Dating Amber: Review and Perspective
Abstract
1. Introduction
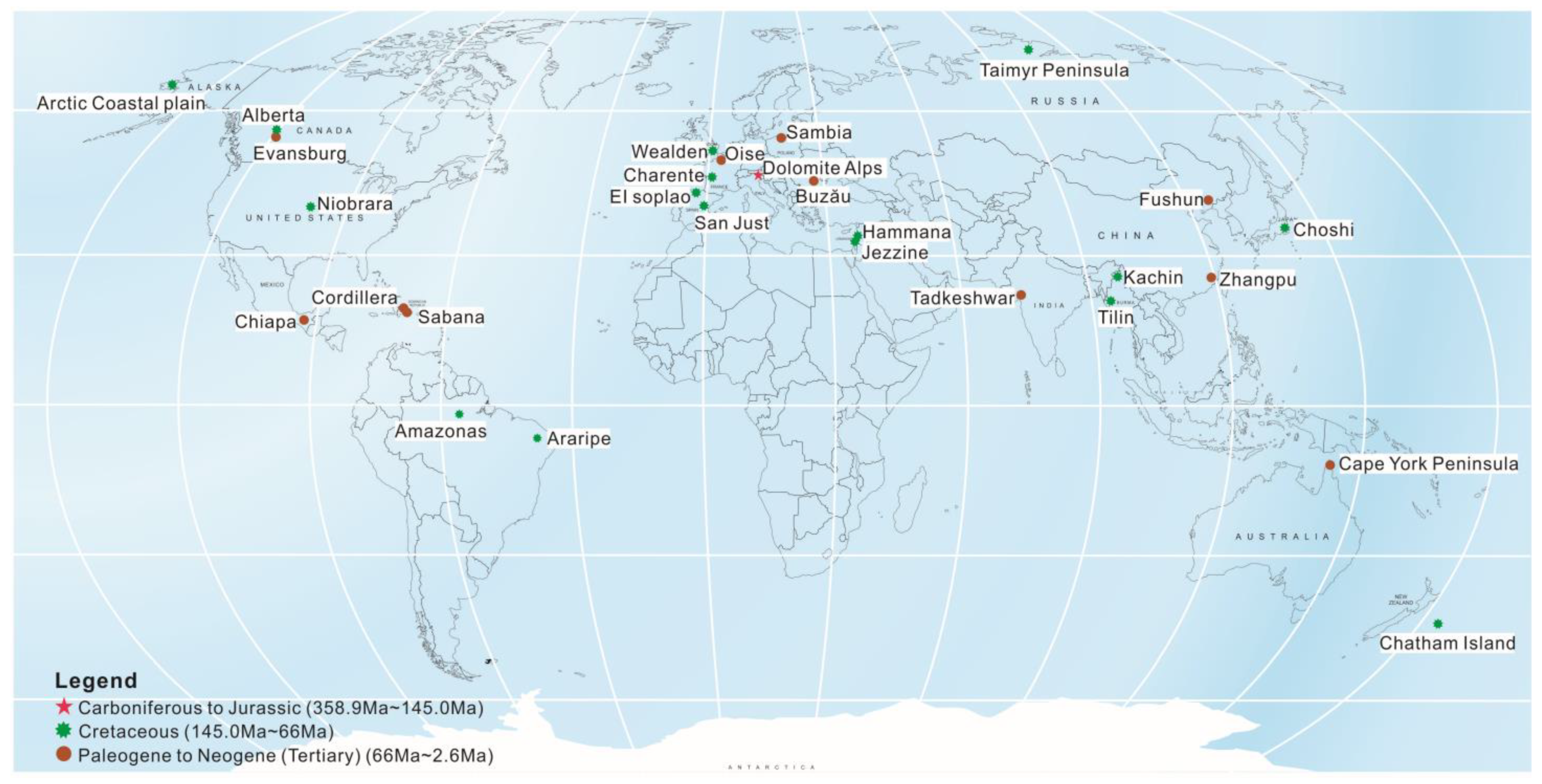
2. The Youngest Reported Amber in China
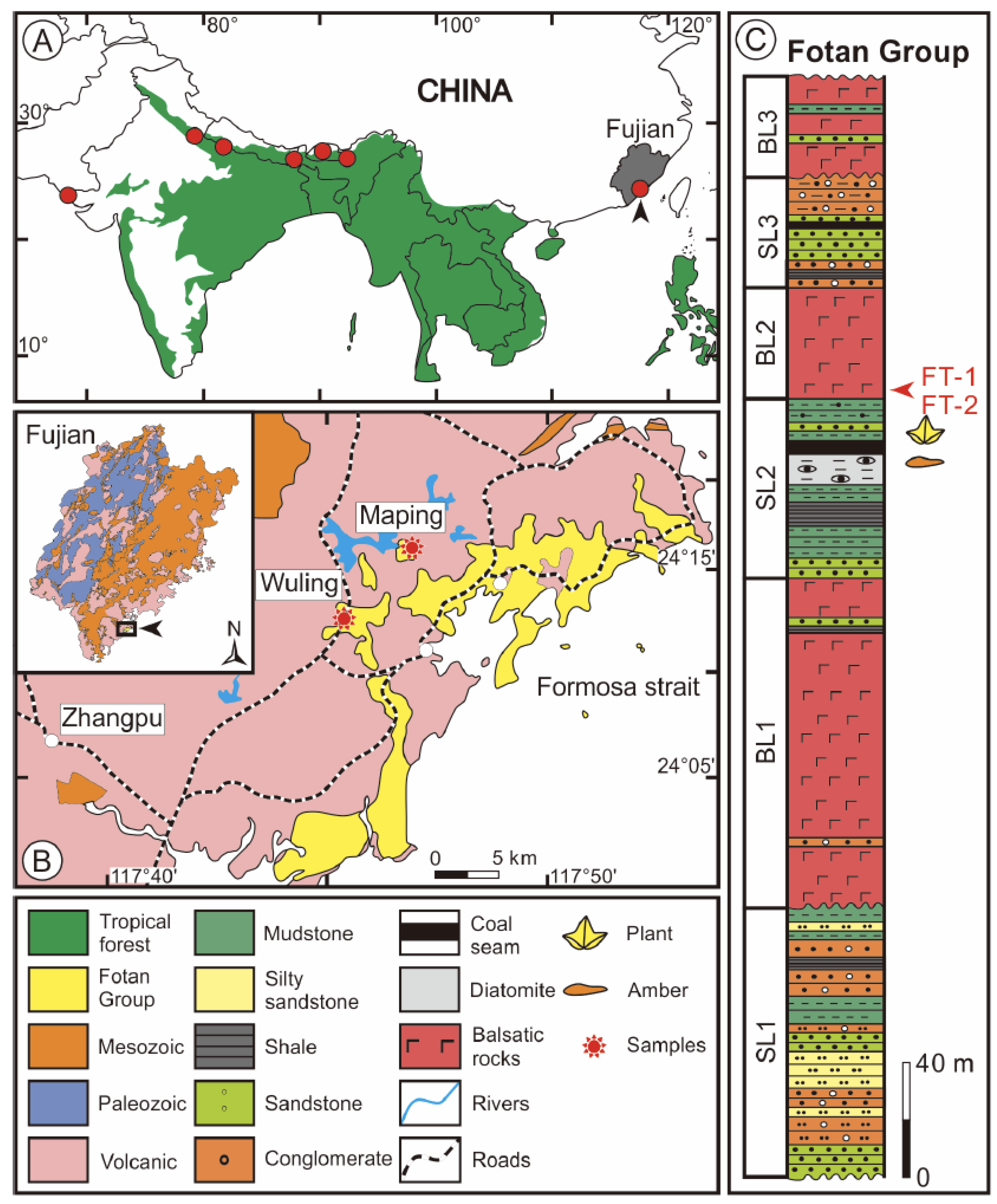
2.1. Zhangpu Amber in Fujian
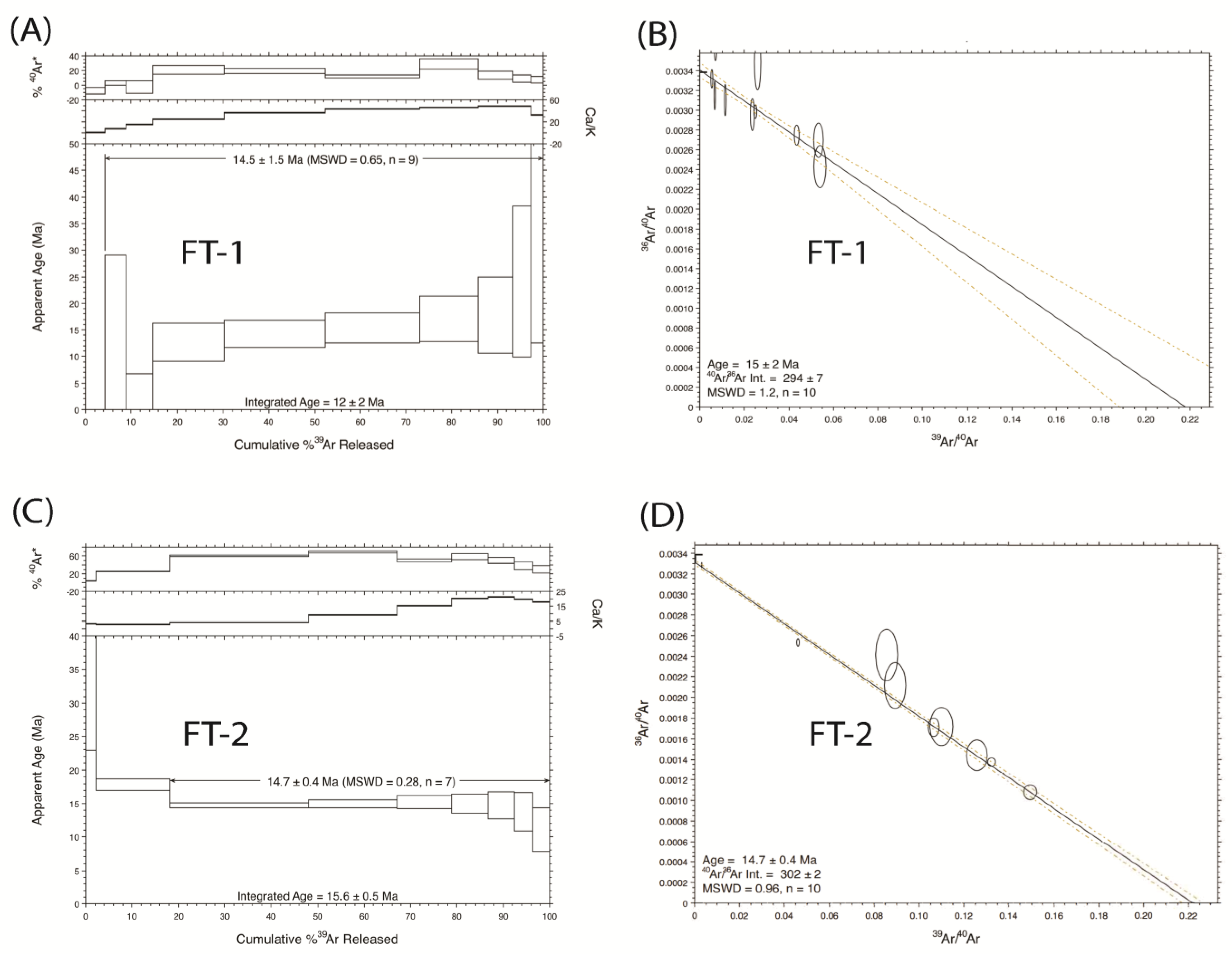
2.2. 40Ar/39Ar Geochronology
2.3. Implications
2.3.1. Miocene Tropical Paradise
2.3.2. Zhangpu Biomass and the Middle Miocene Climatic Optimum
3. The Oldest Known Amber in China
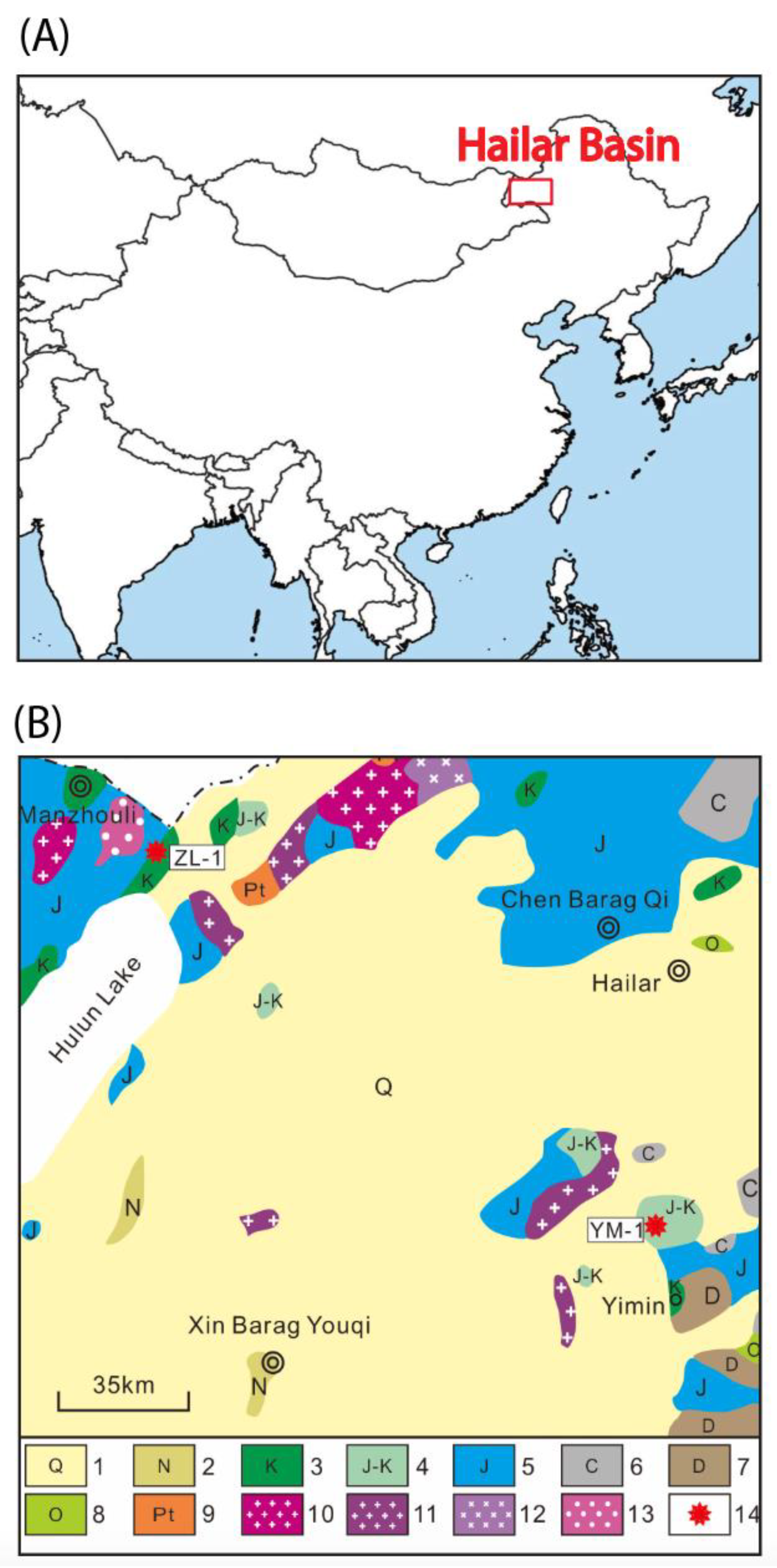
3.1. Hailar Amber in Inner Mongolia
3.2. U–Pb Geochronology
3.3. Clues to Cretaceous Greenhouse Climate
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D. Pushing back amber production. Science 2009, 326, 51–52. [Google Scholar] [CrossRef]
- Grimaldi, D.A. Amber. Curr. Biol. 2019, 29, R861–R862. [Google Scholar] [CrossRef]
- Riddle, J.M. AMBER in ancient pharmacy: The transmission of information about a single drug: A case study. Pharm. Hist. 1973, 15, 3–17. [Google Scholar] [PubMed]
- Causey, F. Amber and the Ancient World; Getty Publications: Los Angeles, CA, USA, 2011. [Google Scholar]
- Creamer, P. A Comparison of Resinous Artifacts in the Ancient Near East. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2014. [Google Scholar]
- De Navarro, J.M. Prehistoric routes between Northern Europe and Italy defined by the amber trade. Geogr. J. 1925, 66, 481–503. [Google Scholar] [CrossRef]
- Todd, J.M.; Eichel, M.H. New evidence of Baltic-Adriatic amber trade. J. Balt. Stud. 1976, 7, 330–342. [Google Scholar] [CrossRef]
- Laufer, B. Historical jottings on amber in Asia. Mem. Am. Anthropol. Assoc. 1906, 1, 211–244. [Google Scholar]
- Qin, C.; Sun, A. A tentative identification and sources investigation of China’s ancient amber beads. Acta Petrol. Mineral. 2016, 35, 127–136, (In Chinese with English Abstract). [Google Scholar]
- Beck, C.W. Authentication and conservation of amber: Conflict of interests. Stud. Conserv. 1982, 27 (Suppl. S1), 104–107. [Google Scholar] [CrossRef]
- Bonfante, G. The word for amber in Baltic, Latin, Germanic, and Greek. J. Balt. Stud. 1985, 16, 316–319. [Google Scholar] [CrossRef]
- Poinar, G.O. The range of life in amber: Significance and implications in DNA studies. Experientia 1994, 50, 536–542. [Google Scholar] [CrossRef]
- Iturralde-Vinent, M.A.; MacPhee, R.D.E. Age and paleogeographical origin of Dominican amber. Science 1996, 273, 1850–1852. [Google Scholar] [CrossRef]
- Ross, A. Palaeontology: Chinese amber insects bridge the gap. Curr. Biol. 2014, 24, R642–R643. [Google Scholar] [CrossRef]
- Rust, J.; Singh, H.; Rana, R.S.; McCann, T.; Singh, L.; Anderson, K.; Sarkar, N.; Nascimbene, P.C.; Stebner, F.; Grimaldi, D.; et al. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. Proc. Natl. Acad. Sci. USA 2010, 107, 18360–18365. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, B.; Jarzembowski, E.A.; Chang, S.C.; Nel, A. Burmadysagrioninae, a new subfamily (Odonata: Zygoptera: Dysagrionidae) from mid-Cretaceous Burmese amber. Cretac. Res. 2016, 67, 126–132. [Google Scholar] [CrossRef]
- Zheng, D. Odonatans in lowermost Cenomanian Kachin amber: Updated review and a new hemiphlebiid damselfly. Cretac. Res. 2021, 118, 104640. [Google Scholar] [CrossRef]
- Wang, B.; Rust, J.; Engel, M.S.; Szwedo, J.; Dutta, S.; Nel, A.; Fan, Y.; Meng, F.; Shi, G.; Zhang, H.; et al. A diverse paleobiota in Early Eocene Fushun amber from China. Curr. Biol. 2014, 24, 1606–1610. [Google Scholar] [CrossRef]
- Xing, L.; McKellar, R.C.; Xu, X.; Li, G.; Bai, M.; Persons, W.S.; Miyashita, T.; Benton, M.J.; Zhang, J.; Currie, P.J.; et al. A feathered dinosaur tail with primitive plumage trapped in mid-Cretaceous amber. Curr. Biol. 2016, 26, 3352–3360. [Google Scholar] [CrossRef]
- Zheng, D.; Nel, A.; Jarzembowski, E.A.; Chang, S.C.; Zhang, H.; Wang, B. Exceptionally well-preserved dragonflies (Insecta: Odonata) in Mexican amber. Alcheringa Australas. J. Palaeontol. 2019, 43, 157–164. [Google Scholar] [CrossRef]
- Yu, T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.; Zhang, H.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Natl. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef]
- Zheng, D.; Nel, A.; Jarzembowski, E.A.; Chang, S.C.; Zhang, H.; Xia, F.; Wang, B.; Dilcher, D.; Wang, B. Extreme adaptations for probable visual courtship behaviour in a Cretaceous dancing damselfly. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Zheng, D.; Chang, S.C.; Jarzembowski, E.A.; Wang, B. The first aeshnoid dragonfly (Odonata: Anisoptera: Telephlebiidae) from mid-Cretaceous Burmese amber. Cretac. Res. 2017, 72, 105–109. [Google Scholar] [CrossRef]
- Jiang, T.; Szwedo, J.; Wang, B. A unique camouflaged mimarachnid planthopper from mid-Cretaceous Burmese amber. Sci. Rep. 2019, 9, 13112. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, D.; Agosti, D. A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proc. Natl. Acad. Sci. USA 2000, 97, 13678–13683. [Google Scholar] [CrossRef] [PubMed]
- Perrichot, V.; Marion, L.; Néraudeau, D.; Vullo, R.; Tafforeau, P. The early evolution of feathers: Fossil evidence from Cretaceous amber of France. Proc. R. Soc. B Biol. Sci. 2008, 275, 1197–1202. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Peñalver, E. A mantidfly in Cretaceous Spanish amber provides insights into the evolution of integumentary specialisations on the raptorial foreleg. Sci. Rep. 2019, 9, 13248. [Google Scholar] [CrossRef]
- Zhao, Z.; Shih, C.; Gao, T.; Ren, D. Termite communities and their early evolution and ecology trapped in Cretaceous amber. Cretac. Res. 2021, 117, 104612. [Google Scholar] [CrossRef]
- Grimaldi, D.A. The age of Dominican amber. In Amber, Resinite, and Fossil Resins, ACS Symposium Series; Anderson, K.B., Crelling, J.C., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 203–217. [Google Scholar]
- Poinar, G.O.; Poinar, R. The Amber Forest: A Reconstruction of a Vanished World; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Smejkal, G.B.; Poinar, G.O.; Righetti, P.G. Will amber inclusions provide the first glimpse of a Mesozoic proteome? Expert Rev. Proteom. 2009, 6, 1–4. [Google Scholar] [CrossRef]
- Daza, J.D.; Stanley, E.L.; Wagner, P.; Bauer, A.M.; Grimaldi, D.A. Mid-Cretaceous amber fossils illuminate the past diversity of tropical lizards. Sci. Adv. 2016, 2, e1501080. [Google Scholar] [CrossRef]
- Stigall, A.L.; Bauer, J.E.; Lam, A.R.; Wright, D.F. Biotic immigration events, speciation, and the accumulation of biodiversity in the fossil record. Glob. Planet. Chang. 2017, 148, 242–257. [Google Scholar] [CrossRef]
- Poinar, G., Jr. Palaeoecological perspectives in Dominican amber. Ann. De La Société Entomol. De Fr. 2010, 46, 23–52. [Google Scholar] [CrossRef]
- Iturralde-Vinent, M.A.; MacPhee, R.D. Remarks on the age of Dominican amber. Palaeoentomology 2019, 2, 236–240. [Google Scholar] [CrossRef]
- Alonso, J.; Arillo, A.; Barrón, E.; Corral, J.C.; Grimalt, J.; López, J.F.; López, R.; Martínez-Delclòs, X.; Ortuño, V.; Trincão, P.R.; et al. A new fossil resin with biological inclusions in Lower Cretaceous deposits from Álava (Northern Spain, Basque-Cantabrian Basin). J. Paleontol. 2000, 74, 158–178. [Google Scholar] [CrossRef]
- Azar, D.; Nel, A.; Solignac, M.; Paicheler, J.C.; Bouchet, F. New genera and species of psychodoid flies from the Lower Cretaceous amber Lebanon. Palaeontology 1999, 42, 1101–1136. [Google Scholar] [CrossRef]
- Bouju, V.; Perrichot, V. A review of amber and copal occurrences in Africa and their paleontological significance. Bull. Société Géologique Fr. 2020, 191, 17. [Google Scholar] [CrossRef]
- Chou, C.Y.; Xing, L.D. Vertebrate remains in amber around the world. Acta Palaeontol. Sin. 2020, 59, 30–42. (In Chinese) [Google Scholar]
- Dutta, S.; Mallick, M.; Bertram, N.; Greenwood, P.F.; Mathews, R.P. Terpenoid composition and class of Tertiary resins from India. Int. J. Coal Geol. 2009, 80, 44–50. [Google Scholar] [CrossRef]
- McKellar, R.C.; Wolfe, A.P.; Penney, D. Canadian amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Siri Scientific Press: Rochdale, UK, 2010; pp. 149–166. [Google Scholar]
- Melinte-Dobrinescu, M.C.; Brustur, T.; Jipa, D.; Macaleţ, R.; Ion, G.; Ion, E.; Popa, A.; Stănescu, I.; Briceag, A. The geological and palaeontological heritage of the Buzău Land Geopark (Carpathians, Romania). Geoheritage 2017, 9, 225–236. [Google Scholar] [CrossRef]
- Nel, A.; De Ploëg, G.; Millet, J.; Menier, J.J.; Waller, A. The French ambers: A general conspectus and the Lowermost Eocene amber deposit of Le Quesnoy in the Paris Basin. Geol. Acta Int. Earth Sci. J. 2004, 2, 3–8. [Google Scholar]
- Nel, A.; de Plöeg, G.; Dejax, J.; Dutheil, D.; de Franceschi, D.; Gheerbrant, E.; Godinot, M.; Hervet, S.; Menier, J.-J.; Rage, J.C.; et al. Un gisement sparnacien exceptionnel à plantes, arthropodes et vertébrés (Éocène basal, MP7): Le Quesnoy (Oise, France). Comptes Rendus De L’académie Des Sci.-Ser. IIA-Earth Planet. Sci. 1999, 329, 65–72. [Google Scholar] [CrossRef]
- Pereira, R.; de Souza Carvalho, I.; Simoneit, B.R.; de Almeida Azevedo, D. Molecular composition and chemosystematic aspects of Cretaceous amber from the Amazonas, Araripe and Recôncavo basins, Brazil. Org. Geochem. 2009, 40, 863–875. [Google Scholar] [CrossRef]
- Zheng, D.; Chang, S.C.; Perrichot, V.; Dutta, S.; Rudra, A.; Mu, L.; Thomson, U.; Li, S.; Zhang, Q.; Wang, B.; et al. A Late Cretaceous amber biota from central Myanmar. Nat. Commun. 2018, 9, 3170. [Google Scholar] [CrossRef] [PubMed]
- Rasnitsyn, A.P.; Bashkuev, A.S.; Kopylov, D.S.; Lukashevich, E.D.; Ponomarenko, A.G.; Popov, Y.A.; Vorontsov, D.D. Sequence and scale of changes in the terrestrial biota during the Cretaceous (based on materials from fossil resins). Cretac. Res. 2016, 61, 234–255. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, D.; Sha, J.; Zhang, H.; Denyszyn, S.; Chang, S.C. Lower Cretaceous Hailar amber: The oldest-known amber from China. Cretac. Res. 2023, 145, 105472. [Google Scholar] [CrossRef]
- Wilde, S.A.; Valley, J.W.; Peck, W.H.; Graham, C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 2001, 409, 175–178. [Google Scholar] [CrossRef]
- Cawood, P.A.; Kroner, A.; Pisarevsky, S. Precambrian plate tectonics: Criteria and evidence. GSA Today 2006, 16, 4. [Google Scholar] [CrossRef]
- Clark, J.D.; Beyene, Y.; Wolde Gabriel, G.; Hart, W.K.; Renne, P.R.; Gilbert, H.; Defleur, A.; Suwa, G.; Katoh, S.; White, T.D.; et al. Stratigraphic, chronological and behavioural contexts of Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature 2003, 423, 747–752. [Google Scholar] [CrossRef]
- Coty, D.; Lebon, M.; Nel, A. When phylogeny meets geology and chemistry: Doubts on the dating of Ethiopian 411 amber. Ann. Société Entomol. Fr. 2016, 52, 161–166. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Perrichot, V.; Svojtka, M.; Anderson, K.B.; Belete, K.H.; Bussert, R.; Dörfelt, H.; Jancke, S.; Mohr, B.; Vávra, N.; et al. Cretaceous African life captured in amber. Proc. Natl. Acad. Sci. USA 2010, 107, 7329–7334. [Google Scholar] [CrossRef]
- Perrichot, V.; Boudinot, B.F.; Chény, C. The age and paleobiota of Ethiopian amber revisited. In Proceedings of the IPC5-5th International Palaeontological Congress, Paris, France, 9–13 July 2018; p. 23. [Google Scholar]
- Smith, T.R.; Szawaryn, K. Pastillus aethiopicusn. sp.(Coleoptera: Cybocephalidae), a new fossil beetle from Miocene Ethiopian amber and a taxonomic key to the species of Pastillus Endrödy-Younga. Palaeoworld 2023, in press. [Google Scholar] [CrossRef]
- Kaddumi, H.F. Amber of Jordan: The Oldest Prehistoric Insects in Fossilized Resin; Eternal River Museum of Natural History: Amman, Jordan, 2007; Volume 224, p. 298. [Google Scholar]
- Bouju, V.; Feldberg, K.; Kaasalainen, U.; Schäfer-Verwimp, A.; Hedenäs, L.; Buck, W.R.; Wang, B.; Perrichot, V.; Schmidt, A.R. Miocene Ethiopian amber: A new source of fossil cryptogams. J. Syst. Evol. 2022, 60, 932–954. [Google Scholar] [CrossRef]
- Zheng, D.; Shi, G.; Hemming, S.R.; Zhang, H.; Wang, W.; Wang, B.; Chang, S.C. Age constraints on a Neogene tropical rainforest in China and its relation to the Middle Miocene Climatic Optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 518, 82–88. [Google Scholar] [CrossRef]
- Wang, H.; Dutta, S.; Kelly, R.S.; Rudra, A.; Li, S.; Zhang, Q.Q.; Wu, Y.-X.; Cao, M.-Z.; Wang, B.; Zhang, H.C.; et al. Amber fossils reveal the Early Cenozoic dipterocarp rainforest in central Tibet. Palaeoworld 2018, 27, 506–513. [Google Scholar] [CrossRef]
- Shi, G.; Dutta, S.; Paul, S.; Wang, B.; Jacques, F.M. Terpenoid compositions and botanical origins of Late Cretaceous and Miocene amber from China. PLoS ONE 2014, 9, e111303. [Google Scholar] [CrossRef]
- Shi, G.; Li, H. A fossil fruit wing of Dipterocarpus from the middle Miocene of Fujian, China and its palaeoclimatic significance. Rev. Palaeobot. Palynol. 2010, 162, 599–606. [Google Scholar] [CrossRef]
- Shi, G.; Jacques, F.M.; Li, H. Winged fruits of Shorea (Dipterocarpaceae) from the Miocene of Southeast China: Evidence for the northward extension of dipterocarps during the Mid-Miocene Climatic Optimum. Rev. Palaeobot. Palynol. 2014, 200, 97–107. [Google Scholar] [CrossRef]
- Jacques, F.M.; Shi, G.; Su, T.; Zhou, Z. A tropical forest of the middle Miocene of Fujian (SE China) reveals Sino-Indian biogeographic affinities. Rev. Palaeobot. Palynol. 2015, 216, 76–91. [Google Scholar] [CrossRef]
- Wang, B.; Shi, G.; Xu, C.; Spicer, R.A.; Perrichot, V.; Schmidt, A.R.; Feldberg, K.; Heinrichs, J.; Chény, C.; Engel, M.S.; et al. The mid-Miocene Zhangpu biota reveals an outstandingly rich rainforest biome in East Asia. Sci. Adv. 2021, 7, eabg0625. [Google Scholar] [CrossRef]
- Zheng, Y. Marginipollis (Lecythidaceae) from the upper Tertiary Fotan group in southern Fujian. Acta Palaeontol. Sin. 1984, 23, 764–767, (In Chinese with English Abstract). [Google Scholar]
- Zheng, Y. Fossil pollen grains of Podocarpaceae from Upper Tertiary in Fujian. Acta Palaeontol. Sin. 1987, 26, 604–615, (In Chinese with English Abstract). [Google Scholar]
- Ho, K.S.; Chen, J.C.; Lo, C.H.; Zhao, H.L. 40Ar–39Ar dating and geochemical characteristics of late Cenozoic basaltic rocks from the Zhejiang–Fujian region, SE China: Eruption ages, magma evolution and petrogenesis. Chem. Geol. 2003, 197, 287–318. [Google Scholar] [CrossRef]
- Chang, S.C.; Zhang, H.; Hemming, S.R.; Mesko, G.T.; Fang, Y. Chronological evidence for extension of the Jehol Biota into Southern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 344, 1–5. [Google Scholar] [CrossRef]
- Chang, S.C.; Hemming, S.R.; Gao, K.Q.; Zhou, C.F. 40Ar/39Ar age constraints on Cretaceous fossil-bearing formations near the China–North Korea border. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 396, 93–98. [Google Scholar] [CrossRef]
- Engel, M.S.; Herhold, H.; Davis, S.; Wang, B.; Thomas, J. Stingless bees in Miocene amber of southeastern China (Hymenoptera: Apidae). J. Melittology 2021, 105, 1–83. [Google Scholar] [CrossRef]
- Brazidec, M.; Perrichot, V. The first fossil Parascleroderma (Hymenoptera: Bethylidae): A new species in mid-Miocene Zhangpu amber. Palaeoworld 2022, in press. [Google Scholar] [CrossRef]
- Wang, H.; Lei, X.J.; Luo, C.H.; Dunlop, J.A. First jumping spider (Araneae: Salticidae) from mid-Miocene Zhangpu amber. Palaeoworld 2022, in press. [Google Scholar] [CrossRef]
- Beurel, S.; Bachelier, J.B.; Hammel, J.U.; Shi, G.L.; Wu, X.T.; Rühr, P.T.; Sadowski, E.M. Flower inclusions of Canarium (Burseraceae) from Miocene Zhangpu amber (China). Palaeoworld 2023, in press. [Google Scholar] [CrossRef]
- Holbourn, A.; Kuhnt, W.; Kochhann, K.G.; Andersen, N.; Sebastian Meier, K.J. Global perturbation of the carbon cycle at the onset of the Miocene Climatic Optimum. Geology 2015, 43, 123–126. [Google Scholar] [CrossRef]
- Kasbohm, J.; Schoene, B. Rapid eruption of the Columbia River flood basalt and correlation with the mid-Miocene climate optimum. Sci. Adv. 2018, 4, eaat8223. [Google Scholar] [CrossRef]
- Böhme, M. The Miocene climatic optimum: Evidence from ectothermic vertebrates of Central Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 195, 389–401. [Google Scholar] [CrossRef]
- Croft, D.A.; Carlini, A.A.; Ciancio, M.R.; Brandoni, D.; Drew, N.E.; Engelman, R.K.; Anaya, F. New mammal faunal data from Cerdas, Bolivia, a middle-latitude Neotropical site that chronicles the end of the Middle Miocene Climatic Optimum in South America. J. Vertebr. Paleontol. 2016, 36, e1163574. [Google Scholar] [CrossRef]
- Azar, D.; Maksoud, S.; Cai, C.; Huang, D. A new amber outcrop from the Lower Cretaceous of northeastern China. Palaeoentomology 2019, 2, 345–349. [Google Scholar] [CrossRef]
- Azar, D. Preservation and accumulation of biological inclusions in Lebanese amber and their significance. Comptes Rendus Palevol 2007, 6, 151–156. [Google Scholar] [CrossRef]
- Arillo, A.; Nel, A. Two new fossil cecidomyiids flies from the Lower Cretaceous amber of Alava (Spain) (Diptera, Cecidomyiidae). Bull. De La Soc. Entomol. De Fr. 2000, 105, 285–288. [Google Scholar] [CrossRef]
- Ji, Z.; Meng, Q.A.; Wan, C.B.; Zhu, D.F.; Ge, W.C.; Zhang, Y.L.; Yang, H.; Dong, Y. Geodynamic evolution of flat-slab subduction of Paleo-Pacific Plate: Constraints from Jurassic adakitic lavas in the Hailar Basin, NE China. Tectonics 2019, 38, 4301–4319. [Google Scholar] [CrossRef]
- Min-Na, A.; Zhang, F.Q.; Yang, S.F.; Chen, H.L.; Batt, G.E.; Sun, M.D.; Meng, Q.-A.; Zhu, D.-F.; Cao, R.-C.; Li, J.S. Early Cretaceous provenance change in the southern Hailar Basin, northeastern China, and its implication for basin evolution. Cretac. Res. 2013, 40, 21–42. [Google Scholar]
- Wang, J.; Chang, S.C.; Chen, Y.; Yan, S. Early Cretaceous transpressional and transtensional tectonics straddling the Sulu orogenic belt, East China. Geosci. Front. 2019, 10, 2287–2300. [Google Scholar] [CrossRef]
- Barron, E.J.; Washington, W.M. Cretaceous climate: A comparison of atmospheric simulations with the geologic record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1982, 40, 103–133. [Google Scholar] [CrossRef]
- Barron, E.J.; Fawcett, P.J.; Peterson, W.H.; Pollard, D.; Thompson, S.L. A “simulation” of mid-Cretaceous climate. Paleoceanography 1995, 10, 953–962. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Brinkman, D.B.; Renne, P.R.; Cottrell, R.D.; Scher, H.; Castillo, P. Evidence for extreme climatic warmth from Late Cretaceous Arctic vertebrates. Science 1998, 282, 2241–2243. [Google Scholar] [CrossRef]
- Haq, B.U.; Hardenbol, J.A.N.; Vail, P.R. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar] [CrossRef]
- Frakes, L.A.; Francis, J.E.; Syktus, J.I. Climate Modes of the Phanerozoic; Cambridge University Press: Cambridge, UK, 1992; p. 286. [Google Scholar]
- Van de Schootbrugge, B.; Föllmi, K.B.; Bulot, L.G.; Burns, S.J. Paleoceanographic changes during the early Cretaceous (Valanginian–Hauterivian): Evidence from oxygen and carbon stable isotopes. Earth Planet. Sci. Lett. 2000, 181, 15–31. [Google Scholar] [CrossRef]
- Wissler, L.; Funk, H.; Weissert, H. Response of Early Cretaceous carbonate platforms to changes in atmospheric carbon dioxide levels. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 200, 187–205. [Google Scholar] [CrossRef]
- Huber, B.T.; Norris, R.D.; MacLeod, K.G. Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 2002, 30, 123–126. [Google Scholar] [CrossRef]
- Berner, R.A.; Kothavala, Z. GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 2001, 301, 182–204. [Google Scholar] [CrossRef]
- Huber, B.T.; MacLeod, K.G.; Watkins, D.K.; Coffin, M.F. The rise and fall of the Cretaceous Hot Greenhouse climate. Glob. Planet. Change 2018, 167, 1–23. [Google Scholar] [CrossRef]
- Bryan, S.E.; Constantine, A.E.; Stephens, C.J.; Ewart, A.; Schön, R.W.; Parianos, J. Early Cretaceous volcano-sedimentary successions along the eastern Australian continental margin: Implications for the breakup of eastern Gondwana. Earth Planet. Sci. Lett. 1997, 153, 85–102. [Google Scholar] [CrossRef]
- Larson, R.L.; Erba, E. Onset of the Mid-Cretaceous greenhouse in the Barremian-Aptian: Igneous events and the biological, sedimentary, and geochemical responses. Paleoceanography 1999, 14, 663–678. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, Y.; Jiang, D.; Lin, S. Rapid change from compression to extension in the North China Craton during the Early Cretaceous: Evidence from the Yunmengshan metamorphic core complex. Tectonophysics 2015, 656, 91–110. [Google Scholar] [CrossRef]
- Pan, Y.; Sha, J.; Fuersich, F.T.; Wang, Y.; Zhang, X.; Yao, X. Dynamics of the lacustrine fauna from the Early Cretaceous Yixian Formation, China: Implications of volcanic and climatic factors. Lethaia 2012, 45, 299–314. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Sun, B.; Quan, C.; Wu, J.; Lin, Z. Paleo-CO2 variation trends and the Cretaceous greenhouse climate. Earth-Sci. Rev. 2014, 129, 136–147. [Google Scholar] [CrossRef]
- Azar, F.; Mullet, E.; Vinsonneau, G. The propensity to forgive: Findings from Lebanon. J. Peace Res. 1999, 36, 169–181. [Google Scholar] [CrossRef]
- Azar, D.; Gèze, R.; Acra, F.; Penney, D. Lebanese amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Siri Scientific Press: Rochdale, UK, 2010; pp. 271–298. [Google Scholar]
- Maksoud, S.; Azar, D.; Granier, B.; Gèze, R. New data on the age of the Lower Cretaceous amber outcrops of Lebanon. Palaeoworld 2017, 26, 331–338. [Google Scholar] [CrossRef]
- Nicholas, C.J.; Henwood, A.A.; Simpson, M. A new discovery of early Cretaceous (Wealden) amber from the Isle of Wight. Geol. Mag. 1993, 130, 847–850. [Google Scholar] [CrossRef]
- Selden, P.A. First British Mesozoic spider, from Cretaceous amber of the Isle of Wight, southern England. Palaeontology 2002, 45, 973–983. [Google Scholar] [CrossRef]
- Austen, P.A.; Batten, D.J. English Wealden fossils: An update. Proc. Geol. Assoc. 2018, 129, 171–201. [Google Scholar] [CrossRef]
- Allen, P.; Alvin, K.L.; Andrews, J.E.; Batten, D.J.; Charlton, W.A.; Cleevely, R.J.; Ensom, P.C.; Evans, S.E.; Francis, J.E.; Banham, G.H.; et al. Purbeck–Wealden (early Cretaceous) climates. Proc. Geol. Assoc. 1998, 109, 197–236. [Google Scholar] [CrossRef]
- Coram, R.A.; Jarzembowski, E.A. Immature Insect Assemblages from the Early Cretaceous (Purbeck/Wealden) of Southern England. Insects 2021, 12, 942. [Google Scholar] [CrossRef]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Vermeesch, P. Dissimilarity measures in detrital geochronology. Earth-Sci. Rev. 2018, 178, 310–321. [Google Scholar] [CrossRef]
- Andersen, T.; Elburg, M.A.; Magwaza, B.N. Sources of bias in detrital zircon geochronology: Discordance, concealed lead loss and common lead correction. Earth-Sci. Rev. 2019, 197, 102899. [Google Scholar] [CrossRef]
- Chang, S.C.; Dassanayake, S.; Wang, J. Preliminary analysis of detrital zircon U–Pb ages from the fossil-rich Tabbowa beds, Sri Lanka. Palaeoworld 2017, 26, 396–402. [Google Scholar] [CrossRef]
- Spicer, R.A.; Su, T.; Valdes, P.J.; Farnsworth, A.; Wu, F.X.; Shi, G.; Spicer, T.E.V.; Zhou, Z. The topographic evolution of the Tibetan Region as revealed by palaeontology. Palaeobiodivers. Palaeoenviron. 2021, 101, 213–243. [Google Scholar] [CrossRef]
- Liu, J.; Su, T.; Spicer, R.A.; Tang, H.E.; Wu, F.X.; Srivastava, G.; Spicer, T.; Van Do, T.; Deng, T.; Zhou, Z.K.; et al. Biotic interchange through lowlands of Tibetan Plateau suture zones during Paleogene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 524, 33–40. [Google Scholar] [CrossRef]
- Deng, T.; Wang, S.; Xie, G.; Li, Q.; Hou, S.; Sun, B. A mammalian fossil from the Dingqing Formation in the Lunpola Basin, northern Tibet, and its relevance to age and paleo-altimetry. Chin. Sci. Bull. 2012, 57, 261–269. [Google Scholar] [CrossRef]
- Perkovsky, E.E.; Zosimovich, V.Y.; Vlaskin, A.P. Rovno Amber; Siri Scientific Press: Manchester, UK, 2010; pp. 116–136. [Google Scholar]
- Jones, E.R.; Zarina, G.; Moiseyev, V.; Lightfoot, E.; Nigst, P.R.; Manica, A.; Pinhasi, R.; Bradley, D.G. The Neolithic transition in the Baltic was not driven by admixture with early European farmers. Curr. Biol. 2017, 27, 576–582. [Google Scholar] [CrossRef]
- Weitschat, W.; Wichard, W.; Penney, D. Baltic amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Siri Scientific Press: Rochdale, UK, 2010; pp. 80–115. [Google Scholar]
- Perkovsky, E.E.; Rasnitsyn, A.P.; Vlaskin, A.P.; Taraschuk, M.V. A comparative analysis of the Baltic and Rovno amber arthropod faunas: Representative samples. Afr. Invertebr. 2007, 48, 229–245. [Google Scholar]
- Wolfe, A.P.; Tappert, R.; Muehlenbachs, K.; Boudreau, M.; McKellar, R.C.; Basinger, J.F.; Garrett, A. A new proposal concerning the botanical origin of Baltic amber. Proc. R. Soc. B Biol. Sci. 2009, 276, 3403–3412. [Google Scholar] [CrossRef]
- Bogri, A.; Solodovnikov, A.; Żyła, D. Baltic amber impact on historical biogeography and palaeoclimate research: Oriental rove beetle Dysanabatium found in the Eocene of Europe (Coleoptera, Staphylinidae, Paederinae). Pap. Palaeontol. 2018, 4, 433–452. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Penney, D. Bitterfeld amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Siri Scientific Press: Rochdale, UK, 2010; pp. 57–68. [Google Scholar]
- Bukejs, A.; Alekseev, V.I.; Pollock, D.A. Waidelotinae, a new subfamily of Pyrochroidae (Coleoptera: Tenebrionoidea) from Baltic amber of the Sambian peninsula and the interpretation of Sambian amber stratigraphy, age and location. Zootaxa 2019, 4664, 261–273. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-C.; Li, Y.; Zheng, D. Dating Amber: Review and Perspective. Minerals 2023, 13, 948. https://doi.org/10.3390/min13070948
Chang S-C, Li Y, Zheng D. Dating Amber: Review and Perspective. Minerals. 2023; 13(7):948. https://doi.org/10.3390/min13070948
Chicago/Turabian StyleChang, Su-Chin, Yuling Li, and Daran Zheng. 2023. "Dating Amber: Review and Perspective" Minerals 13, no. 7: 948. https://doi.org/10.3390/min13070948
APA StyleChang, S.-C., Li, Y., & Zheng, D. (2023). Dating Amber: Review and Perspective. Minerals, 13(7), 948. https://doi.org/10.3390/min13070948






