Study on Chalcopyrite Dissolution Mechanism and Bioleaching Community Behavior Based on Pulp Concentration Gradient at 6 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Mineral
2.2. Bioleaching Experiments
2.3. Characterization of Ore Residues
2.4. Electrochemical Experiments
2.5. Sequencing of Prokaryotic 16S rRNA Gene Sequences
3. Results and Discussion
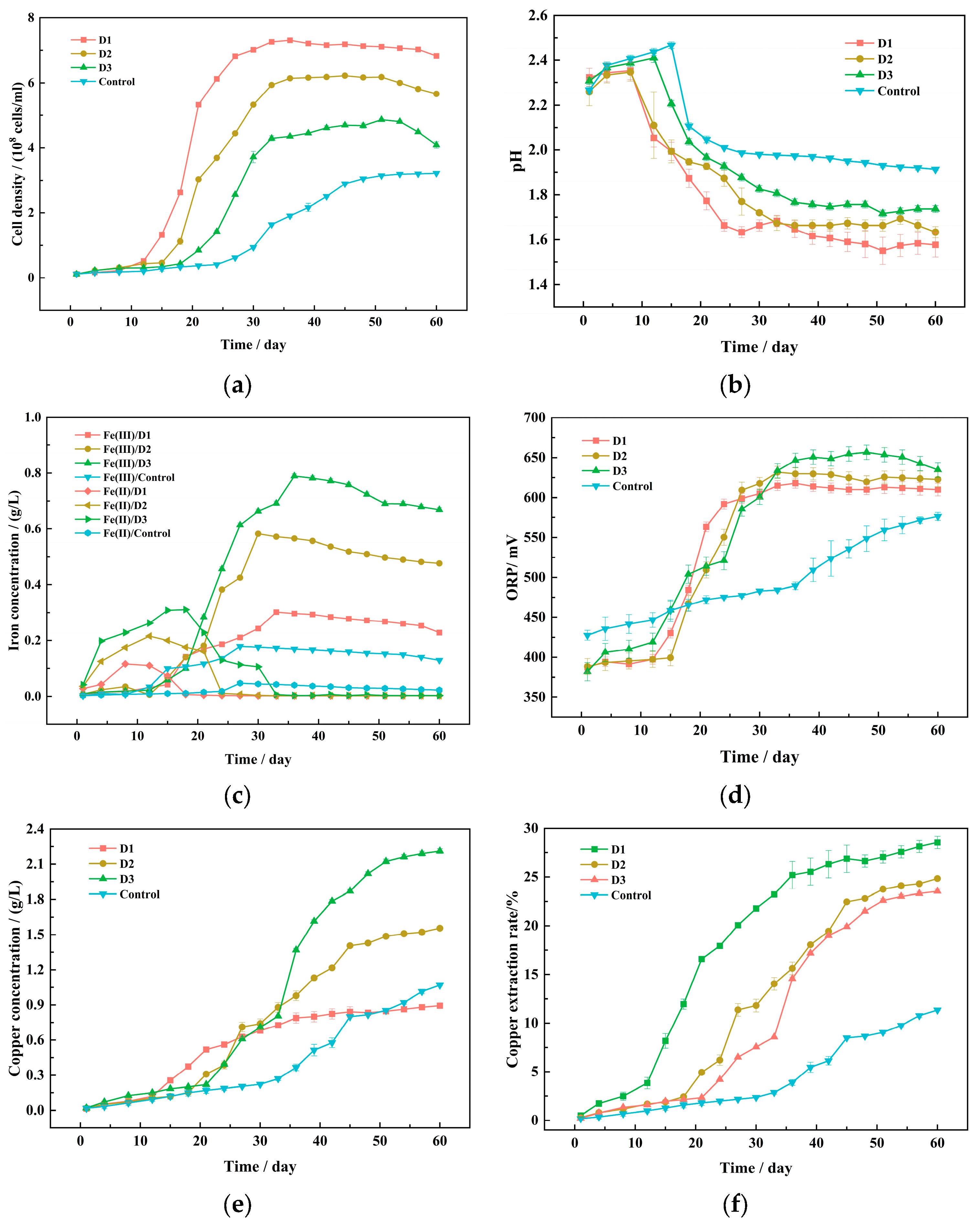
3.1. Bioleaching Experiments
3.2. Surface Morphology of Chalcopyrite
3.3. X-ray Diffraction Analysis
3.4. Electrochemical Analyses
3.4.1. Cyclic Voltammograms
3.4.2. Potentiodynamic Polarization
3.5. Microbial Community Analysis
3.5.1. Alpha-Diversity
3.5.2. Microbial Community Structure
3.5.3. Comparison of Microbial Community Structures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Xue, Z.; Feng, Y.; Li, H.; Zhu, Z.; Xu, C.; Ju, J.; Yang, Y. A comprehensive review on progresses of coal and minerals bioflotation in presence of microorganisms. J. Environ. Chem. Eng. 2023, 11, 111182. [Google Scholar] [CrossRef]
- Ahonen, L.; Tuovinen, O.H. Temperature effects on bacterial leaching of sulfide minerals in shake flask experiments. Appl. Environ. Microbiol. 1991, 57, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Elberling, B.; Schippers, A.; Sand, W. Bacterial and chemical oxidation of pyritic mine tailings at low temperatures. J. Contam. Hydrol. 2000, 41, 225–238. [Google Scholar] [CrossRef]
- Escobar, B.; Buccicardi, S.; Morales, G.; Wiertz, J. Biooxidation of ferrous iron and sulphide at low temperatures: Implications on acid mine drainage and bioleaching of sulphide minerals. Hydrometallurgy 2010, 104, 454–458. [Google Scholar] [CrossRef]
- Langdahl, B.R.; Ingvorsen, K. Temperature characteristics of bacterial iron solubilisation and 14C assimilation in naturally exposed sulfide ore material at Citronen Fjord, North Greenland (83 N). FEMS Microbiol. Ecol. 1997, 23, 275–283. [Google Scholar] [CrossRef]
- Dopson, M.; Halinen, A.K.; Rahunen, N.; Özkaya, B.; Sahinkaya, E.; Kaksonen, A.H.; Lindström, E.B.; Puhakka, J.A. Mineral and iron oxidation at low temperatures by pure and mixed cultures of acidophilic microorganisms. Biotechnol. Bioeng. 2007, 97, 1205–1215. [Google Scholar] [CrossRef]
- Peng, T.; Chen, L.; Wang, J.; Miao, J.; Shen, L.; Yu, R.; Gu, G.; Qiu, G.; Zeng, W. Dissolution and passivation of chalcopyrite during bioleaching by Acidithiobacillus ferrivorans at low temperature. Minerals 2019, 9, 332. [Google Scholar] [CrossRef]
- Li, L.; King, A.; Davis, K.; Yu, B. Electrochemical Kinetics Study of Ultrasound-Assisted Chalcopyrite Oxidation. J. Sustain. Metall. 2023, 9, 678–687. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, B.; Liao, R.; Hong, M.; Yu, S.; Liu, S.; Wang, J.; Qiu, G. Combined effect and mechanism of visible light and Ag+ on chalcopyrite bioleaching. Miner. Eng. 2022, 175, 107283. [Google Scholar] [CrossRef]
- Pan, H.D.; Yang, H.Y.; Tong, L.L.; Zhong, C.B.; Zhao, Y.S. Control method of chalcopyrite passivation in bioleaching. Trans. Nonferrous Met. Soc. China 2012, 22, 2255–2260. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Bioleaching of copper sulphide concentrate using extreme thermophilic bacteria. Miner. Eng. 1999, 12, 893–904. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A.; van Rooyen, J.V. Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture. Int. J. Miner. Process. 2001, 62, 243–255. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Liu, Y.; Jia, H.; Zhou, J.; Wei, P.; Zhou, H. Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: Process optimization and kinetics. Hydrometallurgy 2020, 191, 105227. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Zhou, H.; Chen, M. Electrochemical behaviour of massive chalcopyrite electrodes bioleached by moderately thermophilic microorganisms at 48 °C. Hydrometallurgy 2011, 105, 259–263. [Google Scholar] [CrossRef]
- Zhao, H.B.; Hu, M.H.; Li, Y.N.; Zhu, S.; Qin, W.Q.; Qiu, G.Z.; Wang, J. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium. Trans. Nonferrous Met. Soc. China 2015, 25, 303–313. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Chen, M. Investigation of Cu-S intermediate species during electrochemical dissolution and bioleaching of chalcopyrite concentrate. Hydrometallurgy 2013, 134, 158–165. [Google Scholar] [CrossRef]
- Peng, T.; Liao, W.; Wang, J.; Miao, J.; Peng, Y.; Gu, G.; Wu, X.; Qiu, G.; Zeng, W. Bioleaching and Electrochemical Behavior of Chalcopyrite by a Mixed Culture at Low Temperature. Front. Microbiol. 2021, 12, 663757. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H.; Deveci, H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 2007, 20, 310–318. [Google Scholar] [CrossRef]
- Gu, G.H.; Hu, K.T.; Li, S.K. Bioleaching and electrochemical properties of chalcopyrite by pure and mixed culture of Leptospirillum ferriphilum and Acidthiobacillus thiooxidans. J. Cent. South Univ. 2013, 20, 178–183. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Qin, W.Q.; Wang, J.; Zhen, S.J.; Yang, C.R.; Zhang, J.W.; Nai, S.-s.; Qiu, G.-z. Bioleaching of chalcopyrite by pure and mixed culture. Trans. Nonferrous Met. Soc. China 2008, 18, 1491–1496. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyov, M.I. Two-step biohydrometallurgical technology of copper-zinc concentrate processing as an opportunity to reduce negative impacts on the environment. J. Environ. Manag. 2018, 226, 270–277. [Google Scholar] [CrossRef]
- Lukhele, T.; Selvarajan, R.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Diversity and functional profile of bacterial communities at Lancaster acid mine drainage dam, South Africa as revealed by 16S rRNA gene high-throughput sequencing analysis. Extremophiles 2019, 23, 719–734. [Google Scholar] [CrossRef]
- Romo, E.; Weinacker, D.F.; Zepeda, A.B.; Figueroa, C.A.; Chavez-Crooker, P.; Farias, J.G. Bacterial consortium for copper extraction from sulphide ore consisting mainly of chalcopyrite. Braz. J. Microbiol. 2013, 44, 523–528. [Google Scholar] [CrossRef]
- Latorre, M.; Paz Cortes, M.; Travisany, D.; Di Genova, A.; Budinich, M.; Reyes-Jara, A.; Hoedar, C.; Gonzalez, M.; Parada, P.; Bobadilla-Fazzini, R.A.; et al. The bioleaching potential of a bacterial consortium. Bioresour. Technol. 2016, 218, 659–666. [Google Scholar] [CrossRef]
- Rios, D.; Bellenberg, S.; Christel, S.; Lindblom, P.; Giroux, T.; Dopson, M. Potential of single and designed mixed cultures to enhance the bioleaching of chalcopyrite by oxidation-reduction potential control. Hydrometallurgy 2024, 224, 106245. [Google Scholar] [CrossRef]
- Halinen, A.K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore: Part II. Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 2009, 98, 101–107. [Google Scholar] [CrossRef]
- Zepeda, V.; Galleguillos, F.; Castillo, D.; Lastra, M.; Demergasso, C. Bacterial activity at low temperature in cultures derived from a low-grade copper sulphide bioleaching heap at the Escondida Mine, Chile. Adv. Mater. Res. 2007, 20, 543–546. [Google Scholar] [CrossRef]
- Liljeqvist, M.; Rzhepishevska, O.I.; Dopson, M. Gene identification and substrate regulation provide insights into sulfur accumulation during bioleaching with the psychrotolerant acidophile Acidithiobacillus ferrivorans. Appl. Environ. Microbiol. 2013, 79, 951–957. [Google Scholar] [CrossRef]
- Johnson, D.B.; Rolfe, S.; Hallberg, K.B.; Iversen, E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ. Microbiol. 2001, 3, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ghahremaninezhad, A.; Dixon, D.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Shen, L.; Li, J.; Yu, R.; Liu, Y.; Qiu, G.; Zeng, W. Whole genome sequencing and comparative genomics analyses of Pandoraea sp. XY-2, a new species capable of biodegrade tetracycline. Front. Microbiol. 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, Y.; Dong, W.; Liang, Y.; Fan, F.; Zhang, X.; Zhang, X.; Niu, J.; Ma, L.; She, S. The complicated substrates enhance the microbial diversity and zinc leaching efficiency in sphalerite bioleaching system. Appl. Microbiol. Biotechnol. 2015, 99, 10311–10322. [Google Scholar] [CrossRef]
- Rinke, C.; Lee, J.; Nath, N.; Goudeau, D.; Thompson, B.; Poulton, N.; Dmitrieff, E.; Malmstrom, R.; Stepanauskas, R.; Woyke, T. Obtaining genomes from uncultivated environmental microorganisms using FACS-based single-cell genomics. Nat. Protoc. 2014, 9, 1038–1048. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.; Qiu, G.; Chen, X.; Zhou, H. A Moderately Thermophilic Mixed Microbial Culture for Bioleaching of Chalcopyrite Concentrate at High Pulp Density. Appl. Environ. Microbiol. 2014, 80, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Borja, D.; You, J.; Hong, G.; Jung, H.; Kim, H. Chalcopyrite Bioleaching Using Adapted Mesophilic Microorganisms: Effects of Temperature, Pulp Density, and Initial Ferrous Concentrations. Mater. Trans. 2018, 59, 1860–1866. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef]
- Kocaman, A.T.; Cemek, M.; Edwards, K.J. Kinetics of pyrite, pyrrhotite, and chalcopyrite dissolution by Acidithiobacillus ferrooxidans. Can. J. Microbiol. 2016, 62, 629–642. [Google Scholar] [CrossRef]
- Zhang, R.; Bellenberg, S.; Castro, L.; Neu, T.R.; Sand, W.; Vera, M. Colonization and biofilm formation of the extremely acidophilic archaeon Ferroplasma acidiphilum. Hydrometallurgy 2014, 150, 245–252. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Chen, M. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles. Miner. Eng. 2013, 48, 31–35. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, J.L.; Yang, Y.; Nie, Z.Y.; Zheng, L.; Ma, C.Y.; Zhang, R.Y.; Peng, A.A.; Tang, L.; Qiu, G.Z. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite. Bioresour. Technol. 2011, 102, 3877–3882. [Google Scholar] [CrossRef]
- Nicol, M.J. The electrochemistry of chalcopyrite in alkaline solutions. Hydrometallurgy 2019, 187, 134–140. [Google Scholar] [CrossRef]
- Gu, G.; Hu, K.; Zhang, X.; Xiong, X.; Yang, H. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum. Electrochim. Acta 2013, 103, 50–57. [Google Scholar] [CrossRef]
- Biegler, T.; Horne, M. The electrochemistry of surface oxidation of chalcopyrite. J. Electrochem. Soc. 1985, 132, 1363. [Google Scholar] [CrossRef]
- Arce, E.A.; González, I. A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution. Int. J. Miner. Process. 2002, 67, 17–28. [Google Scholar] [CrossRef]
- Velásquez, P.; Leinen, D.; Pascual, J.; Ramos-Barrado, J.R.; Cordova, R.; Gómez, H.; Schrebler, R. XPS, SEM, EDX and EIS study of an electrochemically modified electrode surface of natural chalcocite (Cu2S). J. Electroanal. Chem. 2001, 510, 20–28. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, J.; Li, Y.; Liao, R.; Wang, X.; Qiu, X.; Xiong, Y.; Qin, W.; Qiu, G. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite. Miner. Eng. 2017, 109, 153–161. [Google Scholar] [CrossRef]
- O’Connor, G.; Lepkova, K.; Eksteen, J.; Oraby, E. Electrochemical behaviour of copper in alkaline glycine solutions. Hydrometallurgy 2018, 181, 221–229. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Luo, X.; Teng, T.; Jiang, C.; Drewniak, L.; Yang, Z.; Yin, H. An integrated insight into bioleaching performance of chalcopyrite mediated by microbial factors: Functional types and biodiversity. Bioresour. Technol. 2021, 319, 124219. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, W.; Wan, M.; Zhang, Y.; Zou, L.; Huang, J.; Qiu, G.; Liu, X. Diversity of bacterial communities in acid mine drainage from the Shen-bu copper mine, Gansu province, China. Electron. J. Biotechnol. 2008, 11, 1–12. [Google Scholar] [CrossRef][Green Version]
- Tavakoli, H.Z.; Abdollahy, M.; Ahmadi, S.J.; Darban, A.K. Kinetics of uranium bioleaching in stirred and column reactors. Miner. Eng. 2017, 111, 36–46. [Google Scholar] [CrossRef]
- Wang, X.; Liao, R.; Zhao, H.; Hong, M.; Huang, X.; Peng, H.; Wen, W.; Qin, W.; Qiu, G.; Huang, C.; et al. Synergetic effect of pyrite on strengthening bornite bioleaching by Leptospirillum ferriphilum. Hydrometallurgy 2018, 176, 9–16. [Google Scholar] [CrossRef]
- Huo, X.; Liu, J.; Hong, X.; Bai, H.; Chen, Z.; Che, J.; Yang, H.; Tong, Y.; Feng, S. Enhancing column bioleaching of chalcocite by isolated iron metabolism partners Leptospirillum ferriphilum/Acidiphilium sp. coupling with systematically utilizing cellulosic waste. Bioresour. Technol. 2024, 394, 130193. [Google Scholar] [CrossRef]
- Ccorahua-Santo, R.; Eca, A.; Abanto, M.; Guerra, G.; Ramirez, P. Physiological and comparative genomic analysis of Acidithiobacillus ferrivorans PQ33 provides psychrotolerant fitness evidence for oxidation at low temperature. Res. Microbiol. 2017, 168, 482–492. [Google Scholar] [CrossRef]
- Bellenberg, S.; Salas, B.; Ganji, S.; Jorquera-Roman, C.; Valenzuela, M.L.; Buetti-Dinh, A.; Unelius, C.R.; Dopson, M.; Vera, M. Diffusible signal factor signaling controls bioleaching activity and niche protection in the acidophilic, mineral-oxidizing leptospirilli. Sci. Rep. 2021, 11, 16275. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Ballester, A.; Blázquez, M.L.; González, F.; Muñoz, J.A. Study of bacterial attachment during the bioleaching of pyrite, chalcopyrite, and sphalerite. Geomicrobiol. J. 2003, 20, 131–141. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Hou, H.; Chen, G.; Liu, H.; Liu, X.; Shen, L. Effect of Introduction of Exogenous Strain Acidithiobacillus thiooxidans A01 on Structure and Function of Adsorbed and Planktonic Microbial Consortia during Bioleaching of Low-Grade Copper Sulfide. Front. Microbiol. 2020, 10, 3034. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.; Acevedo, F. Are bioleaching rates determined by the available particle surface area concentration? World J. Microbiol. Biotechnol. 2009, 25, 101–106. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Wu, J.; Xiao, Y.; Tao, J.; Liu, X. Effective bioleaching of low-grade copper ores: Insights from microbial cross. Bioresour. Technol. 2020, 308, 123273. [Google Scholar] [CrossRef] [PubMed]







| System | Name | Pulp Density | Microbial Consortium |
|---|---|---|---|
| Experimental groups | D1 | 1% | original community |
| D2 | 2% | D1 | |
| D3 | 3% | D2 | |
| Control group | Control | 3% | original community |
| System | Ecorr/mV | Icorr/((uA/cm2) | ba/(mV/decade) | bc/(mV/decade) |
|---|---|---|---|---|
| Before bioleaching | 260 | 3.79 | 213.6 | 234.6 |
| D1 | 184 | 9.83 | 211.9 | 233.4 |
| D2 | 196.9 | 10.58 | 210.8 | 208.9 |
| D3 | 202 | 11.01 | 199.1 | 205 |
| Control | 218.8 | 12.99 | 179.3 | 150.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Man, M.; Zeng, W. Study on Chalcopyrite Dissolution Mechanism and Bioleaching Community Behavior Based on Pulp Concentration Gradient at 6 °C. Minerals 2024, 14, 698. https://doi.org/10.3390/min14070698
Jiang X, Man M, Zeng W. Study on Chalcopyrite Dissolution Mechanism and Bioleaching Community Behavior Based on Pulp Concentration Gradient at 6 °C. Minerals. 2024; 14(7):698. https://doi.org/10.3390/min14070698
Chicago/Turabian StyleJiang, Xiao, Meilian Man, and Weimin Zeng. 2024. "Study on Chalcopyrite Dissolution Mechanism and Bioleaching Community Behavior Based on Pulp Concentration Gradient at 6 °C" Minerals 14, no. 7: 698. https://doi.org/10.3390/min14070698
APA StyleJiang, X., Man, M., & Zeng, W. (2024). Study on Chalcopyrite Dissolution Mechanism and Bioleaching Community Behavior Based on Pulp Concentration Gradient at 6 °C. Minerals, 14(7), 698. https://doi.org/10.3390/min14070698





