‘Rhythmite’, Ca29(SiO4)8Cl26, an Anthropogenic Phase from the Chelyabinsk Coal Basin (Ural, Russia) with a Complex Modular Structure Related to α-Ca3SiO4Cl2 (‘Albovite’): Crystal Structure, Raman Spectra, and Thermal Expansion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Raman Spectroscopy
2.3. X-Ray Diffraction Data
2.4. Chemical Analysis
2.5. Structural Complexity
| Atom | x | y | z | BVS 1 | BVS 2 EcoN21 | U(eq) |
|---|---|---|---|---|---|---|
| Ca1 | 0.4396(1) | 1/4 | 0.1858(2) | 1.95 | 1.96 | 0.0341(5) |
| Ca2 | 0.55835(6) | 0.40634(7) | 0.45282(8) | 2.10 | 2.04 | 0.0127(2) |
| Ca3 | 0.3723(1) | 1/4 | 0.4963(1) | 2.54 | 2.45 | 0.0177(4) |
| Ca4 | 0.21231(7) | 0.40009(8) | 0.27646(8) | 1.95 | 1.94 | 0.0168(2) |
| Ca5 | 0.0945(2) | 1/4 | 0.5009(2) | 0.96 | 1.03 | 0.0169(7) |
| Ca6 | 0.23618(7) | 0.44771(8) | 0.59401(8) | 1.88 | 1.89 | 0.0168(2) |
| Ca7 | 0.3535(1) | 1/4 | 0.7576(1) | 1.72 | 1.85 | 0.0157(3) |
| Ca8 | 0.40536(7) | 0.42937(8) | 0.92237(8) | 1.94 | 2.02 | 0.0164(2) |
| Ca9 | 0.22429(10) | 1/4 | 0.96939(13) | 1.89 | 1.93 | 0.0184(4) |
| Ca10 | 0.07294(7) | 0.0911(1) | 0.78615(9) | 1.84 | 1.85 | 0.0243(3) |
| Si1 | 0.40373(8) | 0.4265(1) | 0.6563(1) | 3.90 | 3.94 | 0.0107(3) |
| Si2 | 0.23326(9) | 0.4294(1) | 0.8711(1) | 3.98 | 3.97 | 0.0107(3) |
| Cl1 | 0.63220(1) | 1/4 | 0.3546(2) | 0.92 | 0.93 | 0.0216(4) |
| Cl2 | 0.41432(9) | 0.1321(1) | 0.3501(1) | 0.92 | 1.00 | 0.0223(3) |
| Cl3 | 0.5218(1) | 1/4 | 0.5767(2) | 1.14 | 1.12 | 0.0209(4) |
| Cl4 | 0.23090(1) | 1/4 | 0.4031(2) | 1.12 | 1.21 | 0.0274(5) |
| Cl5 | 0.0535(1) | 0.1223(1) | 0.3420(1) | 0.81 | 0.84 | 0.0378(4) |
| Cl6 | −0.0520(1) | 1/4 | 0.5339(2) | 1.12 | 1.19 | 0.0232(4) |
| Cl7 | 0.07256(9) | 0.4414(1) | 0.5794(1) | 1.18 | 1.22 | 0.0294(4) |
| Cl8 | 0.1572(2) | 1/4 | 0.6994(2) | 0.91 | 0.66 | 0.0374(6) |
| Cl9 | 0.18480(8) | 0.6365(1) | 0.6142(1) | 1.01 | 1.03 | 0.0197(3) |
| O1 | 0.3439(2) | 0.3509(3) | 0.6119(3) | 2.10 | 2.00 | 0.0153(8) |
| O2 | 0.4804(2) | 0.4490(2) | 0.5884(3) | 2.06 | 2.02 | 0.0143(8) |
| O3 | 0.3518(2) | 0.5176(2) | 0.6587(3) | 2.00 | 1.97 | 0.0133(8) |
| O4 | 0.4382(2) | 0.3890(2) | 0.7623(3) | 1.89 | 1.89 | 0.0118(8) |
| O5 | 0.2040(2) | 0.4646(2) | 0.7615(3) | 1.94 | 1.89 | 0.0144(8) |
| O6 | 0.2948(2) | 0.3475(2) | 0.8683(3) | 2.11 | 2.06 | 0.0132(8) |
| O7 | 0.2822(2) | 0.5115(2) | 0.9230(3) | 1.94 | 1.86 | 0.0143(8) |
| O8 | 0.1573(2) | 0.3928(2) | 0.9316(3) | 1.89 | 1.93 | 0.0146(8) |
| Si1―O1 | 1.625(4) | Si2―O5 | 1.623(4) |
| ―O2 | 1.628(4) | ―O6 | 1.624(4) |
| ―O3 | 1.638(4) | ―O7 | 1.629(4) |
| ―O4 | 1.642(4) | ―O8 | 1.647(4) |
| <Si1―O> | 1.633 | <Si2―O> | 1.631 |
| Ca1―Cl2 | 2.850(2)2X | Ca2―O2 | 2.332(4) |
| ―Cl5 | 2.763(3)2X | ―O2 | 2.347(4) |
| ―Cl6 | 2.924(3) | ―O3 | 2.422(4) |
| ―Cl9 | 2.891(2)2X | ―O8 | 2.292(4) |
| <Ca1―Cl> | 2.847 | ―Cl1 | 2.978(2) |
| Ca3―O1 | 2.218(4)2X | ―Cl2 | 2.872(2) |
| ―Cl2 | 2.731(2)2X | ―Cl3 | 2.946(2) |
| ―Cl3 | 2.767(3) | <Ca2―O,Cl> | 2.598 |
| ―Cl4 | 2.713(3) | Ca4―O3 | 2.279(4) |
| <Ca3―O,Cl> | 2.563 | ―O5 | 2.501(4) |
| Ca5―Cl4 | 2.667(4) | ―O7 | 2.364(4) |
| ―Cl5 | 2.944(3)2X | ―Cl1 | 3.169(2) |
| ―Cl6 | 2.539(4) | ―Cl4 | 2.841(2) |
| ―Cl7 | 3.096(2)2X | ―Cl5 | 2.868(2) |
| ―Cl8 | 2.848(4) | ―Cl9 | 2.836(2) |
| <Ca5―Cl> | 2.876 | <Ca4―O,Cl> | 2.694 |
| Ca7―O1 | 2.470(4)2X | Ca6―O1 | 2.361(4) |
| ―O4 | 2.550(4)2X | ―O3 | 2.398(4) |
| ―O6 | 2.309(4)2X | ―O5 | 2.308(4) |
| ―Cl6 | 3.206(1) | ―O7 | 2.375(4) |
| <Ca7―O,Cl> | 2.552 | ―Cl7 | 2.802(2) |
| Ca9―O6 | 2.329(4)2X | ―Cl9 | 2.996(2) |
| ―O8 | 2.492(4)2X | <Ca6―O,Cl> | 2.540 |
| ―Cl1 | 2.818(3) | Ca8―O4 | 2.283(4) |
| ―Cl9 | 3.009(2)2X | ―O6 | 2.369(4) |
| <Ca9―O,Cl> | 2.639 | ―O7 | 2.441(4) |
| Ca10―O2 | 2.376(4) | ―Cl5 | 3.181(1) |
| ―O4 | 2.408(4) | ―Cl6 | 2.865(1) |
| ―O5 | 2.413(4) | ―Cl7 | 2.861(2) |
| ―O8 | 2.423(4) | ―Cl7 | 2.882(2) |
| ―Cl3 | 3.138(2) | ―Cl9 | 3.140(2) |
| ―Cl7 | 2.792(2) | <Ca8―O,Cl> | 2.753 |
| ―Cl8 | 3.026(2) | ||
| <Ca10―O,Cl> | 2.653 |
3. Results and Discussion
3.1. Raman Spectroscopy
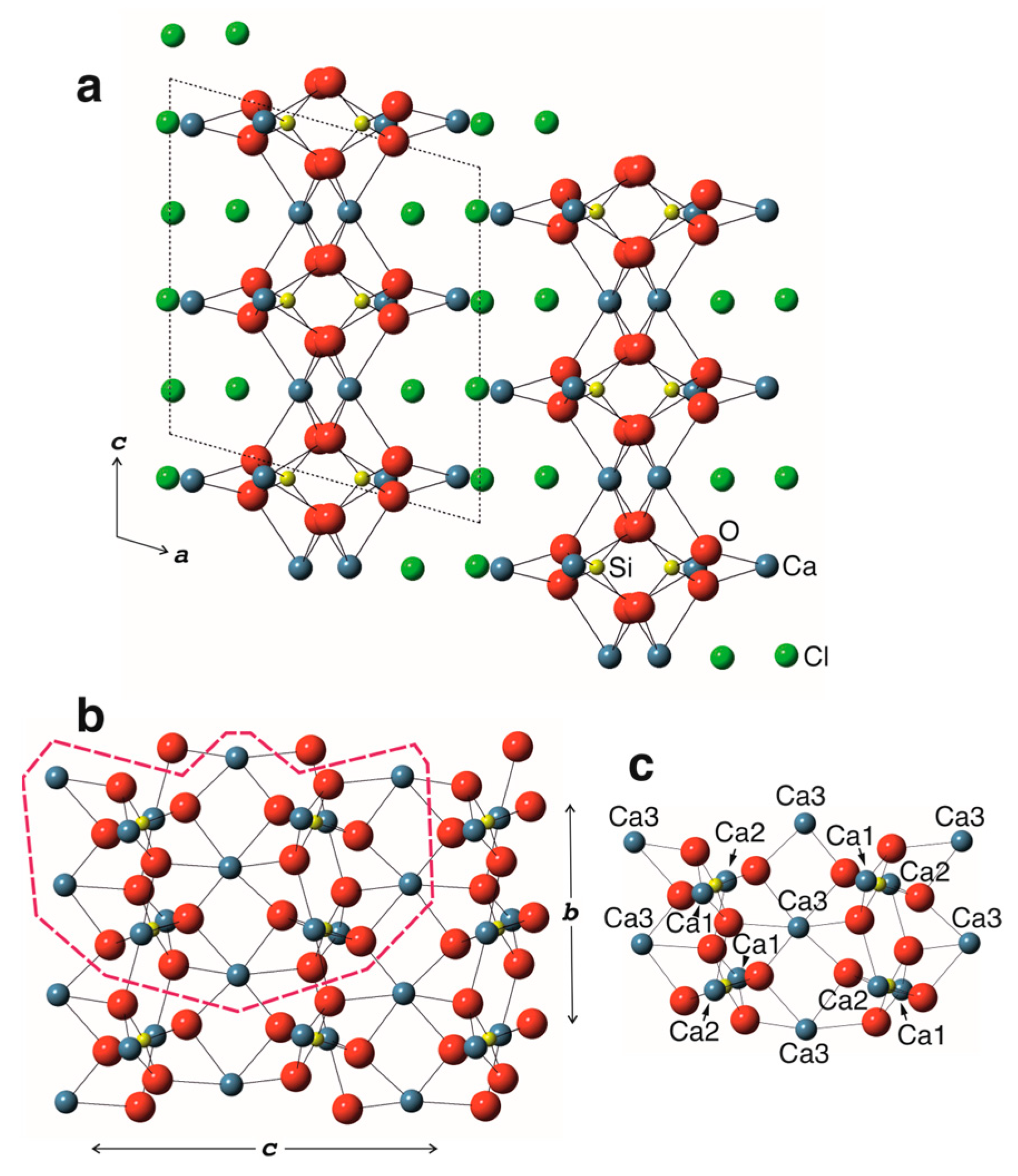
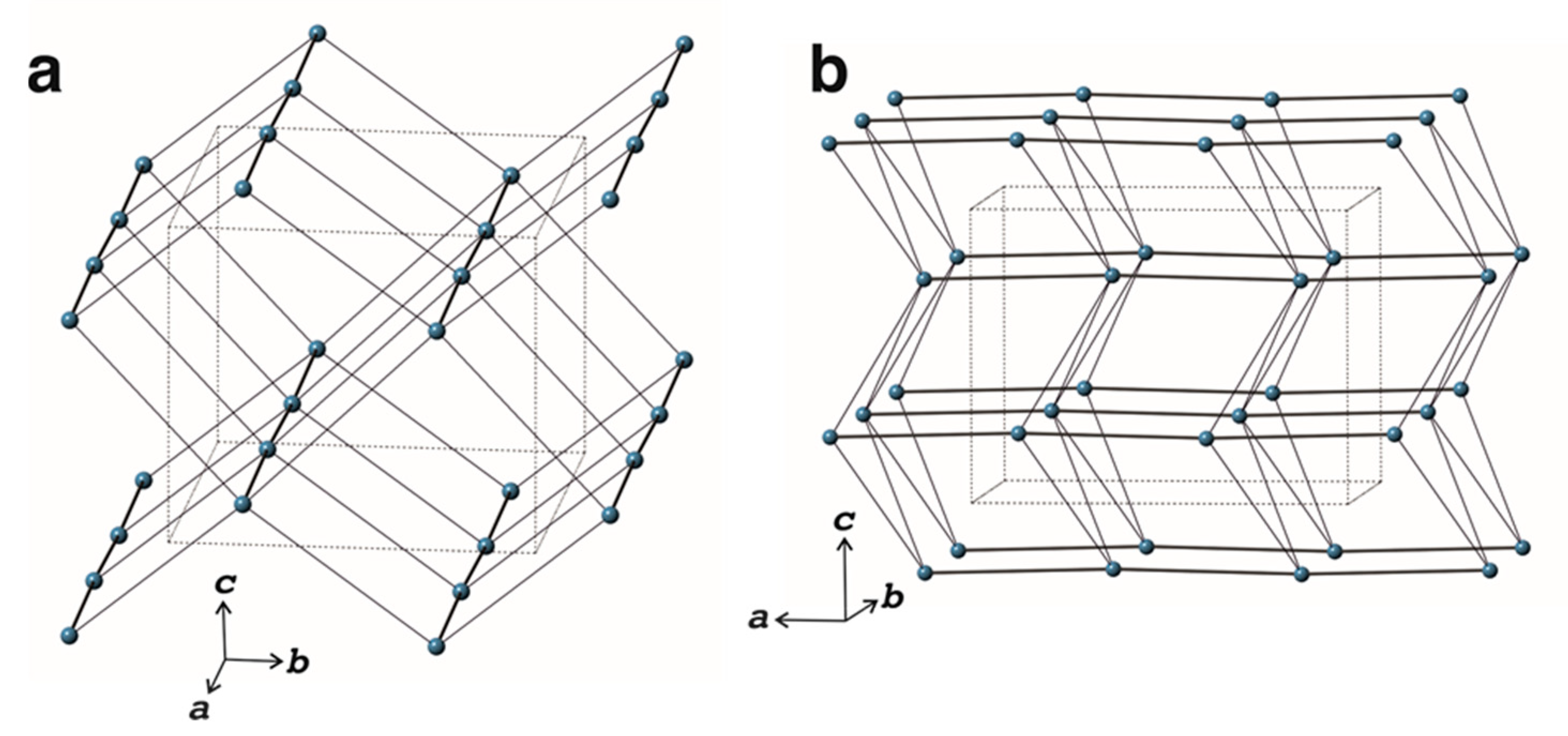
3.2. Crystal Structure
3.2.1. Cation Coordination
3.2.2. Structure Description
3.3. Chemical Composition
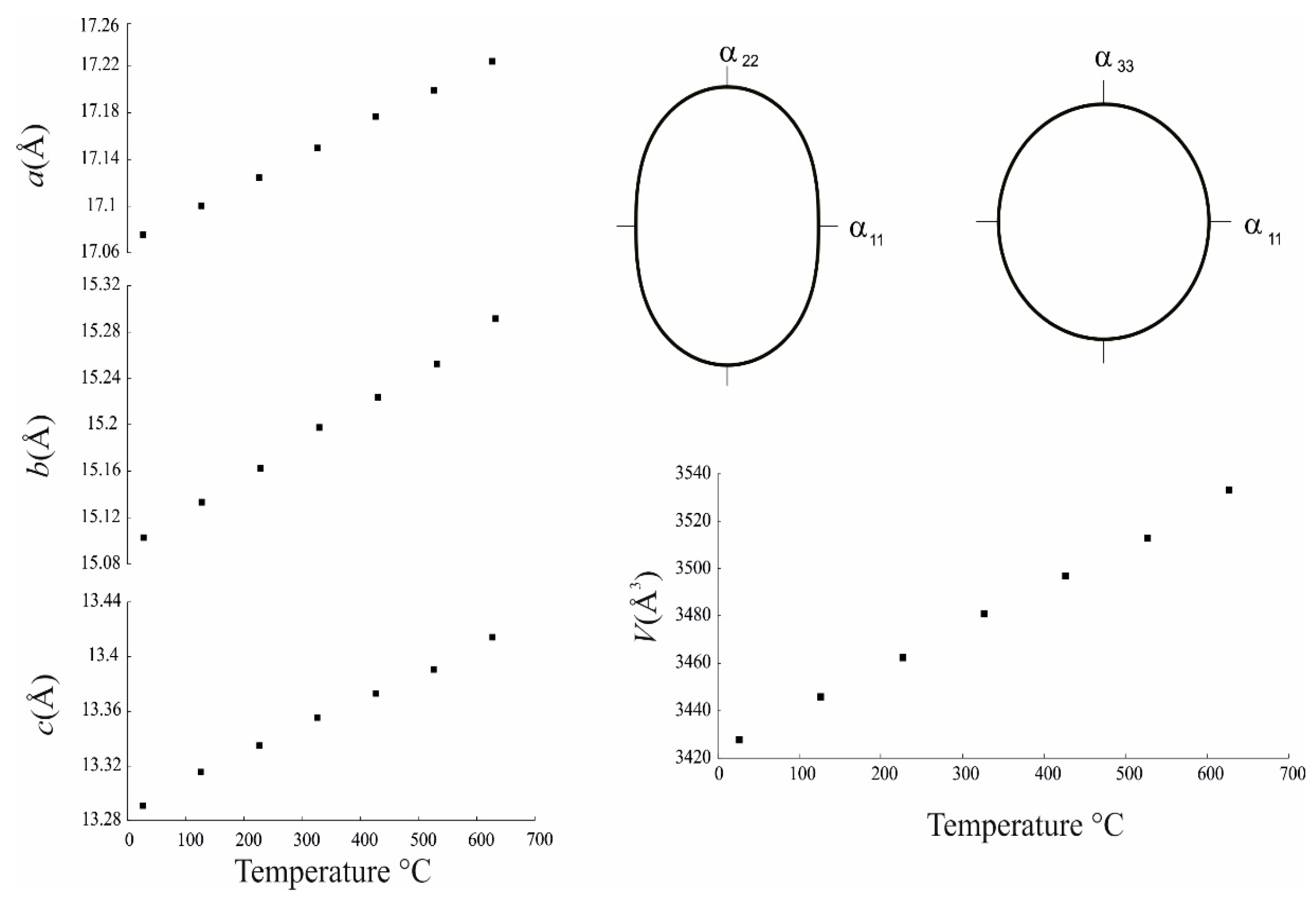
3.4. Thermal Behaviour
3.5. Structural Complexity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chesnokov, B.V.; Shcherbakova, E.P.; Nishanbaev, T.P. Minerals of Burnt Dumps of the Chelyabinsk Coal Basin; Ural Branch of RAS: Miass, Russia, 2008; pp. 1–139. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Bazhenova, L.B. Srebrodolskite Ca2Fe2O5—A new mineral. Zap. Vseross. Miner. Obs. 1985, 114, 195–199. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Polyakov, V.O.; Bushmakin, A.F. Bazhenovite CaS5·CaS2O3·6Ca(OH)2·20H2O—A new mineral. Zap. Vseross. Miner. Obs. 1987, 116, 737–743. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Bazhenova, L.F.; Bushmakin, A.F. Fluorellestadite Ca10[(SO4),(SiO4)]6F2—A new mineral. Zap. Vseross. Miner. Obs. 1987, 116, 743–746. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Lotova, E.V.; Nigmatullina, E.N.; Pavlutchenko, V.S.; Bushmakin, A.F. Dmisteinbergite CaAl2Si2O8 (hexagonal)—A new mineral. Zap. Vseross. Miner. Obs. 1990, 119, 43–46. (In Russian) [Google Scholar]
- Chesnokov, B.V.; Nishanbaev, T.P.; Bazhenova, L.P. Rorisite CaFCl—A new mineral. Zap. Vseross. Miner. Obs. 1990, 119, 73–76. (In Russian) [Google Scholar]
- Shcherbakova, Y.P.; Bazhenova, L.F.; Chesnokov, B.V. Godovikovite-NH4(Al,Fe)(SO4)2 a new ammonium-bearing sulfate. Zap. Vseross. Miner. Obs. 1988, 117, 208–211. (In Russian) [Google Scholar]
- Shcherbakova, Y.P.; Bazhenova, L.F. Efremovite (NH4)2Mg2(SO4)3—Ammonium analogue of langbeinite—A new mineral. Zap. Vseross. Miner. Obs. 1989, 118, 84–87. (In Russian) [Google Scholar]
- Parafiniuk, J.; Hatert, F. New IMA CNMNC guidelines on combustion products from burning coal dumps. Eur. J. Mineral. 2020, 32, 215–217. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Shcherbakova, E.P.; Nishanbaev, T.P. The crystal structure of β-CaMg2(SO4)3, a mineral phase from coal dumps of the Chelyabinsk coal basin. Can. Mineral. 2010, 48, 1469–1475. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Shcherbakova, E.P.; Nishanbaev, T.P. The crystal structure of svyatoslavite and evolution of complexity during crystallization of a CaAl2Si2O8 melt: A structural automata description. Can. Mineral. 2012, 50, 585–592. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Panikorovskii, T.L.; Gurzhiy, V.V.; Bocharov, V.N.; Rassomakhin, M.A. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals 2019, 9, 570. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Zhitova, E.S.; Krzhizhanovskaya, M.G.; Rassomakhin, M.A.; Shilovskikh, V.V.; Krivovichev, S.V. Crystal chemistry and high-temperature behaviour of ammonium phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the burned dumps of the Chelyabinsk Coal Basin. Minerals 2019, 9, 486. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Avdontceva, M.S.; Shilovskikh, V.V.; Rassomakhin, M.A.; Yapaskurt, V.O.; Pekov, I.V. Crystal chemistry of alkali–aluminum–iron sulfates from the burnt mine dumps of the Chelyabinsk Coal Basin, South Urals, Russia. Crystals 2020, 10, 1062. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Avdontceva, M.S.; Zhitova, E.S.; Shchipalkina, N.V.; Pekov, I.V. Crystal chemistry of “Malakhovite”, an anthropogenic analog of khesinite from burnt dumps of the Chelyabinsk Coal Basin (South Urals). Cryst. Rep. 2021, 66, 66–75. [Google Scholar] [CrossRef]
- Zolotarev, A.A., Jr.; Avdontceva, M.S.; Sheveleva, R.M.; Pekov, I.V.; Vlasenko, N.S.; Bocharov, V.N.; Krzhizhanovskaya, M.G.; Zolotarev, A.A.; Rassomakhin, M.A.; Krivovichev, S.V. Zn(NH3)2Cl2, a Mineral-like Anthropogenic Phase with Ammine Complexes from the Burned Dumps of the Chelyabinsk Coal Basin, South Urals, Russia: Crystal structure, Spectroscopy and Thermal Evolution. Minerals 2023, 13, 1109. [Google Scholar] [CrossRef]
- Zolotarev, A.A., Jr.; Avdontceva, M.S.; Krivovichev, S.V.; Sokol, E.V.; Zhitova, E.S.; Chen, J.; Li, Y.; Zolotarev, A.A.; Vlasenko, N.S.; Rassomakhin, M.A. Burned coal dumps as a source of new compounds: The novel mixed-valent iron oxysulfide Ca4Fe2+3Fe3+2O6S4 from the Chelyabinsk Coal Basin, South Ural. ACS Earth Space Chem. 2024, 8, 1429–1439. [Google Scholar] [CrossRef]
- Avdontceva, M.S.; Zolotarev, A.A., Jr.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Sokol, E.V.; Kokh, S.N.; Bocharov, V.N.; Rassomakhin, M.A.; Zolotarev, A.A. Fluorellestadite from burned coal dumps: Crystal structure refinement, vibrational spectroscopy data and thermal behavior. Mineral. Petrol. 2021, 115, 271–281. [Google Scholar] [CrossRef]
- Avdontceva, M.S.; Zolotarev, A.A.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Bocharov, V.N.; Shilovskikh, V.V.; Zolotarev, A.A.; Rassomakhin, M.A. Rapidcreekite of anthropogenic origin—‘Korkinoite’ from burnt mine dump in the Chelyabinsk coal basin, South Urals, Russia: Crystal structure refinement, thermal behaviour and spectroscopic characterization. J. Geosci. 2021, 66, 147–156. [Google Scholar] [CrossRef]
- Brazhnikova, A.S.; Avdontceva, M.S.; Zolotarev, A.A.; Krzhizhanovskaya, M.G.; Bocharov, V.N.; Shilovskikh, V.V.; Rassomakhin, M.A.; Gurzhiy, V.V.; Krivovichev, S.V. Ca3SiO4Cl2—An Anthropogenic phase from burnt Mine Dumps of the Chelyabinsk Coal Basin: Crystal Structure refinement, Spectroscopic Study and Thermal Evolution. Minerals 2023, 13, 668. [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Velisov, V.A.; Bushmakin, A.F.; Kotlyarov, V.A.; Belogub, E.V. New minerals from Burned Dumps of Chelyabinsk Coal basin. In Uralskii Mineralogicheskii. Sbornik; Institute of Mineralogy UrO RAS: Miass, Russian, 1994; pp. 3–34. (In Russian) [Google Scholar]
- CrysAlisPro Software System, Version 1.171.39.44; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with It SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. It OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (Theta to Tensor-TTT). Glass Phys. Chem. 2013, 39, 347–351. [Google Scholar] [CrossRef]
- Downs, R.T. Analysis of harmonic displacement factors. Rev. Mineral. Geochem. 2000, 41, 61–187. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. Sect. A 2012, 68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and chemical complexity of minerals: An update. Miner. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Ilinca, G. Charge Distribution and Bond Valence Sum Analysis of Sulfosalts—The ECoN21 Computer Program. Minerals 2022, 12, 924. [Google Scholar] [CrossRef]
- Frezotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Pauling, L. The principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which nets are the most common? CrystEngComm 2024, 26, 1245–1251. [Google Scholar] [CrossRef]
- Hazen, R.M.; Grew, E.S.; Origlieri, M.J.; Downs, R.T. On the Mineralogy of the “Anthropocene Epoch”. Am. Miner. 2017, 102, 595–611. [Google Scholar] [CrossRef]
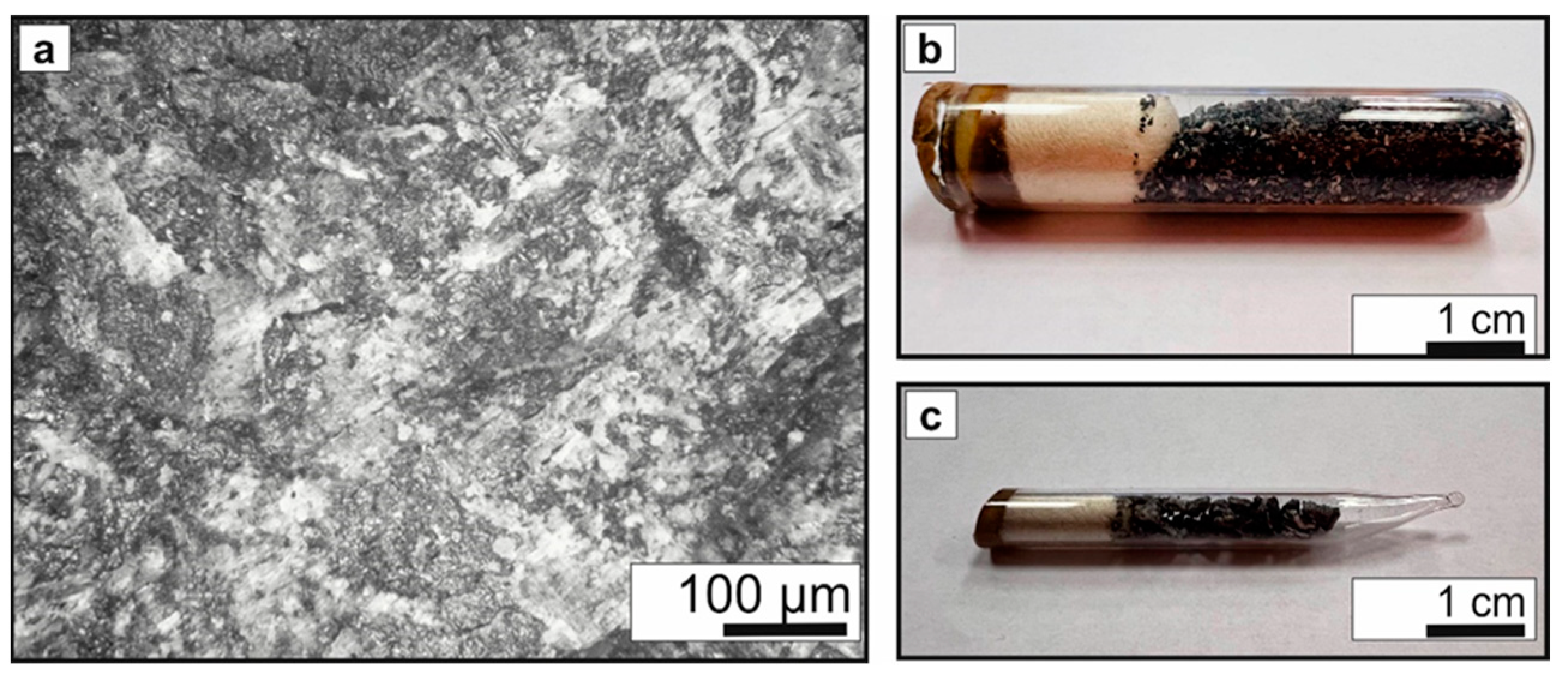
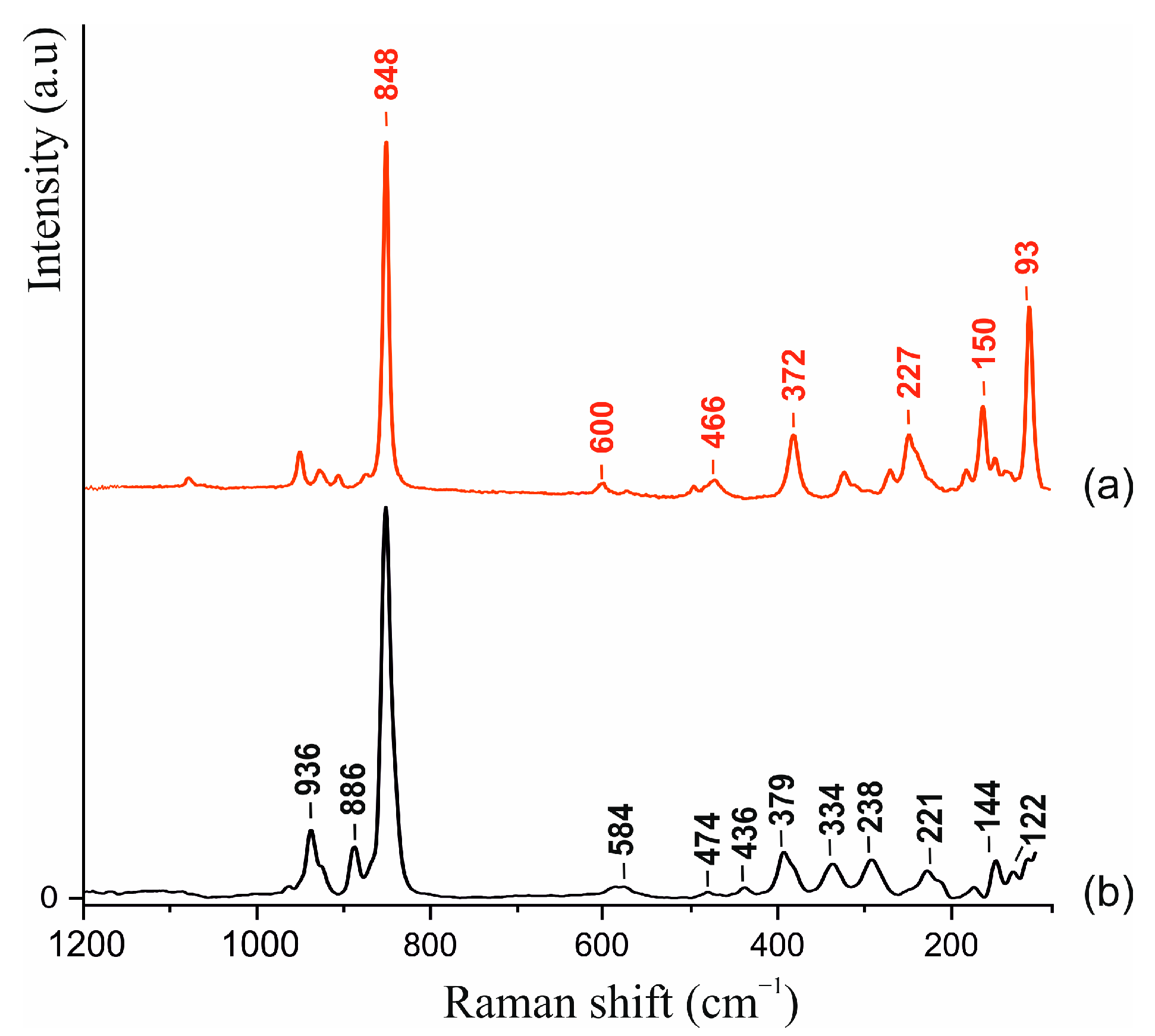

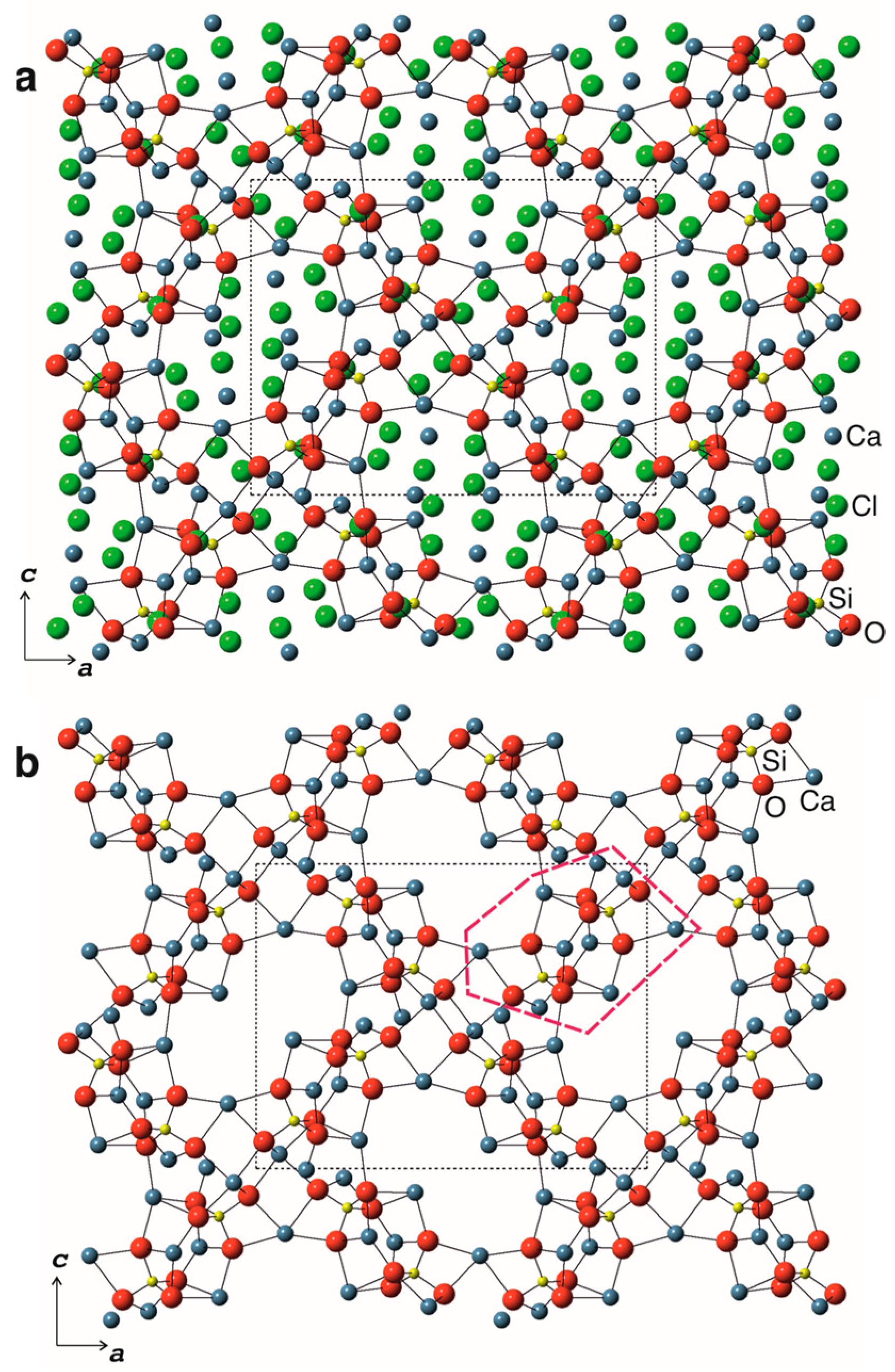
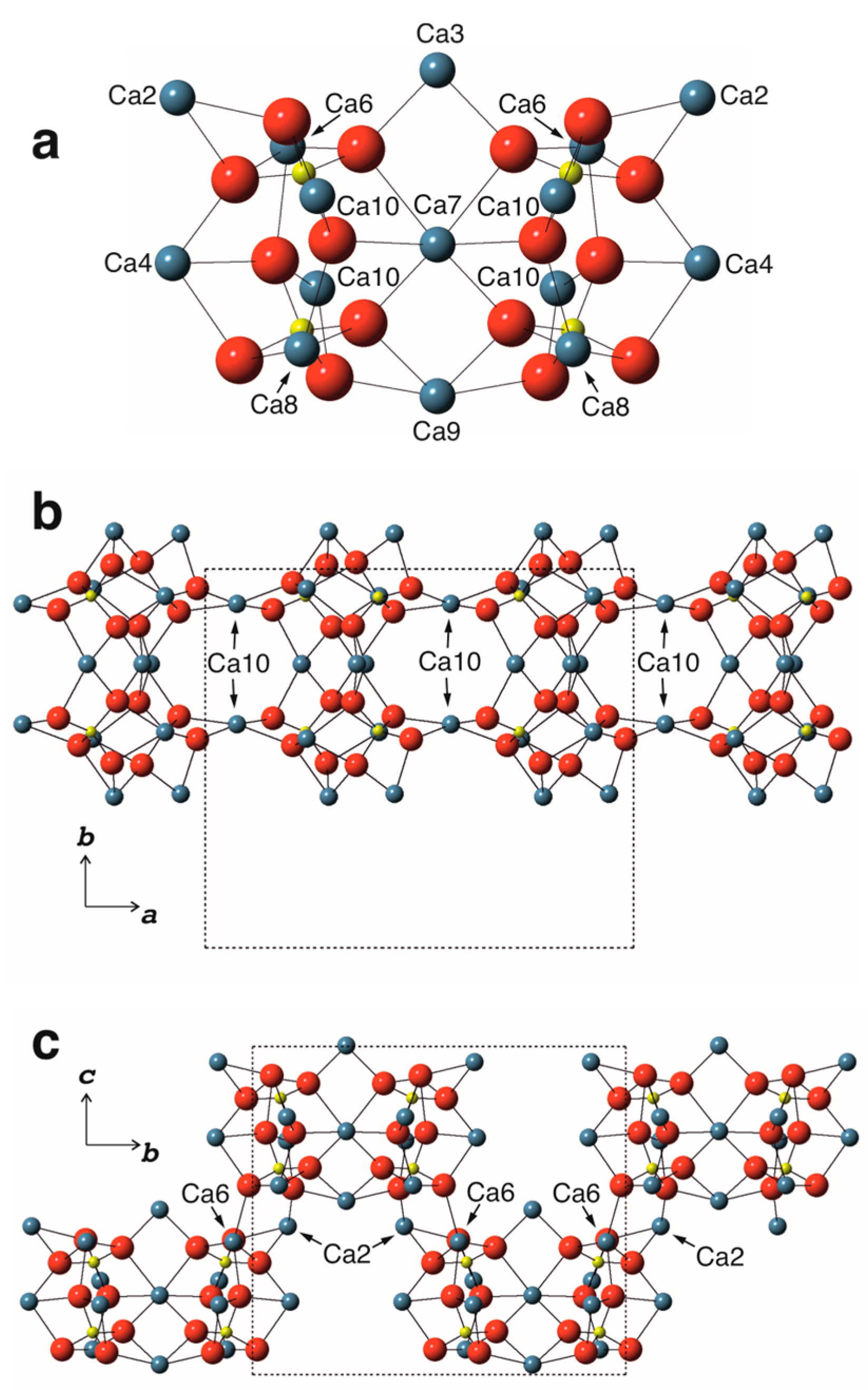
| Sample | 1 | 2 |
|---|---|---|
| Crystal system | orthorhombic | orthorhombic |
| Temperature (°C) | 27 | 327 |
| Space group | Pnma | |
| a, Å | 17.0749(6) | 17.1494(7) |
| b, Å | 15.1029(5) | 15.1980(7) |
| c, Å | 13.2907(4) | 13.3550(5) |
| Volume, Å3 | 3427.42(18) | 3480.8(3) |
| Z | 2 | |
| Dcalc (g/cm3) | 2.736 | 2.691 |
| μ/mm−1 | 3.423 | 3.365 |
| F(000) | 2783.0 | 2780.0 |
| Radiation | MoKα (λ = 0.71073) | |
| 2Θ range for data collection/° | 6.58 to 59.418 | 6.548 to 59.408 |
| Index ranges | −16 ≤ h ≤ 21, −19 ≤ k ≤ 20, −16 ≤ l ≤ 12 | |
| Reflections collected | 18,310 | 18,679 |
| Independent reflections | 4179 [Rint = 0.0623, Rsigma = 0.0610] | 4245 [Rint = 0.0843, Rsigma = 0.0894] |
| Data/restraints/parameters | 4179/0/232 | 4245/0/232 |
| Goodness-of-fit on F2 | 1.066 | 1.072 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0449, wR2 = 0.0875 | R1 = 0.0569, wR2 = 0.1007 |
| Final R indexes [all data] | R1 = 0.0869, wR2 = 0.1112 | R1 = 0.1348, wR2 = 0.1359 |
| Largest diff. peak/hole/e Å−3 | 2.30/−1.19 | 1.55/−1.10 |
| Component | 1 | 2 | Component | 2 | Apfu (Cl + O = 58) |
|---|---|---|---|---|---|
| Ca | 41.22 | 40.11 | CaO | 57.13 | 29.02 |
| Fe | 0.38 | - | |||
| Si | 8.05 | 7.79 | SiO2 | 16.65 | 7.89 |
| P | - | 0.09 | P2O5 | 0.125 | 0.05 |
| Al | 0.22 | 0.05 | Al2O3 | 0.09 | 0.05 |
| Cl | 31.06 | 30.16 | Cl | 32.32 | 26.00 |
| F | 0.22 | - | –O=Cl2 | 7.283 | |
| Total | 99.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdontceva, M.S.; Zolotarev, A.A.; Brazhnikova, A.S.; Bocharov, V.N.; Vlasenko, N.S.; Rassomakhin, M.A.; Krivovichev, S.V. ‘Rhythmite’, Ca29(SiO4)8Cl26, an Anthropogenic Phase from the Chelyabinsk Coal Basin (Ural, Russia) with a Complex Modular Structure Related to α-Ca3SiO4Cl2 (‘Albovite’): Crystal Structure, Raman Spectra, and Thermal Expansion. Minerals 2024, 14, 1048. https://doi.org/10.3390/min14101048
Avdontceva MS, Zolotarev AA, Brazhnikova AS, Bocharov VN, Vlasenko NS, Rassomakhin MA, Krivovichev SV. ‘Rhythmite’, Ca29(SiO4)8Cl26, an Anthropogenic Phase from the Chelyabinsk Coal Basin (Ural, Russia) with a Complex Modular Structure Related to α-Ca3SiO4Cl2 (‘Albovite’): Crystal Structure, Raman Spectra, and Thermal Expansion. Minerals. 2024; 14(10):1048. https://doi.org/10.3390/min14101048
Chicago/Turabian StyleAvdontceva, Margarita S., Andrey A. Zolotarev, Anastasia S. Brazhnikova, Vladimir N. Bocharov, Natalia S. Vlasenko, Mikhail A. Rassomakhin, and Sergey V. Krivovichev. 2024. "‘Rhythmite’, Ca29(SiO4)8Cl26, an Anthropogenic Phase from the Chelyabinsk Coal Basin (Ural, Russia) with a Complex Modular Structure Related to α-Ca3SiO4Cl2 (‘Albovite’): Crystal Structure, Raman Spectra, and Thermal Expansion" Minerals 14, no. 10: 1048. https://doi.org/10.3390/min14101048
APA StyleAvdontceva, M. S., Zolotarev, A. A., Brazhnikova, A. S., Bocharov, V. N., Vlasenko, N. S., Rassomakhin, M. A., & Krivovichev, S. V. (2024). ‘Rhythmite’, Ca29(SiO4)8Cl26, an Anthropogenic Phase from the Chelyabinsk Coal Basin (Ural, Russia) with a Complex Modular Structure Related to α-Ca3SiO4Cl2 (‘Albovite’): Crystal Structure, Raman Spectra, and Thermal Expansion. Minerals, 14(10), 1048. https://doi.org/10.3390/min14101048








