Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments
Abstract
1. Introduction
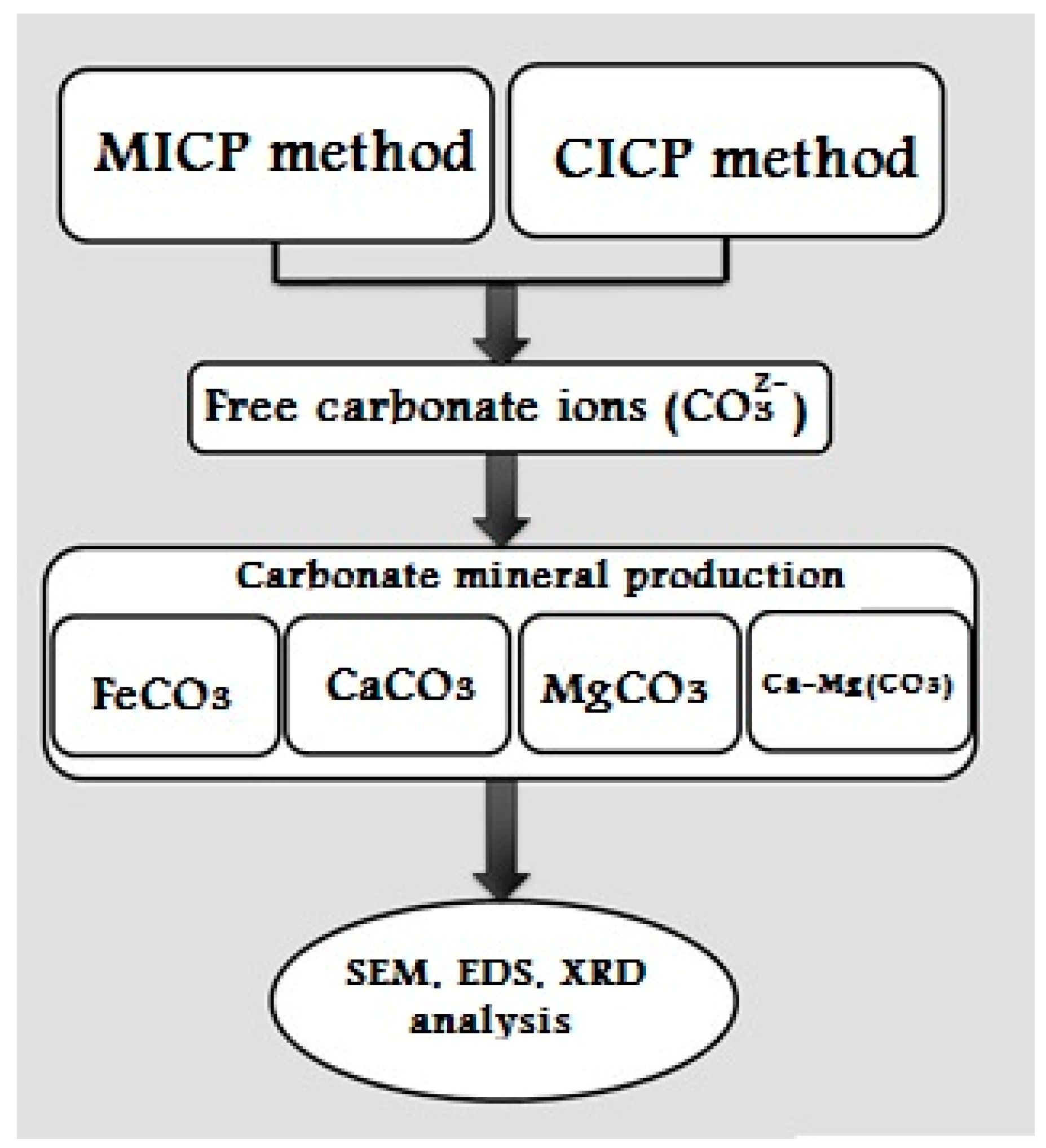
2. Materials and Methods
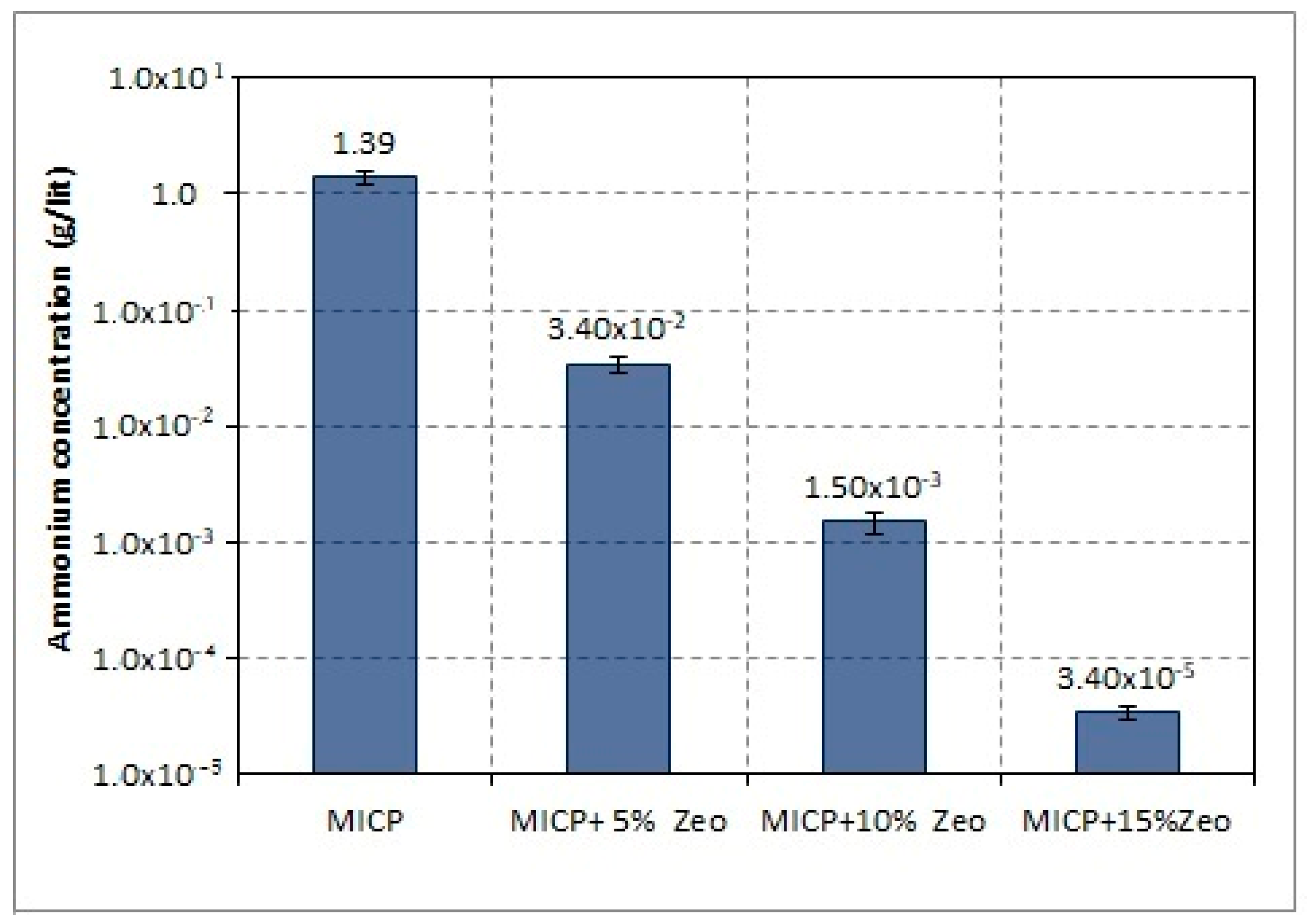
2.1. Microbial Induced-Ammonium Free Carbonate Production
2.2. CO2 Induced-Carbonate Production
2.3. Carbonate Minerals Production
2.4. SEM, EDS and XRD Analysis
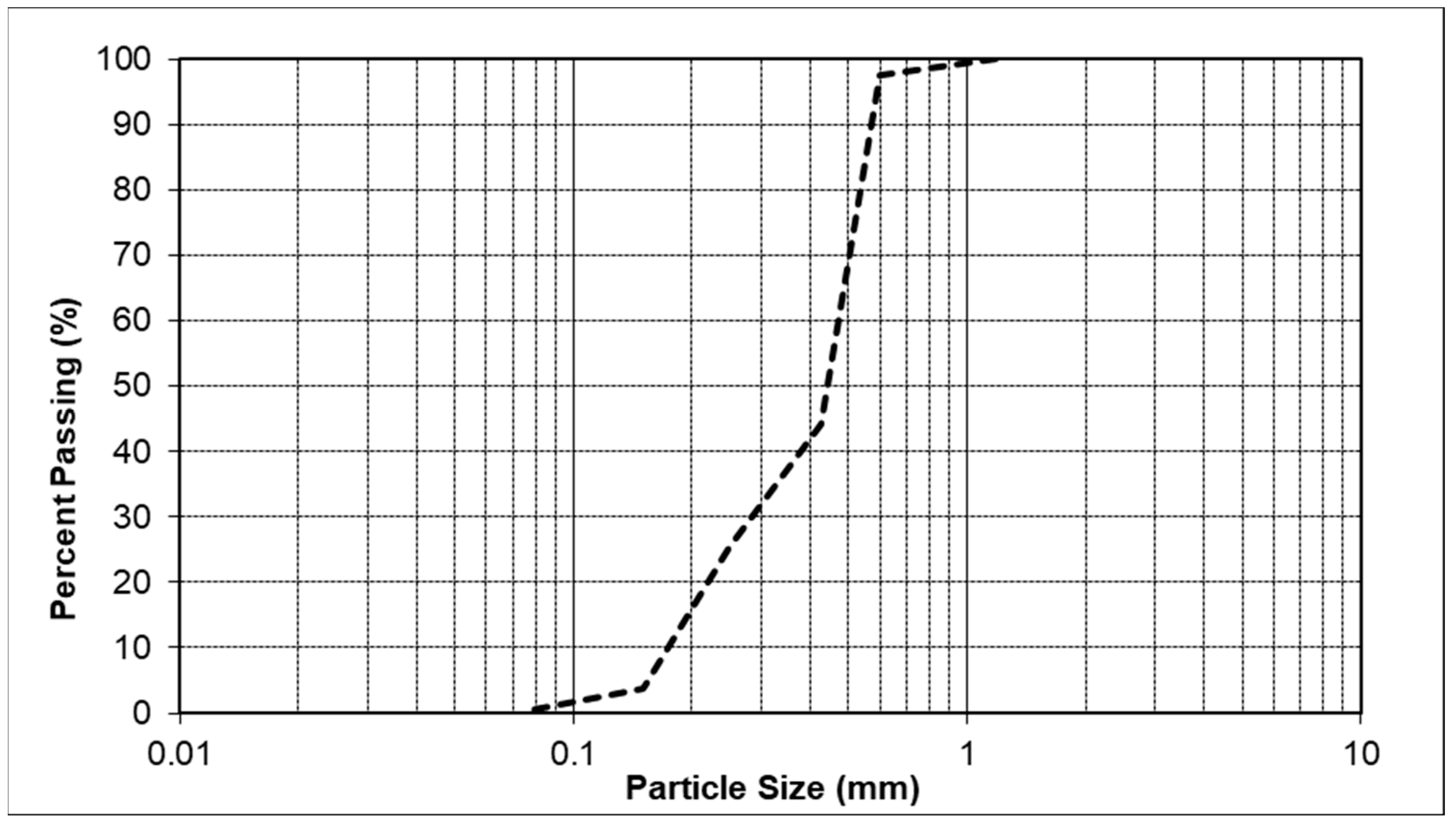
2.5. Green Soil Improvement Using Carbonate Minerals
Unconfined Compressive Strength Testing
3. Results and Discussion
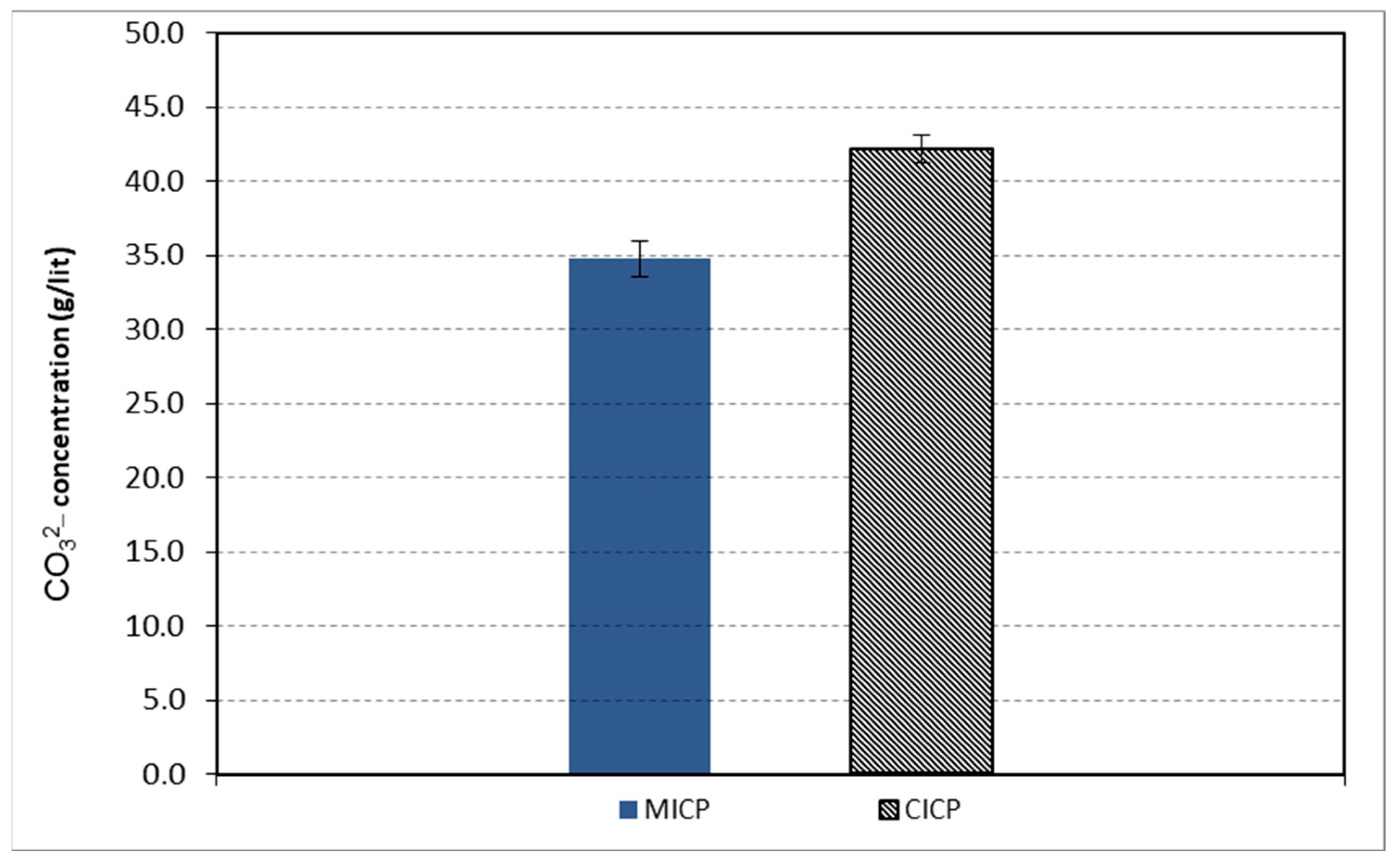
3.1. Induced Carbonate Production (CO32−)
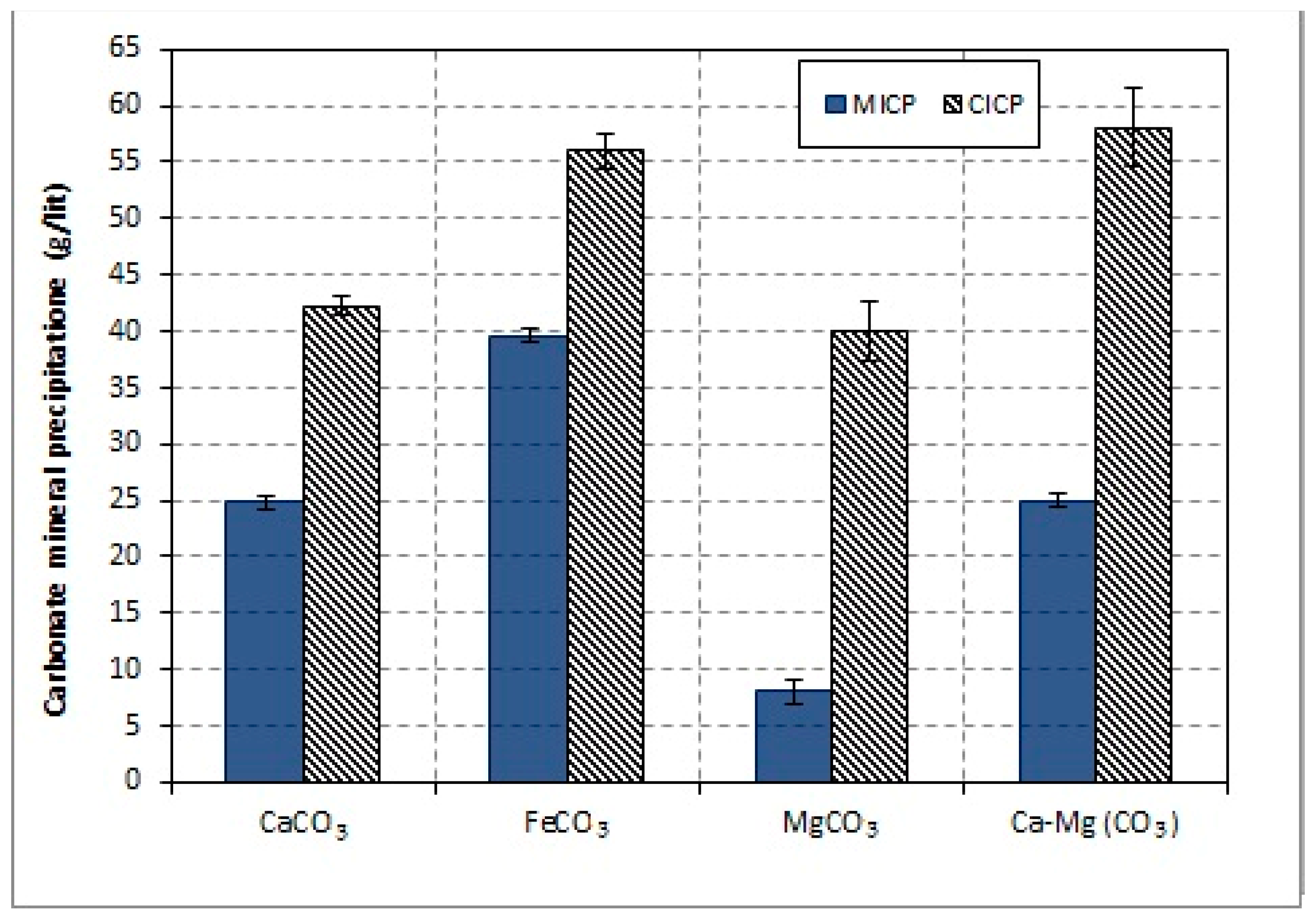
3.2. Carbonate Mineral Production
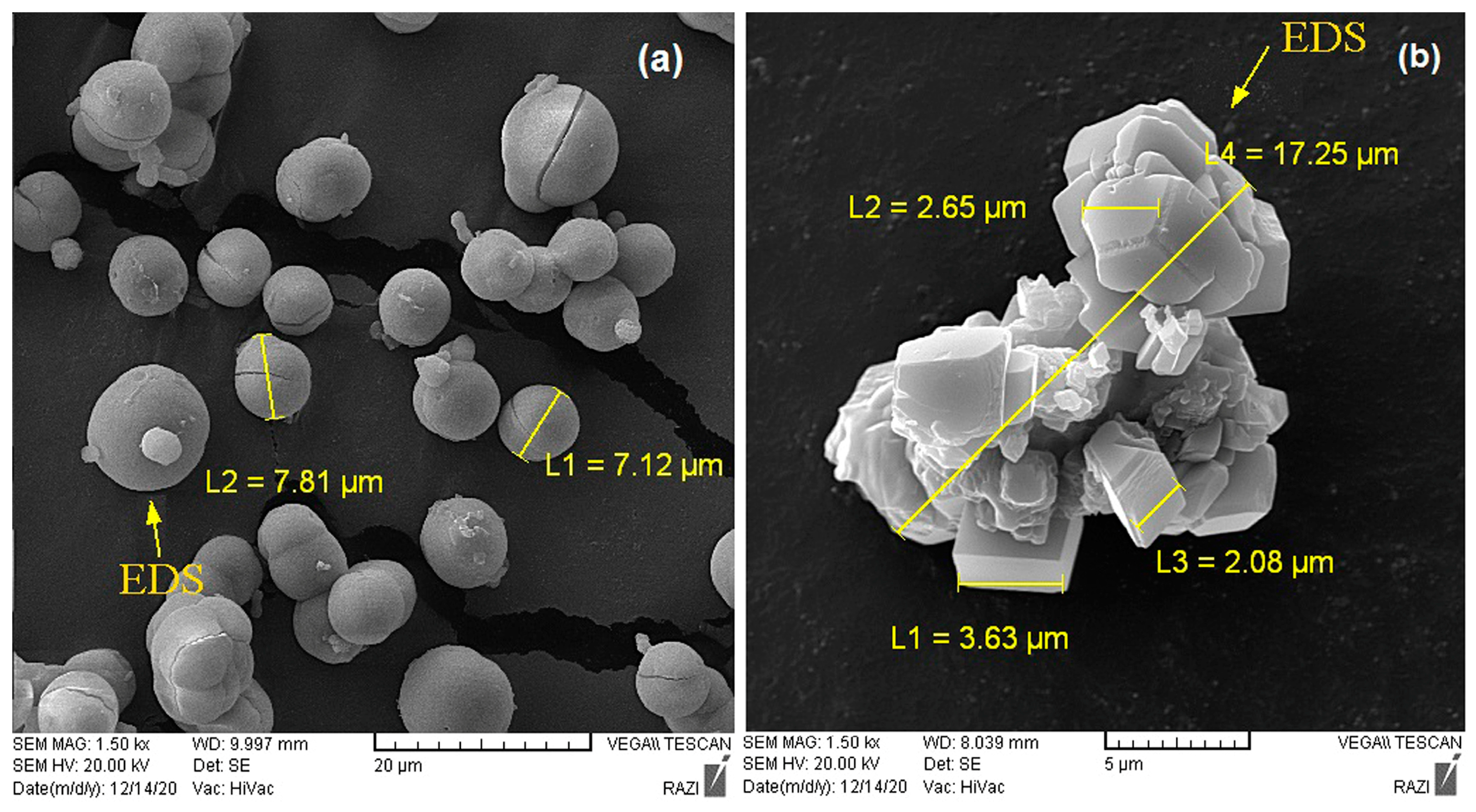
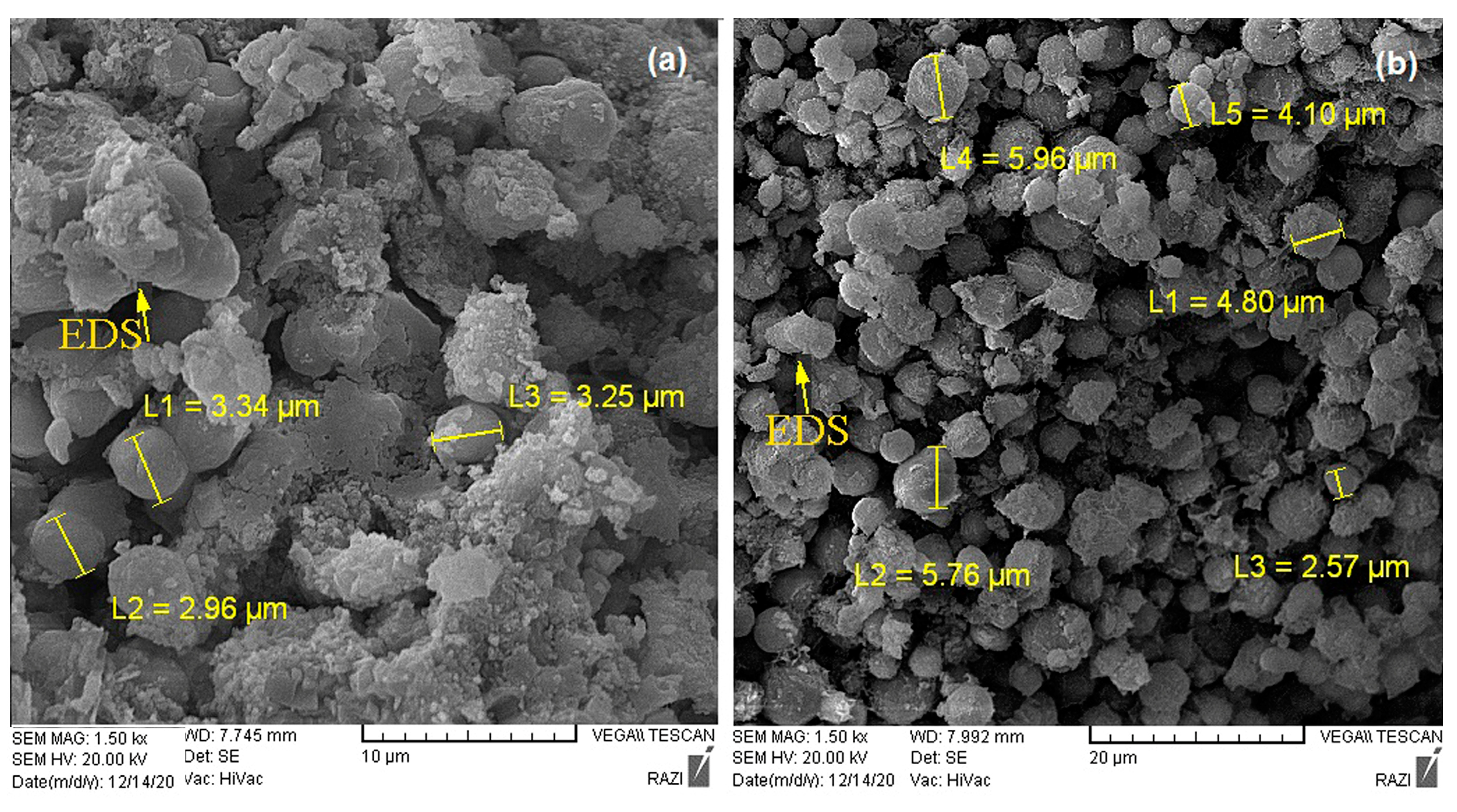
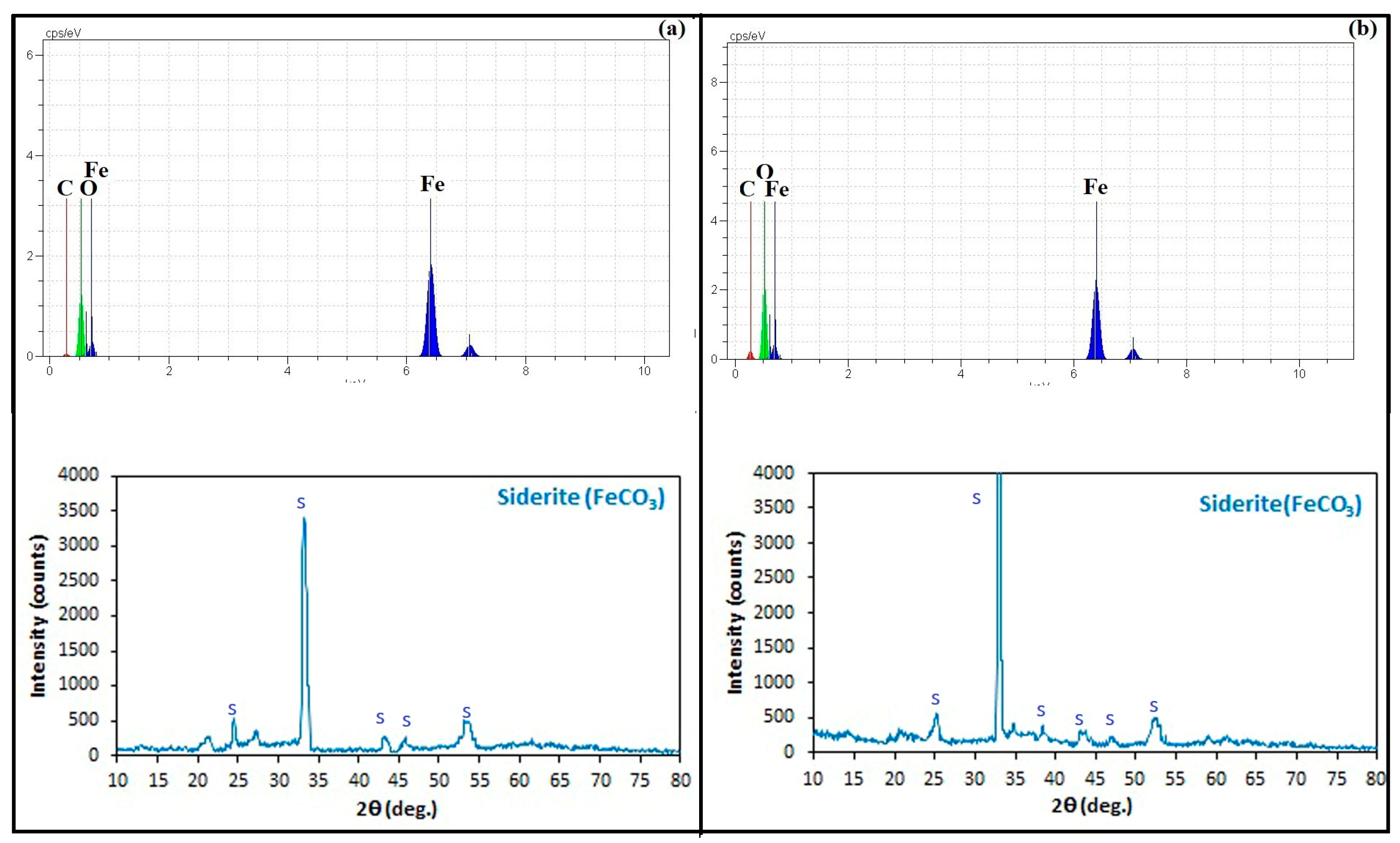
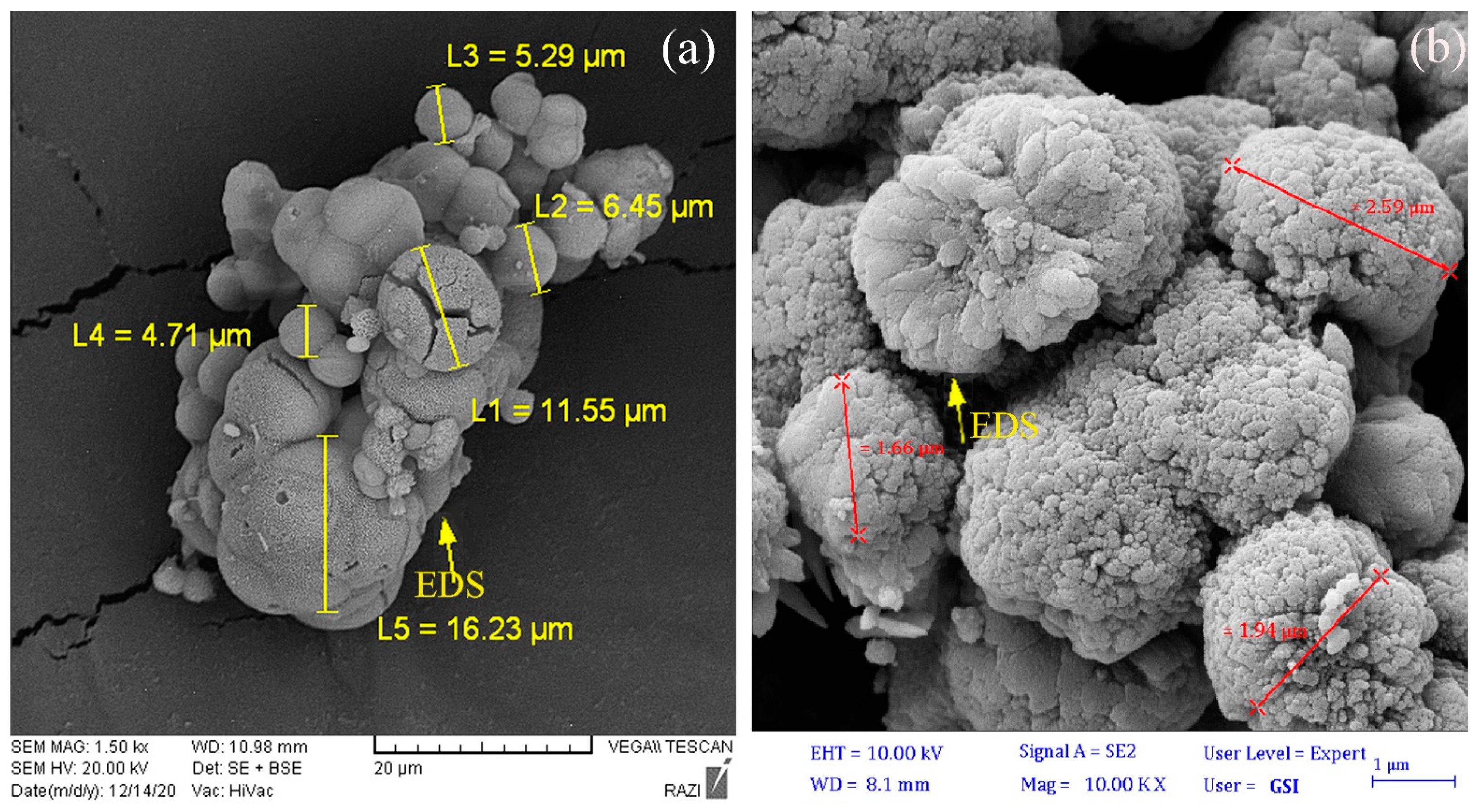
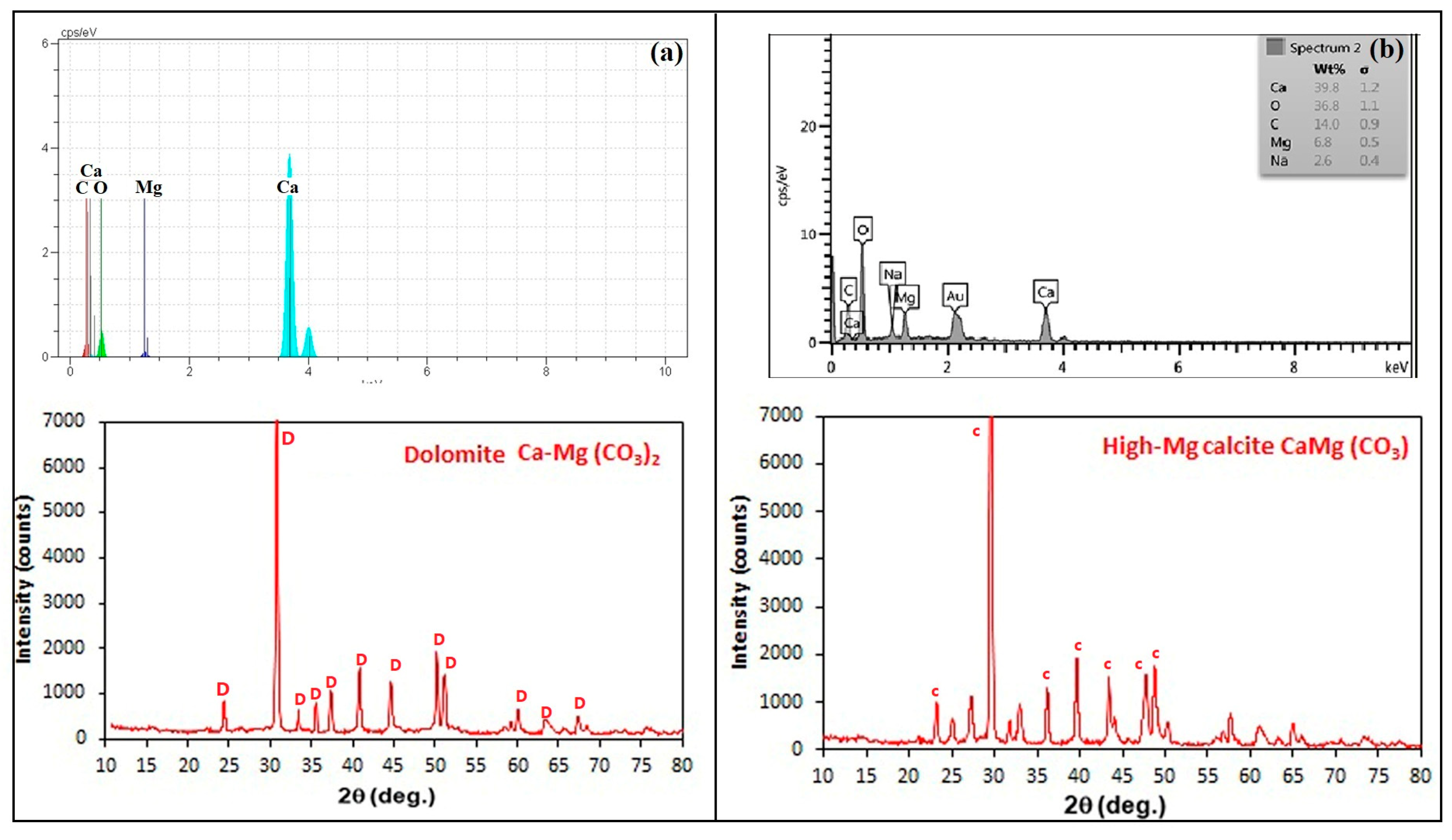
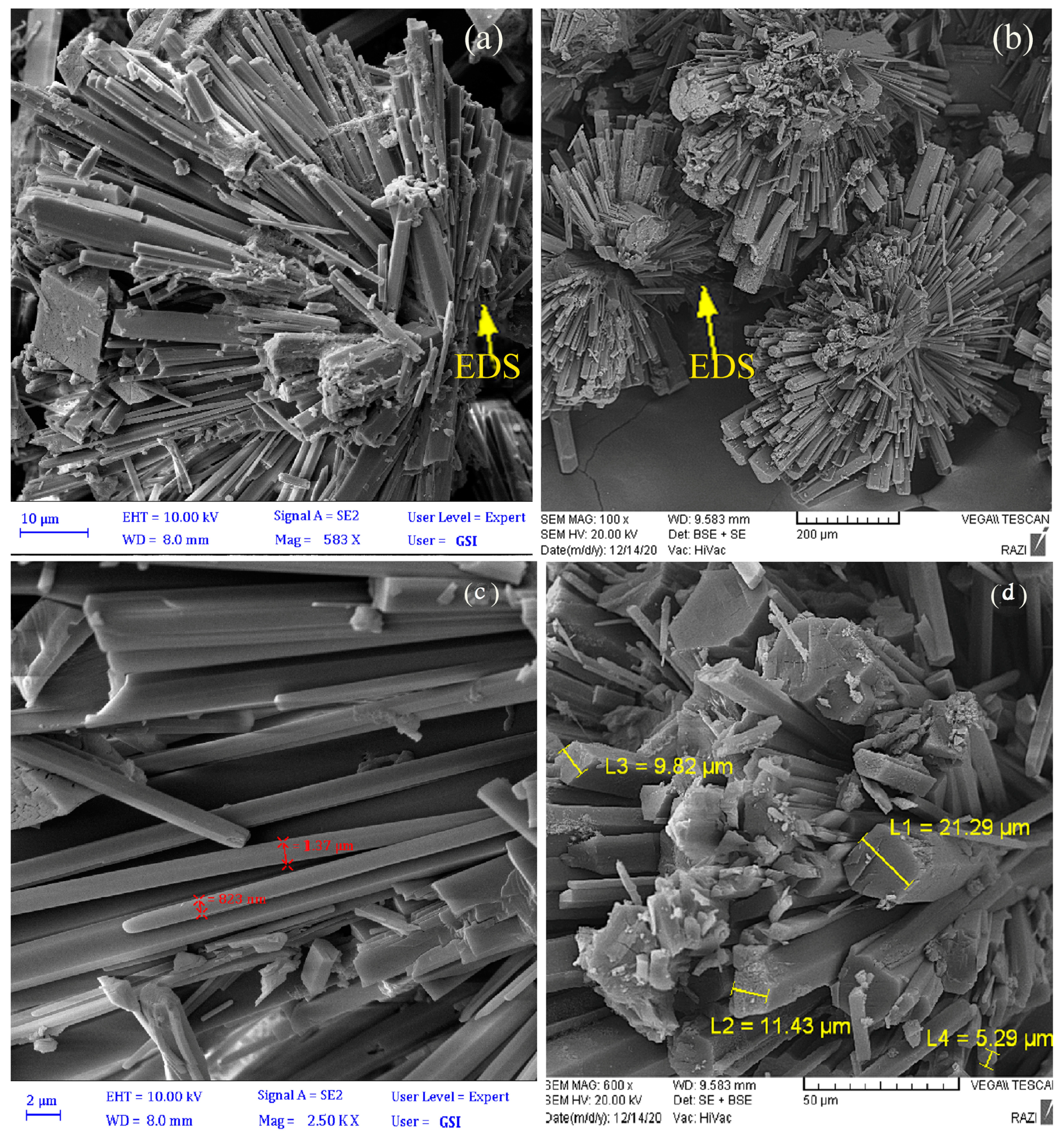
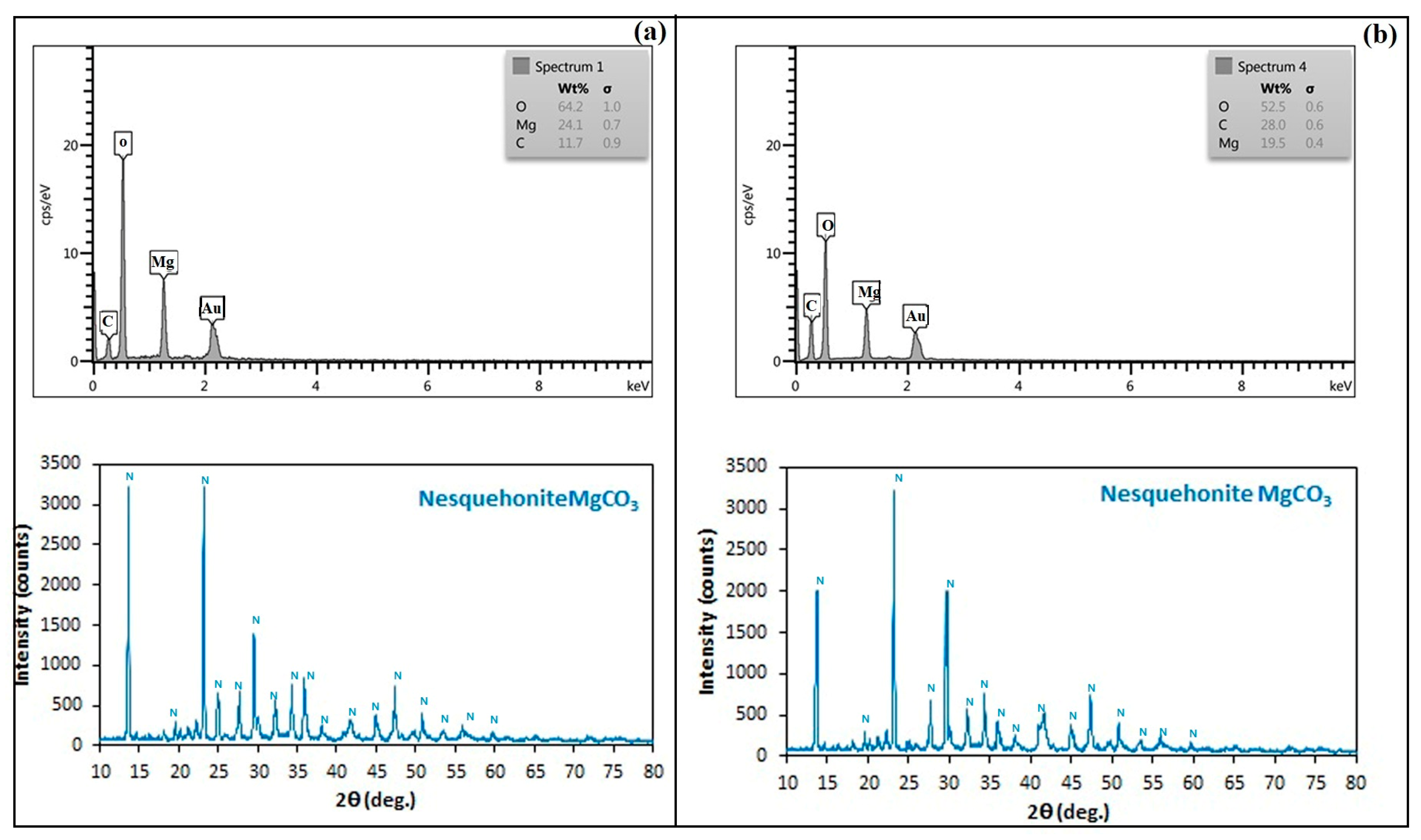
3.3. Carbonate Mineral Characterization
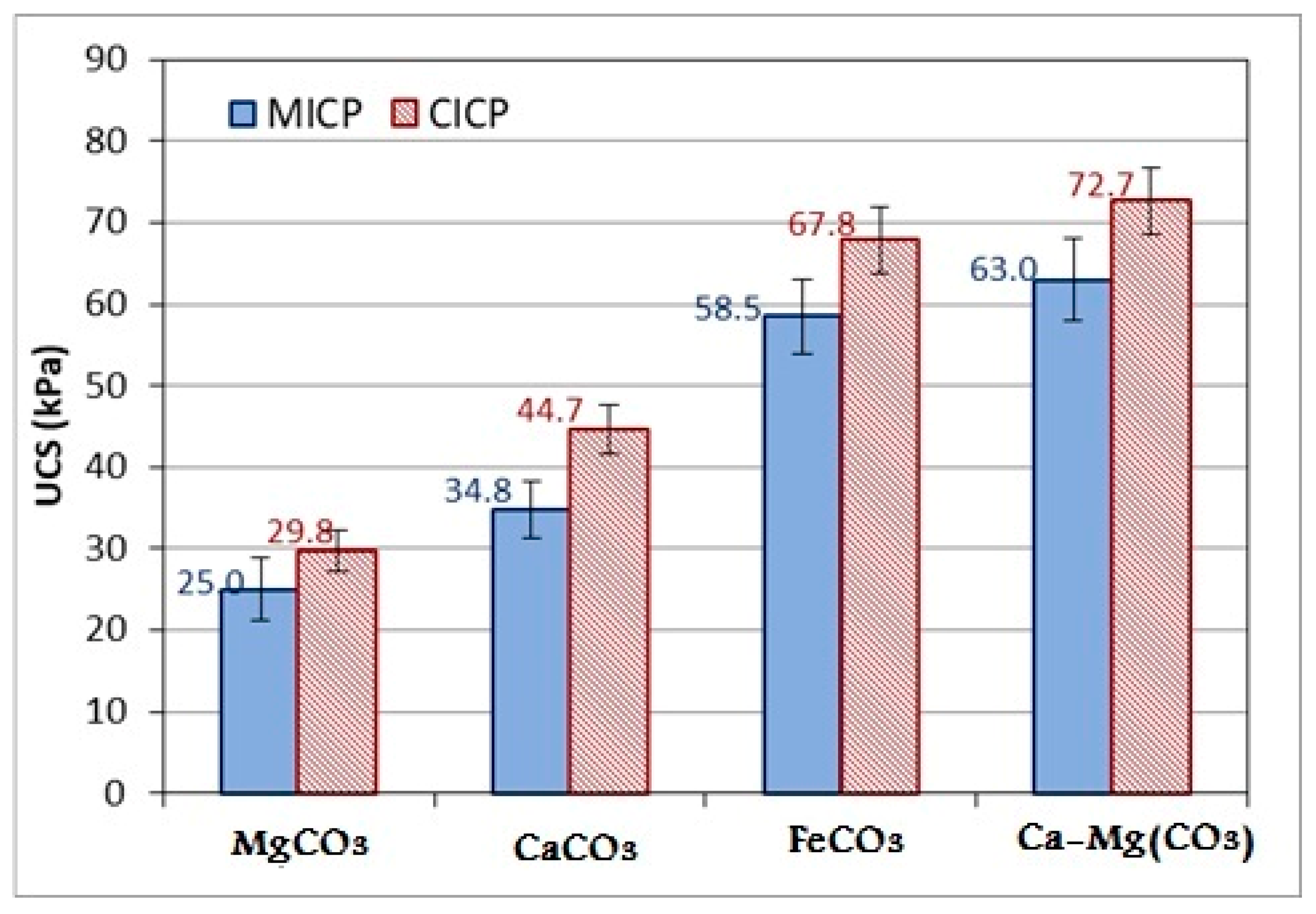
3.4. Unconfined Compressive Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keykha, H.A.; Asadi, A.; Zareian, M. Environmental factors affecting the compressive strength of microbiologically induced calcite precipitation-treated soil. Geomicrobiol. J. 2017, 34, 889–894. [Google Scholar] [CrossRef]
- Keykha, H.A.; Asadi, A.; Huat, B.B.; Kawasaki, S. Microbial induced calcite precipitation by Sporosarcina pasteurii and Sporosarcina aquimarina. Environ. Geotech. 2018, 6, 562–566. [Google Scholar] [CrossRef]
- Romiani, H.M.; Keykha, H.A.; Talebi, M.; Asadi, A.; Kawasaki, S. Green soil improvement: Using carbon dioxide to enhance the behaviour of clay. Proc. Inst. Civ. Eng.-Ground Improv. 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.C.; Xue, Z.F.; Xie, Y.X.; Lv, X.J. Feasibility study of applying electrokinetic technology coupled with enzyme-induced carbonate precipitation treatment to Cu-and Pb-contaminated loess remediation. J. Clean. Prod. 2023, 401, 136734. [Google Scholar] [CrossRef]
- Xue, Z.F.; Cheng, W.C.; Xie, Y.X.; Wang, L.; Hu, W.; Zhang, B. Investigating immobilization efficiency of Pb in solution and loess soil using bio-inspired carbonate precipitation. Environ. Pollut. 2023, 322, 121218. [Google Scholar] [CrossRef]
- Ali, A.; Li, M.; Su, J.; Li, Y.; Wang, Z.; Bai, Y.; Ali, E.F.; Shaheen, S.M. Brevundimonas diminuta isolated from mines polluted soil immobilized cadmium (Cd2+) and zinc (Zn2+) through calcium carbonate precipitation: Microscopic and spectroscopic investigations. Sci. Total Environ. 2022, 813, 152668. [Google Scholar] [CrossRef] [PubMed]
- Keykha, H.A.; Romiani, H.M.; Zebardast, E.; Asadi, A.; Kawasaki, S. CO2-induced carbonate minerals as soil stabilizing agents for dust suppression. Aeolian Res. 2021, 52, 100731. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Jin, Y.; Li, Q.; Xu, J. Application of microbially induced calcium carbonate precipitation (MICP) in sand embankments for scouring/erosion control. Mar. Georesour. Geotechnol. 2021, 39, 1459–1471. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Ferris, F.G.; Stehmeier, L.G.; Kantzas, A.; Mourits, F.M. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1997, 36, 56–61. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Tang, C.S.; Yin, L.Y.; Jiang, N.J.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e0271761. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, H.; Li, M.; Deng, X. Bio-cementation improvement via CaCO3 cementation pattern and crystal polymorph: A review. Constr. Build. Mater. 2021, 297, 123478. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; DeJong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Y.; Xie, A.; Huang, B.; Jia, R.; Guo, R.; Tang, W. Bacteria-mediated synthesis of metal carbonate minerals 552 with unusual morphologies and structures. Cryst. Growth Des. 2009, 9, 743–754. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J. Microbiol. Biotechnol. 2013, 23, 707–714. [Google Scholar] [CrossRef]
- Al-Thawadi, S.M. Consolidation of sand particles by aggregates of calcite nanoparticles synthesized by ureolytic bacteria under non-sterile conditions. J. Chem. Sci. Technol. 2013, 2, 141–146. [Google Scholar]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 17. [Google Scholar] [CrossRef]
- Yoo, Y.; Kim, I.; Lee, D.; Choi, W.Y.; Choi, J.; Jang, K.; Park, J.; Kang, D. Review of contemporary research on inorganic CO2 utilization via CO2 conversion into metal carbonate-based materials. J. Ind. Eng. Chem. 2022, 116, 60–74. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 29093. [Google Scholar] [CrossRef]
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; World Scientific, Imperial College Press: London, UK, 2014; Volume 1. [Google Scholar]
- Department of Economic and Social Affairs. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Inoue, R.; Ueda, S.; Wakuta, K.; Sasaki, K.; Ariyama, T. Thermodynamic consideration on the absorption properties of carbon dioxide to basic oxide. ISIJ Int. 2010, 50, 1532–1538. [Google Scholar] [CrossRef]
- ASTM D2166; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2010.
- Zhong, X.; Qi, J.; Li, H.; Dong, L.; Gao, D. Seasonal distribution of microbial activity in bioaerosols in the outdoor environment of the Qingdao coastal region. Atmos. Environ. 2016, 140, 506–513. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Moskowitz, B.M. Microbial biomineralization of magnetic iron minerals: Microbiology, magnetism and environmental significance. Geomicrobiology 2018, 17, 181–224. [Google Scholar] [CrossRef]
- Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Insights into the influence of cell concentration in design and development of microbially induced calcium carbonate precipitation (MICP) process. PLoS ONE 2021, 16, e0254536. [Google Scholar] [CrossRef]
- Lead, C. Mineral carbonation and industrial uses of carbon dioxide. Carbon Dioxide Capture Storage 2005, 10, 319. [Google Scholar]
- Kralj, D.; Kontrec, J.; Brečević, L.; Falini, G.; Nöthig-Laslo, V. Effect of inorganic anions on the morphology and structure of magnesium calcite. Chem.-A Eur. J. 2004, 10, 1647–1656. [Google Scholar] [CrossRef]
- Rahman, M.A.; Oomori, T. In vitro regulation of CaCO3 crystal growth by the highly acidic proteins of calcitic sclerites in soft coral, Sinularia polydactyla. Connect. Tissue Res. 2009, 50, 285–293. [Google Scholar] [CrossRef]
- Marvasi, M.; Gallagher, K.L.; Martinez, L.C.; Molina Pagan, W.C.; Rodríguez Santiago, R.E.; Castilloveitía Vega, G.; Visscher, P.T. Importance of B4 medium in determining organomineralization potential of bacterial environmental isolates. Geomicrobiol. J. 2012, 29, 916–924. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef]
- Bielak, K.; Różycka, M.O.; Zoglowek, A.; Ożyhar, A.; Dobryszycki, P. Counter-Diffusion System as an in Vitro Model in the Investigation of Proteins Involved in the Formation of Calcium Carbonate Biominerals. Cryst. Growth Des. 2021, 21, 1389–1400. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Influence of the Primary Structure of Enzymes on the Formation of CaCO3 Polymorphs: A Comparison of Plant (Canavalia e nsiformis) and Bacterial (Bacillus p asteurii) Ureases. Langmuir 2005, 21, 8876–8882. [Google Scholar] [CrossRef]
- French, B.M. Stability relations of siderite (FeCO3) in the system Fe-CO. Am. J. Sci. 1971, 271, 37–78. [Google Scholar]
- Isambert, A.; Valet, J.P.; Gloter, A.; Guyot, F. Stable Mn-magnetite derived from Mn-siderite by heating in air. J. Geophys. Res. Solid Earth 2003, 108, 2283. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial dolomite precipitation under aerobic conditions: Results from Brejo do Espinho Lagoon (Brazil) and culture experiments. Perspect. Carbonate Geol. A Tribut. Career Robert Nathan Ginsburg 2009, 41, 167–178. [Google Scholar] [CrossRef]
- Al Disi, Z.A.; Jaoua, S.; Bontognali, T.R.; Attia, E.S.; Al-Kuwari, H.A.A.S.; Zouari, N. Evidence of a role for aerobic bacteria in high magnesium carbonate formation in the evaporitic environment of Dohat Faishakh Sabkha in Qatar. Front. Environ. Sci. 2017, 5, 1. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Gregg, J.M.; Bish, D.L.; Machel, H.G.; Fouke, B.W.; MacNeil, A.; Lonnee, J.; Wood, R.J. Dolomite, very-high magnesium calcite, and microbes: Implications for the microbial model of dolomitization. In Characterization and Modeling of Carbonates–Mountjoy Symposium; SEPM (Society for Sedimentary Geology), 2017; Volume 1, pp. 7–20. Available online: https://www.researchgate.net/publication/320042867 (accessed on 1 June 2017).
- Dupraz, S.; Parmentier, M.; Ménez, B.; Guyot, F. Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem. Geol. 2009, 265, 44–53. [Google Scholar] [CrossRef]
- González-Muñoz, M.T.; Rodriguez-Navarro, C.; Martínez-Ruiz, F.; Arias, J.M.; Merroun, M.L.; Rodriguez-Gallego, M. Bacterial biomineralization: New insights from Myxococcus-induced mineral precipitation. Geol. Soc. Lond. Spec. Publ. 2010, 336, 31–50. [Google Scholar] [CrossRef]
- Morse, J.W.; Wang, Q.; Tsio, M.Y. Influences of temperature and Mg: Ca ratio on CaCO3 precipitates from seawater. Geology 1997, 25, 85–87. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Zhang, J.; Zhang, Q.; Chen, J.; Liu, Z.; Liang, X. Synthesis and shape evolution of monodisperse basic magnesium carbonate microspheres. Cryst. Growth Des. 2007, 7, 337–342. [Google Scholar] [CrossRef]
- Chen, Q.; Hui, T.; Sun, H.; Peng, T.; Ding, W. Synthesis of magnesium carbonate hydrate from natural talc. Open Chem. 2020, 18, 951–961. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H. Biomineralization of Calcium and Magnesium Carbonate Minerals Induced by Bacillus licheniformis and Its Application in Water Softening. Preprints.org 2017, 2017010096. [Google Scholar] [CrossRef]
- Glasser, F.P.; Jauffret, G.; Morrison, J.; Galvez-Martos, J.L.; Patterson, N.; Imbabi, M.S.E. Sequestering CO2 by mineralization into useful nesquehonite-based products. Front. Energy Res. 2016, 4, 3. [Google Scholar] [CrossRef]
- Bäuerlein, E. Biomineralization of unicellular organisms: An unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angew. Chem. Int. Ed. 2003, 42, 614–641. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef]
- Huijgen, W.J.; Witkamp, G.J.; Comans, R.N. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates. Environ. Implic. Appl. Miner. 2022, 12, 1562. [Google Scholar]
- Turchyn, A.V.; Bradbury, H.J.; Walker, K.; Sun, X. Controls on the precipitation of carbonate minerals within marine sediments. Front. Earth Sci. 2021, 9, 618311. [Google Scholar] [CrossRef]
- Farsang, S.; Louvel, M.; Zhao, C.; Mezouar, M.; Rosa, A.D.; Widmer, R.N.; Feng, X.; Liu, J.; Redfern, S.A. Deep carbon cycle constrained by carbonate solubility. Nat. Commun. 2021, 12, 4311. [Google Scholar] [CrossRef]
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A review of flotation separation of Mg carbonates (dolomite and magnesite). Minerals 2018, 8, 354. [Google Scholar] [CrossRef]
- Long, X.; Ma, Y.; Qi, L. Biogenic and synthetic high magnesium calcite—A review. J. Struct. Biol. 2014, 185, 1–14. [Google Scholar] [CrossRef]
- Sun, W.; Nešić, S.; Woollam, R.C. The effect of temperature and ionic strength on iron carbonate (FeCO3) solubility limit. Corros. Sci. 2009, 51, 1273–1276. [Google Scholar] [CrossRef]
- Xiao, Y.; Stuedlein, A.W.; Ran, J.; Evans, T.M.; Cheng, L.; Liu, H.; Van Paassen, L.A.; Chu, J. Effect of particle shape on strength and stiffness of biocemented glass beads. J. Geotech. Geoenviron. Eng. 2019, 145, 06019016. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Department of Civil Engineering and Geosciences, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]

| Reagents | Concentration | Cation:Carbonate Ratios | |
|---|---|---|---|
| FeSO4·7H2O | 1 M | Fe2+:CO32− | 1:1 |
| MgSO4·7H2O | 1 M | Mg2+:CO32− | 1:1 |
| CaCl2·2H2O | 1 M | Ca2+:CO32− | 1:1 |
| MgSO4/CaCl2 | 1 M | Mg2+/Ca2+:CO32− | 1:1 |
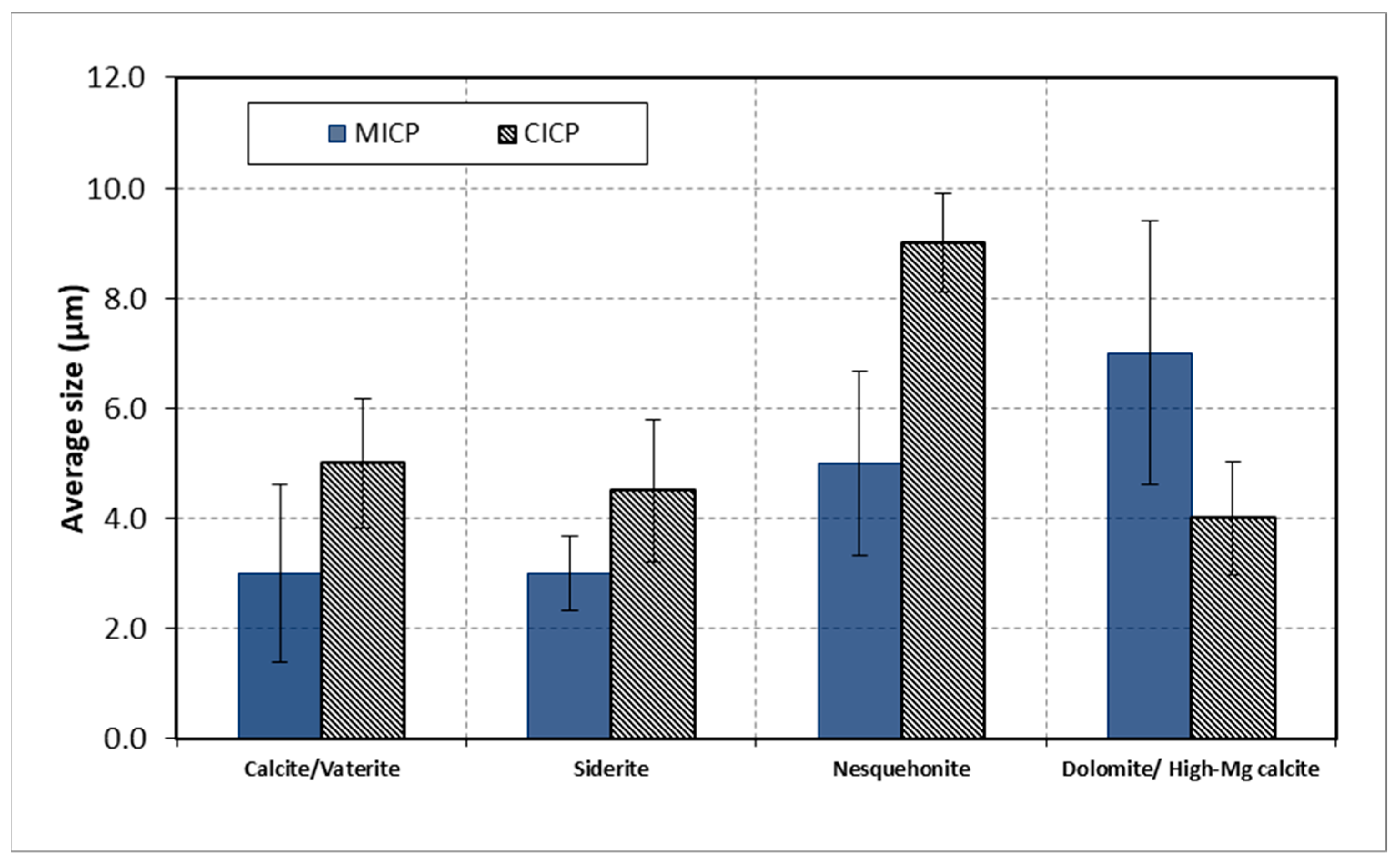
| Name | Method | Type of Mineral | Size (µm) | Mineral Shape |
|---|---|---|---|---|
| Calcium carbonate | MICP | Vaterite | 2–7 | Spherical |
| CICP | Calcite | 2–6 | Rhombohedral | |
| Ferrous carbonate | MICP | Siderite | 0.5–3 | Spherical |
| CICP | Siderite | 2–6 | Spherical | |
| Magnesium calcium carbonate | MICP | Dolomite | 5–16 | Botryoidal |
| CICP | High-Mg calcite | 2–5 | Botryoidal | |
| Magnesium carbonate | MICP | Nesquehonite | 1–10 | Radial-needle |
| CICP | Nesquehonite | 5–20 | Radial-needle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keykha, H.A.; Zangani, A.; Romiani, H.M.; Asadi, A.; Kawasaki, S.; Radmanesh, N. Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments. Minerals 2023, 13, 976. https://doi.org/10.3390/min13070976
Keykha HA, Zangani A, Romiani HM, Asadi A, Kawasaki S, Radmanesh N. Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments. Minerals. 2023; 13(7):976. https://doi.org/10.3390/min13070976
Chicago/Turabian StyleKeykha, Hamed Abdeh, Alireza Zangani, Hadi Mohamadzadeh Romiani, Afshin Asadi, Satoru Kawasaki, and Niloofar Radmanesh. 2023. "Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments" Minerals 13, no. 7: 976. https://doi.org/10.3390/min13070976
APA StyleKeykha, H. A., Zangani, A., Romiani, H. M., Asadi, A., Kawasaki, S., & Radmanesh, N. (2023). Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments. Minerals, 13(7), 976. https://doi.org/10.3390/min13070976








