Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis
2.2. X-ray Spectral Analysis
2.3. Single-Crytsal X-ray Diffraction
3. Results and Discussion
3.1. Chemical Composition
3.2. Crystal Structure Solution
3.3. Na2CoSiO4, a Structural Analog of the Mineral Liberite
3.3.1. The Structural Description and Analysis of Interatomic Distances
3.3.2. Na2CoSiO4 and the Liberite Structural Family of Ionic Conductors
3.4. Na2Cu3O(Cu0.8Na0.2)(PO4)2Cl with Oxo-Centered Pyroxene-like Chains
3.4.1. Analysis of Interatomic Distances and Description of the Crystal Structure
3.4.2. Na2Cu3O(Cu0.8Na0.2)(PO4)2Cl in the Series of Compounds with Oxo-Centered Pyroxene-like Chains
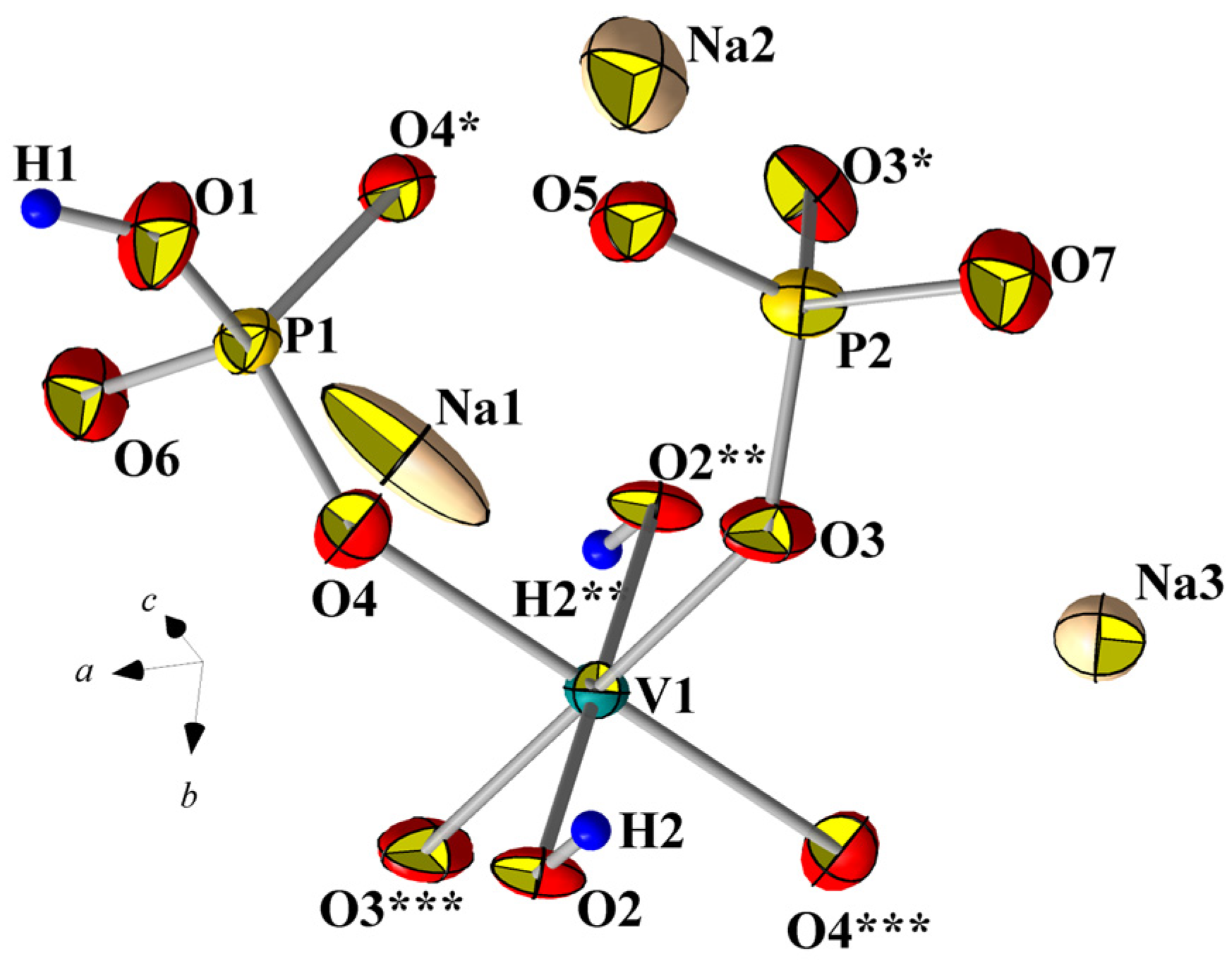
3.5. Mineralogically Probable Na3V(OH)(HPO4)(PO4)
3.5.1. Description of Crystal Structure and Analysis of Interatomic Distances
3.5.2. Magnetic Properties of Na3V(OH)(HPO4)(PO4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, G.; Abeysinghe, D.; Felder, J.B.; Egodawatte, S.; Ferreira, T.; zur Loye, H.-C. Where do new materials come from? Neither the stork nor the birds and the bees! In search of the next “first material”. J. South Carolina Acad. Sci. 2017, 15, 2. [Google Scholar]
- McMillen, C.D.; Kolis, J.W. Hydrothermal synthesis as a route to mineralogically-inspired structures. Dalton Trans. 2016, 45, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Yakubovich, O.V.; Steele, I.M.; Kiriukhina, G.V.; Dimitrova, O.V. A microporous potassium vanadyl phosphate analogue of mahnertite: Hydrothermal synthesis and crystal structure. Z. Kristallog. Cryst. Mater. 2015, 230, 337–344. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Kiriukhina, G.V.; Dimitrova, O.V.; Shvanskaya, L.V.; Volkova, O.S.; Vasiliev, A.N. A novel cobalt sodium phosphate hydroxide with the ellenbergerite topology: Crystal structure and physical properties. Dalton Trans. 2015, 44, 11827–11834. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Khasanova, N.R.; Antipov, E.V. Mineral-Inspired Materials: Synthetic Phosphate Analogues for Battery Applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, B69, 249–259. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Han-ching, C. The crystal structure of liberite. Kexue Tongbao 1966, 17, 425–428. [Google Scholar]
- Armstrong, A.R.; Kuganathan, N.; Islam, M.S.; Bruce, P.G. Structure and lithium transport pathways in Li2FeSiO4 cathodes for lithium batteries. JACS 2011, 133, 13031–13035. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, M.; Mohamed, Z.; Ling, C.D. Magnetic structures of βI-Li2CoSiO4 and γ0-Li2MnSiO4: Crystal structure type vs. magnetic topology. J. Solid State Chem. 2014, 216, 42–48. [Google Scholar] [CrossRef]
- Nalbandyan, V.B.; Zvereva, E.A.; Shukaev, I.L.; Gordon, E.; Politaev, V.V.; Whangbo, M.H.; Petrenko, A.A.; Denisov, R.S.; Markina, M.M.; Tzschoppe, M.; et al. A2MnXO4 Family (A = Li, Na, Ag; X = Si, Ge): Structural and Magnetic Properties. Inorg. Chem. 2017, 56, 14023–14039. [Google Scholar] [CrossRef] [PubMed]
- Treacher, J.C.; Wood, S.M.; Islam, M.S.; Kendrick, E. Na2CoSiO4 as a cathode material for sodium-ion batteries: Structure, electrochemistry and diffusion pathways. Phys. Chem. Chem. Phys. 2016, 18, 32744–32752. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, J.; Zhao, Y.; Wu, Y.; Zhang, X.; Wang, L.; Liu, X.; Rui, Y.; Xu, J. Na2CoSiO4 as a novel positive electrode material for sodium-ion capacitors. Mater. Lett. 2015, 158, 300–303. [Google Scholar] [CrossRef]
- Wang, J.; Hoteling, G.; Shepard, R.; Wahila, M.; Wang, F.; Smeu, M.; Liu, H. Reaction Mechanism of Na-Ion Deintercalation in Na2CoSiO4. J. Phys. Chem. C 2022, 126, 16983–16992. [Google Scholar] [CrossRef]
- Trabelsi, K.; Karoui, K.; Jomni, F.; Rhaiem, A.B. Optical and AC conductivity behavior of sodium orthosilicate Na2CoSiO4. J. Alloys Compd. 2021, 867, 159099. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Agakhanov, A.A.; Polekhovsky, Y.S. Aleutite [Cu5O2](AsO4)(VO4)·(Cu0.5□0.5)Cl, a new complex salt-inclusion mineral with Cu2+ substructure derived from Kagome-net. Mineral. Mag. 2019, 83, 847–853. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Kiriukhina, G.V.; Simonov, S.V.; Volkov, A.S.; Dimitrova, O.V. (Na,Li)3(Cl,OH)[Cu3OAl(PO4)3]: A first salt-inclusion aluminophosphate oxocuprate with a new type of crystal structure. Acta Crystallogr. 2023, B79, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V.; Filatov, S.K. Structural principles for minerals and inorganic compounds containing anion-centered tetrahedra. Am. Mineral. 1999, 84, 1099–1106. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Mentre, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Etheredge, K.M.; Hwu, S.J. A novel copper (I/II) oxophosphate chloride with a quasi-one-dimensional μ4-oxo-bridged copper (II) chain. Crystal structure and magnetic properties of [Na2CuII3(PO4)2][CuIOCl]. Inorg. Chem. 1996, 35, 5278–5282. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Krivovichev, S.V. Na2Cu+[Cu2+3O](AsO4)2Cl and Cu3[Cu3O]2(PO4)4Cl2: Two new structure types based upon chains of oxocentered tetrahedra. Z. Kristallogr. Cryst. Mater. 2022, 237, 343–350. [Google Scholar] [CrossRef]
- Jin, T.T.; Liu, W.; Chen, S.; Prots, Y.; Schnelle, W.; Zhao, J.T.; Kniep, R.; Hoffmann, S. Crystal structure and magnetic properties of NaCuII[(CuII3O)(PO4)2Cl]. J. Solid State Chem. 2012, 192, 47–53. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Shvanskaya, L.V.; Kiriukhina, G.V.; Volkov, A.S.; Dimitrova, O.V.; Vasiliev, A.N. Hydrothermal synthesis and a composite crystal structure of Na6Cu7BiO4(PO4)4[Cl,(OH)]3 as a candidate for quantum spin liquid. Inorg. Chem. 2021, 60, 11450–11457. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K.; Seraphimova, Y.K.; Varaksina, T.V. Refractive indices of minerals and synthetic compounds. Zap. Vses. Mineral. Obshch. 1988, 117, 459–461. (In Russian) [Google Scholar]
- Varaksina, T.V.; Fundamensky, V.S.; Filatov, S.K.; Vergasova, L.P. The crystal structure of kamchatkite, a new naturally occurring oxychloride sulphate of potassium and copper. Mineral. Mag. 1990, 54, 613–616. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Krivovichev, S.V.; Semenova, T.F.; Filatov, S.K.; Ananiev, V.V. Chloromenite, Cu9O2(SeO3)4Cl6, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Eur. J. Mineral. 1999, 11, 119–123. [Google Scholar] [CrossRef]
- Berlepsch, P.; Armbruster, T.; Brugger, J.; Bykova, E.Y.; Kartashov, P.M. The crystal structure of vergasovaite Cu3O[(Mo,S)O4SO4], and its relation to synthetic Cu3O[MoO4]2. Eur. J. Mineral. 1999, 11, 101–110. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Zaitsev, A.N.; Polekhovsky, Y.S.; Wenzel, T.; Spratt, J. Dokuchaevite, Cu8O2(VO4)3Cl3, a new mineral with remarkably diverse Cu2+ mixed-ligand coordination environments. Mineral. Mag. 2019, 83, 749–755. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Zelenski, M.E.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Fadeeva, O.A.; Pushcharovsky, D.Y. Yaroshevskite, Cu9O2(VO4)4Cl2, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Mineral. Mag. 2013, 77, 107–116. [Google Scholar] [CrossRef]
- Zelenski, M.E.; Zubkova, N.V.; Pekov, I.V.; Polekhovsky, Y.S.; Pushcharovsky, D.Y. Cupromolybdite, Cu3O(MoO4)2, a new fumarolic mineral from the Tolbachik volcano, Kamchatka Peninsula, Russia. Eur. J. Mineral. 2012, 24, 749–757. [Google Scholar] [CrossRef]
- Ferdov, S.; Reis, M.S.; Lin, Z.; Ferreira, R.A.S. Hydrothermal synthesis, crystal structure, and magnetic properties of a new inorganic vanadium (III) phosphate with a chain structure. Inorg. Chem. 2008, 47, 10062–10066. [Google Scholar] [CrossRef] [PubMed]
- Lii, K.H.; Wang, S.L. Na3M(OH)(HPO4)(PO4), M = Al, Ga: Two phosphates with a chain structure. J. Solid State Chem. 1997, 128, 21–26. [Google Scholar] [CrossRef]
- Kolitsch, U.; Taylor, M.; Fallon, G.; Pring, A. Springcreekite, BaV3+3(PO4)2(OH,H2O)6, a new member of the crandallite group, from the Spring Creek mine, South Australia: The first natural V3+-member of the alunite family. Neues Jahrb. Miner. Monatsh. 1999, 1999, 529–544. [Google Scholar]
- Pring, A.; Kolitsch, U.; Birch, W.D.; Beyer, B.D.; Elliott, P.; Ayyappan, P.; Ramanan, A. Bariosincosite, a new hydrated barium vanadium phosphate, from the Spring Creek Mine, South Australia. Mineral. Mag. 1999, 63, 735–741. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, W.C.; Wang, S.L.; Lii, K.H. Hydrothermal Synthesis and Structural Characterization of Four Layered Vanadyl(IV) Phosphate Hydrates A(VO)2(PO4)2·4H2O (A = Co, Ca, Sr, Pb). Inorg. Chem. 1992, 31, 4743–4748. [Google Scholar] [CrossRef]
- Roca, M.; Marcos, M.D.; Amoros, P.; Alamo, J.; Beltran-Porter, A.; Beltran-Porter, D. Synthesis and crystal structure of a novel lamellar barium derivative: Ba(VOPO4)2·4H2O. Synthetic pathways for layered oxovanadium phosphate hydrates M(VOPO4)2·nH2O. Inorg. Chem. 1997, 36, 3414–3421. [Google Scholar] [CrossRef]
- Franke, W.A.; Luger, P.; Weber, M.; Ivanova, T.I. Low hydrothermal growth of sincosite Ca(VO/PO4)2·4H2O. Zap. Vseross Miner. Obs. 1997, 126, 85–86. (In Russian) [Google Scholar]







| (I) | (II) | (III) |
|---|---|---|
| Na2CoSiO4 | Na2(Cu+0.8Na0.2)Cl[Cu2+3O(PO4)2] | Na3V(OH)(HPO4)(PO4) |
 |  |  |
| T 450 °C, P 500 atm | T 450 °C, P 500 atm | T 270 °C, P 70 atm |
| 2 g CoCl2 (15.4 mmol) 1 g SiO2 (16.7 mmol) NaOH solution | 3 g CuCl2 (22.3 mmol) 1 g CuCl (10.1 mmol) 2 g (12.2 mmol) Na3PO4 HCl solution | 1 g V2O3 (6.6 mmol) 2 g Na3PO4 (12 mmol) 0.2 g Li2CO3 (3 mmol) H2O |
| pH 12 | pH 1.5 | pH 3 |
| Copper-lined chrome-nickel autoclave, V 14 mL Autoclave filling 50% | Copper-lined chrome-nickel autoclave, V 14 mL Autoclave filling 50% | Steel autoclave lined with fluoroplastic, V 7 mL Autoclave filling 72% |
| Structural Formula | (I) | (II) | (III) |
|---|---|---|---|
| Na2CoSiO4 | Na2Cu3O(Cu0.8Na0.2)(PO4)2Cl | Na3V(OH)(HPO4)(PO4) | |
| Mr | 197.00 | 531.00 | 327.87 |
| Space group, Z | Pn, 2 | Cmcm, 4 | C2/m, 4 |
| Temperature (K) | 170 | 150 | 293 |
| Unit cell parameters a, b, c (Å) | 5.2271(5) | 13.6243(2) | 15.4157(10) |
| 5.4198(4) | 10.3531(2) | 7.3107(4) | |
| 7.0466(6) | 6.3586(1) | 7.0556(4) | |
| β (°) | 90.011(7) | 90 | 96.702(6) |
| V (Å3) | 199.63(3) | 896.90(3) | 789.73 (8) |
| Radiation | Mo Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 4.69 | 9.56 | 1.86 |
| Crystal size (mm) | 0.15 × 0.06 × 0.02 | 0.17 × 0.11 × 0.07 | 0.19 × 0.09 × 0.06 |
| Diffractometer | Oxford Diffraction Gemini | Xcalibur Sapphire3 | |
| Number of reflections: measured independently based on [I > 2σ(I)] | 1800, 909, 894 | 8798, 732, 727 | 1352, 1352, 1043 |
| Rint | 0.016 | 0.016 | - |
| (sin θ/λ)max (Å−1) | 0.703 | 0.703 | 0.594 |
| R [F2 > 2σ(F2)], wR(F2), S | 0.025, 0.064, 1.03 | 0.018, 0.046, 1.29 | 0.043, 0.109, 1.03 |
| Number of refined parameters | 74 | 57 | 91 |
| Δρmax, Δρmin (e Å−3) | 0.46, −0.45 | 0.74, −0.91 | 0.64, −0.57 |
| Flack parameter * | 0.01(3) * | - | - |
| Co–Tetrahedron | Si–Tetrahedron | ||
| Co–O2 | 1.928(6) | Si1–O4 | 1.618(8) |
| –O3 | 1.944(5) | –O3 | 1.627(4) |
| –O1 | 1.958(4) | –O1 | 1.631(7) |
| –O4 | 1.969(5) | –O2 | 1.644(8) |
| <Co–O> | 1.95 | <Si–O> | 1.63 |
| Na1–Tetrahedron | Na2–Tetrahedron | ||
| Na1–O2 | 2.245(10) | Na2–O2 | 2.325(6) |
| –O3 | 2.272(10) | –O4 | 2.344(12) |
| –O4 | 2.279(5) | –O1 | 2.364(11) |
| –O1 | 2.331(10) | –O3 | 2.407(10) |
| <Na1–O> | 2.28 | <Na2–O> | 2.36 |
| Na1 | Na2 | Co | Si | Σ | |
|---|---|---|---|---|---|
| O1 | 0.203, 0.052 | 0.189 | 0.487 | 0.981 | 1.91 |
| O2 | 0.242 | 0.205 | 0.528 | 0.947 | 1.92 |
| O3 | 0.229 | 0.173, 0.061 | 0.506 | 0.992 | 1.96 |
| O4 | 0.226 | 0.197 | 0.473 | 1.016 | 1.91 |
| Σ | 0.95 | 0.83 | 1.99 | 3.94 |
| Cu1 Octahedron | Cu2 Tetragonal Pyramid | Cu3 * Trigonal Bipyramid | |||
| Cu1–O3 × 2 | 1.852(1) | Cu2–O3 | 1.911(2) | Cu3–O2 × 2 | 2.032(2) |
| –O4 × 4 | 2.254(2) | –O1 | 1.921(2) | –Cl | 2.394(5) |
| –O4 × 2 | 2.022(2) | –Cl × 2 | 3.1875(1) | ||
| <Cu1–O> | 2.12 | –Cl1 | 2.5789(8) | ||
| <Cu2–O> | 1.97 | <Cu3–O> | 2.03 | ||
| Na Octahedron | P Tetrahedron | ||||
| Na–O2 × 2 | 2.309(2) | P–O2 | 1.532(2) | ||
| –O1 × 2 | 2.460(2) | –O1 | 1.544(2) | ||
| –O4 × 2 –Cl × 2 | 2.884(2) 3.270(2) | –O4 × 2 | 1.544(2) | ||
| <Na–O> | 2.55 | <P–O> | 1.54 | ||
| Cu1 | Cu2 | Cu3 ** | P | Na | ∑ | |
|---|---|---|---|---|---|---|
| O1 | 0.520 | 1.212 | 0.155 ×2↓ ×2→ | 2.04 | ||
| O2 | 0.182 ×2↓ | 1.251 | 0.212 ×2↓ ×2→ | 1.98 | ||
| O3 | 0.626 ×2↓ ×2→ | 0.534 ×2→ | 2.32 | |||
| O4 | 0.212 ×4↓ | 0.395 ×2↓ | 1.213 ×2↓ | 0.064×2↓ | 1.88 | |
| Cl | 0.209 ×2→ | 0.144 0.02 ×2↓ ×2→ | 0.073 ×2↓ ×4→ | 1.04 | ||
| ∑ | 2.10 | 2.05 | 0.95 | 4.89 | 1.01 |
| D–H···A | D–H, Å | H···A, Å | D···A, Å | D–H···A, ° |
|---|---|---|---|---|
| O1–H1···O6 | 0.82(1) | 1.80(2) | 2.616(7) | 172(9) |
| O2–H2···O1 | 0.82(1) | 2.53(3) | 3.319(7) | 164(8) |
| V-Centered Octahedron | P1–Tetrahedron | P2–Tetrahedron | |||
| V–O3 –O3 –O4 –O4 –O2 –O2 <V–O> | 1.966(3) 1.966(3) 2.020(3) 2.020(3) 2.039(2) 2.039(2) 2.008 | P1–O6 –O4 –O4 –O1 <P1–O> | 1.526(5) 1.538(3) 1.538(3) 1.582(5) 1.544 | P2–O5 –O7 –O3 –O3 <P2–O> | 1.508(5) 1.510(5) 1.558(3) 1.558(3) 1.534 |
| Na1–Octahedron | Na2–Polyhedron | Na3–Octahedron | |||
| Na1–O5 –O5 –O4 –O4 –O1 –O1 <Na1–O> | 2.259(3) 2.259(3) 2.448(3) 2.448(3) 2.747(4) 2.747(4) 2.485 | Na2–O7 –O5 –O2 –O4 –O4 <Na2–O> | 2.175(6) 2.238(6) 2.435(5) 2.550(3) 2.550(3) 2.390 | Na2–O3 –O3 –O6 –O6 –O7 –O7 <Na3–O> | 2.427(3) 2.427(3) 2.460(4) 2.460(4) 2.482(4) 2.482(4) 2.456 |
| Na1 | Na2 | Na3 | V | P1 | P2 | H1 | H2 | Σ | |
|---|---|---|---|---|---|---|---|---|---|
| O1 | 0.086 ×2↓ ×2→ | 1.093 | 0.71 | 0.06 | 2.04 | ||||
| O2 | 0.163 | 0.449 ×2↓ ×2→ | 0.94 | 2.00 | |||||
| O3 | 0.166 ×2↓ | 0.547 ×2↓ | 1.167 ×2↓ | 1.88 | |||||
| O4 | 0.159 ×2↓ | 0.129 ×2↓ | 0.473 ×2↓ | 1.231 ×2↓ | 1.99 | ||||
| O5 | 0.235 ×2↓ ×2→ | 0.246 | 1.335 | 2.05 | |||||
| O6 | 0.155 ×2↓ ×2→ | 1.272 | 0.29 | 1.87 | |||||
| O7 | 0.28 | 0.148 ×2↓ ×2→ | 1.328 | 1.90 | |||||
| Σ | 0.96 | 0.95 | 0.94 | 2.94 | 4.83 | 5.00 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiriukhina, G.; Yakubovich, O.; Verchenko, P.; Volkov, A.; Shvanskaya, L.; Dimitrova, O.; Simonov, S. Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry. Minerals 2024, 14, 46. https://doi.org/10.3390/min14010046
Kiriukhina G, Yakubovich O, Verchenko P, Volkov A, Shvanskaya L, Dimitrova O, Simonov S. Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry. Minerals. 2024; 14(1):46. https://doi.org/10.3390/min14010046
Chicago/Turabian StyleKiriukhina, Galina, Olga Yakubovich, Polina Verchenko, Anatoly Volkov, Larisa Shvanskaya, Olga Dimitrova, and Sergey Simonov. 2024. "Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry" Minerals 14, no. 1: 46. https://doi.org/10.3390/min14010046
APA StyleKiriukhina, G., Yakubovich, O., Verchenko, P., Volkov, A., Shvanskaya, L., Dimitrova, O., & Simonov, S. (2024). Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry. Minerals, 14(1), 46. https://doi.org/10.3390/min14010046







