Genesis of the Large-Scale Kamado Magnesite Deposit on the Tibetan Plateau
Abstract
:1. Introduction
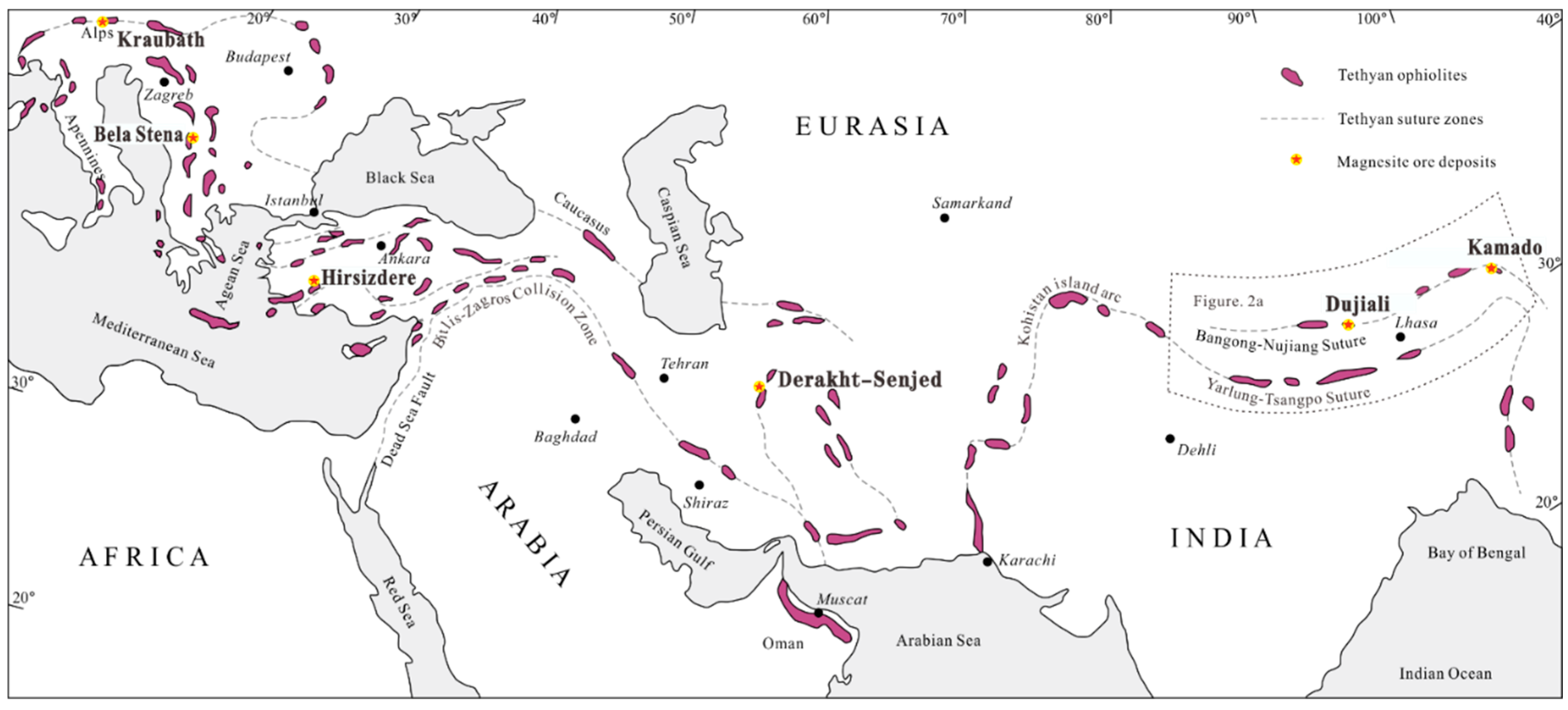
2. Geological Setting
2.1. Regional Geology
2.2. Geology of the Kamado Magnesite Deposit
2.3. Hydromagnesite in Lake Dujiali
3. Sampling and Analytical Methods
3.1. Scanning Electron Microscopy (SEM)
3.2. Whole-Rock Geochemistry Analysis
3.3. Carbon and Oxygen Isotope Analysis
4. Results
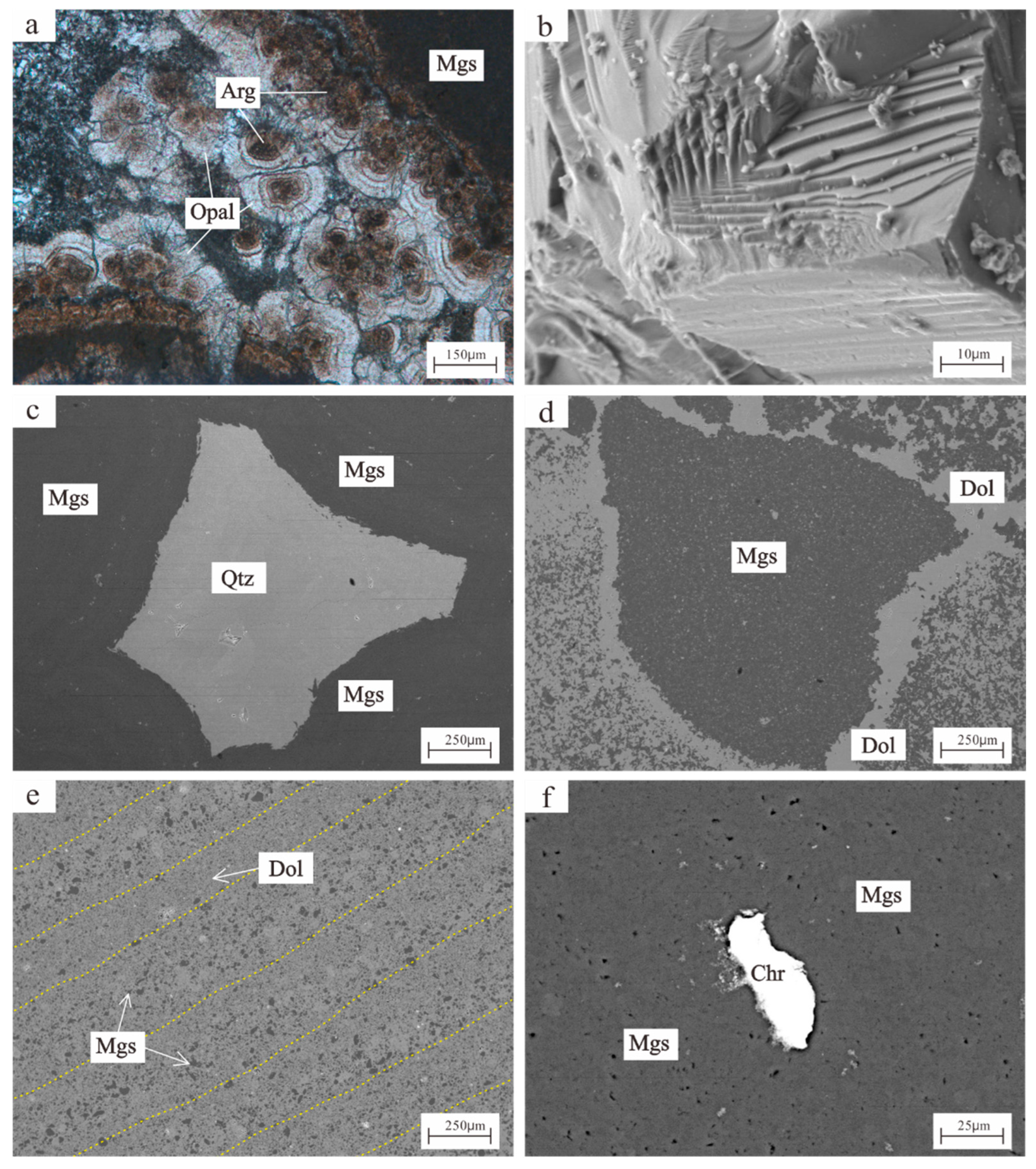
4.1. Scanning Electron Microscopy
4.2. Whole-Rock Geochemistry
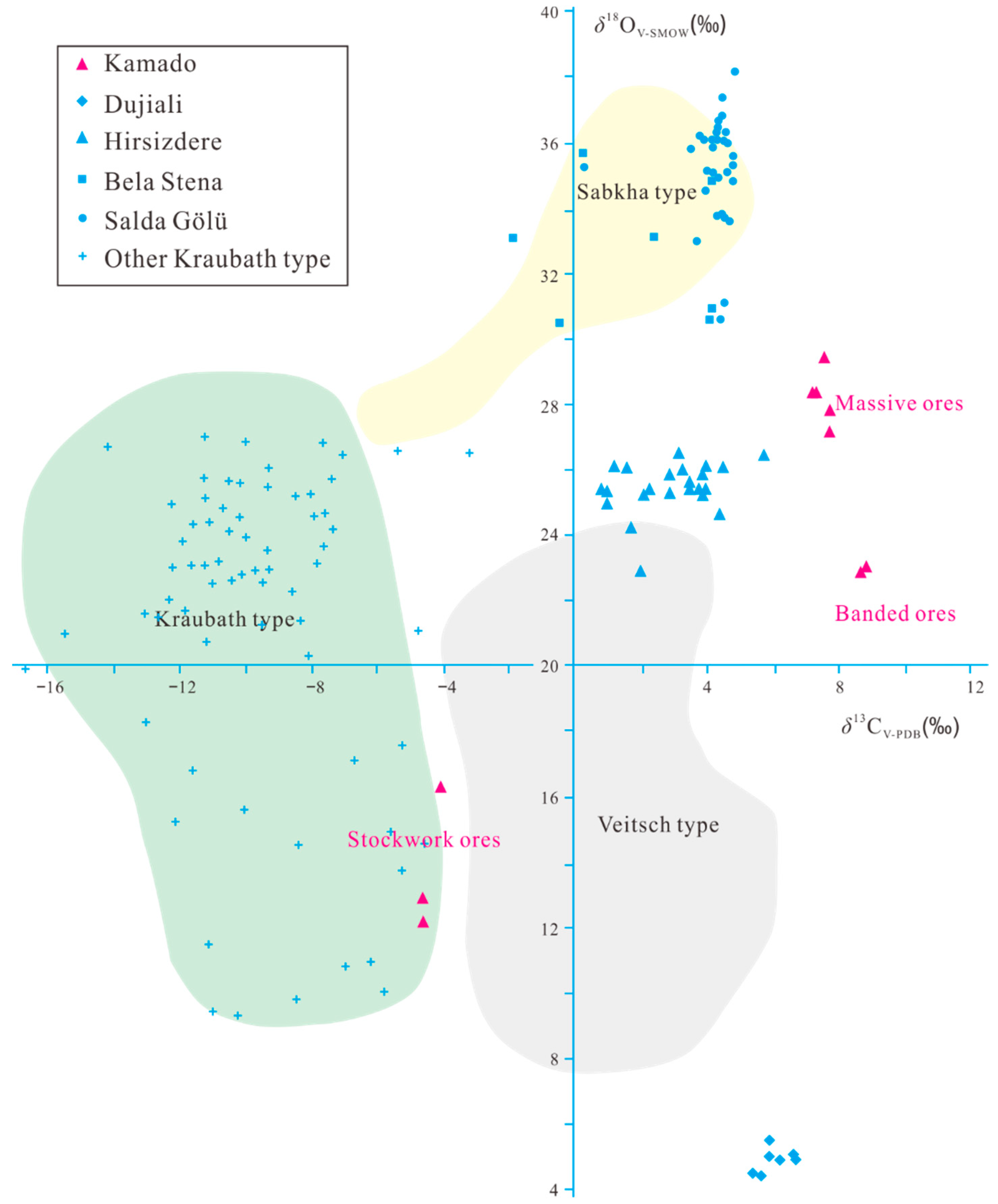
4.3. Carbon and Oxygen Isotopes
5. Discussion
5.1. Magnesium Sources of the Kamado Magnesite Deposit
5.2. Carbon Sources of the Kamado Magnesite Deposit
5.3. Genesis of the Kamado Magnesite Deposit
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drnek, T.L.; Moraes, M.N.; Neto, P.B. Overview of magnesite. J. Refractory Innov. RHIM Bull. 2018, 1, 14–22. [Google Scholar]
- Redlich, K.A. Die Typen der Magnesitlagerstatten. Z. Prakt. Geol. 1909, 17, 300–310. [Google Scholar]
- Nishihara, H. Origin of the bedded magnesites of Manchuria. Econ. Geol. 1956, 51, 698–711. [Google Scholar] [CrossRef]
- Zhang, Q.S. Early proterozoic tectonic styles and associated mineral deposits of the North China platform. Precambrian Res. 1988, 39, 1–29. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Fallick, A.E.; Medvedev, P.V.; Makarikhin, V.V. Palaeoproterozoic magnesite: Lithological and isotopic evidence for playa/sabkha environments. Sedimentology 2001, 48, 379–397. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Chen, C.X.; Chen, Y.Q.; Jiang, Y.H.; Dai, B.Z.; Ni, P. Geochemistry and genetic model for the giant magnesite deposits in the eastern Liaoning Province, China. Acta Petrol. Sin. 2004, 20, 765–772. [Google Scholar]
- Power, I.M.; Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Mccutcheon, J.; Southam, G.; Kenward, P.A. A depositional model for hydromagnesite-magnesite playas near Atlin, British Columbia, Canada. Sedimentology 2014, 61, 1701–1733. [Google Scholar] [CrossRef]
- Dong, A.G.; Zhu, X.K.; Li, S.Z.; Kendall, B.; Wang, Y.; Gao, Z.F. Genesis of a giant Paleoproterozoic strata-bound magnesite deposit: Constrains from Mg isotopes. Precambrian Res. 2016, 281, 673–683. [Google Scholar] [CrossRef]
- Velasco, F.Q.; Pesquera, A.; Arce, R.; Olmedo, F. A contribution to the ore genesis of the magnesite deposit of Eugui, Navarra (Spain). Miner. Depos. 1987, 22, 33–41. [Google Scholar] [CrossRef]
- Aharon, P. A stable-isotope study of magnesites from the Rum Jungle uranium field, Australia: Implications for the origin of strata-bound massive magnesites. Chem. Geol. 1988, 69, 127–145. [Google Scholar] [CrossRef]
- Joshi, P.; Pant, P.D.; Upadhyaya, R.C. Petrography and geochemistry of magnesite and talc deposits of Jhiroli, Kumaun Lesser Himalaya. In Magmatism, Tectonism and Mineralization; Macmillan Publishers India Ltd.: New Delhi, India, 2009; pp. 215–229. [Google Scholar]
- Barnes, I.; O’Neil, J.R.; Trescases, J.J. Present day serpentinization in New Caledonia, Oman and Yugoslavia. Geochim. Cosmochim. Acta 1978, 42, 144–145. [Google Scholar] [CrossRef]
- Abu-Jaber, N.S.; Kimberley, M.M. Origin of ultramafic-hosted vein magnesite deposits. Ore Geol. Rev. 1992, 7, 155–191. [Google Scholar] [CrossRef]
- Alcicek, H. Late Miocene nanmarine sedimentation and formation of magnesites in the Acigol Basin, southwestern Anatolia, Turkey. Sediment. Geol. 2009, 219, 115–135. [Google Scholar] [CrossRef]
- Oskierski, H.C.; Bailey, J.G.; Kennedy, E.M.; Jacobsen, G.; Ashley, P.M.; Dlugogorski, B.Z. Formation of weathering-derived magnesite deposits in the New England Orogen, New South Wales, Australia: Implications from mineralogy, geochemistry and genesis of the Attunga magnesite deposit. Miner. Depos. 2013, 48, 525–541. [Google Scholar] [CrossRef]
- Oskierski, H.C.; Dlugogorski, B.Z.; Jacobsen, G. Sequestration of atmospheric CO2 in a weathering-derived, serpentinite-hosted magnesite deposit: 14C tracing of carbon sources and age constraints for a refined genetic model. Geochim. Cosmochim. Acta 2013, 122, 226–246. [Google Scholar] [CrossRef]
- Oskierski, H.C.; Beinlich, A.; Mavromatis, V.; Altarawneh, M.; Dlugogorski, B.Z. Mg isotope fractionation during continental weathering and low temperature carbonation of ultramafic rocks. Geochim. Cosmochim. Acta 2019, 262, 60–77. [Google Scholar] [CrossRef]
- Rielli, A.; Boschi, C.; Dini, A. Tectonically driven carbonation of serpentinite by mantle CO2: Genesis of Castiglioncello magnesite deposit in the Ligurian ophiolite of central Tuscany (Italy). Ore Geol. Rev. 2022, 149, 105022. [Google Scholar] [CrossRef]
- Pohl, W.; Siegl, W. Sediment-hosted magnesite deposits. In Handbook of Strata-Bound and Stratiform Ore Deposits; Wolf, K.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 14, pp. 223–310. [Google Scholar]
- Kralik, M.; Aharon, P.; Zachmann, D.W. Carbon and oxygen isotope systematics of magnesites: A review. Monopraph Ser. Miner. Depos. 1989, 28, 197–223. [Google Scholar]
- Beinlich, A.; Austrheim, H. In situ sequestration of atmospheric CO2 at low temperature and surface cracking of serpentinized peridotite in mine shafts. Chem. Geol. 2012, 332–333, 32–44. [Google Scholar] [CrossRef]
- Boschi, C.; Dini, A.; Dallai, L.; Ruggieri, G.; Gianelli, G. Enhanced CO2-mineral sequestration by cyclic hydraulic fracturing and Si-rich fluid infiltration into serpentinites at Malentrata (Tuscany, Italy). Chem. Geol. 2009, 265, 209–226. [Google Scholar] [CrossRef]
- De Deckker, P. Groundwater interactions control dolomite and magnesite precipitation in saline playas in the Western District Volcanic Plains of Victoria, Australia. Sediment. Geol. 2019, 380, 105–126. [Google Scholar] [CrossRef]
- Fallick, A.E.; Ilich, M.; Russell, M.J. A stable isotope study of the magnesite deposits associated with the Alpine-type ultramafic rocks of Yugoslavia. Econ. Geol. 1991, 86, 847–861. [Google Scholar] [CrossRef]
- Russell, M.J. The generation at hot spring of sedimentary ore deposits, microbialites and life. Ore Geol. Rev. 1996, 10, 199–214. [Google Scholar] [CrossRef]
- Zedef, V.; Russell, M.J.; Fallick, A.E.; Hall, A.J. Genesis of vein stockwork and sedimentary magnesite and hydromagnesite deposits in the ultramafic terranes of southwestern Turkey: A stable isotope study. Econ. Geol. 2000, 95, 429–445. [Google Scholar] [CrossRef]
- Schroll, E. Genesis of magnesite deposits in the view of isotope geochemistry. Bol. Parana. Geociências 2002, 50, 59–68. [Google Scholar] [CrossRef]
- Pohl, W. Genesis of magnesite deposits—Models and trends. Geol. Rundsch. 1990, 79, 291–299. [Google Scholar] [CrossRef]
- del Real, P.G.; Maher, K.; Kluge, T.; Bird, D.K.; Brown, G.E., Jr.; John, C.M. Clumped-isotope thermometry of magnesium carbonates in ultramafic rocks. Geochim. Cosmochim. Acta 2016, 193, 222–250. [Google Scholar] [CrossRef]
- Falk, E.S.; Kelemen, P.B. Geochemistry and petrology of listvenite in the Samail ophiolite, Sultanate of Oman: Complete carbonation of peridotite during ophiolite emplacement. Geochim. Cosmochim. Acta 2015, 160, 70–90. [Google Scholar] [CrossRef]
- Bucher, K.; Grapes, R. Metamorphism of Ultramafic Rocks. In Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 2023; pp. 209–247. [Google Scholar]
- Chen, Y.C.; Wang, D.H.; Xu, Z.G. Important Minerals and Regional Metallogenic Regularity in China; Geological Publishing House: Beijing, China, 2015. (In Chinese) [Google Scholar]
- Song, Y.; Zeng, Q.G.; Liu, H.Y.; Liu, Z.B.; Li, H.F.; Dexi, Y.Z. An innovative perspective for the evolution of Bangong-Nujiang Ocean: Also discussing the Paleo- and Neo-Tethys conversion. Acta Petrol. Sin. 2019, 35, 625–641, (In Chinese with English Abstract). [Google Scholar]
- Ding, J.H.; Chen, Z.H.; Yang, G.J.; Deng, F.; Lou, D.B. Metallogeny and resource potential of magnesite deposits in China. Geol. China 2013, 40, 1699–1711, (In Chinese with English Abstract). [Google Scholar]
- Zhao, Z.; Bai, G.; Wang, D.H.; Chen, Y.C.; Xu, Z.G. The Metallogenic Belts of Chinese Magnesite Deposits and Key Scientific Issues. Acta Geol. Sin. 2014, 88, 2326–2338, (In Chinese with English Abstract). [Google Scholar]
- Ilich, M. Hydrothermal-Sedimentary Magnesite Deposit of Nevade, Gornji Milanovac (Western Serbia): Zbornik Radova Rudarsko-Geoloskog Fakulteta; University of Beograde, Faculty of Mining and Geology Transactions: Belgrade, Serbia, 1976; Volume 19, pp. 307–329. [Google Scholar]
- Dilek, Y.; Furnes, H. Structure and geochemistry of Tethyan ophiolites and their petrogenesis in subduction rollback systems. Lithos 2009, 113, 1–20. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wan, B.; Zhao, L.; Xiao, W.J.; Zhu, R.X. Tethyan geodynamics. Acta Petrol. Sin. 2020, 36, 1627–1674, (In Chinese with English Abstract). [Google Scholar]
- Zhu, R.X.; Zhao, P.; Zhao, L. Tectonic evolution and geodynamics of the Neo-Tethys Ocean. Sci. China Earth. Sci. 2022, 65, 1–24, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yin, A.; Harrison, T.M. Geologic evolution of the Himalayan-Tibetan orogen. Annu. Rev. Earth Planet. Sci. 2000, 28, 211–280. [Google Scholar] [CrossRef]
- Xiong, F.H.; Yang, J.S.; Dilek, Y.; Xu, X.Z.; Zhang, Z.M. Origin and significance of diamonds and other exotic minerals in the Dingqing ophiolite peridotites, eastern Bangong-Nujiang suture zone, Tibet. Lithosphere 2017, 10, 142–155. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.S.; Lian, D.Y.; Li, G.L. Geological features of Neothyan ophiolites in Tibetan Plateau and its tectonic evolution. Acta Petrol. Sin. 2020, 36, 2913–2945, (In Chinese with English Abstract). [Google Scholar]
- Tapponnier, P.; Xu, Z.Q.; Roger, F.; Meyer, B.; Arnaud, N.; Wittlinger, G.; Yang, J.S. Oblique stepwise rise and growth of the Tibet Plateau. Science 2001, 294, 1671–1677. [Google Scholar] [CrossRef]
- Zheng, M.P.; Xiang, J.; Wei, X.J.; Zheng, Y. Saline Lakes on the Qinghai-Xizang(Tibet) Plateau; Beijing Science and Technology Press: Beijing, China, 1989; pp. 1–431, (In Chinese with English Abstract). [Google Scholar]
- Zhang, W.; Tan, H.; Xu, W.; Huang, J. Boron source and evolution of the Zabuye salt lake, Tibet: Indication from boron geochemistry and isotope. Appl. Geochem. 2023, 148, 105516. [Google Scholar] [CrossRef]
- Eleng, H.I.; Tan, H.B.; Su, J.B.; Yang, J.Y. Origin of the enrichment of B and alkali metal elements in the geothermal water in the Tibetan Plateau: Evidence from B and Sr isotopes. Geochemistry 2021, 81, 125797. [Google Scholar] [CrossRef]
- Tan, H.B.; Shi, Z.W.; Cong, P.X.; Xue, F.; Chen, G.H. The spatial distribution law of B, Li, Rb and Cs elements and supernormal enrichment mechanism in Tibet geothermal system. Sediment. Geol. Tethyan Geol. 2023, 43, 404–415. [Google Scholar]
- Tang, Y.; Zhai, Q.G.; Chung, S.L.; Hu, P.Y.; Wang, J.; Xiao, X.C.; Song, B.; Wang, H.T.; Lee, H.Y. First mid-ocean ridge-type ophiolite from the Meso-Tethys suture zone in the north-central Tibetan plateau. Geol. Soc. Am. Bull. 2020, 132, 2202–2220. [Google Scholar] [CrossRef]
- Jiang, S.; Jiang, Y.; Liu, Y.; Li, S.; Zhang, W.; Wang, G.; Lu, L.; Somerville, I. The Bangong-Nujiang Suture Zone, Tibet Plateau: Its role in the tectonic evolution of the eastern Tethys Ocean. Earth-Sci. Rev. 2021, 218, 103656. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhang, M.G.; Xu, X.; Li, B.X. Saline Lakes of China; Science Press: Beijing, China, 2002; pp. 1–415. (In Chinese) [Google Scholar]
- O’Neil, J.R. Stable isotopes in mineralogy. Phys. Chem. Miner. 1977, 2, 105–123. [Google Scholar] [CrossRef]
- Das Sharma, S.; Patil, D.J.; Gopalan, K. Temperature dependence of oxygen isotope fractionation of CO2 from magnesite-phosphoric acid reaction. Geochim. Cosmochim. Acta 2002, 66, 589–593. [Google Scholar] [CrossRef]
- Kim, S.-T.; Coplen, T.B.; Horita, J. Normalization of stable isotope data for carbonate minerals: Implementation of IUPAC guidelines. Geochim. Cosmochim. Acta 2015, 158, 276–289. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zheng, M.P.; Ye, C.Y. Hydromagnesite precipitation in the Alkaline Lake Dujiali, central Qinghai-Tibetan Plateau: Constraints on hydromagnesite precipitation from hydrochemistry and stable isotopes. Appl. Geochem. 2017, 78, 139–148. [Google Scholar] [CrossRef]
- Jurković, I.; Palinkaš, L.A.; Garašić, V.; Strmić Palinkaš, S. Genesis of vein-stockwork cryptocrystalline magnesite from the Dinaride ophiolites. Ofioliti 2012, 37, 13–26. [Google Scholar]
- Hoke, L.; Lamb, S.; Hilton, D.R.; Poreda, R.L. Southern limit of mantle-derived geothermal helium emissions in Tibet: Implications for lithospheric structure. Earth Planet. Sci. Lett. 2000, 180, 297–308. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Li, Z.Q. Possible location for underthrusting front of the Indus continent: Constraints from Helium isotope of the geothermal gas in the southern Tibet and eastern Tibet. Acta Geol. Sin. 2004, 78, 382–393, (In Chinese with English Abstract). [Google Scholar]
- Klemperer, S.L.; Zhao, P.; Shi, D.N.; Crossey, L.J.; Karlstrom, K.E.; Winn, C.L.; Liu, T.Z.; Xiong, Z.Y.; Guo, X.D.; Zeng, D.; et al. Detection of a widespread mantle component of 3He in thermal springs of Lhasa Block and Tethyan Himalaya, eastern Tibet: Evidence for roll-back of the Indian-Asian mantle suture. In Proceedings of the International Symposium on Deep Earth Exploration and Practices, Beijing, China, 24–26 October 2018. [Google Scholar]
- Su, J.B.; Tan, H.B.; Chen, X. The groundwater deep circulation and large-scale geothermal deposition in response to the extension of the Yadong-Gulu rift, South Tibet, China. J. Volcanol. Geotherm. Res. 2020, 395, 106836. [Google Scholar] [CrossRef]
- Wang, S.Q.; Lu, C.; Nan, D.W.; Hu, X.C.; Shao, J.L. Geothermal resources in Tibet of China: Current status and prospective development. Environ. Earth Sci. 2017, 76, 239. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zheng, M.P.; Ye, C.Y.; Power, I.M. Trace and rare earth element geochemistry of Holocene hydromagnesite from Dujiali Lake, central Qinghai–Tibetan Plateau, China. Carbonates Evaporites 2017, 3–4, 1–15. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, K.J.; Yan, L.L. Hydrothermal metasomatism and solid-phase transfer in petrogenesis of listvenite: The Meso-Tethyan ophiolite, central Tibet, China. Contrib. Mineral. Petrol. 2023, 178, 4. [Google Scholar] [CrossRef]
- Hannigan, R.E. Rare earth, major and trace elements geochemistry of surface and geothermal waters from the Tanpo volcanic zone, north island New Zealand. Rare Earth Elem. Groundw. Flow Syst. 2005, 51, 67–88. [Google Scholar]
- Wang, M.M.; Zhou, X.; Liu, Y.; Xu, H.F.; Wu, Y.Q.; Zhuo, L.Y. Major, trace and rare earth elements geochemistry of geothermal waters from the Rehai high-temperature geothermal field in Tengchong of China. Appl. Geochem. 2020, 119, 1–12. [Google Scholar] [CrossRef]
- Chudaev, O.V.; Chelnokov, G.A.; Bragin, I.V.; Kharitonova, N.A.; Rychagov, S.N.; Nuzhdaev, A.A.; Nuzhdaev, I.A. Rare earth and major elements geochemistry of geothermal waters from Mutnovsky Volcano, Kamchatka. Procedia Earth Planet. Sci. 2017, 17, 92–95. [Google Scholar] [CrossRef]
- Morteani, G.; Moeller, P.; Schley, F. The rare earth element contents and the origin of the sparry magnesite mineralizations of Tux-Lanersbach, Entachen Alm, Spiessnaegel, and Hochfilzen, Austria, and the lacustrine magnesite deposits of Aiani-Kozani, Greece, and Bela Stena, Yugoslavia. Econ. Geol. 1982, 77, 617–631. [Google Scholar] [CrossRef]
- Morteani, G.; Schley, F.; Moller, P. On the formation of magnesite. In Mineral Deposits of the Alpine Epoch in Europe; Schneider, H.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 237–266. [Google Scholar]
- Braithwaite, C.J.R.; Zedef, V. Hydromagnesite stromatolites and sediments in an alkaline lake, Salda Golu, Turkey. J. Sediment. Res. 1996, 66, 991–1002. [Google Scholar]
- Zedef, V. The Origin of Magnesite in Turkey, a Stable Isotope Study. Unpublished. Ph.D. Thesis, Glasgow University, Glasgow, UK, 1994. [Google Scholar]
- Veizer, J.; Hoefs, J. The nature of 18O/16O and 13C/12C secular trends in sedimentary carbonate rocks. Geochim. Cosmochim. Acta 1976, 40, 1387–1395. [Google Scholar] [CrossRef]
- Falk, E.S.; Guo, W.; Paukert, A.N.; Matter, J.M.; Mervine, E.M.; Kelemen, P.B. Controls on the stable isotope compositions of travertine from hyperalkaline springs in Oman: Insights from clumped isotope measurements. Geochim. Cosmochim. Acta 2016, 192, 1–28. [Google Scholar] [CrossRef]
- Bekker, A.; Karhu, J.A.; Eriksson, K.A.; Kaufman, A.J. Chemostratigraphy of Paleoproterozoic carbonate successions of the Wyoming Craton: Tectonic forcing of biogeochemical change? Precambrian Res. 2003, 120, 279–325. [Google Scholar] [CrossRef]
- Bekker, A.; Eriksson, K.A. A Paleoproterozoic drowned carbonate platform on the southeastern margin of the Wyoming Craton: A record of the Kenorland breakup. Precambrian Res. 2003, 120, 327–364. [Google Scholar] [CrossRef]
- Bekker, A.; Karhu, J.A.; Kaufman, A.J. Carbon isotope record for the onset of the Lomagundi carbon isotope excursion in the Great Lakes area, North America. Precambrian Res. 2006, 148, 145–180. [Google Scholar] [CrossRef]
- Schidlowski, M.; Eichmann, R.; Junge, C.E. Carbon isotope geochemistry of the Precambrian Lomagundi carbonate province, Rhodesia. Geochim. Cosmochim. Acta 1976, 40, 449–455. [Google Scholar] [CrossRef]
- Karhu, J.A.; Holland, H.D. Carbon isotopes and the rise of atmospheric oxygen. Geology 1996, 24, 867–870. [Google Scholar] [CrossRef]
- Tang, H.S.; Chen, Y.J.; Santosh, M.; Zhong, H.; Wu, G.; Lai, Y. C–O isotope geochemistry of the Dashiqiao magnesite belt, North China Craton: Implications for the Great Oxidation Event and ore genesis. Geol. J. 2013, 48, 467–483. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. The onset and early evolution of life. In Evolution of Early Earth’s Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits: Geological Society of America Memoir; Kesler, S.E., Ohmoto, H., Eds.; Geological Society of America location: Boulder, CO, USA, 2006; Volume 198, pp. 1–32. [Google Scholar]
- Hoefs, J. Isotope fractionation processes of selected elements. In Stable Isotope Geochemistry; Springer: Cham, Switzerland, 2021; pp. 58–63. [Google Scholar]
- Irwin, H.; Curtis, C.; Coleman, M.L. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 1977, 269, 209–213. [Google Scholar] [CrossRef]
- Wenner, D.B.; Taylor, H.P. Oxygen and hydrogen isotope studies of the serpentinization of ultramafic rocks in oceanic environments and continental ophiolite complexes. Am. J. Sci. 1973, 273, 207–239. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zheng, M.P.; Ye, C.Y.; Power, I.M. Rare earth element and strontium isotope geochemistry in Dujiali Lake, central Qinghai-Tibet Plateau, China: Implications for the origin of hydromagnesite deposits. Geochemistry 2019, 79, 337–346. [Google Scholar] [CrossRef]
- Zheng, M.P.; Zhang, Y.S.; Liu, X.F.; Nie, Z.; Kong, F.J.; Qi, W.; Jia, Q.X.; Pu, L.Z.; Hou, X.H.; Wang, H.L.; et al. Progress and Prospects of Salt Lake Research in China. Acta Geol. Sin.-Engl. 2016, 90, 1195–1235. [Google Scholar] [CrossRef]
- Koçyigit, A. Güneybati Tütkiye ve yakin dolayinda levha içi yeni tektonik gelisim. Geol. Soc. Turk. Bull. 1984, 27, 1–16. [Google Scholar]
- Braithwaite, C.J.R.; Zedef, V. Living hydromagnesite stromatolites from Turkey. Sediment. Geol. 1994, 92, 1–5. [Google Scholar] [CrossRef]
- Tapponnier, P.; Molnar, P. Slip-line field theory and large-scale continental tectonics. Nature 1976, 264, 319–324. [Google Scholar] [CrossRef]
- Qin, X.W.; Ma, H.Z.; Zhang, X.Y.; Hu, X.S.; Li, G.R.; Cheng, H.D.; Han, J.B.; Li, Y.S.; Miao, W.L.; Han, W.H.; et al. Origin and Circulation of Springs in the Nangqen and Qamdo Basins, Southwestern China, Based on Hydrochemistry and Environmental Isotopes. Geofluids 2022, 2022, 7190994. [Google Scholar] [CrossRef]
- Zhu, J.L.; Hu, K.Y.; Lu, X.L.; Huang, X.X.; Liu, K.T.; Wu, X.J. A Review of Geothermal Energy Resources, Development, and Applications in China: Current Status and Prospects. Energy 2015, 93, 466–483. [Google Scholar] [CrossRef]
- Nelson, C.E.; Giles, D.L. Hydrothermal eruption mechanisms and hot spring gold deposits. Econ. Geol. 1985, 80, 1633–1639. [Google Scholar] [CrossRef]
- Shettel, D.L. Solubility of quartz in H2O-CO2 fluids at 5 kb and 500–900 C. Am. Geophys. Union Trans. 1973, 54, 480. [Google Scholar]



| Sample No. | TD20-40-4 | TD20-40-5 | TD20-42-1 | TD20-43-1 | TD20-47-1 | TD20-47-2 | TD20-47-3 | TD20-48-1 | TD20-45-8 | TD20-45-11 | TD20-45-4 | TD20-45-6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock Type | Banded Magnesite | Massive Magnesite | Dolomite | Siliceous Sinter | ||||||||
| SiO2 (%) | 5.40 | 3.50 | <0.01 | <0.01 | 0.76 | <0.01 | 0.13 | 1.10 | 5.12 | 3.31 | 98.91 | 98.80 |
| Al2O3 (%) | 0.85 | 0.66 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.30 | 0.12 | 0.12 | 0.74 | 0.96 |
| CaO (%) | 2.98 | 4.32 | 0.59 | 1.73 | 1.06 | 0.66 | 0.57 | 0.95 | 18.90 | 17.84 | 0.17 | 0.15 |
| TFe2O3 (%) | 0.53 | 0.44 | 0.03 | 0.03 | 0.05 | 0.02 | 0.04 | 0.04 | 0.17 | 0.13 | 0.09 | 0.07 |
| FeO (%) | 0.32 | 0.28 | <0.01 | <0.01 | 0.04 | <0.01 | 0.04 | 0.04 | 0.07 | 0.11 | 0.05 | 0.05 |
| K2O (%) | 0.12 | 0.21 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.02 | <0.01 | 0.03 | 0.03 |
| MgO (%) | 42.19 | 43.55 | 48.53 | 47.32 | 47.37 | 48.33 | 48.03 | 47.00 | 30.07 | 33.27 | 0.19 | 0.11 |
| MnO (%) | 0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.06 | <0.01 | <0.01 |
| Na2O (%) | 0.07 | 0.12 | 0.07 | 0.10 | 0.09 | 0.09 | 0.07 | 0.09 | 0.02 | 0.01 | <0.01 | <0.01 |
| P2O5 (%) | 0.42 | 0.35 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| TiO2 (%) | 0.06 | 0.05 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | <0.01 | 0.02 | 0.01 |
| CO2 (%) | 45.48 | 44.36 | 49.19 | 49.19 | 49.67 | 50.64 | 50.46 | 50.24 | 44.20 | 44.62 | 0.34 | 0.34 |
| H2O+ (%) | 0.90 | 1.10 | 1.14 | 0.42 | 0.70 | 0.32 | 0.70 | 0.26 | 1.12 | 1.06 | 0.36 | 0.14 |
| LOI (%) | 46.72 | 45.88 | 50.82 | 50.35 | 50.61 | 51.30 | 51.13 | 50.76 | 45.13 | 45.84 | 0.49 | 0.48 |
| Total | 99.67 | 99.38 | 100.13 | 99.62 | 100.07 | 100.49 | 100.1 | 100.35 | 99.65 | 100.69 | 100.69 | 100.66 |
| B (ppm) | 24.5 | 23.4 | 32.0 | 68.1 | 52.5 | 52.9 | 42.4 | 50.0 | <2.00 | <2.00 | 2.66 | 12.4 |
| Li (ppm) | 13.5 | 15.2 | 14.7 | 23.4 | 20.1 | 21.1 | 18.5 | 20.4 | 4.41 | 3.19 | 52.4 | 87.3 |
| La (ppm) | 1.85 | 1.32 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.07 | 0.13 | 0.23 | 0.23 |
| Ce (ppm) | 4.00 | 3.25 | 0.09 | 0.10 | 0.07 | <0.05 | <0.05 | <0.05 | 0.15 | 0.28 | 0.40 | 0.39 |
| Pr (ppm) | 0.42 | 0.30 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Nd (ppm) | 1.39 | 0.82 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.10 | 0.16 | 0.13 | 0.13 |
| Sm (ppm) | 0.23 | 0.26 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.06 | <0.05 | <0.05 |
| Eu (ppm) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Gd (ppm) | 0.22 | 0.15 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.11 | 0.12 | <0.05 | <0.05 |
| Tb (ppm) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Dy (ppm) | 0.20 | 0.22 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.09 | 0.10 | <0.05 | <0.05 |
| Ho (ppm) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Er (ppm) | 0.11 | 0.18 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Tm (ppm) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Yb (ppm) | 0.12 | 0.16 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Lu (ppm) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Y (ppm) | 1.12 | 0.92 | 0.10 | 0.12 | 0.11 | 0.10 | 0.06 | <0.05 | 0.60 | 0.59 | 0.75 | 0.95 |
| Sample No. | Sample Type | Mineralization Stage | δ13CV-PDB (‰) | δ18OV-PDB (‰) | δ18OV-SMOW (‰) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single | Mean | 2SD | Single | Mean | Single | Mean | 2SD | |||
| TD22-5-7 | Stockwork magnesite | Early stage | −4.5 | −4.6 | 0.3 | −18.0 | −18.1 | +12.4 | +12.3 | 0.3 |
| TD22-5-7′ | −4.7 | −18.2 | +12.1 | |||||||
| TD22-5-8 | −4.8 | −4.7 | 0.3 | −17.6 | −17.4 | +12.8 | +13.0 | 0.6 | ||
| TD22-5-8′ | −4.6 | −17.2 | +13.2 | |||||||
| TD22-5-9 | −3.9 | −4.1 | 0.6 | −14.2 | −14.2 | +16.3 | +16.3 | 0.1 | ||
| TD22-5-9′ | −4.3 | −14.1 | +16.4 | |||||||
| TD20-40-4 | Banded magnesite | Middle stage | +8.7 | +8.8 | 0.3 | −8.0 | −7.7 | +22.7 | +23.0 | 1.0 |
| TD20-40-4′ | +8.9 | −7.3 | +23.4 | |||||||
| TD20-40-5 | +8.5 | +8.7 | 0.4 | −8.1 | −7.9 | +22.6 | +22.8 | 0.7 | ||
| TD20-40-5′ | +8.8 | −7.6 | +23.1 | |||||||
| TD20-42-1 | Massive magnesite | Late stage | +7.2 | +7.4 | 0.4 | −2.5 | −2.4 | +28.3 | +28.4 | 0.3 |
| TD20-42-1′ | +7.5 | −2.3 | +28.5 | |||||||
| TD20-43-1 | +7.2 | +7.3 | 0.3 | −2.5 | −2.5 | +28.3 | +28.4 | 0.1 | ||
| TD20-43-1′ | +7.4 | −2.4 | +28.4 | |||||||
| TD20-47-1 | +7.4 | +7.7 | 0.7 | −3.2 | −3.1 | +27.6 | +27.8 | 0.4 | ||
| TD20-47-1′ | +7.9 | −2.9 | +27.9 | |||||||
| TD20-47-3 | +7.7 | +7.7 | 0.0 | −3.8 | −3.7 | +27.0 | +27.1 | 0.4 | ||
| TD20-47-3′ | +7.7 | −3.5 | +27.3 | |||||||
| TD22-2-1 | +7.5 | +7.6 | 0.3 | −1.3 | −1.4 | +29.6 | +29.5 | 0.3 | ||
| TD22-2-1′ | +7.7 | −1.5 | +29.4 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Hu, G.; Chen, Y.; Xu, Y.; Chen, H.; Wang, D.; Huang, F.; You, S.; Liu, H.; He, L.; et al. Genesis of the Large-Scale Kamado Magnesite Deposit on the Tibetan Plateau. Minerals 2024, 14, 45. https://doi.org/10.3390/min14010045
Yu X, Hu G, Chen Y, Xu Y, Chen H, Wang D, Huang F, You S, Liu H, He L, et al. Genesis of the Large-Scale Kamado Magnesite Deposit on the Tibetan Plateau. Minerals. 2024; 14(1):45. https://doi.org/10.3390/min14010045
Chicago/Turabian StyleYu, Xuhui, Guyue Hu, Yuchuan Chen, Ying Xu, Han Chen, Denghong Wang, Fan Huang, Shuisheng You, Haiyong Liu, Liang He, and et al. 2024. "Genesis of the Large-Scale Kamado Magnesite Deposit on the Tibetan Plateau" Minerals 14, no. 1: 45. https://doi.org/10.3390/min14010045
APA StyleYu, X., Hu, G., Chen, Y., Xu, Y., Chen, H., Wang, D., Huang, F., You, S., Liu, H., He, L., & Li, Y. (2024). Genesis of the Large-Scale Kamado Magnesite Deposit on the Tibetan Plateau. Minerals, 14(1), 45. https://doi.org/10.3390/min14010045






