Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Sample and Reagents
2.2. Characterization Methods
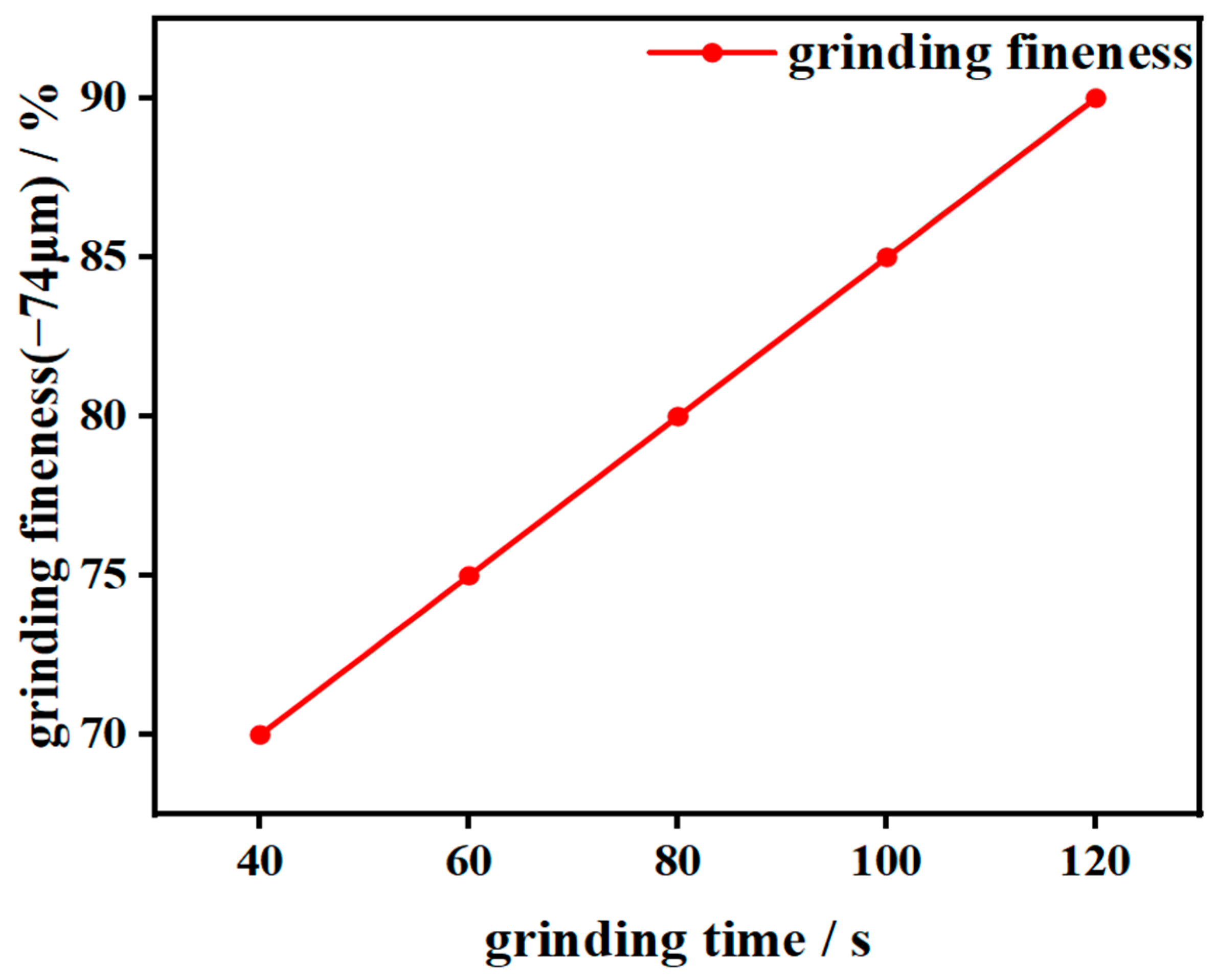
2.3. Flotation Tests
3. Results and Discussion
3.1. Process Mineralogy Analysis
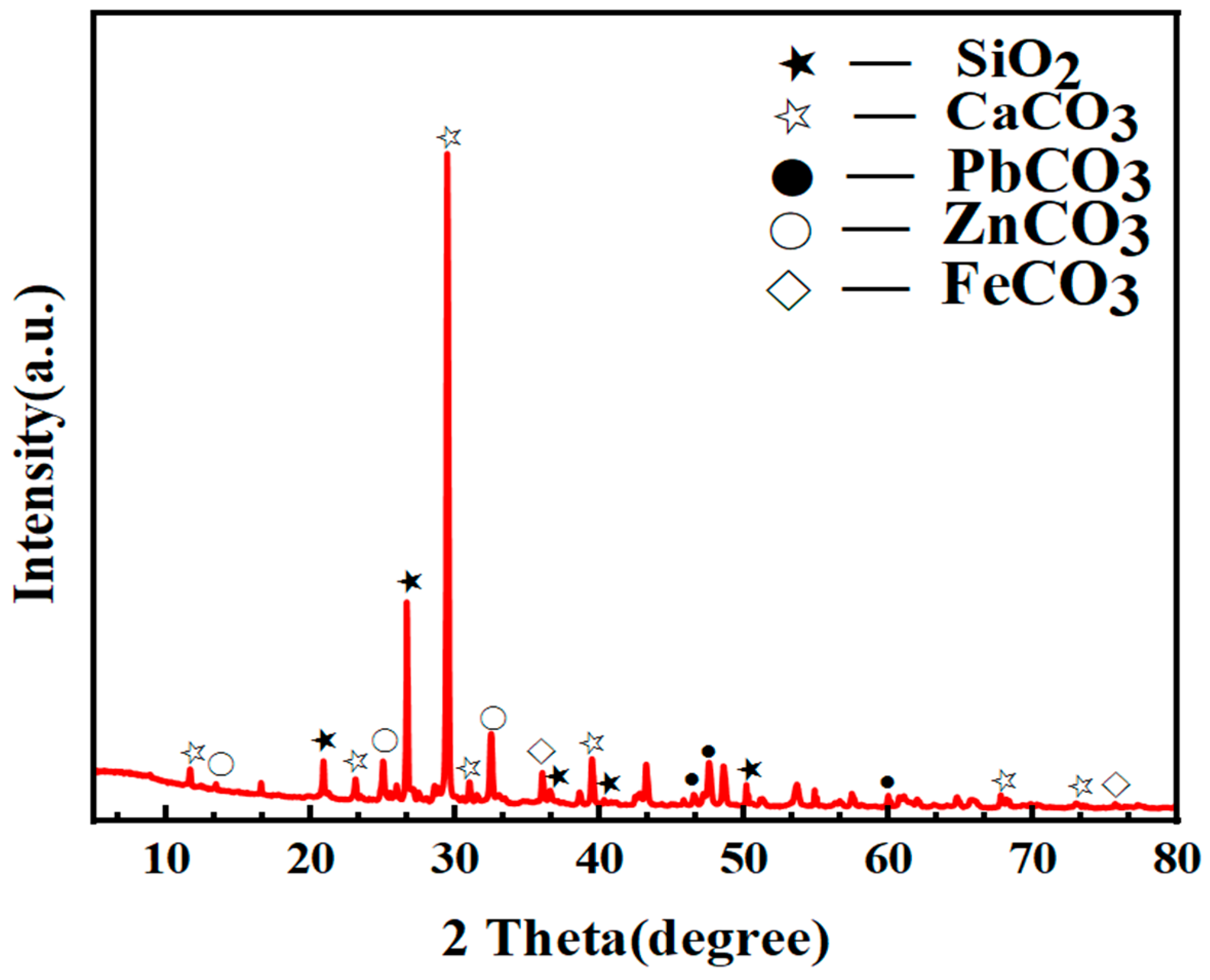
3.1.1. Mineral Composition and Element Occurrence
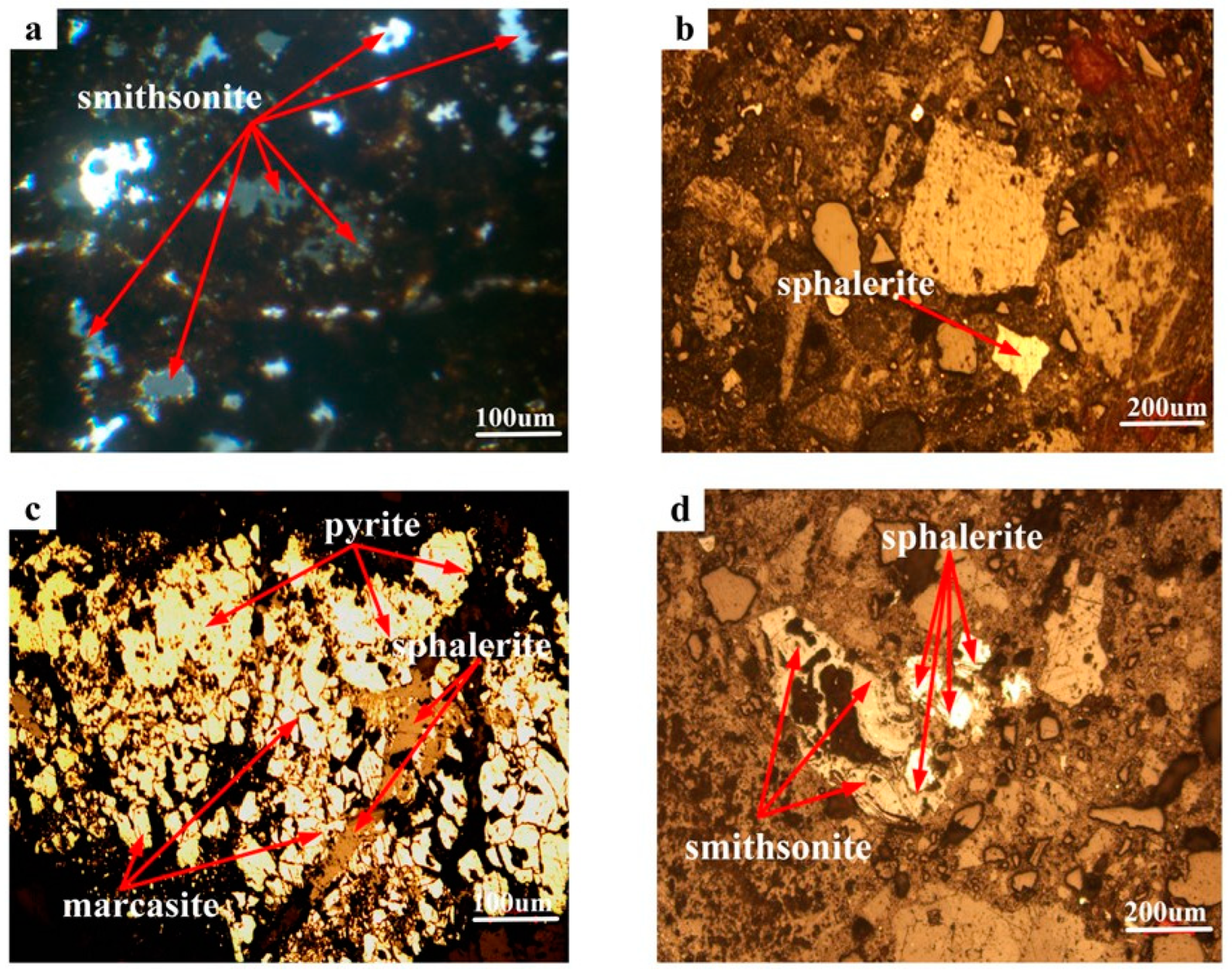
3.1.2. Zinc Minerals Embedded Characteristic
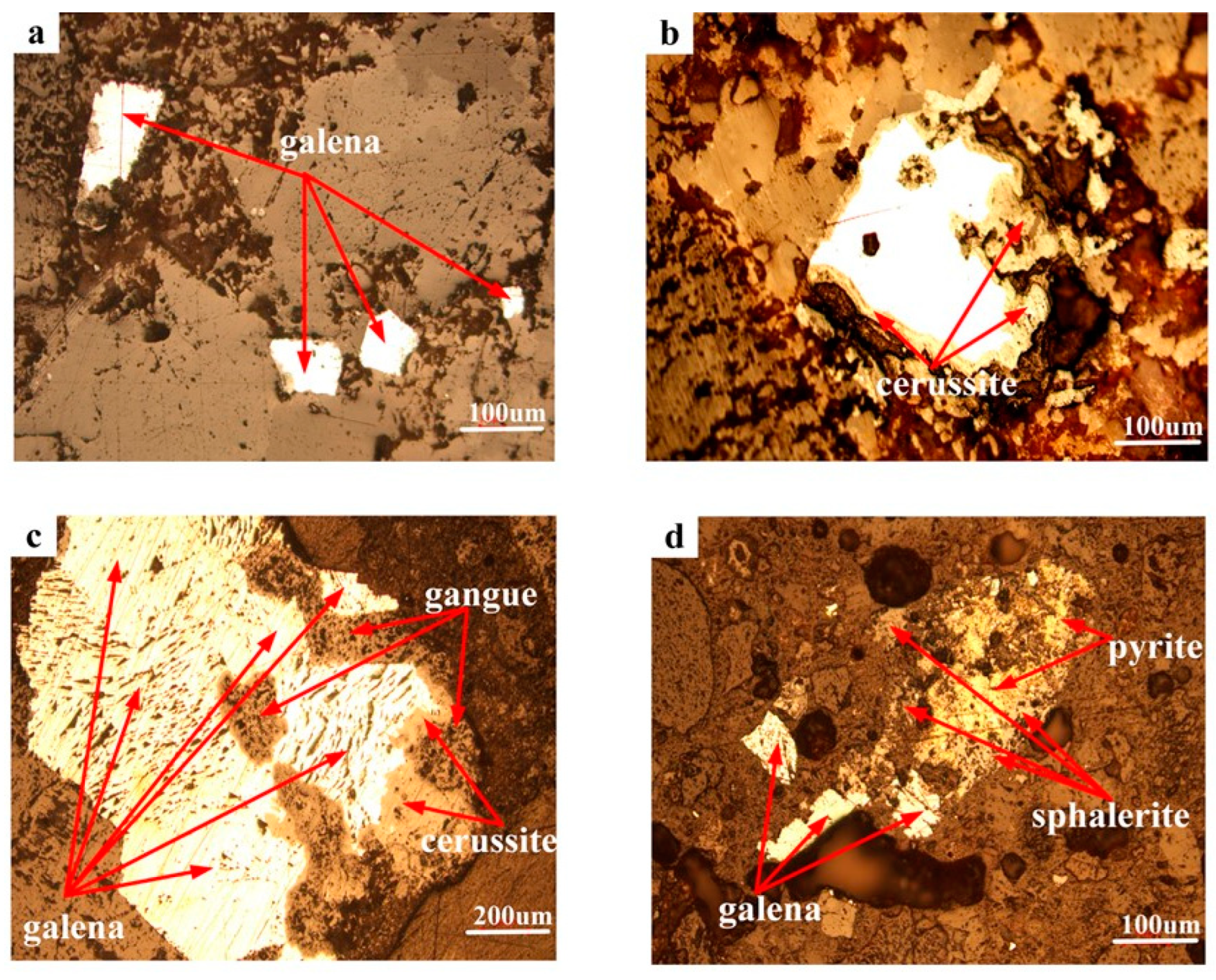
3.1.3. Lead Minerals Embedded Characteristic
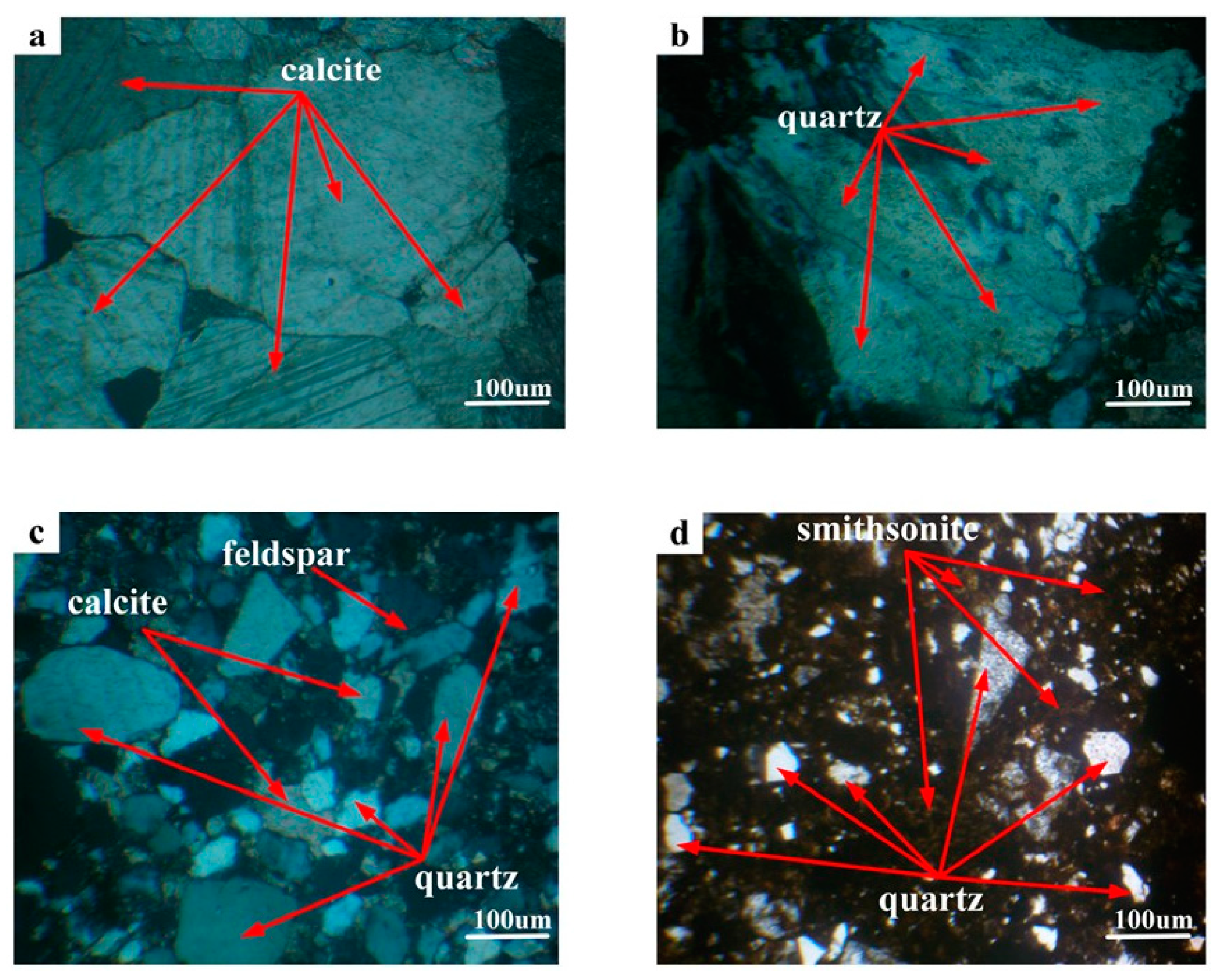
3.1.4. Gangue Minerals Embedded Characteristic
3.2. Flotation Experiments
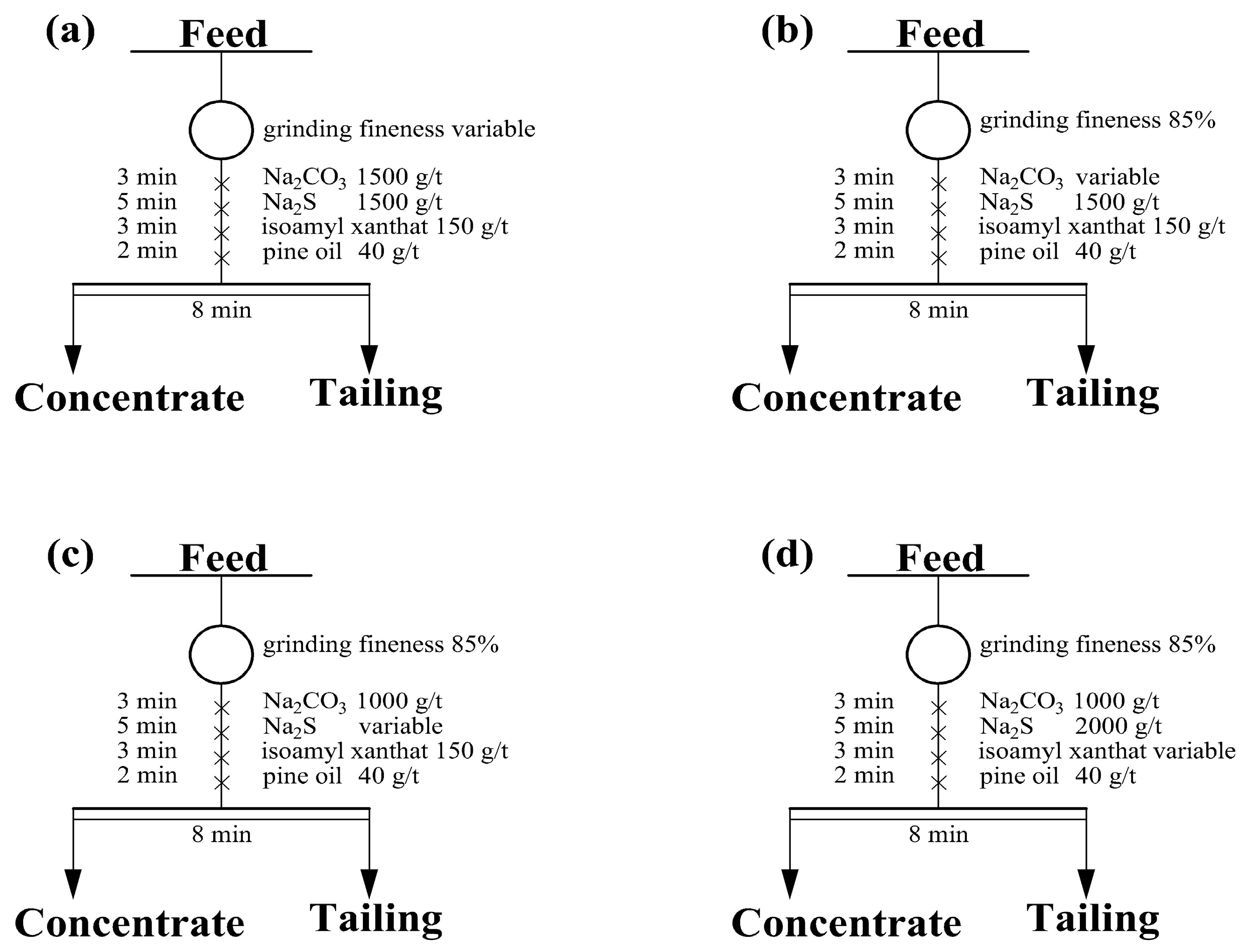
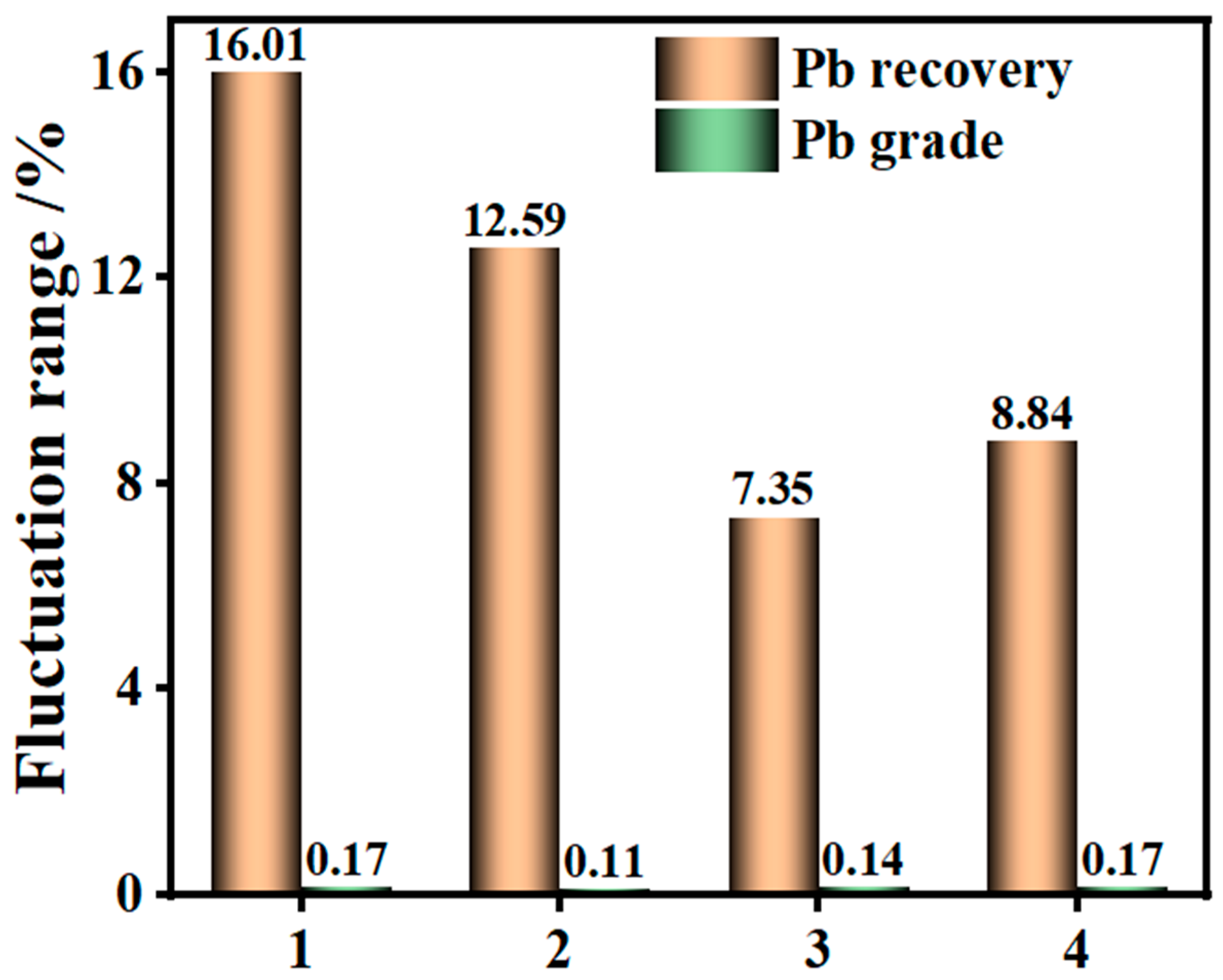
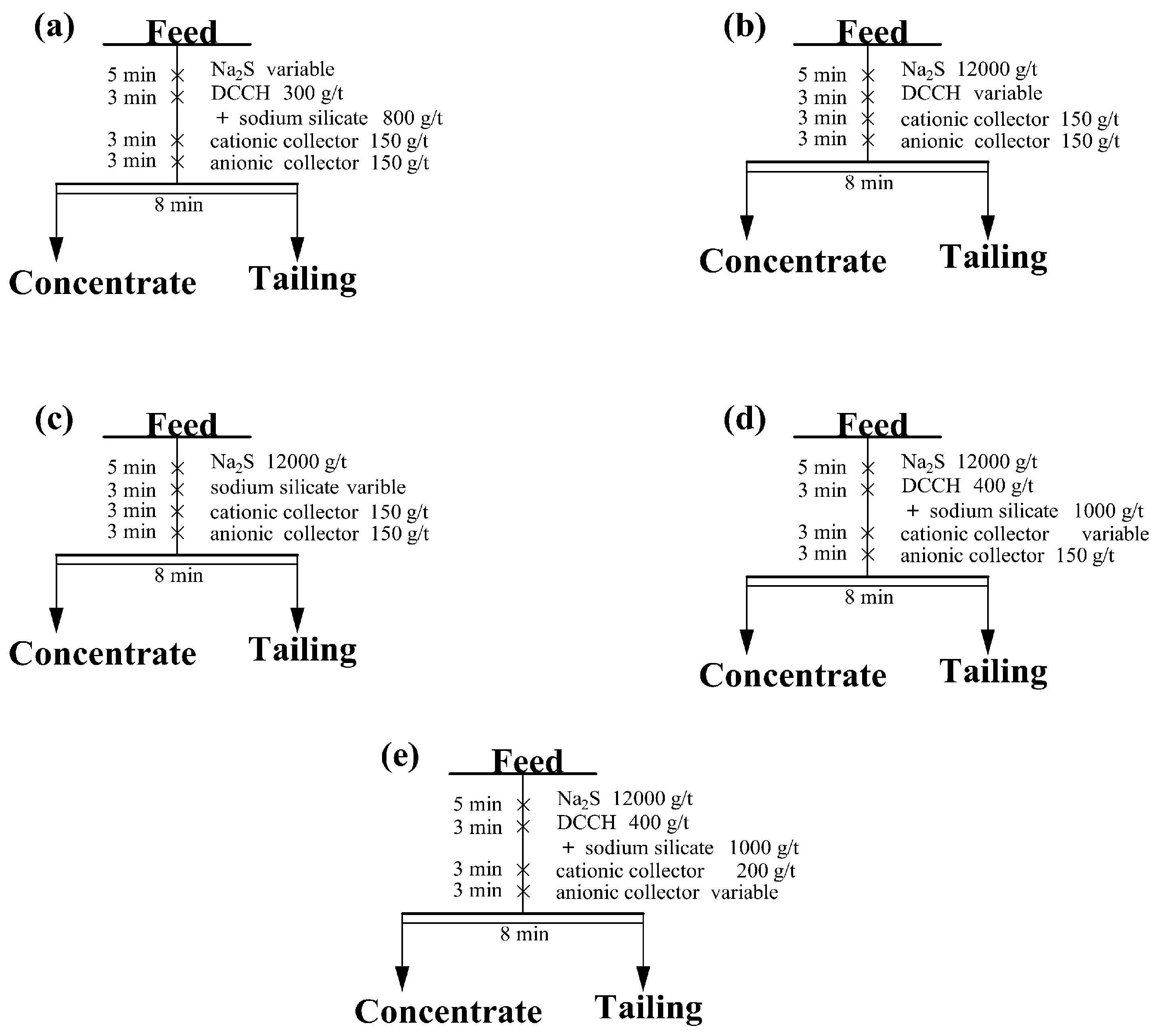
3.2.1. Flotation Conditional Experiments
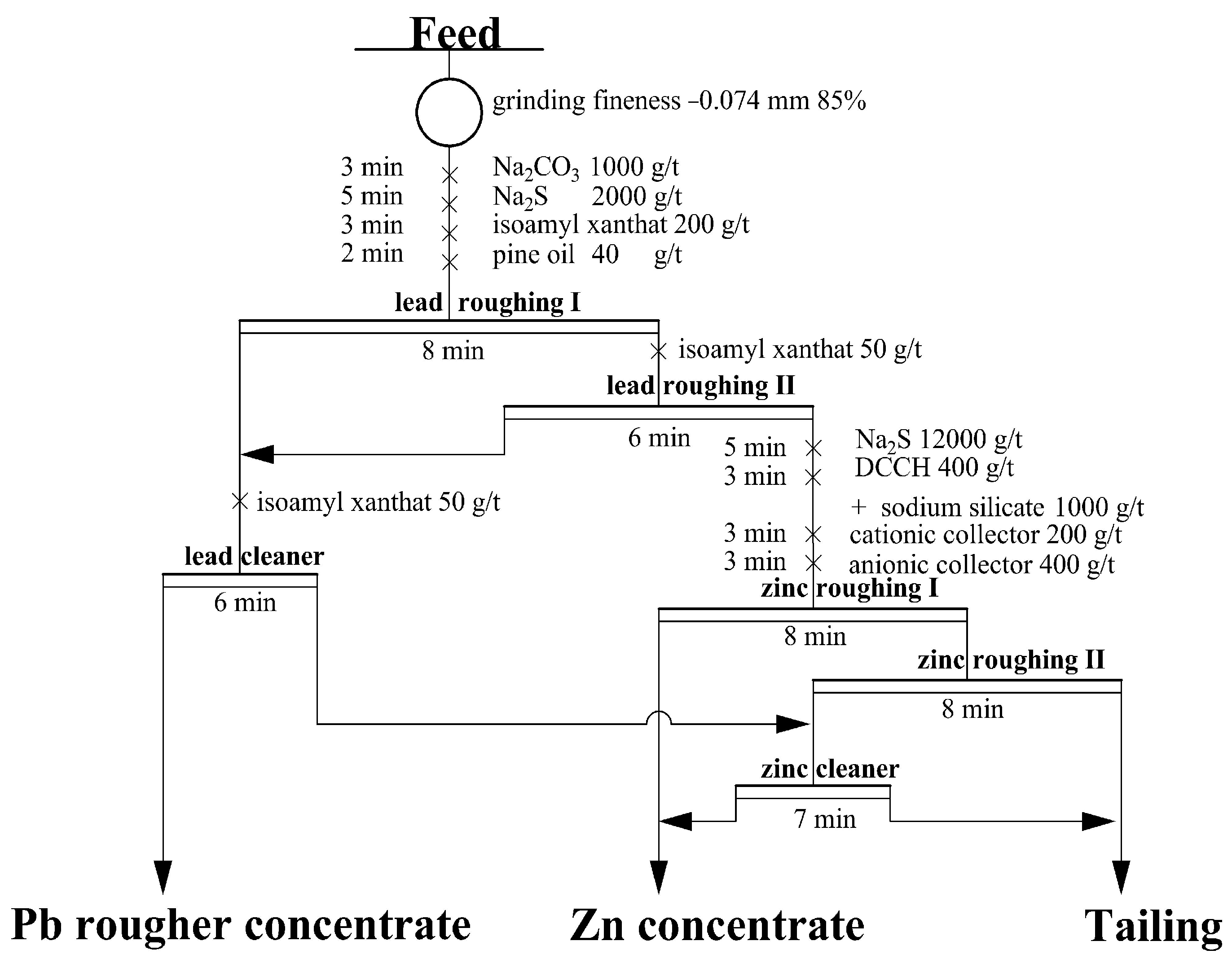
3.2.2. Flotation Flowchart Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moradi, S.; Monhemius, A.J. Mixed sulphide–oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076. [Google Scholar] [CrossRef]
- Saidi, M.; Kadkhodayan, H. Experimental and theoretical evaluation of zinc recovery from zinc oxide ore: Process optimization and simulation using Aspen Plus software. Int. J. Chem. React. Eng. 2020, 18, 103772. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nayl, A.A.; Daoud, J.A. Leaching and Recovery of Zinc and Copper from Brass Slag by Sulfuric Acid. J. Saudi Chem. Soc. 2016, 20, S280–S285. [Google Scholar] [CrossRef]
- Brough, C.P.; Warrender, R.; Bowell, R.J.; Barnes, A.; Parbhakar-Fox, A. The process mineralogy of mine wastes. Miner. Eng. 2013, 52, 125–135. [Google Scholar] [CrossRef]
- Lotter, N.O.; Baum, W.; Reeves, S.; Arruéd, C.; Bradshaw, D.J. The business value of best practice process mineralogy. Miner. Eng. 2018, 116, 226–238. [Google Scholar] [CrossRef]
- Cabri, L.J.; Beattie, M.; Rudashevsky, N.S.; Rudashevsky, V.N. Process mineralogy of Au, Pd and Pt ores from the Skaergaard intrusion, Greenland, using new technology. Miner. Eng. 2005, 18, 887–897. [Google Scholar] [CrossRef]
- Bastien, D.; Deyan, M.; Hassan, B.; Stoyan, G. Mineralogical study of electrum grain size, shape and mineral chemistry in process streams from the Krumovgrad mine, Bulgaria. Miner. Eng. 2023, 198, 108080. [Google Scholar]
- Bahrami, A.; Abdollahi, M.; Mirmohammadi, M.; Kazemi, F.; Shokrzadeh, M. A Process mineralogy approach to study the efficiency of milling of molybdenite circuit processing. Sci. Rep. 2020, 10, 21211. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Wang, M.; Wang, J.; Wu, S.; Yao, X.; Li, L. Separation of elemental sulfur from zinc concentrate direct leaching residue by vacuum distillation. Sep. Purif. Technol. 2014, 138, 41–46. [Google Scholar] [CrossRef]
- Muravyov, M. Zinc leaching from sulfidic concentrate in two-step biohydrometallurgical process. J. Biotechnol. 2019, 305, S51. [Google Scholar] [CrossRef]
- Tsogtkhankhai, D.; Mamyachenkov, S.V.; Anisimova, O.S.; Naboichenko, S.S. Thermodynamics of reactions during nitric acid leaching of minerals of a copper concentrate. Russ. J. Non-Ferr. Met. 2011, 52, 135–139. [Google Scholar] [CrossRef]
- Chaudhury, G.R.; Sukla, L.B.; Das, R.P. Kinetics of biochemical leaching of sphalerite concentrate. Metall. Trans. B 1985, 16, 667–670. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Tang, H.; Wang, L.; Han, H.; Sun, W.; Qu, Y.; Yang, Y. Copper recovery from copper slags through flotation enhanced by sodium carbonate synergistic mechanical activation. J. Environ. Chem. Eng. 2022, 10, 107671. [Google Scholar]
- Hosseini, S.H.; Forssberg, E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector. Miner. Eng. 2007, 20, 621–624. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.; Zhou, W.; Zhang, H. The role of sodium phytate in the flotation separation of smithsonite from calcite. Miner. Eng. 2022, 23, 107775. [Google Scholar] [CrossRef]
- Wen, S.M.; Feng, Q.C. Formation of zinc sulfide species on smithsonite surfaces and its response to flotation performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar]
- Li, Y.; Wang, J.K.; Wei, C.; Liu, C.X.; Jiang, J.B.; Wang, F.C. Sulfidation roasting of low grade lead–zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Da, J.; Ruofan, S.; Guoyong, W.; Jiushuai, D.; Xi, Z. Flotation separation of fluorite and calcite using anhydrous glucose and aluminum sulfate as a combined depressant. Appl. Surf. Sci. 2023, 624, 157089. [Google Scholar]
- Wang, M.T.; Zhang, G.F.; Zhao, L.; Chen, Y.F.; Liu, D.Z.; Li, C.B. Application of eco-friendly tetrasodium iminodisuccinate for separation of smithsonite from zinc ions activated quartz. Miner. Eng. 2022, 181, 107545. [Google Scholar] [CrossRef]
- Salum, M.J.G.; De Araujo, A.C.; Peres, A.E.C. The role of sodium sulphide in amine flotation of silicate zinc minerals. Miner. Eng. 1992, 5, 411–419. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.C.; Song, S.X.; Li, H.Q.; Jiao, X.K. A novel insight of the effect of sodium chloride on the sulfidization flotation of cerussite. Powder Technol. 2019, 344, 103–107. [Google Scholar] [CrossRef]
- Atani, S.E.; Tekir, U.; Sebitla, L.D. Effects of additives in froth flotation of silicate zinc ore; a study by zeta potential measurement and infrared spectroscopy. Chiang Mai J. Sci. 2007, 34, 191–200. [Google Scholar]
- Wang, Y.H.; Sun, D.X.; Wang, L.G.; Zhou, Y.L. Effects of sodium tripolyphosphate and sodium carbonate on the selective flocculation of diasporic-bauxite in the presence of calcium and magnesium ions. Miner. Eng. 2011, 24, 1031–1037. [Google Scholar]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Cheng, L.; Feng, Q.M.; Zhang, G.F.; Chen, W.; Chen, Y.F. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar]
- Kupka, N.; Martin, R. Role of sodium carbonate in scheelite flotation—A multi-faceted reagent. Miner. Eng. 2018, 129, 120–128. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Wang, M.; Shi, Q.; Liu, D.; Li, Q. Utilization of sodium carbonate to eliminate the adverse effect of Ca2+ on smithsonite sulphidisation flotation. Miner. Eng. 2019, 132, 121–125. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Marion, C.; Li, R.; Waters, K.E. A review of reagents applied to rare-earth mineral flotation. Adv. Colloid Interface Sci. 2020, 279, 102142. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.Q.; Hu, J.J.; Zhu, H.L.; Jiao, F.; Jia, W.H.; Han, J.W.; Chen, W.Q. Effect of depressants on flotation separation of magnesite from dolomite and calcite. Int. J. Min. Sci. Technol. 2023, 33, 83–91. (In Chinese) [Google Scholar] [CrossRef]
- Qin, W.Q.; Hu, J.J.; Zhu, H.L.; Jiao, F.; Pan, Z.C.; Jia, W.H.; Han, J.W.; Chen, C. Selective inhibition mechanism of PBTCA on flotation separation of magnesite from calcite. Colloids Surf. A 2021, 630, 127597. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]




| Element | Pb | Zn | K2O | Au * | Cu |
| Content | 0.84 | 7.4 | 0.81 | 0.06 | 0.0099 |
| Element | Fe | As | CaO | MgO | SiO2 |
| Content | 5.37 | 0.042 | 26.02 | 0.79 | 18.96 |
| Element | Elemental Phase | Content (%) | Distribution Ratio (%) |
|---|---|---|---|
| Pb | Cerussite | 0.43 | 51.19 |
| Galena | 0.12 | 14.29 | |
| Anglesite | 0.16 | 19.05 | |
| Plumbojarosite and others | 0.13 | 15.48 | |
| Total | 0.84 | 100.00 | |
| Zn | Sulfides | 0.66 | 8.92 |
| Zinc sulfate | 0.05 | 0.68 | |
| Oxide | 6.59 | 89.05 | |
| Franklinite and others | 0.10 | 1.35 | |
| Total | 7.40 | 100.00 |
| Mineral | Molecular Formula | Content/% | Mineral | Molecular Formula | Content/% |
|---|---|---|---|---|---|
| Quartz | SiO2 | 15.02 | Smithsonite | ZnCO3 | 10.49 |
| Calcite | CaCO3 | 40.97 | Siderite | FeCO3 | 9.85 |
| Dolomite | CaMg(CO3)2 | 3.07 | Cerussite | PbCO3 | 0.74 |
| Sphalerite | ZnS | 0.88 | Pyrite | FeS2 | 1.09 |
| Galena | PbS | 0.44 | Hemimorphite | Zn4Si2O7(OH)2(H2O) | 2.42 |
| Goethite | FeO(OH) | 1.27 | Barite | BaSO4 | 1.36 |
| Celestine | SrSO4 | 1.46 | Gypsum | CaSO4∙2H2O | 2.08 |
| Muscovite | KAl2Si3AlO10(OH)2 | 0.82 | Kaolinite | Al2Si2O5(OH)4 | 0.96 |
| Potassium feldspar | KAlSi3O8 | 2.08 | Others | - | 5.00 |
| Product | Yield/% | Grade/% | Recovery/% | ||
|---|---|---|---|---|---|
| Pb | Zn | Pb | Zn | ||
| Pb rougher concentrate | 17.29 | 2.83 | 5.14 | 57.56 | 11.77 |
| Zn concentrate | 22.00 | 0.23 | 28.64 | 5.59 | 83.45 |
| Tailing | 60.71 | 0.51 | 0.59 | 36.49 | 4.78 |
| Total | 100.00 | 0.85 | 7.55 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Ding, Z.; Yuan, J.; Feng, Q.; Wen, S.; Bai, S. Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine. Minerals 2023, 13, 1167. https://doi.org/10.3390/min13091167
Yu A, Ding Z, Yuan J, Feng Q, Wen S, Bai S. Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine. Minerals. 2023; 13(9):1167. https://doi.org/10.3390/min13091167
Chicago/Turabian StyleYu, Anmei, Zhan Ding, Jiaqiao Yuan, Qicheng Feng, Shuming Wen, and Shaojun Bai. 2023. "Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine" Minerals 13, no. 9: 1167. https://doi.org/10.3390/min13091167
APA StyleYu, A., Ding, Z., Yuan, J., Feng, Q., Wen, S., & Bai, S. (2023). Process Mineralogy Characteristics and Flotation Optimization of a Low-Grade Oxidized Lead and Zinc Ore from Lanping Mine. Minerals, 13(9), 1167. https://doi.org/10.3390/min13091167








