Grinding of Australian and Brazilian Iron Ore Fines for Low-Carbon Production of High-Quality Oxidised Pellets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Grindability
2.2.2. Settling Characteristics
2.2.3. Filtering Characteristics
2.2.4. Pellet Preparation
3. Results and Discussion
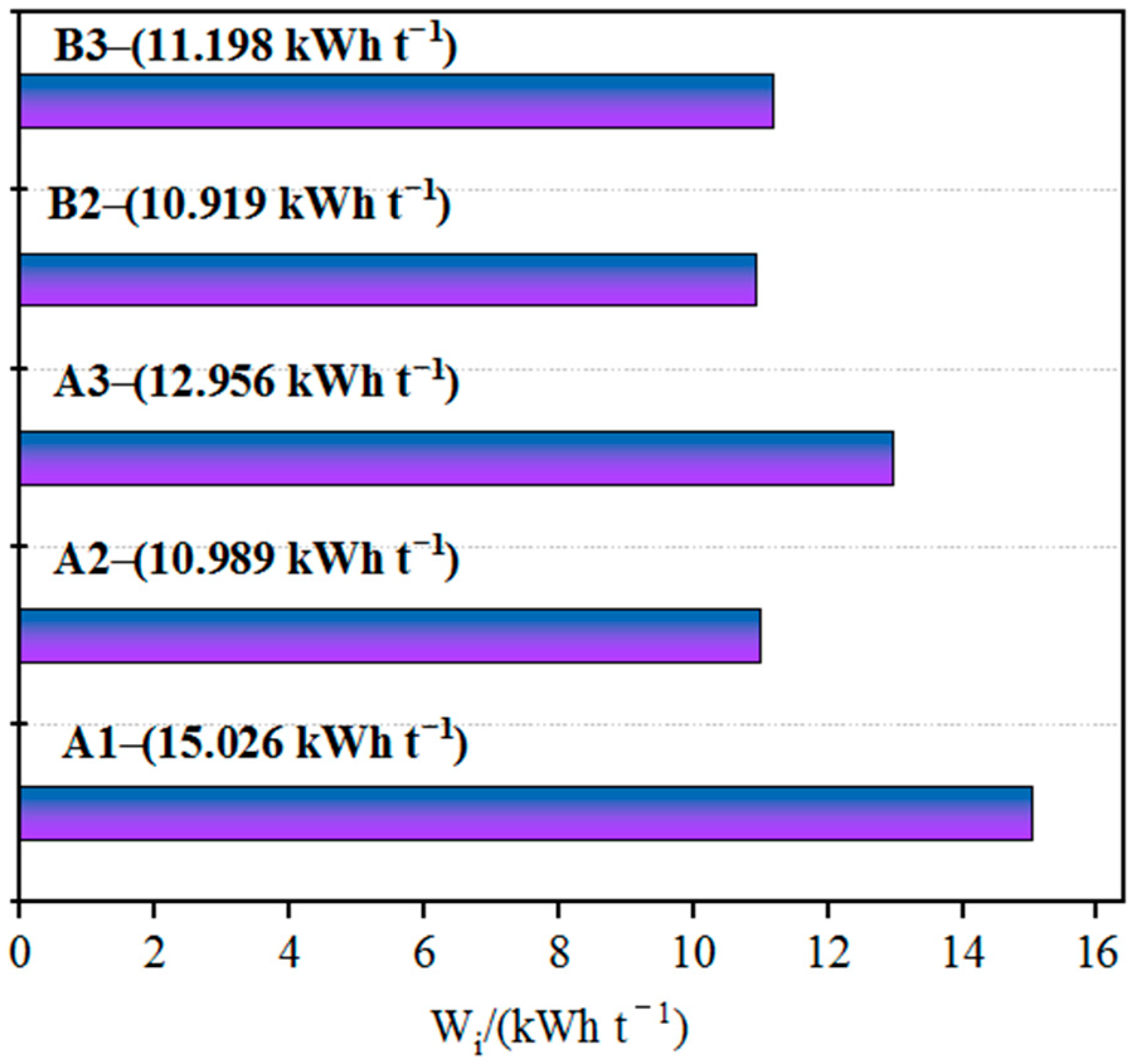
3.1. Grindability
Analysis of the Relationship between Grindability and Ore Mineralogy
3.2. Settling and Filtration Characteristics
3.2.1. Settling Characteristics
3.2.2. Analysis of the Relationship between Settling Performance and Ore Mineralogy
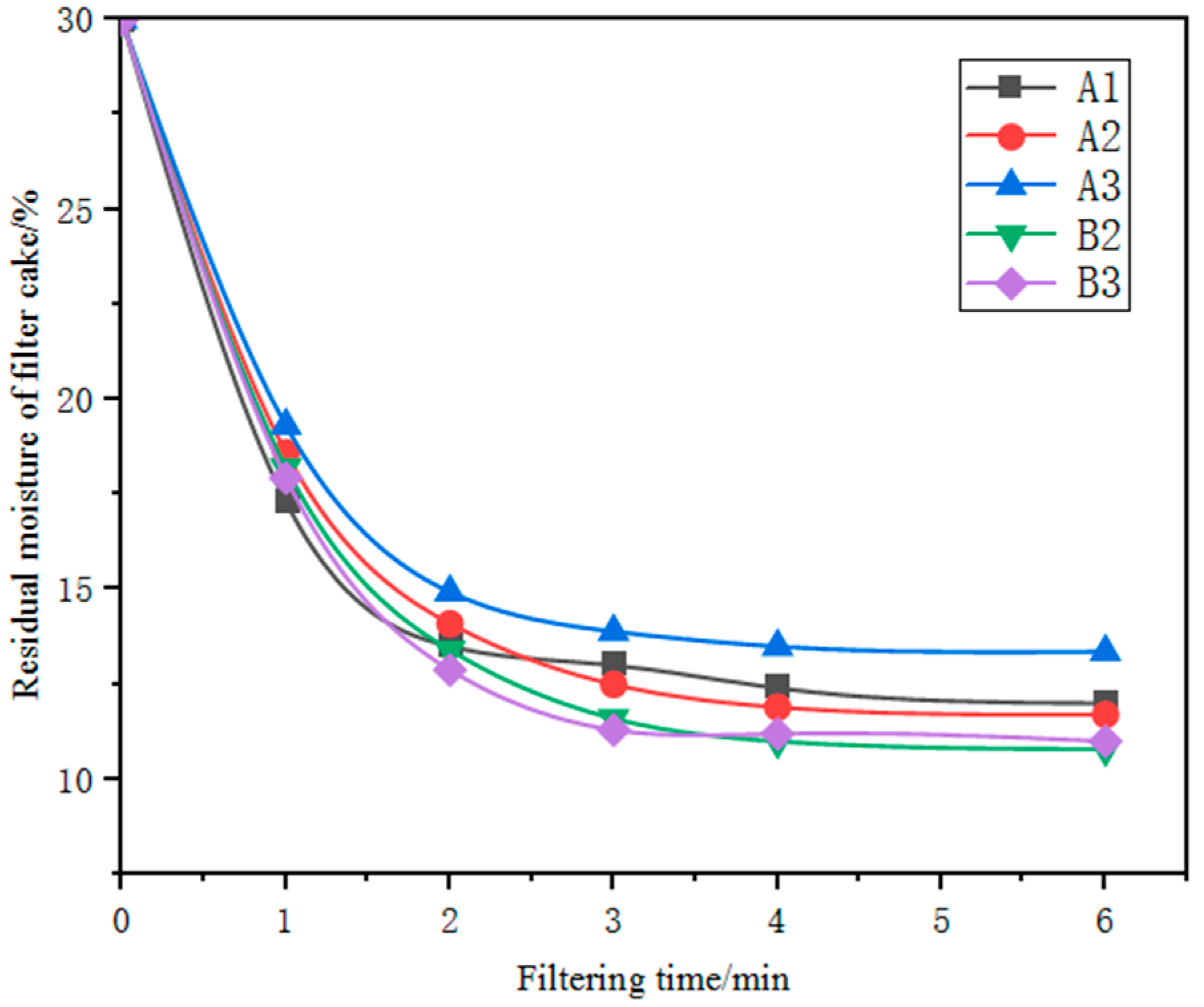
3.2.3. Filtering Characteristics
3.2.4. Analysis of the Relationship between Filtering Performance and Ore Mineralogy
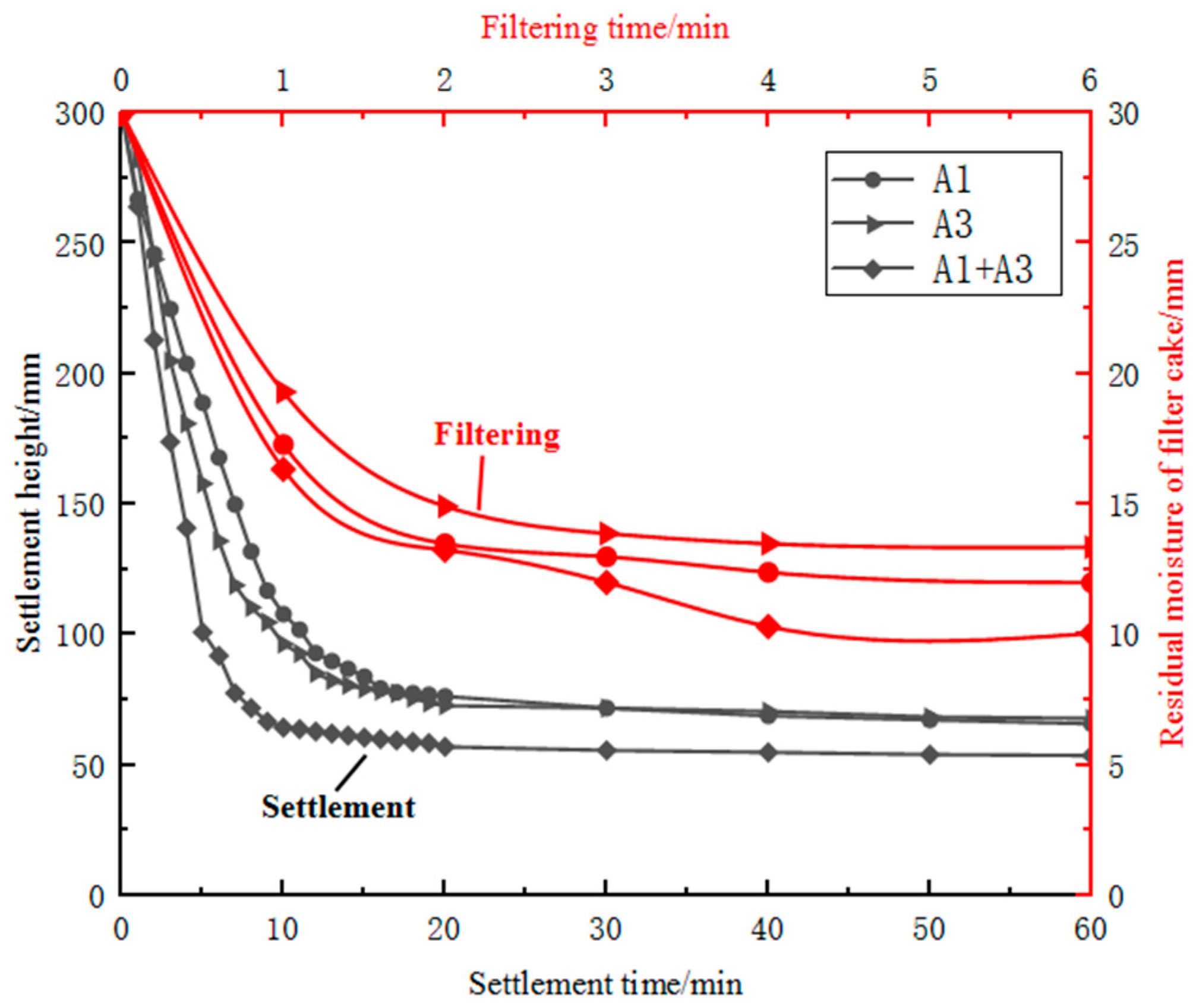
3.2.5. The Effect of Combined Ore Blending on Settlement and Filtration
3.3. Effect on Pellet Preparation
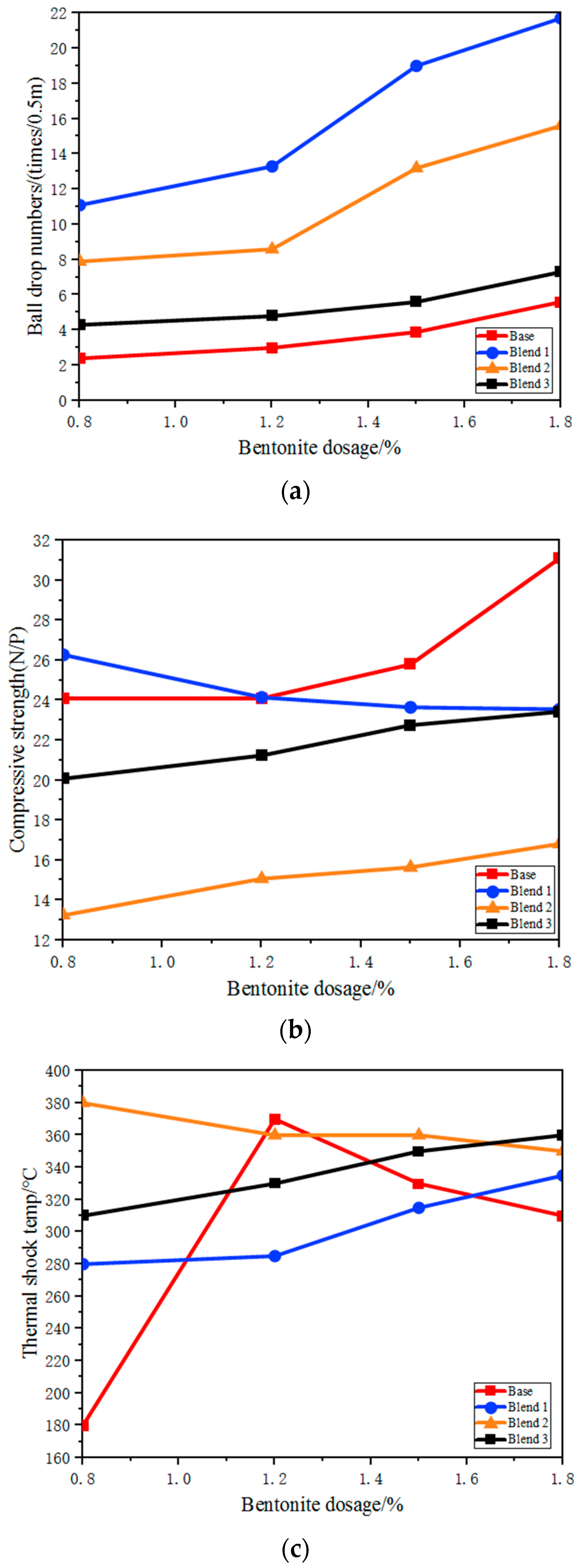
3.3.1. Effect on the Quality of Green Balls
3.3.2. Effect on the Quality of the Fired Pellets
3.3.3. Effect on the Metallurgical Properties of Pellets
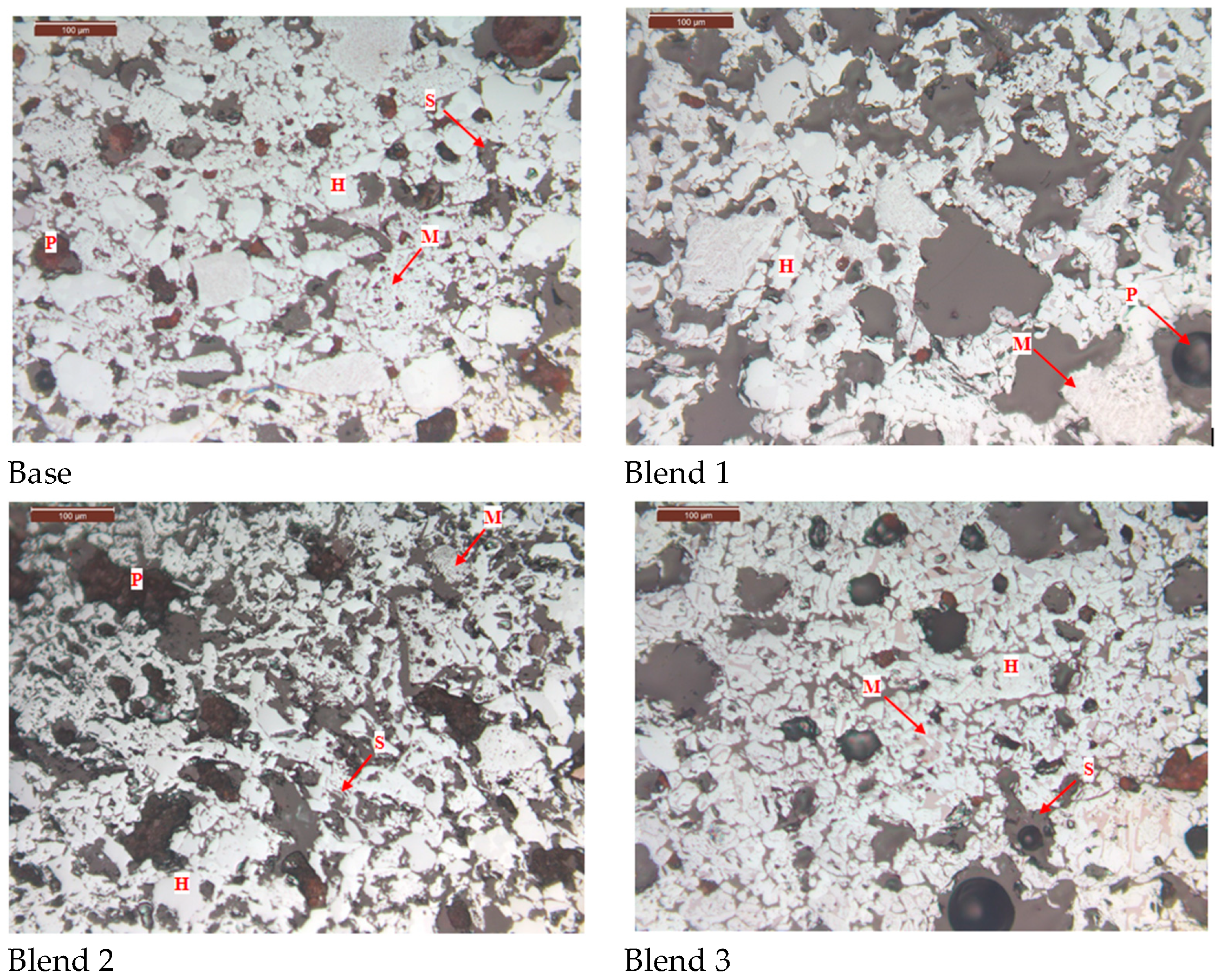
3.3.4. Effect on the Microstructure of Fired Pellets
4. Conclusions
- By improving the technology of fine grinding, various imported ore resources can be well adapted and matched, providing more resource options for pellet feed blending. The Bond work index of the five iron ore fines samples investigated ranged from 10 to 15 kW·h/t, indicating good grindability and they can all be considered as materials that are relatively easy to grind. The static settling performance of the three Australian iron ore fines studied in this article was poorer than that of the Brazilian iron ore fines.
- There was reasonably clear relationship between the inherent mineralogical characteristics of the ores and their grinding, settling and filtration performance. Grindability was largely determined by the hardness of the iron ore and in general, the harder the ore, the more grinding energy required. Generally, iron ore with fewer goethite minerals, fewer soft minerals (such as kaolinite) and coarse particle size has good settling and filtration performance.
- By smart ore blending, it is possible to obtain pellet feeds with excellent settling and filtration performance, which can meet the requirements of the grinding and selection process, maximise the production rate of equipment, reduce operating costs and produce high-quality finely ground iron ore concentrate.
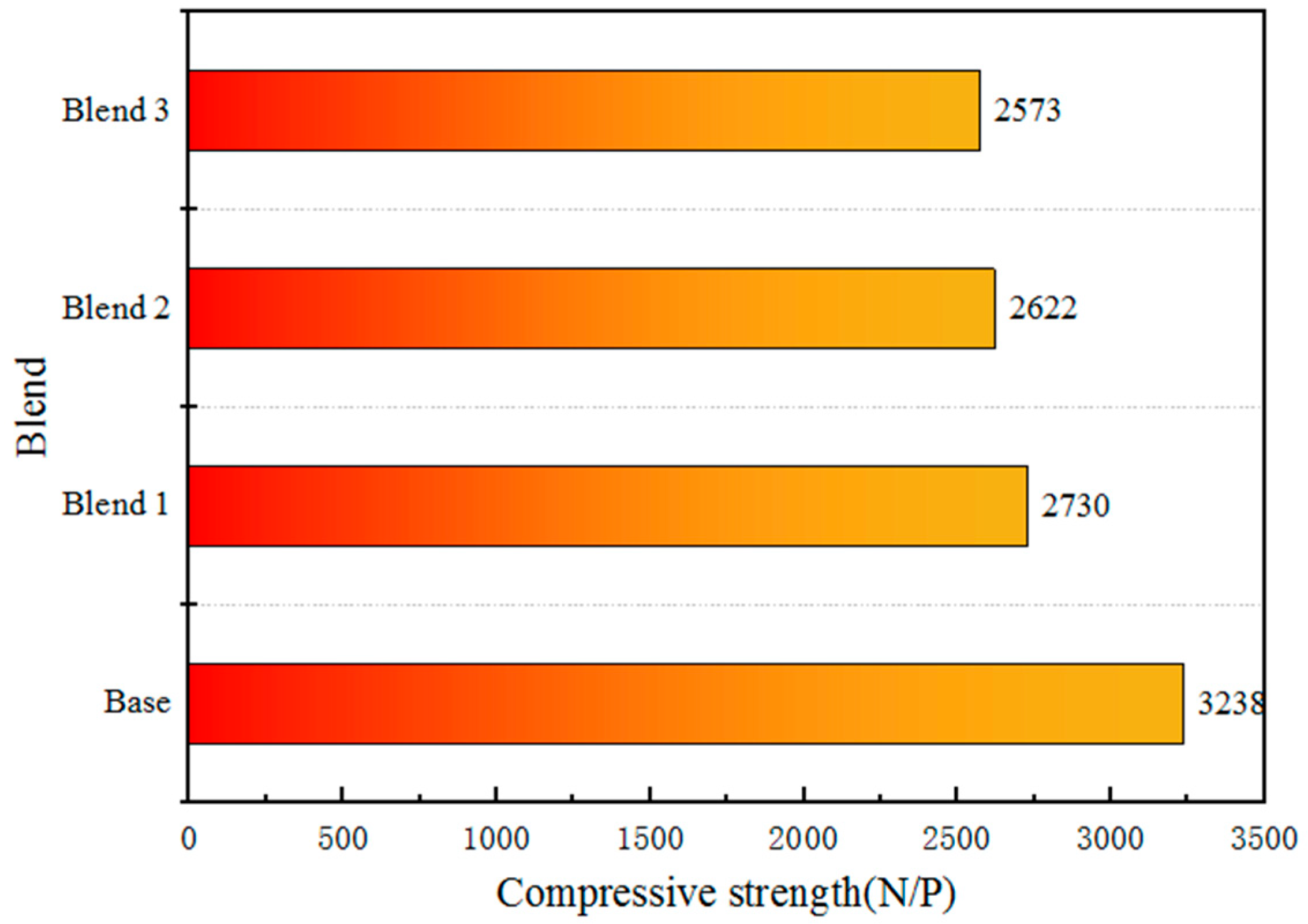
- The addition of finely ground Australian ores improve the balling performance of pellet mixtures. The addition of 40% finely-ground Australian iron ores is capable of improving the metallurgical performance of pellet products. And the fired pellets obtained from blend 1, blend 2 and blend 3 exhibit good metallurgical properties, with reduction index (RI) of 67–68%, low-temperature reduction disintegration index (RDI+3.15) generally greater than 95% and reduction swelling index (RSI) less than 15%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yie, K.W. The present state and the forecasting of our country pelletizing industry. Sinter. Pelletizing 2003, 1, 14. [Google Scholar]
- Mohamed, O.A.; Shalabi, M.E.H.; El-Hussiny, N.A.; Khedr, M.H.; Khedr, F.M. Effect of some additives on flowability and compressibility of lactose powders. Powder Technol. 2003, 130, 277–282. [Google Scholar] [CrossRef]
- Fu, J.Y.; Jiang, T.; Zhu, D.Q. Sintering and Pelletizing; Central South University of Technology Press: Changsha, China, 1996; pp. 313–315. [Google Scholar]
- Peltoniemi, M.; Kallio, R.; Tanhua, A.; Luukkanen, S.; Perämäki, P. Mineralogical and surface chemical characterization of flotation feed and products after wet and dry grinding. Miner. Eng. 2020, 156, 106500. [Google Scholar] [CrossRef]
- Gao, E.X.; Zhang, C.; Li, Y.P.; Luo, J.H.; Gao, R.Z.; Rang, J.C. Effects of Grinding Methods on the Flotation Kinetics of Sphalerite and Pyrite. Metal. Mine 2022, 9, 100–106. [Google Scholar]
- Wills, B.A.; Napier-Munn, T.J. Will’s Mineral Processing Technology, 7th ed.; Elsevier Inc.: Oxford, UK, 2006; pp. 108–117, 267–352. [Google Scholar]
- Zhu, D.Q.; Guo, Z.Q.; Pan, J.; Wang, Z.Y. Insights on pretreatment of Indian hematite fines in grate–kiln pelletizing process: The choice of grinding processes. J. Iron Steel Res. Int. 2018, 25, 506–514. [Google Scholar] [CrossRef]
- Ma, L.; Qing, G.L. Experimental study on pelletizing proportioned with grinded MAC fines. Sinter. Pelletizing 2019, 44, 39–41. [Google Scholar]
- Yang, Y.B.; Zhang, J.; Zhong, Q. Effect of pretreatment on the ballability of hematite in preparation of a fluxed pellet. Min. Metall. Eng. 2019, 39, 83–88. [Google Scholar]
- Ahmadi, R.; Shahsavari, S. Investigation of laboratory conditions effect on prediction accuracy of size distribution of industrial ball mill discharge by using a perfect mixing model. Miner. Eng. 2009, 22, 104–106. [Google Scholar] [CrossRef]
- Gent, M.; Menendez, M.; Torano, J.; Torno, S. Effect of grinding aids on the grinding energy consumed during grinding of calcite in a stirred ball mill. Powder Technol. 2012, 224, 217–222. [Google Scholar] [CrossRef]
- Magdalinovic, N.; Trumic, M.; Trumic, G.; Magdalinovic, S.; Trumic, M. Grinding of hematite ore with a high specific surface area using a planetary ball mill. Int. J. Miner. Process. 2012, 114–117, 48–50. [Google Scholar] [CrossRef]
- Ipek, H.; Ucbas, Y.; Hosten, C. Effect of particle size distribution on grinding kinetics in dry and wet ball milling operations. Miner. Eng. 2005, 18, 981–983. [Google Scholar] [CrossRef]
- GB/T 13241-2017; Iron Ore–Determination of Reducibility. China National Standardization Administration: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF.aspx/GBT13241-2017 (accessed on 14 October 2017).
- GB/T 13240-2018; Iron Ore Pellets for Blast Furnace Feedstocks—Determination of the Free-Swelling Index. China National Standardization Administration: Beijing, China, 2018. Available online: https://www.codeofchina.com/standard/GBT13240-2018.html (accessed on 1 February 2019).
- GB/T 13242-2017; Iron Ores—Low-Temperature Disintegration Test—Method Using Cold Tumbling after Static Reduction. China National Standardization Administration: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT13242-2017 (accessed on 14 October 2017).
- Long, H.M.; Chun, T.J.; Wang, P.; Meng, Q.M.; Di, Z.X.; Li, J.X. Grinding kinetics of vanadium-titanium magnetite concentrate in a damp mill and its properties. Metall. Mater. Trans. B 2017, 48, 815–822. [Google Scholar] [CrossRef]
- Xing, W.; Yang, H.L.; Xi, Z.W. Study on Fine Grinding Technology of Imported Iron Concentrate. Min. Eng. 2020, 18, 43–46. [Google Scholar]
- De Bakker, J. Energy Use of Fine Grinding in Mineral Processing. Metall. Mater. Trans. E 2013, 1, 8–19. [Google Scholar] [CrossRef]
- Zhou, C.M.; Shao, G.Y. Weighted Vickers Hardness and Grindability of Granite. Stone 1997, 1, 18–20; 30. [Google Scholar]
- Su, G.H.; Wang, Z.Q.; Li, H.Y. Application of modifier to improve sedimentation and filtration performance of superfine slurry of laterite nickel ore. World Nonferrous Met. 2017, 16, 247–248. [Google Scholar]
- Cai, F.W.; Zhou, X.T.; Xia, J.P.; Luo, Z.Q.; Man, L.; Zhu, L. Study on Settling Character of Bauxite Tailings Slurry. Bull. Chin. Ceram. Soc. 2014, 33, 1544–1549. [Google Scholar]
- He, J.W.; An, G.; Wang, K.; Kang, H.J.; Zhao, W.C.; Liu, C.J. Shougang Jingtang imported fine grinding technology. Hebei Metall. 2023, 2, 33–36. [Google Scholar]
- Peng, D.S.; Pan, J.; Li, J.; Tian, H.Y.; Yang, C.C.; Shi, X.J.; Sheng, W.J. Experimental study on the preparation of fluxed pellets from fine grinding carajas powder. Sinter. Pelletizing 2021, 4, 50–57. [Google Scholar]
- Yi, L.J.; Jiang, L.H.; Li, J.; Niu, C.S.; Chen, J.H.; Zhu, D.Q.; Yang, C.C.; Tian, H.Y. Technology development and application of diversified low cost clean production of high quality pellet products. Sinter. Pelletizing 2022, 47, 95–103. [Google Scholar]
- Yang, C.C.; Zhu, D.Q.; Pan, J. Oxidation and Induration Characteristics of Pellets Made from Western Australian Ultrafine Magnetite Concentrates and Its Utilization Strategy. J. Iron Steel Res. (Int.) 2016, 23, 924–932. [Google Scholar] [CrossRef]







| Sample | T.Fe | FeO | SiO2 | Al2O3 | CaO | MgO | P | S | LOI |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 60.64 | 0.66 | 3.33 | 2.23 | 0.06 | 0.10 | 0.085 | 0.026 | 6.46 |
| A2 | 62.31 | 0.51 | 4.43 | 1.94 | 0.15 | 0.12 | 0.110 | 0.015 | 4.03 |
| A3 | 60.18 | 0.59 | 4.87 | 2.22 | 0.21 | 0.09 | 0.083 | 0.036 | 7.35 |
| B1 | 67.11 | 0.28 | 3.51 | 0.47 | 0.062 | 0.011 | 0.013 | 0.013 | 0.017 |
| B2 | 62.15 | 0.92 | 4.80 | 1.57 | 0.2 | 0.13 | 0.077 | 0.011 | 3.75 |
| B3 | 65.71 | 1.56 | 1.91 | 1.02 | 0.22 | 0.71 | 0.059 | 0.044 | 2.41 |
| M1 | 64.75 | 26.41 | 8.07 | 0.32 | 0.20 | 0.40 | 0.045 | 0.013 | −2.03 |
| Sample | Size Distribution/mm | ||||||
|---|---|---|---|---|---|---|---|
| +8 | −8 + 4 | −4 + 1 | −1 + 0.5 | −0.5 + 0.25 | −0.25 + 0.074 | −0.074 | |
| A1 | 11.4 | 27.21 | 28.35 | 3.99 | 8.19 | 15.02 | 5.85 |
| A2 | 8.33 | 27.17 | 37.41 | 7.45 | 8.67 | 7.30 | 3.67 |
| A3 | 10.28 | 19.48 | 34.94 | 11.27 | 11.47 | 10.07 | 2.49 |
| B1 | 0 | 0 | 0 | 1.37 | 0.9 | 8.34 | 89.39 |
| B2 | 8.72 | 13.02 | 31.77 | 12.40 | 13.03 | 16.69 | 4.37 |
| B3 | 10.73 | 28.58 | 17.45 | 13.14 | 3.41 | 24.57 | 2.12 |
| M1 | 0 | 0 | 0 | 0.51 | 2.14 | 22.57 | 74.78 |
| Samples | Microstructure | Analysis |
|---|---|---|
| A1 |  | The major minerals in sample A1 are hematite and goethite, accounting for 34 per cent and 60 per cent of the sample, respectively. Sample A1 also contains a relatively high proportion of kaolinite (3 per cent) and quartz (3 per cent). The goethite and magnetite in A1 are interwoven with each other, and there are many internal pores, which often contain magnetite, quartz and kaolinite particles. |
| A2 |  | The major minerals in sample A2 are hematite (68 per cent), goethite (29 per cent), quartz (1 per cent) and kaolinite (2 per cent). In sample A2, the goethite and magnetite are mainly interwoven with fine-grained hematite to form cluster-like aggregates. The hematite occurs as individual bean-shaped particles with large inter-particle pores. The quartz is distributed within the pores of the ore. |
| A3 |  | The major minerals in sample A3 are hematite (46 per cent) and goethite (51 per cent). In sample A3, the goethite occurs as large continuous patches and is intercalated with hematite, magnetite and other ores. There are many internal pores within the grains of goethite. The hematite occurs as individual particles and some flattened particles embedded within the goethite grains. The magnetite mainly exists within the large grains of hematite, and there are many pores. |
| B1 |  | The major minerals in sample B1 are hematite and specularite, with some quartz. The hematite occurs as coarse-grained or platy crystal aggregates with a dense structure and is mostly an independent mineral. Some hematite contains speckled specularite inclusions in a symbiotic and encased structure, while some specularite is interwoven with hematite in a reticular pattern. The gangue minerals consist mainly of quartz, occurring as independent minerals or filling intergrowths with hematite in a banded structure. |
| B2 |  | The major mineral phase in sample B2 is hematite, with a content of up to 79 per cent, mainly occurring as granules and flakes. Sample B2 also contains a certain amount of goethite, typically closely symbiotic with hematite, with a content of around 13 per cent. The presence of goethite is the reason for the relatively high loss on ignition (LOI) of sample B2. Generally, the LOI of iron ore fines should be controlled within a certain range to avoid adverse effects on the pelletisation and product mechanical strength during the firing process. In addition, B2 contains small amounts of quartz and magnetite, mainly occurring as granules, with content of about 4 per cent and 2 per cent, respectively. |
| B3 |  | The major minerals in sample B3 are hematite and goethite, accounting for 90 per cent and 8 per cent, respectively, with small amounts of magnetite, kaolinite and quartz. The goethite occurs as large particles, typically surrounded by kaolinite and hematite, while the hematite particles vary in size, with larger particles typically occurring together with quartz and magnetite and smaller particles usually coexisting with kaolinite or embedded within the goethite. The quartz and kaolinite are typically distributed within the pores between the goethite and hematite particles. |
| Blends | Base | Blend 1 | Blend 2 | Blend 3 |
|---|---|---|---|---|
| Magnetite | 80 | 40 | 40 | 40 |
| B3 | 20 | 20 | 20 | 20 |
| A1 | 0 | 40 | 0 | 0 |
| A2 | 0 | 0 | 40 | 0 |
| A3 | 0 | 0 | 0 | 40 |
| Total | 100 | 100 | 100 | 100 |
| Metallurgical Performance | Reduction Index (RI) | Reduction Swelling Index (RSI) | Low-Temperature Reduction Disintegration Index (LTRDI) |
|---|---|---|---|
| Standards | GB-13241 [14] | GB-13240 [15] | GB-13242 [16] |
| Ore Blend | Base | Blend 1 | Blend 2 | Blend 3 | |
|---|---|---|---|---|---|
| Updraft drying | Air flow rate/m·s−1 | 1.2 | 1.2 | 1.2 | 1.2 |
| Duration/min | 3 | 3 | 3 | 3 | |
| Temperature/°C | 200 | 200 | 200 | 200 | |
| Downdraft drying | Air flow rate/m·s−1 | 1.2 | 1.2 | 1.2 | 1.2 |
| Time/min | 4 | 4 | 4 | 4 | |
| Temperature/°C | 240 | 230 | 220 | 220 | |
| TPH (transit preheating) | Air flow rate/m·s−1 | 2.4 | 2.4 | 2.6 | 2.6 |
| Time/min | 3 | 3 | 3 | 3 | |
| Temperature/°C | 650 | 650 | 650 | 650 | |
| Preheating | Air flow rate/m·s−1 | 2.4 | 2.4 | 2.6 | 2.6 |
| Time/min | 6 | 6 | 6 | 6 | |
| Temperature/°C | 900 | 900 | 950 | 950 | |
| Firing | Air flow rate/m·s−1 | 2.4 | 2.4 | 2.6 | 2.6 |
| Time/min | 12 | 15 | 12 | 15 | |
| Temperature/°C | 1200 | 1250 | 1200 | 1250 | |
| Soaking | Air flow rate/m·s−1 | 1.8 | 1.8 | 1.8 | 1.8 |
| Time/min | 5 | 5 | 5 | 5 | |
| Temperature/°C | 950 | 950 | 950 | 950 | |
| Blend | RI | RSI | LTRDI | ||
|---|---|---|---|---|---|
| +6.3 mm | +3.15 mm | −0.5 mm | |||
| Base | 56.01 | 2.62 | 99.37 | 99.49 | 0.43 |
| Blend 1 | 68.35 | 5.91 | 92.21 | 97.90 | 1.50 |
| Blend 2 | 67.46 | 11.72 | 99.24 | 99.24 | 1.10 |
| Blend 3 | 67.86 | 8.35 | 93.60 | 97.67 | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhou, Q.; Pan, J.; Zhu, D.; Yang, C. Grinding of Australian and Brazilian Iron Ore Fines for Low-Carbon Production of High-Quality Oxidised Pellets. Minerals 2024, 14, 236. https://doi.org/10.3390/min14030236
Zhang W, Zhou Q, Pan J, Zhu D, Yang C. Grinding of Australian and Brazilian Iron Ore Fines for Low-Carbon Production of High-Quality Oxidised Pellets. Minerals. 2024; 14(3):236. https://doi.org/10.3390/min14030236
Chicago/Turabian StyleZhang, Wuju, Qi Zhou, Jian Pan, Deqing Zhu, and Congcong Yang. 2024. "Grinding of Australian and Brazilian Iron Ore Fines for Low-Carbon Production of High-Quality Oxidised Pellets" Minerals 14, no. 3: 236. https://doi.org/10.3390/min14030236
APA StyleZhang, W., Zhou, Q., Pan, J., Zhu, D., & Yang, C. (2024). Grinding of Australian and Brazilian Iron Ore Fines for Low-Carbon Production of High-Quality Oxidised Pellets. Minerals, 14(3), 236. https://doi.org/10.3390/min14030236









