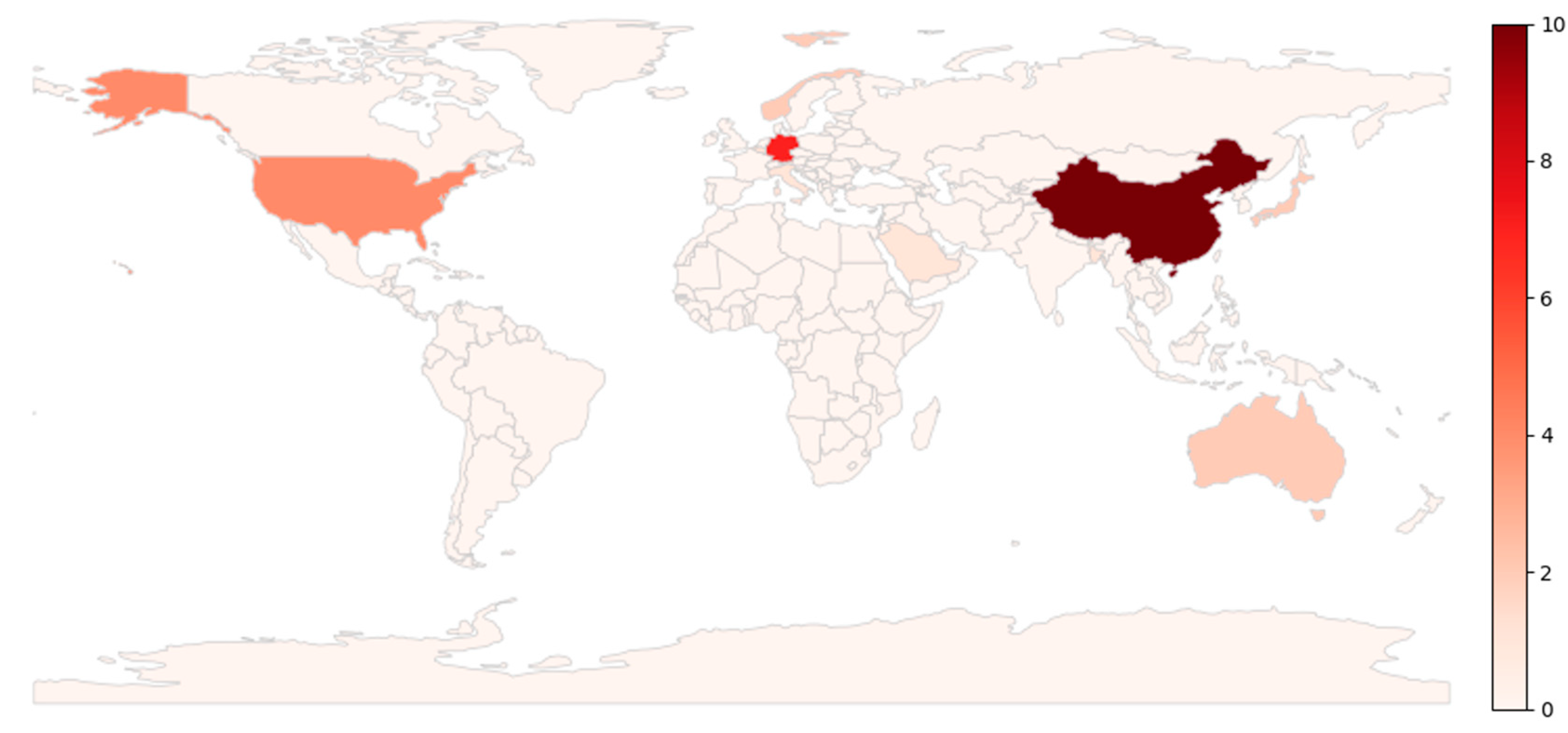
- A geographic distribution map based on the lead author’s country to identify regional leadership in the field (Figure 7).
- (4)
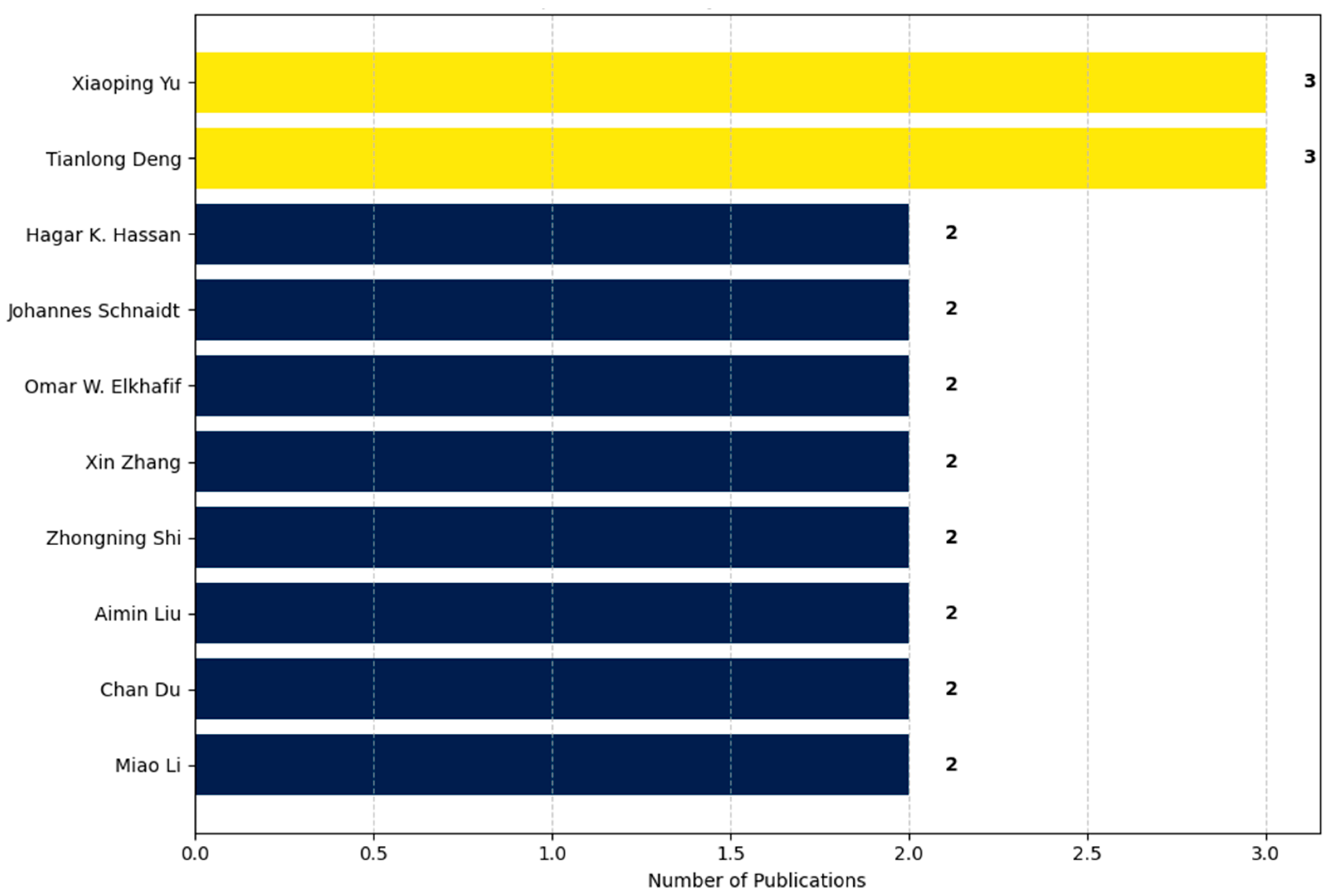
- A breakdown of publications by author, highlighting individual research contributions (Figure 8).
3.2. Findings per Analysis Category
3.2.1. Use of Ionic Liquids as Electrolytes
- Interactions Mg-ILs
| Reference | IL Used | IL Abbreviation | Benefits | Limitations |
|---|---|---|---|---|
| [9] | 1-butyl-3-methylimidazol-2-ylidene borane | BBH3MIm | High reductive stability (−1.66 V), avoids passivation, reversible Mg deposition (~96% CE), and low viscosity | No oxidative stability > 3.56 V; sensitive to trace water; synthesis not trivial |
| [10] | 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide | Pyr14TFSI | High oxidative stability (~6.0 V); stabilizes electrolyte | Increases overpotential (>±1.6 V); does not form active complexes alone; low MgCl2 solubility |
| [11] | 1-methyl-1-propylpiperidinium bis(trifluoromethanesulfonyl)imide | MPPip-TFSI | High oxidative stability (~4.2 V), good conductivity (8.53 mS/cm), and low volatility. | High viscosity (124 mPa·s); Requires co-solvents and additives. |
| [12] | 1-(2-(2-(2-Methoxyethoxy)ethoxy)- ethyl)-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide | MPEGxPyrTFSI | High reductive stability; Coulombic Efficiency up to 90%; displaces TFSI− and BH4− from Mg2+ coordination. | Complex synthesis, moderate viscosity, lower conductivity than ether solvents. |
| [8] | Poly(1-butyl-3-methylimidazolium styrenesulfonyl(trifluoromethanesulfonyl)imide) | BMIPSTFSI + Mg(PSTFSI)2 | High thermal stability; plasticization improves segmental mobility. | BMI, not Mg2, dominates transport+; Mg deposition is limited or non-uniform. |
| Magnesium bis(styrenesulfonyl(trifluoromethanesulfonyl)imide) | ||||
| [13] | 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide | BMP-TFSI | High thermal stability; well-known structure; enables systematic analysis. | Mg deposition is inhibited by strong interactions between TFSI− anions and Mg2+; TFSI− decomposition is enhanced; low CE. |
| [14] | Choline chloride + urea (DES) | ChCl:Urea | Low toxicity and a good electrochemical window (~−1.4 V) enable induced co-deposition. | Mg2+ cannot be reduced directly; it requires a catalytic Co2+ pathway. |
| [15] | Aluminum chloride–1-ethyl-3-methylimidazolium chloride | AlCl3–EMIC | Enables direct Al deposition; supports Al–Mg alloy formation. | Mg does not reduce alone; the incorporation of Mg limits it. solubility constraints. |
| [16] | 1,3-Dimethyl-2-imidazolidinone–aluminum chloride | DMI–AlCl3 (solvate ionic system) | Forms electroactive species ([MgCl(DMI)n]+); dense deposits. | Mg co-deposition is dependent on Al; no independent Mg2+ reduction occurs. |
| [17] | 1-butyl-3-methylimidazolium chloride + glycerin (1:1) | BMIC/GL | Non-toxic; good thermal stability; supports Ni–Mg alloy formation. | Mg cannot be reduced alone; conductivity is limited (~0.21 mS/cm). |
| [18] | Choline chloride + urea (DES) | ChCl:Urea | Enables induced co-deposition of Mg via catalytic Co2+. | Mg2+ not reducible independently in DES. |
| [19] | Choline chloride + urea (DES) | ChCl:Urea | Facilitates Zn plating on Mg alloys; suppresses H2 evolution. | No Mg deposition reported. |
| [20] | 1-(2-hydroxyethyl)pyridinium TFSI + derivatives | OH-PyrTFSI | Wide electrochemical window; strong coordination features. | No Mg deposition achieved; coordination only studied spectroscopically. |
| [21] | 1-ethyl-3-methylimidazolium tetrafluoroborate | EMImBF4 | Forms complex anions; enhances Mg2+ carrier density; 0.007 S/cm. | Mg not electroplated; coordination/mobility studied only. |
| [22] | Multiple TFSI-based ILs | Various | High-voltage stability (>5.5 V); good conductivity; improved cathode compatibility. | No Coulombic efficiency is reported for Mg; only the insertion of Mg2+ into Cathodes are evaluated. |
| [23] | N-methyl-N-butylpiperidinium bis(trifluoromethanesulfonyl)imide | [C4mpyr][TFSI] | High conductivity (~2.8 mS/cm); excellent oxidative stability (>3 V); improves thermal properties. | The TFSI− anion coordinates strongly with Mg2+, leading to passivation. It generates high overpotentials. |
| [24] | Magnesium bis(triglyme) bis(bis(trifluoromethanesulfonyl)imide) + Tetrabutylammonium chloride | Mg(G3)2(Tf2N)2 + TBACl | CE >87%; reversible stripping; conductivity enhanced with Cl− | Poor performance without Cl−; viscosity challenges. |
| [25] | N-butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate | PyrrFAP y otros | Mg deposited with CE up to ~85%; enhanced crystallinity. | Most ILs inhibit stripping without Cl− or Grignard Species. |
| [26] | N-methyl-N-propylpiperidinium bis[(trifluoromethyl)sulfonyl]amide | PP13TFSI | Low volatility; high oxidative stability (~+4 V); Mg deposition with Coulombic efficiency up to 95%. | Degradation observed with the G3 system; requires glyme coordination. |
| [27] | Methyltrioctylammonium neodecanoate | [A336][V10] | Extracts Mg2+ selectively (60.98%); facilitates direct Electrodeposition. | Requires dehydration with MgCl2/tamper to prevent Mg(OH)2 formation. |
| [28] | Tributylhexylphosphonium bis(trifluoromethanesulfonyl)imide | [P4446][NTf2] | Enhances the conductivity of the extractant system; stable with diluents. | Viscosity increases above ~10% content; no direct reactivity. |
| [29] | N-allyl-N-methylpyrrolidinium chloride | AMPyrrCl | High CE (~100%); avoids dendrites; prevents passivation. | Low solubility in THF; unstable at excess ratios. |
| [30] | Methyltrioctylammonium di-(2-ethylhexyl)phosphate | [A336][P204] | High Mg2+ extraction rate (~83.99%); good conductivity. | High viscosity at large volumes; conductivity decreases by more than 60% with increased content. |
| [31] | Choline chloride + urea (0.68:1) | ChCl:Urea (DES) | Enables uniform Mg deposition via pulse technique (~20 nm layer). | Affects deposition morphology in DC mode; sensitive to water/air. |
| [32] | 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | BMP-TFSI | Low volatility, wide electrochemical stability window. Enables Mg plating/stripping when combined with Mg(BH4)2 and 18-crown-6. | Strong Mg2+–TFSI− coordination promotes reductive decomposition of TFSI−. Without additives, Mg plating/stripping is inefficient. |
| [33] | 1-methyl-1-propylpiperidinium bis[(trifluoromethyl)sulfonyl]imide | MPPip-TFSI | Hydrophobic ILs with wide electrochemical stability windows. Good ionic conductivity, thermal stability, and non-flammability. After drying (<1 mg·L−1 water), improved reversibility of Mg deposition/dissolution and reduced passivation. | Strongly hygroscopic, easily contaminated with trace water. Residual water induces passivation (MgO, Mg(OH)2), shifts deposition potential by ~0.9 V, lowers conductivity, and reduces stability. |
| 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | BMP-TFSI | |||
| [34] | N,N-diethyl-N-methyl-N-(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide | DEME·TFSI | Improves ionic conductivity. Enhances current density and reversibility of Mg deposition/dissolution. Compatible with Mg(HMDS)2–MgCl2/THF electrolyte, preserves high anodic stability. | At high concentration, minor decomposition (TFSI− traces detected in deposits). |
| [35] | 1-butyl-3-methylimidazolium tetrafluoroborate | [BMIM][BF4] | Supports reversible Mg plating/stripping when combined with Mg(CF3SO3)2; moderate viscosity; less reductive decomposition compared to TFSI− systems. | Still viscous; efficiency strongly depends on co-solvent addition; hygroscopic, requires careful drying. |
| [36] | 1-ethyl-3-methylimidazolium tetrachloroaluminate | [EMIM][AlCl4] or [C2mim][AlCl4] | Enables reversible Mg plating/stripping at room temperature; high anodic stability; Lewis-neutral chloroaluminate species are less corrosive and compatible with high-voltage cathodes. | Requires THF as co-solvent; performance strongly depends on molar ratio (1:2 is optimal). |
| [37] | N,N,N-tri-(2-(2-methoxyethoxy)ethyl)-N-(2-methoxyethyl)ammonium bis(trifluoromethylsulfonyl)imide | N07TFSI | Noncorrosive compared to Pyr14TFSI (avoids F and S contamination in Mg deposits). Supports reversible Mg plating/stripping with high Coulombic efficiency. | Still exhibits moderate initial Coulombic inefficiency (~70%). Long-term cycling stability needs improvement, as degradation occurs after extended cycling. |
| [38] | 1-methyl-1-propylpiperidinium bis(trifluoromethanesulfonyl) amide | PP13TFSA | High thermal stability, wide electrochemical window, and stable up to 350 °C. | Without ether additives, Mg plating is not reversible; it is prone to TFSA− decomposition, leading to impurities (F, N, S) and mossy deposits. |
| [39] | 1,3-Dimethyl-2-imidazolidinone + AlCl3 (chloroaluminate ionic liquid analog). | DMI | Forms a chloroaluminate IL analog; stabilizes Mg as [MgCl(DMI)4]+; enables Mg co-deposition with Al. | Deposits are mixed (Al + Al12Mg17 alloy); efficiency decreases at high MgCl2 concentration due to precipitation. |
- Interactions Mg-Electrolyte-Additives
| Reference | Electrolyte Used | Additives | Effect |
|---|---|---|---|
| [9] | 0.5 M Mg(BH4)2 dissolved in BBH3MIm | Mg(BH4)2 | Scavenges trace H2O, preventing oxide formation. BBH3MIm coordinates Mg2+ via a zwitterionic borane site, enabling high-purity non-passivating deposition (CE ~96%). |
| [10] | 0.25 M Mg(TFSI)2 + 0.5 M MgCl2 in DME, TEGDME or Pyr14TFSI | DME | DME coordinates Mg2+, forming stable [Mg3Cl4]2+·5DME complexes. Improves ion mobility and lowers overpotential; however, the CE is not directly quantified. |
| [11] | 0.1 M Mg(TFSI)2 + 0.01 M Mg(BH4)2 in MPPip-TFSI:diglyme (1:3) | Mg(BH4)2, Diglyme | BH4− acts as a drying agent and improves plating morphology. Diglyme enhances conductivity and solvates Mg2+ through ether oxygen interactions. Together they yield CE ~80%–85%. |
| [12] | Mg(BH4)2 in MPEGxPyrTFSI (up to 0.5 M) | Mg(BH4)2 | BH4− removes water; MPEG chains coordinate Mg2+, displacing TFSI− from the inner sphere. The chelating effect improves deposition efficiency (~90%) and suppresses passivation. |
| [8] | IL1/IL2 blend: Mg(PSTFSI)2 in BMIPSTFSI (up to 25 mol%) | None reported | PIL system transports Mg2+ via polymer chains, but conductivity is low, and deposits are sparse. Mg2+ mobility is limited; no reliable CE measured. |
| [13] | BMP-TFSI with Mg(TFSI)2 | None reported | Mg2+ strongly interacts with TFSI− anions, leading to IL decomposition and poor plating (55% CE). No effective coordination or film formation was observed. |
| [14] | ChCl:urea DES with CoCl2, MgCl2, Nd(NO3)3 | Thiourea | Promotes induced co-deposition of Mg–Nd via Co2+ catalysis. Thiourea adjusts nucleation mode and corrosion resistance. Mg does not deposit alone in the DES system. |
| [15] | AlCl3–EMImCl with MgCl2 | None reported | Mg2+ is incorporated via induced co-deposition with Al, forming Al5.15Mg3.15 alloy. Independent Mg plating not observed. CE ~99% for alloy process. |
| [16] | DMI–AlCl3 in solvate with MgCl2 | None reported | Mg is co-deposited via alloy formation with Al. Mg2+ does not plate independently; deposition requires induced reduction through Al3+. |
| [17] | BMImCl–Glycerin eutectic with NiCl2 and MgCl2 | None reported | Mg2+ is co-deposited with Ni2+. Deposition depends on Ni presence; Mg incorporation confirmed via EDX, but not isolated deposition. |
| [18] | ChCl–Urea DES with CoCl2, MgCl2, Pr(NO3)3 | None reported | Co2+ induces Mg and Pr deposition. Mg2+ does not reduce alone; alloy films confirmed by SEM/XRD. Co is catalytic for Mg inclusion. |
| [19] | Comparative systems using ILs and DES with Mg sources | None reported | Highlights the advantages of ILs over aqueous media for Mg2+ plating. Specific electrolyte not defined; no CE quantified. |
| [20] | Hydroxyl-functionalized pyridinium ILs with Mg2+ | None reported | Hydroxyl groups enhance Mg2+ solvation and stability; study explores Mg–IL coordination via NMR and FTIR. No plating CE reported. |
| [21] | EMImBF4 mixed with δ-MgCl2 | None reported | BF4− and Cl− form complex anionic structures with Mg2+, increasing conductivity and transport. Deposition behavior not demonstrated. |
| [22] | Hybrid electrolyte: IL + DPGDME or acetonitrile + Mg(TFSI)2 | DPGDME, Acetonitrile | DPGDME improves Mg2+ coordination; acetonitrile boosts conductivity. Used for Mg2+ intercalation, not for direct metallic deposition. |
| [23] | 0.3 M Mg(TFSI)2 in [C4mpyr][TFSI]:tetraglyme (1:2 v/v) | Mg(BH4)2 (~18–19 mM) | BH4− removes residual water and suppresses MgO formation; glyme coordinates Mg2+, improving mobility. CE ~87% with stable non-dendritic deposition confirmed by SEM/XRD. |
| [24] | 1:1 molar Mg(G3)2(Tf2N)2 with TBACl | TBACl | Cl− facilitates Mg2+ stripping and improves reversibility; the solvate structure enhances plating uniformity and transport. CE >87% observed. |
| [25] | 90% EtMgBr/THF + 10% IL (e.g., [Py14][FAP]) | None reported | Direct deposition from Grignard reagent; Mg+ acts as source species, IL stabilizes redox medium and morphology. CE up to ~85%. |
| [26] | Mg(TFSI)2 + PP13-TFSI + glymes (G2, G3, G4) in molar ratios 1:7:8 | None reported | G4 provides multidentate ether coordination, yielding crystalline Mg(G4)(Tf2N)2 species. Stable plating behavior confirmed via XRD. CE ~95%. |
| [27] | Organic phase with [A336][V10] + MIBK (extractive system) | MIBK | A336][V10] enables direct deposition of Mg2+ from brine-rich organic phase via coordination. MIBK acts as a phase separation enhancer, increasing conductivity and supporting plating without thermal dehydration. Deposition morphology confirmed by SEM/XRD; CE not quantified. |
| [28] | Organic phase with 5% [P4446][NTf2] + 55% MIBK + 40% P204 (saponified) | MIBK | P4446][NTf2] enhances conductivity (~1500×), enabling electrodeposition from high Mg/Li ratio brine. MIBK improves phase separation and supports ionic mobility in the organic phase. Mg2+ deposition confirmed by XRD; CE not specified. |
| [29] | 0.4 M PhMgCl + 0–0.4 M AMPyrrCl in THF (Grignard + allyl-IL system) | PhMgCl | Grignard reagent supplies Mg+ species for direct deposition. Allyl-functionalized IL (AMPyrrCl) is part of the base electrolyte and enhances oxidative stability (~+1 V), prevents passivation, and improves plating morphology. CE is near 100% in CV tests. |
| [30] | Organic phase composed of 60% [A336][P204] + 40% MIBK (extracted phase, R = 10:1) | MIBK | MIBK enhances phase separation and ionic conductivity, enabling direct Mg2+ deposition from the brine-derived organic phase. Deposition confirmed by XRD; CE not explicitly reported. |
| [31] | Mg(CH3COO)2 dissolved in DMSO and mixed with choline chloride:urea DES | DMSO | DMSO enhances the solubility of Mg(CH3COO)2 and facilitates its transport within the DES matrix. Pulsed deposition yields uniform Mg nanofilms (~20 nm) on TiO2 nanotubes. CE not reported. |
| [32] | BMP-TFSI + 0.4 M Mg(BH4)2 + 0.2 M 18-crown-6 | Mg(BH4)2 and 18-crown-6 | Crown ether complexes Mg2+, reducing its strong interaction with TFSI−. Borohydride stabilizes Mg2+ solvation and improves reductive stability. Together, they significantly enhance the reversibility of Mg plating/stripping. |
| [33] | MPPip-TFSI/tetraglyme (1:1) + 100 mM Mg(TFSI)2 + 10 mM Mg(BH4)2 | Mg(BH4)2 | Drying decreases solution resistance, increases capacitance, suppresses passivation, and shifts Mg deposition to less negative potentials. |
| [34] | 0.625 M Mg(HMDS)2–MgCl2 in THF + DEME·TFSI | DEME·TFSI (13.3–53.2 mol%) | Linear increase in ionic conductivity with DEME·TFSI concentration. Reversible Mg deposition/dissolution. Smaller, microporous Mg deposits with higher surface area. |
| [35] | 1 M Mg(CF3SO3)2 in [BMIM][BF4] + [PP13][TFSI] | Ether solvents | Addition of ethers decreases viscosity and increases ionic conductivity by 1–2 orders of magnitude, enabling higher reversibility and coulombic efficiency of Mg plating/stripping. |
| [36] | 1 M [Mg(DIPA)2] + 2 M [C2mim][AlCl4] + 2.5 mL anhydrous THF | THF | A 1:2 ratio shows the lowest overpotential and the highest CE. Overpotential decreased from 375 mV (1st cycle) to 225 mV (100th cycle). |
| [37] | [Mg(BH4)2]0.2[N07TFSI]0.8 and [Mg(BH4)2]0.3[N07TFSI]0.7 | Mg(BH4)2 | Displacement of TFSI− from Mg2+ coordination sphere. CE: 70.3% (1st cycle) → ~98% from 2nd cycle onward. |
| [38] | Mg(TFSA)2 + PP13TFSA | 18-crown-6 and Diglyme | Improved anti-oxidation capability up to 3.5 V vs. Mg2+/Mg. Thermal stability up to 350 °C in 1:1:5 ratio solutions. |
| [39] | DMI–AlCl3 (molar ratio 1.6) with 0.2 M MgCl2 | MgCl2 | Shifts equilibrium from [Al2Cl7]− → [AlCl4]−; generates [MgCl(DMI)4]+ as electroactive species; supports Mg co-deposition with Al, improving current density. |
3.2.2. Magnesium Electrodeposition
- Design of the electrochemical cell
| Reference | Cell Design | Anode | Cathode | Reference Electrode | Cell Chamber Separator | Temp. (°C) | Setup Benefits |
|---|---|---|---|---|---|---|---|
| [9] | Swagelok cell, glass fiber separator, glovebox | Mg metal | Stainless steel 316 | Mg metal (pseudo-reference) | Not reported | Ambient | Sealed cell, high reversibility, no passivation |
| [10] | Coin and flooded PEEK cells; symmetric Mg configuration | Mg metal | Mg metal/Pt (LSV) | Mg metal (pseudo-reference) | Whatman Grade GF/A Binder-Free Glass Microfiber Filter | 20–40 | Enables comparison of solvents; low overpotential in DME |
| [11] | BOLA cell (Teflon); Mg symmetric cells | Mg metal | Pt/WS2–PANI | Mg metal (pseudo-reference) | Whatman Grade GF/A Binder-Free Glass Microfiber Filter | 25 | Compatible with voltammetry and full cells; inert atmosphere (<0.5 ppm H2O/O2) |
| [12] | Three-electrode setup in a 25 mL round-bottom flask | Mg metal | Au | Mg metal (pseudo-reference) | No separator | 25 | Enables clean galvanostatic deposition on gold; smooth morphology |
| [8] | Coin cell (2032) with asymmetric configuration | Mg foil | Cu foil with PIL coating | Mg foil (pseudo-reference) | 53 μm glass microfiber spacer | 80–160 | Solid-state-like evaluation; enhanced thermal stability |
| [13] | DEMS-integrated cell without a membrane | Mg foil | GC/Au | Ag/AgCl | No separator | 25 | Coupled voltammetry and mass spectrometry; detailed decomposition tracking |
| [14] | 3-electrode cell in a round-bottom flask under argon | Mg foil | Cu | Ag (pseudo-reference) | No separator | 80 | Induced co-deposition with Co2+ and Nd3+; thiourea adjusts nucleation |
| [15] | Three-compartment Pyrex glass cell | Al metal | Cu/Au | Al metal | No separator | 25–70 | Enables alloy formation (Al5.15Mg3.15); independent Mg plating not observed |
| [16] | Three-electrode cell | Al metal | Cu | Al metal | No separator | 50 | Solvate species [MgCl(DMI)n]+ formed; dense alloy films |
| [17] | Three-electrode cell | Graphite | GC | Ag (pseudo-reference) | No separator | 80 | Ni–Mg alloy formation via induced reduction; 9.6% Mg reported |
| [18] | Three-electrode flask | Pt/Cu | Cu | Ag (pseudo-reference) | No separator | 80 | Co2+ induces Mg–Pr deposition; improved adhesion and homogeneity. |
| [19] | Open beaker setup | Pt | Mg–RE alloy (substrate) | None used | No separator | 60–90 | Zn plating onto Mg alloys; low H2 evolution in DES. |
| [20] | Three-electrode flask in glovebox | Pt | GC | Ag/AgCl | No separator | 25 | Mg–IL coordination studied spectroscopically; no Mg0 plating. |
| [21] | Spectroscopic setup | - | - | - | - | 20–90 | Ionic mobility and coordination evaluated; no metallic Mg deposition |
| [22] | Three-electrode cell in glovebox | Pt/C | Mg0.07V2O5, Mg–MnO2 on Al | Ag/AgNO3 | No separator | 25 | Mg2+ intercalation explored; no direct deposition of Mg0 |
| [23] | Three-electrode dry cell | Pt | GC | Scraped Mg foil | No separator | 25 | ~87% CE; dendrite-free Mg deposits; stable cycling over 280 cycles |
| [24] | Three-electrode cell | Mg foil | Pt/Au | Fc/Fc+ | No separator | 80 | Reversible plating from solvate IL; Cl− is critical to performance |
| [25] | Three-electrode cell | Mg foil | Al/Pt | Mg (pseudo-RE)/Fc/Fc+ | No separator | 25 | Grignard-based system; high Mg0 purity and crystallinity with IL |
| [26] | Three-electrode cell in glovebox | Mg metal | Pt/Cu | Mg RE (external) + Mg pseudo-RE | No separator | 30 | Enables coordination studies with G4; deposition verified via crystallography; ~95% CE |
| [27] | Three-electrode cell; dry N2 atmosphere | Graphite | Ag | Mg reference in EtMgBr/THF via frit | No separator | 23–25 | Direct Mg0 deposition from organic phase; avoids thermal dehydration; SEM/XRD confirmed |
| [28] | Three-electrode cell; dry N2 atmosphere | Graphite | Ag/Pt | Mg RE (external) | No separator | 23–25 | Saponified IL improves Mg extraction; high conductivity with MIBK; pure deposit confirmed. |
| [29] | Three-electrode cell in Ar | Mg metal | Ni/Pt/Ag | Mg metal (pseudo-reference) | No separator | 25 | Allyl-functionalized IL improves stability and morphology; near-100% CE with PhMgCl. |
| [30] | Three-electrode cell under dry N2 | Graphite | Ag | Mg RE external in EtMgBr/THF | No separator | 23–25 | Strong Mg2+ extraction (~83.99%); phase separation and conductivity enhanced by MIBK. |
| [31] | Two-electrode cell; dry box, ambient RH < 30% | Pt | TiO2 nanotubes on Ti foil | None | No separator | 50 | Pulsed electrolysis yields ~20 nm Mg nanofilm; uniform plating confirmed via HRTEM/XPS. |
| [32] | Three-electrode glass cell | Mg foil | W microelectrode | Mg wire | No separator, glass chamber | ~25 | Minimizes current density effects and highlights nucleation/overpotential. |
| [33] | Three-electrode BOLA-type cell (KelF, 0.25 cm3 volume) | Pt foil | Au(111) or Pt sheet (12 mm) | Mg wire | Two glass fiber separators (Whatman GF/B) | ~25 | Small volume, high stability single-crystal electrodes, reliable EIS/CV characterization. |
| [34] | Coin cell CR2016 and three-electrode Swagelok-type cells | Mg foil | Mo6S8 Chevrel phase | Mg wire | Glass fiber separator | Ambient and 55 | Elevated T° improves Mg deposition kinetics and cell stability. |
| [35] | Three-electrode Swagelok-type and coin-cell setups | Mg foil | Mo6S8 Chevrel phase | Mg wire | Glass fiber filters | Room temperature and 55 | Coin cells enable realistic cycling tests; elevated temperatures reduce viscosity, enhancing Mg kinetics. |
| [36] | T-type three-electrode cell | Mg disk | Mo, graphite, or SS316 | Mg strip | No separator | Ambient | Allowed evaluation of collector corrosion and overpotentials. |
| [37] | Three-electrode cell | Mg metal | V2O5 aerogel | Mg ribbon | Glass fiber | 20 | Prototype Mg/V2O5 cell demonstrated reversible cycling with high capacity. |
| [38] | Three-electrode cell | Mg plate | Pt plate | Ag wire | Porous glass separates the reference chamber | 80 | A three-electrode cell allowed evaluation of Mg plating reversibility and additive effects. |
| [39] | Three-electrode system | Al wire | Pt wire or Cu sheets | Al wire | No separator | ~30 | Pre-electroplating the electrolyte removes impurities, reducing overpotentials and enhancing current density for Al/Mg deposition. |
- Monitoring and data analysis
3.2.3. Raw Materials Used as Magnesium Source
- Chemical form, purity and required pretreatment
| Reference | Mg Source 1 | Purity | Mg Source 2 (If Any) | Purity | Purification and Drying |
|---|---|---|---|---|---|
| [9] | Mg(BH4)2 | Not specified; highly moisture-sensitive | - | - | Manipulated in glovebox; reacts with water as an in situ drying agent. |
| [10] | Mg(TFSI)2 | 99.5% | MgCl2 | 99.99% | Both dried at 110 °C for 24 h under vacuum; glovebox handling |
| [11] | Mg(TFSI)2 | 99.5% | Mg(BH4)2 | 95% | Mg(BH4)2 dried for one week in a glovebox with drying of air over a 4 Å molecular sieve. |
| [12] | Mg(BH4)2 | Not quantified | - | - | IL dried ≥ 17 h at 80 °C under vacuum; mixed under dry conditions |
| [8] | Mg(PSTFSI)2 (polymeric) | Not specified | MgCl2 | Unspecified | Gradual drying (RT → 160 °C); cell aged at 160 °C pre-polarization |
| [13] | Mg(TFSI)2 | 99.5% | Mg foil | Not specified | IL dried at 80 °C for 17 h; glovebox assembly (<25 ppm H2O) |
| [14] | MgCl2 | Not specified | - | - | Dried at 80 °C under argon before mixing into DES |
| [15] | MgCl2 | 99.9% | - | - | Mixed with acidic AlCl3–EMIC IL; used directly |
| [16] | MgCl2 | 99.9% | - | - | Used under inert gas; no purification reported |
| [17] | MgCl2 | 99.99% | - | - | Dried 10 h at 120 °C; glyme dehydrated over 4 Å sieve |
| [18] | MgCl2·6H2O | Analytical grade | - | - | Dehydrated 22 h at 160 °C under vacuum |
| [19] | Mg–RE alloys | Not specified | - | - | No precursor salt; Mg is the substrate |
| [20] | - | - | - | - | No Mg precursor used; coordination studied spectroscopically |
| [21] | MgCl2 (bound polymer) | Verified by TGA/FTIR | - | - | Spectroscopic drying; no electrodeposition |
| [22] | Mg(TFSI)2 | Not specified | - | - | Titrated to <50 ppm H2O; handled in glovebox |
| [23] | Mg(TFSI)2 | 99.5% | Mg(BH4)2 | 95% | Dried 48 h at 80 °C; glovebox manipulation |
| [24] | Mg(G3)2 [Tf2N]2 (solvate IL) | Stoichiometric | - | - | Dried under vacuum; TBACl was dried for 48 h at 110 °C |
| [25] | EtMgBr/THF | 1 M (commercial) | - | - | Used under an inert atmosphere; THF dried with sieves |
| [26] | Mg(TFSI)2 | High purity, battery grade | - | - | Dried ≥ 24 h at 80 °C; glymes purified beforehand |
| [27] | Mg2+ from brine | ICP-confirmed | MgCl2 (anhydrous) | High purity | Extractive phase dried with MgCl2 and a 4 Å sieve |
| [28] | Mg2+ from natural brine (bischofite) | ~76.87 g·L−1 | MgCl2 | Unspecified | Saponified organic phase; dried with sieves and MgCl2 |
| [29] | PhMgCl (Grignard reagent) | 2.0 M in THF | - | - | Used as received; handled under inert atmosphere in glovebox |
| [30] | EtMgBr/THF | 1 M or 0.095 M | - | - | THF dried using 4 Å molecular sieves; all components manipulated in a glovebox |
| [31] | Mg(CH3COO)2 (magnesium acetate) | Commercial grade | - | - | Dissolved in DMSO; dried in oven at 60 °C before use |
| [32] | Mg(BH4)2 | ≥95% | Mg foil | 99.9% | Electrolytes prepared in an Ar-filled glovebox (O2 and H2O < 1 ppm). Solids are dried under vacuum before use. IL used as received |
| [33] | Mg(TFSI)2 | 99.5% | Mg(BH4)2 | 95% | ILs dried with molecular sieves (3–4 Å) at 80 °C, vacuum (10−3 mbar, 20 h). Tetraglyme dried over 4 Å sieves for 1 week. Electrolytes prepared in glovebox (O2/H2O < 1 ppm). |
| [34] | Mg(HMDS)2–MgCl2 | Not specified | Mg metal foil and Mg wire | 99.9% | Electrolytes prepared in glovebox (O2, H2O < 0.1 ppm). DEME·TFSI dried under vacuum (80 °C, 48 h). THF is distilled over sodium/benzophenone before use. |
| [35] | Mg(CF3SO3)2 | Not specified | Mg metal foil | 99.9% | ILs dried under vacuum at 80 °C for 48 h. Ether solvents distilled over sodium/benzophenone. Electrolyte preparation inside glovebox (H2O, O2 < 0.1 ppm). |
| [36] | Mg(DIPA)2 | ≥99.9% | Mg disks and strips | >99.9% | Mg foils/disks polished with P800 emery paper and rinsed with dry THF. Electrolytes were stirred for 24 h in an Ar-filled glovebox. THF anhydrous (99.9%), all operations under Ar (O2/H2O < 0.1 ppm). |
| [37] | Mg(BH4)2 | 95% | Mg foil | 99.9% | ILs synthesized and extensively purified (anion exchange, filtration, drying under vacuum, glovebox storage). Mg(BH4)2 used as received. |
| [38] | Mg(TFSA)2 | High | Metallic Mg plate | High purity | All reagents used as received; preparation in Ar atmosphere (glovebox); electrochemical measurements under Ar. |
| [39] | MgCl2 | ≥ 99.99% | - | - | All work under Ar in glovebox (O2, H2O < 0.1 ppm); electrolyte pre-electroplated before experiments to eliminate impurities. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Coulombic efficiency |
| DES | Deep eutectic solvent |
| FTIR | Fourier-transform infrared spectroscopy |
| IL | Ionic liquid |
| ILs | Ionic liquids |
| PIL | Poly (ionic liquid) |
| SEM | Scanning electron microscopy |
| SR | Systematic Review |
| XRD | X-ray diffraction |
References
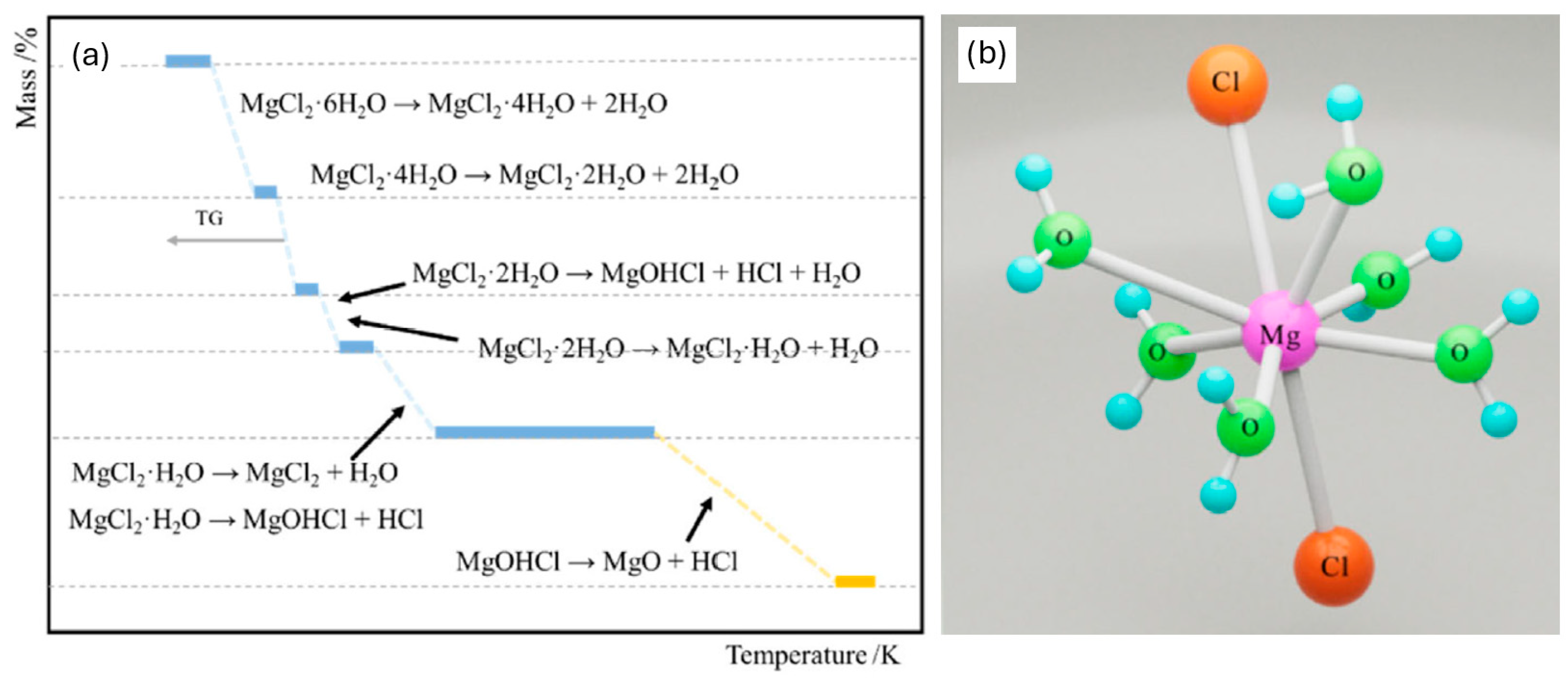
- Ming, H.; Zhang, X.; Huang, X.; Cheng, L.; Zhang, L. Advances in the preparation process and mechanism study of high-purity anhydrous magnesium chloride from magnesium chloride hexahydrate. Chin. J. Chem. Eng. 2025, 78, 1–23. [Google Scholar] [CrossRef]
- Löw, M.; Maroni, F.; Zaubitzer, S.; Dongmo, S.; Marinaro, M. Nucleation mechanisms of electrodeposited magnesium on metal substrates. Batter. Supercaps 2024, 7, e202400250. [Google Scholar] [CrossRef]
- Liu, G.S.; Song, X.F.; Yu, J.G. Density functional theoretical study on dehydration process of MgCl2·6H2O. Mater. Sci. Forum 2005, 488–489, 53–56. [Google Scholar] [CrossRef]
- Lodovico, L.; Martins, V.L.; Benedetti, T.M.; Torresi, R.M. Electrochemical behavior of iron and magnesium in ionic liquids. J. Braz. Chem. Soc. 2013, 24, 225–233. [Google Scholar] [CrossRef]
- Lu, H.M.; Jia, W.; Liao, C.F.; Ma, R.X.; Yuan, W.H. Pilot experiments of magnesia direct electrolysis in a five kA magnesium reduction cell. In Essential Readings in Magnesium Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 101–105. [Google Scholar]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of ionic liquids in electrochemistry—Recent advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Cheek, G.T.; O’Grady, W.E.; El Abedin, S.Z.; Moustafa, E.M.; Endres, F. Studies on the electrodeposition of magnesium in ionic liquids. J. Electrochem. Soc. 2008, 155, D91. [Google Scholar] [CrossRef]
- Park, B.; Ford, H.O.; Merrill, L.C.; Liu, J.; Murphy, L.P.; Schaefer, J.L. Dual cation exchanged poly(ionic liquid)s as magnesium conducting electrolytes. ACS Appl. Polym. Mater. 2019, 1, 2907–2913. [Google Scholar] [CrossRef]
- Tröger-Müller, S.; Liedel, C. A zwitterionic liquid electrolyte for magnesium batteries. Batter. Supercaps 2019, 2, 223–228. [Google Scholar] [CrossRef]
- Küpers, V.; Weintz, D.; Mück-Lichtenfeld, C.; Bieker, P.; Winter, M.; Kolek, M. Approaching electrochemical limits of MgxClyz+ complex-based electrolytes for Mg batteries by tailoring the solution structure. J. Electrochem. Soc. 2020, 167, 160505. [Google Scholar] [CrossRef]
- Elkhafif, O.W.; Hassan, H.K.; Hashmi, M.; Arya, N.; Anjass, M.; Jacob, T. Boosting Mg deposition and dissolution from ionic liquids—The role of different additives for applications in Mg-ion batteries. J. Energy Storage 2025, 105, 114636. [Google Scholar] [CrossRef]
- Watkins, T.; Kumar, A.; Buttry, D.A. Designer ionic liquids for reversible electrochemical deposition/dissolution of magnesium. J. Am. Chem. Soc. 2016, 138, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Alwast, D.; Schnaidt, J.; Hancock, K.; Yetis, G.; Behm, R.J. Effect of Li+ and Mg2+ on the electrochemical decomposition of the ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide and related electrolytes. ChemElectroChem 2019, 6, 3009–3019. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, B.; Li, M.; Du, C.; Zhu, Y.; Zhang, S.; Zhao, J. Effect of thiourea on electrodeposited Co–Mg–Nd alloy coating in deep eutectic solvents. Russ. J. Electrochem. 2021, 57, 51–61. [Google Scholar] [CrossRef]
- Rostom Ali, M.; Abbott, A.P.; Ryder, K.S. Electrodeposition of Al-Mg alloys from acidic AlCl3–EMIC–MgCl2 room temperature ionic liquids. J. Electrochem. 2015, 21, 172–180. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Liu, F.; Shi, Z.; Zhang, B.; Wang, X. Electrodeposition of aluminum–magnesium alloys from an aluminum-containing solvate ionic liquid at room temperature. Electrochem. Commun. 2021, 133, 107160. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Hua, Y.; Zhang, Q. Electrodeposition of Ni-Mg alloys from 1-butyl-3-methylimidazolium chloride/glycerin eutectic-based ionic liquid. J. Solid State Electrochem. 2016, 20, 793–800. [Google Scholar] [CrossRef]
- Li, M.; Chen, B.Q.; Xiong, T.T.; Gao, L.X.; Du, C.; Zhu, Y.N.; Zhang, S.M. Electrodeposition of Pr-Mg-Co ternary alloy films from the choline chloride-Urea ionic liquids and their corrosion properties. J. Dispers. Sci. Technol. 2020, 41, 941–947. [Google Scholar] [CrossRef]
- Bakkar, A. Examples of the superiority of ionic liquids and deep eutectic solvents over aqueous solutions in electrodeposition processes. ChemEngineering 2025, 9, 16. [Google Scholar] [CrossRef]
- Lethesh, K.C.; Evjen, S.; Raj, J.J.; Roux, D.C.D.; Venkatraman, V.; Jayasayee, K.; Fiksdahl, A. Hydroxyl functionalized pyridinium ionic liquids: Experimental and theoretical study on physicochemical and electrochemical properties. Front. Chem. 2019, 7, 625. [Google Scholar] [CrossRef]
- Bertasi, F.; Vezzù, K.; Nawn, G.; Pagot, G.; Di Noto, V. Interplay between structure and conductivity in 1-ethyl-3-methylimidazolium tetrafluoroborate/(δ-MgCl2)f electrolytes for magnesium batteries. Electrochim. Acta 2016, 219, 152–162. [Google Scholar] [CrossRef]
- Huie, M.M.; Cama, C.A.; Smith, P.F.; Yin, J.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Ionic liquid hybrids: Progress toward non-corrosive electrolytes with high-voltage oxidation stability for magnesium-ion based batteries. Electrochim. Acta 2016, 219, 267–276. [Google Scholar] [CrossRef]
- Ma, Z.; Forsyth, M.; MacFarlane, D.R.; Kar, M. Ionic liquid/tetraglyme hybrid Mg[TFSI]2 electrolytes for rechargeable Mg batteries. Green Energy Environ. 2019, 4, 146–153. [Google Scholar] [CrossRef]
- Geysens, P.; Fransaer, J.; Binnemans, K. Reversible electrodeposition and stripping of magnesium from solvate ionic liquid–tetrabutylammonium chloride mixtures. RSC Adv. 2020, 10, 42021–42029. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, S.; Liu, Y.; Horne, M.; Bond, A.M.; Zhang, J. Room temperature electrodeposition of metallic magnesium from ethylmagnesium bromide in tetrahydrofuran and ionic liquid mixtures. J. Electrochem. Soc. 2016, 163, H3043–H3051. [Google Scholar] [CrossRef]
- Kitada, A.; Kang, Y.; Matsumoto, K.; Fukami, K.; Hagiwara, R.; Murase, K. Room temperature magnesium electrodeposition from glyme-coordinated ammonium amide electrolytes. J. Electrochem. Soc. 2015, 162, D389–D396. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Wang, W.; Liu, C.; Deng, T.; Yu, X. Separation and production of metallic magnesium from salt lake brine by extraction-electrodeposition technology. Sep. Purif. Technol. 2025, 357, 130062. [Google Scholar] [CrossRef]
- Yu, X.; Cui, J.; Liu, C.; Yuan, F.; Guo, Y.; Deng, T. Separation of magnesium from high Mg/Li ratio brine by extraction with an organic system containing ionic liquid. Chem. Eng. Sci. 2021, 229, 116019. [Google Scholar] [CrossRef]
- Lee, B.; Cho, J.-H.; Seo, H.R.; Na, S.B.; Kim, J.H.; Cho, B.W.; Yim, T.; Oh, S.H. Strategic combination of Grignard reagents and allyl-functionalized ionic liquids as an advanced electrolyte for rechargeable magnesium batteries. J. Mater. Chem. A 2018, 6, 3126–3133. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Wan, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of functional ionic liquids with high extraction rate and electroconductivity for lithium-magnesium separation and metallic magnesium production from salt lake brine. Chem. Eng. J. 2023, 452, 139610. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhao, S.; Zheng, L.; Huang, Y.; Hong, Z. Uniform nanoplating of metallic magnesium film on titanium dioxide nanotubes as a skeleton for reversible Na metal anode. Int. J. Miner. Metall. Mater. 2023, 30, 1868–1877. [Google Scholar] [CrossRef]
- Weber, I.; Ingenmey, J.; Schnaidt, J.; Kirchner, B.; Behm, R.J. Influence of complexing additives on the reversible deposition/dissolution of magnesium in an ionic liquid. ChemElectroChem 2021, 8, 390–402. [Google Scholar] [CrossRef]
- Elkhafif, O.W.; Hassan, H.K.; Ceblin, M.U.; Farkas, A.; Jacob, T. Influence of residual water traces on the electrochemical performance of hydrophobic ionic liquids for magnesium-containing electrolytes. ChemSusChem 2023, 16, e202300421. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lau, K.C.; Vaughey, J.T.; Zhang, L.; Zhang, Z.; Liao, C. Ionic liquid as an effective additive for rechargeable magnesium batteries. J. Electrochem. Soc. 2017, 164, A902–A906. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, K.; Wu, F.; Bai, Y.; Wu, C. Ionic liquid-based electrolytes for aluminum/magnesium/sodium-ion batteries. Energy Mater. Adv. 2021, 2021, 1–29. [Google Scholar] [CrossRef]
- Chellappan, L.K.; Kvello, J.; Tolchard, J.R.; Dahl, P.I.; Hanetho, S.M.; Berthelot, R.; Fiksdahl, A.; Jayasayee, K. Non-nucleophilic electrolyte based on ionic liquid and magnesium bis(diisopropyl)amide for rechargeable magnesium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 9585–9593. [Google Scholar] [CrossRef]
- Gao, X.; Mariani, A.; Jeong, S.; Liu, X.; Dou, X.; Ding, M.; Moretti, A.; Passerini, S. Prototype rechargeable magnesium batteries using ionic liquid electrolytes. J. Power Sources 2019, 423, 52–59. [Google Scholar] [CrossRef]
- Sagane, F.; Ogi, K.; Konno, A.; Kanamura, K. The effect of cyclic ethers on Mg plating/stripping reaction in ionic liquid electrolytes. J. Electrochem. Soc. 2019, 166, A5054–A5058. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, L.; Liu, A.; Shi, Z. The influence of the molar ratio of AlCl3 to DMI and the electrochemical behavior of aluminum and magnesium in the 1,3-dimethyl-2-imidazolidinone-AlCl3-MgCl2 system. J. Alloys Compd. 2025, 1032, 181170. [Google Scholar] [CrossRef]
| Database | Search Algorithm |
|---|---|
| WoS | electrodeposition OR electroplating OR “electrochemical deposition” OR electrodepositing OR “cathodic deposition” |
| AND | |
| magnesium OR mg AND Ionic liquids OR ILs OR “deep eutectic solvents” | |
| Scopus | electrodeposition OR electroplating OR “electrochemical deposition” OR electrodepositing OR “cathodic deposition” AND magnesium OR mg AND Ionic liquids OR ILs OR “deep eutectic solvents” |
| Dimension | Inclusion Criteria | Exclusion Criteria | Justification |
|---|---|---|---|
| Magnesium production technology | Electrodeposition OR electroplating OR “electrochemical deposition” OR electrodepositing OR “cathodic deposition”: to identify studies that use electrodeposition as a metal recovery method. | Metal production technologies other than electrodeposition. | Focuses the review on electrochemical methods relevant to the research objective. |
| Target metal | Magnesium OR Mg: to ensure magnesium is the metal of interest. | Studies focused on metals other than magnesium. | Ensures thematic alignment with the research question. |
| Electrolyte used | Ionic liquids OR ILs OR “deep eutectic solvents”: to identify the use of ionic liquids as electrolytes in magnesium electrodeposition. | Electrolytes other than ionic liquids. | Targets the specific class of electrolytes under investigation. |
| Accessibility | All accessible publications are included. | No exclusion criterion. | Maximizes the comprehensiveness of the review. |
| Language | Publications written in English are limited due to the limited number of studies in other languages. | Publications in languages other than English. | Ensures interpretability and consistency in data extraction. |
| Resource type | Journal articles and reviews. | All resource types other than journal articles and reviews. | Prioritizes peer-reviewed scientific literature. |
| Time frame | Publications from 2015 to 2025, to prioritize recent research. | Publications before 2015. | Focuses on the most current developments in the field. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arancibia-Zúñiga, A.; Carlesi, C. Electrodeposition of Metallic Magnesium in Ionic Liquids: A Systematic Review. Minerals 2025, 15, 1021. https://doi.org/10.3390/min15101021
Arancibia-Zúñiga A, Carlesi C. Electrodeposition of Metallic Magnesium in Ionic Liquids: A Systematic Review. Minerals. 2025; 15(10):1021. https://doi.org/10.3390/min15101021
Chicago/Turabian StyleArancibia-Zúñiga, Agustín, and Carlos Carlesi. 2025. "Electrodeposition of Metallic Magnesium in Ionic Liquids: A Systematic Review" Minerals 15, no. 10: 1021. https://doi.org/10.3390/min15101021
APA StyleArancibia-Zúñiga, A., & Carlesi, C. (2025). Electrodeposition of Metallic Magnesium in Ionic Liquids: A Systematic Review. Minerals, 15(10), 1021. https://doi.org/10.3390/min15101021








