Removal of Low Concentrations of Er(III) from Water Using Heptadecyl-1,1-bisphosphonic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Removal Experiments
3. Results
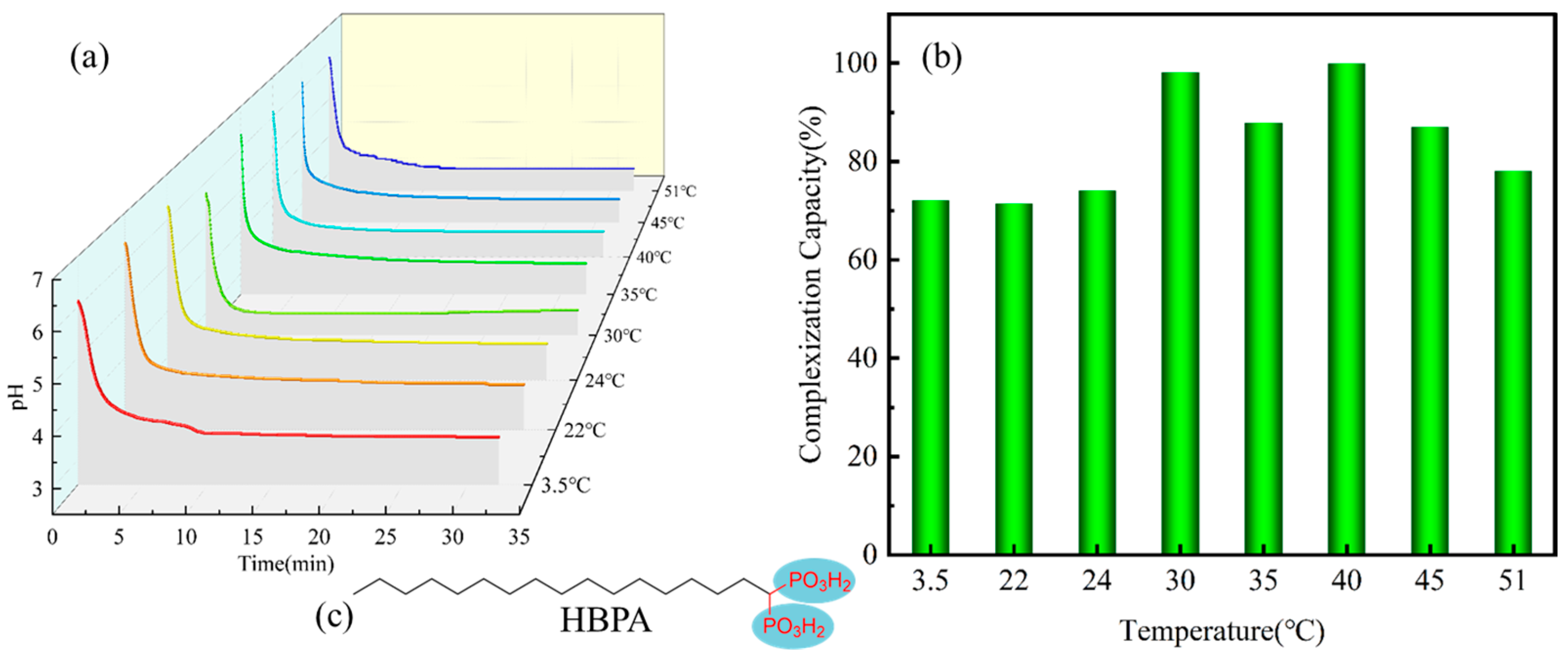
3.1. Effect of Temperature on Er(III) Removal and Complexation Capacities
| Element | Ca2+ | Mg2+ | Sr2+ | Ba2+ | SO42− | Er3+ |
|---|---|---|---|---|---|---|
| Initial con. (mg/L) | 39.48 | 27.46 | 0.46 | 0.03 | 129.06 | 4.76 |
| Final con. (mg/L) | 11.78 | 5.62 | 0.19 | 0.005 | 128.56 | 0.008 |
| Expulsion (%) | 71.6% | 80.5% | 60.9% | 83.3% | - | 99.8% |
| Temperature (°C) | Final Con. (mg/L) a | Complexation Capacity (mg/g) |
|---|---|---|
| 3.5 | 12.311 | 265.3 (72.1%) |
| 22 | 12.632 | 262.6 (71.4%) |
| 24 | 11.418 | 272.7 (74.1%) |
| 30 | 0.847 | 360.8 (98.1%) |
| 35 | 5.383 | 323.0 (87.8%) |
| 40 | 0.044 | 367.5 (99.9%) |
| 45 | 5.731 | 320.1 (87.0%) |
| 51 | 9.663 | 287.4 (78.1%) |
3.2. Effect of the HBPA Dosage on Er(III) Removal
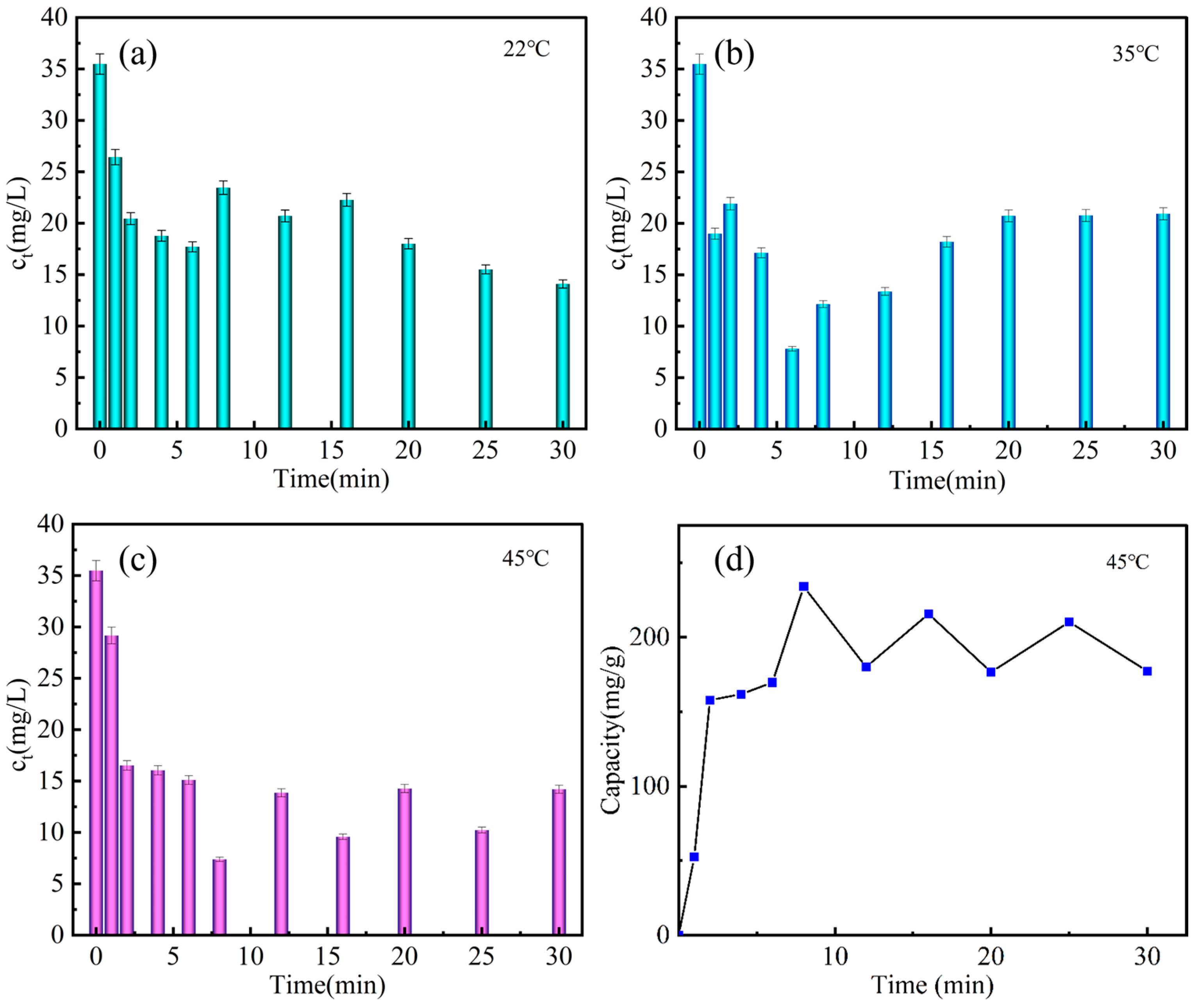
3.3. Er(III) Removal from Aqueous Solution over Time at Different Temperatures
3.4. Removal of Er(III) from Tap Water
3.5. Mechanism of Er(III) Removal from Aqueous Solution by HBPA
4. Discussion
5. Conclusions
- (1)
- Over a wide pH range (3–8) and at different temperatures, HBPA is an effective Er(III) chelator, and, especially at neutral to alkaline conditions, Er(III) ions could be removed efficiently using HBPA, which is expected to be further developed in the future, as a simple method for the removal of low concentrations of Er(III) ions in aqueous solutions on a large scale.
- (2)
- Low concentrations of Er(III) ions in aqueous solutions were treated by the addition of HBPA, followed by the addition of NaHCO3 (adjusting the pH to 6–8) and activated carbon. Er(III) removal is typically achieved by filtration after ~30 min. Such a method of ion-exchange and complexation-assisted filtration using HBPA may be a very useful tool for the separation and removal of low concentrations of REEs from mineral-processing wastewater to drinking water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Du, Y.; Zhang, H. A brief summary of research progress on the application of rare earth materials in heterogeneous catalysis. Acta Chim. Sin. 2020, 78, 625–633. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Z.; Shi, X.; Du, Y. In-depth study on the structures and properties of rare-earth-containing perovskite materials. Nanoscale 2021, 13, 13976–13994. [Google Scholar] [CrossRef]
- Kιrkgecit, R.; Torun, H.; Dokan, F.; Oztürk, E. Optical and electrical conductivity properties of rare earth elements (Sm, Y, La, Er) co-doped CeO2. J. Rare Earth 2022, 40, 1619–1627. [Google Scholar] [CrossRef]
- Zhan, W.; Guo, Y.; Gong, X.; Guo, Y.; Wang, Y.; Lu, G. Current status and perspectives of rare earth catalytic materials and catalysis. Chin. J. Catal. 2014, 35, 1238–1250. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jing, H.; Geng, L.; Qiu, S.; Zou, H.; Liang, M.; Deng, D. Research progress of rare earth composite shielding materials. J. Rare Earth 2023, 41, 32–41. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H. Special issue: Rare earth luminescent materials. Light Sci. Appl. 2022, 11, 260. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhen, Z. Research advances of rare earth catalysts for catalytic purification of vehicle exhausts—Commemorating the 100th anniversary of the birth of Academician Guangxian Xu. J. Rare Earth 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Hu, P.; Sun, S.; Shi, L.; Sun, L. Facile synthesis of Er3+/Tm3+ co-doped magnetic/luminescent nanosystems for possible bioimaging and therapy applications. J. Rare Earth 2020, 40, 11–19. [Google Scholar] [CrossRef]
- Liang, T.; Li, K.; Wang, L. State of rare earth elements in different environmental components in mining areas of China. Environ. Monit. Assess. 2014, 186, 1499–1513. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Donadio, C.; Ferrara, L.; Stanislao, C.; Toscanesi, M.; Arienzo, M. Levels of pollution of rare earth elements in the surface sediments from the Gulf of Pozzuoli (Campania, Italy). Mar. Pollut. Bull. 2018, 136, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, M.; Liu, C.; Yuan, M.; Chen, X.; Huot, H.; Zhao, C.; Tang, Y.; Morel, J.; Qiu, R. Water, sediment and agricultural soil contamination from an ion-adsorption rare earth mining area. Chemosphere 2019, 216, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rim, K. Effects of rare earth elements on the environment and human health: A literature review. Toxicol. Environ. Health Sci. 2016, 8, 189–200. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds—A review. Compos. Part B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Zaichick, S.; Zaichick, V.; Karandashev, V.; Nosenko, S. Accumulation of rare earth elements in human bone within the lifespan. Metallomics 2011, 3, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological toxicity of lanthanide elements on algae. Chemosphere 2010, 80, 1031. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health effects and toxicity mechanisms of rare earth elements-Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, J.; Zhu, W.; Liu, C.; Shao, P.; Gu, J. Chronic toxicity of rare-earth elements on human beings. Biol. Trace Elem. Res. 2000, 73, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Castro, F.D.; Prasad, S.; Rtimi, S. Emerging technologies for the recovery of rare earth elements (REEs) from the end-of-life electronic wastes: A review on progress, challenges, and perspectives. Environ. Sci. Pollut. R. 2020, 27, 36052–36074. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; Huang, K.; Liu, W.; Zhao, Z.; Liu, H. Instability behavior of bubble supported organic liquid membrane in extraction of low-concentration rare earths from in-situ leaching solutions of ion-adsorption ores. Miner. Eng. 2020, 159, 106645. [Google Scholar] [CrossRef]
- Ashour, R.M.; El-sayed, R.; Abdel-Magied, A.F.; Abdel-khalek, A.A.; Ali, M.; Forsberg, K.; Uheida, A.; Muhammed, M.; Dutta, J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: Kinetic and thermodynamic studies. Chem. Eng. J. 2017, 327, 286–296. [Google Scholar] [CrossRef]
- Qi, X.; Du, K.; Feng, M.; Gao, Y.; Huang, X.; Kanatzidis, M.G. Layered A2Sn3S7·1.25H2O (A=Organic cation) as efficient ion-exchanger for rare earth element recovery. J. Am. Chem. Soc. 2017, 139, 4314–4317. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Safarbali, R.; Yaftian, M.; Zamani, A. Solvent extraction-separation of La(III), Eu(III) and Er(III) ions from aqueous chloride medium using carbamoyl-carboxylic acid extractants. J. Rare Earth 2016, 34, 91–98. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.; Rabie, K.; Elwakeel, K.; Zayed, Y.; Hamza, M.; Guibal, E. Development of phosphoryl-functionalized algal-PEI beads for the sorption of Nd(III) and Mo(VI) from aqueous solutions-Application for rare earth recovery from acid leachates. Chem. Eng. J. 2021, 412, 127399. [Google Scholar] [CrossRef]
- He, C.L.; Salih, K.A.; Wei, Y.Z.; Mira, H.; Abdel-Rahman, A.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient recovery of rare earth elements (Pr(III) and Tm(III)) from mining residues using a new phosphorylated hydrogel (Algal biomass/PEI). Metals 2021, 11, 294. [Google Scholar] [CrossRef]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of rare earth elements with ionic liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- Fu, L.; Wang, J.; Su, Y. Removal of low concentrations of hardness ions from aqueous solutions using electrodeionization process. Sep. Purif. Technol. 2009, 68, 390–396. [Google Scholar] [CrossRef]
- Gergoric, M.; Ekberg, C.; Steenari, B.M.; Retegan, T. Separation of heavy rare-earth elements from light rare-earth elements via solvent extraction from a neodymium magnet leachate and the effects of diluents. J. Sustain. Metall. 2017, 3, 601–610. [Google Scholar] [CrossRef]
- Li, D. Development course of separating rare earths with acid phosphorus extractants: A critical review. J. Rare Earth 2019, 37, 468–486. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent extraction of rare-earth elements with ionic liquids: Toward a selective and sustainable extraction of these valuable elements. Curr. Opin. Green Sust. 2021, 27, 100428. [Google Scholar] [CrossRef]
- Somayeh, D.; Farzaneh, S.; Sina, S.; Fereshteh, R.; Ahmad, G. Separation and solvent extraction of rare earth elements (Pr, Nd, Sm, Eu, Tb, and Er) using TBP and Cyanex 572 from a chloride medium. Miner. Eng. 2021, 161, 106694. [Google Scholar]
- Hérès, X.; Blet, V.; Natale, P.D.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective extraction of rare earth elements from phosphoric acid by ion exchange resins. Metals 2018, 8, 682. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Tanaka, M. Rapid and selective recovery of heavy rare earths by using an adsorbent with diglycol amic acid group. Hydrometallurgy 2015, 155, 105–109. [Google Scholar] [CrossRef]
- Elkhansa, E.; Afnan, M.; MhdAmmar, H.; Alaa, H.H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar]
- Negrea, A.; Gabor, A.; Davidescu, C.M.; Ciopec, M.; Negrea, P.; Duteanu, N.; Barbulescu, A. Rare earth elements removal from water using natural polymers. Sci. Rep. 2018, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, L.; Long, Z.; Feng, Z.; Wang, L. Adsorption ability of rare earth elements on clay minerals and its practical performance. J. Rare Earth 2016, 34, 543–548. [Google Scholar] [CrossRef]
- Liu, E.; Zheng, X.; Xu, X.; Zhang, F.; Liu, E.; Wang, Y.; Li, C.; Yan, Y. Preparation of diethylenetriamine-modified magnetic chitosan nanoparticles for adsorption of rare-earth metal ions. New J. Chem. 2017, 41, 7739–7750. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earth 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Zou, W.; Shu, Q.; Xu, B. Adsorption characteristics of spirulina to rare earth erbium ions. China Environ. Sci. 2019, 39, 674–683. [Google Scholar]
- Zhao, X.; Cui, K.; Huang, K. Enhanced interfacial salt effect on extraction and separation of Er(III) from Mg(II), Al(III), Fe(III) sulfate aqueous solutions using bubble-supported organic liquid membrane. Sep. Purif. Technol. 2022, 285, 120344. [Google Scholar] [CrossRef]
- Trivunac, K.; Stevanovic, S. Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef]
- Borrini, J.; Bernier, G.; Pellet-Rostaing, S.; Favre-Reguillon, A.; Lemaire, M. Separation of lanthanides(iii) by inorganic nanofiltration membranes using a water soluble complexing agent. J. Membr. Sci. 2010, 348, 41–46. [Google Scholar] [CrossRef]
- Hu, Y.; Florek, J.; Larivière, D.; Fontaine, F.G.; Kleitz, F. Recent advances in the separation of rare earth elements using mesoporous hybrid materials. Chem. Rec. 2018, 18, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Manawi, Y.; McKay, G.; Ismail, N.; Fard, A.K.; Kochkodan, V.; Atieh, M.A. Enhancing lead removal from water by complex-assisted filtration with acacia gum. Chem. Eng. J. 2018, 3352, 828–836. [Google Scholar] [CrossRef]
- Cojocar, C.; Zakrzewska-Trznadel, G.; Jaworska, A. Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. part 1: Optimization of complexation conditions. J. Hazard. Mater. 2009, 16, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Nie, Z.; Xi, X.; Li, X. Theoretical simulation and experimental study on nickel, cobalt, manganese separation in complexation-precipitation system. Sep. Purif. Technol. 2013, 108, 124–132. [Google Scholar] [CrossRef]
- Nie, Z.; Ma, L.; Xi, X. “Complexation–precipitation” metal separation method system and its application in secondary resources. Rare Met. 2014, 33, 369–378. [Google Scholar] [CrossRef]
- Anneli, S.; Torbjörn, K.; Staffan, S.; Per, P. Complexation and precipitation reactions in the ternary As(V)–Fe(III)–Om (organic matter) system. Geochim. Cosmochim. Acta 2014, 145, 297–314. [Google Scholar]
- Ho, T.L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Kolarik, Z. Complexation and separation of lanthanides(III) and actinides(III) by heterocyclic N-donors in solutions. Chem. Rev. 2008, 108, 4208–4252. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, C.; Lan, J.; Wu, Q.; Zhao, Y.; Chai, Z.; Nie, C.; Shi, W. Theoretical studies on the complexation of Eu(III) and Am(III) with HDEHP: Structure, bonding nature and stability. Sci. China Chem. 2016, 59, 324–331. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar]
- Graillot, A.; Monge, S.; Faur, C.; Bouyer, D.; Robin, J.J. Synthesis by RAFT of innovative well-defined (co)polymers from a novel phosphorus-based acrylamide monomer. Polym. Chem. 2013, 4, 795–803. [Google Scholar] [CrossRef]
- Gomes Rodrigues, D.; Monge, S.; Dacheux, N.; Pellet-Rostaing, S.; Faur, C. Highlighting the selective properties of carbamoylmethylphosphonated hydrosoluble polymers for Gd(III)/Th(IV)/U(VI) separation. Sep. Purif. Technol. 2021, 254, 117260. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Sun, S.; Cherian, P.; Roshandel, S.; Neighbors, J.D.; Hu, H.; Dunford, D.E.; Sedghizadeh, P.P.; McKenna, C.E.; Srinivasan, V.; et al. Bisphosphonates: The role of chemistry in understanding their biological actions and structure-activity relationships, and new directions for their therapeutic use. Bone 2022, 156, 116289. [Google Scholar] [CrossRef]
- Li, G.; Bai, C.; Cheng, C.; Zheng, S. NMR and TOF-SIMS investigation of alkylbisphosphonic acid binding to Ca2+ on CaCO3 surface. Sci. Sin. Chim. 2019, 49, 1491–1496. [Google Scholar]
- Li, G.; Zheng, S.; Bai, C.; Li, G.; Cheng, C. Self-assembled monolayer of mica coating using organobisphosphonic acid. Appl. Surf. Sci. 2018, 457, 449–455. [Google Scholar]
- Zhu, Y.; Ma, T.; Liu, Y.; Ren, T.; Yuan, Z. Metal phosphonate hybrid materials: From densely layered to hierarchically nanoporous structures. Inorg. Chem. Front. 2014, 1, 360–383. [Google Scholar] [CrossRef]
- Alanne, A.L.; Tuikka, M.; Tõnsuaadu, K.; Ylisirniö, M.; Hämäläinen, L.; Turhanen, P.; Vepsäläinen, J.; Peräniemi, S. A novel bisphosphonate-based solid phase method for effective removal of chromium(III) from aqueous solutions and tannery effluents. RSC Adv. 2013, 3, 14132–14138. [Google Scholar] [CrossRef]
- Mohammadi, M.; Reinicke, B.; Wawrousek, K. Biosorption and biomagnetic recovery of La3+ by magnetospirillum magneticum AMB-1 biomass. Sep. Purif. Technol. 2022, 303, 122140. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Nada, N.; Furukawa, Y. Anisotropic growth kinetics of ice crystals from water studied by molecular dynamics simulation. J. Cryst. Growth 1996, 169, 587–597. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Yang, J.; Sun, H.; Sun, J. Healable ionic conductors with extremely low-hysteresis and high mechanical strength enabled by hydrophobic domain-locked reversible interactions. Adv. Mater. 2023, 35, e2307990. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.C. Hydrogen-bond networks in supercooled liquid water and amorphous/vitreous ices. J. Mol. Struct. 1990, 237, 221–232. [Google Scholar] [CrossRef]
- Xantheas, S.S. Cooperativity and hydrogen bonding network in water clusters. Chem. Phys. 2000, 258, 225–231. [Google Scholar] [CrossRef]
- Xian, D.; Zhou, W.; Wang, J.; Pan, D.; Li, X.; Li, Y.; Shi, Y.; Wu, W.; Tan, Z.; Liu, C. Multiple investigations of aqueous Eu(III)-oxalate complexes: The reduction in coordination number and validation of spectral linear correlation. Dalton Trans. 2021, 50, 9388–9398. [Google Scholar] [CrossRef]
- Taboada-Serrano, P.; Chin, C.J.; Yiacoumi, S.; Tsouris, C. Modeling aggregation of colloidal particles. Curr. Opin. Colloid Interface Sci. 2005, 10, 123–132. [Google Scholar] [CrossRef]
- Li, B.; Zhou, D.; Han, Y. Assembly and phase transitions of colloidal crystals. Nat. Rev. Mater. 2016, 1, 249–324. [Google Scholar] [CrossRef]
- Kind, M. Colloidal aspects of precipitation processes. Chem. Eng. Sci. 2002, 57, 4287–4293. [Google Scholar] [CrossRef]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkove, M. Aggregation and charging of colloidal silica particles: Effect of particle size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.; Neale, N.; Chen, T.; Kortshagen, U.R. Hypervalent surface interactions for colloidal stability and doping of silicon nanocrystals. Nat. Commun. 2013, 4, 2197. [Google Scholar] [CrossRef] [PubMed]
- Fakari, S.; Nezamzadeh-Ejhieh, A. Synergistic effects of ion exchange and complexation processes in cysteine-modified clinoptilolite nanoparticles for removal of Cu(II) from aqueous solutions in batch and continuous flow systems. New J. Chem. 2017, 41, 3811–3820. [Google Scholar] [CrossRef]
- Alabi, A.; AlHajaj, A.; Cseri, L.; Szekely, G.; Budd, P.; Zou, L. Review of nanomaterials-assisted ion exchange membranes for electromembrane desalination. NPJ Clean Water 2018, 1, 10. [Google Scholar] [CrossRef]
- Sun, C.; Li, K.; Wang, J.; Xue, D. Searching for novel materials via 4f chemistry. J. Rare Earth 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Xue, D.; Sun, C.; Chen, X. Hybridized valence electrons of 4f0−145d0−16s2: The chemical bonding nature of rare earth elements. J. Rare Earth 2017, 35, 837–843. [Google Scholar] [CrossRef]
- Jia, Y. Crystal radii and effective ionic radii of the rare earth ions. J. Solid State Chem. 1991, 95, 184–187. [Google Scholar] [CrossRef]
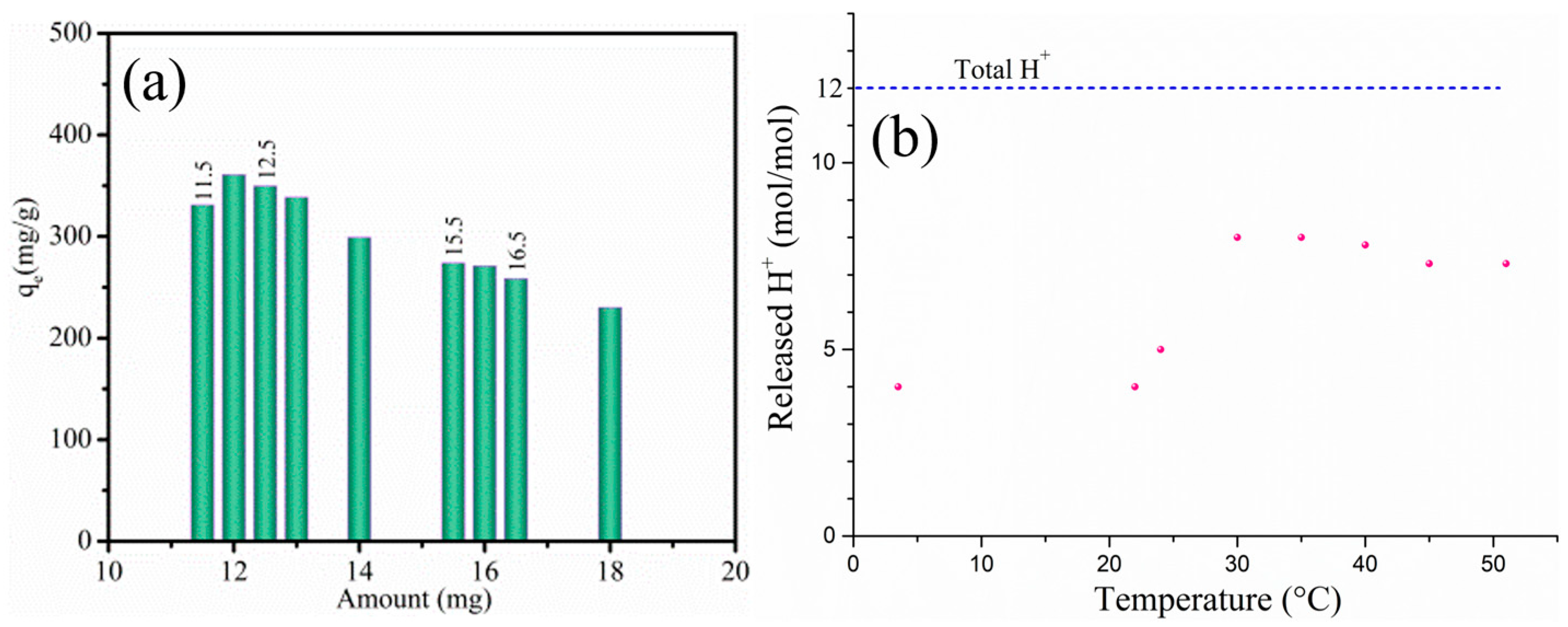


| Amount (mg) | Final Con. (mg/L) | Complexation Capacity (mg/g) | Final pH |
|---|---|---|---|
| 11.5 | 6.082 | 331.0 (86.2%) | 3.24 |
| 12.0 | 0.847 | 360.8 (98.1%) | 3.14 |
| 12.5 | 0.437 | 349.7 (99.0%) | 3.26 |
| 13.0 | 0.149 | 338.4 (99.7%) | 3.13 |
| 14.0 | 2.285 | 299.0 (94.8%) | 3.07 |
| 15.5 | 1.697 | 273.9 (96.2%) | 3.07 |
| 16.0 | 0.784 | 271.0 (98.2%) | 3.08 |
| 16.5 | 1.495 | 258.5 (96.6%) | 3.07 |
| 18.0 | 2.736 | 230.1 (93.8%) | 2.93 |
| NaOH (mL) | pH | NaOH (mL) | pH |
|---|---|---|---|
| 0 | 2.02 | 10.8 | 6.03 |
| 4.8 | 2.50 | 11.4 | 6.49 |
| 7.0 | 3.04 | 12.7 | 7.00 |
| 8.5 | 3.51 | 14.7 | 7.99 |
| 9.3 | 4.04 | 15.3 | 8.97 |
| 9.6 | 4.58 | 15.9 | 9.98 |
| 10.0 | 5.10 | 17.6 | 10.80 |
| 10.3 | 5.48 | 28.0 | 11.50 |
| Ion | Initial con. (mg/L) | Final con. (Expulsion, %) | Final con. (Expulsion, %) * |
|---|---|---|---|
| Ca | 46.53 | 13.72 (70.5%) | 6.43 (86.2%) |
| Mg | 26.67 | 9.98 (62.6%) | 7.73 (71.0%) |
| Sr | 0.70 | 0.16 (76.9%) | 0.076 (89.1%) |
| Ba | 0.048 | 0.0046 (90.5%) | 0.0026 (94.6%) |
| Er | 0.016 | 0.0038 (77.0%) | 0.0037 (77.6%) |
| Pr | 0.018 | 0.0016 (91.2%) | 0.0008 (95.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, C.; Yang, X.; Li, G. Removal of Low Concentrations of Er(III) from Water Using Heptadecyl-1,1-bisphosphonic Acid. Minerals 2024, 14, 534. https://doi.org/10.3390/min14060534
Bai C, Yang X, Li G. Removal of Low Concentrations of Er(III) from Water Using Heptadecyl-1,1-bisphosphonic Acid. Minerals. 2024; 14(6):534. https://doi.org/10.3390/min14060534
Chicago/Turabian StyleBai, Chunhua, Xiaoning Yang, and Guanghui Li. 2024. "Removal of Low Concentrations of Er(III) from Water Using Heptadecyl-1,1-bisphosphonic Acid" Minerals 14, no. 6: 534. https://doi.org/10.3390/min14060534
APA StyleBai, C., Yang, X., & Li, G. (2024). Removal of Low Concentrations of Er(III) from Water Using Heptadecyl-1,1-bisphosphonic Acid. Minerals, 14(6), 534. https://doi.org/10.3390/min14060534








