Abstract
The flotation separation of smithsonite and calcite is difficult due to their similar surface properties. In this study, cupferron was applied as a collector to realize the separation of smithsonite and calcite. Micro-flotation experiment results indicated that smithsonite and calcite express different floatability after treatment with cupferron. The maximum recovery difference was 63%, from a cupferron concentration of 2 × 10−4 mol/L at pH 8. Based on a series of tests, including an adsorption test, Fourier-transform infrared (FTIR), zeta potential and X-ray photoelectron spectroscopy (XPS), the selective collection mechanism of cupferron was studied. It was found that the cupferron was more easily adsorbed on the surface of smithsonite and the reaction was violent. The adsorption capacity of the cupferron on the surface of smithsonite was higher than that of calcite, and the surface potential shift was greater. The cupferron chelated with the exposed Zn sites on the smithsonite surface to form a N-O-Zn ring structure. This special chelate structure caused the smithsonite surface to be more hydrophobic, which confirmed that the cupferron can selectively collect smithsonite instead of calcite.
1. Introduction
As an important nonferrous metal, zinc is widely used in electrical, mechanical, and metallurgical applications due to its excellent abrasion and corrosion resistance [1]. In the last few decades, most of the world’s zinc has been produced from zinc sulfide (ZnS) concentrates [2,3]. With the gradual depletion of zinc sulfide, the proportion of zinc extracted from zinc oxide concentrates has been increasing. Hence, the flotation of smithsonite, one of the major minerals in zinc oxide ore, has attracted considerable attention [4,5].
At present, the most common flotation method for smithsonite is sulfidation-amine or sulfidation-xanthate [4], which is widely used in industry. The method applies to most zinc oxide ores [6,7,8,9]. For this method, the surface of smithsonite is converted to a sulfide surface by using sulfiding agents. Many studies have already explored the mechanism of sulfidation in order to improve the recovery of smithsonite [10]. Surface sulfidation flotation has been extensively studied, including screening sulfurizing reagents, characterizing sulfide films on smithsonite surfaces and promoting the sulfidation process of smithsonite [11,12,13,14,15]. However, this method has drawbacks, such as environmental pollution and excessive reagent consumption. Sulfidation flotation is not the only way to recover smithsonite; fatty acids or chelating agents can also be used to recover smithsonite directly.
Fatty acids are usually used in direct flotation for zinc oxide ore if the gangue is clay or silica minerals [16]. New collectors development has also been reported, such as with lauryl phosphate, N,N′-dilauroyl ethylenediamine dipropionate and benzohydroxamic acid [17,18,19]. The central problem for direct flotation of oxidized zinc ores is poor selectivity, which is needed to separate gangue minerals into concentrate. Calcite is a common gangue mineral and is frequently associated with smithsonite in zinc oxide deposits. Both smithsonite and calcite belong to the group of carbonate minerals, and the properties of their surfaces are remarkably similar [20]. For this reason, the semi-solubility of the mineral results in adverse effects from calcite on the smithsonite surface. Some approaches for separating smithsonite from calcite pay more attention to calcite depressants, such as starch, water glass, calcium lignosulphonate or carboxymethyl cellulose [21,22,23], but the effectiveness of these depressants is facing more challenges as the average calcite content in worldwide raw zinc oxide ore has begun to increase.
Chelating agents are a class of specific complexing agents that can interact with certain metal ions to form a ring structure [24]. The formation of water-insoluble metal chelates on the surfaces of various minerals enhances the hydrophobicity of these mineral surfaces. Cupferron, a well-known analytical reagent, is widely used in the detection of trace metal ions. It is also considered to be an effective collector of oxide and sulfide minerals. Some investigations have reported that cupferron can form monodentate or bidentate complexes with active metal sites on mineral surfaces, such as chalcopyrite, uranium oxide and malachite [25,26,27]. Cupferron is used as the ligand in ion flotation to separate gallium and aluminum, demonstrating its high selectivity [28]. Bottei has studied the thermal stability and infrared spectra of different cupferron complexes, and this work showed that zinc complexes have higher stability than other complexes [29]. The cupferron has good chelating properties for zinc, it could be used as a potential collector for the flotation of smithsonite.
In this study, with the aim of providing an effective chelating agent for smithsonite flotation, we evaluated cupferron as a chelate collector in the separation of smithsonite from calcite. Different flotation behaviors of smithsonite and calcite were observed through micro-flotation and adsorption experiments. Furthermore, the adsorption mechanism of cupferron on the smithsonite surface was illustrated with Fourier-transform infrared spectra (FTIR), zeta potential and X-ray photoelectron spectroscopy (XPS).
2. Materials and Methods
2.1. Samples and Reagents
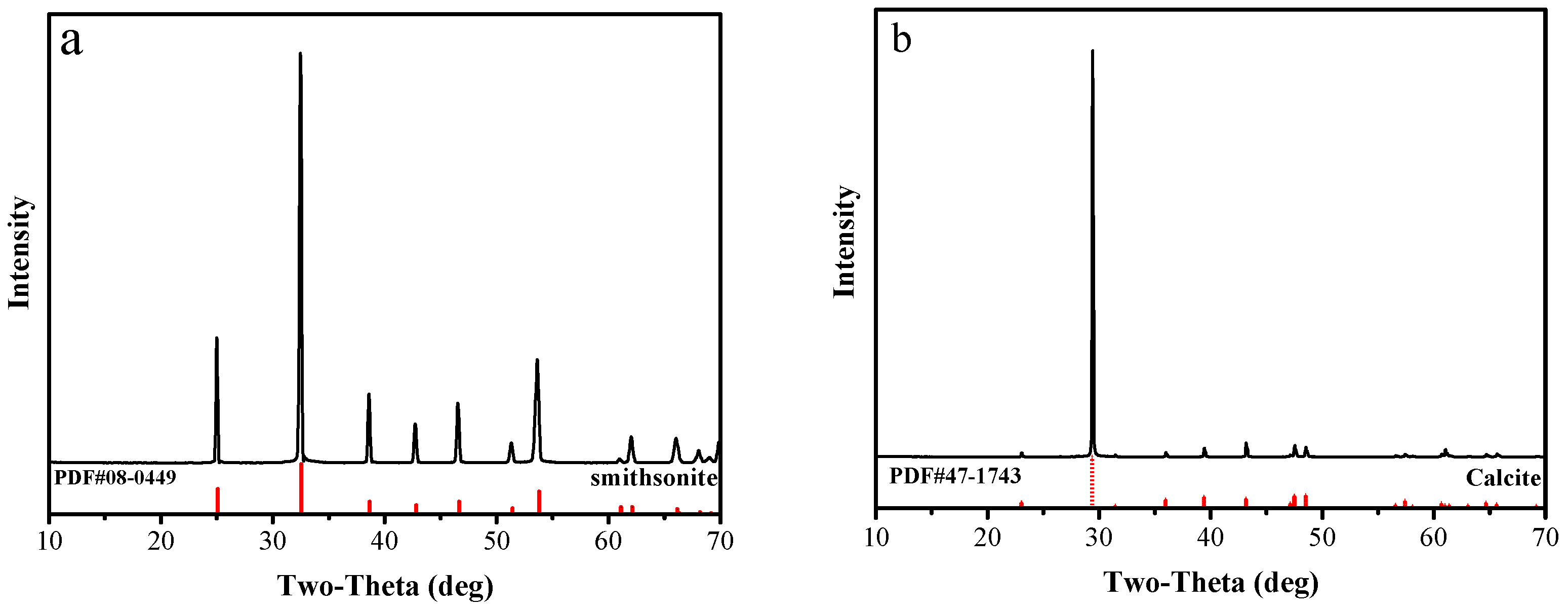
High-purity samples of smithsonite and calcite minerals were obtained from Yunnan Province, China. Samples were crushed in a laboratory jaw crusher and ground in a porcelain ball mill to reduce the particle size. Then, standard sieves were used to obtain samples of several different particle sizes. The ground −74 + 38 μm was used for micro-flotation and adsorption experiments. The −5 μm fraction samples were utilized for FTIR and XPS analysis. The XRD spectra of the smithsonite and calcite particles are shown in Figure 1. Table 1 shows that the purities of the smithsonite and calcite were 96.02% and 97.68%, respectively.
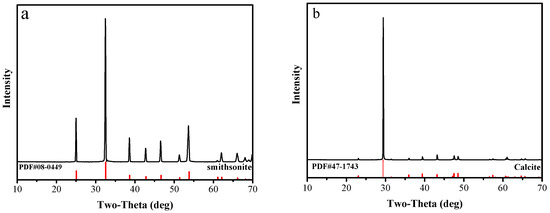
Figure 1.
XRD of (a) smithsonite samples and (b) calcite samples.

Table 1.
The chemical composition of smithsonite and calcite samples (wt.%).
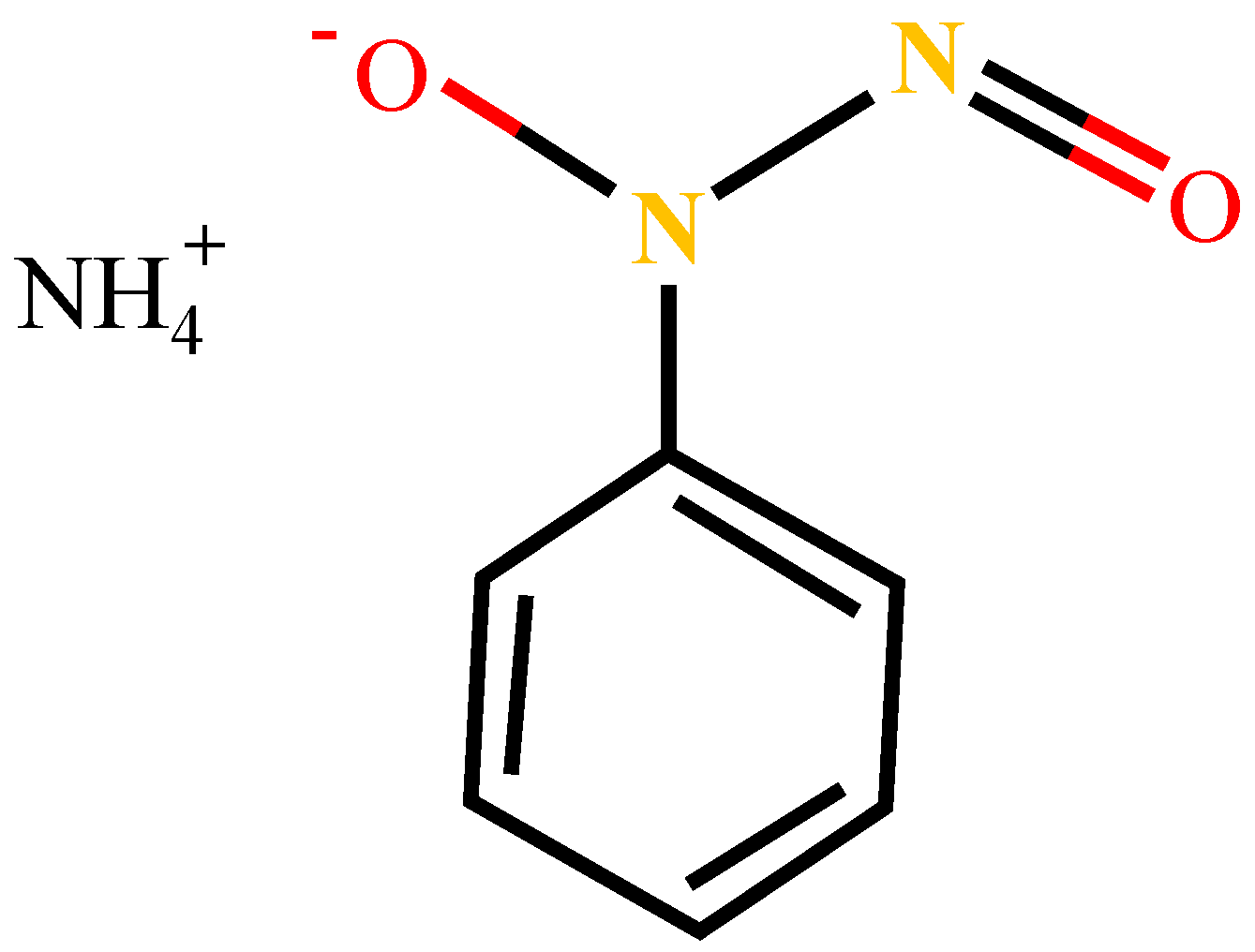
Analytical grade (98%) cupferron and terpineol used in this study were purchased from Macklin Biochemical Company (Shanghai, China). The molecular structure of cupferron is shown in Figure 2 and its chemical name is N-nitroso-N-phenylhydroxylamine ammonium salt. Cupferron and terpineol were used as collectors and frothers, respectively. Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were used as pH regulators. Deionized water (DI) was used throughout the experimental work.
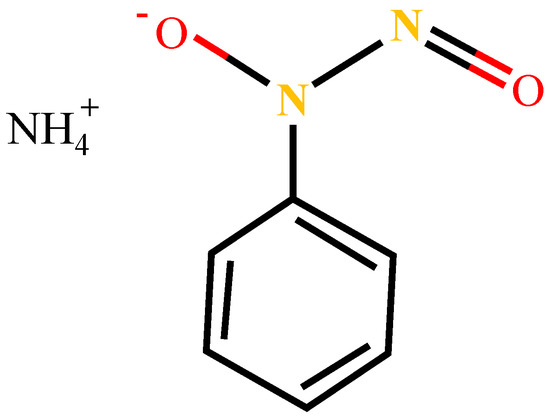
Figure 2.
The molecular structure formula of cupferron.
2.2. Micro-Flotation Experiments
The single-mineral and mixed-mineral micro-flotation tests were performed in an XFG flotation machine with a fixed speed of 1650 rpm. For the mixed-mineral micro-flotation tests, the sample was fully mixed with a ratio of 1:1 smithsonite and calcite. For each test, 2 g of mineral samples and 38 mL of DI water were added to the flotation cell. The pH value was adjusted by adding HCl or NaOH until the required value was attained. Cupferron was added as a collector to the pulp and agitated for 5 min. Subsequently, terpineol was added to the pulp for 1 min more of agitation. Finally, the flotation stage was performed for 5 min. Both floated and non-floated samples were filtered, dried, and weighed to calculate the flotation recovery. To ensure the reliability of the flotation results, each test was repeated three times under the same conditions. The standard deviation was calculated and presented as an error bar in the flotation results.
2.3. Adsorption Experiments
For the adsorption experiments, the adsorption quantity of reagents on the mineral’s surface was detected by using an ultraviolet-visible spectrum analyzer (UV2600, Shimadzu Corporation, Kyoto, Japan). For this analysis, 2 g mineral samples were used in each test, and the samples were treated in the same way as in the flotation test. After the agent reacted with the mineral, the solution was centrifuged by the TG16-WS machine (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 9000 r/min for 15 min, and then the centrifuged solution was sent to the ultraviolet-visible spectrophotometer for residual concentration detection. The specific surface area of the minerals was measured by N2 adsorption on a specific surface analyzer (TriStar II 3flex, micro, Norcross, GA, USA). The smithsonite surface area was 0.52 m2/g, and the calcite surface area was 0.46 m2/g. The adsorption capacity was calculated as follows:
represents the adsorption quantity of cupferron on the mineral surface. C0 and C are the initial and supernatant cupferron concentrations. V is the volume of the solution, and M is the weight of the mineral sample. S is the specific surface area of the smithsonite or calcite sample.
2.4. FTIR Spectroscopy Measurements
The smithsonite and calcite samples before and after the adsorption were measured by an infrared spectrometer (Nicolet 6700, Thermo Fisher, Waltham, MA, USA). We tested the FTIR spectra in the range of 400 cm−1~4000 cm−1 wavenumber. In a beaker, 2 g of minerals (<5 μm) and 38 mL of DI water were magnetically stirred. The specific concentration of cupferron was then added to the beaker after adjusting the pH of the slurry. After stirring for 30 min, the samples were filtered and placed in a low-temperature vacuum-drying oven. Finally, 100 mg of KBr and 5 mg of the mineral samples were mixed for infrared analysis.
2.5. Zeta Potential Measurement
The zeta potential measurements of smithsonite and calcite were performed on the Malvern Zetasizer instrument (Nano-ZS900, Malvern Inc., Malvern, Worcestershire, UK). The 50 mg sample below 5 μm was dispersed in 1 × 10−3 mol/L KCl electrolyte solution with magnetic stirring at room temperature. The pH was adjusted to the expected value with HCl or NaOH. According to the test conditions, a certain amount of cupferron was selectively added to the slurry. After the cupferron fully reacted with the mineral, it was left for 10 min and 2 mL of supernatant was removed to measure zeta potential. For each sample, the average of three independent measurements was taken as the final value.
2.6. XPS
The XPS measurement was performed on the ESCALAB 250Xi (Thermo Fisher, Waltham, MA, USA) using monochromatic Al Kα radiation. The mineral sample (−5 μm) was treated with cupferron concentrations of 2 × 10−4 mol/L, the blank sample was treated with DI water, and the stirring time was 30 min. After the reaction, the samples were filtered and vacuum-dried for XPS analysis. The C 1s peak of 284.8 eV was used to calibrate the spectra.
3. Results and Discussion
3.1. Micro-Flotation Tests
3.1.1. Single-Mineral Micro-Flotation Test
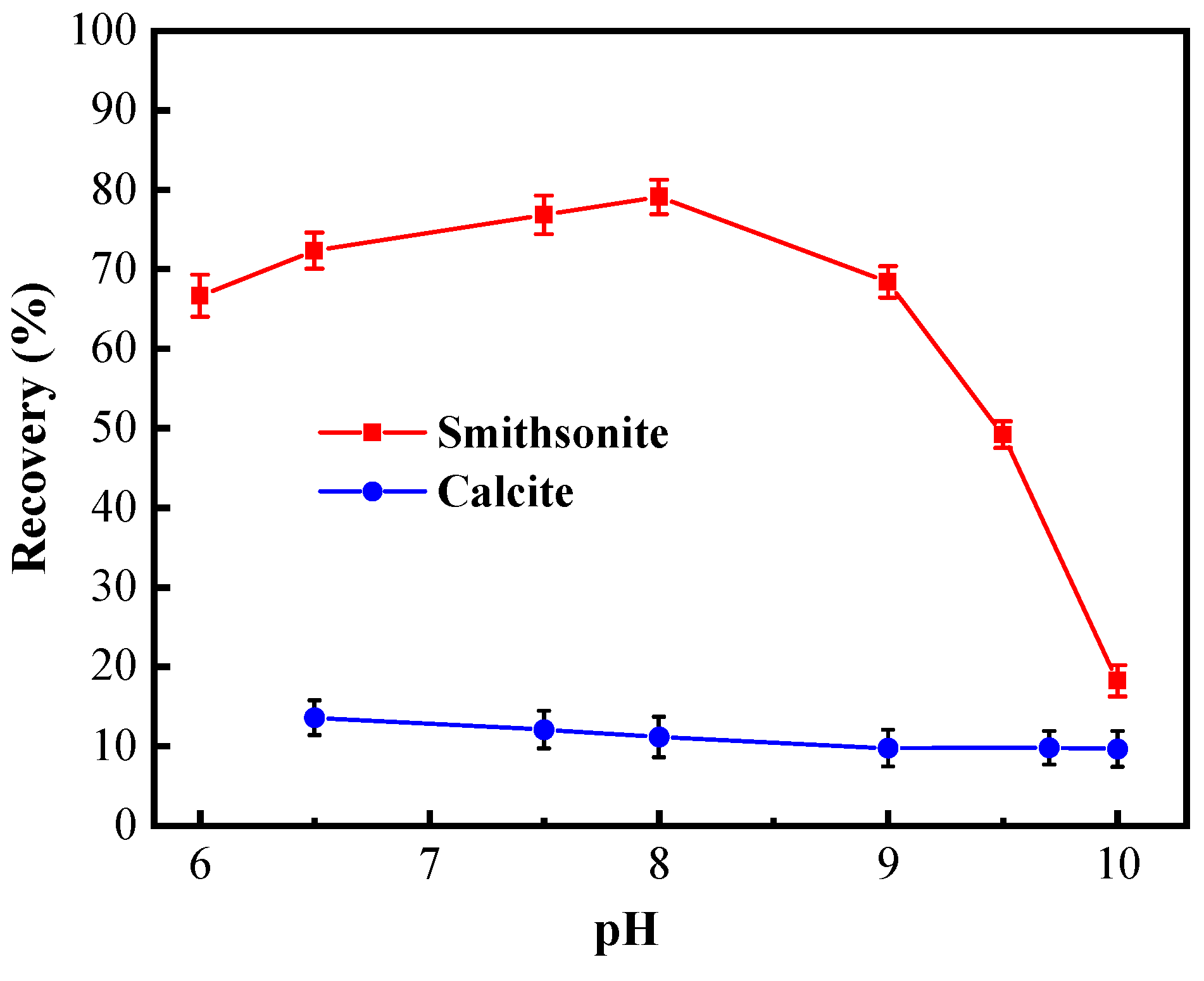
The pH condition test started at the neutral pH, considering the self-solubility of minerals. As shown in Figure 3, the pH value of pulp had different influences on the floatability of smithsonite and calcite. In the presence of cupferron, the recovery of smithsonite slowly increased in the range of pH 6 to 8, and the recovery of smithsonite reached its maximum of 79.08% at pH value 8. When the pH value exceeded 8.0, the recovery of smithsonite began to decline sharply, and the recovery was only 18.23% at pH 10. Compared with the maximum recovery rate, this is a decrease of 60.85%. Over the entire pH range, the recovery of calcite was low and remained stable in a small range around 15%. The chemical equilibrium of the smithsonite solution has been discussed in previous research, which showed that the species Zn2+ predominates until pH 8.5 [30]. Under this condition, cupferron can better chelate with smithsonite surface. Therefore, the optimum condition for separating smithsonite and calcite is pH 8.
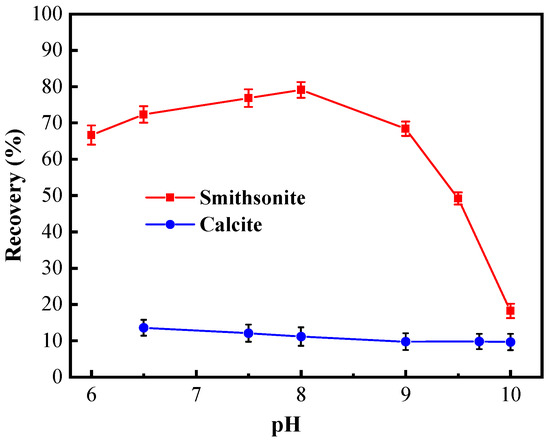
Figure 3.
Flotation recovery of smithsonite and calcite as a function of pH using 2 × 10−4 mol/L cupferron.
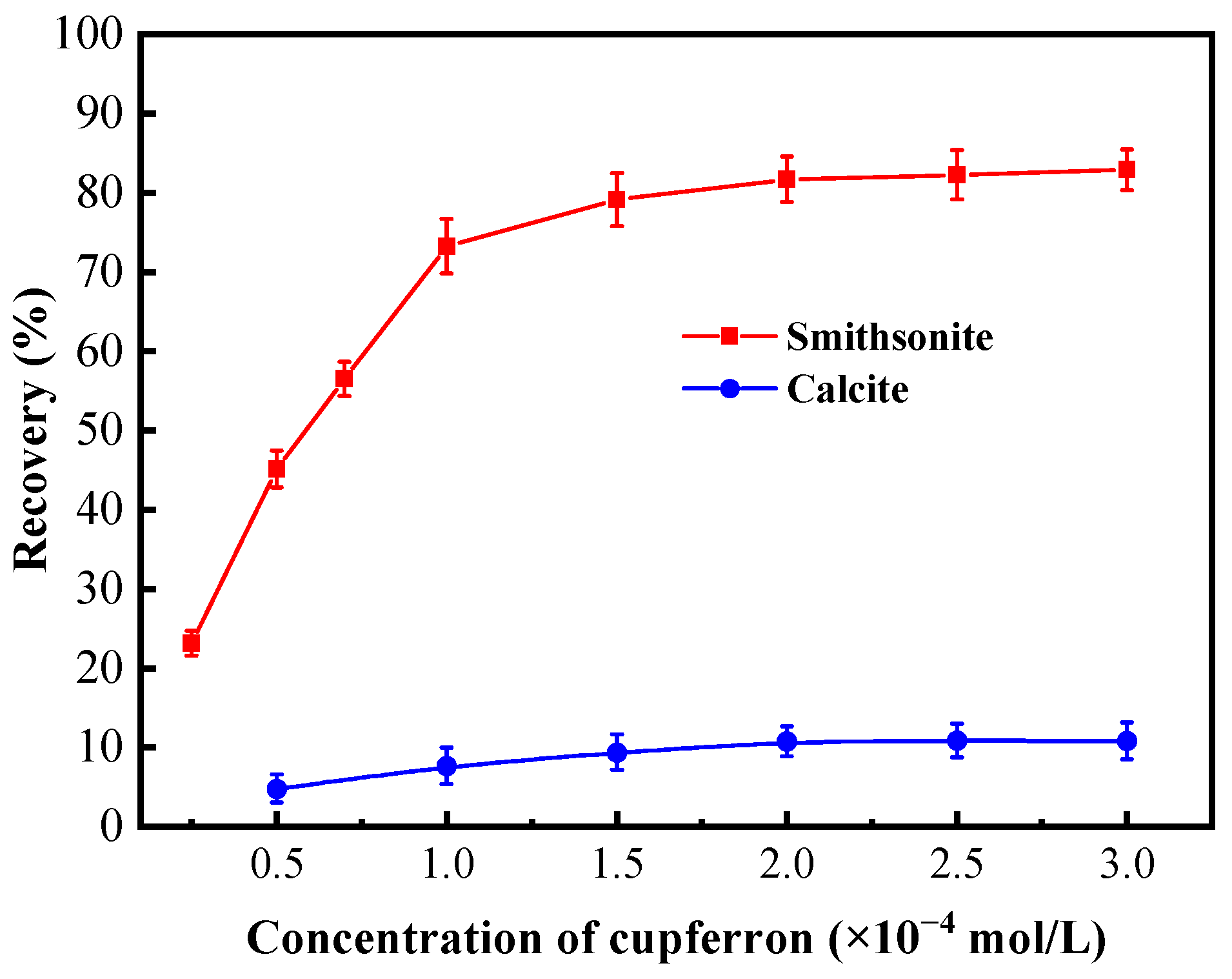
Figure 4 presents the effects of the cupferron concentration on the flotation performance of smithsonite and calcite at pH 8. As is shown in this figure, the recovery rates of smithsonite and calcite were different when the concentration of cupferron increased. The recovery rate of smithsonite increased rapidly when the concentration of cupferron was in the range of 0 to 2 × 10−4 mol/L. The recovery of smithsonite achieved 80.67% at a cupferron concentration of 2 × 10−4 mol/L. As the concentration of cupferron exceeded 2 × 10−4 mol/L, the smithsonite recovery fluctuated around 80%. In contrast, the recovery of calcite was about 15% in the entire cupferron concentration range.
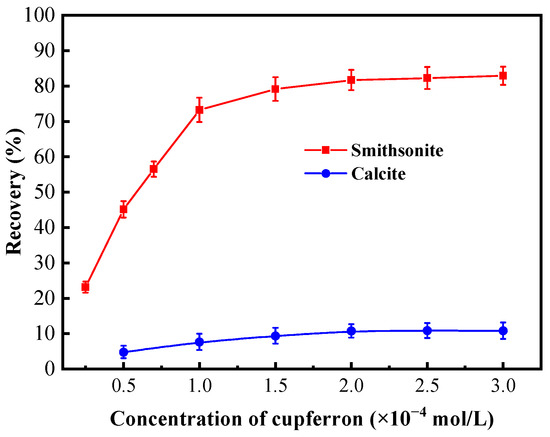
Figure 4.
Flotation recovery of smithsonite and calcite as a function of the concentration of cupferron at pH 7.5~8.0.
3.1.2. Mixed-Minerals Micro-Flotation Test
The micro-flotation test of mixed minerals was performed further to verify the specific collecting performance of cupferron on smithsonite. The pulp pH was adjusted to 8, and the concentration of cupferron was 2 × 10−4 mol/L. The test results are shown in Table 2.

Table 2.
The results of mixed-minerals micro-flotation test/%.
Table 2 shows that the recovery rate of smithsonite could reach 78.53% and that the recovery of calcite was 25.70% in the mixed-mineral flotation test. Compared with the single-mineral flotation test, the recovery of calcite was slightly improved. Previous studies have shown that mineral interactions and dissolved ions in mixed-mineral systems interfere with mineral recovery. Zn ions can disrupt the interactions between reagents and calcite by forming insoluble surface precipitates [31]. Furthermore, the presence of Ca2+ prevents the adsorption of sulfur ions onto smithsonite surface due to hydrolysis and precipitation, thus affecting the floatability of mineral [10]. Despite the interference of these influencing factors, on the whole, cupferron can be used to separate smithsonite from calcite.
3.2. Adsorption Experiments
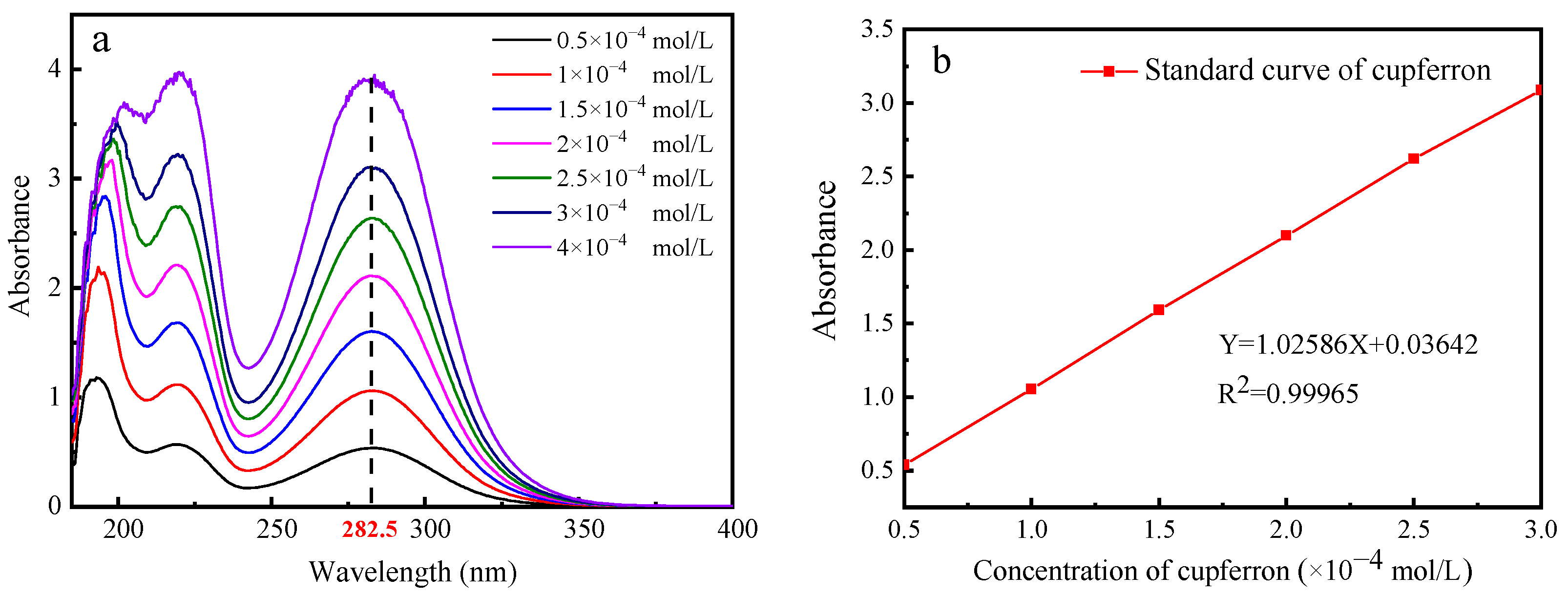
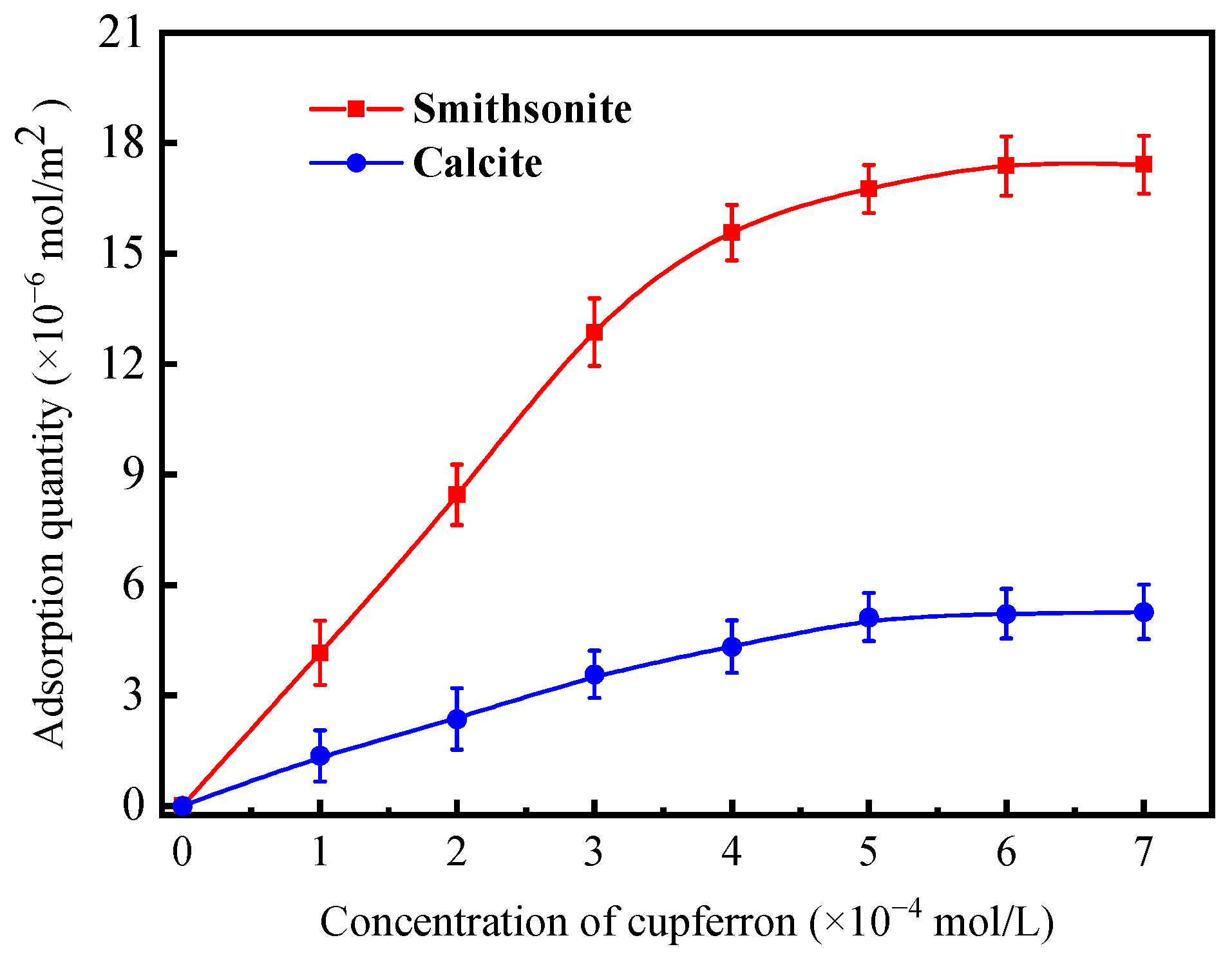
From Figure 5, it is obvious that a fixed absorption peak centered at 282.5 nm can be found by measuring the absorbance of cupferron at several different concentrations. The fitted curve indicates a linear relationship between absorbance and concentration in the range of 0.5 to 3 × 10−4 mol/L. Figure 6 illustrates that the adsorption capacities of smithsonite and calcite to cupferron are quite different, and the adsorption amount of smithsonite was significantly higher than that of calcite. The surface adsorption amount of smithsonite increased rapidly at a concentration of 0 to 5 × 10−4 mol/L and reached a maximum of 17.36 × 10−6 mol/m2 at a concentration of 5 × 10−4 mol/L, tending to stabilize as the cupferron increased. For calcite, the adsorption capacity of calcite increased slowly and was lower than 6 × 10−6 mol/m2 over the whole cupferron concentration range. It can be concluded that cupferron was adsorbed on the surface of smithsonite but hardly on the surface of calcite. These results were consistent with those of the micro-flotation tests.
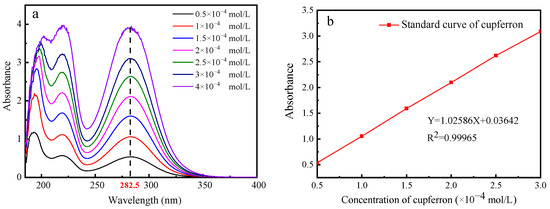
Figure 5.
The UV absorption spectra of cupferron as a function of cupferron concentration (a) and the standard curve of cupferron with different concentrations (b).
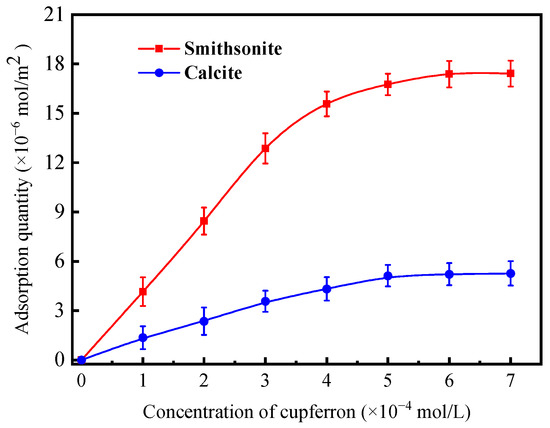
Figure 6.
Adsorption quantity of cupferron on smithsonite and calcite surfaces as a function of concentration at pH 8.
3.3. FTIR Analysis
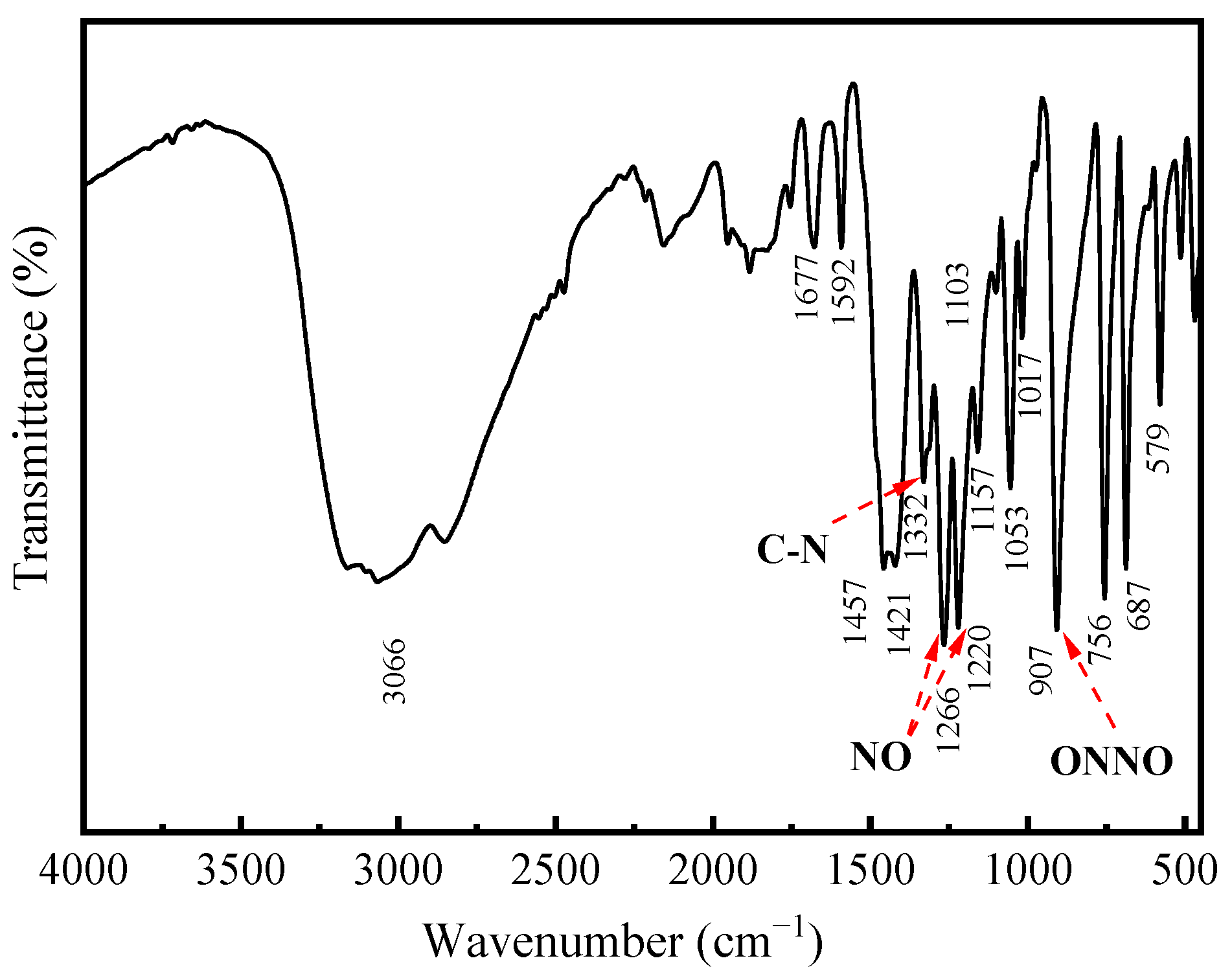
The infrared spectra of cupferron are shown in Figure 7. The strong absorption band around 3060 cm−1 can be attributed to the stretching mode of the NH4 group [29], and the aromatic ring vibrations were consistent with monosubstituted benzene ring vibrations. The bands at 1592, 1457 and 1421 cm−1 were due to C=C stretching modes, and the four bands at 1157, 1103, 1054 and 1017 cm−1 in the spectra of cupferron corresponded to the C-H in-plane bending modes of the benzene ring. The only remaining C-H vibrational modes were assigned to a band at 687 cm−1. The C-N stretching vibration should come around 1332 cm−1, and the bands at 1266 and 1220 cm−1 were ascribed to the N=O stretching modes. Finally, the bands around 1332 cm−1 and 907 cm−1 of cupferron were assigned to the C-N and the N-O stretching modes [32].
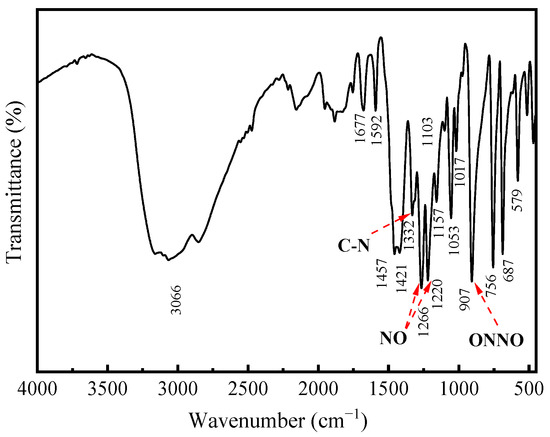
Figure 7.
FTIR spectra of cupferron.
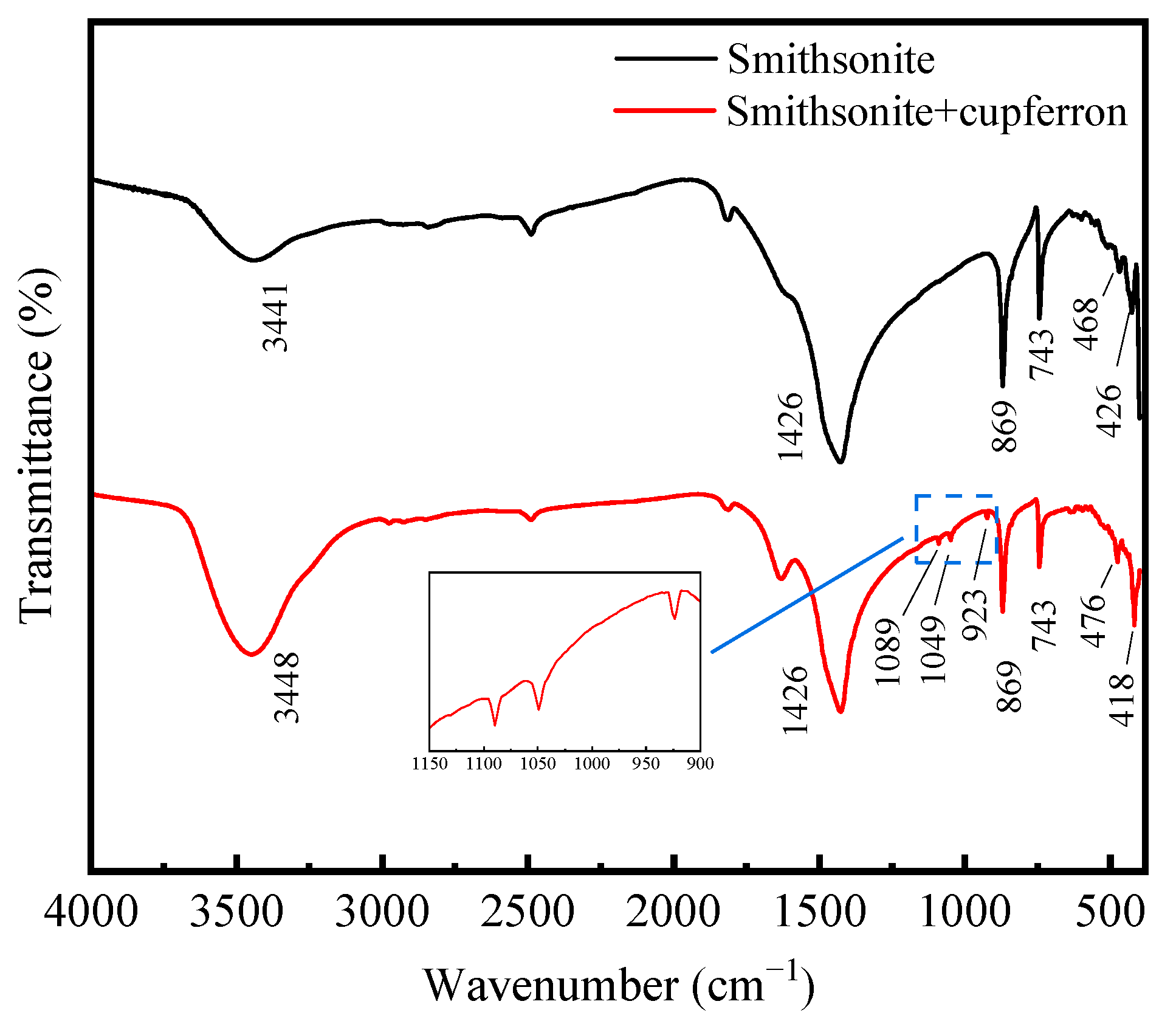
The FTIR spectra of smithsonite and cupferron-treated smithsonite are illustrated in Figure 8. The IR spectra of smithsonite showed an intense broad band at 1426 cm−1 that could have been from the stretching modes [33,34], and the peaks at 869 and 743 cm−1 were assigned to bending modes. Compared to the FTIR spectra of cupferron-treated smithsonite, the band at 426 cm−1 was ascribed to the Zn-O modes that shifted to 418 cm−1 [35,36]. Except for the unique groups of smithsonite, we could also see three new bands at 1089, 1049 and 923 cm−1. The bands at 923 cm−1 could be ascribed to the single-bonded N-O absorption. Bottei and Schneggenburger have shown that the N-O group shifts at a high frequency in metal complexes [29]. The C-H of the phenyl group should be observed at around 1000 to 1200 cm−1, and the bonds were indeed found at 1089 and 1049 cm−1 in our experiment. We concluded that cupferron adsorbed on the surface of smithsonite, and the N-O group of cupferron was found in the FTIR spectra of cupferron-treated smithsonite. This provided evidence for the chemical adsorption of cupferron on the surface of smithsonite.
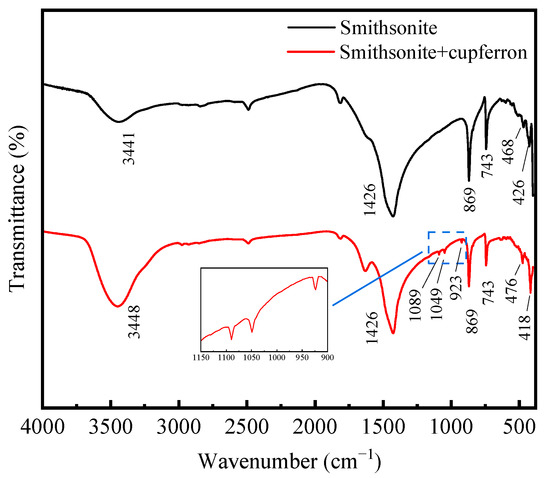
Figure 8.
FTIR spectra of smithsonite and cupferron-treated smithsonite.
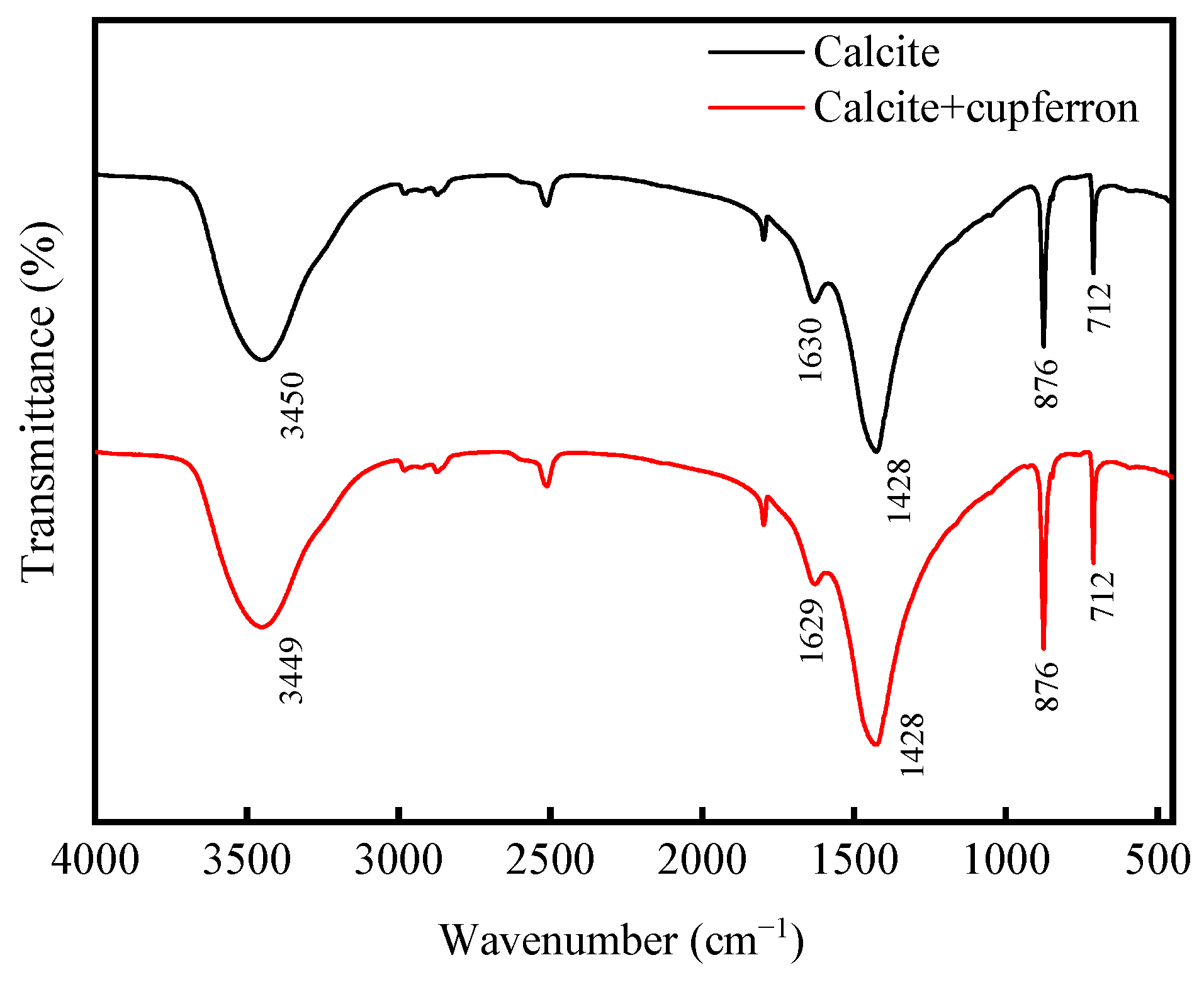
Figure 9 shows the FTIR spectra of calcite and cupferron-treated calcite. As with smithsonite, the spectra of calcite were characterized by the vibration mode of the molecule. There were three main peaks in the spectrum located at 1428, 876 and 712 cm−1 that were ascribed to vibration mode. Compared with the FTIR spectra of calcite, no new IR bands were observed for cupferron-treated calcite, especially in the region of 800 to 1300 cm−1. These results indicated that no chemisorption occurred on the calcite surface.
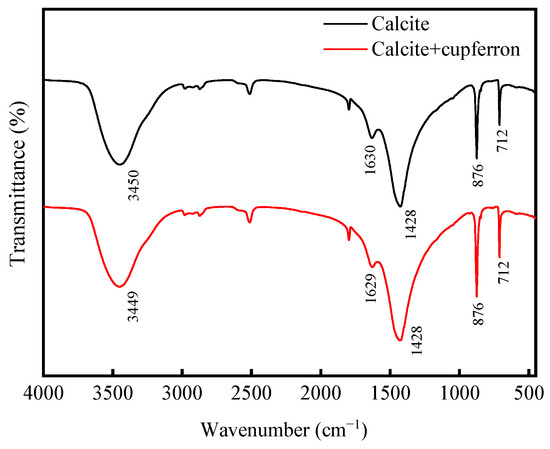
Figure 9.
FTIR spectra of calcite and cupferron-treated calcite.
3.4. Zeta Potential
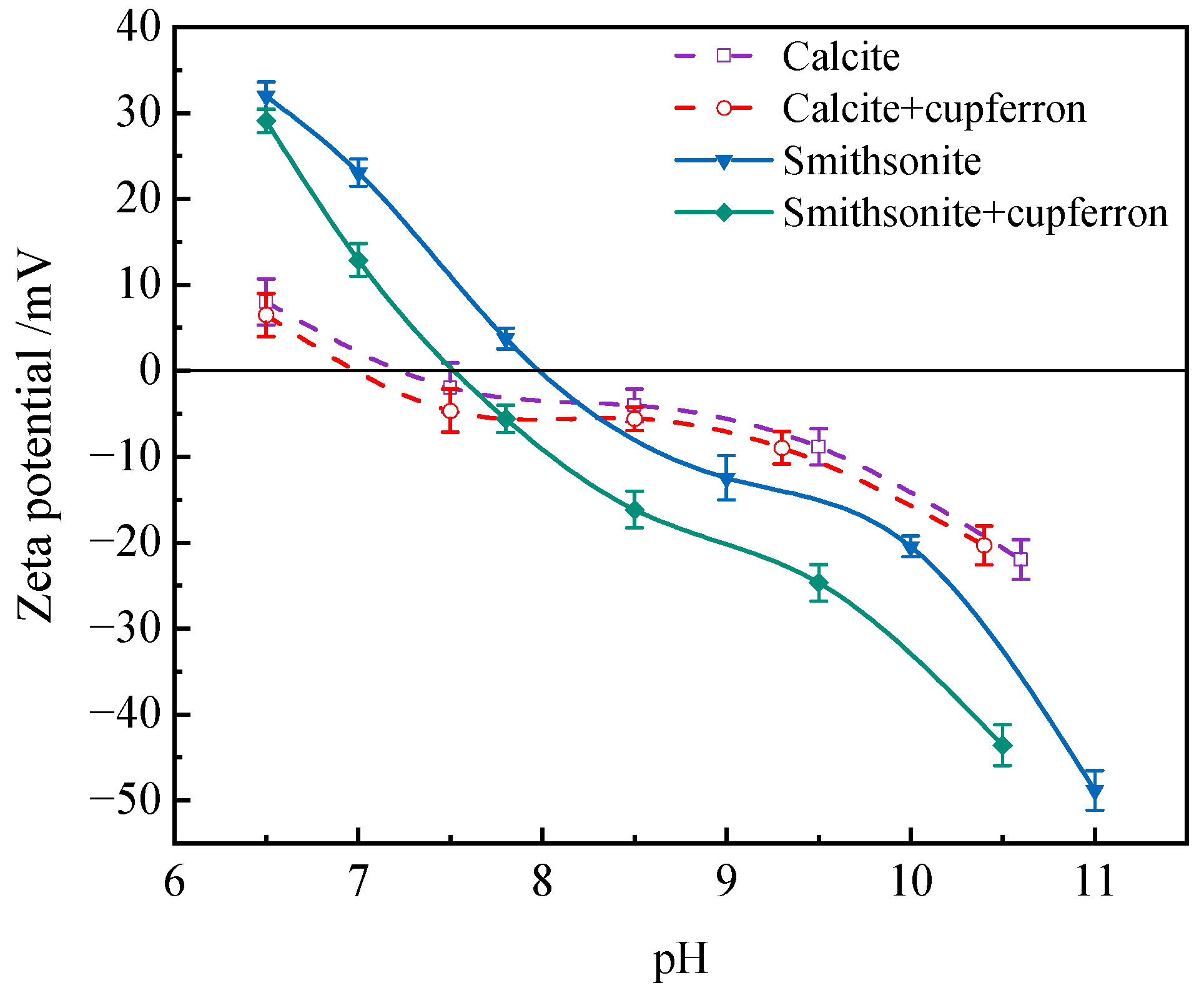
When minerals react with reagents, the surface potential of the minerals will change. The zeta potential of mineral surface is measured to reveal the adsorption behavior of reagents on mineral surface. The zeta potential of calcite and smithsonite before and after cupferron treatment is shown in Figure 10. The results showed that the isoelectric points of calcite and smithsonite were at pH 7.2 and 8.0, which agreed with previous reports [37,38]. With the addition of cupferron, the zeta potential of smithsonite and calcite were both shifted negatively, indicating a certain adsorption of cupferron on the two minerals’ surfaces. It is more obvious that the zeta potential of smithsonite decreases more than that of calcite. There may be some differences in the adsorption methods of smithsonite and calcite. The adsorption of cupferron by smithsonite is a strong chemical adsorption, mainly due to the chemical adsorption of nitrosohydroxylamine ions on the surface of smithsonite, which leads to a high zeta potential shift, while a small amount of cupferron is physically adsorbed on the surface of calcite. This adsorption is weak and unstable, so it will cause a slight shift in the zeta potential of calcite.
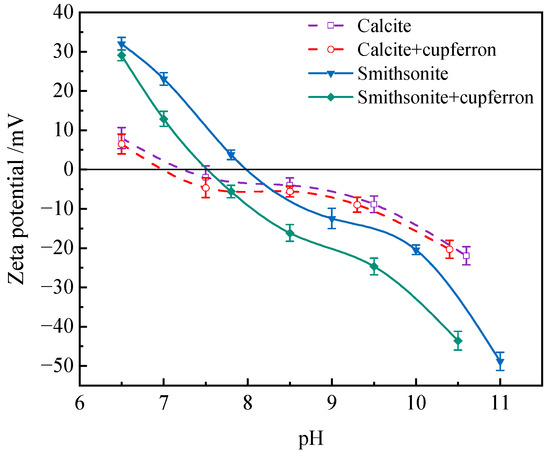
Figure 10.
Influences of cupferron addition on the zeta potential of smithsonite and calcite.
3.5. XPS Analysis
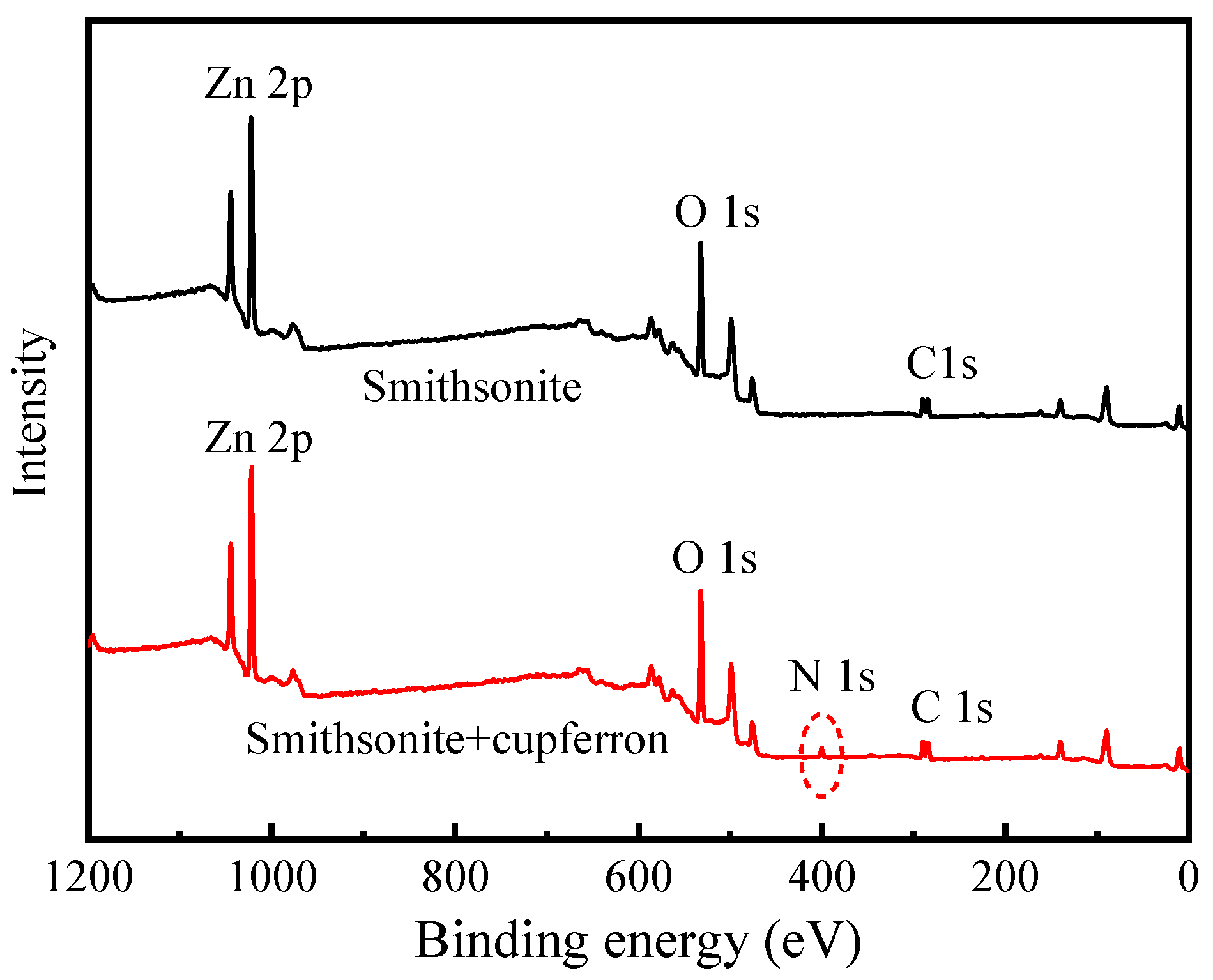
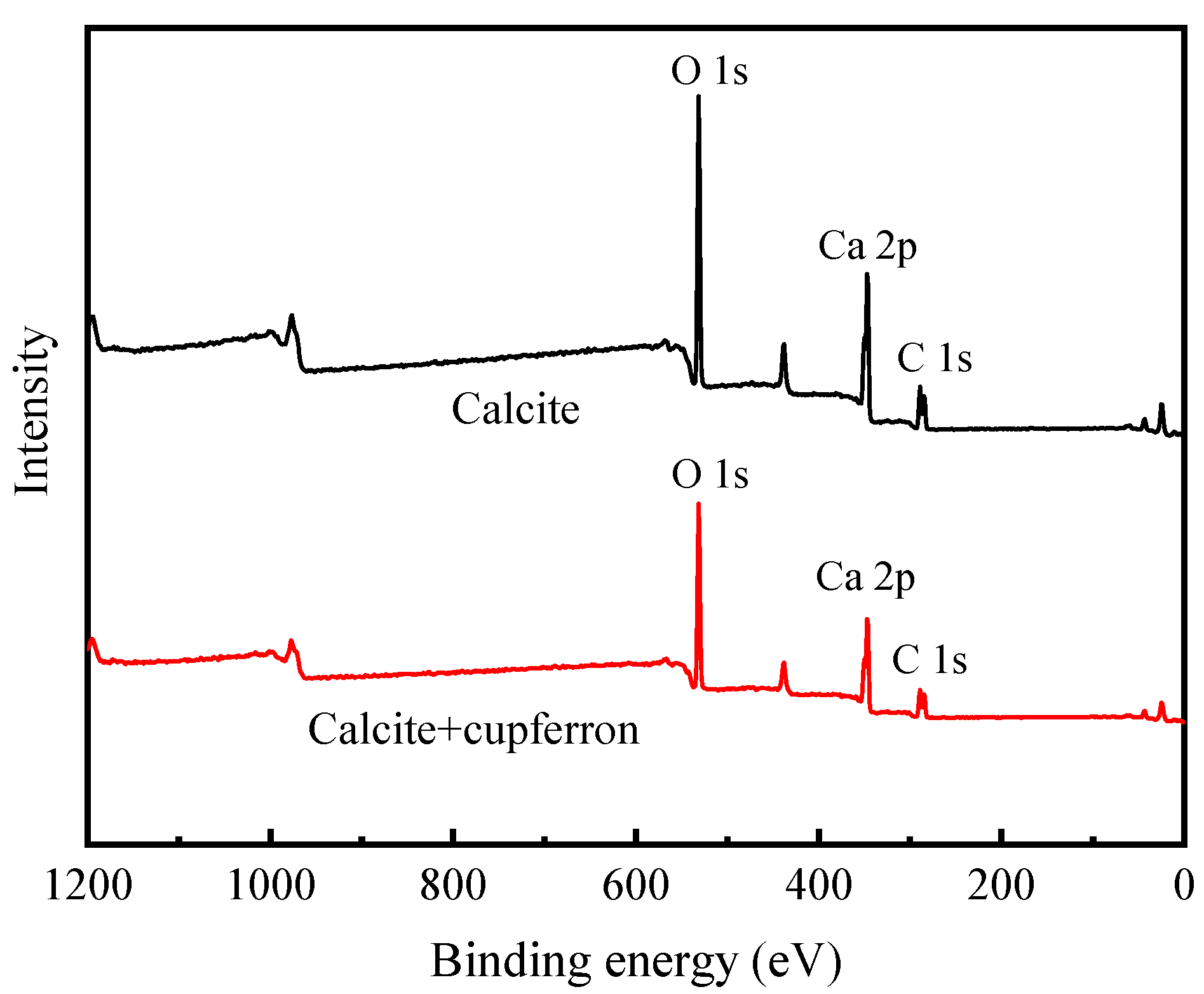
The XPS survey spectra of smithsonite and cupferron-treated smithsonite are shown in Figure 11. In the XPS of smithsonite, there were no other peaks observed except for the peculiar Zn 2p, O 1s and C 1s peaks, demonstrating the purity of the sample. New peaks at N 1s were observed in the XPS survey spectra of cupferron-treated smithsonite, indicating that the cupferron adsorbed on the mineral surface. However, as shown in Figure 12, although specific peaks of Ca 2p, O 1s and C 1s were observed in both calcite and cupferron-treated calcite, no obvious peaks appeared around 400 eV related to N 1s in the XPS of cupferron-treated calcite. It can be concluded that the cupferron tended to adsorb on the surface of smithsonite but not calcite.
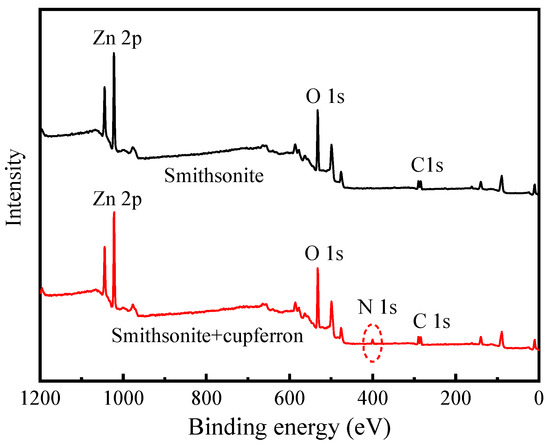
Figure 11.
Survey scan XPS spectra of smithsonite and cupferron-treated smithsonite.
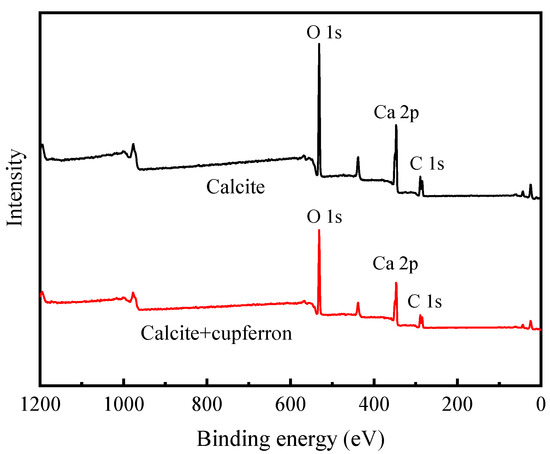
Figure 12.
Survey scan XPS spectra of calcite and cupferron-treated calcite.
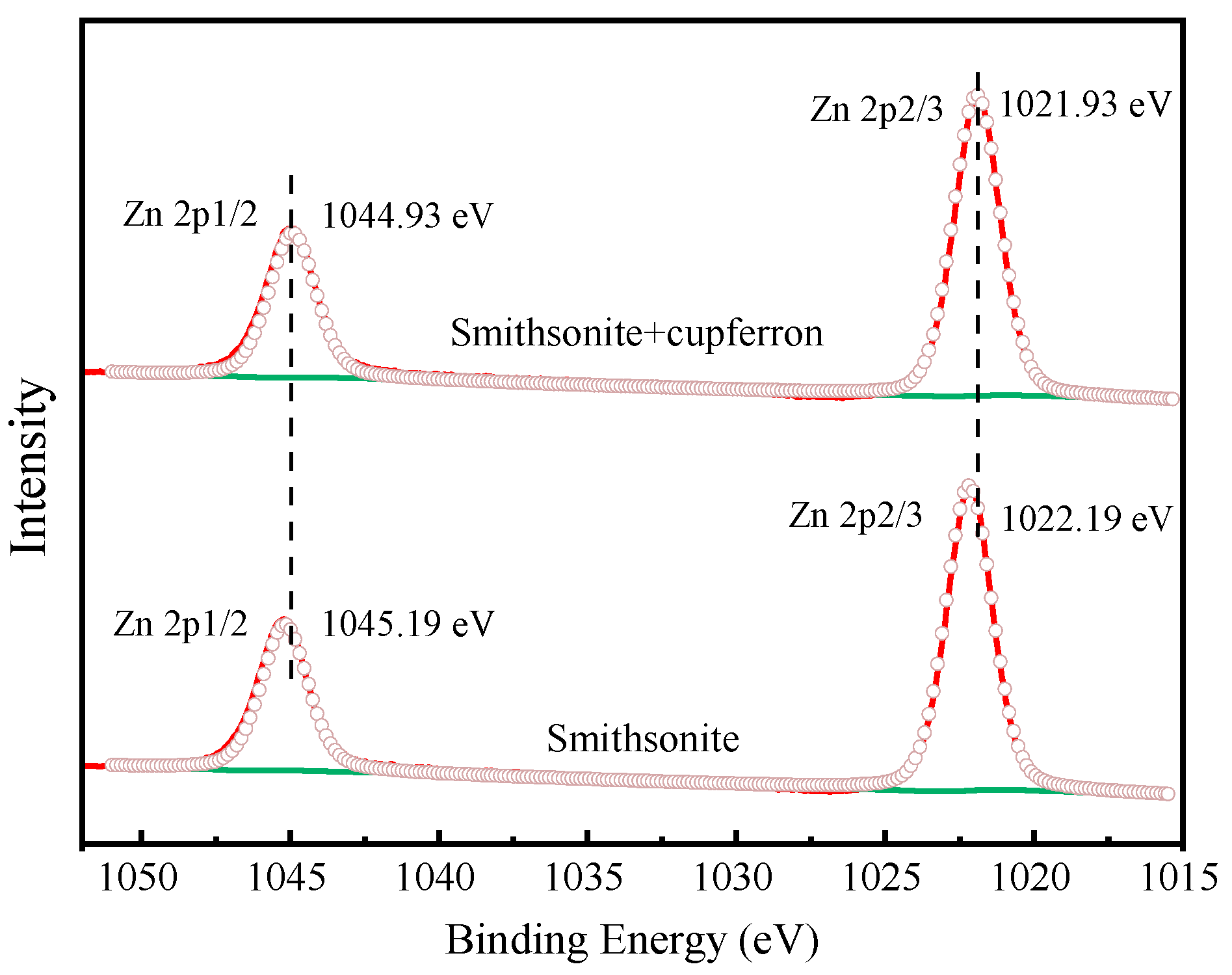
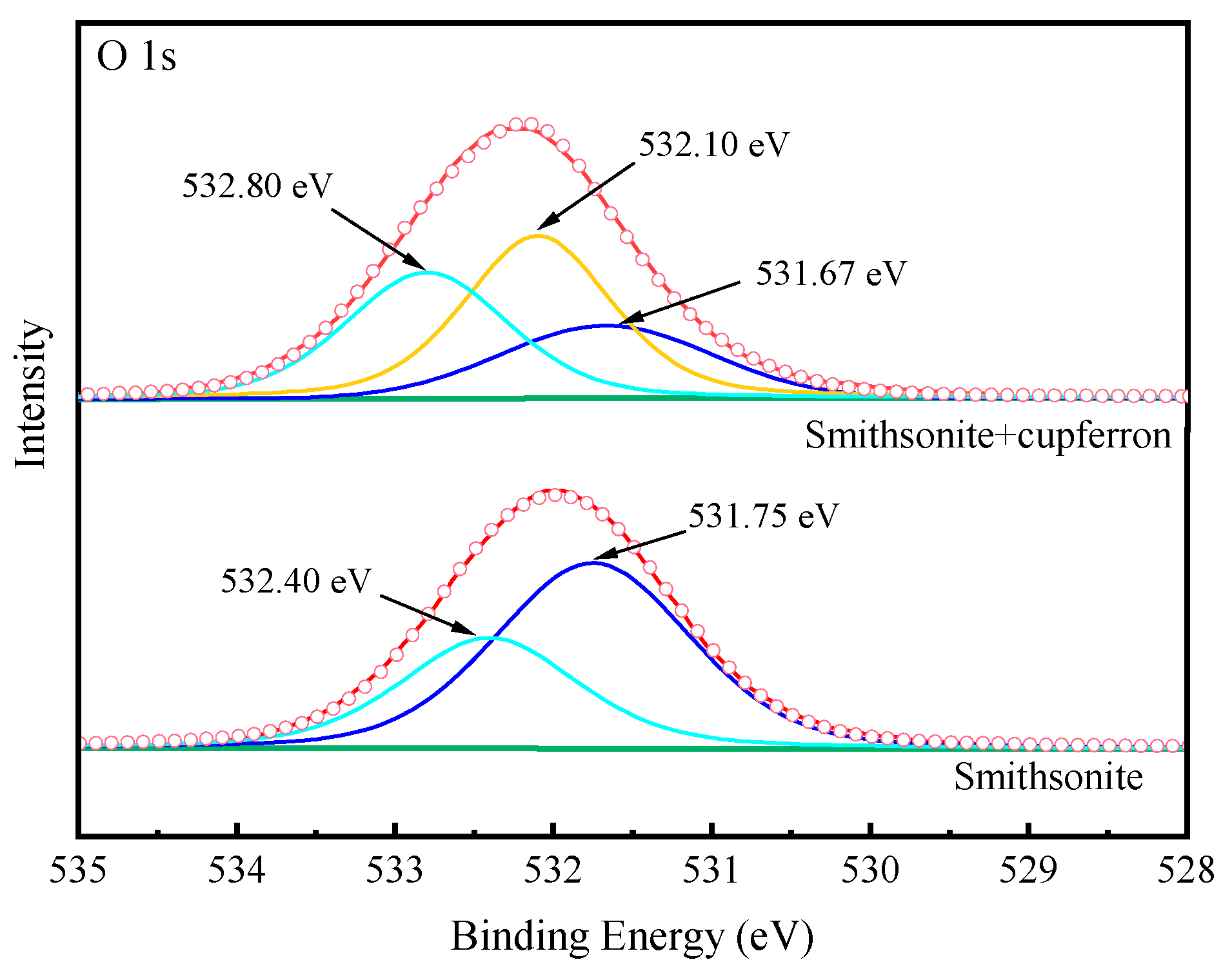
Figure 13 exhibits the high-resolution spectra of Zn 2p. The peaks positioned at 1022.19 eV and 1045.19 eV corresponded to the binding energies of Zn 2p3/2 and Zn 2p1/2. After the smithsonite was treated with cupferron, the two peaks of Zn 2p3/2 and Zn 2p1/2 shifted 0.26 eV towards the lower binding energy. Figure 14 shows the high-resolution O1s XPS spectra of smithsonite treated with cupferron. For bare smithsonite samples, this peak can be divided into two peaks centered at the binding energies of 531.75 eV and 532.40 eV, which are assigned to Zn hydroxide and Zn carbonate on the surface of the smithsonite [39,40]. After treatment with cupferron, this peak was divided into three peaks centered at the binding energies of 531.67 eV, 532.10 eV and 532.80 eV. The peaks were offset at Zn carbonate and Zn hydroxide, and there was an extra peak at 532.10 eV that could be ascribed to the N-O group [41].
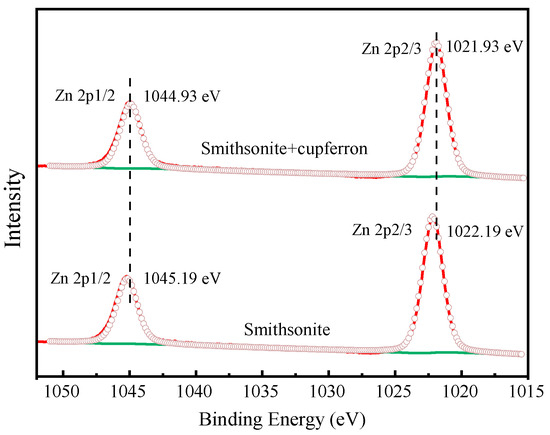
Figure 13.
Zn 2p XPS analysis of smithsonite and cupferron-treated smithsonite.
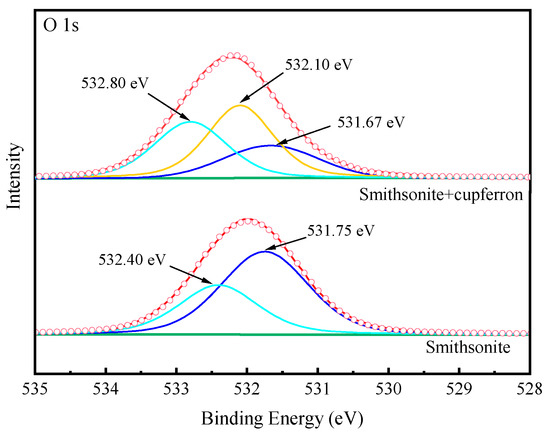
Figure 14.
O 1s XPS analysis of cupferron-treated smithsonite.
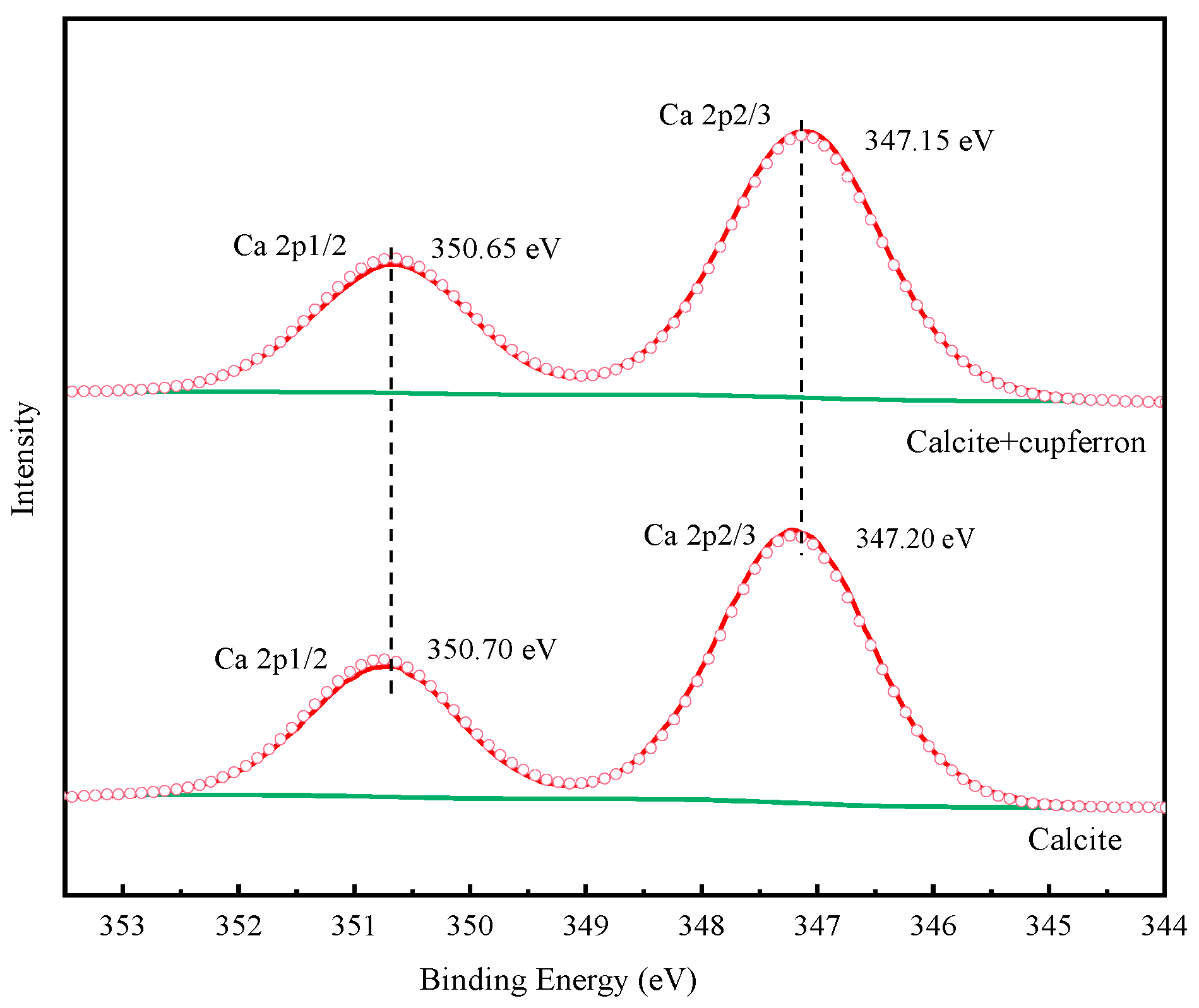
Table 3 illustrates the relative atomic concentrations on the surface of smithsonite and calcite before and after cupferron treatment. After the surface of smithsonite was treated with cupferron, the relative abundance of C and N atoms increased compared with that of bare smithsonite, while the relative content of O and Zn atoms decreased. Compared with the surface of calcite, the overall relative atomic content changed little—less than 0.1%. This indicated that cupferron was easily adsorbed on the surface of smithsonite and interacted with the surface Zn sites, resulting in a decrease in the relative content of Zn. The surface of calcite hardly adsorbed cupferron. Figure 15 shows the high-resolution spectra of Ca 2p. The binding energies of 347.20 eV and 350.70 eV are ascribed to Ca 2p1/2 and Ca 2p3/2. When calcite was treated with cupferron, the two peaks of Ca 2p3/2 and Ca 2p1/2 merely decreased, and the peak only shifted to 0.05 eV. From this, we concluded that cupferron chelated with smithsonite does not interact with calcite.

Table 3.
The relative atomic content changes on the surface of smithsonite and calcite.
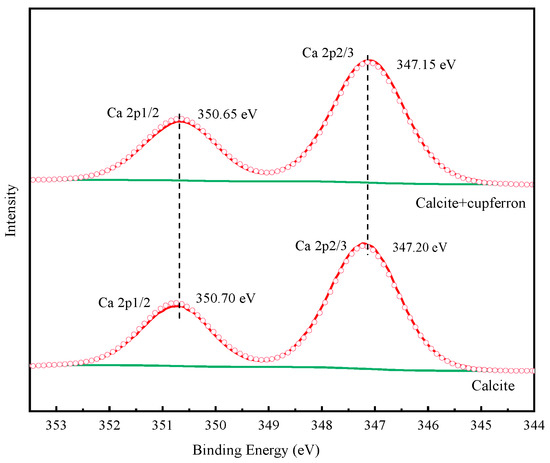
Figure 15.
Ca 2p XPS analysis of calcite and cupferron-treated calcite.
3.6. The Mechanism of the Flotation of Smithsonite with Cupferron as the Collector
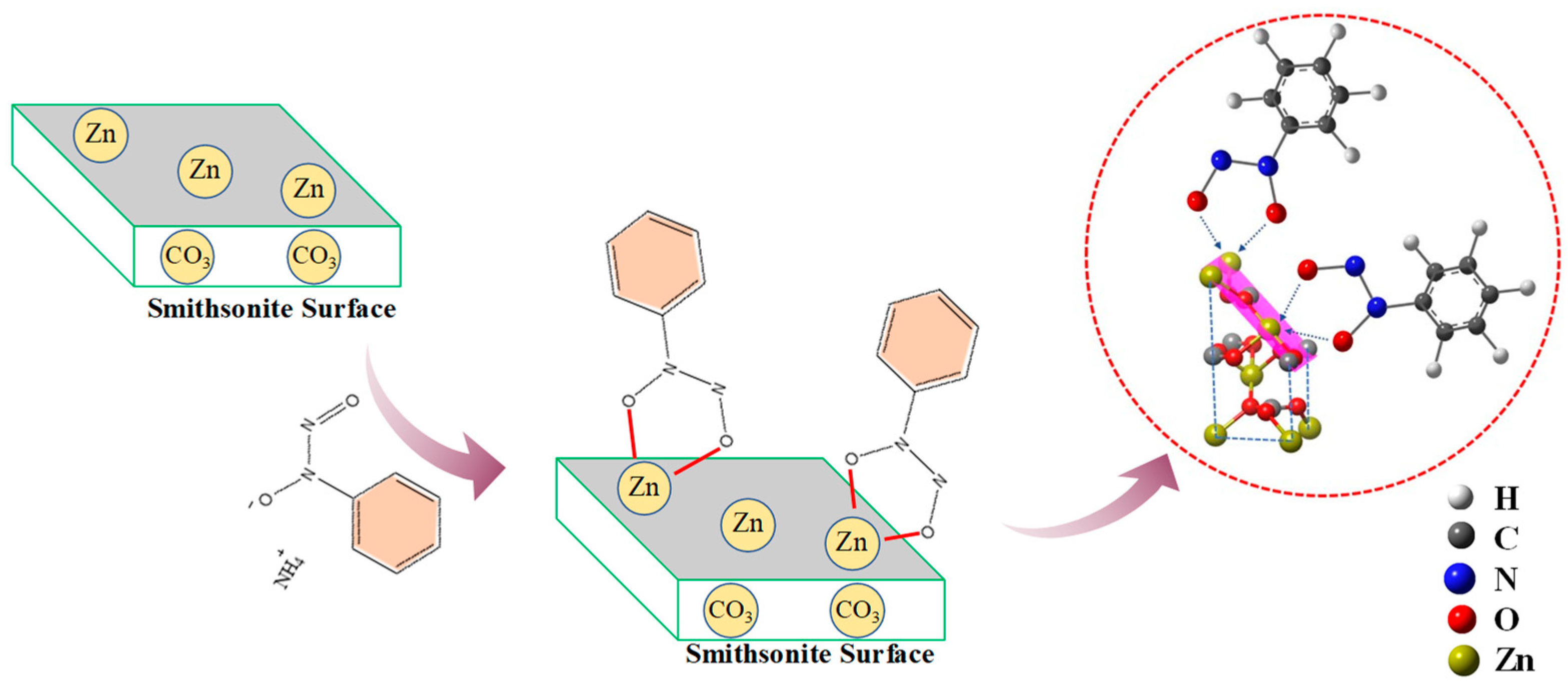
From these flotation experiments and characterization results, the adsorption model of cupferron adsorption on the smithsonite surface can be constructed. As shown in Figure 16, compared with calcite, the cupferron was more easily adsorbed on the surface of smithsonite and altered the surface hydrophobicity of smithsonite. Infrared results showed that new chemical bond vibration peaks were observed in the spectrum of cupferron-treated smithsonite. These vibration peaks belonged to the characteristic peaks of cupferron, including N-O functional group and C-H functional group of the benzene ring. The Zn-O bond on the surface of smithsonite also shifted. A new peak N 1s was found in the XPS analysis results. The infrared results were further validated by the high-resolution spectra of Zn 2p and O 1s. The O 1s spectra of the cupferron-treated smithsonite samples were fitted by three well-separated peaks, the O peaks in zinc carbonate and zinc hydroxide were shifted, and there was an extra peak that could be ascribed to the N-O group. The results clearly indicated that cupferron as a chelating reagent can form a five-membered ring complex with metal ions [26,27,28]. On the basis of these findings, cupferron was mostly chelated with the smithsonite surface zinc sites to form the insoluble species.
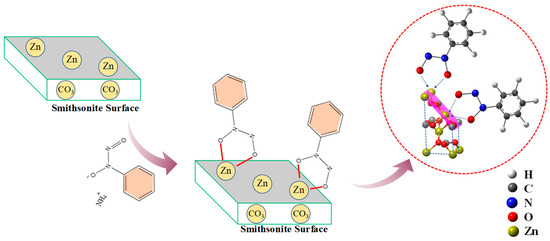
Figure 16.
The adsorption mechanism of cupferron on the surface of smithsonite and calcite.
4. Conclusions
This study demonstrated the effective separation of smithsonite from calcite using cupferron as a chelating collector. Mineral flotation and adsorption experiments revealed significant differences in the flotation behavior of calcite and smithsonite in the presence of cupferron. Smithsonite exhibited a higher adsorption capacity for cupferron, resulting in a recovery of 79.08% at a pH of 8.0. Zeta potential measurements indicated a greater decrease in zeta potential for smithsonite compared to calcite when treated with cupferron, suggesting a stronger effect of cupferron on smithsonite. Furthermore, IR and XPS spectra analysis demonstrated that cupferron chemically adsorbed onto the surface of smithsonite, primarily interacting with Zn2+ ions to form hydrophobic chelate complexes. These findings highlighted cupferron as a potential collector for smithsonite flotation, offering an alternative to sulfidation flotation methods and improving selectivity while mitigating environmental pollution.
Author Contributions
Formal analysis, investigation, writing–original draft, Q.W.; formal analysis, data curation, writing—review and editing, Y.C. and X.W.; investigation, data curation, formal analysis, L.S.; methodology, project administration, resources, supervision, writing—review and editing, Y.Q. and M.X.; conceptualization, methodology, funding acquisition, G.L. and W.S. All authors have read and agreed to the published version of the manuscript.
Funding
All authors of this work acknowledged the financial support from the National Key Technology Research and Development Program of China (2020YFC1909202), and the Major Science and Technology Program of Yunnan Province, China (202202AB080012).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We sincerely thank the editor and reviewers for their constructive comments which helped in improving our paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, M.; Zhang, X.; Wang, S.; Cao, W.; Jiang, Y. Life Cycle Assessment on Lead–Zinc Ore Mining and Beneficiation in China. J. Clean. Prod. 2019, 237, 117833. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.S.; Rashchi, F.; Naazeri, M. Review of the Hydrometallurgical Processing of Non-Sulfide Zinc Ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Balarini, J.C.; Polli, L.d.O.; Miranda, T.L.S.; de Castro, R.M.Z.; Salum, A. Importance of Roasted Sulphide Concentrates Characterization in the Hydrometallurgical Extraction of Zinc. Miner. Eng. 2008, 21, 100–110. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A Review of Zinc Oxide Mineral Beneficiation Using Flotation Method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, W.; Duan, H.; Wang, X.; Fang, P.; Liu, W.; Zhou, X.; Shen, Y. Design and Selection of Flotation Collectors for Zinc Oxide Minerals Based on Bond Valence Model. Miner. Eng. 2021, 160, 106681. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Chen, J.; Li, Y.; Zhao, C.; Mu, X. A Density Functional Based Tight Binding (DFTB+) Study on the Sulfidization-Amine Flotation Mechanism of Smithsonite. Appl. Surf. Sci. 2018, 458, 454–463. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Physicochemical Studies of Smithsonite Flotation Using Mixed Anionic/Cationic Collector. Miner. Eng. 2007, 20, 621–624. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The Effect of Reagents on Selective Flotation of Smithsonite–Calcite–Quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Zarei, H. Smithsonite Flotation from Zinc Oxide Ore Using Alkyl Amine Acetate Collectors. Sep. Sci. Technol. 2014, 49, 445–457. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Wang, M.; Shi, Q.; Liu, D.; Li, Q. Utilization of Sodium Carbonate to Eliminate the Adverse Effect of Ca2+ on Smithsonite Sulphidisation Flotation. Miner. Eng. 2019, 132, 121–125. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Bai, X.; Chang, W.; Cui, C.; Zhao, W. Surface Modification of Smithsonite with Ammonia to Enhance the Formation of Sulfidization Products and Its Response to Flotation. Miner. Eng. 2019, 137, 1–9. [Google Scholar] [CrossRef]
- Lan, Z.; Lai, Z.; Zheng, Y.; Lv, J.; Pang, J.; Ning, J. Thermochemical Modification for the Surface of Smithsonite with Sulfur and Its Flotation Response. Miner. Eng. 2020, 150, 106271. [Google Scholar] [CrossRef]
- Li, C.; Bai, S.; Ding, Z.; Yu, P.; Wen, S. Visual MINTEQ Model, ToF–SIMS, and XPS Study of Smithsonite Surface Sulfidation Behavior: Zinc Sulfide Precipitation Adsorption. J. Taiwan Inst. Chem. Eng. 2019, 96, 53–62. [Google Scholar] [CrossRef]
- Luo, B.; Liu, Q.; Deng, J.; Yu, L.; Lai, H.; Song, C.; Li, S. Characterization of Sulfide Film on Smithsonite Surface during Sulfidation Processing and Its Response to Flotation Performance. Powder Technol. 2019, 351, 144–152. [Google Scholar] [CrossRef]
- Cai, J.; Su, C.; Ma, Y.; Yu, X.; Peng, R.; Li, J.; Zhang, X.; Fang, J.; Shen, P.; Liu, D. Role of Ammonium Sulfate in Sulfurization Flotation of Azurite: Inhibiting the Formation of Copper Sulfide Colloid and Its Mechanism. Int. J. Min. Sci. Technol. 2022, 32, 575–584. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Chen, Y.; Zhu, Y. Interaction between Smithsonite and Carboxyl Collectors with Different Molecular Structure in the Presence of Water: A Theoretical and Experimental Study. Appl. Surf. Sci. 2020, 510, 145410. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Wang, X.; Miller, J.D. Smithsonite Flotation with Lauryl Phosphate. Miner. Eng. 2020, 147, 106155. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wang, J.; Wang, L.; Xiao, J. A Comparison Study of Adsorption of Benzohydroxamic Acid and Amyl Xanthate on Smithsonite with Dodecylamine as Co-Collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.; Duan, H.; Yang, T.; Li, Z.; Zhou, S. Sodium Carbonate Effects on the Flotation Separation of Smithsonite from Quartz Using N,N′-Dilauroyl Ethylenediamine Dipropionate as a Collector. Miner. Eng. 2018, 126, 1–8. [Google Scholar] [CrossRef]
- Dong, L.; Qiao, L.; Zheng, Q.; Shen, P.; Qin, W.; Jiao, F.; Liu, D. Enhanced Adsorption of Citric Acid at the Calcite Surface by Adding Copper Ions: Flotation Separation of Scheelite from Calcite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131036. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation Separation of Smithsonite from Calcite Using 2-Phosphonobutane-1,2,4-Tricarboxylic Acid as a Depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, G.; Mai, Q.; Liu, H.; Li, C.; Feng, H. Flotation Separation of Smithsonite from Calcite Using Depressant Sodium Alginate and Mixed Cationic/Anionic Collectors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124227. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, G.; Li, C.; Mai, Q.; Liu, H.; Zhou, H.; Shi, Q. Flotation Separation of Smithsonite from Calcite Using a New Depressant Fenugreek Gum. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123794. [Google Scholar] [CrossRef]
- Marabini, A.M.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating Reagents for Flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Muthuswami, S.V.; Vijayan, S.; Woods, D.R. Flotation of Uranium from Uranium Ores in Canada: Part II—Cupferron Adsorption on Uranium Oxide, Quartz, Illite and a Uranium Ore from Elliot Lake. Can. J. Chem. Eng. 1985, 63, 650–661. [Google Scholar] [CrossRef]
- Prabhakar, S.; Khangaonkar, P.R. Flotation and Adsorption Studies of Chalcopyrite with Cupferron. Int. J. Miner. Process. 1982, 9, 87–95. [Google Scholar] [CrossRef]
- Sheng, Q.; Yang, B.; Cao, S.; Yin, W.; Sun, H.; Ma, Y.; Chen, K. Adsorption of Cupferron on Malachite (−201) Surface and Implication for Flotation. Miner. Eng. 2021, 169, 106954. [Google Scholar] [CrossRef]
- Bahri, Z.; Rezai, B.; Kowsari, E. Evaluation of Cupferron on the Selective Separation of Gallium from Aluminum by Flotation: The Separation Mechanism. Miner. Eng. 2016, 98, 194–203. [Google Scholar] [CrossRef]
- Bottei, R.S.; Schneggenburger, R.G. Thermal and Spectral Study of Some Divalent Metal Chelates of Cupferron and Dicupferron. J. Inorg. Nucl. Chem. 1970, 32, 1525–1545. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Deng, H. Effect of Solution Chemistry on the Flotation System of Smithsonite and Calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Ou, L.; Lu, Y. Effect of the Lattice Ions on the Calcite Flotation in Presence of Zn(II). Miner. Eng. 2013, 40, 24–29. [Google Scholar] [CrossRef]
- Thakur, N.V.; Kartha, V.B.; Kanekar, C.R.; Marathe, V.R. Infrared Spectra of Cupferron and Some Rare Earth Cupferrates. J. Inorg. Nucl. Chem. 1972, 34, 2831–2836. [Google Scholar] [CrossRef]
- Frost, R.L.; Reddy, B.J.; Wain, D.L.; Hales, M.C. An Application of near Infrared Spectroscopy to the Study of Carbonate Minerals—Smithsonite, Rhodochrosite, Sphaerocobaltite and Cadmium Smithsonite. J. Near Infrared Spectrosc. 2006, 14, 317–324. [Google Scholar] [CrossRef]
- Hales, M.C.; Frost, R.L. Synthesis and Vibrational Spectroscopic Characterisation of Synthetic Hydrozincite and Smithsonite. Polyhedron 2007, 26, 4955–4962. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; Zakaria, A.; Kassim, A.; Norleha Basri, S. Novel Conductive Polypyrrole/Zinc Oxide/Chitosan Bionanocomposite: Synthesis, Characterization, Antioxidant, and Antibacterial Activities. IJN 2014, 10, 217. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Song, M.; Shin, H.-S. The Role of PH Variation on the Growth of Zinc Oxide Nanostructures. Appl. Surf. Sci. 2009, 255, 4891–4896. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Chen, Y. Using Phytic Acid as a Depressant for the Selective Flotation Separation of Smithsonite from Calcite. Sep. Purif. Technol. 2022, 302, 122104. [Google Scholar] [CrossRef]
- Xie, X.; Li, B.; Xie, R.; Tong, X.; Li, Y.; Zhang, S.; Li, J.; Song, Q. Al3+ Enhanced the Depressant of Guar Gum on the Flotation Separation of Smithsonite from Calcite. J. Mol. Liq. 2022, 368, 120759. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, F. Understanding the Correlation of Crystal Atoms with Photochemistry Property: Zn5(OH)6(CO3)2 vs. ZnCO3. ChemistrySelect 2018, 3, 8886–8894. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Pan, S.; Nong, P.; Kong, Q.; Wang, X.; Zhang, L.; Tan, S.; Zhu, Z. Dissolution of the Smithsonite–Rhodochrosite (ZnCO3-MnCO3) Solid Solutions in Aqueous Solution at 25 °C. Chem. Geol. 2022, 602, 120886. [Google Scholar] [CrossRef]
- Gomez-Bolivar, J.; Mikheenko, I.P.; Orozco, R.L.; Sharma, S.; Banerjee, D.; Walker, M.; Hand, R.A.; Merroun, M.L.; Macaskie, L.E. Synthesis of Pd/Ru Bimetallic Nanoparticles by Escherichia coli and Potential as a Catalyst for Upgrading 5-Hydroxymethyl Furfural Into Liquid Fuel Precursors. Front. Microbiol. 2019, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).