Determining the Metabolic Processes of Metal-Tolerant Fungi Isolated from Mine Tailings for Bioleaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation and Identification
2.2. Screening for Organic Acid Production and Metal Tolerance
2.2.1. Qualitative Assay
2.2.2. Quantitative Analysis
- Growth rate assays
2.3. Carbon Source for Organic Acid Production
2.3.1. Screening for Carbon Source
2.3.2. Organic Acid and Metabolites Identification
3. Results
3.1. Microbial Isolation and Identification
3.2. Qualitative Screening
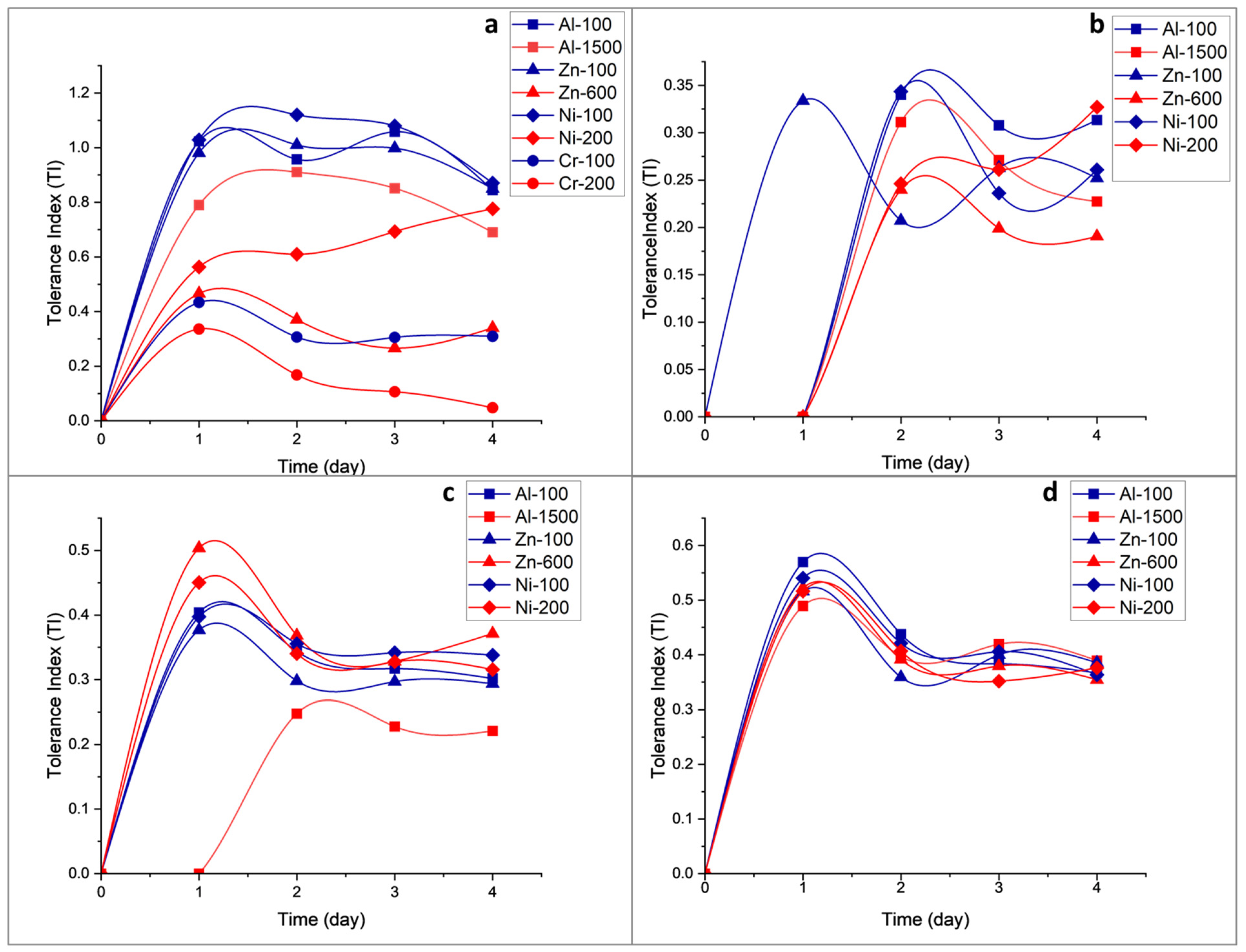
3.3. Quantitative Screening
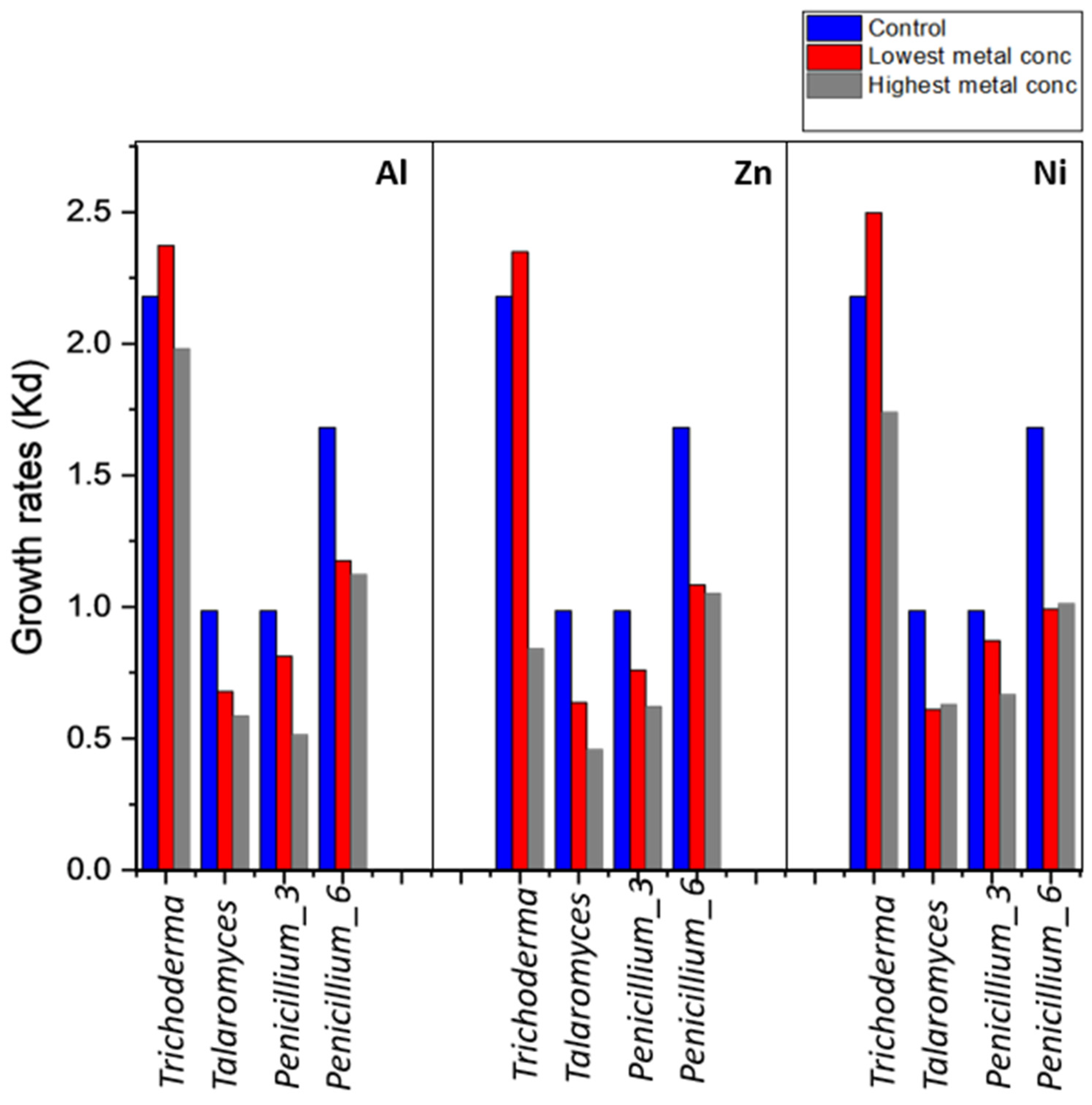
3.3.1. Effect of Heavy Metal Concentration on Fungal Growth Phases
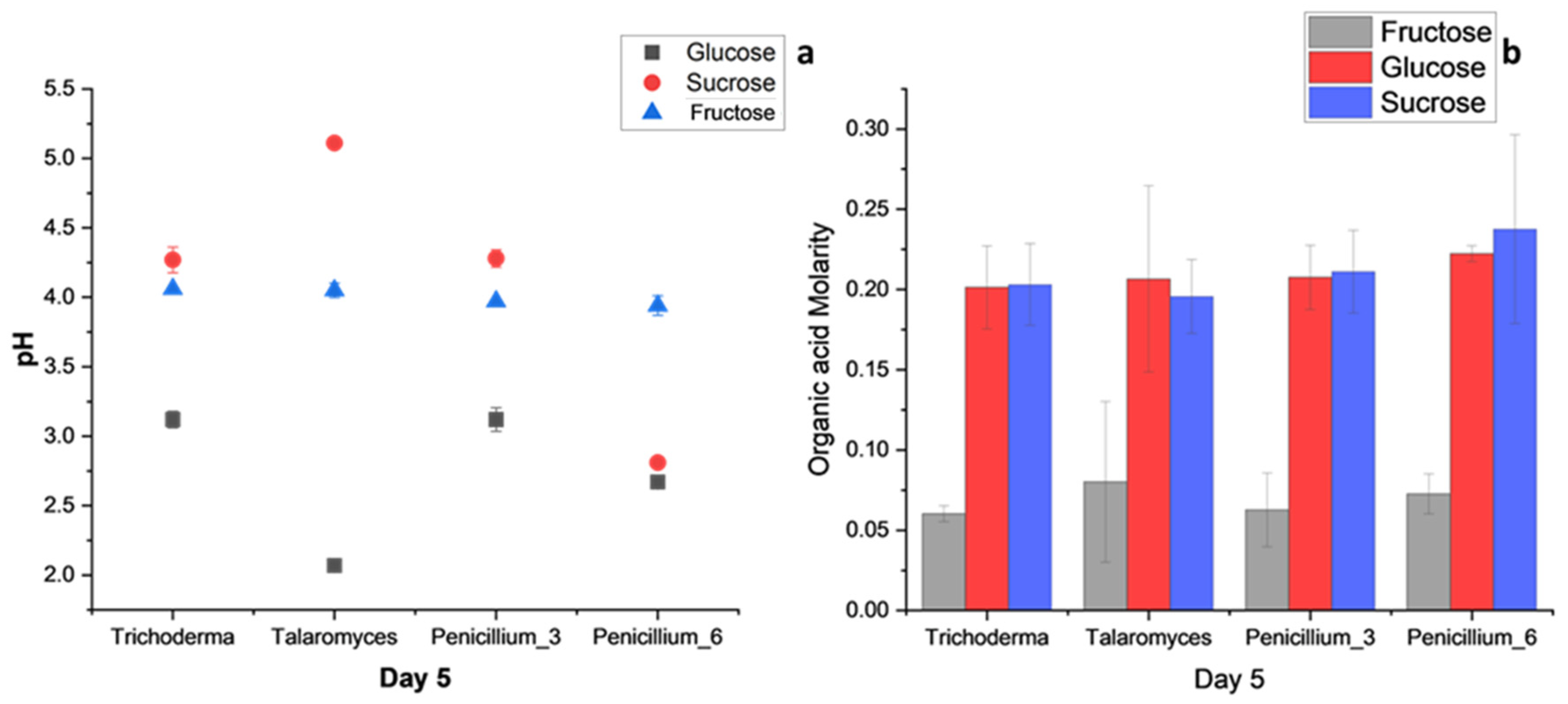
3.3.2. Carbon Utilization
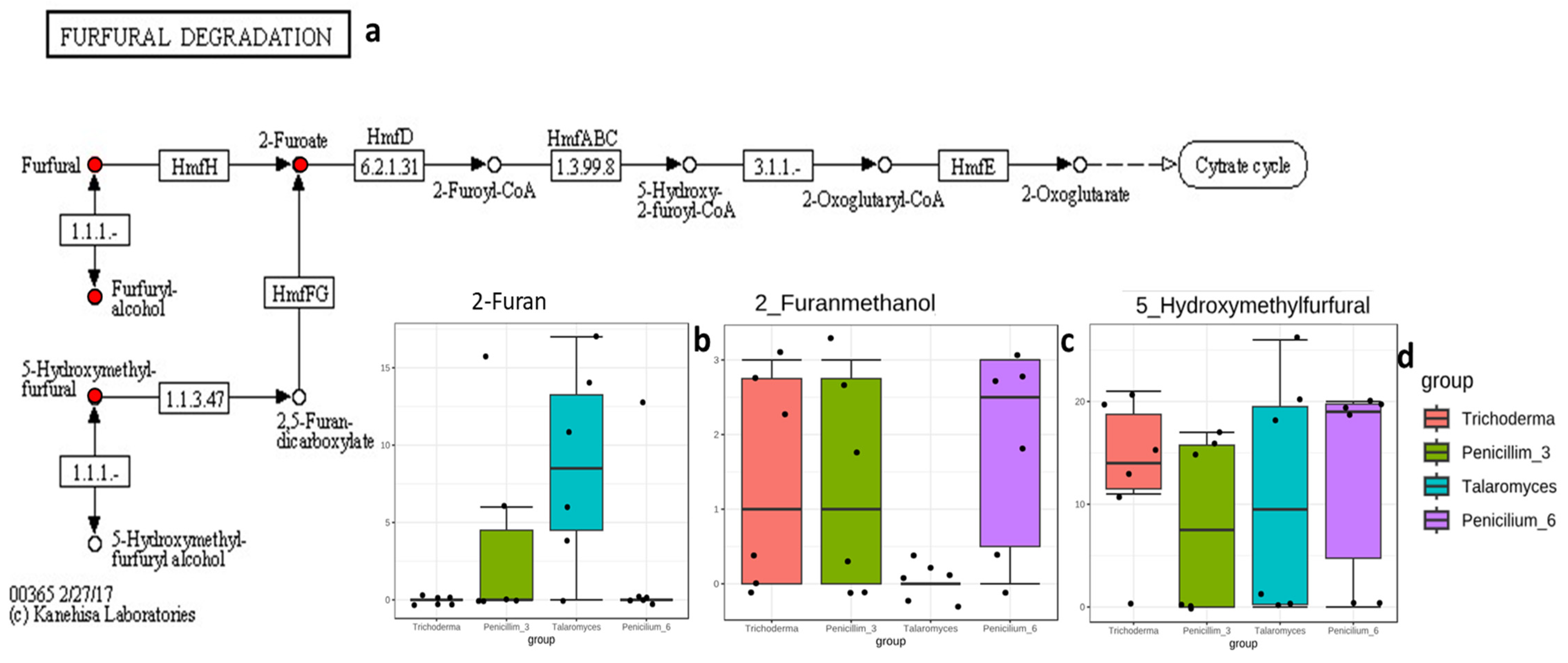
3.3.3. Compounds/Metabolites Screening
4. Discussion
4.1. Qualitative and Quantitative Assay: Organic Acid and Metal Tolerance
4.2. Growth Rates and Effects of Metal on Fungal Growth Phases
4.3. Carbon Source Utilization: Glucose, Sucrose, and Fructose
4.4. Comparison of Organic Acid Production using Glucose as the Carbon Source
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Russo, F.; Ceci, A.; Pinzari, F.; Siciliano, A.; Guida, M.; Malusà, E.; Tartanus, M.; Miszczak, A.; Maggi, O.; Persiani, A.M. Bioremediation of Dichlorodiphenyltrichloroethane (DDT)-Contaminated Agricultural Soils: Potential of Two Autochthonous Saprotrophic Fungal Strains. Appl. Environ. Microbiol. 2019, 85, e01720-19. [Google Scholar] [CrossRef] [PubMed]
- Shourie, A.; Vijayalakshmi, U. Fungal Diversity and Its Role in Mycoremediation. Geomicrobiol. J. 2022, 39, 426–444. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous Fungi for the Production of Enzymes, Chemicals and Materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological Avenues in Mineral Processing: Fundamentals, Applications and Advances in Bioleaching and Bio-Beneficiation. Miner. Process. Extr. Metall. Rev. 2023, 44, 22–51. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential techno-economic challenges: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Wu, H.Y.; Ting, Y.P. Metal Extraction from Municipal Solid Waste (MSW) Incinerator Fly Ash—Chemical Leaching and Fungal Bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Dhanasekaran, D.; Vinothini, G.; Thajuddin, N. Extraction and Recovery of Precious Metals from Electronic Waste Printed Circuit Boards by Bioleaching Acidophilic Fungi. Int. J. Environ. Sci. Technol. 2018, 15, 119–132. [Google Scholar] [CrossRef]
- Kolenčík, M.; Urík, M.; Čerňanský, S.; Molnárová, M.; Matúš, P. Leaching of Zinc, Cadmium, Lead and Copper from Electronic Scrap Using Organic Acids and the Aspergillus niger Strain. Fresenius Environ. Bull. 2013, 22, 3673–3679. [Google Scholar]
- Ren, W.X.; Li, P.J.; Geng, Y.; Li, X.J. Biological Leaching of Heavy Metals from a Contaminated Soil by Aspergillus niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef]
- Din, G.; Hassan, A.; Rafiq, M.; Hasan, F.; Badshah, M.; Khan, S.; Chen, G.; Ripp, S.; Shah, A.A. Characterization of Organic Acid Producing Aspergillus Tubingensis FMS1 and Its Role in Metals Leaching from Soil. Geomicrobiol. J. 2020, 37, 336–344. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic Potential of Selected Metal Ions for Bioleaching, and Potential Techno-Economic and Environmental Issues: A Critical Review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium Bioleaching: An Emerging Approach for the Recovery of Li from Spent Lithium Ion Batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.J.; Cao, B.; Madhavi, S. A Review on the Recycling of Spent Lithium-Ion Batteries (LIBs) by the Bioleaching Approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, W.; Zheng, G.; Din, G.; Ma, X.; Rafiq, M.; Xu, W. Metals Extraction from Sulfide Ores with Microorganisms: The Bioleaching Technology and Recent Developments. Trans. Indian Inst. Met. 2019, 72, 559–579. [Google Scholar] [CrossRef]
- Qayyum, S.; Meng, K.; Pervez, S.; Nawaz, F.; Peng, C. Optimization of PH, Temperature and Carbon Source for Bioleaching of Heavy Metals by Aspergillus flavus Isolated from Contaminated Soil. Main Group Met. Chem. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Keshavarz, S.; Faraji, F.; Rashchi, F.; Mokmeli, M. Bioleaching of Manganese from a Low-Grade Pyrolusite Ore Using Aspergillus niger: Process Optimization and Kinetic Studies. J. Environ. Manag. 2021, 285, 112153. [Google Scholar] [CrossRef]
- Muddanna, M.H.; Baral, S.S. Leaching of Nickel and Vanadium from the Spent Fluid Catalytic Cracking Catalyst by Reconnoitering the Potential of Aspergillus niger Associating with Chemical Leaching. J. Environ. Chem. Eng. 2019, 7, 103025. [Google Scholar] [CrossRef]
- Alavi, N.; Partovi, K.; Majlessi, M.; Rashidi, M.; Alimohammadi, M. Bioleaching of Metals from Cellphones Batteries by a Co-Fungus Medium in Presence of Carbon Materials. Bioresour. Technol. Rep. 2021, 15, 100768. [Google Scholar] [CrossRef]
- Santhiya, D.; Ting, Y.P. Bioleaching of Spent Refinery Processing Catalyst Using Aspergillus niger with High-Yield Oxalic Acid. J. Biotechnol. 2005, 116, 171–184. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Yang, J.; Guo, Z.; Shi, J.; Deng, Y.; Liu, H.; Jiang, H.; Liang, Y.; et al. A Review on the Bioleaching of Toxic Metal(Loid)s from Contaminated Soil: Insight into the Mechanism of Action and the Role of Influencing Factors. Front. Microbiol. 2022, 13, 1049277. [Google Scholar] [CrossRef] [PubMed]
- Shekhar Samanta, N.; Das, P.P.; Dhara, S.; Purkait, M.K. An Overview of Precious Metal Recovery from Steel Industry Slag: Recovery Strategy and Utilization. Ind. Eng. Chem. Res. 2023, 62, 9006–9031. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current Nature-Based Biological Practices for Rare Earth Elements Extraction and Recovery: Bioleaching and Biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Metabolomic Analysis Reveals Contributions of Citric and Citramalic Acids to Rare Earth Bioleaching by a Paecilomyces Fungus. Front. Microbiol. 2020, 10, 3008. [Google Scholar] [CrossRef] [PubMed]
- Valix, M.; Loon, L.O. Adaptive Tolerance Behaviour of Fungi in Heavy Metals. Min. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Rose, P.K.; Devi, R. Heavy Metal Tolerance and Adaptability Assessment of Indigenous Filamentous Fungi Isolated from Industrial Wastewater and Sludge Samples. Beni Suef Univ. J. Basic Appl. Sci. 2018, 7, 688–694. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological Advances in Bioremediation of Heavy Metals Contaminated Ecosystems: An Overview with Special Reference to Phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Anahid, S.; Yaghmaei, S.; Ghobadinejad, Z. Heavy Metal Tolerance of Fungi. Sci. Iran. 2011, 18, 502–508. [Google Scholar] [CrossRef]
- Thavamani, P.; Samkumar, R.A.; Satheesh, V.; Subashchandrabose, S.R.; Ramadass, K.; Naidu, R.; Venkateswarlu, K.; Megharaj, M. Microbes from Mined Sites: Harnessing Their Potential for Reclamation of Derelict Mine Sites. Environ. Pollut. 2017, 230, 495–505. [Google Scholar] [CrossRef]
- Guo, D.; Hou, H.; Long, J.; Guo, X.; Xu, H. Underestimated Environmental Benefits of Tailings Resource Utilization: Evidence from a Life Cycle Perspective. Environ. Impact Assess. Rev. 2022, 96, 106832. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Zyukin, D.A.; Karlina, A.I. Prospects for Return of Valuable Components Lost in Tailings of Light Metals Ore Processing. Metallurgist 2023, 67, 96–103. [Google Scholar] [CrossRef]
- Yu, Y.H.; Du, C.M. Leaching of Phosphorus from Phosphate Tailings and Extraction of Calcium Phosphates: Toward Comprehensive Utilization of Tailing Resources. J. Environ. Manag. 2023, 347, 119159. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, X.; Gu, J.; Qian, L.; Wang, Z.; Nie, Y.; Han, X.; An, L.; Jiang, H. Metals Recovery from Polymetallic Sulfide Tailings by Bioleaching Functional Bacteria Isolated with the Improved 9K Agar: Comparison between One-Step and Two-Step Processes. Environ. Res. 2024, 240, 117511. [Google Scholar] [CrossRef] [PubMed]
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S. Applying EDTA in Chelating Excess Metal Ions to Improve Downstream DNA Recovery from Mine Tailings for Long-Read Amplicon Sequencing of Acidophilic Fungi Communities. J. Fungi 2022, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, R.; Cloete, E.; Khasa, D. Isolation and Identification of Iron Ore-Solubilising Fungus. S. Afr. J. Sci. 2010, 106, 1–6. [Google Scholar] [CrossRef]
- Chen, A.; Hao, X.; Xiao, Y.; Zou, K.; Liu, H.; Liu, X. Responses of Acidithiobacillus thiooxidans A01 to Individual and Joint Nickel (Ni2+) and Ferric (Fe3+). Minerals 2019, 9, 82. [Google Scholar] [CrossRef]
- Martínez, P.; Gálvez, S.; Ohtsuka, N.; Budinich, M.; Cortés, M.P.; Serpell, C.; Nakahigashi, K.; Hirayama, A.; Tomita, M.; Soga, T.; et al. Metabolomic Study of Chilean Biomining Bacteria Acidithiobacillus Ferrooxidans Strain Wenelen and Acidithiobacillus thiooxidans Strain Licanantay. Metabolomics 2012, 9, 247–257. [Google Scholar] [CrossRef]
- Ilyas, S.; Bhatti, H.N.; Bhatti, I.A.; Sheikh, M.A.; Ghauri, A. Bioleaching of Metal Ions from Low Grade Sulphide Ore: Process Optimization by Using Orthogonal Experimental Array Design. Afr. J. Biotechnol. 2010, 9, 2801–2810. [Google Scholar]
- Kishimoto, N.; Tano, T. Acidophilic Heterotrophic Bacteria Isolated from Acidic Mine Drainage, Sewage, and Soils. J. Gen. Appl. Microbiol. 1987, 33, 11–25. [Google Scholar] [CrossRef]
- Amiri, F.; Mousavi, S.M.; Yaghmaei, S. Enhancement of Bioleaching of a Spent Ni/Mo Hydroprocessing Catalyst by Penicillium Simplicissimum. Sep. Purif. Technol. 2011, 80, 566–576. [Google Scholar] [CrossRef]
- Shaikh, Z.; Qureshi, P. Screening and Isolation of Organic Acid Producers from Samples of Diverse Habitats. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 39–44. [Google Scholar]
- Valix, M.; Usai, F.; Malik, R. Fungal Bio-Leaching of Low Grade Laterite Ores. Min. Eng. 2001, 14, 197–203. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of Adapted Metal Tolerant Aspergillus niger to Enhance Bioleaching Efficiency of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Le, L.; Tang, J.; Ryan, D.; Valix, M. Bioleaching Nickel Laterite Ores Using Multi-Metal Tolerant Aspergillus foetidus Organism. Min. Eng. 2006, 19, 1259–1265. [Google Scholar] [CrossRef]
- Dutta, S. Valorization of Biomass-Derived Furfurals: Reactivity Patterns, Synthetic Strategies, and Applications. Biomass Convers. Biorefin. 2023, 13, 10361–10386. [Google Scholar] [CrossRef]
- Kallio, H. Aroma of birch syrup. J. Agric. Food Chem. 1989, 37.5, 1367–1371. [Google Scholar] [CrossRef]
- Etemadzadeh, S.S.; Emtiazi, G.; Etemadifar, Z. Heterotrophic Bioleaching of Sulfur, Iron, and Silicon Impurities from Coal by Fusarium Oxysporum FE and Exophiala Spinifera FM with Growing and Resting Cells. Curr. Microbiol. 2016, 72, 707–715. [Google Scholar] [CrossRef]
- Martinez, P.; Vera, M.; Bobadilla-Fazzini, R.A.; Haghshenas, D.F.; Alamdari, E.K.; Bonakdarpour, B.; Darvishi, D.; Nasernejad, B.; Elissawy, A.; Ebada, S.S.; et al. Omics on Bioleaching: Current and Future Impacts. Appl. Microbiol. Biotechnol. 2015, 99, 8337–8350. [Google Scholar] [CrossRef]
- Tansengco, M.; Tejano, J.; Coronado, F.; Gacho, C.; Barcelo, J. Heavy Metal Tolerance and Removal Capacity of Trichoderma Species Isolated from Mine Tailings in Itogon, Benguet. Environ. Nat. Resour. J. 2018, 16, 39–57. [Google Scholar] [CrossRef]
- Vásquez, C.; Colmenárez, Y.; Morales-Sánchez, J.; Valera, N.; Sandoval, M.F.; Balza, D. Current and Potential Use of Phytophagous Mites as Biological Control Agent of Weeds. Weed Biol. Control 2015, 109, 110–126. [Google Scholar]
- Yin, X.; Shan, X.; Shao, W.; Liu, L.; Liang, G.; Zhou, Q.; Lin, W. Copper from Waste Printed Circuit Boards Was Effectively Bioleached Using Newly Isolated Microorganisms and Subsequently Recovered by Microbial Fuel Cell. Sustainability 2023, 15, 9996. [Google Scholar] [CrossRef]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and Cellular Mechanisms Sustaining Metal Tolerance in Ectomycorrhizal Fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Duncan, J.R.; Burgess, J.E. Fungal Bioaccumulation of Copper, Nickel, Gold and Platinum. Min. Eng. 2008, 21, 55–60. [Google Scholar] [CrossRef]
- Lobos, A.; Harwood, V.J.; Scott, K.M.; Cunningham, J.A. Tolerance of Three Fungal Species to Lithium and Cobalt: Implications for Bioleaching of Spent Rechargeable Li-Ion Batteries. J. Appl. Microbiol. 2021, 131, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Q.; Wang, Q.; Wu, T. Heavy Metals Extraction from Municipal Solid Waste Incineration Fly Ash Using Adapted Metal Tolerant Aspergillus niger. Bioresour. Technol. 2009, 100, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C.; Valix, M. Overcoming the Bacteriostatic Effects of Heavy Metals on Acidithiobacillus thiooxidans for Direct Bioleaching of Saprolitic Ni Laterite Ores. Hydrometallurgy 2017, 168, 21–25. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect Bioleaching of Low-Grade Nickel Limonite and Saprolite Ores Using Fungal Metabolic Organic Acids Generated by Aspergillus niger. Hydrometallurgy 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, H.; Shi, Q.; Meng, X.; Zhao, Y.; Qiu, G.; Zhang, X.; Yu, H.; He, X.; He, H.; et al. Comparative Chemical and Non-Contact Bioleaching of Ion-Adsorption Type Rare Earth Ore Using Ammonium Sulfate and Metabolites of Aspergillus niger and Yarrowia Lipolytica to Rationalise the Role of Organic Acids for Sustainable Processing. Hydrometallurgy 2023, 216, 106019. [Google Scholar] [CrossRef]
- Park, L.K.E.; Liu, J.; Yiacoumi, S.; Borole, A.P.; Tsouris, C. Contribution of Acidic Components to the Total Acid Number (TAN) of Bio-Oil. Fuel 2017, 200, 171–181. [Google Scholar] [CrossRef]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Environmentally Sustainable and Cost-Effective Bioleaching of Aluminum from Low-Grade Bauxite Ore Using Marine-Derived Aspergillus niger. Hydrometallurgy 2020, 195, 105368. [Google Scholar] [CrossRef]
- Zuo, J.; Tang, J.; Lu, M.; Zhou, Z.; Li, Y.; Tian, H.; Liu, E.; Gao, B.; Liu, T.; Shao, P. Glycolysis Rate-Limiting Enzymes: Novel Potential Regulators of Rheumatoid Arthritis Pathogenesis. Front. Immunol. 2021, 12, 779787. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Heitman, J. Chlamydospore Formation during Hyphal Growth in Cryptococcus Neoformans. Eukaryot. Cell 2005, 4, 1746–1754. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary Metabolites from Mangrove-Associated Fungi: Source, Chemistry and Bioactivities. Nat. Prod. Rep. 2021, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ren, H.; Mousa, J.J.; Rangel, D.E.N.; Zhang, Y.; Bruner, S.D.; Keyhani, N.O. The PacC Transcription Factor Regulates Secondary Metabolite Production and Stress Response, but Has Only Minor Effects on Virulence in the Insect Pathogenic Fungus Beauveria Bassiana. Environ. Microbiol. 2017, 19, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, F.K.H.; Al-Sadi, A.M.; Al-Riyamy, B.Z.; Maharachchikumbura, S.S.N.; Al-Sabahi, J.N.; Velazhahan, R. Endophytic Fungi from the Medicinal Plant Aloe dhufarensis Lavranos Exhibit Antagonistic Potential against Phytopathogenic Fungi. S. Afr. J. Bot. 2022, 147, 1078–1085. [Google Scholar] [CrossRef]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis Elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef]
- Legiša, M.; Mattey, M. Changes in Primary Metabolism Leading to Citric Acid Overflow in Aspergillus niger. Biotechnol. Lett. 2007, 29, 181–190. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Y.; Zhang, R.; Ning, Y.; Yu, Y.; Xu, P.; Deng, L.; Wang, F. Recent Advances and Perspectives on Production of Value-Added Organic Acids through Metabolic Engineering. Biotechnol. Adv. 2023, 62, 108076. [Google Scholar] [CrossRef]
- Morin, N.; Cescut, J.; Beopoulos, A.; Lelandais, G.; Le Berre, V.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Transcriptomic Analyses during the Transition from Biomass Production to Lipid Accumulation in the Oleaginous Yeast Yarrowia Lipolytica. PLoS ONE 2011, 6, e27966. [Google Scholar] [CrossRef]
- Yang, Q.; Qian, X.; Routledge, M.N.; Wu, X.; Shi, Y.; Zhu, Q.; Zhang, H. Metabonomics Analysis of Postharvest Citrus Response to Penicillium Digitatum Infection. LWT 2021, 152, 112371. [Google Scholar] [CrossRef]




| Name | Composition | ||
|---|---|---|---|
| General | |||
| Basic bioleaching media composition | 3.0 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.5 g/L K2HPO4, 0.1 g/L KCl, FeSO4·7H2O or sulfur and 1.5% Agar bacteriological | ||
| Compounds Different in Each Media | pH | References | |
| Silverman and Lundgren 9 K | 0.01 g/L Ca (NO3)2 | 1.8 | [37] |
| KDM | 0.145 g/L NaH2PO4, 0.021 g/L CaCL2 | 1.8 | [38] |
| Iron-tryptone soya broth (FeTSB) | 0.05 g/L Ca (NO3)2, 0.25 g/L Tryptone soy broth | 1.8 | [39] |
| Glucose yeast extract medium (GYEM), when prepared as broth—GYEB, and when prepared as agar—GYEA | 5 g/L glucose, 0.05 g/L yeast extract | 3 | [40] |
| Yeast sucrose media (YSM) | 100 g/L sucrose, 1.5g/l NaNO3, 1.6 g/L yeast extract | 3 | [41] |
| Organism | Organic Acid | |||
|---|---|---|---|---|
| Qualitative | Quantitative | |||
| Colony Zone (mm) | Yellow Zone (mm) | Acid Unitage (AU) | pH | |
| Penicillium_7 | 27.91 | 49.04 | 1.75 ± 0.06 | 6.25 ± 0.10 |
| Coniochaeta | 28.06 | 44.68 | 1.59 ± 0.03 | 6.16 ± 0.14 |
| Acidiella | 0.00 | 0.00 | 0.00 | 6.15 ± 0.14 |
| Penicillium_4 | 23.91 | 44.74 | 1.87 ± 0.06 | 6.013 ± 0.02 |
| Penicillium_1 | 23.55 | 34.42 | 1.46 ± 0.03 | 6.04 ± 0.03 |
| Acidomyces | 0.00 | 0.00 | 0.00 | 5.965 ± 0.01 |
| Penicillium_2 | 21.76 | 44.99 | 2.03 ± 0.03 | 5.88 ± 0.4 |
| Talaromyces_2 | 25.34 | 36.67 | 1.45 ± 0.14 | 5.76 ± 0.02 |
| Fodinomyces | 0.00 | 0.00 | 0.00 | 5.53 ± 0.06 |
| Penicillium_5 | 26.21 | 46.55 | 1.77 ± 0.35 | 5.07 ± 0.0 |
| Fusarium | 32.50 | 37.75 | 1.16 ± 0.05 | 4.14 ± 0.0 |
| Penicillium_6 | 21.50 | 28.81 | 1.33 ± 0.17 | 4.16 ± 0.03 |
| Penicillium_3 | 17.86 | 37.97 | 2.13 ± 0.11 | 4.08 ± 0.01 |
| Trichoderma | 35.71 | 48.81 | 1.37 ± 0.007 | 3.70 ± 0.003 |
| Talaromyces_1 | 8.56 | 15.62 | 1.82 ± 0.02 | 3.46 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkuna, R.; Matambo, T.S. Determining the Metabolic Processes of Metal-Tolerant Fungi Isolated from Mine Tailings for Bioleaching. Minerals 2024, 14, 235. https://doi.org/10.3390/min14030235
Nkuna R, Matambo TS. Determining the Metabolic Processes of Metal-Tolerant Fungi Isolated from Mine Tailings for Bioleaching. Minerals. 2024; 14(3):235. https://doi.org/10.3390/min14030235
Chicago/Turabian StyleNkuna, Rosina, and Tonderayi S. Matambo. 2024. "Determining the Metabolic Processes of Metal-Tolerant Fungi Isolated from Mine Tailings for Bioleaching" Minerals 14, no. 3: 235. https://doi.org/10.3390/min14030235
APA StyleNkuna, R., & Matambo, T. S. (2024). Determining the Metabolic Processes of Metal-Tolerant Fungi Isolated from Mine Tailings for Bioleaching. Minerals, 14(3), 235. https://doi.org/10.3390/min14030235







